Abstract
Quorum sensing (QS) has been previously shown to play an important role in the development of Pseudomonas aeruginosa biofilms (D. G. Davies et al., Science 280:295-298, 1998). Although QS regulation of swarming and DNA release has been shown to play important roles in biofilm development, regulation of genes directly involved in biosynthesis of biofilm matrix has not been described. Here, transcription of the pel operon, essential for the production of a glucose-rich matrix exopolysaccharide, is shown to be greatly reduced in lasI and rhlI mutants. Chemical complementation of the lasI mutant with 3-oxo-dodecanoyl homoserine lactone restores pel transcription to the wild-type level and biofilm formation ability. These findings thus connect QS signaling and transcription of genes responsible for biofilm matrix biosynthesis.
Bacteria form biofilms, matrix-enclosed multicellular assemblages that appear to provide increased survival ability under various stress conditions (3, 4). Cell-cell communication or quorum sensing (QS) has been shown to be involved in the formation of biofilm in several bacterial species (reviewed in references 11 and 12). Pseudomonas aeruginosa, a gram-negative and opportunistic human pathogen, possesses two hierarchical QS systems known as las and rhl (10, 15, 19). Upon increase in the population density, the concentration of the signaling molecule 3-oxo-dodecanoyl (3-O-C12) homoserine lactone increases and is able to form a complex with LasR, a transcriptional regulator. This complex activates the transcription of lasI, rhlR, and a number of other genes. In a seminal paper that first indicated a role for cell-cell signaling in biofilm formation, Davies et al. showed that mutants defective in the Las QS system yielded unstructured and frail biofilms (5). Recent studies have demonstrated that QS-dependent regulation of swarming and DNA release play important roles in biofilm development of P. aeruginosa (1, 16). Because mutants lacking the Las QS system form defective biofilms that are completely flat and detergent sensitive and because the Las QS system controls expression of a large set of genes, it is highly likely that the biofilm-defective phenotype of the mutants is multifactorial. This includes above-mentioned defects in swarming, DNA release, and possibly defects in the production of other structural components of biofilm matrix.
Recently, our laboratory identified a cluster of P. aeruginosa genes, termed pel, whose products are responsible for the production of glucose-rich biofilm matrix exopolysaccharide in strain PA14 (7). Later, an independent study by Vasseur et al. showed that this was also the case in strain PAK (18). The glucose-rich polysaccharide is essential for the formation of a wrinkled colony and a surface-associated biofilm; thus, it is a major component of biofilm in this strain (7). The pel operon contains seven genes, pelA to pelG, that display sequence similarity with genes that encode sugar-processing enzymes. These include oligogalacturonide lyase (pelA), glycosyltransferases (pelC and pelF), sucrose synthase (pelE), and transmembrane proteins (pelD and pelG). Transposon insertion into or deletion of these genes resulted in severe defects in biofilm formation (7, 18).
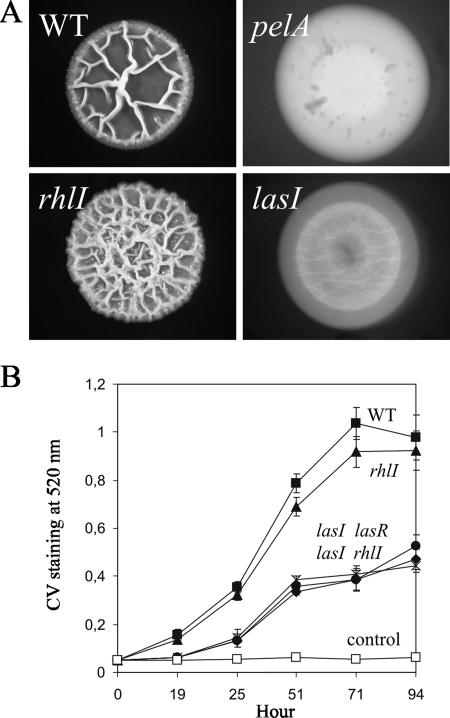
