Abstract
The genome of Lactobacillus salivarius UCC118 includes a 242-kb megaplasmid, pMP118. We now show that 33 strains of L. salivarius isolated from humans and animals all harbor a megaplasmid, which hybridized with the repA and repE replication origin probes of pMP118. Linear megaplasmids that did not hybridize with the pMP118 repA probe were also found in some strains of L. salivarius, showing for the first time that a lactic acid bacterium has multiple megaplasmids. Phylogenetic analysis of the repE and groEL sequences of 28 L. salivarius strains suggested similar evolutionary paths for the chromosome and megaplasmid. Although the replication origin of circular megaplasmids in L. salivarius was highly conserved, genotypic and phenotypic comparisons revealed significant variation between megaplasmid-encoded traits. Furthermore, megaplasmids of sizes ranging from 120 kb to 490 kb were present in seven strains belonging to six other Lactobacillus species from among 91 strains and 47 species tested. The discovery of the widespread presence of megaplasmids in L. salivarius, and restricted carriage by other Lactobacillus species, provides an opportunity to study the contribution of large extrachromosomal replicons to the biology of Lactobacillus.
Lactic acid bacteria (LAB) are a diverse group of gram-positive bacteria that are found in a wide variety of nutrient-rich environments (29). LAB have been extensively exploited because of their ability to preserve food, beverages, and forage (43). Many LAB have been subjected to comprehensive genetic and genomic analyses (33, 40, 42) to provide a molecular basis for understanding their distinctive properties, some of which are determined extrachromosomally. Plasmids are commonly found in many members of the lactic acid bacteria (25). Many of these plasmids are cryptic (61), and their contribution to the biology of the strain harboring them is unclear. However, it is recognized that plasmid-borne traits are major accessories that are key to the phenotypes of industrially important groups such as the lactococci (reviewed in reference 45). Noteworthy lactococcal properties that are plasmid encoded include the production of the PrtP protease (12), abortive infection mechanisms to prevent bacteriophage attack (6, 49), exopolysaccharide biosynthesis (69), and bacteriocin production (66). DNA sequencing of the four plasmids harbored by Lactococcus lactis strain SK11, a widely used dairy strain, identified a broad repertoire of novel genes that significantly enhance or expand the metabolism, fitness, and stress resistance of the bacterium (62). The ability of plasmids to undergo dissemination by conjugation or other processes underlines their potential importance for contributing significant but variable traits to LAB.
Comprising over a hundred species, the genus Lactobacillus represents the largest group within the family Lactobacillaceae (15, 60). The lactobacilli, like LAB in general, are associated with foodstuffs, plants, and animals (27), and many species are used in industrial applications such as food production (reviewed in reference 63). Some members of this genus are also attributed with “probiotic” properties (reviewed in references 17, 18, 26, 31, 32, 57, and 58), meaning the conferring of benefits to the consumer over and above inherent nutrition (26). This has contributed to heightened interest in genomics of the lactobacilli, and 10 genome sequences from nine Lactobacillus species have now been determined (1, 9, 13, 34, 42, 50, 67). As for other LAB, plasmids are commonly found in lactobacilli, and multiple extrachromosomal replicons are often present in a single strain (70). The lactobacilli whose genomes were sequenced are therefore somewhat anomalous for their paucity of plasmid content. Lactobacillus plantarum WCFS1 and Lactobacillus salivarius UCC118 each harbor three plasmids (13, 34), while the sequenced strains of Lactobacillus casei and Lactobacillus brevis contain one and two plasmids, respectively (42). However, the genomes of the sequenced strains of Lactobacillus acidophilus (1), Lactobacillus johnsonii (50) and Lactobacillus sakei (9) and two strains of Lactobacillus bulgaricus (42, 67) all lack any plasmids.
The three plasmids of L. plantarum WCFS1 have been functionally analyzed (68). The two smaller plasmids had no annotated genes related to functions other than replication. The largest plasmid, pWCFS103 (36 kb), conferred resistance to arsenate/arsenite and was shown to be conjugative. Lactobacillus plasmids from nonsequenced strains/species confer properties similar to those described previously for other plasmids of LAB (reviewed in reference 70), such as carbohydrate utilization (10), bacteriocin production (48), and exopolysaccharide biosynthesis (35). Interestingly, the sequence of the plasmid genome of Lactobacillus paracasei strain NFBC338 identified genes related to collagen adhesion and biotin utilization (16), which may be relevant for the probiotic properties of this strain.
The genome of L. salivarius strain UCC118 includes three plasmids (13), pSF20 (20 kb), pSF44 (44 kb), and pMP118 (242 kb). Plasmids pSF20 and pSF44 are almost exclusively cryptic (22), and their contribution to phenotype is currently unclear. Megaplasmid pMP118 is the largest sequenced plasmid in LAB. The annotation of pMP118 suggested that it conferred a range of additional metabolic capabilities upon L. salivarius UCC118, such as rhamnose and sorbitol utilization. Furthermore, it completed the genetic complement for encoding the pentose phosphate pathway (13), which allowed it to utilize ribose. pMP118 also harbored genes that are likely to contribute to host colonization or probiotic properties such as a bile salt hydrolase and the production of the broad-spectrum two-component bacteriocin Abp118 (23). Hybridization analysis of S1 nuclease-treated genomic DNA of nine other L. salivarius strains identified related megaplasmids in all strains.
We noted in the sequence description of pMP118 that plasmids greater than 100 kb had previously been suggested for L. acidophilus (48) and Lactobacillus gasseri (56) but that those and other analyses of Lactobacillus plasmid content predated pulsed-field gel electrophoresis (PFGE) and the usage of conditions to separate megaplasmids. In the present study, we examined the distribution and relatedness of megaplasmids in a large and diverse panel of L. salivarius strains as well as in other members of the genus.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The 33 strains of Lactobacillus salivarius used in this study are listed in Table 1. Ninety-one strains belonging to 47 species of Lactobacillus used in this study are listed in Table 2. Unless otherwise indicated, lactobacilli were routinely cultured at 37°C under microaerobic conditions (5% CO2) in de Man-Rogosa-Sharpe (MRS) medium (Oxoid Ltd., Basingstoke, Hampshire, United Kingdom).
TABLE 1.
L. salivarius strains used in this studya
| Strain | Origin | Reference(s) |
|---|---|---|
| UCC118 | Human ileal-cecal region | 13, 65 |
| AH4231 | Human ileal-cecal region | 65 |
| AH4331 | Human ileal-cecal region | 65 |
| AH43310 | Human ileal-cecal region | 65 |
| AH43324 | Human ileal-cecal region | 65 |
| AH43348 | Human ileal-cecal region | 65 |
| DSM20492 | Human saliva | |
| DSM20554T | Human saliva | 53 |
| DSM20555T | Human saliva | 53 |
| NCIMB8816 | Italian human saliva | |
| NCIMB8817 | Turkey feces | |
| NCIMB8818 | St. Ivel cheese | |
| NCIMB702343 | Unknown | |
| CCUG27530B | Human abdomen, abscess | |
| CCUG38008 | Human gall, 73-year-old male | |
| CCUG43299 | Human blood | |
| CCUG45735 | Human blood | |
| CCUG47825 | Human blood, 55-year-old female | |
| CCUG44481 | Bird | |
| CCUG47171 | Human tooth plaque | |
| CCUG47826 | Human blood, 55-year-old female | |
| JCM1040 | Human intestine | 46 |
| JCM1042 | Human intestine | 46 |
| JCM1044 | Human intestine | 46 |
| JCM1045 | Human intestine | 46 |
| JCM1046 | Swine intestine | 46 |
| JCM1047 | Swine intestine | 46 |
| JCM1230 | Chicken intestine | 46 |
| UCC119 | Chicken cecum | |
| 01M14315 | Human gallbladder pus | 71 |
| LMG14476 | Cat with myocarditis | |
| LMG14477 | Parakeet with sepsis | |
| L21 | Human feces | 45 |
CCUG, Culture Collection University Göteborg; DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH; JCM, Japan Collection of Microorganisms; LMG, Laboratorium voor Microbiologie, Universiteit Gent; NCIMB, National Collections of Industrial Food and Marine Bacteria. Type strains are indicated by a superscript T.
TABLE 2.
