Abstract
Sequence analysis of the bacterial luminescence (lux) genes has proven effective in helping resolve evolutionary relationships among luminous bacteria. Phylogenetic analysis using lux genes, however, is based on the assumptions that the lux genes are present as single copies on the bacterial chromosome and are vertically inherited. We report here that certain strains of Photobacterium leiognathi carry multiple phylogenetically distinct copies of the entire operon that codes for luminescence and riboflavin synthesis genes, luxCDABEG-ribEBHA. Merodiploid lux-rib strains of P. leiognathi were detected during sequence analysis of luxA. To define the gene content, organization, and sequence of each lux-rib operon, we constructed a fosmid library of genomic DNA from a representative merodiploid strain, lnuch.13.1. Sequence analysis of fosmid clones and genomic analysis of lnuch.13.1 defined two complete, physically separate, and apparently functional operons, designated lux-rib1 and lux-rib2. P. leiognathi strains lelon.2.1 and lnuch.21.1 were also found to carry lux-rib1 and lux-rib2, whereas ATCC 25521T apparently carries only lux-rib1. In lnuch.13.1, lelon.2.1, lnuch.21.1, and ATCC 25521T, lux-rib1 is flanked upstream by lumQ and putA and downstream by a gene for a hypothetical multidrug efflux pump. In contrast, transposase genes flank lux-rib2 of lnuch.13.1, and the chromosomal location of lux-rib2 apparently differs in lnuch.13.1, lelon.2.1, and lnuch.21.1. Phylogenetic analysis demonstrated that lux-rib1 and lux-rib2 are more closely related to each other than either one is to the lux and rib genes of other bacterial species, which rules out interspecies lateral gene transfer as the origin of lux-rib2 in P. leiognathi; lux-rib2 apparently arose within a previously unsampled or extinct P. leiognathi lineage. Analysis of 170 additional strains of P. leiognathi, for a total of 174 strains examined from coastal waters of Japan, Taiwan, the Philippine Islands, and Thailand, identified 106 strains that carry only a single lux-rib operon and 68 that carry multiple lux-rib operons. Strains bearing a single lux-rib operon were obtained throughout the geographic sampling range, whereas lux-rib merodiploid strains were found only in coastal waters of central Honshu. This is the first report of merodiploidy of lux or rib genes in a luminous bacterium and the first indication that a natural merodiploid state in bacteria can correlate with geography.
Luminescence in Photobacterium leiognathi and other luminous bacteria is the product of bacterial luciferase, a mixed-function oxidase that uses oxygen, reduced flavin mononucleotide, and a long-chain fatty aldehyde as substrates to produce blue-green luminescence. The genes for bacterial light production are present as an operon, luxCDABEG: luxA and luxB encode the α and β subunits of luciferase; luxC, luxD, and luxE specify the enzymatic components of a fatty acid reductase complex necessary for synthesis and recycling of the aldehyde substrate; and luxG encodes a flavin reductase (14). Most luminous Photobacterium species, i.e., Photobacterium phosphoreum, Photobacterium kishitanii, and Photobacterium mandapamensis, also carry luxF, which encodes a nonfluorescent flavoprotein, with a lux operon gene order of luxCDABFEG (2, 3, 4, 23, 28, 37). Linked to the luminescence genes in some Photobacterium species, and apparently cotranscribed with them, are genes involved in the synthesis of riboflavin, forming an operon of 10 or 11 genes, luxCDAB(F)EG-ribEBHA, which we refer to here as the lux-rib operon (23, 26, 27, 34, 37; this study). Upstream of the lux-rib operon in P. mandapamensis, a species closely related to P. leiognathi, are lumQ and lumP (encoding proteins of the lumazine operon), and these genes are located adjacent to the putA gene (encoding proline dehydrogenase) (29, 30, 31).
Phylogenetic analysis of lux and rib genes, together with housekeeping genes such as the16S rRNA gene, gyrB, pyrH, recA, rpoA, and rpoD, has proven helpful in defining evolutionary relationships among luminous bacteria and in the identification of new species (2, 3, 4, 12, 23). Phylogenetic analysis based on lux and other genes has also proven effective in providing the bacterial species- and clade-level resolution necessary for testing hypotheses of symbiont-host specificity and evolutionary codivergence in bioluminescent symbioses (16, 23). These studies reveal that the evolutionary divergence of symbiotic luminous bacteria has not followed the evolutionary divergence of their host animals.
