Abstract
Two environmental strains, Delftia acidovorans C17 and Delftia tsuruhatensis A90, were found to carry class 3 integrons, which have seldom been reported and then only from pathogens in which they are associated with antibiotic resistance genes. The Delftia integrons comprised a highly conserved class 3 integrase gene, upstream and oppositely oriented from a set of three or four gene cassettes that encoded unidentified functions. The A90 integron had one more gene cassette than the C17 integron, but the two were otherwise the same; furthermore, they were located within regions of sequence identity in both strains and linked to chromosomal genes. A screen of other Delftia and related strains did not reveal the presence of additional class 3 integrons. The observations suggest that these integrons were horizontally transferred to Delftia as part of a larger region and reside as chromosomal elements that probably predate transposon dissemination, as has been proposed for certain class 1 integrons.
Integrons constitute a diverse family of genetic elements that are found in many gram-negative and some gram-positive bacteria. As defined by Hall and Collis (18), these elements encode a site-specific recombination system that is able to capture gene cassettes in tandem arrays. Integron-associated gene cassettes are found on a variety of larger genetic elements such as transposons or plasmids that permit horizontal gene transfer between different bacterial genera; such elements may also be integrated into chromosomes. The most studied integrons are the resistance integrons (RIs), in which the cassettes are antibiotic resistance determinants. More than 80 different antibiotic-encoding cassettes have been identified in RI clusters (27). RIs are widely distributed in the Enterobacteriacae, where they are the major factors involved in the development of multidrug resistance (13, 17).
The key elements of all integrons are the integrase gene (intI), which encodes a tyrosine recombinase (2) responsible for the insertion and assortment of the gene cassettes, and an associated integration site (attI). The integrase gene incorporates a strong promoter sequence within its 5′-terminal sequence that is responsible for the transcription of gene cassettes that have been recombined into the attI site.
Three classes of RIs have been identified on the basis of the amino acid sequence of the integrase (14). Class 1 is widely distributed, is often associated with Tn402-like transposons, and is most commonly observed. The related but less frequently detected class 2 possesses a defective integrase gene and is usually embedded in a Tn7 family transposon (36). Class 3 integrons are rare; to date, only two have been studied in detail (1, 7-9), although fragments of others have been detected by PCR in studies of clinical isolates from Japan (42, 43). The class 3 integrons that have been characterized are both RIs and encode a metallo-β-lactamase (1, 34) and an aminoglycoside acetyltransferase; they have essentially the same organization as class 1 and 2 integrons.
A number of related genetic elements have been found in bacteria, such as the superintegrons, which encode much larger clusters (∼100) of gene cassettes that are almost exclusively unidentified open reading frames (28), and the resistance gene clusters found in strains of Vibrio cholerae (20). Recent metagenomic analyses of soils and sediments have revealed many integron-related gene cassettes encoding unidentified functions (32, 46). It has been proposed that integron-associated cassettes are genetic elements that played roles in bacterial chromosome evolution (19, 21, 27, 31).
During an investigation into the use of RIs for tracking the spread of resistant bacteria in aquatic environments, we detected class 3 integrase (intI3) sequences in DNA isolated from water samples, and by using colony hybridization (3), we isolated a strain of Delftia tsuruhatensis that carried the integrase within an integron-like structure. Delftia spp. are rod-shaped, nitrate-reducing, gram-negative bacteria with a G+C content in the range of 66% that are widely distributed in the environment and capable of degrading a variety of xenobiotics such as chlorinated aromatic compounds (10, 40). They were formerly considered to be members of the genus Comamonas or Pseudomonas (54). There have been no previous reports of integrons in Delftia; however, superintegrons are commonly found in related bacterial genera such as Pseudomonas (52). This prompted us to inspect other Delftia strains, and we identified a second class 3 integron, in Delftia acidovorans. The detailed genetic organization of the two Delftia integrons was determined by cloning and nucleotide sequencing and revealed two closely related chromosomal elements with gene cassettes but no known antibiotic resistance determinants.
MATERIALS AND METHODS
Detection of class 3 integrons in aquatic samples.
Water samples were collected from the Joint Abbotsford-Mission Environmental System Water Pollution Control Centre, Abbotsford, British Columbia, Canada, and filtered through a sterile 47-mm-diameter membrane (GVHP 04700; pore size, 0.22 μm; Millipore Corp.) to retain bacteria. Membranes were placed in 100 ml Luria-Bertani broth and incubated by shaking at 37°C overnight. Total DNA was extracted using a QIAamp DNA minikit (QIAGEN Inc.) and used as a template for PCR amplification with primers that distinguish among the integrases. Primer pairs intI1F-intI1R (5′-GTTCGGTCAAGGTTCTGG-3′ and 5′-CGTAGAGACGTCGGAATG-3′, derived from GenBank accession no. Y18050), intI2F-intI2R (5′-CAAGCATCTCTAGGCGTA-3′ and 5′-AGAAGCATCAGTCCATCC-3′, from accession no. L10818), and intI3F-intI3R (5′-CATCAAGCTGCTCGA TCA-3′ and 5′-ACAACTCTTGCACCGTTC-3′, from accession no. D50438) were each used to amplify a portion of an integrase gene: 890 bp of intI1, 1,056 bp of intI2, or 878 bp of intI3, respectively. A fourth primer pair, int1.F-int1.R (5′-GGGTCAAGGATCTGGATTTCG-3′ and 5′-ACATGCGTGTAAATCATCGTCG-3′ [29]), which generates a 484-bp amplicon from class 1 and class 3 integrons, was also used sometimes; in these cases, a secondary assay with intI3F-intI3R was also done. Thirty-five cycles of amplification were conducted (95°C for 20 s, 54°C for 30 s, and 70°C for 30 s) after 2 min at 95°C to denature template DNA. Plasmids with In1 (pJR88 [23, 55]), In2 (pIP1100 [4]), or In3 (pSMB731 [1]) were used as positive controls for PCR.