Other Lactobacillus strains used in this study
| Strain | Growth temp (°C) | Origin | Reference or source |
|---|---|---|---|
| L. sobrius DSM16698T | 37 | Feces, piglet intestine; The Netherlands | 36 |
| L. amylovorus DSM20552 | 37 | Intestine of adult | |
| L. kitasatonis DSM16761T | 37 | Chicken, intestine; Japan | 47 |
| L. ultunensis DSM16047T | 37 | Gastric biopsies, human stomach mucosa; Kalix, Sweden | 54 |
| L. crispatus DSM20356 | 37 | Turkey feces | |
| L. acidophilus ATCC4356T | 37 | Human | 59 |
| L. helveticus NCDO87 | 37 | ||
| L. helveticus NCDO1243 | 37 | ||
| L. gallinarum DSM10532T | 37 | Chicken crop | |
| L. acetotolerans DSM20749T | 30 | Fermented vinegar broth | |
| L. hamsteri DSM5661T | 37 | Feces of hamster | |
| L. intestinalis DSM6629T | 37 | Intestine of rat | 24 |
| L. kalixensis DSM16043T | 37 | Gastric biopsies, human stomach mucosa | 54 |
| L. delbrueckii subsp. bulgaricus DSM20081T | 37 | Bulgarian yogurt | |
| L. delbrueckii subsp. lactis DSM20073 | 37 | Saliva | |
| L. johnsonii DSM20553 | 37 | Sour milk | |
| L. oris DSM4864T | 37 | Human saliva | 20 |
| L. antri DSM16041T | 37 | Gastric biopsies, human stomach mucosa; Kalix, Sweden | 54 |
| L. reuteri DSM20016T | 37 | Intestine of adult | |
| L. reuteri DSM17509 | 37 | Rat gut; New Zealand | |
| L. reuteri DSM20015 | 37 | Manure | |
| L. reuteri DSM20053 | 37 | Human feces | |
| L. reuteri DSM20056 | 37 | Rat feces | |
| L. reuteri NCDO1359 | 37 | ||
| L. ingluviei DSM15946T | 37 | Pigeon, crop; Belgium | 2 |
| L. ingluviei DSM14792 | 42 | Chicken feces; Thailand | |
| L. fermentum DSM20055 | 30 | Saliva | |
| L. gastricus DSM16045T | 37 | Gastric biopsies, human stomach mucosa; Kalix, Sweden | 54 |
| L. mucosae DSM13345T | 37 | Pig small intestine; Sweden | 55 |
| L. vaccinostercus DSM20634T | 30 | Cow dung | |
| L. paracasei subsp. paracasei NCDO151 | 30 | ||
| L. paracasei subsp. paracasei 43362 | 30 | Human ileocecum | |
| L. paracasei subsp. paracasei 43332 | 30 | Human ileocecum | |
| L. paracasei subsp. paracasei 43338 | 30 | Human ileocecum | |
| L. casei DSM20011T | 30 | Cheese | |
| L. casei NCDO1202 | 30 | Cheese starter | |
| L. bifermentans DSM20003T | 30 | Blown cheese | |
| L. pantheris DSM15945T | 37 | Jaguar, feces; China | 39 |
| L. parabuchneri DSM5707T | 28 | Human saliva | |
| L. brevis DSM20054T | 30 | Feces | |
| L. brevis NCDO1058 | 30 | Farmhouse red Cheshire cheese 1955 | |
| L. malefermentans DSM5705T | 28 | Beer | |
| L. mindensis DSM14500T | 30 | Sourdough; Germany | 19 |
| L. plantarum NCDO326 | 30 | Isolated from dental carries | |
| L. plantarum NCDO340 | 30 | Isolated from silage | |
| L. plantarum NCDO704 | 30 | Isolated from starter | |
| L. plantarum 14-E10 | 30 | Sauerkraut | 41 |
| L. plantarum 14-F3 | 30 | Sauerkraut | 41 |
| L. plantarum 22-E2 | 30 | Sauerkraut | 41 |
| L. plantarum NCIMB8826 | 30 | Human saliva | |
| L. pentosus DSM20314T | 30 | ||
| L. paraplantarum 14-H4 | 30 | 41 | |
| L. gramini DSM20719T | 30 | Grass silage | |
| L. curvatus NCDO2739 | 30 | Isolated from milk | |
| L. curvatus UCC7017 | 30 | ||
| L. sakei DSM20100 | 30 | Stool of breast-fed infant | |
| L. sakei DSM6333 | 30 | Pork, vacuum packaged; produces bacteriocin sakacin A | |
| L. sakei UCC7012 | 30 | ||
| L. sakei UCC7016 | 30 | ||
| L. sakei NCDO2714 | 30 | Isolated from “moto,” starter for sake | |
| L. sakei LMG2313 | 30 | ||
| L. aviarius subsp. araffinosus DSM20653T | 37 | Intestine of chicken | |
| L. aviarius subsp. aviarius DSM20655T | 37 | Feces of chicken | |
| L. cypricasei DSM15353T | 37 | Cheese, Halloumi; Cyprus | |
| L. acidipiscis DSM15836T | 30 | Fermented fish; Thailand | |
| L. equi DSM15833T | 37 | Feces of horses; Japan | |
| L. agilis DSM20509T | 37 | Municipal sewage | |
| L. murinus DSM20452T | 37 | Intestine of rat | |
| L. animalis DSM20602T | 37 | Dental plaque of baboon | |
| L. saerimneri DSM16049T | 37 | Pig feces; Sweden | |
| L. ruminis ATCC 25644 | 37 | Bovine rumen | |
| L. ruminis ATCC 27780T | 37 | Bovine rumen | |
| L. ruminis ATCC 27781 | 37 | Bovine rumen | |
| L. ruminis ATCC 27782 | 37 | Bovine rumen | |
| L. ruminis L5 | 37 | Human feces | 64 |
| L. ruminis Subject 21 | 37 | G. W. Tannock | |
| L. ruminis Subject 23 | 37 | G. W. Tannock | |
| L. ruminis Subject 36 | 37 | G. W. Tannock | |
| L. ruminis Subject 38 | 37 | G. W. Tannock | |
| L. gasseri SR21 | 37 | Human gastric biopsy | K. A. Ryan et al., unpublished data |
| L. gasseri SR23 | 37 | Human gastric biopsy | Ryan et al., unpublished |
| L. gasseri SR26 | 37 | Human gastric biopsy | Ryan et al., unpublished |
| L. gasseri SR27 | 37 | Human gastric biopsy | Ryan et al., unpublished |
| L. gasseri SR29 | 37 | Human gastric biopsy | Ryan et al., unpublished |
| L. gasseri SR210 | 37 | Human gastric biopsy | Ryan et al., unpublished |
| L. gasseri SR211 | 37 | Human gastric biopsy | Ryan et al., unpublished |
| L. gasseri SR212 | 37 | Human gastric biopsy | Ryan et al., unpublished |
| L. gasseri SR214 | 37 | Human gastric biopsy | Ryan et al., unpublished |
| L. vaginalis SR28 | 37 | Human gastric biopsy | Ryan et al., unpublished |
| L. vaginalis SR213 | 37 | Human gastric biopsy | Ryan et al., unpublished |
| L. fermentum SR22 | 37 | Human gastric biopsy | Ryan et al., unpublished |
ATCC, American Type Culture Collection; DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH; NCDO, National Collection of Dairy Organisms; NCIMB, National Collection of Industrial and Marine Bacteria. Type strains are indicated by a superscript T.
Chemicals.
Low-melting-pointing (LMP) agarose, PFGE certified agarose, and λ DNA PFGE marker were purchased from Bio-Rad Laboratories (Hercules, CA). Sarkosyl (N-lauroylsarcosine), lysozyme, proteinase K, mutanolysin, and phenylmethylsulfonyl fluoride (PMSF) were purchased from Sigma-Aldrich (St. Louis, MO). Aspergillus oryzae S1 nuclease was purchased from Roche (Mannheim, Germany). All reagents were of analytical grade or high quality.
PFGE plug preparations.
Agarose gel plugs of high-molecular-weight DNA for PFGE were prepared according to a protocol described previously (3), with some small modifications, as outlined below. All Lactobacillus strains were grown in MRS broth supplemented with 0.5 g/liter cysteine in an anaerobic jar, at a temperature specified in Table 2, to early stationary phase. A volume of culture containing approximately 109 bacteria (equivalent to an optical density at 600 nm of 2.0) was centrifuged (20,000 × g for 1 min), washed once with 1 ml NT buffer (1 M NaCl, 10 mM Tris-HCl [pH 7.6]), and repelleted (20,000 × g for 1 min). The cell pellet was resuspended in 450 μl NET buffer (1 M NaCl, 100 mM EDTA, 10 mM Tris-Cl [pH 7.6]). An equal volume of melted 2% (wt/vol) LMP agarose, prepared in 0.125 M EDTA (pH 7.6) and maintained at 50°C, was added. The cell suspension and LMP agarose were mixed carefully without introducing bubbles. Gel plugs were formed by pipetting 300-μl volumes into plug molds and were allowed to solidify at 4°C for 10 min. Up to three plugs per strain were added to 2 ml of NET buffer containing 1% (wt/vol) sarkosyl, 10 mg/ml lysozyme, and 40 U/ml mutanolysin and then incubated at 37°C for 24 h. The lysozyme solution was replaced with 5 ml of 0.5 M EDTA (pH 8.0) containing 1% (wt/vol) sarkosyl and 0.5 mg/ml proteinase K and then incubated at 37°C for 24 h. This step was repeated with a fresh proteinase K solution. Plugs were then washed with 5 ml TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]) containing 1 mM PMSF (freshly prepared) at 37°C for 1 h to inactivate the proteinase K. This was followed by two 30-min incubations in 5 ml TE buffer at room temperature to remove the PMSF. Plugs were stored in 10 mM Tris-HCl-100 mM EDTA (pH 8.0) at 4°C.
S1 nuclease treatment.
Single slices (2 mm by 2 mm) were soaked in 200 μl S1 buffer (50 mM NaCl, 30 mM sodium acetate [pH 4.5], 5 mM ZnSO4) at room temperature for 30 min. The S1 buffer was replaced with another 200 μl S1 buffer containing 1 unit of A. oryzae S1 nuclease and then incubated at 37°C for 45 min. The reaction was stopped by replacing the S1 buffer with 200 μl of 0.5 M EDTA (pH 8.0) and held stationary at room temperature for 10 min. The 0.5 M EDTA was replaced with 200 μl TE and left still at room temperature for at least 30 min before loading onto a gel.
PFGE.
Plug slices were loaded directly into the wells of a 1% (wt/vol) PFGE agarose gel melted in 0.5× TBE (89 mM Tris-borate, 2 mM EDTA [pH 8.3]) buffer. The wells were sealed with molten 1% LMP agarose in 0.5× TBE buffer. DNA fragments were resolved using a CHEF-DR III pulsed-field system (Bio-Rad Laboratories, Hercules, CA) at 6 V/cm for 20 h with 0.5× TBE running buffer maintained at 14°C. Linear ramped pulse times were selected depending on the size of DNA fragments to be resolved; for routine analyses, a linear ramped pulse time of 3 s to 50 s was employed. Gels were stained in distilled water containing 0.5 μg/ml ethidium bromide for 120 min under light-limited conditions.