Little is known, however, about the evolution of the bacterial luminescence system itself. Based on amino acid sequence identities, a tandem duplication of the ancestral luxA gene, followed by sequence divergence in the duplicated gene, is thought to have given rise to luxB, leading to the formation of the heterodimeric luciferase present in modern-day luminous bacteria. Similarly, a tandem duplication of luxB is thought to have given rise to the luxF gene (6, 14, 37). The subsequent loss of luxF from the lineage that gave rise to P. leiognathi might reflect the evolutionary divergence of this species from other Photobacterium species (2). Recently, strains of P. mandapamensis bearing nonsense mutations in luxF have been isolated from nature, the first report of naturally occurring mutations in lux structural genes (23). The presence of strains bearing luxF mutations in nature suggests that luxF is less functionally constrained than other lux genes and indicates that this gene does not play an essential role in the free-living ecology and symbiosis of this species.
Relevant to both phylogenetic analysis based on lux genes and evolution of the luminescence system is the question of the lux gene copy number. The lux and rib genes are tacitly assumed to be present as single copies in P. leiognathi and other luminous bacteria. In contradiction of that assumption, we report here that certain strains of P. leiognathi carry two complete, physically separate, and apparently functional lux-rib operons, one closely associated with putA and the other located elsewhere on the chromosome. The presence of multiple copies of each of the lux and rib genes would presumably provide opportunities for the accumulation of mutations leading to sequence divergence in one or the other copy of each lux and rib gene and opportunities for recombination between the two operons. Instead, we find that both operons are stably inherited and show little or no evidence of mutation or recombination in different merodiploid strains.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Bacterial strains used in this study are listed in Table 1 and in the supplemental material. Strains of P. leiognathi were isolated from light organs of bacterially luminous marine animals and from seawater collected in various locations in Honshu, Shikoku, and Okinawa, Japan; Taiwan; the Philippine Islands; and Thailand (2, 8, 15, 16, 18; this study) (see the supplemental material for collection details). Bacteria were grown in LSW-70 broth (15), which contained (per liter) 10 g tryptone, 5 g yeast extract, 350 ml double-strength artificial seawater (38), 650 ml deionized water, and, for solid medium, 15 g agar. Genomic DNA was purified from cultures of strains grown overnight in LSW-70 broth using the DNeasy tissue extraction kit (QIAGEN). Strains were identified to the species level by phylogenetic analysis of lux and other genes (2, 15, 16, 23; this study).
TABLE 1.
Bacterial strains used in this study
| Species | Strain | Habitat, geographic source, and isolation yra | Reference(s) and/or source |
|---|---|---|---|
| P. leiognathi | ATCC 25521T | LO, Gulf of Thailand, ca. 1967 | 8 |
| lelon.2.1 | LO, Sagami Bay, Honshu, Japan, 1983 | 16; this study | |
| lnuch.13.1 | LO, Wakasa Bay, Honshu, Japan, 2003 | This study | |
| lnuch.21.1 | LO, Suruga Bay, Honshu, Japan, 2004 | This study | |
| P. kishitanii | ATCC BAA-1194T | LO, Sagami Bay, Honshu, Japan, 1982 | 3, 4 |
| P. mandapamensis | ATCC 27561T | SW, Banda Island, Indonesia, before 1970 | 21, 43 |
| PL-721b | FS, Sulu Sea, 1975 | 2, 39 | |
| P. phosphoreum | ATCC 11040T | FS, Delft, The Netherlands, 1934 | 3 |
| Vibrio orientalis | ATCC 33934T | SW, Yellow Sea, China, before 1983 | 53 |
| Vibrio splendidus | ATCC 33125T | FS, North Sea, before 1955 | 47 |
LO, light organ of fish; SW, seawater; FS, skin of marine fish.
Strain PL-721 may be incorrectly designated PL-741 in some records.
DNA amplification and sequencing.
