Abstract
Chlamydia trachomatis is a major pathogen throughout the world, and preventive measures have focused on the production of a vaccine using the major outer membrane protein (MOMP). Here, in elementary bodies and in preparations of the outer membrane, we identified native trimers of the MOMP. The trimers were stable under reducing conditions, although disulfide bonds appear to be present between the monomers of a trimer and between trimers. Cross-linking of the outer membrane complex demonstrated that the MOMP is most likely not in a close spatial relationship with the 60- and 12-kDa cysteine-rich proteins. Extraction of the MOMP from Chlamydia isolates under nondenaturing conditions yielded the trimeric conformation of this protein as shown by cross-linking and analysis by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis with different concentrations of acrylamide. Using circular dichroism spectroscopy, we determined that the trimers were formed mainly of β-pleated sheet structures in detergent micelles. Using a liposomal swelling assay, the MOMP was found to have porin activity, and the size of the pore was estimated to be approximately 2 nm in diameter. The trimers were found to be stable in SDS at temperatures ranging from 4 to 37°C and over a pH range of 5.0 to 8.0. In addition, the trimers of MOMP were found to be resistant to digestion with trypsin. In conclusion, these results show that the native conformation of the MOMP of C. trachomatis is a trimer with predominantly a β-sheet structure and porin function.
Chlamydia strains are ubiquitous pathogens in nature (1, 31, 33, 70). These bacteria have two distinct morphological and functional forms, the elementary bodies (EB) and the reticulate bodies (RB) (33). The EB measure approximately 300 nm in diameter and have a rigid cell membrane, while the RB measure from 1,000 to 1,200 nm in diameter and have a highly pliable membrane. The EB infect eukaryotic cells and become transformed into RB that replicate inside a cytoplasmic inclusion. As a result of replication, the cytoplasmic inclusion progressively enlarges. Following 1 to 3 days of replication, the RB transform again into EB, and cell lysis releases the infectious EB.
Unlike other gram-negative bacteria, Chlamydia strains may lack a peptidoglycan layer (50). As a substitute for peptidoglycan, it has been postulated that the 60-kDa (OmcB) and the 12-kDa (OmcA) cysteine-rich proteins (CRPs), and maybe the major outer membrane protein (MOMP) and other membrane components, form a supramolecular structure that provides rigidity to the EB (16, 21, 37, 50, 63). The Chlamydia trachomatis MOMP belongs to a family of proteins found in the outer membranes of gram-negative bacteria that have a molecular mass of approximately 40 kDa and function as porins (40). Porins, such as OmpF and PhoE of Escherichia coli, form homotrimers held together by hydrophobic and electrostatic interactions (40, 48). Due to the unusual binding of sodium dodecyl sulfate (SDS), the trimers migrate on 10% SDS-polyacrylamide gel electrophoresis (PAGE) gels with an apparent molecular mass of only 65 to 70 kDa, and other conformational forms have been described for some of these proteins (9, 13, 26, 54).
Based on results of two-dimensional (2-D) gel electrophoresis, Newhall and Jones (38) previously proposed that the Chlamydia MOMP is a trimeric structure stabilized by disulfide bonds. Subsequently, Bavoil et al. (6) tested a preparation of the outer membrane of Chlamydia and tentatively ascribed the porin activity to the MOMP. DNA sequencing of the MOMP from various serovars of C. trachomatis demonstrated that this protein has four variable domains (VD) and five constant domains (59). The sequence variability of the VD has been considered to be a mechanism for the bacteria to escape the immunological surveillance of the host (3, 59). Using algorithms for the prediction of the secondary structure, models for the topology of the C. trachomatis mouse pneumonitis (MoPn) MOMP have been proposed (18, 51, 69). In the model proposed previously by Rodriguez-Marañon et al. (51), the MOMP has 16 antiparallel β-sheets forming a barrel-like structure consistent with that of other porins. The VD are located in four of the loops facing the cell exterior, and disulfide bridges located at the base of two of the VD have been mapped (72). Several studies have shown that the MOMP is a strong antigen and potentially a good vaccine candidate (3, 5, 11, 14, 45, 46). Thus, characterization of this protein may help not only to gain an understanding of the immunopathogenesis of this organism but also to develop preventive and therapeutic measures. Due to the difficulties in obtaining adequate quantities of Chlamydia, limited progress has been made in this area. Here, we extracted and purified the MOMP directly from C. trachomatis MoPn using nondenaturing methods, analyzed its structural conformation, and assayed its porin function. We chose to analyze the MOMP from C. trachomatis MoPn because this isolate is the most commonly used strain for the characterization of the immunopathogenesis of chlamydial infections and for testing of vaccine candidates in animal models.
MATERIALS AND METHODS
Preparation of stocks of C. trachomatis MoPn.
C. trachomatis serovar MoPn (strain Nigg II; ATCC VR 123 [also called Chlamydia muridarum]) was purchased from the American Type Culture Collection (Manassas, VA) (39). To prepare stocks, Chlamydia was grown in tissue culture flasks using L-929 (ATCC CCL 1) cells. Eagle's minimal essential medium was supplemented with 10% fetal bovine serum and 1 μg/ml of cycloheximide (10). The stocks were stored at −70°C in SPG (0.2 M sucrose, 0.02 M sodium phosphate [pH 7.2], and 0.005 M glutamic acid).
Purification of C. trachomatis MoPn EB.
To prepare stocks of purified EB, a method described previously by Caldwell et al. (10) was used. Infected HeLa-229 cells were ruptured by sonication and pelleted by centrifugation, and the crude preparation of Chlamydia was first purified by pelleting through a 35% Hypaque-76 (66% [wt/vol] diatrizoate meglumine and 10% [wt/vol] diatrizoate sodium; Nycomed Inc., Princeton, NJ) gradient. The pellet was collected, and the EB were further purified by ultracentrifugation at 260,000 × g on a 30%-35%-40%-step Hypaque-76 gradient. The EB banding in the interface between 35 and 40% Hypaque-76 were collected and stored at −70°C in SPG.
Isolation of C. trachomatis MoPn outer membrane complex.
The Chlamydia outer membrane complex (COMC) was extracted as previously described (10, 11). Approximately 2 to 3 mg of EB was resuspended in 5 ml of phosphate-buffered saline (PBS) (pH 8.0) containing 1.5 mM EDTA and 2% N-lauroylsarcosine sodium salt (Sarkosyl; International Biotechnologies, Inc., New Haven, CT). The suspension was sonicated, incubated at 37°C for 1 h, and centrifuged at 100,000 × g for 60 min at room temperature. The insoluble pellet was resuspended in the same Sarkosyl buffer and centrifuged as described above. The pellet was washed twice in PBS (pH 8.0) and resuspended in 500 μl of 0.02 M sodium phosphate (pH 8.0) containing 10 mM MgCl2 and 25 μg each of DNase I and RNase A (Sigma, St. Louis, MO). The suspension was incubated for 2 h at 37°C and centrifuged as described above. The insoluble pellet was washed twice with PBS to remove any remaining nucleases from the COMC.
Purification of C. trachomatis MoPn native MOMP.
The extraction and purification of the native C. trachomatis MoPn MOMP were described previously (46). Briefly, infected cell monolayers were centrifuged and washed with PBS (pH 7.4). The pellet was resuspended in a solution containing 0.02 M Tris (pH 7.4), 1.0 M NaCl, 0.012 M MgCl2, and 1 mM phenylmethylsulfonyl fluoride (PMSF; Calbiochem, La Jolla, CA); sonicated to resuspend the EB; and incubated with 25 μg of DNase/ml for 2 h on ice with constant mixing. Following ultracentrifugation (100,000 × g), the pellet was resuspended in 0.2 M sodium phosphate buffer (pH 5.5) containing 0.001 M each of EDTA and PMSF. The pellet was further extracted with 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS; Anatrace, Maumee, OH) and dithiothreitol (DTT; Roche Applied Sciences, Indianapolis, IN) at final concentrations of 2% and 0.1 M, respectively, for 2 h at 37°C. Following centrifugation (100,000 × g), the pellet was extracted a second time with CHAPS for 1 h at 37°C. The pellet of the second CHAPS extraction was reextracted with 2% n-tetradecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (Z3-14; Anatrace) (8, 27). After incubation at 37°C for 2 h with constant mixing, the sample was centrifuged (100,000 × g), and the MOMP was recovered in the supernatant.
The MOMP was further purified using a 1- by 20-cm hydroxyapatite column, as described previously by Caldwell et al. (10), with the following modifications. The column was equilibrated with 0.02 M phosphate buffer (pH 5.5) containing 0.1% Z3-14 and 0.001 M each of EDTA, PMSF, and DTT. The column was eluted with a linear gradient of phosphate buffer from 0.02 M to 0.5 M containing 0.1% Z3-14 and EDTA, PMSF, and DTT as indicated above. The purity of the MOMP preparation was assessed by gel electrophoresis and amino acid sequencing.
Western blots.
Western blot analyses were performed using nitrocellulose membranes as previously described (43). C. trachomatis MoPn EB, COMC, or MOMP was loaded onto a 7.5-cm-wide minislab gel and resolved by SDS-PAGE. Ascites fluid containing monoclonal antibodies (mAbs) was diluted with PBS containing 0.05% Tween 20 and 10% fetal bovine serum. The following mAbs to the C. trachomatis MoPn were used: mAb MoPn-13-2, which recognizes the linear epitope MTTWNPTISGSGI in VD4 of MOMP; mAb MoPn-40, which binds to the linear epitope TGDADLTTAPTP in VD1; mAb MoPn-18b, which binds to a conformational epitope of the MOMP; and mAb MoPn-32, which was used to detect the lipopolysaccharide (LPS) (44, 72).