Probe preparation and Southern hybridization.
Gels were depurinated for 10 min in 0.2 M HCl, denatured for 30 min in 0.5 M NaOH-1.5 M NaCl, neutralized for 45 min in 0.5 M Tris (pH 7.5)-1.5 M NaCl, transferred by capillary overnight to Hybond-N+ nylon membranes (Amersham Biosciences, United Kingdom), and cross-linked to the membrane with UV light. The primers used to generate PCR amplicons that were used as probes are listed in Table S1 in the supplemental material.
To detect genes present on megaplasmids from L. salivarius strains, membranes were probed with PCR products as detailed in Table S1 in the supplemental material. Five hundred nanograms of probe DNA was labeled with the enzyme horseradish peroxidase according to the instructions for the ECL direct nucleic acid labeling and detection kit (Amersham Biosciences, United Kingdom). Membranes were prehybridized in 20 ml ECL hybridization buffer containing 5% blocking agent and 0.3 M NaCl at 42°C for 30 min, prior to the addition of the labeled probe. Hybridization was performed at 42°C for 16 h. Membranes were washed three times for 20 min in 6 M urea-0.4% sodium dodecyl sulfate-0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) (pH 7.0) at 42°C and three times for 5 min in 2× SSC at room temperature. Autoradiographs were produced by exposing Hyperfilm ECL for 1 h to 16 h at room temperature.
Stripping the membrane and rehybridization.
A previously hybridized membrane was rinsed thoroughly in double-distilled water. The membrane was washed three times for 30 min in 0.2 M NaOH containing 0.1% sodium dodecyl sulfate at 37°C to remove the bound probe. The membrane was further washed for 5 min in 2× SSC and stored in 2× SSC before hybridization with a second probe.
DNA sequencing.
The sequences of LSL_1740 (repE) from each of the 28 L. salivarius strains were generated by sequencing a 2.0-kb PCR amplicon amplified by using primers 1739_F1 and 1740_R1 (see Table S1 in the supplemental material). Sequencing was performed by MWG Biotech (Ebersberg, Germany). In addition, the fidelity of all probes used for Southern hybridization was confirmed by DNA sequencing.
Phylogenetic analysis.
Two different phylogenetic trees corresponding to chromosomal groEL- and repA-type megaplasmid repE genes for 28 L. salivarius strains were analyzed. Maximum likelihood trees were built using the best model (TrN+I for groEL and TIM+I for repE) with the Web-based tool MULTIPHYL (30) with a bootstrap value of 100. Gap regions were excised manually. The groEL sequences used in this study were reported in our previous study (38).
Carbohydrate utilization assay.
The ability of various L. salivarius strains to ferment ribose, sorbitol, and rhamnose was tested using API 50 CH strips in conjunction with API 50 CHL medium (bioMerieux). Details of this method were described in a previous study (38).
Bacteriocin assay.
L. salivarius strains were grown on MRS plates at appropriate temperatures for 16 h and then flooded with MRS sloppy agar (MRS broth plus 0.75% agar) containing Listeria monocytogenes EGDe as an indicator, with L. salivarius UCC118 as a positive control. Colonies that produced a halo against L. monocytogenes EGDe were recorded as being bacteriocin-positive strains.
Nucleotide sequence accession numbers.
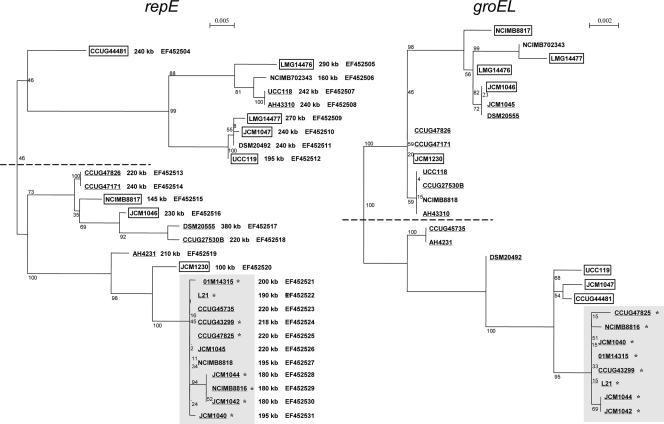
The sequences of the repE gene from 28 L. salivarius strains were deposited in GenBank under accession numbers EF452504 to EF452531 (specified by strain in Fig. 4).
FIG. 4.
Phylogenetic analysis of the L. salivarius repE genes of the repA-type megaplasmids and groEL gene phylogeny of the same strains. The size of the individual repA-type circular megaplasmids is shown to the right of the respective strain name in the repE tree. GenBank accession numbers for repE sequences are shown to the right of the megaplasmid sizes. Boxed and underlined strain labels represent animal origin and human origin, respectively. Gray shading indicates clusters that are relatively conserved between trees; strains located in this area in both phylogenies are labeled with an asterisk.
RESULTS
The presence of megaplasmids is a general feature of L. salivarius.
Ten L. salivarius strains were previously shown to harbor a megaplasmid whose size ranged from 100 kb to 380 kb (13). We therefore hypothesized that the presence of megaplasmids might be a general feature of L. salivarius. To test this, we collected another 23 L. salivarius strains from a variety of origins, including gastrointestinal tract isolates and clinical strains (Table 1). The presence of megaplasmids in these 23 strains was analyzed by the S1 PFGE protocol combined with Southern analysis that was described in our previous study (13).
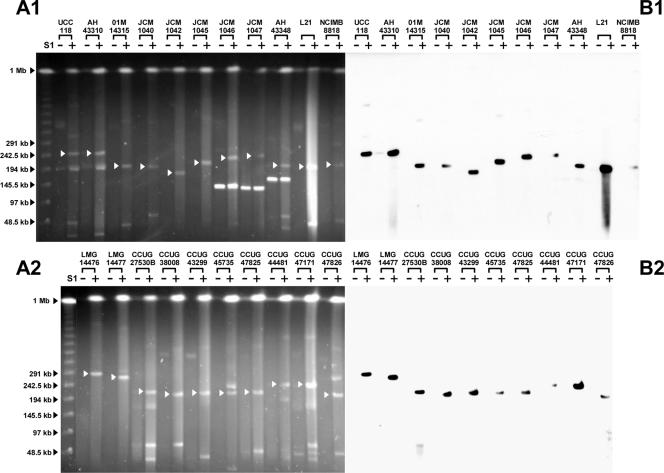
A survey of the additional 23 strains revealed that all strains harbor a megaplasmid that hybridized to the LSL_1739 (repA) probe derived from the sequenced megaplasmid pMP118 from L. salivarius strain UCC118 (Fig. 1). We therefore designated the megaplasmids in the 23 strains used in this study, and the 10 strains described in our previous study (13), as repA-type megaplasmids. We also noted the variable presence of DNA bands migrating close to the respective repA-type megaplasmids, e.g., in strains UCC118, AH43310, CCUG27530B, CCUG45735, CCUG44481, and CCUG47826 (Fig. 1). By Southern hybridization, we established that the apparently 195-kb band in the UCC118 lane was actually another form of the 20-kb plasmid pSF20 in strain UCC118 (data not shown). This suggests that the supercoiled or open circular forms of small plasmids migrate much slower than the corresponding linear form in PFGE. The presence of small plasmids may therefore complicate establishing if a given strain has megaplasmids, presenting a technical obstacle in surveying the presence of megaplasmids. To circumvent this, all genomic DNA plugs of L. salivarius strains were studied by multiple PFGE experiments under various running conditions. The linearized DNA fragment would thus be expected to migrate to the same position relative to linear DNA markers, while supercoiled or open circular forms of a plasmid should display inconsistent migration relative to linear DNA markers. A comparison between Fig. 1 and Fig. 2A exemplifies the behavior of repA-type megaplasmids, which migrate to a constant position relative to the linear DNA marker, while the other bands close to the corresponding megaplasmids migrate differently under two running conditions. This suggests that strains UCC118, AH43310, CCUG 45735, and CCUG47826 each harbor only one circular megaplasmid of the repA type. Similar data were obtained for strains CCUG27530B and CCUG44481 (data not shown).
FIG. 1.
repA-type megaplasmids are widely present in L. salivarius. (A1 and A2) PFGE of genomic DNA of 23 L. salivarius strains with strain UCC118 as a positive control. (B1 and B2) Corresponding Southern hybridization with the pMP118 repA probe. + and − indicate presence and absence of treatment with S1 nuclease, respectively. PFGE was run at 6 V/cm at 14°C for 20 h using a linear ramped pulse time of 3 s to 50 s. Black arrows indicate DNA size standards. White arrows indicate the S1 nuclease-linearized megaplasmid band that hybridized with the pMP118 repA probe. The prominent smear in the lane for strain L21 indicates genomic DNA degradation due to overtreatment with S1 nuclease.
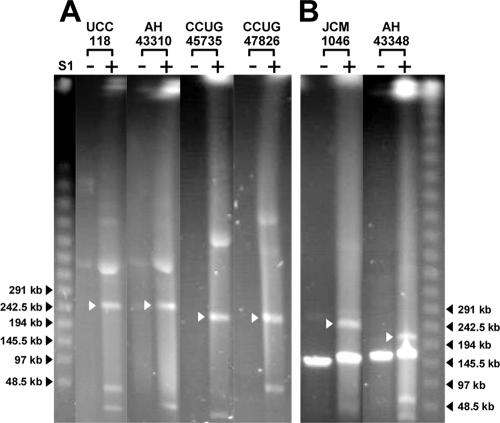
FIG. 2.