For DNA amplification by PCR, MasterTaq polymerase (Eppendorf) and the following protocol were used: a 95°C initial denaturing step for 2 min; 35 cycles with a 94°C denaturing step for 20 s, a variable temperature annealing step for 15 s, and an extension step at 68°C for 1 min; a 7-min final extension step at 68°C; and snap cooling to 4°C. PCR primers, annealing temperatures, and exceptions to this protocol can be found at http://www-personal.umich.edu/∼pvdunlap/supplementalinfo.html. PCR products were visualized using electrophoresis on 1% agarose gels stained with ethidium bromide and were purified using a QIAquick PCR purification kit (QIAGEN) or a Montage PCR filter kit (Millipore). PCR products were sequenced using the respective PCR primers. Sequencing was carried out by the staff of the University of Michigan Sequencing Core using dye terminator cycle sequencing on a Perkin-Elmer ABI 3730 or 3700 DNA analyzer. Specific primers for species of luminous bacteria other than P. leiognathi were designed based on existing publicly available sequences (see http://www-personal.umich.edu/∼pvdunlap/supplementalinfo.html). Sequences not obtained in this laboratory were downloaded from public databases.
Cloning of luxA and lux-rib operons.
The luxA genes of P. leiognathi lnuch.13.1, amplified using primers CWLAPlfor and CWLAPlrev, were cloned using the TOPO 4.0 cloning kit (Invitrogen). A fosmid library of genomic DNA from strain lnuch.13.1 was constructed using a CopyControl Fosmid Library Production kit (Epicenter). The library consists of approximately 3,500 clones with inserts of 35 to 40 kb. The library was screened for fosmids containing lux-rib sequences by pooling transformant colonies into groups that were subsequently analyzed by PCR using lux-rib primers. From these groups, two colonies that each contained a fosmid with a complete lux-rib operon were identified, clones B7-25 and C30-24. Fosmids were recovered from colonies using a WizardPlus Miniprep kit (Promega) and sequenced directly using vector-specific and lux-rib-specific primers. See http://www-personal.umich.edu/∼pvdunlap/supplementalinfo.html for the sequencing strategy. Recovered fosmids were also used as templates for long-range PCR using the TripleMaster PCR system (Eppendorf) to determine the orientation of the inserted DNA and the approximate position of lux-rib in the inserted DNA relative to the vector ends.
Plasmid profiling and pulsed-field gel electrophoresis (PFGE).
Small plasmids in P. leiognathi (15) were purified from strains lelon.2.1, lnuch.13.1, lnuch.21.1, and ATCC 25521T grown overnight in LSW-70 broth by using a WizardPlus Miniprep kit (Promega). The resulting DNA was electrophoresed through a 0.7% agarose gel (Bio-Rad) and stained with ethidium bromide. Plasmids smaller than 25 kb were present in lnuch.13.1, lelon.2.1, and lnuch.21.1 (data not shown); these plasmids were found to be smaller than the lux-rib-containing fosmid clones B7-25 and C30-24.
To visualize plasmids larger than 25 kb, we used PFGE and a Bio-Rad CHEF mapper-chiller system. Genomic DNA in agarose plugs was prepared according to a method described previously by Lucangeli et al. (35), with modifications. Before lysis of the cells embedded in agarose, the plugs were first washed for 1 h at 37°C in buffer (6 mM Tris-HCl [pH 7.5], 100 mM EDTA [pH 8.0], 1 M NaCl, 0.5% Brij 58, 0.2 M sodium deoxycholate, 0.5% N-laurosylsarcosine NL-97) with 0.5 mg·ml−1 of lysozyme. Genomic DNA was run in 1% SeaKem agarose gels in 1× Tris-acetate-EDTA buffer. Electrophoresis was done for 15 h at 14°C with a gradient of 6 V·cm−1, an angle of 120°, an initial pulse switch time of 5 s, and a final pulse switch time of 15 s, with linear ramping. Two plasmids of approximately 180 to 200 kb, sufficiently large to account for a cosmid clone bearing a lux-rib operon, were present in strain lelon.2.1, whereas plasmids of 35 to 40 kb or greater were not detected in lnuch.13.1 or lnuch.21.1.