Cultures that were positive in the PCR assays were diluted to 10−6, and 100-μl aliquots were spread onto LB plates. After overnight incubation at 37°C, the plates were replicated onto nylon membranes (Nylon Membranes for Colony and Plaque Hybridization; Roche Diagnostics) and hybridized at 42°C with digoxigenin-labeled probes obtained by using intI-specific primers (described above) and a PCR DIG labeling kit (Roche Diagnostics) according to the manufacturer's instructions. The colonies detected by hybridization were then purified.
Detection of class 3 integrons in other strains.
Strains (Table 1) were screened by PCR using the primers and conditions described above. DNA was extracted using a ChargeSwitch gDNA Mini Bacteria kit (Invitrogen Inc.). PCR for 16S rRNA genes (described below) was conducted as a positive control for DNA template quality during screening.
TABLE 1.
Bacterial strains and sources
| Species | Straina | Origin | Reference | 16S rRNA accession no. |
|---|---|---|---|---|
| Delftia acidovorans | NBRC14950T | The Netherlandsb | 54 | AB021417 |
| Delftia acidovorans | SPH-1 | Germanyc | 40 | AB021417e |
| Delftia acidovorans | C17 | United Statesc | 10d | This study |
| Delftia acidovorans | EEZ23 | Spainb | 38 | This study |
| Delftia acidovorans | WDL34 | Belgiumb | 11 | AF538930 |
| Delftia acidovorans | NBRC13582 | United Kingdomb | 15 | AB020186 |
| Delftia sp. | EH2-1 | United Statesb | 51 | AY3677007 |
| Delftia tsuruhatensis | NBRC16741T | Japanc | 44 | AB075017 |
| Delftia tsuruhatensis | A90 | Canadac | This study | This study |
| Delftia sp. | AN3 | Chinac | 25 | AY052781 |
| Delftia sp. | 8 | Canadab | 24 | AF181575 |
| Acidovorax avenae subsp. citrulli | AAC00-1 | United States | 53 | AASX01000007f |
| Acidovorax sp. | JS42 | United Statesb | 16 | AASD01000022f |
| Comamonas testosteroni | I2gfp | Belgiumc | 5 | This study |
| Comamonas testosteroni | KF-1 | Switzerlandc | 40 | M11224e |
NBRC strains were obtained from the National Biological Resource Center, Japan. Other strains were obtained from colleagues: C17, EEZ23, WDL34, and I2gfp from E. Top, University of Idaho; KF-1 and SPH-1 from D. Schleheck, University of New South Wales; EH2-1 from W. Hickey, University of Wisconsin; AN3 from S.-J. Liu, Research Center of Microbial Technology, Beijing, China; strain 8 from J. Lawrence, Environment Canada, Saskatoon; and AAC00-1 and JS42 from D. Stahl, University of Washington.
Isolated from soil or groundwater.
Isolated from wastewater or activated sludge.
Isolated from material described in this reference, but not specifically identified by name (E. Top, personal communication).
Sequence is identical to that of the type strain.
Draft genome sequence.
Southern blot analysis.
Genomic DNA, extracted as for test strains, was either left untreated or digested with HindIII and then size fractionated by agarose gel electrophoresis. The DNA was blotted onto a nylon membrane (Roche Diagnostics), hybridized at 42°C to a digoxigenin-labeled intI3 probe generated by PCR using primers intI3F and intI3R, and washed as directed by the manufacturer.
Antibiotic sensitivity tests.
Disk diffusion assays to determine the antibiotic resistance of source strains and Escherichia coli hosts carrying library clones were conducted on Mueller-Hinton agar (Difco). All antibiotics were obtained from Sigma-Aldrich.
16S rRNA analysis.
Molecular typing of A90, C17, and other strains was conducted using 16S rRNA. Primers 16S.0007F21 (5′-GAGAGTTTGATCCTGGCTCAG-3′) and 16S.1511R21 (5′-CGGCTACCTTGTTACGACTTC-3′) were used for PCR of the 16S rRNA gene from genomic DNA and for sequencing (MacroGen, Inc.). The PCR program comprised an initial incubation at 96°C for 3 min, followed by 35 cycles of 96°C for 30 s, 60°C for 45 s, and 72°C for 90 s. Sequence assembly, alignment of a ca. 1,300 nucleotide region, and dendrogram construction (neighbor-joining method, default parameters) were done with MacVector (Accelrys).
Cloning and sequencing of integrons and flanking DNA.
Genomic DNA of A90 and C17 was isolated as for test strains, partially digested with Sau3A1, and cloned into SuperCos1 (Stratagene) as directed by the manufacturer. A90 genomic DNA was also completely digested with HindIII and cloned into pUC19. The libraries were screened by hybridization with the intI3-specific probe, and positive clones were wholly (pAV3.5, a pUC19 clone of A90) or partially (cosmids CA90-6 and CC17-15 from A90 and C17, respectively) sequenced to include the integron and adjacent DNA. The C17 contig was generated by primer-walking cosmid DNA, except for a 1.1-kb gap (nucleotides 2738 to 3843) closed by sequencing a PCR product. A90 sequences were derived from cosmid primer walking, and PCR products were generated to close gaps. A 2.7-kb region (nucleotides 1 to 2708) extending beyond CA90-6 was sequenced from amplicons obtained from A90 genomic DNA with primer pairs 5′-AGGCACTGGAKGCMGCYTCG-3′-5′-AACACCCGTGCGCTATATGG-3′ and 5′-ATAGGTAGCTGTGAACGACG-3′-5′-CCTCGCACAGTCGCTGAACG-3′. These were determined from regions of identity between C17 and presumptive homologs. Sequences were compared to known genes by BLAST.
Nucleotide sequence accession numbers.
New 16S rRNA sequences were deposited as GenBank accession no. EF421404 to EF421407. Sequences of contigs determined in this study were deposited in GenBank as accession no. EF467661 and EF469602.
RESULTS AND DISCUSSION
Isolation and characterization of strains.
