Abstract
The cyclic AMP (cAMP)-dependent biosynthesis of N-acylphenylalanine antibiotics by NasP, an environmental DNA-derived N-acyl amino acid synthase, is controlled by an NasP-associated cyclic nucleotide-binding domain and is independent of the global cAMP signal transducer, cAMP receptor protein. A 16S rRNA gene sequence found on the same environmental DNA cosmid as NasP is most closely related to 16S sequences from β-proteobacteria.
High-throughput sequencing of DNA extracted directly from environmental samples indicates that uncultured bacteria are likely to be a very rewarding source of interesting new proteins (26). While it is easy to infer the existence of a large number of novel proteins from the data generated by environmental DNA sequencing projects, it has been much more challenging to functionally access these new proteins (12, 13). Screening environmental DNA clones using simple assays that respond to a wide array of stimuli should increase the likelihood of functionally cloning new proteins from environmental DNA and, in turn, identifying new biological phenomena from the genomes of uncultured bacteria. Large-scale screening of environmental DNA libraries for antibacterially active cosmid clones has identified a number of new N-acyl amino acid synthases that confer the production of long-chain N-acyl amino acid antibiotics on Escherichia coli (4-7). The identification of multiple unique N-acyl amino acid-producing clones in environmental DNA libraries suggests that these compounds could play an important, if undefined, role for soil microbes and that their biosynthesis might therefore be highly regulated. Here we report the discovery and characterization of an environmental DNA-derived N-acyl amino acid synthase (NasP) that is directly regulated by cyclic AMP (cAMP), defining a new role for this widely studied universal second messenger.
The first insight into how N-acyl amino acid biosynthesis is regulated in some uncultured bacteria comes from NasP, an environmental DNA-derived N-acyl amino acid synthase that confers the production of long-chain N-acylphenylalanines on Escherichia coli (Fig. 1). While all previously described N-acyl amino acid synthases are predicted to contain one domain, a conserved-domain search with NasP indicated the presence of two domains, an N-terminal N-acyl amino acid synthase domain and a C-terminal cyclic nucleotide-binding domain that is related to both eukaryotic and prokaryotic cAMP-binding domains (14). Signal transduction pathways present in both eukaryotes and prokaryotes employ cAMP as a second messenger. In prokaryotes cAMP is biosynthesized from ATP by an adenylate cyclase and its presence is then detected by cAMP receptor proteins, a small family of transcription factors found in the gamma subdivision of the proteobacteria (2). Although cAMP receptor proteins are thought to be the main cAMP signal transducer in prokaryotes, non-cAMP receptor protein-associated cAMP-binding domains have been found in the genomes of many sequenced bacteria (10, 15, 17). If these domains prove to be functional, it would indicate that cAMP directly regulates a broad range of enzymatic activities in bacteria. The functional cloning of NasP from environmental DNA provides an opportunity to confirm a new role for cAMP-binding domains in bacteria and to investigate the mechanism by which N-acyl amino acid biosynthesis is regulated in some uncultured bacteria.
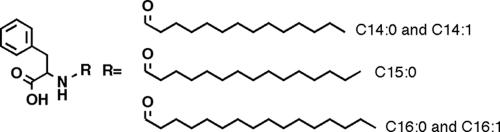
FIG. 1.
Ethyl acetate extracts derived from cultures of E. coli that express NasP contain long-chain N-acylphenylalanines derivatized with both fully saturated and monounsaturated fatty acid side chains ranging from 14 to 16 carbons in length.
The environmental DNA cosmid clone CSL142, from which NasP was cloned, was originally identified in a high-throughput screen for antibacterially active environmental DNA clones (3). One- and two-dimensional nuclear magnetic resonance analysis of the antibacterially active material purified from ethyl acetate extracts of this clone indicated that the active material was a mixture of long-chain N-acylphenylalanines (see the supplemental material). The major clone-specific compounds present in the extracts were analyzed by high-resolution mass spectrometry and found to contain both fully saturated and monounsaturated fatty acid side chains ranging from 14 to 16 carbons in length (Fig. 1). Sequencing of transposed cosmids that no longer conferred antibacterial activity on E. coli indicated that a single open reading frame (NasP) with an N-terminal N-acyl amino acid synthase domain and a C-terminal cyclic nucleotide-binding domain was responsible for the production of these compounds. Sequences that show significant sequence identity (>20%) to NasP in a BLAST search are all hypothetical proteins of unknown function (NP_924045 and NP_924043 from Gloeobacter violaceus PCC 7421 and ZP_01079393 from Synechococcus sp. strain RS9917).