Confirmation of the presence of circular and linear megaplasmids in selected strains. + and − indicate the presence and absence of treatment with S1 nuclease, respectively. PFGE was run at 6 V/cm at 14°C for 20 h using a linear ramped pulse time of 30 s to 60 s. (A) Confirmation of the presence of a single circular megaplasmid in L. salivarius strains (refer also to the migration patterns in Fig. 1). (B) Confirmation of the coexistence of a circular megaplasmid and a linear megaplasmid in L. salivarius strains. White arrows indicate linearized repA-type circular megaplasmids. A and B were grouped from different parts of the same gel.
Apart from the universal presence of the repA-type megaplasmids in L. salivarius, S1 PFGE revealed that genomic DNA of L. salivarius strains JCM1046, JCM1047, and AH43348 included a prominent band whose running behavior was not affected by S1 nuclease treatment. This is indicative of the topology expected for a linear megaplasmid. Changing the PFGE running conditions further confirmed that strains JCM1046, JCM1047 (data not shown), and AH43348 harbor linear megaplasmids (Fig. 2B). To our knowledge, this is the first time that a lactic acid bacterium is reported to contain multiple megaplasmids. For clarity and consistency of nomenclature, we designated the L. salivarius circular repA-type megaplasmids pMP followed by the strain name, while the linear megaplasmid was designated pLMP followed by the strain name. None of the linear megaplasmids in strains JCM1046, JCM1047, and AH43348 hybridized with the repA probe, showing that the replication of these linear megaplasmids is different from that of the repA-type circular megaplasmids, However, the linear megaplasmid pLMP43348 in strain AH43348 had a sequence that hybridized to the probe for the presumptive chromosome partitioning ATPase (parA [LSL_1741]), while pLMP1046 and pLMP1047 did not (Fig. 3). Because the 33 strains that we tested all contain at least one repA-type megaplasmid, we conclude that the presence of megaplasmids is a general genomic feature of this species.
FIG. 3.
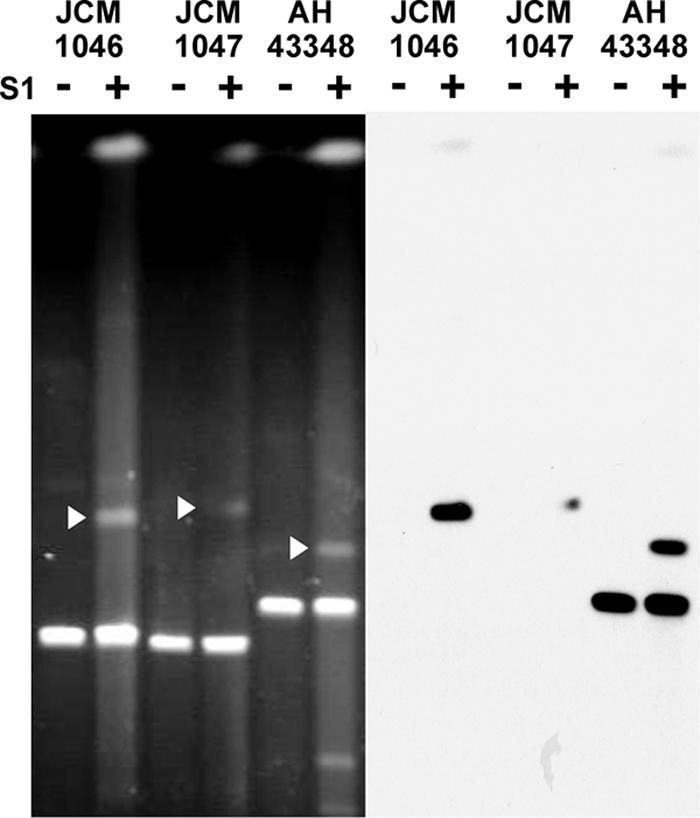
pLMP43348 in strain AH43348 contains sequences that hybridize to the parA probe of pMP118. + and − indicate the presence and absence of treatment with S1 nuclease, respectively. PFGE was run at 6 V/cm at 14°C for 20 h using a linear ramped pulse time of 3 s to 50 s. White arrows indicate linearized repA-type circular megaplasmids. The gel pattern in A is the same as that shown in Fig. 1, while that in B was generated by stripping the membrane used in Fig. 1 and rehybridizing it with the parA probe.
Phylogenetic analysis of repA-type megaplasmids in L. salivarius.
Further Southern analysis revealed that all L. salivarius repA-type megaplasmids also hybridized with the LSL_1740 (repE) and LSL_1741 (parA) probes (Table 3), which are located downstream of the repA gene. Primers spanning the repA and repE genes amplified a similarly sized amplicon in 28 L. salivarius strains tested (data not shown). This suggests that all L. salivarius repA-type megaplasmids have a highly conserved replication backbone. In order to compare the evolutionary histories of chromosomes and megaplasmids in L. salivarius, the groEL and repE genes from the respective replicons were subjected to molecular phylogeny. The groEL gene has been extensively used as an alternative molecular marker in phylogenetic analyses since it is a highly conserved housekeeping gene (38). repE was selected as a megaplasmid molecular marker, as it is universally present, and sequencing of the 2-kb repAE amplicon revealed that repE is more divergent than repA (data not shown). This analysis was performed for 28 of the 33 L. salivarius strains, since PFGE analysis of chromosomal digests identified two pairs of presumptively identical strains (AH43310-AH43324 and AH4331-AH4231), which were previously shown to have near-identical phenotypic profiles (38). In addition, PCRs for the repA-repE amplicon failed for strains DSM20554 and CCUG38008, presumably due to a 3′ mismatch, since they were shown by hybridization to harbor the repA and repE genes on their respective megaplasmids. Finally, strain AH43348 was a late addition to the strain panel and was not used for repE gene phylogeny. Maximum likelihood trees were constructed for the groEL and repE genes of the 28 strains (Fig. 4). Both trees clearly show two major branches although with a different level of confidence, as indicated by the bootstrap values. Although not totally concordant, there was a strong agreement between trees constructed based upon repE of the megaplasmid and the corresponding chromosomal groEL gene of the host strain. Longer tree branch lengths indicated by a higher relative scale in the repE tree are the consequence of a higher mutation rate of this gene than that of groEL. Interestingly, sequences from human-derived strains clustered better with each other than with those of animal origin, which is particularly evident in the lower branch. Moreover, despite the approximately 2:1 ratio (18/10) of human isolates to animal isolates, it is apparent that when the repE tree is analyzed, the major branches more consistently represent animal-derived strains for the upper branch and human-derived strains for the lower branch.
TABLE 3.
Distribution of genes related to replication, sugar metabolism, and bacteriocin production on the repA-type megaplasmids in 33 strains of L. salivariusa
| repA-type megaplasmid | Strain | Size of megaplasmid (kb) | LSL_1739 (repA) | LSL_1740 (repE) | LSL_1741 (parA) | Sorbitol utilization
|
Rhamnose utilization
|
Ribose utilization
|
Bacteriocin production
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype | LSL_1894 | Phenotype | LSL_1752 (rhaB) | Phenotype | LSL_1888 (mipB) | LSL_1946 (tktA) | Phenotype | LSL_1916 + LSL_1917 (Abp118α + Abp118β) | ||||||
| pMP20555 | DSM20555 | 380b | + | + | + | F | + | F | + | NF | + | + | NBA | N |
| pMP14476 | LMG14476 | 290 | + | + | + | F | + | F | + | NF | + | + | NBA | + |
| pMP14477 | LMG14477 | 270 | + | + | + | F | + | F | + | NF | + | + | NBA | + |
| pMP20554 | DSM20554 | 260b | + | + | + | F | + | NF | N | NF | + | + | NBA | N |
| pMP118 | UCC118 | 242b | + | + | + | F | + | F | + | F | + | + | BA | + |
| pMP43310 | AH43310 | 240 | + | + | + | F | + | F | + | F | + | + | BA | + |
| pMP43324 | AH43324 | 240 | + | + | + | F | + | F | + | F | + | + | BA | + |
| pMP20492 | DSM20492 | 240b | + | + | + | F | + | WF | + | NF | + | + | NBA | N |
| pMP44481 | CCUG44481 | 240 | + | + | + | F | + | NF | N | NF | + | N | NBA | + |
| pMP1047 | JCM1047 | 240 | + | + | + | F | + | NF | N | NF | + | N | NBA | + |
| pMP47171 | CCUG47171 | 240 | + | + | + | F | + | F | + | NF | + | + | NBA | + |
| pMP1046 | JCM1046 | 230 | + | + | + | F | + | F | + | NF | + | + | NBA | + |
| pMP1045 | JCM1045 | 220 | + | + | + | F | + | F | + | NF | + | + | NBA | + |
| pMP47825 | CCUG47825 | 220 | + | + | + | F | + | F | + | WF | + | + | NBA | + |
| pMP45735 | CCUG45735 | 220 | + | + | + | F | + | F | + | WF | + | + | NBA | + |
| pMP27530B | CCUG27530B | 220 | + | + | + | F | + | NF | + | WF | + | + | NBA | + |
| pMP47826 | CCUG47826 | 220 | + | + | + | F | + | F | + | NF | + | + | NBA | + |
| pMP43299 | CCUG43299 | 218 | + | + | + | F | + | NF | N | WF | + | + | NBA | N |
| pMP38008 | CCUG38008 | 215 | + | + | + | F | + | NF | N | WF | + | N | NBA | + |
| pMP4231 | AH4231 | 210b | + | + | + | F | + | NF | + | NF | + | + | BA | + |
| pMP4331 | AH4331 | 210 | + | + | + | F | + | NF | + | NF | + | + | BA | + |
| pMP14315 | 01M14315 | 200 | + | + | + | F | + | NF | N | NF | + | + | NBA | N |
| pMP119 | UCC119 | 195b | + | + | + | NF | N | F | + | NF | N | N | BA | + |
| pMP43348 | AH43348 | 195 | + | + | + | F | + | NF | + | WF | + | + | ND | + |
| pMP8818 | NCIMB8818 | 195 | + | + | + | F | + | WF | + | NF | + | + | NBA | + |
| pMP1040 | JCM1040 | 195 | + | + | + | NF | N | NF | N | NF | + | + | NBA | N |
| pMP21 | L21 | 190 | + | + | + | F | + | F | + | NF | + | + | NBA | + |
| pMP8816 | NCIMB8816 | 180b | + | + | + | F | + | NF | N | NF | + | N | NBA | N |
| pMP1042 | JCM1042 | 180 | + | + | + | F | + | NF | N | NF | + | N | NBA | N |
| pMP1044 | JCM1044 | 180 | + | + | + | F | + | NF | N | NF | + | N | NBA | N |
| pMP702343 | NCIMB702343 | 160b | + | + | + | NF | N | WF | + | NF | N | N | NBA | + |
| pMP8817 | NCIMB8817 | 145b | + | + | + | NF | N | NF | + | NF | N | + | NBA | N |
| pMP1230 | JCM1230 | 100b | + | + | + | F | + | NF | N | NF | + | + | NBA | + |
+, gene detected by hybridization; N, no hybridization signal detected; F, carbon source fermented; WF, carbon source weakly fermented; NF, carbon source not fermented; BA, bacteriocin activity detected; NBA, no bacteriocin activity detected; ND, not determined. Boldface type indicates that the gene is present but has a conflicting phenotype; italic type indicates that the gene is absent and has a corresponding phenotype. Data for carbohydrate-fermenting tests were obtained from our previous study (38). Indicator strains used in this study for determining the bacteriocin activity were L. monocytogenes EGDe and L. sake LMG2313.