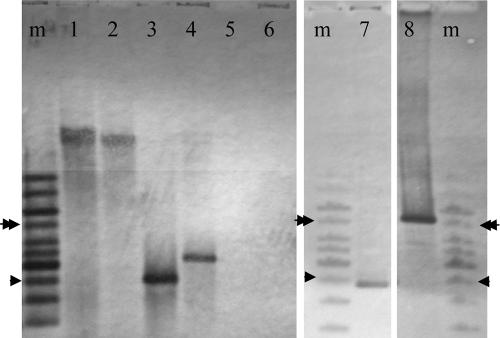
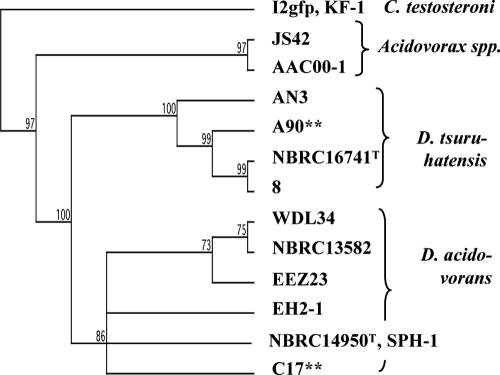
A90, a strain containing class 3 integrase-encoding sequences, was isolated from a wastewater treatment plant in British Columbia by dilution plating of wastewater on a nonselective medium and colony hybridization with an intI3-specific probe. Sequencing of an amplicon generated by PCR with intI3-specific primers and probing of isolated genomic DNA with an intI3-specific probe confirmed that A90 was the source (Fig. 1). Hybridization of the probe to uncut genomic DNA suggested that the integron was located in the chromosome or on a large plasmid. Molecular typing by 16S rRNA gene sequencing indicated that A90 was most likely a strain of Delftia tsuruhatensis (Fig. 2).
FIG. 1.
Southern blot analyses of Delftia strains and cloned DNA using an intI3-specific probe. Lanes 1, 2, 5, and 6, uncut genomic DNA of D. acidovorans C17, D. tsuruhatensis A90, D. acidovorans NBRC14950T, and D. tsuruhatensis NBRC16741T, respectively. Lanes 3, 4, 7, and 8, HindIII-digested DNA of C17, A90, and cosmids CC17-15 and CA90-6, respectively. The hybridizing fragment in CA90-6 is 5.2 kb, larger than that in A90 genomic DNA (3.1 kb), because the latter is interrupted by cloning. A GeneRuler 1-kb ladder (Fermentas) in lanes m is used as a marker. Single arrowhead, 2.5 kb; double arrowhead; 5 kb.
FIG. 2.
16S rRNA gene phylogeny of Delftia and related strains and distribution of integrons. Significant bootstrap support values for 500 replicates are shown. Class 3 intI-positive strains are indicated by asterisks. The 16S rRNA sequence of C. testosteroni I2gfp is the same as that of strain KF-1; the 16S rRNA sequence of D. acidovorans SPH-1 is identical to that of NBRC14959 T (40). A90 and C17 are 99.5% identical across a 1,315-nucleotide region.
To date, intI3 sequences are known only from Serratia marcescens, Klebsiella pneumoniae, Pseudomonas aeruginosa, Pseudomonas putida, and Alcaligenes xylosoxidans (1, 9, 42, 43). This prompted examination of available strains of Delftia and related genera in the Comamonadaceae to determine the prevalence of intI3 in this new host group (Table 1). The only one of 14 strains tested that was positive for intI3 by a PCR assay was another Delftia strain: Delftia acidovorans C17 was confirmed by Southern blot analysis to possess intI3 sequences (Fig. 1 and 2). Strain C17 also originated from an activated-sludge community (10), but the source was a wastewater treatment facility in Idaho that was geographically distant from the source of A90 in Canada. Since class 3 integrons (1, 9), or portions thereof (42, 43), have previously been reported only from Japan and Portugal, strains A90 and C17 represent the first intI3-bearing strains from North America. The lack of association of intI3 with a phylogenetic lineage among the representatives of the group and the geographic separation of the source habitats suggest that the intI3 sequences were introduced into the two Delftia strains horizontally, on two occasions.
Integrons often have associated gene cassettes encoding resistance to a variety of antibiotics and play a role in the dissemination of resistance in hospitals. The two class 3 integrons characterized, one from S. marcescens AK9373 (1), designated In3-1 here for convenience, and one from K. pneumoniae FFUL 22K (9), referred to below as In3-2, were both isolated from clinical strains and were associated with gene cassettes for resistance to broad-spectrum β-lactams and other antibiotics. Delftia strains A90 and C17 are distinct from these in originating from an environmental rather than a clinical setting; it was therefore of interest to examine the organization of the integrons associated with the intI3 sequences in Delftia.
Isolation and characterization of Delftia class 3 integrons.
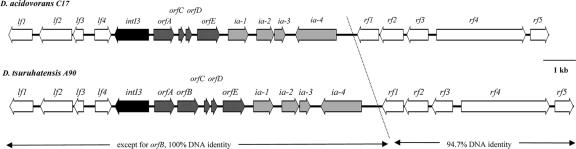
To isolate the entire integrons, genomic libraries of the two Delftia strains were constructed and screened by hybridization, and candidate clones were partially sequenced. An integron of 3,213 bp, designated In3-3, was identified in cosmid CC17-15 from D. acidovorans C17, and another integron, of 3,964 bp, designated In3-4, was found in cosmid CA90-6 from D. tsuruhatensis A90 (Fig. 3).
FIG. 3.
Chromosomal regions containing class 3 integrons in D. acidovorans C17 and D. tsuruhatensis A90.