To produce polyclonal sera to the 12-kDa CRP, the gene encoding this protein (GenBank accession number AE002341), without the leader sequence, was amplified by PCR, and the product was cloned into the pET-45b vector (Novagen, Madison, WI) and expressed in E. coli BL21(DE3). A six-histidine tag was fused at the N terminus, and the protein was purified using a Ni2+ column according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Female BALB/c mice were inoculated intramuscularly with 10 μg of protein/mouse in Freund's adjuvant. Following two boost immunizations, the serum was collected. Polyclonal serum to the 60-kDa CRP was obtained by immunizing mice with this protein, recovered from an SDS-polyacrylamide gel of the COMC, and emulsified in Freund's adjuvant (43). All animal protocols were approved by the Animal Care and Use Committee, University of California, Irvine.
For Western blot analysis, the mAbs and polyclonal antibodies were incubated for 2 h at room temperature and washed extensively, the membranes were treated with a 1:500 dilution of horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G, and the bands were visualized with 4-chloro-1-naphthol.
CD spectroscopy.
The secondary structure of the purified MOMP trimer was analyzed by circular dichroism (CD) spectroscopy in the far-UV (190- to 260-nm) region (32). Solutions of the protein were dialyzed against a buffer containing 100 mM sodium phosphate (pH 7.4) and 0.05% Z3-14. The MOMP samples containing 4.4 mg/ml of protein were analyzed at 1.0-nm wavelength intervals using a JASCO (Easton, MD) model 720 CD spectropolarimeter at a scan speed of 50 nm/min and an average response time of 8 s. A total of 10 consecutive scans were accumulated for analysis. The samples were analyzed at 25°C using a 0.1-mm-path-length cell (NSG Precision Cells, Inc., Farmingdale, NY).
Determination of pore-forming activity by liposome swelling.
The liposome swelling assay was done according to a method described previously by Nikaido et al. (41). C. trachomatis MoPn native MOMP in 300 mM sodium phosphate buffer (pH 5.5), 0.05% Z3-14, 1 mM EDTA, 1 mM PMSF, and 1 mM DTT was concentrated by using a centrifugal concentrator (Vivaspin 15R, 10,000-molecular-weight [MW] cutoff; Vivascience Inc.) and was then dialyzed against a solution containing 20 mM sodium phosphate buffer (pH 6.4), 0.1% Z3-14, 1 mM EDTA, 1 mM PMSF, and 1 mM DTT overnight at 4°C. Different amounts of the native MOMP preparation were reconstituted into liposomes by adding the protein to a dried film containing 2.4 μmol egg phosphatidylcholine and 0.1 μmol dicetylphosphate, and proteoliposomes were made in 15% dextran T-40 (Amersham Biosciences, Sweden). As a routine measure of pore-forming activity, swelling rates of isosmotic l-arabinose were used because the influx of this small sugar was rapid, and the swelling rates could be determined more accurately. The pore size was inferred from the dependence of swelling rates on the size of the permeating solutes as described previously (41).
Cross-linking of the C. trachomatis MoPn COMC and MOMP.
To determine the association between different components of the COMC, the Sarkosyl-extracted pellet was cross-linked using bis(sulfosuccinimidyl)suberate (BS3; Pierce, Rockford, IL) as follows (47, 53). Briefly, 8 μl of freshly made 20 mM BS3 in PBS buffer (pH 7.4) was added to 50 μl (0.3 mg/ml) of COMC in PBS buffer (pH 8.0) and incubated at room temperature for 2 h. To quench the reaction, 5 μl of 1 M Tris-HCl (pH 7.5) was added at room temperature. The BS3-treated and nontreated COMC were separated on a 5% SDS-PAGE gel and analyzed by silver staining and by immunoblotting.
Assessment of the trimeric conformation of MOMP was performed by cross-linking with dithiobis(succinimidylpropionate) (DSP; Pierce, Rockford, IL) (2). DSP was dissolved in dimethyl sulfoxide and added to final molar ratios of MOMP/DSP of 1:50, 1:100, and 1:300. Following 1 h of incubation at room temperature, the reaction was quenched with Tris-HCl (pH 7.5), and the samples were characterized by 8% SDS-PAGE and immunoblotting.
Determination of the MW of the C. trachomatis MoPn MOMP by SDS-PAGE.
The apparent MW of the MOMP was determined by Tricine-SDS-PAGE using various concentration of acrylamide ranging from 5% to 16% (26, 53). The MOMP was mixed with 2× sample buffer (0.125 M Tris [pH 8.0], 4% SDS, 20% glycerol, 50 mM DTT, and 0.01% bromophenol blue). The samples were either boiled or loaded directly onto the gels. The gels were stained with Coomassie blue. The MW of the MOMP was calculated using the Ferguson plot for proteins with anomalous mobility on SDS gels (4, 25, 47). Gels used to characterize the MOMP trimer were run in a cold room at low voltage to minimize heat buildup.
Trypsin digestion of the C. trachomatis MoPn MOMP.
To test the resistance of the MOMP to protease digestion, 5 μg of the MOMP was heated at 100°C for 10 min or incubated at room temperature for 10 min and was subsequently treated with trypsin (Sigma, St. Louis, MO) at molar ratios of MOMP to trypsin ranging from 30:1 to 12:1 (52, 60, 65). The reaction mixtures were incubated in 50 mM Tris-HCl (pH 8.0) with 100 mM NaCl at 37°C for 20 h. The products of the digestion were loaded in a 10% SDS-PAGE gel, visualized using a silver stain kit (Sigma), and analyzed by Western blotting.
Thermal stability of the trimeric conformation of the MOMP.
Thermal stability was investigated by incubating the trimer of the MOMP at different temperatures (52, 55). The MOMP was mixed with an equal volume of 2× sample buffer (pH 8.4) (with and without 4% SDS and with and without 50 mM DTT) and incubated from 4 to 65°C for 1 h or boiled for 10 min and analyzed in a 10% SDS-PAGE gel followed by immunoblotting.
Effect of pH on the conformation of the MOMP trimers.
To assess the stability of the trimers of the MOMP over a pH range, the following buffers were prepared: 20 mM sodium acetate buffer adjusted to a pH of 3.0, 4.0, and 5.0 with glacial acetic acid; 20 mM sodium phosphate buffer prepared at pH 6.0, 7.0, and 8.0; and 20 mM sodium carbonate buffer, pH 9.0 and 10.0. The purified MOMP was diluted 1:20 with each of the above-described buffers (29). Prior to analyses by Western blot, the preparations were incubated at room temperature for 3 h.
RESULTS
Characterization of the structure of the MOMP from C. trachomatis MoPn EB.
To initiate the structural characterization of the native MOMP, we ran a 10% SDS-PAGE gel of purified EB under different conditions. EB were aliquoted into four different fractions and boiled, or incubated at room temperature, for 5 min in loading buffer with and without DTT. The Coomassie blue stain of this gel is shown in Fig. 1A. A single band with a molecular mass of 40 kDa was clearly identifiable only in the sample boiled in the presence of DTT. Heating the sample without DTT resulted in a poorly defined band in the 40-kDa region. No band in the 40-kDa region was observed in the nonboiled samples with or without DTT.
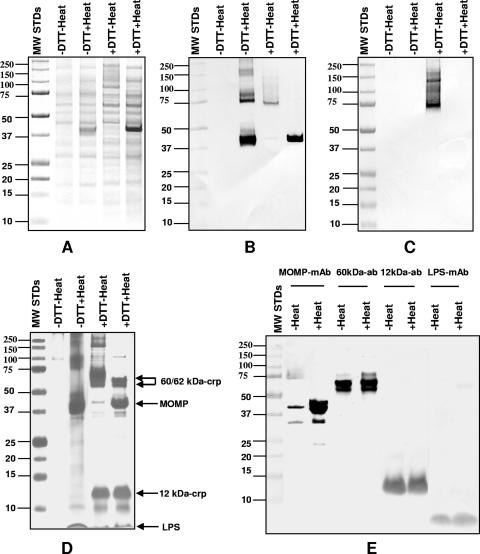
FIG. 1.
SDS-PAGE (10%) and Western blotting of C. trachomatis MoPn EB and COMC. (A) Coomassie blue R stain of 10% SDS-PAGE of C. trachomatis MoPn EB. The EB were incubated at room temperature or were boiled in the presence or absence of 25 mM DTT as indicated at the top of the lanes. (B and C) Western blot of same samples probed with mAb MoPn-13-2 (B) or with mAb MoPn-18b (C). (D) The COMC was extracted from EB with 2% Sarkosyl, and the insoluble pellet was resuspended in PBS buffer and incubated at room temperature or boiled in the presence (25 mM) or absence of DTT, as indicated. (E) Western blot of the same boiled, or nonboiled, COMC preparations in the presence of 25 mM DTT probed with mAb MoPn-13-2 to the MOMP, mAb MoPn-32 to LPS, and mouse polyclonal antibodies to the 60- and 12-kDa CRPs as indicated. MW standards (MW STDs) (in thousands) were loaded onto the first lane of each gel as shown.
A Western blot of this gel, probed with mAb MoPn 13-2, which recognizes a linear epitope in VD4, showed a single band at 40 kDa in the sample boiled in the presence of DTT (Fig. 1B). From the EB boiled without DTT, bands with molecular masses of approximately 40 and 80 kDa and other fainter bands of higher molecular masses were detected. The sample that was incubated at room temperature with DTT showed a faint band at approximately 67 kDa with a weak trail of higher molecular masses. A very weak band was also observed at 40 kDa. No bands were observed when the sample was incubated at room temperature without DTT.
