Abstract
The bacterium Aggregatibacter actinomycetemcomitans is a common commensal of the human oral cavity and the putative causative agent of the disease localized aggressive periodontitis. A. actinomycetemcomitans is a slow-growing bacterium that possesses limited metabolic machinery for carbon utilization. This likely impacts its ability to colonize the oral cavity, where growth and community composition is mediated by carbon availability. We present evidence that in the presence of the in vivo relevant carbon substrates glucose, fructose, and lactate A. actinomycetemcomitans preferentially metabolizes lactate. This preference for lactate exists despite the fact that A. actinomycetemcomitans grows faster and obtains higher cell yields during growth with carbohydrates. The preference for lactate is mediated by a novel exclusion mechanism in which metabolism of lactate inhibits carbohydrate uptake. Coculture studies reveal that A. actinomycetemcomitans utilizes lactate produced by the oral bacterium Streptococcus gordonii, suggesting the potential for cross-feeding in the oral cavity.
Aggregatibacter (Actinobacillus) actinomycetemcomitans is a nonmotile, facultative anaerobic gram-negative bacterium found in the mammalian oral cavity (27). A. actinomycetemcomitans is the putative causative agent of localized aggressive periodontitis, a disease characterized by tissue destruction and tooth loss (38, 49). Within the oral cavity, A. actinomycetemcomitans resides in the gingival crevice, the area around the tooth bounded by the tooth surface on one side and the epithelium lining the gingiva on the other. The gingival crevice contains a robust microbial population that is consistently bathed in crevicular fluid, which differentiates it from the tooth surface, which is immersed in saliva. Crevicular fluid is a plasma exudate that passes through the gingiva and flows along the teeth (20) and contains a number of potential carbon sources including sugars, small acids, and amino acids (6, 11, 20, 48). A. actinomycetemcomitans primarily resides in the “moderate” pockets (4 to 6 mm in depth) in the gingival crevice instead of the deeper portions (9). Moderate pockets differ from deeper subgingival pockets by containing oxygen (19), and controlled studies in the laboratory indicate that A. actinomycetemcomitans exhibits enhanced growth in the presence of oxygen (32). Although the importance of A. actinomycetemcomitans as a common commensal and a potential pathogen has been appreciated for some time, virtually nothing is known about the growth and physiology of this bacterium.
The mammalian oral cavity is a diverse environment that contains an estimated 500 bacterial species (17). Although the microbiology of the oral cavity has been the subject of study for many years, surprisingly little is known about the basic nutritional preference of oral bacteria. Compared to many common bacteria within the oral cavity, such as the streptococci, A. actinomycetemcomitans grows slowly and possesses the enzymatic capabilities to utilize only a small number of carbon sources, including glucose, fructose, maltose, mannose, and lactate (5). Streptococci on the other hand, are fast-growing bacteria capable of quickly transporting and consuming a diversity of carbohydrates to produce small organic acids, primarily lactate. Importantly, cultural studies and investigations using specific DNA methods (18, 40) provide evidence for multiple species of streptococci in the gingival crevice. Depending upon the subject and method of sampling, the concentration of streptococci capable of producing lactate in the gingival crevice can vary from approximately 5% (26) to more than 60% (43) of the recoverable flora.
The first step in carbon catabolism is solute transport into the cell by active or passive means. In many bacteria, transport is accomplished by carbon-specific systems including the phosphoenolpyruvate (PEP)-dependent phosphotransferase systems (PTS) (33). PTS utilize phosphoryl transfer proteins to ultimately transfer phosphate from PEP to the transported carbohydrate. Aside from solute transport, PTS also function to exclude other non-PTS carbohydrates in a process termed inducer exclusion (35). Inducer exclusion involves the inhibition of proteins involved in transport and/or metabolism of non-PTS carbon sources by PTS system proteins, thus providing the bacterium with a molecular preference mechanism for PTS carbon sources.
The slow growth rate and limited carbon catabolic capabilities of A. actinomycetemcomitans present theoretical problems for the survival of this bacterium in the oral cavity. For example, A. actinomycetemcomitans possesses overlapping carbon substrate utilization profiles with many faster-growing bacteria in the oral cavity (such as streptococci). This is important in the oral cavity since metabolizable carbon likely limits growth and composition of the oral microbiota (12, 20, 22, 42). One potential survival mechanism for A. actinomycetemcomitans is to evolve carbon usage preferences that reduce competition with faster-growing members of the community. In many environments, competition for nutrients often leads to the evolution of resource (niche) partitioning, a phenomenon by which resources (such as catabolizable carbon sources) are subdivided such that competition is minimized. We present evidence here that, when provided with the physiologically relevant carbon sources glucose, fructose, and lactate, A. actinomycetemcomitans prefers lactate. This occurs despite the fact that A. actinomycetemcomitans grows faster and obtains higher cell yields during growth with carbohydrates. This preference for lactate is mediated by a novel exclusion mechanism in which metabolism of lactate to pyruvate inhibits the uptake of PTS carbohydrates.
MATERIALS AND METHODS
Bacterial strains and growth media.
A. actinomycetemcomitans strains VT1169 (24) and Y4 (3), along with Streptococcus gordonii strain Challis DL1.1 (ATCC 49818), were used in these studies. Liquid cultures were grown in the chemically defined Socransky's medium (39) lacking dl-mevalonic acid and hemin (referred to as chemically defined medium [CDM]). Cultures were grown with shaking at 165 rpm in a 37°C incubator with a 10% CO2 atmosphere. For growth curves, overnight cultures grown in CDM supplemented with 20 mM glucose, fructose, or l-lactate were diluted to an optical density at 600 nm (OD600) of 0.015 in CDM supplemented with 20 mM carbon source.
DNA manipulations and construction of A. actinomycetemcomitans mutants.