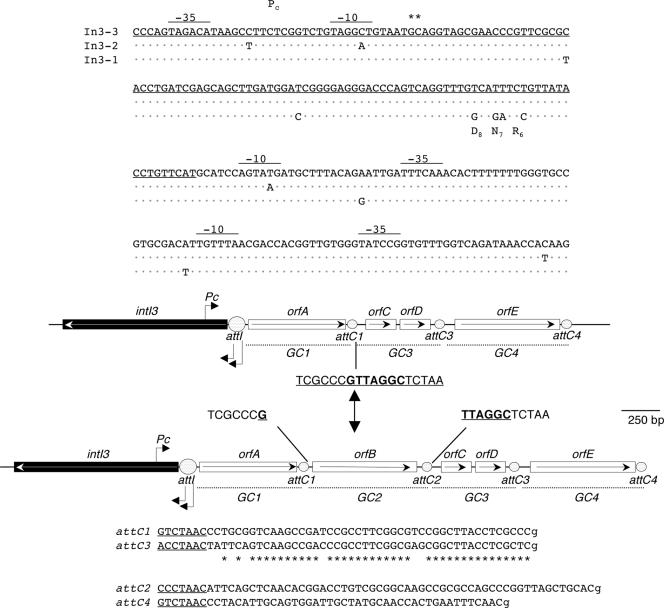
Detailed analyses showed that, except for the presence of an extra gene cassette, GC2, in In3-4, the integrons from the two Delftia strains were identical in structure and sequence (Fig. 3 and 4; Table 2). The key elements, intI3, Pc, and attI3, were highly similar to those from the only two previously characterized class 3 integrons. The intI3 gene in Delftia is 99% identical to the two known intI3 genes. Four of the differences in the intI3 gene of Delftia relative to intI3 in In3-1 were associated with substitutions (G6R, S7N, and A8D), but none of them was in a position conserved among the tyrosine recombinases (30, 33). A region identical to the Pc promoter of In3-1 studied by Collis et al., 2002 (8), including nucleotides identified as transcription starts, is found in Delftia. Next to intI3, in the attI3 region, two differences were found, including a G/A transition in the more proximal of two possible Pint promoters proposed for In3-1 (8) (Fig. 4). The attI3 region in Delftia is identical to that of In3-1 (7) and is divided by the insertion of gene cassettes (see below) such that the remaining nine or ten 3′ nucleotides, including the remainder of the simple site, lie beyond the last gene cassette. In In3-1, the region beyond the last gene cassette can be aligned with the left end of Tn402-type class 1 integrons and terminates in an inverted repeat (IRi) (8) that provides part of the evidence that In3-1 has a Tn402-like backbone. The Delftia integrons lack the distal sequences and the IRi, but the proximal 44 bp of this region are completely conserved among all the class 3 integrons (Fig. 5). The far edge of this short tract of identity matches a “deletion endpoint” observed in some environmental class 1 integrons (47), so called because it marks a location beyond which conserved sequences that are typical for mobile class 1 integrons are missing. This location was noted as being within 1 bp of an insertion site for P. aeruginosa IsPa7 in some class 1 integrons (47).
FIG. 4.
Fine structure of integrons In3-3 (top) and In3-4 (bottom). Tandem gene cassettes (GC) are indicated by double-headed arrows; genes (filled arrows) in each GC are named as in Table 2. The attI and attC sites are represented by boxes. Pc and two possible Pint promoters (8) are indicated by angled arrows above or below the integron. (Upper box) Alignment of promoter and attI regions of In3-1 (GenBank accession AF416297), In3-2 (AY219651), and In3-3. The coding region (opposite strand) of intI is underlined. Features such as −10, −35, and transcription starts after Pc (marked with double asterisks) as originally determined for In3-1 by Collis et al. (8) are labeled; the attI region as delimited in In3-1 by Collis and Hall (7) is highlighted. (Lower box) attC sites, starting from the inverse core (underlined) and including the first G at the possible sites of recombination (“GTTRRRY” motif) are shown; bases conserved between attC1 and attC3 are indicated by asterisks.
TABLE 2.
ORFs in class 3 integrons and flanking DNA
| ORF | % G+C
|
No. of amino acids | Most similar CDSsa | GenBank accession no. | % Identity
|
||
|---|---|---|---|---|---|---|---|
| A90 | C17 | A90 | C17 | ||||
| lf1 | 60 | 60 | 288 | Hypothetical protein, Acidovorax sp. strain JS42 (AJS228) | ZP_01384761 | 83 | 83 |
| lf2 | 59 | 59 | 329 | Hypothetical protein, Acidovorax sp. strain JS42 (AJS229) | ZP_01384762 | 96 | 96 |
| lf3 | 59 | 59 | 107 | Hypothetical protein, Dechloromonas aromatica RCB | YP_284873 | 50 | 50 |
| lf4 | 62 | 62 | 171 | Hypothetical protein, Novosphingobium aromaticivorans DSM 12444 | YP_497478 | 40 | 40 |
| intI3 | 55 | 55 | 346 | IntI3 integrase, Klebsiella pneumoniae | AAO32355 | 99 | 99 |
| orfA | 49 | 49 | 208 | Hypothetical protein, Vibrio splendidus V12 | ZP_00990410 | 46 | 46 |
| orfB | 59 | 217 | Hypothetical protein, Comamonas testosteroni KF-1 | ZP_01521201 | 79 | ||
| orfC | 45 | 45 | 63 | Hypothetical protein, Burkholderia vietnamiensis G4 | ZP_00426308 | 39 | 39 |
| orfD | 48 | 48 | 65 | No significant hit | |||
| orfE | 38 | 38 | 224 | Hypothetical protein, Yersinia frederiksenii ATCC 33641 | ZP_00827899 | 45 | 45 |
| ia-1 | 52 | 52 | 207 | Putative integrase/recombinase, Acidovorax sp. strain MUL2G8 | ABE73721 | 96 | 96 |
| ia-2 | 58 | 58 | 177 | Putative transcriptional regulator in pB8 (IncP-1β) | YP_358816 | 70 | 70 |
| ia-3 | 55 | 55 | 110 | Small multidrug resistance protein in pB8 (IncP-1β) | YP_358817 | 83 | 83 |
| ia-4 | 54 | 54 | 413 | Phage P4-type tyrosine recombinase, Acidovorax sp. strain JS42 (AJS3904) | ZP_01381320 | 95 | 95 |
| rf1 | 62 | 63 | 221 | Phosphoglycolate phosphatase, Delftia acidovorans SPH-1 (5344); phosphoglycolate phosphatase, Acidovorax sp. strain JS42 (AJS3903) | ZP_01578007, ZP_01381319 | 90, 63 | 100, 65 |
| rf2 | 61 | 62 | 240 | Ubiquinone biosynthesis OMTase, Delftia acidovorans SPH-1 (5343); ubiquinone biosynthesis OMTase, Acidovorax sp. strain JS42 (AJS3902) | ZP_01578006, ZP_01381318 | 97, 75 | 100, 77 |
| rf3 | 62 | 62 | 219 | OmpA/MotB, Delftia acidovorans SPH-1 (5342); OmpA/MotB, Acidovorax sp. strain JS42 (AJS3901) | ZP_01578005, ZP_01381317 | 98, 87 | 100, 88 |
| rf4 | 64 | 65 | 888 | DNA gyrase, A subunit, Delftia acidovorans SPH-1 (5341); DNA gyrase, A subunit, Acidovorax sp. strain JS42 (AJS3900) | ZP_01578004, ZP_01381316 | 99, 91 | 100, 92 |
| rf5 | 66 | 66 | 188 | Phosphoserine aminotransferase, Delftia acidovorans SPH-1 (5340); phosphoserine aminotransferase, Acidovorax sp. strain JS42 (AJS3899) | ZP_01578003, ZP_01381315 | 97, 88 | 100, 87 |
Two database hits are shown for rf1 to rf5 to illustrate the conserved synteny (as indicated by consecutive locus tags in brackets) of this group of genes in related organisms and to emphasize the continuation of this series in Acidovorax sp. strain JS42 by an additional gene encoding a phage P4-type tyrosine recombinase. CDSs, coding sequences; OMTase, o-methyltransferase.