The size of these repA-type megaplasmids were estimated in a previous study (13).
Biological and genetic features encoded on the L. salivarius repA-type megaplasmids.
DNA sequencing (13) identified contingency metabolism genes on pMP118, which were predicted to increase the metabolic flexibility of the host bacterium. The ability to assimilate sorbitol, rhamnose, and ribose by strain UCC118 was experimentally confirmed, verifying observations at the genomic level (38). As the replication backbone of the repA-type megaplasmids is highly conserved, we next investigated the degree of conservation of other genetic or phenotypic features on the other repA-type megaplasmids. The positions of the probes used to investigate the genetic features on the pMP118 map are shown in Fig. S1 in the supplemental material.
Sorbitol-fermenting ability was considered to be a universal feature for all strains in the original description of L. salivarius (53) and in the current API50 biochemical test profile. However, 4 strains out of 33 tested were unable to ferment sorbitol (Table 3). Interestingly, all 29 strains that fermented sorbitol harbored an LSL_1894 gene homolog encoding sorbitol-6-phosphate 2-dehydrogenase on their respective repA-type megaplasmids, as revealed by Southern hybridization (Table 3), while in the four strains that did not ferment sorbitol, this gene appears to be absent.
Historically, a rhamnose-fermenting ability was considered to be a criterion to distinguish L. salivarius subsp. salivarius from L. salivarius subsp. salicinius (53). We unified these two subspecies into a single species in a previous study, since not all L. salivarius subsp. salivarius strains can ferment rhamnose, while some L. salivarius subsp. salicinius strains can also ferment rhamnose (38). The rhamnose fermentation pathway in UCC118 involves LSL_1752 (rhaB [rhamnulokinase]), LSL_1754 (l-rhamnose isomerase), and LSL_1755 (araD [rhamnulose-1-phosphate aldolase]). As shown in Table 3, 11 strains that do not have an LSL_1752 homolog failed to ferment rhamnose. In addition, there are five strains that appear to possess an LSL_1752 homolog but that do not ferment rhamnose, suggesting gene silencing or a lack of other genes that are essential for completing the pathway.
L. salivarius was considered to be homofermentative when it was described in 1953 (53). This was corrected in our previous study (13), as pMP118 encodes transketolase and transaldolase, which complete the pentose phosphate pathway. Despite this, the ability to ferment pentose, e.g., ribose, is very rare among L. salivarius strains (38). Using LSL_1888 (encoding transaldolase) and LSL_1946 (encoding transketolase) as probes, we found that these two homologs are actually widely present on the repA-type megaplasmids (Table 3). Nearly half of the strains have sequences that hybridize with the LSL_1888 and LSL_1946 probes but lack the ability to ferment ribose.
The production of the bacteriocin Abp118 is megaplasmid encoded, and bacteriocin production might be a competitive advantage for commensal organisms. It may also be a useful trait for bacterial strains used as probiotic ingredients. Significantly, we have recently shown that Abp118 production is the major mechanism whereby L. salivarius UCC118 dramatically reduces Listeria monocytogenes infection in a mouse model (14). Sequences hybridizing to a probe for the Abp118 locus were detected in 20 strains. However, only 6 out of these 20 isolates produce detectable levels of bacteriocin against L. monocytogenes EGDe (Table 3).
Presence of megaplasmids in other lactobacilli.
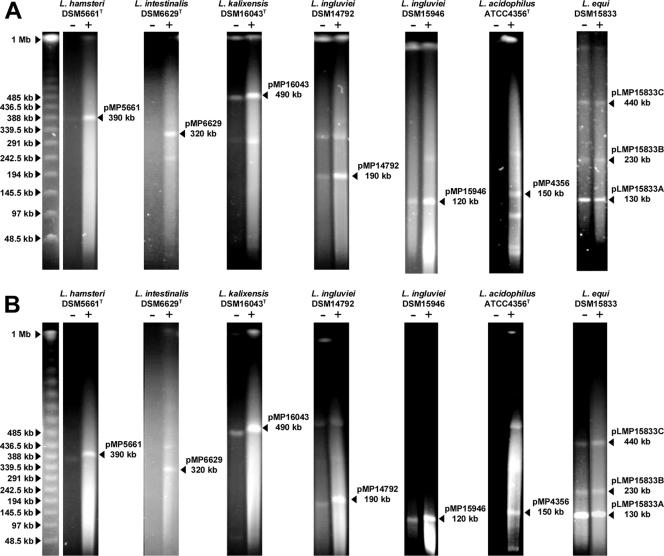
The discovery of megaplasmids in all L. salivarius strains examined prompted us to investigate the presence of megaplasmids across the genus Lactobacillus. We collected 91 strains belonging to 47 species (excluding L. salivarius) and screened them for the presence of megaplasmids by the S1 PFGE protocol. The phylogenetic positions of the species investigated are shown in Fig. S2 in the supplemental material. Megaplasmids of sizes ranging from 120 kb to 490 kb were detected in 7 strains belonging to 6 species from among the 91 strains and 47 species examined. These seven strains are as follows: Lactobacillus hamsteri DSM5661, L. intestinalis DSM6629, L. kalixensis DSM16043, L. ingluviei DSM14792, L. ingluviei DSM15946, L. acidophilus ATCC 4356, and L. equi DSM15833. Figure 5 shows the PFGE patterns for megaplasmid-containing strains run under two different conditions as described above. All megaplasmid-containing strains represent isolates from the gastrointestinal tract. These megaplasmids failed to hybridize with the pMP118 repA probe, suggesting that the L. salivarius megaplasmids represent a unique megaplasmid group in lactobacilli. L. equi DSM15833 harbored a prominent linear megaplasmid band and two other potential megaplasmids. Within the species that are phylogenetically most closely related to L. salivarius, only one of nine species (L. equi) was shown to have megaplasmids, which were not related to the repA-type megaplasmids. This suggests that the distribution of the Lactobacillus megaplasmids is independent of the phylogeny of the genus.
FIG. 5.
Megaplasmids of various sizes are found in a restricted number of other lactobacilli. Genomic DNA of seven strains belonging to six different Lactobacillus species with (+) or without (−) S1 nuclease treatment was resolved by PFGE at 6 V/cm at 14°C for 20 h using a linear ramped pulse time of 3 s to 50 s (A) or from 30 s to 60 s (B). Arrowheads to the left indicate size standards. Brightly smeared material below the pMP15946 band (120 kb) from L. ingluviei DSM15946 represents degraded genomic DNA. Arrowheads to the right of individual photos indicate the sizes of the megaplasmids confirmed by two different PFGE running conditions. Originally, the PFGE of L. equi DSM15833 was run in the loading order of S1 +/−, but we cropped it from the original gel picture and regrouped it in an order of S1 −/+ for consistency with other strains.
DISCUSSION
The occurrence of megaplasmids in lactic acid bacteria was not widely recognized until pMP118 was identified in L. salivarius by genome sequencing (13). At that time, we identified circular megaplasmids in nine other L. salivarius strains. We previously defined pMP118 as a megaplasmid because it contains neither tRNA nor rRNA genes; it has plasmid-related replication and partition proteins, and it does not contain the only copy of any known essential gene in the genome (13). In the present study, L. salivarius megaplasmids that have not been sequenced are functionally defined as those greater than 100 kb in size, by which we confirm the carriage of megaplasmids in 33 strains from very diverse sources. Plasmid pMP118, at 242 kb, constitutes 11% of the L. salivarius genome. The megaplasmid of strain DSM20555 (390 kb) could constitute 17.8% of the genome, assuming that its chromosome is around 1.8 Mb. Among the 33 repA-type circular megaplasmids identified in L. salivarius in this study, the average size was 218 ± 47 kb, with 22 repA-type circular megaplasmids larger than 200 kb. The contribution of a substantial proportion of the L. salivarius genome by a single extrachromosomal replicon is probably a universal phenomenon for this species.