Standard methods were used to manipulate plasmids and DNA fragments (2). Restriction endonucleases and DNA modification enzymes were purchased from New England Biolabs. Chromosomal DNA from A. actinomycetemcomitans was isolated by using DNeasy tissue kits (QIAGEN), and plasmid isolations were performed by using QIAprep spin miniprep kits (QIAGEN). DNA fragments were purified by using QIAquick mini-elute PCR purification kits (QIAGEN), and PCR was performed by using the Expand Long Template PCR system (Roche).
For creation of the A. actinomycetemcomitans ldhA mutant, a 550-bp internal fragment of ldhA was amplified by PCR using the primers ldh-for (GAAGATCTCTGACGGTGGTTCTTATGCAG) and ldh-rev (GGGGTACCCATACCTGAGTGCATATCCCG) and cloned into the T/A cloning vector pGEM-T Easy (Promega) to create pSB100. pSB100 was digested with EcoRI to remove the ldhA internal fragment and ligated into the EcoRI-digested suicide plasmid pVT1461 (23) to create pSB101. pSB101 was transformed into E. coli SM10 λpir and introduced into A. actinomycetemcomitans by conjugation (24). A. actinomycetemcomitans ldhA mutants containing an integrated copy of pSB101 (by Campbell-type recombination) were selected on tryptic soy agar plates containing 0.5% yeast extract and 50 μg of spectinomycin/ml. Inactivation of ldhA was confirmed using PCR.
Microarray studies.
Logarithmic A. actinomycetemcomitans (OD600 = 0.1) were collected and mixed 1:1 with the RNA stabilizing reagent RNALater (Ambion). RNA was isolated by using the Gentra Versagene RNA isolation kit. DNA contamination was assessed with PCR amplification of the clpX gene, and RNA integrity was monitored with agarose gel electrophoresis of glyoxylated samples (Ambion). RNA was prepared for hybridization to a custom A. actinomycetemcomitans Affymetrix GeneChip microarray as previously described (34, 37). Washing, staining, and scanning of the GeneChips was performed at the University of Iowa DNA core facility using an Affymetrix fluidics station. GeneChip analyses were performed in duplicate or triplicate for each condition. The data analysis was performed by using GeneChip Operating Software version 1.4 (GCOS).
Carbohydrate and small-molecule measurements.
A. actinomycetemcomitans organisms grown in CDM supplemented with 20 mM glucose, fructose, or l-lactate were washed with warm CDM containing no carbon source and resuspended to an OD600 of 0.1 in 50 ml of warm CDM supplemented with 2 mM each fructose, glucose, and l-lactate. Then, 1-ml samples were collected at various time points and centrifuged in a tabletop centrifuge at 9,000 × g for 2 min, and the supernatants were filter sterilized through a 0.2-μm-pore-size filter. Supernatants were stored at −20°C until analyzed for the presence of fructose and glucose by using the fructose assay kit and glucose assay kit (Sigma-Aldrich, catalog no. FA-20 and GAHK-20, respectively). Lactate concentrations were determined by using a lactate assay kit (SUNY at Buffalo, catalog no. A-108). For lactate spike studies, overnight cultures of A. actinomycetemcomitans grown in CDM supplemented with 20 mM glucose or fructose were washed with warm CDM containing no carbon source and resuspended to an OD600 of 0.1 in warm CDM containing 2 mM glucose or fructose. After 1 h of incubation, 2 mM l-lactate was added, and the consumption of carbohydrates and l-lactate was monitored over time as described above. For intracellular pyruvate levels, cell extracts were prepared as outlined previously (44) using logarithmic carbohydrate and l-lactate-grown bacteria, and the pyruvate levels were measured as previously described (1) using sodium pyruvate (Sigma) to generate a standard curve.
Radiolabeled substrate studies.
For glucose uptake studies, an overnight culture of A. actinomycetemcomitans grown in CDM supplemented with 20 mM glucose was diluted 1:1 with warm CDM supplemented with 20 mM glucose and then incubated for 1 h shaking at 37°C. The cells were then washed with warm CDM containing no carbon source and resuspended to an OD600 of 0.1 in CDM supplemented with 200 μM or 2 mM l-lactate, 2 mM fructose, or water. The cultures were incubated in a 37°C heating block. After 5 min, 100 μM uniformly 14C-labeled glucose ([U-14C]glucose; Amersham) was added to each sample. At various time points, 100-μl samples were removed and quenched in 2.9 ml of ice-cold CDM containing 20 mM unlabeled glucose. Each sample was filtered through a 0.2-μm-pore-size filter, followed by 3 ml of ice-cold CDM, followed by 10 ml of air. Filters were placed in scintillation vials containing 4 ml of Ecolite scintillation fluid and counted in an LS6500 scintillation counter (Beckman-Coulter). As a control for nonspecific (background) radioactivity, glucose-grown heat-killed A. actinomycetemcomitans (95°C for 10 min) was utilized.
A. actinomycetemcomitans consumption of S. gordonii-produced lactate.
Overnight cultures of S. gordonii were washed and suspended in CDM supplemented with 2 mM sucrose. Growth was monitored, and samples were collected throughout the growth curve and frozen at −20°C until analyzed for sucrose with a sucrose assay kit (Sigma, product code SCA-20) and lactate as described above. Once in stationary phase, cells were removed by centrifugation, the pH of the supernatant was adjusted to 7.0, and the supernatant was filter sterilized through a 0.2-μm-pore-size filter. The supernatant was then inoculated with A. actinomycetemcomitans to an OD600 of 0.025. Samples were removed and analyzed for lactate disappearance.
RESULTS
Growth kinetics of A. actinomycetemcomitans in defined medium with in vivo relevant carbon sources.