FIG. 5.
“Left” boundaries of class 1 (In1) and class 3 (In3-X) integrons. The designation “left” follows the convention in references 8 and 47, from which the first five sequences are adapted; “<” at the start of each line indicates that additional upstream sequence, including the IRi, which is the actual left terminus in some cases, is not shown. Positions of identity with the sequence immediately above are shown as dots. The end of the attI site of the class 3 integrons is underlined, and the stop codon of intI1 is boldfaced (opposite strand). A vertical arrow indicates where ISPa7 is inserted in some class 1 integrons (47). Tn402 and In3-1 sequences flanking the end of intI1 or the last gene cassette, respectively, are related and terminate at the IRi (8). The class 1 integron in strain MUL2G11 has a complete left end like Tn402, but those in MUL2G8 and MUL2G9 are terminally deleted.
In3-3 has three (GC1, GC3, and GC4) and In3-4 has four (GC1 through GC4) tandemly arranged gene cassettes demarcated by potential attC (59-base element) sequences (Fig. 4). The attC sites associated with GC1 and GC3 are >80% identical to each other and to the attC site of a small, conserved gene cassette of unknown function in some class 1 integrons (26, 48). The three cassettes common to both In3-3 and In3-4 have G+C contents (38 to 49%) distinctly lower than that of the Delftia host (∼66%) (Table 2). GC3 is unusual in having two possible open reading frames (ORFs): orfC and orfD are separated by 23 nucleotides. Cassettes with two ORFs are not common. Notable exceptions are those encoding toxin-antitoxin gene pairs in superintegrons in Vibrio (49); however, the two ORFs in Delftia do not resemble these. None of the cassettes carried an obvious resistance gene: orfD has no orthologs in available databases, and the others had low similarities to various unidentified ORFs. The G+C content of orfB in GC2 was higher than that of the other ORFs, and its predicted product had significant similarity to a conserved hypothetical protein in Comamonas testosteroni KF-1.
Characterization of flanking sequences.
Integrons are often components of mosaic structures including various recombination functions; they are themselves not capable of horizontal gene transfer but are frequently associated with mobile elements such as transposons and plasmids. Examination of the DNA context in which an integron is situated is therefore often useful in providing clues as to its history. A region of sequence identity was found to extend beyond the integrons on both sides (Fig. 3; Table 2). This suggests that the integrons shared a recent ancestor prior to the lateral gene transfer event that brought the segment of ancestral DNA, including the integrons, into Delftia. The border of the shared region on the left of the integrons remains to be determined but is at least 3.1 kb away from the end of intI3. In this left flank, lf1 and lf2 have strong similarity to two neighboring genes in Acidovorax sp. strain JS42 (Ajs_228 and Ajs_229) that are not found in related strains. On the right of the integrons, the region of identity includes four integron-adjacent/associated ORFs, ia-1 to ia-4, all of which have some association with transposons or some theoretical role in transposition. The deduced products of ia-1 and ia-4 resemble integrases/recombinases from strains of Acidovorax: in particular, ia-1 is most similar (88% DNA identity; 96% amino acid identity) to a putative integrase/recombinase in the environmental strain Acidovorax sp. strain MUL2G8 that is encoded by a gene adjacent to a class 1 integron (47). Short tracts around ia-1 also have similarity to DNA flanking the MUL2G8 gene: the 50 nucleotides upstream of both share 90% identity, and the 56 nucleotides immediately downstream of both share 87% identity. The ORF for ia-2 encodes a putative TetR family transcriptional repressor (37), and that for ia-3 encodes a putative QacF-like small multidrug efflux protein (35). The published genes most similar to these were found in a transposon inserted into IncP-1β plasmid pB8, where they were comparably arranged and conferred tolerance to quaternary ammonium compounds (41); one might speculate that these genes could have been introduced into the ancestral segment from a plasmid. The proximity of all these genes to In3 is very intriguing, but their functions remain to be demonstrated.