The identification of megaplasmid molecules is facilitated by the S1 nuclease PFGE protocol (3). Definitive megaplasmid identification, particularly in strains that harbor one or more smaller circular plasmids, typically requires multiple PFGE runs under various conditions. This may have confounded the identification of large plasmids in LAB in previous studies. Multiple PFGE runs also contributed robustness to our identification of linear megaplasmids in three L. salivarius strains. The existence of a linear megaplasmid in a Lactobacillus strain was previously indicated by one report on L. gasseri strain CNRZ222 (56), which was in the same size range (150 kb) as the linear megaplasmids in L. salivarius (140 kb, 145 kb, and 175 kb) reported herein. Linear plasmids are well characterized in prokaryotes such as Streptomyces coelicolor (4), Borrelia burgdorferi (21), and Escherichia coli (51). The linear plasmid N15 in E. coli is actually the lysogenic form of a lambdoid phage (52), and linear prophages in Klebsiella oxytoca (8) and Yersinia enterocolitica (28) have also been described. However, these linear plasmid-prophage replicons are considerably smaller than the linear Lactobacillus megaplasmids reported here. It will be interesting to examine the organization of Lactobacillus linear megaplasmids, particularly the mode of replication, and the traits that they carry. Interestingly, the hybridization of LSL_1741 (parA) to pLMP43348 suggested that the partitioning of this linear megaplasmid is dependent upon a protein that is similar to that employed by circular megaplasmids. A similar conclusion was reported previously for the Mycobacterium celatum 23-kb linear plasmid pCLP, which has a genetic locus similar to the maintenance genes (par operon) of a bacterial circular plasmid (37). The pCLP par operon was shown to be important for the stability of this linear plasmid.
All circular megaplasmids in L. salivarius carried repA and repE gene homologs, and the repE gene showed sufficient divergence to allow phylogenetic analyses. It is conceivable that large plasmids such as those analyzed here might have multiple origins and that the resulting lack of selective pressure on the repE genes would make them unsuitable for phylogeny. However, the repA-repE locus is the only identifiable replication region in pMP118. The repE genes sequenced from the other plasmids are also linked to a repA homolog, and both rep genes are very similar to the corresponding genes of pMP118. Finally, none of the repE sequence variations used for phylogeny disrupts the reading frame. Since the pMP118 repE gene is functional, it is highly likely that the repE genes used for phylogeny are also functional. Although concordance was not absolute, the general agreement between trees based upon the repE and groEL genes of the respective strains suggests that the acquisition of a megaplasmid was a relatively early event in the evolution of L. salivarius.
The sorbitol utilization locus was well conserved, with only four of the strains that were analyzed appearing to have lost the corresponding gene from their respective megaplasmids and with the phenotype being consistent with the genotype in all cases. However, almost half the strains tested lacked the rhamnulokinase gene LSL_1752. Conversely, many strains that were unable to ferment ribose harbored both the transaldolase and transketolase genes on their respective megaplasmids. A total of eight strains lacked either or both of these genes. It is plausible that migration from the chromosome to the megaplasmid of part of the genetic information for the pentose phosphate pathway has been followed by the decay of relevant coding sequences in either replicon in particular strains. This makes it more remarkable that a minority of the strains examined have retained the functionality of the pathway.
The bacteriocin Abp118 has broad-spectrum activity (23) that would be expected to contribute to competitive exclusion and strain competitiveness of the producing strain in the gastrointestinal tract. Despite this, exactly one-third of the L. salivarius strains tested failed to hybridize with the abp118 gene probe, and the origin of this trait is unclear. It is also currently unknown if a loss of sequences or gene function, e.g., of induction mechanisms or ancillary genes, is responsible for the lack of bacteriocin production in the 14 out of 20 strains that harbor the genes for the Abp118 peptides.
In addition to L. salivarius, we detected the presence of megaplasmids in 6 other species from among 47 tested across the whole phylogenetic range of this very diverse genus (7). Four of these species are members of group A of the 16S rRNA phylogeny (7) that includes the so-called L. acidophilus complex: L. acidophilus (human gastrointestinal tract), L. hamsteri (hamster feces), L. intestinalis (rat intestine), and L. kalixensis (human stomach mucosa). L. ingluviei, two strains of which harbor megaplasmids, was isolated from pigeon crop, and L. equi was isolated from equine feces. Thus, all of these species are found in the gastrointestinal tract, and while this correlation must be treated with caution, it is clear that megaplasmids are uncommon in extraintestinal lactobacilli, food-associated lactobacilli, and free-living species. The lack of a homolog of the pMP118 repA gene establishes them as genetically distinct, at least at replication level, from the L. salivarius pMP118 replicon. L. equi DSM15833 apparently harbors three linear megaplasmids, representing the most complex genome geometry noted in this study. We note, however, that the intensities of the larger two plasmid bands is significantly lower than those of other linear megaplasmids in this strain and in L salivarius strains. These bands could therefore represent other forms of the linear plasmid pLMP15833A, although they do migrate consistently as linear bands under different switching conditions.
The megaplasmid of L. kalixensis strain DSM16043, at 490 kb, represents the largest plasmid identified in this study and may be considered a minichromosome depending on whether or not it carries essential genes. Recent sequencing projects have uncovered a complicated array of possible bacterial genome architectures (reviewed in reference 5), and the largest bacterial genome sequenced to date (Rhodococcus sp. strain RHA1) (44) includes three linear megaplasmids of 332 kb, 442 kb, and 1.1 Mb. It has been suggested that linear plasmids arose by the recombination of plasmids with bacteriophages, and linear chromosomes arose by the recombination of linear plasmids with circular chromosomes (11). The complexity of genomes such as Rhodococcus sp. strain RHA1 illustrates the possible outcomes of such processes, which may be ongoing in L. salivarius and other lactobacillus species. Further characterization of circular and linear megaplasmids of lactobacilli at the sequence level will elucidate biological traits that may have been selected during megaplasmid evolution and may possibly identify source organisms for the lateral transfer of linear plasmids.
Supplementary Material
Acknowledgments
Work in the laboratory of P.W.O. was supported by Science Foundation Ireland through a Centre for Science, Engineering, and Technology award to the Alimentary Pharmabiotic Centre, UCC, and a Research Frontiers program award and by Enterprise Ireland.
We thank G. W. Tannock for providing strains, P. O'Reilly for advice on culturing a number of species, and B. A. Jones for discussions of PFGE running conditions.
Footnotes
Published ahead of print on 22 June 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Altermann, E., W. M. Russell, M. A. Azcarate-Peril, R. Barrangou, B. L. Buck, O. McAuliffe, N. Souther, A. Dobson, T. Duong, M. Callanan, S. Lick, A. Hamrick, R. Cano, and T. R. Klaenhammer. 2005. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc. Natl. Acad. Sci. USA 102:3906-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baele, M., M. Vancanneyt, L. A. Devriese, K. Lefebvre, J. Swings, and F. Haesebrouck. 2003. Lactobacillus ingluviei sp. nov., isolated from the intestinal tract of pigeons. Int. J. Syst. Evol. Microbiol. 53:133-136. [DOI] [PubMed] [Google Scholar]
- 3.Barton, B. M., G. P. Harding, and A. J. Zuccarelli. 1995. A general method for detecting and sizing large plasmids. Anal. Biochem. 226:235-240. [DOI] [PubMed] [Google Scholar]
- 4.Bentley, S. D., S. Brown, L. D. Murphy, D. E. Harris, M. A. Quail, J. Parkhill, B. G. Barrell, J. R. McCormick, R. I. Santamaria, R. Losick, M. Yamasaki, H. Kinashi, C. W. Chen, G. Chandra, D. Jakimowicz, H. M. Kieser, T. Kieser, and K. F. Chater. 2004. SCP1, a 356,023 bp linear plasmid adapted to the ecology and developmental biology of its host, Streptomyces coelicolor A3(2). Mol. Microbiol. 51:1615-1628. [DOI] [PubMed] [Google Scholar]
- 5.Bentley, S. D., and J. Parkhill. 2004. Comparative genomic structure of prokaryotes. Annu. Rev. Genet. 38:771-792. [DOI] [PubMed] [Google Scholar]
- 6.Boucher, I., E. Emond, M. Parrot, and S. Moineau. 2001. DNA sequence analysis of three Lactococcus lactis plasmids encoding phage resistance mechanisms. J. Dairy Sci. 84:1610-1620. [DOI] [PubMed] [Google Scholar]
- 7.Canchaya, C., M. J. Claesson, G. F. Fitzgerald, D. van Sinderen, and P. W. O'Toole. 2006. Diversity of the genus Lactobacillus revealed by comparative genomics of five species. Microbiology 152:3185-3196. [DOI] [PubMed] [Google Scholar]
- 8.Casjens, S. R., E. B. Gilcrease, W. M. Huang, K. L. Bunny, M. L. Pedulla, M. E. Ford, J. M. Houtz, G. F. Hatfull, and R. W. Hendrix. 2004. The pKO2 linear plasmid prophage of Klebsiella oxytoca. J. Bacteriol. 186:1818-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaillou, S., M. C. Champomier-Verges, M. Cornet, A. M. Crutz-Le Coq, A. M. Dudez, V. Martin, S. Beaufils, E. Darbon-Rongere, R. Bossy, V. Loux, and M. Zagorec. 2005. The complete genome sequence of the meat-borne lactic acid bacterium Lactobacillus sakei 23K. Nat. Biotechnol. 23:1527-1533. [DOI] [PubMed] [Google Scholar]
- 10.Chassy, B., E. M. Gibson, and A. Giuffrida. 1978. Evidence for plasmid-associated lactose metabolism in Lactobacillus casei ssp. casei. Curr. Microbiol. 1:141-144. [DOI] [PubMed] [Google Scholar]
- 11.Chen, C. W., C. H. Huang, H. H. Lee, H. H. Tsai, and R. Kirby. 2002. Once the circle has been broken: dynamics and evolution of Streptomyces chromosomes. Trends Genet. 18:522-529. [DOI] [PubMed] [Google Scholar]
- 12.Christensson, C., C. J. Pillidge, L. J. H. Ward, and P. W. O'Toole. 2001. Nucleotide sequence and characterisation of the cell envelope proteinase plasmid in Lactococcus lactis subsp. cremoris HP. J. Appl. Microbiol. 91:334-343. [DOI] [PubMed] [Google Scholar]
- 13.Claesson, M. J., Y. Li, S. Leahy, C. Canchaya, J.-P. van Pijkeren, A. M. Cerdeno-Tarraga, J. Parkhill, S. Flynn, G. C. O'Sullivan, J. K. Collins, D. Higgins, F. Shanahan, G. F. Fitzgerald, D. van Sinderen, and P. W. O'Toole. 2006. Multireplicon genome architecture of Lactobacillus salivarius. Proc. Natl. Acad. Sci. USA 103:6718-6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corr, S. C., Y. Li, C. U. Riedel, P. W. O'Toole, C. Hill, and C. G. M. Gahan. 2007. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. USA 104:7617-7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dellaglio, F., and G. E. Felis. 2005. Taxonomy of lactobacilli and bifidobacteria, p. 25-49. In G. W. Tannock (ed.), Probiotics and prebiotics: scientific aspects. Caister Academic Press, Norfolk, United Kingdom.