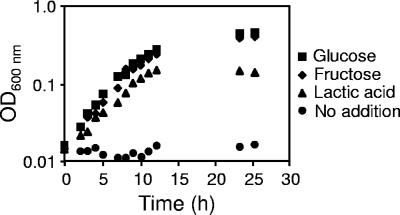
The composition of crevicular fluid is similar to that of plasma and, as such, the fluid contains several potential energy sources for A. actinomycetemcomitans, including glucose, fructose, mannose, and lactate. Two of these energy sources (glucose and lactate) are commonly found at high levels (approximately 1 to 5 mM) (28), while the others are found at low micromolar levels in crevicular fluid and/or plasma (28, 41). We chose to focus on A. actinomycetemcomitans growth using glucose and lactate since they are the most prevalent in vivo and fructose since it is a well-studied PTS-transported carbohydrate present at low levels in vivo (16, 25, 29-31). To examine growth using these in vivo relevant substrates, we utilized a CDM supplemented with these substrates as sole energy sources. The growth kinetics indicate that A. actinomycetemcomitans grows well in CDM supplemented with glucose, fructose, and l-lactate as sole energy sources (Fig. 1). Doubling times were faster in glucose (139 min) and fructose (143 min) than in lactate (204 min), and final cell yields were ∼2-fold higher after growth with carbohydrates compared to growth with lactate (Fig. 1).
FIG. 1.
Growth of A. actinomycetemcomitans in CDM containing glucose, fructose, l-lactate, and no energy source (no addition). Bacteria were grown shaking (165 rpm) at 37°C with a 10% CO2 atmosphere with 20 mM carbon source. Growth curves were determined at least three times, and representative data are provided.
A. actinomycetemcomitans prefers lactate to glucose and fructose.
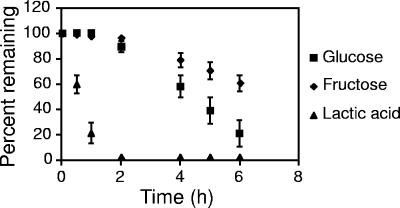
Microorganisms often exhibit catabolic preference for specific carbon sources. Although A. actinomycetemcomitans is capable of growth using glucose, fructose, and lactate, nothing is currently known about carbon preference in this bacterium. To examine this preference, exponential A. actinomycetemcomitans were washed and suspended in CDM containing these three substrates, and the cultures were examined for carbon substrate disappearance. Within 2 h, A. actinomycetemcomitans consumed all detectable lactate whereas most of the glucose (∼90%) and fructose (>90%) remained (Fig. 2). Upon the disappearance of lactate, glucose and fructose were consumed concomitantly, with glucose disappearance occurring at a slightly elevated rate compared to fructose. These results indicate that although A. actinomycetemcomitans doubles approximately 1 h slower with lactate compared to glucose or fructose, this bacterium preferentially catabolizes lactate. The growth substrate of the bacterial inoculum did not impact this preference, since identical carbon consumption profiles were observed when bacteria were grown in glucose, lactate, and fructose before washing and resuspension (data not shown).
FIG. 2.
Glucose, fructose, and l-lactate consumption by A. actinomycetemcomitans. A. actinomycetemcomitans was grown in the presence of equimolar (2 mM) glucose, fructose, and l-lactate, and the disappearance of these carbon substrates was evaluated over time. Experiments were performed in duplicate. Error bars represent the standard deviation and in some cases are too small to be seen.
Lactate rapidly inhibits glucose consumption.
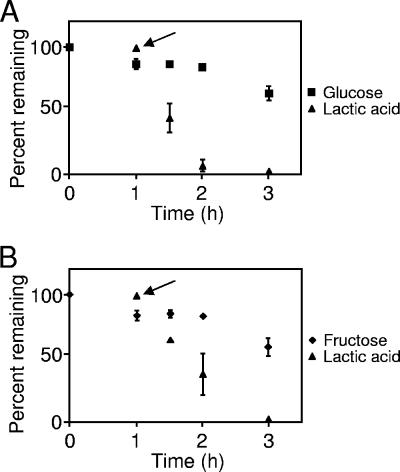
Our carbon consumption experiments indicated that when provided equimolar amounts of glucose, fructose, and lactate A. actinomycetemcomitans will selectively consume all detectable lactate before glucose and fructose. From these experiments, it is not clear whether addition of lactate immediately inhibits glucose consumption or whether the effect is delayed. The kinetics of lactate-mediated inhibition of carbohydrate catabolism is critical, since it will provide clues to the mechanism of this process. To examine the kinetics of this process, A. actinomycetemcomitans was suspended in CDM containing glucose. After 1 h, A. actinomycetemcomitans consumed ca. 20% of the glucose (Fig. 3A). Lactate was then added to the culture, and glucose and lactate levels were measured for the next 2 h. Lactate completely inhibited glucose consumption by A. actinomycetemcomitans, and this inhibition was not alleviated until all detectable lactate was consumed (Fig. 3A). Similar experiments revealed that lactate also inhibited fructose consumption, a finding consistent with its effect on glucose (Fig. 3B).
FIG. 3.
Addition of lactate inhibits glucose and fructose consumption by A. actinomycetemcomitans. A. actinomycetemcomitans was inoculated into CDM containing glucose (A) or fructose (B) at time zero. After growth for 1 h, l-lactate was added (where designated by the arrow), and the consumption of each carbon source was monitored for 3 h. Experiments were performed in duplicate. Error bars represent standard deviation and in some cases are too small to be seen.
Lactate inhibits glucose transport in A. actinomycetemcomitans.
Although it is clear that A. actinomycetemcomitans preferentially metabolizes lactate, the mechanism of lactate-mediated catabolite repression is unknown. In many bacteria, carbon preference is often controlled at the transcriptional level. Since lactate is the preferred carbon source in A. actinomycetemcomitans, one potential mechanism is that lactate represses transcription of genes required for glucose and fructose catabolism. To test this mechanism, a transcriptome analysis of A. actinomycetemcomitans was performed for glucose, fructose, and lactate-grown cells using a custom Affymetrix GeneChip microarray. Results from these experiments revealed that genes involved in transport and catabolism of glucose and lactate are not significantly regulated at the transcriptional level by the different carbon sources (see Table S1 in the supplemental material).