To test whether some genes directly involved in the biosynthesis of a biofilm matrix component might be regulated by QS, we analyzed whether las mutants were defective in the expression of the pel genes. Colony morphology was first observed as an indicator of matrix production (7). Overnight, shaken liquid cultures of P. aeruginosa PA14 and several isogenic mutants, all grown at 37°C, were diluted in tryptone broth without NaCl to a final optical density at 600 nm of 0.0025. Ten microliters of each strain was spotted on tryptone broth lacking NaCl but containing 0.5% agar. After 4 days of incubation at room temperature, the wild-type strain formed a characteristically wrinkled colony (Fig. 1A) that was elastic and resisted breakage when transferred into water and subjected to vigorous vortexing, which is indicative of robust production of the glucose-rich exopolysaccharide produced by the pel gene products (7). The pelA mutant formed a flat colony as previously described (7) (Fig. 1A). The rhlI mutant formed a colony that appeared hyperwrinkled (Fig. 1A); however, the colony easily broke apart into fragments during vigorous vortexing (data not shown), indicating that the matrix structure was altered in the rhlI mutant. In contrast, the lasI mutant formed completely flat colonies, similar to those formed by the pelA mutant (Fig. 1A). We observed these results reproducibly in multiple isolates of these mutants. Because these mutants had been extensively characterized previously, including by genetic complementation, and because of the chemical complementation results described below, we did not carry out genetic complementation analyses here.
FIG. 1.
Biofilm formation of the wild type (WT), pelA mutant, and QS mutants (lasI and rhlI mutants) of P. aeruginosa PA14. (A) Colony morphologies. (B) CV staining of the solid-surface-associated biofilm. The control was medium alone.
A pellicle is a biofilm that assembles at the air-liquid interface of a standing liquid culture and was examined as an independent readout of matrix production. The wild-type strain and the rhlI mutant grown in tryptone broth without NaCl formed thick and visible pellicles by 48 h at room temperature, while the pelA mutant did not form a pellicle, which is consistent with previous results (7) (data not shown). The lasI mutant formed only a thin layer of film that was frail and could easily be broken apart upon gentle shaking (data not shown). These results are in good agreement with colony morphologies and suggested that the lasI mutant is defective in matrix production.
Development of the solid-surface-associated biofilm was examined by using crystal violet (CV) staining (7). Standing cultures in tryptone broth without NaCl in polystyrene microtiter plates were subjected to rigorous washes in water prior to CV staining as described by Friedman and Kolter (7). The wild type showed a dramatic increase of the solid-surface-associated biofilm between 19 h and 71 h (Fig. 1B). The rhlI mutant formed only slightly less solid-surface-associated biofilm than the wild type did (Fig. 1B). On the other hand, the lasI, lasR, and lasI rhlI mutants formed significantly decreased solid-surface-associated biofilm with at least 60% reduction in the CV staining compared to the wild type (Fig. 1B). This level of reduction is very similar to the level of reduction previously reported for the pel mutant (ca. 75%) (7). Taken together, these results indicated that the las QS mutants are defective in biofilm formation characterized by colony morphology, pellicle formation, and the solid-surface-associated biofilm and suggested that the Las QS system could be involved in the regulation of a biofilm matrix component.