The regions of 100% identity in both A90 and C17 are followed on the right flank by rf1 to rf5, a group of housekeeping genes. They are overall 94.7% identical at the DNA level between the strains. These genes, including a gyrA homolog (rf4), are conserved and syntenic in strains from related genera that have been sequenced: Acidovorax sp. strain JS42, Acidovorax avenae subsp. citrulli AAC00-1, D. acidovorans SPH-1, and Comamonas testosteroni KF-1 (http://genome.jgi-psf.org/mic_home.html). Interestingly, Ajs_3904, the ia-4 ortholog in Acidovorax sp. strain JS42, is also part of the syntenic series in its native host. Colinearity with gyrA (rf), together with the results of Southern blot analysis, supports the conclusion that the Delftia class 3 integrons are chromosomally located, in contrast to In3-1 and In3-2, which are plasmid borne (1, 9, 22). The possibility of a preferred site for the transfer event is suggested by linkage of the integrons and their immediately surrounding regions to the same loci (rf1 to rf5) in two distinct Delftia strains, but examination of the sequence in the possible border regions has not yet revealed a particular mechanism. The transferred region might be of Acidovorax-related origin, given the similarities of some of the ORFs to the genome of Acidovorax sp. strain JS42, notably a putative phage P4-type tyrosine recombinase gene that is in the same location as ia-4 relative to the other JS42 chromosomal genes.
Class 3 integrons in the environment.
The results of this study not only provide the first evidence of the presence of class 3 integrons in North America but also show that they have a wider distribution ecologically. The integrons and their contiguous DNA in Delftia have a number of similarities to certain environmental class 1 integrons that have been proposed as chromosomal elements that predate transposon dissemination (47). The resemblance of ORF ia-1 and its flanking DNA to the putative integrase/recombinase coding and intergenic regions in Acidovorax sp. strain MUL2G8 (47) adjacent to the integrase gene of a class I integron is striking. The fact that ia-1 follows the last gene cassette, GC4, rather than intI3, is consistent with the model that class 1 and class 3 integrons have opposite orientations (8). In addition, the Delftia integrons are bounded by precisely the same deletion endpoint after GC4 as are the integrase genes in the environmental class 1 integrons of strains MUL2G8 and MUL2G9 relative to Tn402 (8, 47).
The organization of the new class 3 integrons includes elements that would allow the capture and assortment of gene cassettes. The possibility exists that they may be related to the superintegrons that are commonly found in microbial communities in soils, sediments, and aqueous environments. Superintegrons are chromosomally located, are associated with cassettes of varied organization and largely unknown function (6, 39, 52), and have been hypothesized to be the progenitors of mobile integrons (27). Although superintegrons typically contain many genes, the Shewanella oneidensis superintegron (12), with only three cassettes, provides a comparison to the Delftia integrons.
The discovery of In3-3 and In3-4 in wastewater treatment facilities may be important for understanding the ecology of both the host organisms and the integrons in future studies. Wastewater treatment plants have been proposed as important reservoirs of antibiotic resistance gene cassettes, e.g., on IncP plasmids (41, 50). Introduction of a new integron into a member of the local bacterial population such as Delftia spp. (some of which are known to carry IncP plasmids [45]) could not only lead to proliferation of the integron per se but also may add a new role for this group of organisms in the acquisition of resistance genes from antibiotic-resistant microbes in specialized wastewater input from hospitals or agriculture (10). Both effects will enhance the evolutionary and transmission dynamics of integron-associated antibiotic resistance and ultimately contribute to its persistence and horizontal spread in the environment.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research (Water Source Tracking study, directed by A. Mazumdar).
We thank P. Keen for water samples, I. Villanueva for archiving sequences, D. Rowe-Magnus and W. Kwong for reviewing a draft of the manuscript, and our colleagues who generously provided strains.
Footnotes
Published ahead of print on 15 June 2007.
REFERENCES
- 1.Arakawa, Y., M. Murakami, K. Suzuki, H. Ito, R. Wacharotayankun, S. Ohsuka, N. Kato, and M. Ohta. 1995. A novel integron-like element carrying the metallo-beta-lactamase gene blaIMP. Antimicrob. Agents Chemother. 39:1612-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argos, P., A. Landy, K. Abremski, J. B. Egan, E. Haggard-Ljungquist, R. H. Hoess, M. L. Kahn, B. Kalionis, S. V. Narayana, L. S. Pierson III, et al. 1986. The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J. 5:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlow, R. S., J. M. Pemberton, P. M. Desmarchelier, and K. S. Gobius. 2004. Isolation and characterization of integron-containing bacteria without antibiotic selection. Antimicrob. Agents Chemother. 48:838-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biskri, L., and D. Mazel. 2003. Erythromycin esterase gene ere(A) is located in a functional gene cassette in an unusual class 2 integron. Antimicrob. Agents Chemother. 47:3326-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon, N., J. Goris, P. De Vos, W. Verstraete, and E. M. Top. 2000. Bioaugmentation of activated sludge by an indigenous 3-chloroaniline-degrading Comamonas testosteroni strain, I2gfp. Appl. Environ. Microbiol. 66:2906-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark, C. A., L. Purins, P. Kaewrakon, T. Focareta, and P. A. Manning. 2000. The Vibrio cholerae O1 chromosomal integron. Microbiology 146:2605-2612. [DOI] [PubMed] [Google Scholar]