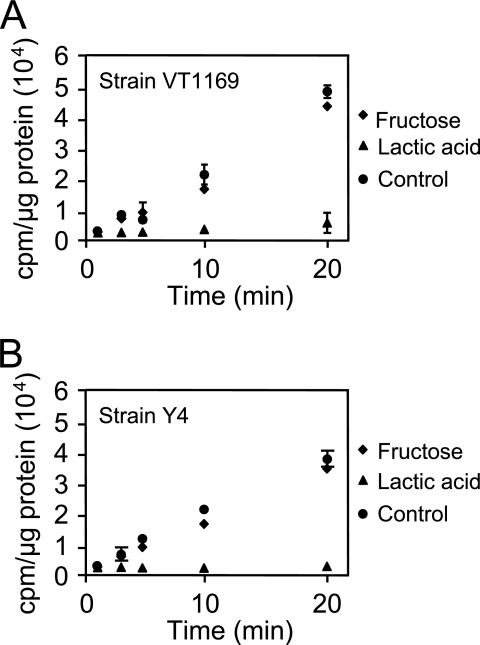
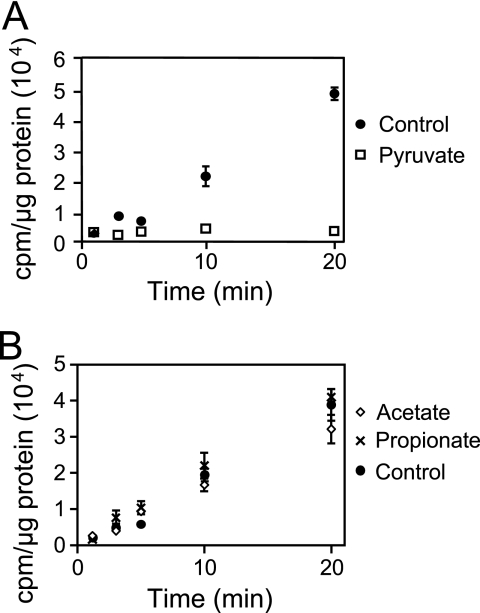
In silico examination of the A. actinomycetemcomitans genome reveals that transport of four of the five known growth substrates (glucose, fructose, mannose, and maltose) likely proceeds via PTS (although alternative transport mechanisms may exist for maltose), whereas transport of lactate likely proceeds via a proton-driven transporter (encoded by lctP) (www.genome.ou.edu/act.html). Thus, a potentially effective mechanism for lactate preference is for lactate to inhibit uptake of PTS carbohydrates into the cell. This mechanism is supported by our observation that addition of lactate rapidly and completely abrogates consumption of the PTS-transported carbohydrates glucose or fructose (Fig. 3). To examine the impact of lactate on carbohydrate transport, exponential glucose-grown A. actinomycetemcomitans was washed and resuspended in CDM containing no catabolizable carbon source. Lactate, fructose, or water (as a control) was added to the cell suspensions, followed by 14C-labeled glucose, and cell-associated radioactivity examined. Linear uptake of glucose was observed in bacteria treated with water and fructose (Fig. 4A); however, no radioactivity (compared to the background) was found associated with bacteria exposed to lactate. These results indicate that lactate addition inhibits uptake of glucose by A. actinomycetemcomitans. The inhibition of glucose transport was not specific to the concentration of lactate utilized since experiments with 10-fold less lactate (200 μM) exhibited similar results (data not shown). This inhibition of glucose transport is also not strain specific since lactate inhibited glucose transport in another common laboratory strain of A. actinomycetemcomitans (Fig. 4B).
FIG. 4.
Lactate inhibits uptake of glucose in A. actinomycetemcomitans strains VT1169 (A) and Y4 (B). A. actinomycetemcomitans was suspended in CDM containing fructose, l-lactate, or no energy source (control). Radiolabeled glucose ([U-14C]glucose) was then added, and uptake was examined as outlined in Materials and Methods. Uptake of radiolabeled glucose is expressed as cpm/μg of A. actinomycetemcomitans protein. Experiments were performed in triplicate. Error bars represent the standard deviation and in some cases are too small to be seen. The background radioactivity using heat-killed cells was 187 ± 27 cpm/μg of protein.
Pyruvate also inhibits glucose transport in A. actinomycetemcomitans.
Our results indicate that addition of lactate inhibits transport of glucose; however, it is not clear whether this effect is mediated by lactate itself or a downstream product of lactate metabolism. The first step in lactate catabolism is the conversion of lactate to pyruvate by the enzyme lactate dehydrogenase. There are several potential fates of pyruvate, but the primary products formed during lactate fermentation are mixtures of propionate, acetate, formate, and succinate (15, 47). Thus, it is plausible that one of these products, and not lactate itself, is responsible for the inhibition of glucose transport and consumption. To test this, the impact of pyruvate, propionate, acetate, formate, and succinate on glucose transport was assessed. Results from these experiments reveal that pyruvate, but not the other organic acids, inhibit glucose uptake by A. actinomycetemcomitans (Fig. 5).
FIG. 5.
Pyruvate inhibits uptake of glucose in A. actinomycetemcomitans. A. actinomycetemcomitans was suspended in CDM and incubated with no energy source (control) or pyruvate (A) or with no energy source (control), acetate, or propionate (B). Radiolabeled glucose ([U-14C]glucose) was then added, and uptake was examined as outlined in Materials and Methods. The uptake of radiolabeled glucose is expressed as cpm/μg of A. actinomycetemcomitans protein. Formate and succinate also had no effect on glucose transport (data not shown). Experiments were performed in duplicate. Error bars represent the standard deviation and in some cases are too small to be seen.
Lactate catabolism is required for inhibition of glucose transport.