On the Western blot probed with MoPn-18b, a mAb specific for a conformational epitope of MOMP, a positive signal was detected only with the EB that were incubated at room temperature with DTT (Fig. 1C). A band with a molecular mass of approximately 67 kDa followed by a trail with a higher molecular mass was observed.
Determination of the components present in the C. trachomatis MoPn outer membrane complex.
To characterize the components of the COMC, C. trachomatis MoPn EB were extracted with Sarkosyl without DTT. The Sarkosyl-insoluble pellet, corresponding to the COMC, was resuspended in loading buffer, aliquoted into four different fractions, and resolved by 10% SDS-PAGE (Fig. 1D). The sample that was incubated at room temperature without DTT showed only very faint bands with a molecular mass of approximately 100 kDa. Boiling the sample without DTT yielded a 40-kDa band and several bands at 80 kDa and above. Incubation at room temperature in the presence of DTT resulted in good separation of the 12- and 60-kDa CRPs and a very faint band with a molecular mass of 40 kDa. Bands with molecular masses of 150 kDa or higher were also observed. Boiling the sample in the presence of DTT resulted in four major bands corresponding to the 60-kDa CRP doublet, MOMP, the 12-kDa CRP, and LPS.
A Western blot using mAb MoPn 13-2 to MOMP and mAb MoPn-32, which binds to the LPS, and polyclonal antibodies to the 60- and 12-kDa CRPs is shown in Fig. 1E. Equal aliquots of the COMC preparation were loaded onto the eight lanes. All samples were treated with 25 mM DTT, but half of the preparation was incubated at room temperature, while the other half was boiled. The antibodies to the 60- and 12-kDa CRPs and to the LPS identified similar bands whether the samples were boiled or not. On the other hand, the MOMP migrated only with a molecular mass of 40 kDa when the sample was boiled, while only a weak band was present at 40 kDa when the sample was incubated at room temperature. Faint bands, probably corresponding to degradation products of the MOMP, were observed at 32 and 24 kDa. A poorly defined band was also detected in the 67-kDa region of the nonboiled sample.
Assessment of the spatial relationships of the components of the COMC.
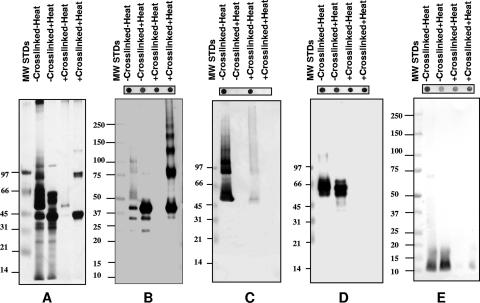
To determine the spatial relationships between the different components of the COMC, the Sarkosyl-insoluble pellet was incubated with the cross-linking reagent BS3. BS3 has a spacer arm length of 1.1 nm and is not cleavable with reducing reagents. Aliquots of cross-linked and non-cross-linked COMC were incubated at room temperature, or boiled, in loading buffer containing DTT. A silver stain and Western blots of these preparations resolved by 5% SDS-PAGE are shown in Fig. 2. To ensure that the proteins were detectable by their respective antibodies following cross-linking, dot blots were performed for all preparations and are shown above the gels.
FIG. 2.
SDS-PAGE (5%) and Western blotting of cross-linked C. trachomatis MoPn COMC. The COMC was cross-linked using BS3, or not cross-linked, and mixed with 2× sample buffer containing 50 mM DTT. Half of the preparation was boiled, and the other half was incubated at room temperature. MW standards (MW STDs) (in thousands) are shown in the first lanes of all the gels as marked. (A) Silver stain gel. The following antibodies were used: mAb MoPn-13-2 (B), mAb MoPn-18b (C), polyclonal serum to the 60-kDa CRP (D), and polyclonal serum to the 12-kDa CRP (E). Dot blots of the cross-linked and non-cross-linked proteins, probed with their respective antibodies, are shown on the top of each gel.
The COMC that was not cross-linked and heated yielded the expected doublet at 60 kDa and the MOMP band at 40 kDa (Fig. 2A). The low percentage of acrylamide used for this gel did not allow the clear separation between the 12-kDa protein and the LPS. When the cross-linked COMC was loaded onto the gel without boiling, the proteins failed to enter the stacking gel. However, if the cross-linked preparation was boiled before loading, two bands at 40 and 80 kDa and a very faint band at 120 kDa were observed.
The non-cross-linked sample, incubated at room temperature and probed with mAb MoPn-13-2, showed a weak band at 40 kDa, fainter bands at 32 and 24 kDa, and diffuse bands at approximately 50, 80, and 100 kDa (Fig. 2B). If the non-cross-linked COMC was boiled, a strong band at 40 kDa and fainter bands at 32 and 24 kDa were observed. No bands were detected with mAb MoPn-13-2 when the cross-linked sample was incubated at room temperature, most likely indicating that the MOMP did not enter the gel. In contrast, when the cross-linked sample was boiled and probed with mAb MoPn-13-2, a ladder of multiple bands with molecular masses of 40, 80, 120, 160, 200, and 240 kDa was detected. If the COMC was not cross-linked, or boiled, and was probed with mAb MoPn-18b, bands with molecular masses of 50, 80, and 120 kDa were observed, although there was a significant amount of trailing (Fig. 2C). Very faint bands with similar molecular masses were detected in the COMC preparation that was cross-linked but not boiled. No signal was detected with mAb MoPn-18b when the samples were boiled before loading. Similarly, the dot blots were negative, indicating that the conformational epitope recognized by MoPn-18b was disrupted.
The boiled or nonboiled cross-linked COMC samples gave no signal with a polyclonal antibody to the 60-kDa CRP, suggesting that this protein formed complexes that were too large to enter the gel (Fig. 2D). However, if the COMC was not cross-linked, the 60-kDa doublet was detected with the antibody whether the sample was boiled or incubated at room temperature before loading. Similar results were obtained when the Western blot was probed with a polyclonal antibody to the 12-kDa CRP (Fig. 2E). If the COMC was cross-linked, the 12-kDa CRP did not enter the gel. On the other hand, the 12-kDa CRP was detected with the antibody in the non-cross-linked, boiled, and nonboiled samples.
SDS-PAGE and Western blotting of the native C. trachomatis MoPn MOMP.
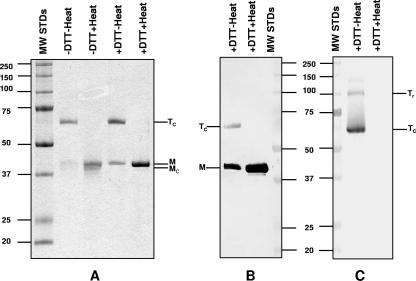
Extraction of the MOMP using two zwitterionic detergents and a hydroxyapatite column yielded a highly purified preparation. N-terminal amino acid analysis revealed only the expected sequence, LPVGNP, of the mature MoPn MOMP, indicating a purity of the protein of greater than 99% (data not shown). Boiling of this preparation in the presence of DTT before analysis by 10% SDS-PAGE yielded only one band with a molecular mass of 40 kDa (Fig. 3A). If the purified MOMP was boiled without DTT, a doublet was detected: one of the bands had a mass of 40 kDa, and a slightly faster, poorly defined band migrated with a molecular mass of approximately 38 kDa. When the purified MOMP preparation was loaded onto the gel without boiling, with or without DTT, two bands were detected: the major band with an apparent molecular mass of 67 kDa and a weak one with a molecular mass of 40 kDa. This latter band was a well-defined singlet in the sample incubated with DTT, while it was poorly defined in the preparation incubated without DTT.
FIG. 3.
SDS-PAGE (10%) and Western blotting of purified C. trachomatis MoPn MOMP. (A) Coomassie blue G staining. (B and C) Western blot probed with mAb MoPn-13-2 (B) and mAb MoPn-18b (C). The samples were incubated at room temperature or boiled in the presence (25 mM) or absence of DTT as indicated on the top of each lane. Tc, compact trimer; Tr, relaxed trimer; M, monomer; Mc, compact monomer; MW STDs, MW standards (in thousands).
By Western blot analysis, mAb MoPn-13-2 recognized the 40- and 67-kDa bands (Fig. 3B). Note that the binding of this mAb to the 67-kDa band was relatively weaker than the binding of the 40-kDa band. mAb MoPn-18b recognized only the 67- and 100-kDa components in the nonboiled preparation (Fig. 3C).
Establishment of the trimeric structure of the native C. trachomatis MoPn MOMP.
To confirm the possibility that the band detected on the 10% SDS-PAGE gel with an apparent molecular mass of 67 kDa, like other porins from gram-negative bacteria, corresponded to a trimer, aliquots of the purified MOMP were prepared and cross-linked with DSP at different molar ratios. DSP is a water-insoluble, thiol-cleavable, homobifunctional N-hydroxysuccinimide ester with a spacer length of 1.2 nm. Half of each preparation was boiled, and the other half was incubated at room temperature in the loading buffer without DTT before loading onto the 8% SDS-PAGE gel. The MOMP was probed by Western blotting with mAb MoPn-13-2 (Fig. 4). The non-cross-linked, boiled MOMP preparation yielded a broad band with a molecular mass of 40 kDa, while the nonboiled preparation had a doublet at 40 kDa and faint bands at 60 and 80 kDa. When the MOMP was cross-linked but not boiled, the 40-kDa band became faint in relation to the non-cross-linked, nonboiled sample, while the 60-kDa band was unchanged. Cross-linking of the MOMP followed by boiling yielded three bands with molecular masses of 40, 89, and 123 kDa; however, if the sample was not boiled, the band at 60 kDa remained, while the 40-kDa band was very faint. Increasing the molar ratio of DSP to MOMP in the boiled sample resulted in an increase in the density of the 123-kDa band and a decrease in the 40- and 60-kDa bands.