Similar PFGE procedures were used to identify the chromosomal locations of lux-rib1 and lux-rib2. Specifically, genomic DNA in agarose plugs was digested for 16 to 18 h with NotI restriction endonuclease (New England Biolabs) at 37°C in the buffer recommended by the manufacturer. PFGE gels were 0.8% SeaKem agarose (Bio Whittaker Molecular Applications) or low-melting-point agarose (Bio-Rad) in 1× Tris-acetate-EDTA buffer. Electrophoresis conditions were as follows: 27 h at 14°C with a gradient of 6 V·cm−1, an angle of 120°, an initial pulse switch time of 2.16 s, and a final pulse switch time of 2 min 26.90 s, with linear ramping. Mid Range PFG marker (New England Biolabs) and Yeast Chromosome marker (New England Biolabs) were used as size markers. Gels were stained with ethidium bromide to visualize DNA.
NotI digestion fragments of DNA were recovered using PFGE carried out in gels of low-melting-point agarose. Individual bands were excised, and the agarose was melted (65°C) and digested using β-agarase (New England Biolabs) as recommended by the manufacturer. DNA fragments were purified using phenol-chloroform extraction, recovered by ethanol precipitation, and suspended in distilled water. NotI digestion of P. leiognathi strain lnuch.13.1 genomic DNA produced seven fragments that ranged in size from approximately 1,500 kb to 220 kb. PCR was performed using the individual DNA fragments as a template and with lux-rib1-specific and lux-rib2-specific luxD-luxA primers.
Phylogenetic analysis.
Sequences of the lux-rib operon genes of Photobacterium strains were aligned to the sequences of lux and rib genes from other luminous species (Table 2), with the nucleotide alignment based on inferred amino acid sequences; data were analyzed simultaneously with the parsimony criterion using PAUP* (48). Spacer regions between genes in the lux and rib operons were omitted from the analysis, and inferred deletions were treated as missing data. Jackknife support percentage values (17) were calculated in PAUP* using 1,000 replicates with 10 heuristic searches per replicate, with a jackknife resampling value of 34%, and emulating jacknife resampling, which resamples characters independently. Housekeeping genes (gapA, gyrB, recA, rpoA, and rpoD) were amplified, sequenced, and analyzed by similar methods to test the relationships within Photobacterium determined by lux-rib analysis.
TABLE 2.
GenBank accession numbers for DNA sequences used in this study
| Species | Strain | Gene(s)a | GenBank accession no. |
|---|---|---|---|
| P. angustum | S14 | AAOJ01000000b | |
| SKA34 | AAOU01000001b | ||
| P. profundum | SS9 | CR378665b | |
| P. leiognathi | ATCC 25521T | putA-luxC | EF536344 |
| luxCDABEG | M63594 | ||
| ribEBH | M90094 | ||
| ribH-orf1 | EF536341 | ||
| lelon.2.1 | lux-rib1 | EF536333 | |
| lux-rib2 | EF536334 | ||
| lnuch.13.1 | lux-rib1 | EF536338c | |
| lux-rib2 | EF536332d | ||
| lnuch.21.1 | lux-rib1 | EF536335 | |
| lux-rib2 | EF536336 | ||
| P. kishitanii | ATCC BAA-1194T | lux-rib | AY341065 |
| P. mandapamensis | ATCC 27561T | putA | EF536343 |
| lux-rib | AY341067 | ||
| orf1 | EF536340 | ||
| PL-741 | putA | U39227 | |
| lumQ | U35231 | ||
| lumP | X65611 | ||
| luxC | X65156 | ||
| luxD | X65612 | ||
| luxE | U66407 | ||
| luxG | AF053227 | ||
| ribEBHA | AF364106 | ||
| lumP-luxC | X56534 | ||
| luxABFE | X08036e | ||
| P. phosphoreum | ATCC 11040T | lux-rib | AY341063 |
| Photorhabdus luminescens subsp. laumondii | TTO1 | luxCDABE, ribE, ribB, ribH, and ribA | NC_005126 |
| Photorhabdus luminescens subsp. luminescens | ATCC 29999T | luxCDABE | M90093 |
| Shewanella hanedai | ATCC 33224T | luxCDABEG | AB058949 |
| Shewanella woodyi | ATCC 51908T | luxA | DQ322063 |
| V. cholerae | NCIMB 41T | luxCDABE | AB115761 |
| V. fischeri | ES114 | luxCDABE | NC_006841 |
| ribEBH | NC_006840 | ||
| MJ-1 | luxCDABE | AF170104 | |
| V. harveyi | B-392 | luxD | J03950 |
| luxAB | M10961 | ||
| luxE | M28815 | ||
| luxG-ribB | M27139f | ||
| Vibrio logei | ATCC 29985T | luxAB | EF576941 |
| V. orientalis | ATCC 33934T | luxDA | AB058948 |
| V. salmonicida | NCIMB 2262T | luxCDABEG | AF452135 |
| V. splendidus | ATCC 33125T | luxAB | EF536342 |
The designation lux-rib indicates the luxCDABEG-ribEBHA genes, and lux-rib1 and lux-rib2 indicate luxCDABEG-ribEBHA operon 1 and operon 2, respectively, of P. leiognathi (see the text).