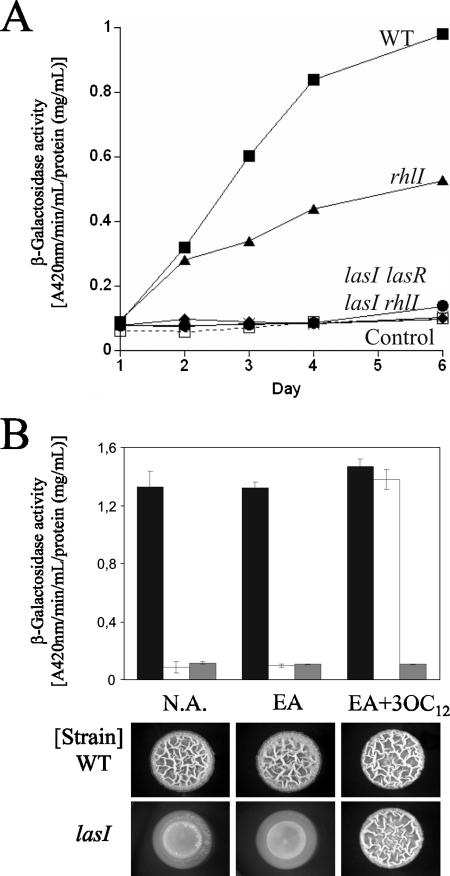
Because the las mutants showed the flat colony morphology, frail thin pellicle, and a complete lack of surface-adhered biofilm similar to those observed for the pelA mutant, we asked whether pelA expression is somehow altered in the las mutants. To test this, a transcriptional fusion composed of the 785-bp upstream sequence of pelA and a promoterless lacZ gene was constructed in the vector mini-CTX-lacZ (2). This construct was introduced as a single copy into the chromosome at the unique attB site (2). Spot colonies were made as described above and harvested at various time points by resuspension in 50 mM sodium phosphate, pH 7.4, and cells were lysed by ultrasonication. β-Galactosidase activities were determined by using cleared supernatants after centrifugation, and the results were normalized to the total protein concentration. The β-galactosidase activity of the wild type increased linearly for the first 4 days and subsequently reached a plateau (Fig. 2A). The rhlI mutant showed a somewhat slower rate of increase of β-galactosidase activity, reaching levels about 50% of those of the wild type (Fig. 2A). In contrast, the lasI and lasR single mutants showed no activity above the background level (Fig. 2A). Likewise, the lasI rhlI double mutant showed no activity (Fig. 2A). These results indicate that transcription of the pel genes is activated by the Las QS signaling system. In addition, it appears that part of the activation is mediated through Rhl QS.
FIG. 2.
Requirement of QS in pelA transcription and wrinkled-colony formation in P. aeruginosa PA14. (A) β-Galactosidase activities of the pel promoter-lacZ transcriptional fusion in the wild type (WT) and QS mutants (lasI, lasR, rhlI, and lasI rhlI mutants). (B) β-Galactosidase activities of the pel promoter-lacZ transcriptional fusion (top panel) and colony morphologies (bottom panel) of the wild type and the lasI mutant in the presence of 10 μM 3-O-C12 homoserine lactone (3OC12). The promoter-less lacZ construct was used as a control. N.A., no autoinducer added; EA, ethyl acetate-glacial acetic acid (1,000:1, vol/vol).
To further confirm the requirement of Las QS for transcription of the pel genes, we carried out a chemical complementation experiment. Synthetic 3-O-C12 homoserine lactone, the autoinducer of the Las QS signaling system, was dissolved in ethyl acetate-glacial acetic acid (1,000:1, vol/vol) and added to a final concentration of 10 μM in tryptone broth agar without NaCl and buffered with 5 mM 3-(N-morpholino)-propanesulfonic acid-NaOH, pH 7.0. Cell suspensions were then spotted on these plates and allowed to grow for 4 days at room temperature. The lasI mutant formed wrinkly colonies only when 3-O-C12-HSL was present in the medium (Fig. 2B). At the same time, β-galactosidase activity was restored to wild-type levels (Fig. 2B). Taken together, these results indicate that the Las QS signaling system activates transcription of the pel genes and in so doing influences biofilm formation by Pseudomonas aeruginosa PA14.