FIG. 4.
Western blot of an 8% SDS-PAGE gel of the C. trachomatis MoPn MOMP cross-linked with DSP and probed with mAb MoPn-13-2. The first lane shows MW standards (MW STDs) (in thousands). The MOMP was cross-linked at different molar ratios with DSP (as labeled on the top of the lanes) or not-cross-linked and boiled or not boiled in 2× buffer without DTT, as indicated for each lane. T, trimer; Tc, compact trimer; D, dimer; M, monomer; Mc, compact monomer.
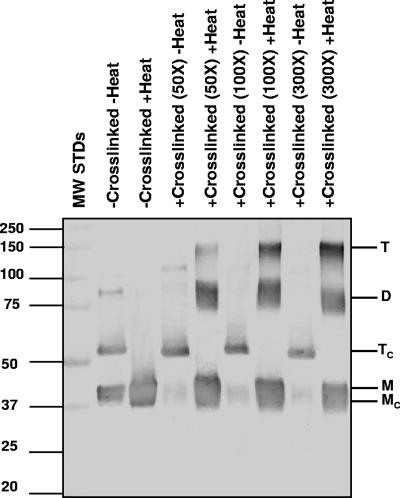
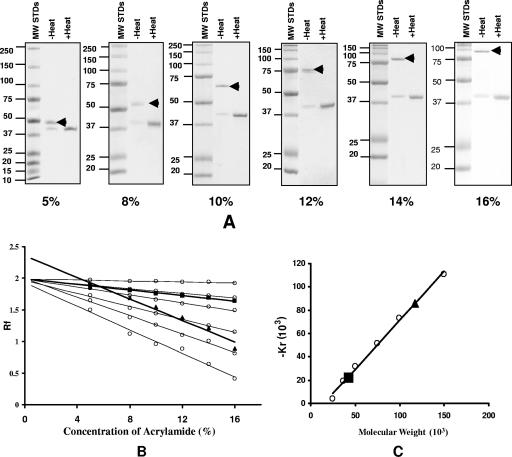
To further confirm the trimeric structure of the MOMP, we performed SDS-PAGE with different concentrations of acrylamide (Fig. 5). In SDS gels with a low concentration of acrylamide, proteins migrate in the electric field mainly according to the charges that result from bound SDS, while in gels with a high concentration of acrylamide, protein migration is affected most by molecular sieving, e.g., independent of charges (26). As shown in Fig. 5A, the monomer of MOMP consistently migrated with the same apparent molecular mass of 40 kDa in relation to the MW standards in SDS-PAGE with acrylamide concentrations ranging from 5% to 16%. On the other hand, the putative trimeric MOMP migrated with different apparent molecular masses depending on the concentrations of acrylamide. Using the Ferguson plot, we calculated the MW of the MOMP monomer and trimer using the retardation coefficient versus the MW for the marker proteins (Fig. 5B and C). The data showed that the monomer of MOMP has a molecular mass of 40 kDa, while the faster-migrating band has an estimated molecular mass of 117 kDa, corresponding to the expected molecular mass of the MOMP trimer.
FIG. 5.
Determination of the MW of the monomer and trimer of the C. trachomatis MoPn MOMP. (A) Coomassie blue staining of an SDS-PAGE gel of the MOMP resolved at different concentrations of acrylamide. Each gel has MW standards (MW STDs) (in thousands) in the first lane. The MOMP was boiled or not boiled before loading as indicated (1 μg/lane). The arrowhead marks the band corresponding to the trimer. (B) Ferguson plot of the undenatured (i.e., unboiled) MOMP trimer (triangle) and monomer (square). The logarithms of the relative mobilities of the marker protein bands from A were graphed against the acrylamide concentrations. Molecular mass standards, from top to bottom, are as follows: 25, 37, 50, 75, 100, and 150 kDa. The mobility of proteins relative to that of dye front (Rf) was plotted against the total acrylamide concentration on a log scale. (C) Plot of retardation coefficient (Kr) versus MW. The values for the retardation coefficient (Kr) were calculated by linear regression analysis of the lines in B and multiplications times 103. The triangle corresponds to the MOMP trimer, and the square corresponds to the monomer.
CD characterization.
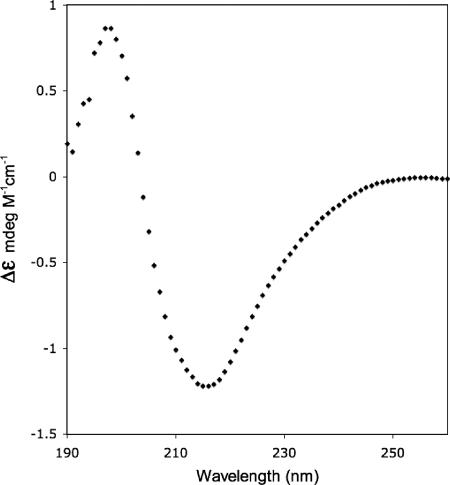
We performed CD measurements to assay for the presence of secondary structures of purified MOMP in Z3-14. To minimize the light-scattering effects of the detergent and allow us to collect low-wavelength data, we used a small-path-length cell (0.1 mm) and a highly concentrated MOMP solution (4.4 mg/ml). The CD spectrum of MOMP shown in Fig. 6 is consistent with the presence of a β-strand structure. We used this spectrum to estimate secondary-structure content with three algorithms, CONTINLL (49, 68), CDSSTR (12), and SELCON3 (56, 57), at the DICHROWEB website (71). Although secondary-structure algorithms designed to deconvolute CD data can be biased by the reference set of protein spectra used in the calculation, it has recently been shown that the soluble-protein reference spectra used in these algorithms perform well in analyzing CD data of membrane proteins (58). These algorithms predict the secondary structure of MOMP to be between 38 and 44% β-sheet, 5% or less helical, and the remainder is turned/disordered. Therefore, the CD data show that the purified native MOMP remains folded in Z3-14 detergent.
FIG. 6.
CD spectrum of C. trachomatis MoPn MOMP. Shown is the far-UV CD spectrum of native MOMP at a protein concentration of 4.4 mg/ml in 100 mM NaPO4 (pH 7.4)-0.05% Z3-14 at 25°C.
Pore-forming activity.
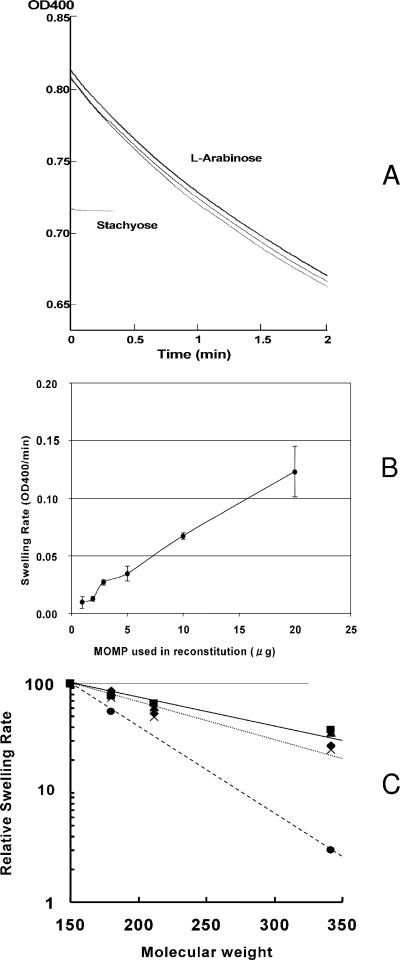
When the purified C. trachomatis MoPn native MOMP was reconstituted into proteoliposomes, it allowed the rapid penetration of l-arabinose, causing the swelling of liposomes (Fig. 7A). The rate of swelling was approximately proportional to the amount of protein added (Fig. 7B). Although specific pore-forming activity of C. trachomatis MOMP ([7.6 ± 1.6] × 10−3 OD/min/μg protein) was significantly lower (∼50-fold) than that of the E. coli trimeric porin OmpF, this protein appears to function as a slow porin like E. coli OmpA and Pseudomonas aeruginosa OprF (40, 41). In order to determine the size of the pore, we tested rates of penetration of sugars of different sizes through the MOMP. As shown in Fig. 7C, the penetration rates were clearly dependent on solute size, and the rate of sucrose (Mr of 342) penetration was 33.3% of that of arabinose (Mr of 150), a result that is similar to that obtained with P. aeruginosa OprF (41, 42). This result suggests that the pore size of C. trachomatis MOMP is larger than that of E. coli OmpF and similar to that of P. aeruginosa OprF, estimated to have a channel diameter of about 2 nm (41).
FIG. 7.
Pore-forming function of native MOMP. (A) Example of proteoliposome swelling data. Proteoliposomes containing 20 μg of native MoPn MOMP were prepared as described in Materials and Methods, and a portion of the suspension was diluted in isotonic solutions of either a tetrasaccharide, stachyose (lower curve), or a pentose, l-arabinose (upper curves). The time-dependent swelling (and the consequent decrease in scattering or optical density) is evident in the l-arabinose solution (three consecutive traces are shown), whereas in stachyose (terminated early), very little changes in optical density occurred. The absolute values of optical density are lower in stachyose because of the higher refractive index of the isotonic stachyose solution. OD400, optical density at 400 nm. (B) Dependence of swelling rates on the amount of MOMP used in reconstitution. Proteoliposomes were reconstituted with different amounts of native MOMP, and the swelling rates in l-arabinose solution were measured. (C) Dependence of swelling rate on the size of solutes. Swelling was measured in isotonic solutions of l-arabinose, d-glucose, N-acetyl-d-glucosamine, and sucrose, with proteoliposomes reconstituted with native MoPn MOMP. Data obtained in three experiments are shown (▪, ▴, and ♦). For comparison, P. aeruginosa OprF porin was used in reconstitution in the same experiment (×). Data reported for the E. coli OmpF porin (41) are also included (•).