P. angustum and P. profundum do not have lux genes; these sequences were used to demonstrate homology to sequences found flanking the lux-rib1 operon.
Sequence from fosmid B7-25.
Sequence from fosmid C30-24.
luxF is designated luxG in this record.
ribB is designated luxH in this record.
Analysis of recombination.
To test for recombination between the genes of lux-rib1 and lux-rib2 in lnuch.13.1, lelon.2.1, and lnuch.21.1, we developed a partition congruence analysis, as described here (script to produce PAUP* commands are available on request). Included in the analysis were lux-rib1 of P. leiognathi ATCC 25521T, the lux-rib operon of P. mandapamensis ATCC 27561T, and, as an outgroup, the lux-rib operon of P. kishitanii ATCC BAA-1194T (also known as pjapo.1.1T) (4). Omitted from the analysis were noncoding intergenic spacer regions, the luxF gene in P. mandapamensis and P. kishitanii, and the first 341 bp of the luxC sequence, which is not available for the lux-rib2 operons of lelon.2.1 and lnuch.21.1 (Fig. 1). The resulting aligned lux-rib1 and lux-rib2 coding regions of each strain, 8,732 nucleotides, were analyzed in a progressive 200-nucleotide window that shifted 50 nucleotides with each analytical run (171 200-character partitions and 1 partition of 182 characters). Each partition was subjected to an exhaustive search using PAUP*, with gaps treated as a fifth nucleotide. Each phylogenetic analysis resulted in up to four equally parsimonious trees, yielding a total of 258 trees. The topology of each tree was then compared with that predicted from phylogenetic analysis of entire lux-rib1 and lux-rib2 operons. Six of the 172 partitions yielded an incongruent tree, and the alignments of these partitions were then visually inspected. Five of the incongruent topologies (four overlapping partitions within the luxA-luxB region and one in luxD) were ascribed to a lack of character change rather than recombination, whereas one showed a possible recombination event between the luxB1 and luxB2 genes of strain lnuch.21.1 (see Results).
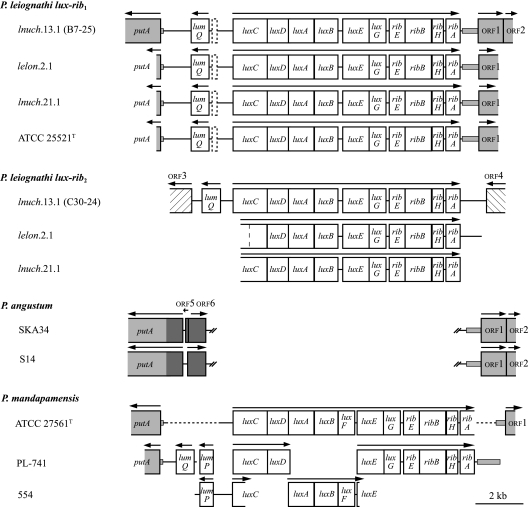
FIG. 1.