- 7.Collis, C. M., and R. M. Hall. 2004. Comparison of the structure-activity relationships of the integron-associated recombination sites attI3 and attI1 reveals common features. Microbiology 150:1591-1601. [DOI] [PubMed] [Google Scholar]
- 8.Collis, C. M., M. J. Kim, S. R. Partridge, H. W. Stokes, and R. M. Hall. 2002. Characterization of the class 3 integron and the site-specific recombination system it determines. J. Bacteriol. 184:3017-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correia, M., F. Boavida, F. Grosso, M. J. Salgado, L. M. Lito, J. M. Cristino, S. Mendo, and A. Duarte. 2003. Molecular characterization of a new class 3 integron in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 47:2838-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Gelder, L., F. P. Vandecasteele, C. J. Brown, L. J. Forney, and E. M. Top. 2005. Plasmid donor affects host range of promiscuous IncP-1β plasmid pB10 in an activated-sludge microbial community. Appl. Environ. Microbiol. 71:5309-5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dejonghe, W., E. Berteloot, J. Goris, N. Boon, K. Crul, S. Maertens, M. Hofte, P. De Vos, W. Verstraete, and E. M. Top. 2003. Synergistic degradation of linuron by a bacterial consortium and isolation of a single linuron-degrading Variovorax strain. Appl. Environ. Microbiol. 69:1532-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drouin, F., J. Melancon, and P. H. Roy. 2002. The IntI-like tyrosine recombinase of Shewanella oneidensis is active as an integron integrase. J. Bacteriol. 184:1811-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fluit, A. C., and F. J. Schmitz. 1999. Class 1 integrons, gene cassettes, mobility, and epidemiology. Eur. J. Clin. Microbiol. Infect. Dis. 18:761-770. [DOI] [PubMed] [Google Scholar]
- 14.Fluit, A. C., and F. J. Schmitz. 2004. Resistance integrons and super-integrons. Clin. Microbiol. Infect. 10:272-288. [DOI] [PubMed] [Google Scholar]
- 15.Gray, P. H. 1928. The formation of indigotin from indol by soil bacteria. Proc. R. Soc. Lond. Ser. B 102:263-280. [Google Scholar]
- 16.Haigler, B. E., W. H. Wallace, and J. C. Spain. 1994. Biodegradation of 2-nitrotoluene by Pseudomonas sp. strain JS42. Appl. Environ. Microbiol. 60:3466-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall, R. M., and C. M. Collis. 1998. Antibiotic resistance in gram-negative bacteria: the role of gene cassettes and integrons. Drug Resist. Updat. 1:109-119. [DOI] [PubMed] [Google Scholar]
- 18.Hall, R. M., and C. M. Collis. 1995. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol. Microbiol. 15:593-600. [DOI] [PubMed] [Google Scholar]
- 19.Hall, R. M., C. M. Collis, M. J. Kim, S. R. Partridge, G. D. Recchia, and H. W. Stokes. 1999. Mobile gene cassettes and integrons in evolution. Ann. N. Y. Acad. Sci. 870:68-80. [DOI] [PubMed] [Google Scholar]
- 20.Hochhut, B., Y. Lotfi, D. Mazel, S. M. Faruque, R. Woodgate, and M. K. Waldor. 2001. Molecular analysis of antibiotic resistance gene clusters in Vibrio cholerae O139 and O1 SXT constins. Antimicrob. Agents Chemother. 45:2991-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes, A. J., M. R. Gillings, B. S. Nield, B. C. Mabbutt, K. M. Nevalainen, and H. W. Stokes. 2003. The gene cassette metagenome is a basic resource for bacterial genome evolution. Environ. Microbiol. 5:383-394. [DOI] [PubMed] [Google Scholar]
- 22.Ito, H., Y. Arakawa, S. Ohsuka, R. Wacharotayankun, N. Kato, and M. Ohta. 1995. Plasmid-mediated dissemination of the metallo-beta-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob. Agents Chemother. 39:824-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kagan, S. A., and J. E. Davies. 1980. Enzymatic modification of aminocyclitol antibiotics: mutations affecting the expression of aminocyclitol acetyltransferase-3. Plasmid 3:312-318. [DOI] [PubMed] [Google Scholar]
- 24.Laramee, L., J. R. Lawrence, and C. W. Greer. 2000. Molecular analysis and development of 16S rRNA oligonucleotide probes to characterize a diclofop-methyl-degrading biofilm consortium. Can. J. Microbiol. 46:133-142. [DOI] [PubMed] [Google Scholar]
- 25.Liu, Z., H. Yang, Z. Huang, P. Zhou, and S. J. Liu. 2002. Degradation of aniline by newly isolated, extremely aniline-tolerant Delftia sp. AN3. Appl. Microbiol. Biotechnol. 58:679-682. [DOI] [PubMed] [Google Scholar]
- 26.Llanes, C., C. Neuwirth, F. El Garch, D. Hocquet, and P. Plesiat. 2006. Genetic analysis of a multiresistant strain of Pseudomonas aeruginosa producing PER-1 beta-lactamase. Clin. Microbiol. Infect. 12:270-278. [DOI] [PubMed] [Google Scholar]
- 27.Mazel, D. 2006. Integrons: agents of bacterial evolution. Nat. Rev. Microbiol. 4:608-620. [DOI] [PubMed] [Google Scholar]
- 28.Mazel, D., B. Dychinco, V. A. Webb, and J. Davies. 1998. A distinctive class of integron in the Vibrio cholerae genome. Science 280:605-608. [DOI] [PubMed] [Google Scholar]
- 29.Mazel, D., B. Dychinco, V. A. Webb, and J. Davies. 2000. Antibiotic resistance in the ECOR collection: integrons and identification of a novel aad gene. Antimicrob. Agents Chemother. 44:1568-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messier, N., and P. H. Roy. 2001. Integron integrases possess a unique additional domain necessary for activity. J. Bacteriol. 183:6699-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michael, C. A., M. R. Gillings, A. J. Holmes, L. Hughes, N. R. Andrew, M. P. Holley, and H. W. Stokes. 2004. Mobile gene cassettes: a fundamental resource for bacterial evolution. Am. Nat. 164:1-12. [DOI] [PubMed] [Google Scholar]