The mature C. trachomatis MoPn MOMP contains eight cysteine residues. Our previous study demonstrated that purified and refolded MOMP contained four cysteines that were involved in intramolecular disulfide bonds (72). MOMP (20 μg) was reconstituted into liposomes, and pore-forming activity was measured in the presence of 0, 3, and 10 mM DTT. Under these conditions, no significant difference was found in the channel activity of MOMP (data not shown). However, we did not examine whether significant amounts of disulfide bonds were reduced during the assay.
We tested two mAbs for their possible effect on the channel activity of MOMP. One of them, mAb MoPn-40, recognizes a linear epitope in VD1, and mAb MoPn-18b binds to a conformational epitope present only in the trimer. Proteoliposomes were made by incubating the mixture containing 3.16 μg of MOMP with 11.85 μg of mAb (1:1 molar ratio between the monomer of MOMP and the mAb) in 40 μl of 15% dextran solution at room temperature for 30 min. When these proteoliposomes were used for the swelling assay, the binding of mAb MoPn-40 to MOMP inhibited the transmembrane diffusion of l-arabinose by 40% (data not shown). MoPn-18b, on the other hand, did not affect the penetration of arabinose through the MOMP.
Resistance of the MOMP trimer to trypsin digestion.
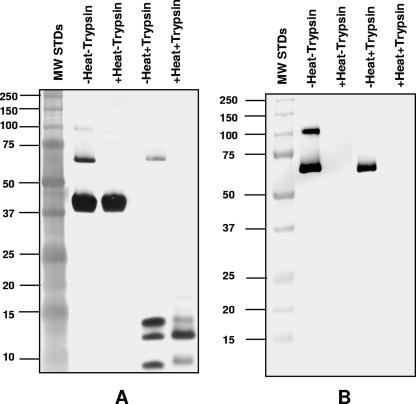
To determine the susceptibility of the MoPn MOMP trimers to protease digestion, a preparation of the purified MOMP containing both the monomer and the trimer was incubated at room temperature or boiled and was subjected to trypsin digestion. The products of the reaction were analyzed by 10% SDS-PAGE and stained with silver or probed by Western blotting with mAb MoPn-18b. As described in Materials and Methods, the digestion was performed under a variety of conditions. Figure 8 shows that even under very harsh conditions, a 12-to-1 molar ratio of MOMP to trypsin incubated at 37°C for 20 h, the 67-kDa trimer was resistant to digestion, while the MOMP monomer and the relaxed trimer (100 kDa) were susceptible to trypsin cleavage. Trypsin digested the trimer only if the MOMP was boiled before it was incubated with the protease.
FIG. 8.
SDS-PAGE and Western blotting of trypsin digestion of the MOMP. (A) Silver stain of a 10% SDS-PAGE gel. The first lane shows the MW standard (MW STDs) (in thousands). MOMP was not heated or heated, which was followed by trypsin digestion (1 μg of MOMP/lane). (B) Western blot of same samples shown in A probed with mAb MoPn-18b.
Thermal stability of the MOMP trimer.
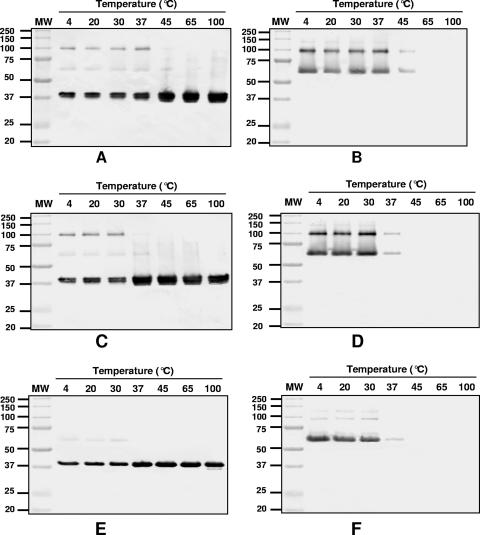
To determine the thermal stability of the trimer, purified MOMP was incubated for 1 h at different temperatures in loading buffer with or without 25 mM DTT in the presence or absence of 2% SDS. Following incubation, the MOMP was analyzed by Western blotting using mAb MoPn-13-2 and MoPn-18b. As shown in Fig. 9A and B, the MOMP trimer was stable from 4°C to 37°C when incubated without SDS and DTT. At 45°C, the trimer started to dissociate into monomers and was completely dissociated following incubation at 65°C. As expected, in the absence of DTT, the 40-kDa band appeared as a doublet. In the presence of SDS without DTT, the trimer started to dissociate at 37°C and was completely dissociated at 45°C (Fig. 9C and D). The monomer migrated as a doublet. In Fig. 9E and F, the sample was incubated in SDS with DTT. In this case, the trimer started to dissociate at 37°C, and at 45°C, it was completely dissociated into monomers. Under these incubation conditions, the monomer migrated as a single band.
FIG. 9.
Immunoblot of the MOMP incubated at various temperatures. The MOMP was incubated at different temperatures for 1 h, except for 10 min at 100°C, and was separated (1 μg/lane) on a 10% SDS-PAGE gel before it was probed with mAb MoPn-13-2 (A, C, and E) and mAb MoPn-18b (B, D, and F). The first lanes show MW standards (in thousands). (A and B) Samples incubated without SDS or DTT. (C and D) Samples incubated with 2% SDS without DTT. (E and F) Samples incubated with 2% SDS and 25 mM DTT.
Effect of pH on the stability of the MOMP trimer.
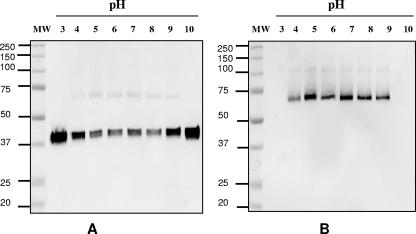
To test the stability of the trimer at a pH range of 3 to 10, a preparation of MOMP was incubated for 3 h in various buffers without DTT. Following incubation, the preparations were separated by 10% SDS-PAGE followed by Western blots probed with mAb MoPn-13-2 and MoPn-18b. As shown in Fig. 10, the MOMP trimer was stable between pH 5.0 and 8.0. At pH 4.0 and pH 9.0, the trimer was partially dissociated, and at pH 3.0 and pH 10.0, the trimer was completely dissociated into monomers.
FIG. 10.
Immunoblot of the MOMP incubated at different pH values. The MOMP was incubated for 3 h at room temperature at pH values ranging from 3 to 10 and was then analyzed (100 ng/lane) by 10% SDS-PAGE followed by an immunoblot probed with mAb MoPn-13-2 (A) and mAb MoPn-18b (B). The first lanes show MW standards (in thousands).
DISCUSSION
Characterization of the structure of the native MOMP has been hampered due to the limitations in obtaining adequate quantities of Chlamydia and difficulties in purifying this protein. Here, for the first time, we have characterized the structure and function of the native MOMP of Chlamydia. Several conclusions can be reached from our studies: (i) in EB, the MOMP is not disulfide bonded to, nor does it appear to be in close physical association with, the 60- and 12-kDa CRPs; (ii) in EB, MOMP forms trimers that are not held by disulfide bonds, although disulfide bridges exist within and between the monomers of a trimer and between trimers; (iii) MOMP trimers in Z3-14 are approximately 40% β-sheet; (iv) the native MOMP trimers showed porin-like channel-forming activity; (v) at room temperature, the trimers of MOMP are stable in SDS; (vi) the trimers can be dissociated to monomers by heat and by low and high pH; and (vii) the trimers of MOMP are resistant to trypsin digestion.
The apparent lack of peptidoglycan in the membrane of Chlamydia has led several investigators to propose that the rigidity of the envelope of the EB is due to disulfide bonds formed between three CRPs, MOMP and the 60- and the 12-kDa proteins (16, 21, 23, 34, 37, 50). Caldwell et al. (10) and Hatch et al. (24) first developed methods for the isolation of the MOMP from C. trachomatis L2 and Chlamydia psittaci 6BC, respectively. Following the extraction of EB with Sarkosyl, Caldwell et al. (10) showed by electron microscopy that the double-tract-unit outer membrane remained intact. Incubation of the COMC at increasing temperatures in the presence of SDS under nonreducing conditions resulted in the release of the MOMP and the progressive loss of membrane integrity. Based on these studies, Caldwell et al. (10) concluded that in EB of the L2 serovar, the MOMP was not linked by disulfide bonds to other components of the membrane. Similarly, Birkelund et al. (7) cross-linked EB from the L2 serovar and, using a mAb to MOMP, detected a 42.5-kDa band and doublet bands with molecular masses of 100 and 110 kDa. They concluded that the 42.5-kDa band was the monomer of MOMP with LPS, while the 100- to 110-kDa bands corresponded to the trimers of MOMP.