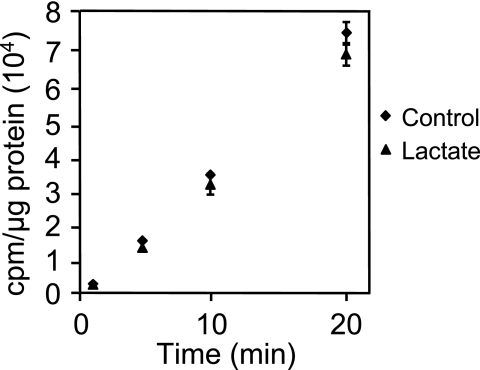
Two possibilities exist to explain the observations that both lactate and pyruvate inhibit A. actinomycetemcomitans glucose transport: (i) both pyruvate and lactate effectively inhibit glucose transport, and (ii) pyruvate alone is responsible for this transport inhibition. To discriminate between these possibilities, we genetically inactivated the l-lactate dehydrogenase gene (ldhA) in A. actinomycetemcomitans. LdhA is required for the conversion of l-lactate to pyruvate; thus, the ldhA mutant is unable to grow using l-lactate as a sole energy source but grows normally on glucose (data not shown). We then examined the impact of lactate on glucose transport in the ldhA mutant, with the rationale that the inability of this strain to convert lactate to pyruvate will provide clues to the direct effect of lactate on glucose transport. Results from these experiments reveal that lactate does not inhibit glucose transport in the ldhA mutant (Fig. 6), indicating that the ability to catabolize lactate is required for lactate-mediated inhibition of glucose transport. It should be noted that, as in the wild type, pyruvate inhibits glucose transport in the ldhA mutant (data not shown). These data strongly support that pyruvate, and not lactate, is critical for inhibition of glucose transport during growth with lactate.
FIG. 6.
Lactate dehydrogenase is required for the inhibition of glucose uptake. The A. actinomycetemcomitans l-lactate dehydrogenase (ldhA) mutant was suspended in CDM and incubated with no energy source (control) or l-lactate. Radiolabeled glucose was then added, and uptake was examined as outlined in Materials and Methods. Uptake of radiolabeled glucose is expressed as cpm/μg of A. actinomycetemcomitans protein. Experiments were performed in duplicate. Error bars represent the standard deviation and in some cases are too small to be seen.
Growth with lactate increases intracellular levels of pyruvate.
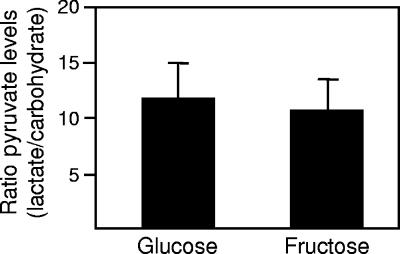
Although it is clear that pyruvate inhibits glucose transport in A. actinomycetemcomitans, the molecular mechanism is not known. Obviously, pyruvate will be generated during growth on all substrates; however, it is possible that the levels of intracellular pyruvate generated are substrate specific. Based on previous studies showing that high levels of pyruvate significantly inhibit PTS carbohydrate uptake (14, 35, 36), we hypothesized that intracellular levels of pyruvate increase during A. actinomycetemcomitans growth with lactate. To test this hypothesis, intracellular pyruvate levels were measured during growth with lactate and the PTS-transported sugars glucose and fructose. The results from these experiments indicate that the intracellular levels of pyruvate increase significantly (>10-fold) during growth using lactate as opposed to PTS carbohydrates (Fig. 7).
FIG. 7.
Lactate-grown A. actinomycetemcomitans contain higher levels of pyruvate. Intracellular levels of pyruvate were measured for logarithmic glucose-, fructose-, and l-lactate-grown A. actinomycetemcomitans as previously described (1) and are presented as fold increases during growth with l-lactate. Error bars represent the standard deviation, and experiments were performed in triplicate. Intracellular volume was calculated based on normal size measurements of A. actinomycetemcomitans (1.6 μm by 0.5 μm by 0.5 μm).
The biological significance of lactate preference.
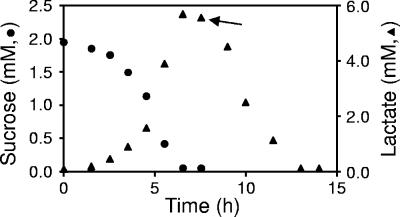
A. actinomycetemcomitans resides in the human oral cavity in a multispecies consortium that includes numerous species of the genus Streptococcus. Streptococcus spp. generally possess fast doubling times (<1 h) and comprise a large percentage of the total bacteria within the oral cavity (8, 13). Most streptococci produce significant amounts of lactate which cause localized decreases in pH. Based on the A. actinomycetemcomitans preference for lactate, we hypothesized that A. actinomycetemcomitans would consume lactate produced by streptococci. To test this hypothesis, S. gordonii, a prevalent streptococcus within the oral cavity, was grown in CDM containing sucrose. As expected, S. gordonii consumed the sucrose with a concomitant production of lactate (Fig. 8). Upon addition of A. actinomycetemcomitans, lactate was consumed (Fig. 8). These results indicate that although A. actinomycetemcomitans does not grow using sucrose, this bacterium is capable of growing on lactate produced by sucrose-grown S. gordonii.
FIG. 8.
A. actinomycetemcomitans utilizes lactate produced by S. gordonii. S. gordonii was grown in CDM containing sucrose, and sucrose and/or lactate levels were measured throughout the growth period. After 8 h, A. actinomycetemcomitans was added (as designated by the arrow), and lactate levels were measured. Experiments were performed in duplicate. Standard deviations are <5% of the mean for sucrose and <9% of the mean for lactate and were not included for the sake of clarity.