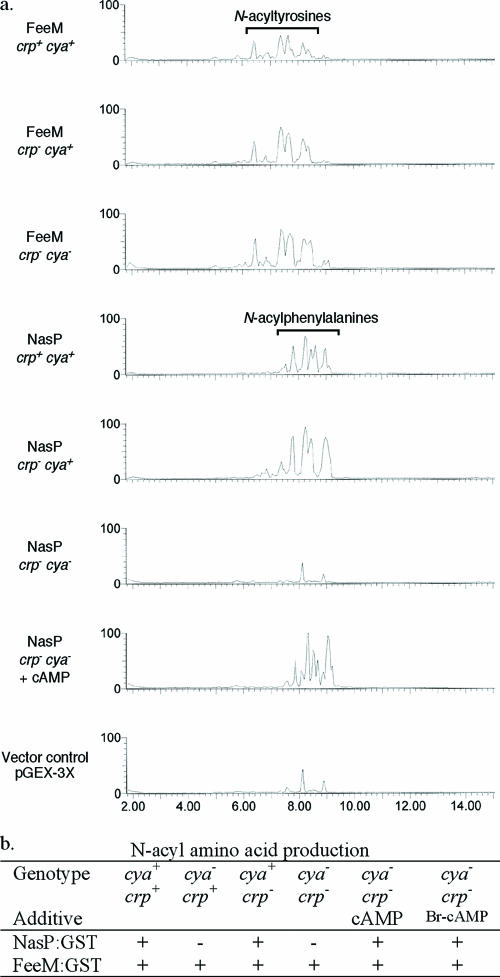
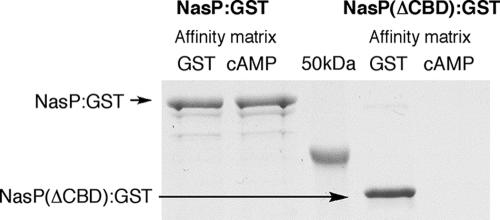
The role of the NasP-associated cAMP-binding domain in the regulation of N-acylphenylalanine biosynthesis was examined using NasP expressed as a glutathione S-transferase fusion protein (NasP:GST) in adenylate cyclase (cya)- and cAMP receptor protein (crp)-deficient strains of E. coli (Fig. 2). The GST fusion construct of an N-acyl amino acid synthase that does not contain a cAMP-binding domain, FeeM:GST, was used as the positive control in these studies (5). Both constructs confer the production of N-acyl amino acids on E. coli strains lacking the cAMP receptor protein; however, in adenylate cyclase mutants where no cAMP is present, only the control construct continues to confer the production of N-acyl amino acids on E. coli (Fig. 2). The addition of cAMP (1 mM) to cya crp double mutants transformed with NasP:GST restored the ability of this construct to confer the production of N-acylphenylalanines on the E. coli host (Fig. 2). The biosynthesis of N-acyl amino acids is therefore cAMP dependent yet independent of the cAMP receptor protein. A direct interaction between NasP and cAMP was confirmed by affinity chromatography using cAMP-presenting affinity resin (Fig. 3). Removal of the proposed cyclic nucleotide-binding domain from this construct abrogated binding to the cAMP affinity resin but did not prevent affinity purification using the GST affinity resin (Fig. 3).
FIG. 2.
(a) To assess the role of cAMP and the cAMP receptor protein in the production of N-acyl amino acids, NasP was transformed into cya+ crp+ (EC100), cya crp+ (SP850) (22), cya+ crp (CA8445-1) (8), and cya crp (CA8439) (19) strains of E. coli and then assayed (by liquid chromatography-mass spectrometry) for the ability to confer the production of N-acyl amino acids on the E. coli host. (b) The addition of cAMP to the culture medium activates the production of N-acyl amino acids by NasP in cya-deficient strains of E. coli. No peaks corresponding to N-acylphenylalanines or N-acyltyrosines were detected in vector control extracts.
FIG. 3.
NasP:GST binds both GST and cAMP affinity resins. When the proposed cAMP-binding domain is removed by deleting amino acids 272 to 393, the new construct [NasP(ΔCBD):GST] binds the GST affinity resin but no longer binds the cAMP affinity resin. As is often seen with GST fusion proteins, a 60-kDa protein, likely GroEL, coelutes with GST fusion constructs (24).