We report that boiling the C. trachomatis MoPn EB in the presence of DTT released the MOMP, which migrated on an SDS-PAGE gel as a single band of 40 kDa. Boiling EB without DTT also yielded monomers of MOMP but also bands of higher molecular mass, most likely corresponding to disulfide-bonded oligomers of the MOMP, although associations with other components could not initially be excluded. Treating EB before electrophoresis with DTT without boiling yielded native trimers. To further rule out the association of the C. trachomatis MoPn MOMP with other proteins, we extracted the COMC under nonreducing conditions and subsequently cross-linked it. As expected, in the COMC, we identified MOMP, the 60- and 12-kDa CRPs, and LPS (7, 10, 22). Analysis of the cross-linked COMC using SDS-PAGE showed that as previously reported by Newhall and Jones (38), the MOMP formed complexes with molecular masses of approximately 40, 80, 120, 160, 200, and 240 kDa, most likely corresponding to monomers and oligomers of this protein. On the other hand, neither the 60- nor the 12-kDa CRP entered the gel. Thus, as with the L2 serovar, in the MoPn strain, the MOMP does not appear to be disulfide bonded to, or in proximity of, the 60- and 12-kDa CRPs or to other major membrane components. This finding is in agreement with the model proposed previously by Everett and Hatch (16) for the envelope of chlamydiae. In that model, MOMP is in the outer membrane, while the 60- and 12-kDa CRPs are in the periplasmic space, perhaps providing rigidity to the envelope of the EB. It is, however, possible that, as suggested by the experiments reported previously by Ting et al. (65), a small portion of the amino terminus of the 60-kDa CRP is exposed on the surface of EB. Our results also support the 2-D model proposed previously by Rodriguez-Marañon et al. (51). In that model, all the cysteines, except one that is in the β-sheet, are on the outer side of the membrane, suggesting that they are not forming disulfide bridges with the 60- and/or 12-kDa CRP.
Our purification and reconstitution procedures yielded a protein containing structural features consistent with a correctly folded porin. Three different models defining the secondary structure topology of MOMP have been presented (18, 51, 69). Although those models differ in the assignment of β-strand to sequence, in each case, based on predictions, the total β-strand content is assumed to be in the range of 34 to 43%. Other porins of comparable size have also shown β-strand content of approximately 40% (32). Our CD data for MOMP in Z3-14 detergent micelles also show a high β-sheet content. Using three deconvolution methods, we estimated the secondary structure to be between 38 and 44% β-sheet. The remainder is turned/disordered, with a very small amount (5% or less) of helix predicted.
The native MOMP preparation showed significant porin-like activity when reconstituted into proteoliposomes. The channel was large, as deduced from the dependence of penetration rates on solute size, an approach validated previously by the crystallographic data on E. coli OmpF (40, 42). The large channel size is not surprising, as MOMP was predicted to form a 16-stranded β-barrel (51), and even a 14-stranded barrel of E. coli OmpG was shown to produce a similarly large channel in the absence of channel narrowing caused by the infolding of the loops (17), as verified recently by X-ray crystallography (61, 73). Interestingly, the specific pore-forming activity of MOMP was at least an order of magnitude lower than that of OmpF. This could be due to the closing of the majority of channels (36, 62, 73); however, at present, we cannot exclude the possibility that this was caused by the partial inactivation of the protein population during its purification. In support of our findings, Bavoil et al. (6) previously reported preliminary data for a preparation of the C. trachomatis L2 MOMP. They found that MOMP was 200 times less efficient than the E. coli OmpF and proposed a similarity to a P. aeruginosa porin (42).
We analyzed the porin function in the presence and absence of DTT and found no differences in the channel activities of MOMP. We did not, however, determine whether the disulfide bonds were reduced in the presence of DTT. Bavoil et al. (6) analyzed the porin activity of the outer membrane of C. trachomatis in the presence and absence of reducing agents and found that there was channel activity only under reducing conditions. However, they did not determine whether there were any changes in the disulfide bonds present in the membrane as a result of the reducing agent (6). Therefore, it was not possible to conclude if the channel activity observed was due to a direct effect on MOMP or on other porins present in the membrane or to an indirect effect due to the numerous potential disulfide bonds among the cysteine-rich membrane proteins of Chlamydia. However, it is possible that the results of Bavoil et al. (6) more closely parallel the functional activity in vivo, since their experiments were performed using the outer membrane, which may better mimic the structure of the MOMP in the bacterium, rather than the purified preparation that we analyzed.
To further characterize the structure-function relation of MOMP, the channel activity was analyzed using two mAbs. One of them, mAb MoPn-40, recognizes a linear epitope in VD1. Based on a 2-D model of the structure of the MOMP, the VD1 is located in the second loop (L2) that latches into the pore (51). It is therefore not surprising that the binding of mAb MoPn-40 to the MOMP resulted in a 40% inhibition of the transmembrane diffusion of l-arabinose. On the other hand, mAb MoPn-18b, which recognizes the trimeric structure of MOMP, did not inhibit the porin activity of the channel. Due to the conformational structure of this epitope, we have not been able to map it. The results for porin function suggest that the epitope recognized by mAb MoPn-18b is not in close proximity to the channel of MOMP.
The functional unit of porins like MOMP from E. coli and other gram-negative bacteria is usually a homotrimer that migrates with a molecular mass of approximately 65 to 70 kDa on a 10% SDS-PAGE gel (13, 27, 28, 35, 54, 66). Here, we have shown that the trimer of MOMP under nondenaturing conditions on a 10% SDS-PAGE gel also migrates with an apparent molecular mass of 67 kDa. Rosenbusch (52) first cross-linked a porin from E. coli with glutaraldehyde to establish its trimeric structure. We cross-linked the MOMP, and following SDS-PAGE of the completely denatured protein in an 8% gel, we detected bands with apparent molecular masses of 40, 89, and 123 kDa, most likely corresponding to the monomer, dimer, and trimer, respectively. To further confirm the trimeric structure of the C. trachomatis MoPn MOMP, we electrophoresed the undenatured MOMP in gels with different concentrations of acrylamide and showed that the undenatured trimer had the expected mass of approximately 120 kDa.
Newhall and Jones (38) proposed that, unlike trimers from other porins of gram-negatives, the trimers of the MOMP from C. trachomatis and C. psittaci are held together by disulfide bonds and not by noncovalent interactions. This conclusion was based on the observation that after electrophoresis of EB in the first dimension, without reduction, the MOMP did not enter the gel. Only after the addition of a reducing agent, followed by electrophoresis in the second dimension, did the MOMP enter the gel, and it was shown to band with a molecular mass consistent with monomers, dimers, trimers, and multimers. In agreement with our results, McCafferty et al. (30) and de Sa et al. (15) previously reported that the disulfide bonds are not required to maintain the trimeric structure of MOMP. Thus, although disulfide bonds between the peptides of the trimer of MOMP exist, they are not required to stabilize the trimer. Structural characterization of E. coli OmpF has shown that of the eight surface-exposed loops present in this protein, the second one (L2) connects each peptide with its neighbor by latching into the pore, thus helping to stabilize the trimer (48). To stabilize the trimers, there are hydrophobic and polar interactions between the monomers. Based on the model proposed previously by Rodriguez-Marañon et al. (51), the second loop, corresponding to VD1, may be the latching component that helps hold together the trimer of the C. trachomatis MoPn MOMP. Intramonomer disulfide bonds have been mapped, and our results here show that these bonds can affect the migration of the monomer (72). However, so far, the intermonomer and the intertrimer disulfide bonds have not been determined.
McCafferty et al. (30) first identified the relaxed form of the C. psittaci ovine abortion (strain S26/3) MOMP trimer. Extraction of the COMC with n-octyl-β-d-glucopyranoside yielded a relatively pure preparation of the MOMP. When the MOMP was loaded onto a 12.5% SDS-PAGE gel without boiling, a major band at approximately 100 kDa was identified by a protective mAb. This mAb, however, failed to bind if the MOMP was boiled before loading onto the SDS-PAGE gel. On the other hand, a mAb to a linear epitope recognized the trimer at 100 kDa and the denatured form of MOMP at 38 kDa. Shortly afterwards, similar results were reported by de Sa et al. (15), characterizing the AB7 ovine strain of C. psittaci. Those authors purified the MOMP using SDS and Triton X-100 and identified a 110-kDa band with a neutralizing mAb by Western blot when the samples were run without boiling. Subsequently, de Sa et al. (15) also purified the MOMP by immunoaffinity using the same mAb. After elution, the MOMP was analyzed by SDS-PAGE without boiling and visualized by silver staining. Interestingly, the MOMP migrated with an apparent molecular mass of 78 kDa, and by Western blotting, it was recognized by the same mAb that recognized the 110-kDa band. Those authors attributed the change in migration to possible contamination with LPS, interaction with other proteins, or a conformational modification. Based on our results, the 78-kDa band reported previously by de Sa et al. (15) most likely corresponds to the compact trimer, while the 110-kDa band represents the relaxed trimer.
To gain a better understanding of the stability of the trimers, we tested the preparation of the MOMP in SDS at different temperatures and pH values. Our results showed that the trimers, like those from other porins of gram-negative bacteria, are SDS resistant but heat labile (26, 40, 55, 67). The dissociation of the MOMP trimers at high temperatures may explain the release of the MOMP from the outer membrane described previously by Caldwell et al. (10). The heat-dependent conformation of the MOMP trimer, in addition to structural significance, may also be important for the immunopathogenesis of this organism. mAbs to heat-sensitive conformational epitopes of Chlamydia have been reported previously, and some of them have been shown to neutralize the infectivity of this pathogen (15, 30, 44). Furthermore, these mAbs can bind EB at room temperature, but heating the EB destroyed the epitopes. Our findings suggest that these mAbs may recognize the heat-labile conformational epitope of the MOMP trimer. The heat sensitivity of the MOMP trimers may also explain the loss of binding and infectivity of EB when heated at 56°C for 30 min (33). However, other heat-labile proteins, or components of the outer membrane, may be responsible for the loss of binding and infectivity.