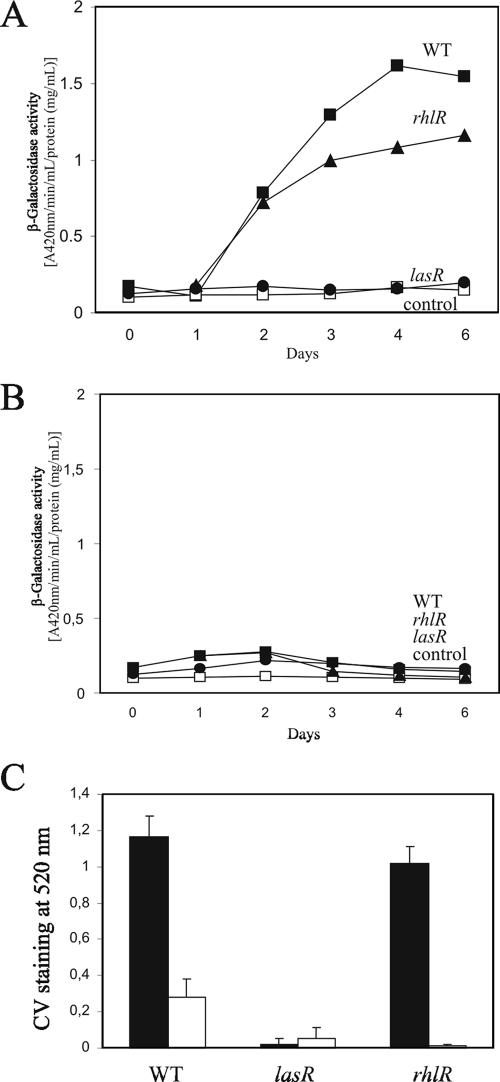
Interestingly, pel transcription was found to be dependent on temperature. β-Galactosidase activity was detected at room temperature (ca. 25°C) (Fig. 3A), whereas little activity was detectable at 37°C in the wild type and in the rhlR mutant (Fig. 3B). No activity was detectable in the lasR mutant at both temperatures (Fig. 3A and B). The formation of the solid-surface-associated biofilm decreased dramatically at 37°C in the wild type and the rhlR mutant (Fig. 3C). These results indicate that pel transcription is subjected to thermocontrol which overrides the transcriptional activation by the Las QS system.
FIG. 3.
Thermoregulation of pel transcription and biofilm formation in P. aeruginosa PA14. (A and B) β-Galactosidase activities of the pel promoter-lacZ transcriptional fusion in the wild type (WT) and QS mutants (lasR and rhlR mutants) at room temperature (A) and at 37°C (B). (C) CV staining of the solid-surface-associated biofilm at room temperature (solid bars) and at 37°C (open bars).
Here we demonstrate that Las QS is involved in transcription of the pel genes and biofilm formation in Pseudomonas aeruginosa PA14, thus providing an additional physical explanation for the involvement of cell-cell signaling in biofilm formation in this bacterium. Aside from swarming and DNA release being dependent on QS (1, 16), our results demonstrate that the expression of the genes whose products are involved in synthesis of a main component of the extracellular matrix, the Pel exopolysaccharide, is regulated by QS.
Our results indicate that Las QS influences pel transcription and that part of the effect, though by no means all, may be through its role in activating the Rhl QS signaling system. Phenotypic outcomes of the rhl mutants, however, were complex. The mutants showed only a slight reduction of the solid-surface-associated biofilms (Fig. 1B) while they formed hyperwrinkled colonies, which were nevertheless frailer than the wild-type colonies (Fig. 1A). It is plausible that, in addition to pel, the Rhl QS system controls as-yet-unknown additional factors that influence biofilm matrix architecture.
We asked whether these QS systems activate pel transcription directly or not. Three pieces of evidence have led us to think it is indirect. First, LasR and RhlR recognition sequences have not been clearly defined, making sequence-based predictions difficult (14). Deletion of a 20-bp sequence that showed significant similarity to the predicted LasR/RhlR binding motif (20) and was located upstream of pelA did not alter its quorum-dependent transcription activity (results not shown). This suggests that the transcription of pel genes may be indirectly regulated by the QS systems, possibly by transcriptional factors whose expression itself is regulated by LasR and RhlR. Second, we observed that pel transcription is under thermoregulation, which overrides the transcriptional activation by the Las QS system (Fig. 3). These results suggest there exists a transcriptional regulator that controls pel expression. Third, results obtained in a recent study suggest a complex regulatory circuit of pel expression, which involves the two-component systems RetS and GacS/GacA, the small RNA molecule RsmZ, and the RNA-binding protein RsmA (8). RetS intercepts the GacS/GacA two-component regulatory circuit (8) that activates transcription of RsmZ (9). RsmZ antagonizes RsmA (9), which is assumed to bind leader sequences of target transcripts and posttranscriptionally represses their expression (6, 9, 17). Interestingly, it has been shown that RsmA negatively regulates lasI and rhlI translation (13). Therefore, it is plausible that pel expression is regulated at two levels, transcriptionally by the as-yet unknown transcriptional regulator that is regulated by the QS systems and posttranscriptionally by RsmA whose activity negatively regulates the QS systems.