DISCUSSION
The human oral cavity is a nutritionally complex environment that supports growth and colonization of more than 500 bacterial species (17). Although multiple carbon substrates exist in this environment, previous studies suggest that competition for carbon and energy is intense in this environment (12, 20, 22, 42); thus it is likely that, to minimize competition, bacteria in the oral cavity have evolved carbon source preferences that do not overlap (resource partitioning). In the presence of multiple carbon substrates, bacteria often utilize only one carbon substrate at a time. Several mechanisms have been identified to allow preferential consumption of energy sources, including transcriptional control of substrate-specific catabolic genes and inducer exclusion (7, 35, 45). Our data illustrate that A. actinomycetemcomitans exhibits little or no differential transcription of catabolic genes based on the carbon substrate (see Table S1 in the supplemental material) but instead mediates preference at the level of carbohydrate transport. Lactate preference in A. actinomycetemcomitans is somewhat analogous to inducer exclusion, a regulatory process whereby one carbon source inhibits transport and/or catabolism of other carbon sources. However, the paradigm for inducer exclusion is PTS sugars inhibiting transport or catabolism of other non-PTS sugars, thereby preventing these sugars from entering cells and transcriptionally activating specific carbon catabolism genes (35). Our results demonstrate the converse scenario, in which a non-PTS carbon substrate (lactate) inhibits the transport of PTS sugars, and that transcriptional induction of specific catabolic genes is not required. Thus, A. actinomycetemcomitans has evolved a novel mechanism for carbon source preference that we refer to as PTS substrate exclusion.
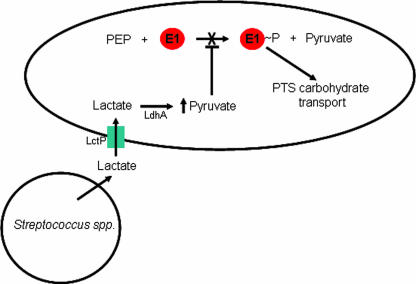
Although the molecular mechanism of PTS substrate exclusion is unknown, our results provide strong evidence that pyruvate and not lactate mediates this process (Fig. 5 and 6). The observation that intracellular levels of pyruvate increase >10-fold during growth with lactate compared to PTS transported sugars (Fig. 7) provides clues to the mechanism. PTS-mediated carbohydrate transport involves transfer of phosphate from PEP through a series of proteins and ultimately to the carbohydrate (33). The first step in PTS sugar transport involves an autophosphorylation reaction in which protein E1 catalyzes the transfer of phosphate from PEP to itself, yielding phosphorylated E1 and pyruvate (Fig. 9). Previous studies of E1 from E. coli (which is 84% similar to the putative E1 from A. actinomycetemcomitans) indicate that high levels of pyruvate (≥20 mM) inhibit autophosphorylation, thereby preventing the flow of phosphate to the carbohydrate (36). Interestingly, our measurements indicate pyruvate levels of >50 mM during A. actinomycetemcomitans growth with lactate, suggesting that these levels are sufficient to significantly inhibit carbohydrate transport via PTS. From these data, we propose a model in which the high levels of intracellular pyruvate produced during growth with lactate inhibit E1 autophosphorylation (Fig. 9). Since E1 phosphorylation is required for the transport of all PTS sugars, this mechanism provides A. actinomycetemcomitans with a single mechanism for exclusion of PTS sugars. Alternative mechanisms exist for lactate-mediated carbohydrate exclusion, including the possibility that, instead of inhibiting E1 autophosphorylation, high levels of intracellular pyruvate initiate the conversion of phosphorylated E1 and pyruvate to E1 and PEP (the reverse reaction of that shown in Fig. 9). Although this reaction has been demonstrated in vitro (46), we do not favor this model since this reaction is thermodynamically unfavorable compared to the forward reaction and therefore not likely to occur in vivo.
FIG. 9.
Model for lactate-mediated inhibition of glucose transport in A. actinomycetemcomitans during growth with streptococci. Lactate produced by Streptococcus spp. is transported into A. actinomycetemcomitans by lactate permease (LctP). Once inside the cell, intracellular pyruvate levels increase due to the conversion of lactate to pyruvate by lactate dehydrogenase (LdhA). Protein E1 normally autophosphorylates using PEP as the phospho-donor; however, elevated intracellular pyruvate levels inhibit this autophosphorylation. Since E1 is the first step in transport of PTS sugars, inhibition of E1 autophosphorylation inhibits the uptake of all PTS carbohydrates.
It is interesting that although A. actinomycetemcomitans grows fastest and obtains higher cell yields during growth with carbohydrates (Fig. 1), this bacterium has evolved to prefer lactate. The paradigm for carbon source preference is that bacteria prefer carbon sources that provide faster growth rates and/or higher growth yields. This is clearly not the case with A. actinomycetemcomitans, suggesting that growth in the oral cavity has selected for the preference for energy sources not ideal for monoculture growth in vitro. Other bacteria in the oral cavity, including Veillonella atypica (10, 21), utilize lactate as a carbon and/or energy source; however, these bacteria do not possess the ability to grow using carbohydrates such as glucose. A. actinomycetemcomitans is unique in that it possesses carbohydrate utilization pathways but has evolved preference for lactate.
The ecological importance of lactate preference in vivo is unknown. An obvious potential benefit is that the ability to catabolize lactate provides A. actinomycetemcomitans with an alternative carbon substrate that is not utilized by most fast-growing community members such as streptococci. A more intriguing question is how inhibition of glucose transport benefits A. actinomycetemcomitans in vivo. One benefit may lie in the interactions of A. actinomycetemcomitans with other oral community members. During fermentation of carbohydrates, streptococci produce lactate from pyruvate to regenerate the NAD necessary for growth and biosynthesis. Recent evidence indicates that high lactate levels inhibit this reaction, and pyruvate is instead converted to acetyl phosphate and H2O2 by pyruvate oxidase (4). Under these conditions, H2O2 can accumulate to levels sufficient for killing many bacterial community members. Thus, it is conceivable that preferential consumption of lactate by A. actinomycetemcomitans within the gingival crevice may reduce H2O2 production by streptococci, thereby protecting A. actinomycetemcomitans from H2O2-mediated stress. These models are currently being tested and should further our understanding of the ecological significance of PTS substrate exclusion in multispecies communities.