- 32.Nemergut, D. R., A. P. Martin, and S. K. Schmidt. 2004. Integron diversity in heavy-metal-contaminated mine tailings and inferences about integron evolution. Appl. Environ. Microbiol. 70:1160-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunes-Duby, S. E., H. J. Kwon, R. S. Tirumalai, T. Ellenberger, and A. Landy. 1998. Similarities and differences among 105 members of the Int family of site-specific recombinases. Nucleic Acids Res. 26:391-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osano, E., Y. Arakawa, R. Wacharotayankun, M. Ohta, T. Horii, H. Ito, F. Yoshimura, and N. Kato. 1994. Molecular characterization of an enterobacterial metallo-beta-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob. Agents Chemother. 38:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ploy, M. C., P. Courvalin, and T. Lambert. 1998. Characterization of In40 of Enterobacter aerogenes BM2688, a class 1 integron with two new gene cassettes, cmlA2 and qacF. Antimicrob. Agents Chemother. 42:2557-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radstrom, P., O. Skold, G. Swedberg, J. Flensburg, P. H. Roy, and L. Sundstrom. 1994. Transposon Tn5090 of plasmid R751, which carries an integron, is related to Tn7, Mu, and the retroelements. J. Bacteriol. 176:3257-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramos, J. L., M. Martinez-Bueno, A. J. Molina-Henares, W. Teran, K. Watanabe, X. Zhang, M. T. Gallegos, R. Brennan, and R. Tobes. 2005. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 69:326-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramos-Gonzalez, M. I., E. Duque, and J. L. Ramos. 1991. Conjugational transfer of recombinant DNA in cultures and in soils: host range of Pseudomonas putida TOL plasmids. Appl. Environ. Microbiol. 57:3020-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowe-Magnus, D. A., and D. Mazel. 2001. Integrons: natural tools for bacterial genome evolution. Curr. Opin. Microbiol. 4:565-569. [DOI] [PubMed] [Google Scholar]
- 40.Schleheck, D., T. P. Knepper, K. Fischer, and A. M. Cook. 2004. Mineralization of individual congeners of linear alkylbenzenesulfonate by defined pairs of heterotrophic bacteria. Appl. Environ. Microbiol. 70:4053-4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schluter, A., H. Heuer, R. Szczepanowski, S. M. Poler, S. Schneiker, A. Puhler, and E. M. Top. 2005. Plasmid pB8 is closely related to the prototype IncP-1β plasmid R751 but transfers poorly to Escherichia coli and carries a new transposon encoding a small multidrug resistance efflux protein. Plasmid 54:135-148. [DOI] [PubMed] [Google Scholar]
- 42.Senda, K., Y. Arakawa, S. Ichiyama, K. Nakashima, H. Ito, S. Ohsuka, K. Shimokata, N. Kato, and M. Ohta. 1996. PCR detection of metallo-beta-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum beta-lactams. J. Clin. Microbiol. 34:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shibata, N., Y. Doi, K. Yamane, T. Yagi, H. Kurokawa, K. Shibayama, H. Kato, K. Kai, and Y. Arakawa. 2003. PCR typing of genetic determinants for metallo-beta-lactamases and integrases carried by gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J. Clin. Microbiol. 41:5407-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shigematsu, T., K. Yumihara, Y. Ueda, M. Numaguchi, S. Morimura, and K. Kida. 2003. Delftia tsuruhatensis sp. nov., a terephthalate-assimilating bacterium isolated from activated sludge. Int. J. Syst. Bacteriol. 53:1479-1483. [DOI] [PubMed] [Google Scholar]
- 45.Sota, M., H. Kawasaki, and M. Tsuda. 2003. Structure of haloacetate-catabolic IncP-1β plasmid pUO1 and genetic mobility of its residing haloacetate-catabolic transposon. J. Bacteriol. 185:6741-6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stokes, H. W., A. J. Holmes, B. S. Nield, M. P. Holley, K. M. Nevalainen, B. C. Mabbutt, and M. R. Gillings. 2001. Gene cassette PCR: sequence-independent recovery of entire genes from environmental DNA. Appl. Environ. Microbiol. 67:5240-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stokes, H. W., C. L. Nesbo, M. Holley, M. I. Bahl, M. R. Gillings, and Y. Boucher. 2006. Class 1 integrons potentially predating the association with Tn402-like transposition genes are present in a sediment microbial community. J. Bacteriol. 188:5722-5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szczepanowski, R., I. Krahn, A. Puhler, and A. Schluter. 2004. Different molecular rearrangements in the integron of the IncP-1β resistance plasmid pB10 isolated from a wastewater treatment plant result in elevated beta-lactam resistance levels. Arch. Microbiol. 182:429-435. [DOI] [PubMed] [Google Scholar]
- 49.Szekeres, S., M. Dauti, C. Wilde, D. Mazel, and D. A. Rowe-Magnus. 22 January 2007. Chromosomal toxin-antitoxin loci can diminish large-scale genome reductions in the absence of selection. Mol. Microbiol. doi: 10.1111/j/1365-2958.2007.05613.x. [DOI] [PubMed]
- 50.Tennstedt, T., R. Szczepanowski, S. Braun, A. Puhler, and A. Schluter. 2003. Occurrence of integron-associated resistance gene cassettes located on antibiotic resistance plasmids isolated from a wastewater treatment plant. FEMS Microbiol. Ecol. 45:239-252. [DOI] [PubMed] [Google Scholar]
- 51.Vacca, D. J., W. F. Bleam, and W. J. Hickey. 2005. Isolation of soil bacteria adapted to degrade humic acid-sorbed phenanthrene. Appl. Environ. Microbiol. 71:3797-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaisvila, R., R. D. Morgan, J. Posfai, and E. A. Raleigh. 2001. Discovery and distribution of super-integrons among pseudomonads. Mol. Microbiol. 42:587-601. [DOI] [PubMed] [Google Scholar]
- 53.Walcott, R. R., A. Fessehaie, and A. C. Castro. 2004. Differences in pathogenicity between two genetically distinct groups of Acidovorax avenae subsp. citrulli on cucurbit hosts. J. Phytopathol. 152:277-285. [Google Scholar]
- 54.Wen, A., M. Fegan, C. Hayward, S. Chakraborty, and L. I. Sly. 1999. Phylogenetic relationships among members of the Comamonadaceae, and description of Delftia acidovorans (den Dooren de Jong 1926 and Tamaoka et al. 1987) gen. nov., comb. nov. Int. J. Syst. Bacteriol. 49:567-576. [DOI] [PubMed] [Google Scholar]
- 55.Wohlleben, W., W. Arnold, L. Bissonnette, A. Pelletier, A. Tanguay, P. H. Roy, G. C. Gamboa, G. F. Barry, E. Aubert, J. Davies, et al. 1989. On the evolution of Tn21-like multiresistance transposons: sequence analysis of the gene (aacC1) for gentamicin acetyltransferase-3-I (AAC(3)-I), another member of the Tn21-based expression cassette. Mol. Gen. Genet. 217:202-208. [DOI] [PubMed] [Google Scholar]