Acknowledgments
We thank E. Peter Greenberg and members of the Kolter lab for helpful discussions.
This work was supported by a grant from the NIH (GM58213) to R.K.
Footnotes
Published ahead of print on 11 May 2007.
REFERENCES
- 1.Allesen-Holm, M., K. B. Barken, L. Yang, M. Klausen, J. S. Webb, S. Kjelleberg, S. Molin, M. Givskov, and T. Tolker-Nielsen. 2006. A characterization of the DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 59:1114-1128. [DOI] [PubMed] [Google Scholar]
- 2.Becher, A., and H. P. Schweizer. 2000. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. BioTechniques 29:948-952. [DOI] [PubMed] [Google Scholar]
- 3.Branda, S. S., S. Vik, and R. Kolter. 2005. Biofilms: the matrix revisited. Trends Microbiol. 13:20-26. [DOI] [PubMed] [Google Scholar]
- 4.Costerton, J. W., K. J. Cheng, G. G. Geesey, T. I. Ladd, J. C. Nickel, M. Dasgupta, and T. J. Marrie. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 5.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 6.Dubey, A. K., C. S. Baker, T. Romeo, and P. Babitzke. 2005. RNA sequence and secondary structure participate in high-affinity CsrA-RNA interaction. RNA 11:1579-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman, L., and R. Kolter. 2004. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilm. Mol. Microbiol. 51:675-690. [DOI] [PubMed] [Google Scholar]
- 8.Goodman, A. L., B. Kulasekara, A. Rietsch, R. S. Smith, and S. Lory. 2004. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell 7:745-754. [DOI] [PubMed] [Google Scholar]
- 9.Heurlier, K., F. Williams, S. Heeb, C. Dormond, G. Pessi, D. Singer, M. Camara, P. Williams, and D. Haas. 2004. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J. Bacteriol. 186:2936-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juhas, M., L. Eberl, and B. Tummler. 2005. Quorum sensing: the power of cooperation in the world of Pseudomonas. Environ. Microbiol. 7:459-471. [DOI] [PubMed] [Google Scholar]
- 11.Kirisits, M. J., and M. R. Parsek. 2006. Does Pseudomonas aeruginosa use intercellular signaling to build biofilm communities? Cell. Microbiol. 8:1841-1849. [DOI] [PubMed] [Google Scholar]
- 12.Parsek, M. R., and E. P. Greenberg. 2005. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 13:27-33. [DOI] [PubMed] [Google Scholar]
- 13.Pessi, G., F. Williams, Z. Hindle, K. Heurlier, M. T. Holden, M. Camara, D. Haas, and P. Williams. 2001. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J. Bacteriol. 183:6676-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuster, M., M. L. Urbanowski, and E. P. Greenberg. 2004. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc. Natl. Acad. Sci. USA 101:15833-15839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuster, M., and E. P. Greenberg. 2006. A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 296:73-81. [DOI] [PubMed] [Google Scholar]
- 16.Shrout, J. D., D. L. Chopp, C. L. Just, M. Hentzer, M. Givskov, and M. R. Parsek. 2006. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 62:1264-1277. [DOI] [PubMed] [Google Scholar]
- 17.Romeo, T. 1998. Global regulation by the small RNA-binding protein CsrA and the non-coding RNA molecule CsrB. Mol. Microbiol. 29:1321-1330. [DOI] [PubMed] [Google Scholar]
- 18.Vasseur, P., I. Vallet-Gely, C. Soscia, S. Genin, and A. Filloux. 2005. The pel genes of Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiology 151:985-987. [DOI] [PubMed] [Google Scholar]
- 19.Venturi, V. 2006. Regulation of quorum sensing in Pseudomonas. FEMS Microbiol. Rev. 30:274-291. [DOI] [PubMed] [Google Scholar]
- 20.Whiteley, M., and E. P. Greenberg. 2001. Promoter specificity elements in Pseudomonas aeruginosa quorum-sensing-controlled genes. J. Bacteriol. 183:5529-5534. [DOI] [PMC free article] [PubMed] [Google Scholar]