In cAMP receptor protein-associated cAMP-binding domains, cAMP binds in an anti conformation with the base oriented away from the sugar phosphate, while in many eukaryotic cAMP-binding domains, cAMP binds predominantly in a syn conformation with the base oriented over the sugar phosphate (21, 23). The cAMP analog 8-Br-cAMP, which adopts a syn conformation, can therefore be used to preferentially activate eukaryotic protein kinase A (PKA)-like cAMP-binding domains over prokaryotic cAMP receptor protein-like cAMP-binding domains (18, 20). Interestingly, the addition of 8-Br-cAMP to cultures of cya crp double mutants transformed with NasP restored the ability of these cultures to produce N-acyl amino acids. Subtle differences in phosphate binding loops are thought to play a major role in altering the conformation of cAMP bound to a cAMP-binding domain. In prokaryotic cAMP receptor proteins, the variable residue in a conserved 3-amino-acid sequence (R-X-A) found in the phosphate-binding loop is a serine which hydrogen bonds to cAMP bound in the anti conformation. In eukaryotic PKA sequences this variable residue is most frequently an amino acid that cannot fulfill this hydrogen bond (i.e., A, N, V, or Q) (10, 21). The cAMP-binding domain from NasP contains a PKA-like R-A-A phosphate-binding loop sequence motif which would suggest that it binds cAMP in the syn conformation and would explain the activation of N-acyl amino acid biosynthesis by 8-Br-cAMP.
Although NasP appears to bind cAMP in the same conformation as many eukaryotic cAMP-binding domains, a full-length 16S rRNA gene found on the same cosmid clone as NasP indicates that it is, in fact, derived from a bacterium. The 16S rRNA gene associated with NasP is most closely related to other 16S rRNA gene sequences isolated directly from environmental samples (>95% identity) (1, 11, 16, 25). The closest 16S rRNA gene sequences from cultured bacteria are those from β-proteobacteria (>90% identity to Nitrosospira spp.) (9, 11).
The functional characterization of cAMP-dependent N-acyl amino acid biosynthesis by NasP and the identification of numerous, as-yet-uncharacterized cAMP-binding domains in bacterial sequencing projects suggest that prokaryotes, like eukaryotes, contain both a global cAMP-dependent signal transducer and numerous more-specific effectors that are directly regulated by cAMP. The large-scale screening of environmental DNA clones in a diverse collection of simple functional assays should be a rewarding strategy for the identification of novel bacterial enzymes and biological phenomena.
Nucleotide sequence accession numbers.
Nucleotide sequences for NasP (accession number DQ224236) and the CSL142 16S rRNA gene (accession number DQ224237) have been deposited with GenBank.
Supplementary Material
Acknowledgments
This work was supported by NIH grants GM077516 (S.F.B.) and CA59021 (J.C.) and the Initiative for Chemical Genetics.
Footnotes
Published ahead of print on 22 June 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Abulencia, C. B., D. L. Wyborski, J. A. Garcia, M. Podar, W. Chen, S. H. Chang, H. W. Chang, D. Watson, E. L. Brodie, T. C. Hazen, and M. Keller. 2006. Environmental whole-genome amplification to access microbial populations in contaminated sediments. Appl. Environ. Microbiol. 72:3291-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botsford, J. L., and J. G. Harman. 1992. Cyclic AMP in prokaryotes. Microbiol. Rev. 56:100-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady, S. F. 2007. Construction of soil environmental DNA cosmid libraries and screening for clones that produce biologically active small molecules. Nat. Protocols 2:1297-1305. [DOI] [PubMed] [Google Scholar]
- 4.Brady, S. F., C. J. Chao, and J. Clardy. 2004. Long-chain N-acyltyrosine synthases from environmental DNA. Appl. Environ. Microbiol. 70:6865-6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady, S. F., C. J. Chao, and J. Clardy. 2002. New natural product families from an environmental DNA (eDNA) gene cluster. J. Am. Chem. Soc. 124:9968-9969. [DOI] [PubMed] [Google Scholar]
- 6.Brady, S. F., and J. Clardy. 2000. Long-chain N-acyl amino acid antibiotics isolated from heterologously expressed environmental DNA. J. Am. Chem. Soc. 122:12903-12904. [Google Scholar]
- 7.Brady, S. F., and J. Clardy. 2005. N-acyl derivatives of arginine and tryptophan isolated from environmental DNA expressed in Escherichia coli. Org. Lett. 7:3613-3616. [DOI] [PubMed] [Google Scholar]