Trimers of the E. coli OmpF in β-octyl-glucoside have been shown to be stable in a pH range of between 2.0 and 7.5 (29). Here, we found that the MoPn MOMP trimers in SDS were stable between pH 5.0 and 8.0 but started to dissociate at pH 4.0 and 9.0. A loss of infectivity of chlamydial EB at high pH has been reported previously (64). In addition to the physiological implications of this finding, our observations can explain some in vitro findings. For example, several methods advise binding proteins to enzyme-linked immunosorbent assay plates using a bicarbonate buffer at pH 9.6. This pH is recommended because it helps to increase the binding of the proteins to the plate. We performed an enzyme-linked immunosorbent assay by binding MoPn EB to plates using bicarbonate buffer at pH 9.6. At this pH, we detected a significant decrease in the binding of mAb to conformational epitopes of the MOMP, while the binding of mAbs to linear epitopes was not affected (our unpublished results).
Dissociation of the trimers as a result of increasing temperature, or changes in pH, in the absence of DTT in the incubation buffer yielded a doublet at 40 and 38 kDa, while in the presence of DTT, only the 40-kDa band was detected. This doublet band probably represents different degrees of oxidation/reduction of the intramonomeric disulfide bonds. Hackstadt et al. (20) also previously described a faster-migrating monomer that is believed to have internal disulfide bridges. In our case, we need to also take into consideration the possibility that at low temperatures or under mild pH conditions, the monomers were released from the trimer while maintaining their β-barrel structure. Monomers of porins with a β-barrel structure migrate faster in SDS-PAGE gels than denatured monomers (9).
Rosenbusch (52) previously described the unique resistance of E. coli porin trimers to enzymatic digestion, a finding that has been confirmed for other porin trimers (27). Digestion of the preparation of the MoPn MOMP with trypsin also showed that the compact trimer was resistant to the protease. In an attempt to determine the surface exposure of the MOMP and its role in infectivity, several authors performed trypsin digestion of EB. For example, Hackstadt and Caldwell (19), working with the L2 serovar, previously showed that trypsin digested the MOMP but that infectivity was not affected. To explain those findings, they proposed that disulfide bonds maintained the supramolecular structure of MOMP. Su et al. (60) previously found that sites in the VD2 and VD4 of serovar D were cleaved by trypsin, resulting in a loss of infectivity. On the other hand, digestion of EB of serovar L2 resulted only in the cleavage of VD4 without a loss of infectivity. Ting et al. (65) incubated EB of C. psittaci GPIC with trypsin and noted that while the 60-kDa CRP was digested, the MOMP was highly resistant. Furthermore, trypsin digestion reduced EB adherence to HeLa cells. Based on those observations, those authors suggested that the 60-kDa CRP may interact with the host eukaryotic cells. In view of the resistance of the MOMP trimer to trypsin digestion, the interpretation of some of the previously published results may need to be reevaluated.
In conclusion, in EB of C. trachomatis MoPn, the MOMP forms trimers that are not disulfide bonded to the 60- or the 12-kDa CRP. Disulfide bonds are present within and between the monomers of a trimer and between trimers. Although disulfide bonds are not required to hold the trimer, they may help to stabilize it. The purified MOMP trimers have a predominant β-sheet structure and function as a porin with a pore size of approximately 2 nm in diameter. The MOMP trimers are stable in SDS and are resistant to digestion with trypsin. Moreover, the trimers are not stable at high temperatures and at high or low pH. These findings have important implications for our understanding of the immunopathogenesis of Chlamydia and for the formulation of preventive and therapeutic measures against this organism.
Acknowledgments
This work was supported by Public Health Service grant AI-32248 (to L. M. de la Maza) and AI-9644 (to H. Nikaido) from the National Institute of Allergy and Infectious Diseases and by the UC Irvine Academic Senate Council on Research, Computing, and Library Resources (to M. J. Cocco and L. M. de la Maza).
We thank Stephen White (UC Irvine) for the use of the circular dichroism spectrophotometer.
Footnotes
Published ahead of print on 29 June 2007.
REFERENCES
- 1.Adler, M. W. 2003. Sexual health: health of the nation. Sex. Transm. Infect. 79:84-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus, B. L., and R. E. W. Hancock. 1983. Outer membrane proteins F, P, and D1 of Pseudomonas aeruginosa and PhoE of Escherichia coli: chemical cross-linking to reveal native oligomers. J. Bacteriol. 155:1042-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baher, W., Y. X. Zhang, T. Joseph, H. Su, F. E. Nano. K. D. E. Everett, and H. D. Caldwell. 1988. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc. Natl. Acad. Sci. USA 85:4000-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banker, G. A., and C. W. Cotman. 1972. Measurement of free electrophoretic mobility and retardation coefficient of protein-sodium dodecyl sulfate complexes by gel electrophoresis. A method to validate molecular weight estimates. J. Biol. Chem. 247:5856-5861. [PubMed] [Google Scholar]
- 5.Batteiger, B. E., R. G. Rank, P. M. Bavoil, and L. S. Soderberg. 1993. Partial protection against genital reinfection by immunization of guinea-pigs with isolated outer-membrane proteins of the chlamydial agent of guinea-pig inclusion conjunctivitis. J. Gen. Microbiol. 139:2965-2972. [DOI] [PubMed] [Google Scholar]
- 6.Bavoil, P., A. Ohlin, and J. Schachter. 1984. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect. Immun. 44:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birkelund, S., A. G. Lundemose, and G. Christiansen. 1988. Chemical cross-linking of Chlamydia trachomatis. Infect. Immun. 56:654-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blake, M. S., and E. C. Gotschlich. 1984. Purification and partial characterization of the opacity-associated proteins of Neisseria gonorrhoeae. J. Exp. Med. 159:452-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolla, J. M., E. Loret, M. Zalewski, and J. M. Pages. 1995. Conformational analysis of the Campylobacter jejuni porins. J. Bacteriol. 177:4266-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldwell, H. D., and J. Schachter. 1982. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect. Immun. 35:1024-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Compton, L. A., and W. C. Johnson, Jr. 1986. Analysis of protein circular dichroism spectra for secondary structure using a simple matrix multiplication. Anal. Biochem. 155:155-167. [DOI] [PubMed] [Google Scholar]
- 13.de Cock, H., R. Hendriks, T. de Vrije, and J. Tommassen. 1990. Assembly of an in vitro synthesized Escherichia coli outer membrane porin into its stable trimeric configuration. J. Biol. Chem. 265:4646-4651. [PubMed] [Google Scholar]
- 14.de la Maza, L. M., and E. M. Peterson. 2002. Vaccines for Chlamydia trachomatis infections. Curr. Opin. Investig. Drugs 3:980-986. [PubMed] [Google Scholar]
- 15.de Sa, C., A. Souriau, F. Bernard, J. Salinas, and A. Rodolakis. 1995. An oligomer of the major outer membrane protein of Chlamydia psittaci is recognized by monoclonal antibodies which protect mice from abortion. Infect. Immun. 63:4912-4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everett, K. D., and T. P. Hatch. 1995. Architecture of the cell envelope of Chlamydia psittaci 6BC. J. Bacteriol. 177:877-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fajardo, D. A., J. Cheung, C. Ito, E. Sugawara, H. Nikaido, and R. Misra. 1998. Biochemistry and regulation of a novel Escherichia coli K-12 porin protein, OmpG, which produces unusually large channels. J. Bacteriol. 180:4452-4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Findlay, H. E., H. McClafferty, and R. H. Ashely. 2005. Surface expression, single-channel analysis and membrane topology of recombinant Chlamydia trachomatis major outer membrane protein. BMC Microbiol. 5:5. doi: 10.1186/1471-2180-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hackstadt, T., and H. D. Caldwell. 1985. Effect of proteolytic cleavage of surface-exposed proteins on infectivity of Chlamydia trachomatis. Infect. Immun. 48:546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hackstadt, T., W. J. Todd, and H. D. Caldwell. 1985. Disulfide-mediated interactions of the chlamydial major outer membrane protein: role in the differentiation of chlamydiae? J. Bacteriol. 161:25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatch, T. P. 1996. Disulfide cross-linked envelope proteins: the functional equivalent of peptidoglycan in chlamydiae? J. Bacteriol. 178:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatch, T. P., I. Allan, and J. H. Pearce. 1984. Structural and polypeptide differences between envelopes of infective and reproductive life cycle form of Chlamydia spp. J. Bacteriol. 157:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatch, T. P., M. Miceli, and J. E. Sublett. 1986. Synthesis of disulfide-bonded outer membrane proteins during the developmental cycle of Chlamydia psittaci and Chlamydia trachomatis. J. Bacteriol. 165:379-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatch, T. P., D. W. Vance, and E. Al-Hossainy. 1981. Identification of a major envelope protein in Chlamydia spp. J. Bacteriol. 146:426-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedrick, J. L., and A. J. Smith. 1968. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch. Biochem. Biophys. 126:155-164. [DOI] [PubMed] [Google Scholar]
- 26.Heller, K. B. 1978. Apparent molecular weights of a heat-modifiable protein from the outer membrane of Escherichia coli in gels with different acrylamide concentrations. J. Bacteriol. 134:1181-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansen, C., A. Wiese, L. Reubsaet, N. Dekker, H. de Cock, U. Seydel, and J. Tommassen. 2000. Biochemical and biophysical characterization of in vitro folded outer membrane porin PorA of Neisseria meningitidis. Biochim. Biophys. Acta 1464:284-289. [DOI] [PubMed] [Google Scholar]