Supplementary Material
Acknowledgments
We thank Charles Schachtele and members of the Whiteley lab for critical discussions.
This study was supported by a grant from the NIH (5P20RR081741 to M.W.) as part of an NIH COBRE award to the University of Oklahoma Health Sciences Center (PI, Joseph J. Ferretti).
Footnotes
Published ahead of print on 22 June 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Anthon, G. E., and D. M. Barrett. 2003. Modified method for the determination of pyruvic acid with dinitrophenylhydrazine in the assessment of onion pungency. J. Sci. Food Agric. 83:1210-1213. [Google Scholar]
- 2.Ausubel, F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1997. Short protocols in molecular biology, 3rd ed. John Wiley & Sons, Inc., New York, NY.
- 3.Baehni, P., C. C. Tsai, W. P. McArthur, B. F. Hammond, and N. S. Taichman. 1979. Interaction of inflammatory cells and oral microorganisms. VIII. Detection of leukotoxic activity of a plaque-derived gram-negative microorganism. Infect. Immun. 24:233-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard, J. P., and M. W. Stinson. 1999. Influence of environmental conditions on hydrogen peroxide formation by Streptococcus gordonii. Infect. Immun. 67:6558-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergey, N., R. Krieg, and J. G. Holt. 1984. Bergey's manual of systematic bacteriology, vol. 4, p. 537. The Williams & Wilkins Co., Baltimore, MD. [Google Scholar]
- 6.Biswas, S., D. F. Duperon, and F. S. Chebib. 1977. Study of crevice fluid in relation to periodontal disease in children. II. Effect of age, sex and gingival inflammation on crevice fluid protein, carbohydrate, total calcium, phosphate and nitrogen. J. Periodontal Res. 12:265-278. [DOI] [PubMed] [Google Scholar]
- 7.Bruckner, R., and F. Titgemeyer. 2002. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol. Lett. 209:141-148. [DOI] [PubMed] [Google Scholar]
- 8.Diaz, P. I., N. I. Chalmers, A. H. Rickard, C. Kong, C. L. Milburn, R. J. Palmer, Jr., and P. E. Kolenbrander. 2006. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl. Environ. Microbiol. 72:2837-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dzink, J. L., A. C. Tanner, A. D. Haffajee, and S. S. Socransky. 1985. Gram-negative species associated with active destructive periodontal lesions. J. Clin. Periodontol. 12:648-659. [DOI] [PubMed] [Google Scholar]
- 10.Egland, P. G., R. J. Palmer, Jr., and P. E. Kolenbrander. 2004. Interspecies communication in Streptococcus gordonii-Veillonella atypica biofilms: signaling in flow conditions requires juxtaposition. Proc. Natl. Acad. Sci. USA 101:16917-16922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ficara, A. J., M. P. Levin, M. F. Grower, and G. D. Kramer. 1975. A comparison of the glucose and protein content of gingival fluid from diabetics and nondiabetics. J. Periodontal Res. 10:171-175. [DOI] [PubMed] [Google Scholar]
- 12.Folke, L. E., T. H. Gawronski, R. H. Staat, and R. S. Harris. 1972. Effect of dietary sucrose on quantity and quality of plaque. Scand. J. Dent. Res. 80:529-533. [DOI] [PubMed] [Google Scholar]
- 13.Frandsen, E. V., V. Pedrazzoli, and M. Kilian. 1991. Ecology of viridans streptococci in the oral cavity and pharynx. Oral Microbiol. Immunol. 6:129-133. [DOI] [PubMed] [Google Scholar]
- 14.Hogema, B. M., J. C. Arents, R. Bader, K. Eijkemans, H. Yoshida, H. Takahashi, H. Aiba, and P. W. Postma. 1998. Inducer exclusion in Escherichia coli by non-PTS substrates: the role of the PEP to pyruvate ratio in determining the phosphorylation state of enzyme IIAGlc. Mol. Microbiol. 30:487-498. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino, E., and M. Sato. 1986. Production and degradation of formate by Veillonella dispar ATCC 17745. J. Dent. Res. 65:903-905. [DOI] [PubMed] [Google Scholar]
- 16.Inoue, T., I. Tanimoto, T. Tada, T. Ohashi, K. Fukui, and H. Ohta. 2001. Fermentable-sugar-level-dependent regulation of leukotoxin synthesis in a variably toxic strain of Actinobacillus actinomycetemcomitans. Microbiology 147:2749-2756. [DOI] [PubMed] [Google Scholar]
- 17.Kolenbrander, P. E., R. N. Andersen, D. S. Blehert, P. G. Egland, J. S. Foster, and R. J. Palmer, Jr. 2002. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 66:486-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroes, I., P. W. Lepp, and D. A. Relman. 1999. Bacterial diversity within the human subgingival crevice. Proc. Natl. Acad. Sci. USA 96:14547-14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loesche, W. J., F. Gusberti, G. Mettraux, T. Higgins, and S. Syed. 1983. Relationship between oxygen tension and subgingival bacterial flora in untreated human periodontal pockets. Infect. Immun. 42:659-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcotte, H., and M. C. Lavoie. 1998. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol. Mol. Biol. Rev. 62:71-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikx, F. H., and J. S. Van der Hoeven. 1975. Symbiosis of Streptococcus mutans and Veillonella alcalescens in mixed continuous cultures. Arch. Oral Biol. 20:407-410. [DOI] [PubMed] [Google Scholar]
- 22.Minah, G. E., E. S. Solomon, and K. Chu. 1985. The association between dietary sucrose consumption and microbial population shifts at six oral sites in man. Arch. Oral Biol. 30:397-401. [DOI] [PubMed] [Google Scholar]