- 8.Brickman, E., L. Soll, and J. Beckwith. 1973. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J. Bacteriol. 116:582-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burrell, P. C., C. M. Phalen, and T. A. Hovanec. 2001. Identification of bacteria responsible for ammonia oxidation in freshwater aquaria. Appl. Environ. Microbiol. 67:5791-5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canaves, J. M., and S. S. Taylor. 2002. Classification and phylogenetic analysis of the cAMP-dependent protein kinase regulatory subunit family. J. Mol. Evol. 54:17-29. [DOI] [PubMed] [Google Scholar]
- 11.Cole, J. R., B. Chai, R. J. Farris, Q. Wang, S. A. Kulam, D. M. McGarrell, G. M. Garrity, and J. M. Tiedje. 2005. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 33:D294-D296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowan, D., Q. Meyer, W. Stafford, S. Muyanga, R. Cameron, and P. Wittwer. 2005. Metagenomic gene discovery: past, present and future. Trends Biotechnol. 23:321-329. [DOI] [PubMed] [Google Scholar]
- 13.Gabor, E., K. Liebeton, F. Niehaus, J. Eck, and P. Lorenz. 2007. Updating the metagenomics toolbox. Biotechnol. J. 2:201-206. [DOI] [PubMed] [Google Scholar]
- 14.Marchler-Bauer, A., J. B. Anderson, C. DeWeese-Scott, N. D. Fedorova, L. Y. Geer, S. He, D. I. Hurwitz, J. D. Jackson, A. R. Jacobs, C. J. Lanczycki, C. A. Liebert, C. Liu, T. Madej, G. H. Marchler, R. Mazumder, A. N. Nikolskaya, A. R. Panchenko, B. S. Rao, B. A. Shoemaker, V. Simonyan, J. S. Song, P. A. Thiessen, S. Vasudevan, Y. Wang, R. A. Yamashita, J. J. Yin, and S. H. Bryant. 2003. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 31:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCue, L. A., K. A. McDonough, and C. E. Lawrence. 2000. Functional classification of cNMP-binding proteins and nucleotide cyclases with implications for novel regulatory pathways in Mycobacterium tuberculosis. Genome Res. 10:204-219. [DOI] [PubMed] [Google Scholar]
- 16.Nercessian, O., E. Noyes, M. G. Kalyuzhnaya, M. E. Lidstrom, and L. Chistoserdova. 2005. Bacterial populations active in metabolism of C1 compounds in the sediment of Lake Washington, a freshwater lake. Appl. Environ. Microbiol. 71:6885-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochoa de Alda, J. A., and J. Houmard. 2000. Genomic survey of cAMP and cGMP signalling components in the cyanobacterium Synechocystis PCC 6803. Microbiology 146:3183-3194. [DOI] [PubMed] [Google Scholar]
- 18.Paces, V., and J. Smrz. 1973. On the specificity of cyclic AMP action in Escherichia coli. FEBS Lett. 31:343-344. [DOI] [PubMed] [Google Scholar]
- 19.Sabourn, D., and J. Beckwith. 1975. Deletion of the Escherichia coli crp gene. J. Bacteriol. 122:338-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholubbers, H. G., P. H. van Knippenberg, J. Baraniak, W. J. Stec, M. Morr, and B. Jastorff. 1984. Investigations on stimulation of lac transcription in vivo in Escherichia coli by cAMP analogues. Biological activities and structure-activity correlations. Eur. J. Biochem. 138:101-109. [DOI] [PubMed] [Google Scholar]
- 21.Shabb, J. B., and J. D. Corbin. 1992. Cyclic nucleotide-binding domains in proteins having diverse functions. J. Biol. Chem. 267:5723-5726. [PubMed] [Google Scholar]
- 22.Shah, S., and A. Peterkofsky. 1991. Characterization and generation of Escherichia coli adenylate cyclase deletion mutants. J. Bacteriol. 173:3238-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su, Y., W. R. Dostmann, F. W. Herberg, K. Durick, N. H. Xuong, L. Ten Eyck, S. S. Taylor, and K. I. Varughese. 1995. Regulatory subunit of protein kinase A: structure of deletion mutant with cAMP binding domains. Science 269:807-813. [DOI] [PubMed] [Google Scholar]
- 24.Thain, A., K. Gaston, O. Jenkins, and A. R. Clarke. 1996. A method for the separation of GST fusion proteins from co-purifying GroEL. Trends Genet. 12:209-210. [DOI] [PubMed] [Google Scholar]
- 25.Tringe, S. G., C. von Mering, A. Kobayashi, A. A. Salamov, K. Chen, H. W. Chang, M. Podar, J. M. Short, E. J. Mathur, J. C. Detter, P. Bork, P. Hugenholtz, and E. M. Rubin. 2005. Comparative metagenomics of microbial communities. Science 308:554-557. [DOI] [PubMed] [Google Scholar]
- 26.Venter, J. C., K. Remington, J. F. Heidelberg, A. L. Halpern, D. Rusch, J. A. Eisen, D. Wu, I. Paulsen, K. E. Nelson, W. Nelson, D. E. Fouts, S. Levy, A. H. Knap, M. W. Lomas, K. Nealson, O. White, J. Peterson, J. Hoffman, R. Parsons, H. Baden-Tillson, C. Pfannkoch, Y. H. Rogers, and H. O. Smith. 2004. Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66-74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.