- 16.Desmond, C., R. P. Ross, G. Fitzgerald, and C. Stanton. 2005. Sequence analysis of the plasmid genome of the probiotic strain Lactobacillus paracasei NFBC338 which includes the plasmids pCD01 and pCD02. Plasmid 54:160-175. [DOI] [PubMed] [Google Scholar]
- 17.Dunne, C., L. Murphy, S. Flynn, L. O'Mahony, S. O'Halloran, M. Feeney, D. Morrissey, G. Thornton, G. Fitzgerald, C. Daly, B. Kiely, E. M. Quigley, G. C. O'Sullivan, F. Shanahan, and J. K. Collins. 1999. Probiotics: from myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. Antonie Leeuwenhoek 76:279-292. [PubMed] [Google Scholar]
- 18.Dunne, C., L. O'Mahony, L. Murphy, G. Thornton, D. Morrissey, S. O'Halloran, M. Feeney, S. Flynn, G. Fitzgerald, C. Daly, B. Kiely, G. C. O'Sullivan, F. Shanahan, and J. K. Collins. 2001. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am. J. Clin. Nutr. 73:386S-392S. [DOI] [PubMed] [Google Scholar]
- 19.Ehrmann, M. A., M. R. Muller, and R. F. Vogel. 2003. Molecular analysis of sourdough reveals Lactobacillus mindensis sp. nov. Int. J. Syst. Evol. Microbiol. 53:7-13. [DOI] [PubMed] [Google Scholar]
- 20.Farrow, J. A. E., and M. D. Collins. 1988. Lactobacillus oris sp. nov. from human oral cavity. Int. J. Syst. Bacteriol. 38:116-118. [Google Scholar]
- 21.Ferdows, M. S., and A. G. Barbour. 1989. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc. Natl. Acad. Sci. USA 86:5969-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flynn, S. 2001. Molecular characterisation of bacteriocin producing genes and plasmid encoded functions of the probiotic strain Lactobacillus salivarius subsp. salivarius UCC118. Ph.D. thesis. University College Cork, Cork, Ireland.
- 23.Flynn, S., D. van Sinderen, G. M. Thornton, H. Holo, I. F. Nes, and J. K. Collins. 2002. Characterization of the genetic locus responsible for the production of ABP-118, a novel bacteriocin produced by the probiotic bacterium Lactobacillus salivarius subsp. salivarius UCC118. Microbiology 148:973-984. [DOI] [PubMed] [Google Scholar]
- 24.Fujisawa, T., K. Itoh, Y. Benno, and T. Mitsuoka. 1990. Lactobacillus intestinalis (ex Hemme 1974) sp. nov., nom. rev., isolated from the intestines of mice and rats. Int. J. Syst. Bacteriol. 40:302-304. [DOI] [PubMed] [Google Scholar]
- 25.Gasson, M. J., and C. A. Shearman. 2003. Plasmid biology, conjugation, and transposition, p. 25-44. In B. J. B. Wood and P. J. Warner (ed.), Genetics of lactic acid bacteria, vol. 3. Kluwer Academic/Plenum Publishers, New York, NY. [Google Scholar]
- 26.Guarner, F., and G. J. Schaafsma. 1998. Probiotics. Int. J. Food Microbiol. 39:237-238. [DOI] [PubMed] [Google Scholar]
- 27.Hammes, W. P., and R. F. Vogel. 1995. The genus Lactobacillus, p. 19-54. In B. J. B. Wood and W. H. Holzapfel (ed.), The genera of lactic acid bacteria, vol. 2. Blackie Academic and Professional, Glasgow, United Kingdom. [Google Scholar]
- 28.Hertwig, S., I. Klein, R. Lurz, E. Lanka, and B. Appel. 2003. PY54, a linear plasmid prophage of Yersinia enterocolitica with covalently closed ends. Mol. Microbiol. 48:989-1003. [DOI] [PubMed] [Google Scholar]
- 29.Kandler, O., and N. Weiss. 1986. Regular, nonsporing gram-positive rods, p. 1208-1234. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. Williams & Wilkins, Baltimore, MD. [Google Scholar]
- 30.Keane, T. M., T. J. Naughton, S. A. Travers, J. O. McInerney, and G. P. McCormack. 2005. DPRml: distributed phylogeny reconstruction by maximum likelihood. Bioinformatics 21:969-974. [DOI] [PubMed] [Google Scholar]
- 31.Klaenhammer, T. R. 1995. Genetics of intestinal lactobacilli. Int. Dairy J. 5:1019-1058. [Google Scholar]
- 32.Klaenhammer, T. R. 2000. Probiotic bacteria: today and tomorrow. J. Nutr. 130:415S-416S. [DOI] [PubMed] [Google Scholar]
- 33.Klaenhammer, T. R., R. Barrangou, B. L. Buck, M. A. Azcarate-Peril, and E. Altermann. 2005. Genomic features of lactic acid bacteria effecting bioprocessing and health. FEMS Microbiol. Rev. 29:393-409. [DOI] [PubMed] [Google Scholar]
- 34.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. P. Kuipers, R. Leer, R. Tarchini, S. A. Peters, H. M. Sandbrink, M. W. Fiers, W. Stiekema, R. M. Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 100:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kojic, M., M. Vujcic, A. Banina, P. Cocconcelli, J. Cerning, and L. Topisirovic. 1992. Analysis of exopolysaccharide production by Lactobacillus casei CG11, isolated from cheese. Appl. Environ. Microbiol. 58:4086-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konstantinov, S. R., E. Poznanski, S. Fuentes, A. D. Akkermans, H. Smidt, and W. M. de Vos. 2006. Lactobacillus sobrius sp. nov., abundant in the intestine of weaning piglets. Int. J. Syst. Evol. Microbiol. 56:29-32. [DOI] [PubMed] [Google Scholar]
- 37.Le Dantec, C., N. Winter, B. Gicquel, V. Vincent, and M. Picardeau. 2001. Genomic sequence and transcriptional analysis of a 23-kilobase mycobacterial linear plasmid: evidence for horizontal transfer and identification of plasmid maintenance systems. J. Bacteriol. 183:2157-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, Y., E. Raftis, C. Canchaya, G. F. Fitzgerald, D. van Sinderen, and P. W. O'Toole. 2006. Polyphasic analysis indicates that Lactobacillus salivarius subsp. salivarius and Lactobacillus salivarius subsp. salicinius do not merit separate subspecies status. Int. J. Sys. Evol. Microbiol. 56:2397-2403. [DOI] [PubMed] [Google Scholar]
- 39.Liu, B., and X. Dong. 2002. Lactobacillus pantheris sp. nov., isolated from faeces of a jaguar. Int. J. Syst. Evol. Microbiol. 52:1745-1748. [DOI] [PubMed] [Google Scholar]
- 40.Liu, M., F. H. van Enckevort, and R. J. Siezen. 2005. Genome update: lactic acid bacteria genome sequencing is booming. Microbiology 151:3811-3814. [DOI] [PubMed] [Google Scholar]
- 41.Lu, Z., F. Breidt, V. Plengvidhya, and H. P. Fleming. 2003. Bacteriophage ecology in commercial sauerkraut fermentations. Appl. Environ. Microbiol. 69:3192-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makarova, K., A. Slesarev, Y. Wolf, A. Sorokin, B. Mirkin, E. Koonin, A. Pavlov, N. Pavlova, V. Karamychev, N. Polouchine, V. Shakhova, I. Grigoriev, Y. Lou, D. Rohksar, S. Lucas, K. Huang, D. M. Goodstein, T. Hawkins, V. Plengvidhya, D. Welker, J. Hughes, Y. Goh, A. Benson, K. Baldwin, J. H. Lee, I. Diaz-Muniz, B. Dosti, V. Smeianov, W. Wechter, R. Barabote, G. Lorca, E. Altermann, R. Barrangou, B. Ganesan, Y. Xie, H. Rawsthorne, D. Tamir, C. Parker, F. Breidt, J. Broadbent, R. Hutkins, D. O'Sullivan, J. Steele, G. Unlu, M. Saier, T. Klaenhammer, P. Richardson, S. Kozyavkin, B. Weimer, and D. Mills. 2006. Comparative genomics of the lactic acid bacteria. Proc. Natl. Acad. Sci. USA 103:15611-15616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayra-Makinen, A., and M. Bigret. 1998. Industrial uses and production of lactic acid bacteria, p. 73-102. In S. Salminen and A. von Wright (ed.), Lactic acid bacteria: microbiology and functional aspects, 2nd ed., vol. 85. Marcel Dekker, New York, NY. [Google Scholar]