- 23.Mintz, K. P., C. Brissette, and P. M. Fives-Taylor. 2002. A recombinase A-deficient strain of Actinobacillus actinomycetemcomitans constructed by insertional mutagenesis using a mobilizable plasmid. FEMS Microbiol. Lett. 206:87-92. [DOI] [PubMed] [Google Scholar]
- 24.Mintz, K. P., and P. M. Fives-Taylor. 2000. impA, a gene coding for an inner membrane protein, influences colonial morphology of Actinobacillus actinomycetemcomitans. Infect. Immun. 68:6580-6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizoguchi, K., H. Ohta, A. Miyagi, H. Kurihara, S. Takashiba, K. Kato, Y. Murayama, and K. Fukui. 1997. The regulatory effect of fermentable sugar levels on the production of leukotoxin by Actinobacillus actinomycetemcomitans. FEMS Microbiol. Lett. 146:161-166. [DOI] [PubMed] [Google Scholar]
- 26.Moore, W. E., L. V. Holdeman, R. M. Smibert, D. E. Hash, J. A. Burmeister, and R. R. Ranney. 1982. Bacteriology of severe periodontitis in young adult humans. Infect. Immun. 38:1137-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norskov-Lauritsen, N., and M. Kilian. 2006. Reclassification of Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus, Haemophilus paraphrophilus and Haemophilus segnis as Aggregatibacter actinomycetemcomitans gen. nov., comb. nov., Aggregatibacter aphrophilus comb. nov., and Aggregatibacter segnis comb. nov., and emended description of Aggregatibacter aphrophilus to include V factor-dependent and V factor-independent isolates. Int. J. Syst. Evol. Microbiol. 56:2135-2146. [DOI] [PubMed] [Google Scholar]
- 28.Nuttall, F. Q., M. A. Khan, and M. C. Gannon. 2000. Peripheral glucose appearance rate following fructose ingestion in normal subjects. Metabolism 49:1565-1571. [DOI] [PubMed] [Google Scholar]
- 29.Ohta, H., K. Fukui, and K. Kato. 1989. Effect of bicarbonate on the growth of Actinobacillus actinomycetemcomitans in anaerobic fructose-limited chemostat culture. J. Gen. Microbiol. 135:3485-3495. [DOI] [PubMed] [Google Scholar]
- 30.Ohta, H., T. Inoue, and K. Fukui. 2001. Energy metabolism of Actinobacillus actinomycetemcomitans during anaerobic and microaerobic growth in low- and high-potassium continuous culture. Microbiology 147:2461-2468. [DOI] [PubMed] [Google Scholar]
- 31.Ohta, H., A. Miyagi, K. Kato, and K. Fukui. 1996. The relationships between leukotoxin production, growth rate and the bicarbonate concentration in a toxin-production-variable strain of Actinobacillus actinomycetemcomitans. Microbiology 142(Pt. 4):963-970. [DOI] [PubMed] [Google Scholar]
- 32.Ohta, H., D. Moriki, A. Miyagi, T. Watanabe, K. Kato, and K. Fukui. 1996. Microaerophilic property of Actinobacillus actinomycetemcomitans in fructose-limited chemostat cultures. FEMS Microbiol. Lett. 136:191-196. [Google Scholar]
- 33.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsey, M. M., and M. Whiteley. 2004. Pseudomonas aeruginosa attachment and biofilm development in dynamic environments. Mol. Microbiol. 53:1075-1088. [DOI] [PubMed] [Google Scholar]
- 35.Saier, M. H., Jr., and M. Crasnier. 1996. Inducer exclusion and the regulation of sugar transport. Res. Microbiol. 147:482-489. [DOI] [PubMed] [Google Scholar]
- 36.Saier, M. H., Jr., M. R. Schmidt, and P. Lin. 1980. Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Kinetic characterization. J. Biol. Chem. 255:8579-8584. [PubMed] [Google Scholar]
- 37.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slots, J., H. S. Reynolds, and R. J. Genco. 1980. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect. Immun. 29:1013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Socransky, S. S., J. L. Dzink, and C. M. Smith. 1985. Chemically defined medium for oral microorganisms. J. Clin. Microbiol. 22:303-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Socransky, S. S., and A. D. Haffajee. 2005. Periodontal microbial ecology. Periodontol. 2000 38:135-187. [DOI] [PubMed] [Google Scholar]
- 41.Soyama, K. 1984. Enzymatic determination of d-mannose in serum. Clin. Chem. 30:293-294. [PubMed] [Google Scholar]
- 42.Staat, R. H., T. H. Gawronski, D. E. Cressey, R. S. Harris, and L. E. Folke. 1975. Effects of dietary sucrose levels on the quantity and microbial composition of human dental plaque. J. Dent. Res. 54:872-880. [DOI] [PubMed] [Google Scholar]
- 43.Syed, S. A., and W. J. Loesche. 1978. Bacteriology of human experimental gingivitis: effect of plaque age. Infect. Immun. 21:821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas, T. D., D. C. Ellwood, and V. M. Longyear. 1979. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J. Bacteriol. 138:109-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ullmann, A. 1985. Catabolite repression 1985. Biochimie 67:29-34. [DOI] [PubMed] [Google Scholar]
- 46.Weigel, N., M. A. Kukuruzinska, A. Nakazawa, E. B. Waygood, and S. Roseman. 1982. Sugar transport by the bacterial phosphotransferase system: phosphoryl transfer reactions catalyzed by enzyme I of Salmonella typhimurium. J. Biol. Chem. 257:14477-14491. [PubMed] [Google Scholar]
- 47.White, D. 2000. The physiology and biochemistry of prokaryotes, 2nd ed., p. 363-383. Oxford University Press, New York, NY.
- 48.Yamaguchi, M., Y. Kawabata, S. Kambe, K. Wardell, F. H. Nystrom, K. Naitoh, and H. Yoshida. 2004. Non-invasive monitoring of gingival crevicular fluid for estimation of blood glucose level. Med. Biol. Eng. Comput. 42:322-327. [DOI] [PubMed] [Google Scholar]
- 49.Zambon, J. J. 1985. Actinobacillus actinomycetemcomitans in human periodontal disease. J. Clin. Periodontol. 12:1-20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.