- 28.Kleivdal, H., R. Benz, and H. B. Jensen. 1995. The Fusobacterium nucleatum major outer-membrane protein (FomA) forms trimeric, water-filled channels in lipid bilayer membranes. Eur. J. Biochem. 233:310-316. [DOI] [PubMed] [Google Scholar]
- 29.Markovic-Housley, Z., and R. M. Garavito. 1986. Effect of temperature and low pH on structure and stability of matrix porin in micellar detergent solutions. Biochem. Biophys. Acta 869:158-170. [DOI] [PubMed] [Google Scholar]
- 30.McCafferty, M. C., A. J. Herring, A. A. Andersen, and G. E. Jones. 1995. Electrophoretic analysis of the major outer membrane protein of Chlamydia psittaci reveals multimers which are recognized by protective monoclonal antibodies. Infect. Immun. 63:2387-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, W. C., C. A. Ford, M. Morris, M. S. Handcock, J. L. Schmitz, M. M. Hubbs, M. S. Cohen, K. M. Harris, and J. R. Udry. 2004. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA 291:2229-2236. [DOI] [PubMed] [Google Scholar]
- 32.Minetti, C. A., J. Y. Tai, M. S. Blake, J. K. Pullen, S. M. Liang, and D. P. Remeta. 1997. Structural and functional characterization of recombinant PorB class 2 protein from Neisseria meningitidis. Conformational stability and porin activity. J. Biol. Chem. 272:10710-10720. [DOI] [PubMed] [Google Scholar]
- 33.Moulder, J. W. 1991. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 55:143-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mygind, P., G. Christiansen, and S. Birkelund. 1998. Topological analysis of Chlamydia trachomatis L2 outer membrane protein 2. J. Bacteriol. 180:5784-5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakae, T., J. Ishii, and M. Tokunaga. 1979. Subunit structure of functional porin oligomers that form permeability channels in the outer membrane of Escherichia coli. J. Biol. Chem. 254:1457-1461. [PubMed] [Google Scholar]
- 36.Nestorovich, E. M., E. Sugawara, H. Nikaido, and S. M. Bezrukov. 2006. Pseudomonas aeruginosa porin OprF. Properties of the channel. J. Biol. Chem. 281:16230-16237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newhall, W. J. 1987. Biosynthesis and disulfide cross-linking of outer membrane components during the growth cycle of Chlamydia trachomatis. Infect. Immun. 55:162-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newhall, W. J., and R. B. Jones. 1983. Disulfide-linked oligomers of the major outer membrane protein of chlamydiae. J. Bacteriol. 154:998-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nigg, C. 1942. An unidentified virus which produces pneumonia and systemic infection in mice. Science 99:49-50. [DOI] [PubMed] [Google Scholar]
- 40.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikaido, H., K. Nikaido, and S. Harayama. 1991. Identification and characterization of porins in Pseudomonas aeruginosa. J. Biol. Chem. 266:770-779. [PubMed] [Google Scholar]
- 42.Nikaido, H., and E. Y. Rosenberg. 1981. Effect of solute size on diffusion rates through the transmembrane pores of the outer membrane of Escherichia coli. J. Gen. Physiol. 77:121-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pal, S., H. L. Davis, E. M. Peterson, and L. M. de la Maza. 2002. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein by use of CpG oligodeoxynucleotides as an adjuvant induces a protective immune response against an intranasal chlamydial challenge. Infect. Immun. 70:4812-4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 1997. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine 15:575-582. [DOI] [PubMed] [Google Scholar]
- 45.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 2001. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect. Immun. 69:6240-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 2005. Vaccination with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect. Immun. 73:8153-8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palva, E. T., and L. L. Randall. 1978. Arrangement of protein I in Escherichia coli outer membrane: cross-linking study. J. Bacteriol. 133:279-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phale, P. S., A. Philippsen, T. Kiefhaber, R. Koebnik, V. P. Phale, T. Schirmer, and J. P. Rosenbusch. 1998. Stability of trimeric OmpF porin: the contributions of the latching loop L2. Biochemistry 37:15663-15670. [DOI] [PubMed] [Google Scholar]
- 49.Provencher, S. W., and J. Glockner. 1981. Estimation of globular protein secondary structure from circular dichroism. Biochemistry 20:33-37. [DOI] [PubMed] [Google Scholar]
- 50.Raulston, J. E. 1995. Chlamydial envelope components and pathogen-host cell interactions. Mol. Microbiol. 15:607-616. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Marañon, M. J., R. M. Bush, E. M. Peterson, T. Schirmer, and L. M. de la Maza. 2002. Prediction of the membrane-spanning-strands of the major outer membrane protein of Chlamydia. Protein Sci. 11:1854-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenbusch, J. P. 1974. Characterization of the major envelope protein from Escherichia coli. J. Biol. Chem. 249:8019-8029. [PubMed] [Google Scholar]
- 53.Schagger, H., and G. Von Jagow. 1987. Tricine-sodium dodecyl sulphate polyacrylamide gel electrophoresis for the separation of protein range 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 54.Schmid, B., M. Kromer, and G. E. Schulz. 1996. Expression of porin from Rhodopseudomonas blastica in Escherichia coli inclusion bodies and folding into exact native structure. FEBS Lett. 381:111-114. [DOI] [PubMed] [Google Scholar]
- 55.Shang, E. S., M. M. Exner, T. A. Aummers, C. Martinich, C. I. Champion, R. E. W. Hancock, and D. A. Haake. 1995. The rare outer membrane protein, OmpL1, of pathogenic Leptospira species is a heat-modifiable porin. Infect. Immun. 63:3174-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sreerama, N., S. Y. Venyaminov, and R. W. Woody. 1999. Estimation of the number of alpha-helical and beta-strand segments in proteins using circular dichroism spectroscopy. Protein Sci. 8:370-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sreerama, N., and R. W. Woody. 1993. A self consistent method for the analysis of protein secondary structure from circular dichroism. Anal. Biochem. 209:32-44. [DOI] [PubMed] [Google Scholar]
- 58.Sreerama, N., and R. W. Woody. 2004. On the analysis of membrane protein circular dichroism spectra. Protein Sci. 13:100-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stephens, R. S., R. Sanchez-Pescador, E. A. Wagar, C. Inouye, and M. S. Urdea. 1987. Diversity of Chlamydia trachomatis major outer membrane protein genes. J. Bacteriol. 169:3879-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su, H., Y.-X. Zhang, O. Barrera, N. G. Watkins, and H. D. Caldwell. 1988. Differential effect of trypsin on infectivity of Chlamydia trachomatis: loss of infectivity requires cleavage of major outer membrane protein variable domains II and IV. Infect. Immun. 56:2094-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Subbarao, G. V., and B. van den Berg. 2006. Crystal structure of the monomeric porin OmpG. J. Mol. Biol. 360:750-759. [DOI] [PubMed] [Google Scholar]
- 62.Sugawara, E., E. M. Nestorovich, S. M. Bezrukov, and H. Nikaido. 2006. Pseudomonas aeruginosa OprF exists in two different conformations. J. Biol. Chem. 281:16220-16229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanzer, R. J., and T. P. Hatch. 2001. Characterization of outer membrane proteins in Chlamydia trachomatis LGV serovar L2. J. Bacteriol. 183:2686-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Theunissen, J. J. H., B. Y. M. van Heust, J. H. T. Wagenvoort, E. Stolz, and M. F. Michel. 1992. Factors influencing the infectivity of Chlamydia pneumoniae elementary bodies on HL cells. J. Clin. Microbiol. 30:1388-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ting L.-M., R.-C. Hsia, C. G. Haidaris, and P. M. Bavoil. 1995. Interaction of outer envelope proteins of Chlamydia psittaci GPIC with the HeLa cell surface. Infect. Immun. 63:3600-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tokunaga, M., H. Tokunaga, Y. Okalima, and T. Nakae. 1979. Characterization of porins from the outer membrane of Salmonella typhimurium. Eur. J. Biochem. 95:441-448. [DOI] [PubMed] [Google Scholar]
- 67.Van Gelder, P., and J. Tommassen. 1996. Demonstration of a folded monomeric form of porin PhoE of Escherichia coli in vitro. J. Bacteriol. 178:5320-5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Stokkum, I. H., H. S. Spoelder, M. Bloemendal, R van Grondelle, and F. C. Groen. 1990. Estimation of protein secondary structure and error analysis from circular dichroism spectra. Anal. Biochem. 191:110-119. [DOI] [PubMed] [Google Scholar]
- 69.Wang, Y., E. A. Berg, X. Feng, L. Shen, T. Smith, C. E. Costello, and Y. X. Zhang. 2006. Identification of surface-exposed components of MOMP of Chlamydia trachomatis serovar F. Protein Sci. 15:122-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Westrom, L., R. Joesoef, G. Reynolds, A. Hagdu, and S. E. Thompson. 1992. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopy. Sex. Transm. Dis. 19:185-192. [PubMed] [Google Scholar]
- 71.Whitmore, L., and B. A. Wallace. 2004. DICROWEB, an online server for protein secondary structure analyses for circular dichroism spectroscopic data. Nucleic Acids Res. 32:W668-W673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yen, T.-T., S. Pal, and L. M. de la Maza. 2005. Characterization of the disulfide bonds and free cysteine residues of the Chlamydia trachomatis mouse pneumonitis major outer membrane protein. Biochemistry 44:6250-6256. [DOI] [PubMed] [Google Scholar]
- 73.Yildiz, O., K. R. Vinothkumar, P. Goswami, and W. Kuhlbrandt. 2006. Structure of the monomeric outer-membrane porin OmpG in the open and closed conformation. EMBO J. 25:3702-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]