- 44.McLeod, M. P., R. L. Warren, W. W. Hsiao, N. Araki, M. Myhre, C. Fernandes, D. Miyazawa, W. Wong, A. L. Lillquist, D. Wang, M. Dosanjh, H. Hara, A. Petrescu, R. D. Morin, G. Yang, J. M. Stott, J. E. Schein, H. Shin, D. Smailus, A. S. Siddiqui, M. A. Marra, S. J. Jones, R. Holt, F. S. Brinkman, K. Miyauchi, M. Fukuda, J. E. Davies, W. W. Mohn, and L. D. Eltis. 2006. The complete genome of Rhodococcus sp. RHA1 provides insights into a catabolic powerhouse. Proc. Natl. Acad. Sci. USA 103:15582-15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mills, S., O. E. McAuliffe, A. Coffey, G. F. Fitzgerald, and R. P. Ross. 2006. Plasmids of lactococci—genetic accessories or genetic necessities? FEMS Microbiol. Rev. 30:243-273. [DOI] [PubMed] [Google Scholar]
- 46.Mitsuoka, T. 1969. Comparative studies on lactobacilli from the faeces of man, swine and chickens. Zentralbl. Bakteriol. 210:32-51. (In German.) [PubMed] [Google Scholar]
- 47.Mukai, T., K. Arihara, A. Ikeda, K. Nomura, F. Suzuki, and H. Ohori. 2003. Lactobacillus kitasatonis sp. nov., from chicken intestine. Int. J. Syst. Evol. Microbiol. 53:2055-2059. [DOI] [PubMed] [Google Scholar]
- 48.Muriana, P. M., and T. R. Klaenhammer. 1987. Conjugal transfer of plasmid-encoded determinants for bacteriocin production and immunity in Lactobacillus acidophilus 88. Appl. Environ. Microbiol. 53:553-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Driscoll, J., F. Glynn, G. F. Fitzgerald, and D. van Sinderen. 2006. Sequence analysis of the lactococcal plasmid pNP40: a mobile replicon for coping with environmental hazards. J. Bacteriol. 188:6629-6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pridmore, R. D., B. Berger, F. Desiere, D. Vilanova, C. Barretto, A. C. Pittet, M. C. Zwahlen, M. Rouvet, E. Altermann, R. Barrangou, B. Mollet, A. Mercenier, T. Klaenhammer, F. Arigoni, and M. A. Schell. 2004. The genome sequence of the probiotic intestinal bacterium Lactobacillus johnsonii NCC 533. Proc. Natl. Acad. Sci. USA 101:2512-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravin, N., and D. Lane. 1999. Partition of the linear plasmid N15: interactions of N15 partition functions with the sop locus of the F plasmid. J. Bacteriol. 181:6898-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ravin, V., N. Ravin, S. Casjens, M. E. Ford, G. F. Hatfull, and R. W. Hendrix. 2000. Genomic sequence and analysis of the atypical temperate bacteriophage N15. J. Mol. Biol. 299:53-73. [DOI] [PubMed] [Google Scholar]
- 53.Rogosa, M., R. F. Wiseman, J. A. Mitchell, M. N. Disraely, and A. J. Beaman. 1953. Species differentiation of oral lactobacilli from man including description of Lactobacillus salivarius nov spec and Lactobacillus cellobiosus nov spec. J. Bacteriol. 65:681-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roos, S., L. Engstrand, and H. Jonsson. 2005. Lactobacillus gastricus sp. nov., Lactobacillus antri sp. nov., Lactobacillus kalixensis sp. nov. and Lactobacillus ultunensis sp. nov., isolated from human stomach mucosa. Int. J. Syst. Evol. Microbiol. 55:77-82. [DOI] [PubMed] [Google Scholar]
- 55.Roos, S., F. Karner, L. Axelsson, and H. Jonsson. 2000. Lactobacillus mucosae sp. nov., a new species with in vitro mucus-binding activity isolated from pig intestine. Int. J. Syst. Evol. Microbiol. 50:251-258. [DOI] [PubMed] [Google Scholar]
- 56.Roussel, Y., C. Colmin, J. M. Simonet, and B. Decaris. 1993. Strain characterization, genome size and plasmid content in the Lactobacillus acidophilus group (Hansen and Mocquot). J. Appl. Bacteriol. 74:549-556. [PubMed] [Google Scholar]
- 57.Sanders, M. E. 1993. Effect of consumption of lactic cultures on human health, p. 67-130. In J. E. Kinsella (ed.), Advances in human nutrition research. Academic Press, San Diego, CA. [DOI] [PubMed]
- 58.Sanders, M. E. 1993. Summary of conclusions from a consensus panel of experts on health attributes of lactic cultures: significance of fluid milk products containing cultures. J. Dairy Sci. 76:1819-1828. [DOI] [PubMed] [Google Scholar]
- 59.Sanders, M. E., and T. R. Klaenhammer. 2001. The scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J. Dairy Sci. 84:319-331. [DOI] [PubMed] [Google Scholar]
- 60.Satokari, R. M., E. E. Vaughan, H. Smidt, M. Saarela, J. Matto, and W. M. de Vos. 2003. Molecular approaches for the detection and identification of bifidobacteria and lactobacilli in the human gastrointestinal tract. Syst. Appl. Microbiol. 26:572-584. [DOI] [PubMed] [Google Scholar]
- 61.Shareck, J., Y. Choi, B. Lee, and C. B. Miguez. 2004. Cloning vectors based on cryptic plasmids isolated from lactic acid bacteria: their characteristics and potential applications in biotechnology. Crit. Rev. Biotechnol. 24:155-208. [DOI] [PubMed] [Google Scholar]
- 62.Siezen, R. J., B. Renckens, I. van Swam, S. Peters, R. van Kranenburg, M. Kleerebezem, and W. M. de Vos. 2005. Complete sequences of four plasmids of Lactococcus lactis subsp. cremoris SK11 reveal extensive adaptation to the dairy environment. Appl. Environ. Microbiol. 71:8371-8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stiles, M. E. 1996. Biopreservation by lactic acid bacteria. Antonie Leeuwenhoek 70:331-345. [DOI] [PubMed] [Google Scholar]
- 64.Tannock, G. W., K. Munro, H. J. Harmsen, G. W. Welling, J. Smart, and P. K. Gopal. 2000. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl. Environ. Microbiol. 66:2578-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thornton, G. M. 1996. Probiotic bacteria: selection of Lactobacillus and Bifidobacterium strains from the healthy human gastrointestinal tract; characterisation of a novel Lactobacillus-derived antibacterial protein. Ph.D. thesis. University College Cork, Cork, Ireland.
- 66.Trotter, M., O. E. McAuliffe, G. F. Fitzgerald, C. Hill, R. P. Ross, and A. Coffey. 2004. Variable bacteriocin production in the commercial starter Lactococcus lactis DPC4275 is linked to the formation of the cointegrate plasmid pMRC02. Appl. Environ. Microbiol. 70:34-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van de Guchte, M., S. Penaud, C. Grimaldi, V. Barbe, K. Bryson, P. Nicolas, C. Robert, S. Oztas, S. Mangenot, A. Couloux, V. Loux, R. Dervyn, R. Bossy, A. Bolotin, J. M. Batto, T. Walunas, J. F. Gibrat, P. Bessieres, J. Weissenbach, S. D. Ehrlich, and E. Maguin. 2006. The complete genome sequence of Lactobacillus bulgaricus reveals extensive and ongoing reductive evolution. Proc. Natl. Acad. Sci. USA 103:9274-9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Kranenburg, R., N. Golic, R. Bongers, R. J. Leer, W. M. de Vos, R. J. Siezen, and M. Kleerebezem. 2005. Functional analysis of three plasmids from Lactobacillus plantarum. Appl. Environ. Microbiol. 71:1223-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Kranenburg, R., M. Kleerebezem, and W. M. de Vos. 2000. Nucleotide sequence analysis of the lactococcal EPS plasmid pNZ4000. Plasmid 43:130-136. [DOI] [PubMed] [Google Scholar]
- 70.Wang, T. T., and B. H. Lee. 1997. Plasmids in Lactobacillus. Crit. Rev. Biotechnol. 17:227-272. [DOI] [PubMed] [Google Scholar]
- 71.Woo, P. C., A. M. Fung, S. K. Lau, and K. Y. Yuen. 2002. Identification by 16S rRNA gene sequencing of Lactobacillus salivarius bacteremic cholecystitis. J. Clin. Microbiol. 40:265-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.