Gene organization of the lux-rib1 and lux-rib2 operons of P. leiognathi. Genes and spacer regions are drawn to scale. Arrows above genes indicate the direction of transcription. Dashed rectangles between lumQ and luxC in P. leiognathi lux-rib1 sequences indicate an approximately 200-bp region that is alignable to the lumP sequence of P. mandapamensis. Genes shaded gray have homologs in P. leiognathi, P. mandapamensis, and P. angustum. Small shaded rectangles outside of genes indicate noncoding intergenic sequences of P. leiognathi and/or P. mandapamensis that are alignable to sequences in P. angustum strains SKA34 and S14, including approximately 80 bp before the P. leiognathi putA start codon that aligns to a sequence within putA in P. angustum. Genes shaded dark gray in P. angustum indicate regions (orf5, orf6, and 759 bp from the start codon of putA) that are not alignable with a gene or intergenic sequence in P. leiognathi or P. mandapamensis. Double hash marks in the P. angustum sequences indicate contiguous sequences that have been separated to indicate the position of the lux-rib operon in P. leiognathi and P. mandapamensis. Blank regions in P. mandapamensis strains indicate that the sequence is not available; dashed lines in P. mandapamensis ATCC 27561T indicate regions where the DNA was amplified in this study but not sequenced. The hashed rectangles indicate ORFs (orf3 and orf4) flanking the lux-rib2 operon of fosmid C30-24 that are apparently homologous to bacterial transposases (see the text). In lux-rib2 of P. leiognathi lelon.2.1, a dashed vertical line in the region homologous to luxC indicates the site of a single nucleotide deletion that causes a frameshift of luxC in this strain, resulting in a stop codon 18 codons later. See the supplemental material for complete maps of the fosmids.
Screening for lux-rib merodiploidy.
In addition to lnuch.13.1, lelon.2.1, lnuch.21.1, and ATCC 25521T, 170 strains of P. leiognathi isolated from various geographic locations (see Fig. 5; also see the supplemental material) were assayed for the presence of single or multiple lux-rib operons using luxA PCR amplification and sequencing. The PCR primers used were those that detected multiple luxA sequencing chromatogram peaks in P. leiognathi lnuch.13.1, lelon.2.1, and lnuch.21.1. The resulting amplicons were then sequenced, and the resulting chromatograms were examined for single or multiple luxA sequence peaks.
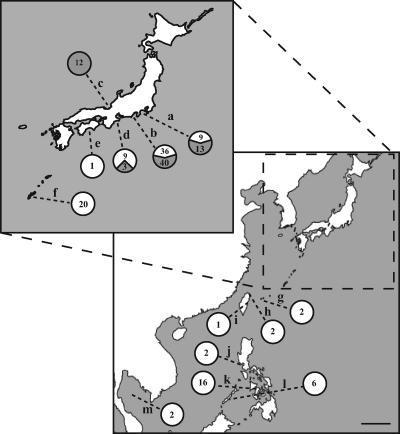
FIG. 5.
Map of Japan and Southeast Asia showing the geographic origins of P. leiognathi strains bearing single or multiple lux-rib operons. The scale bar is approximately 500 km. The inset shows an enlarged map of the main islands of Japan (some landmasses were omitted for clarity). Locations: a, Sagami Bay, Kanagawa Prefecture, Honshu, Japan; b, Suruga Bay, Shizuoka Prefecture, Honshu, Japan; c, Wakasa Bay, Fukui Prefecture, Honshu, Japan; d, Ago Bay, Mie Prefecture, Honshu, Japan; e, Tosa Bay, Kochi Prefecture, Shikoku, Japan; f, Nakagusuku Bay, Okinawa-honto, Okinawa Prefecture, Japan; g, Funauki Bay, Iriomote Island, Okinawa Prefecture, Japan; h, Taipei, Taiwan; i, Dahsi, Taiwan; j, Manila Bay, Luzon, Philippine Islands; k, Iloilo, Panay, Philippine Islands; l, Palawan, Philippine Islands; m, Gulf of Thailand. Numbers next to each location indicate the number of strains identified as bearing single (white area in circle) or multiple (gray area in circle) lux-rib operons.
GenBank accession numbers and sources of sequence data.
GenBank accession numbers for DNA sequence data obtained or used in this study are listed in Table 2. Other sources of DNA sequence data used in the analysis shown in Fig. 1 are luxCDABEG (28) and ribEBHA (27) of P. leiognathi ATCC 25521T; lumP, luxD (9), luxC (29), lumQ (30), putA (31), luxE (32), luxG (33), and ribEBHA (34) of P. mandapamensis PL-741; luxABF (22), lumP to luxC (unpublished GenBank submission) of P. mandapamensis 554; Photobacterium angustum SKA34 (http://www.moore.org/microgenome/detail.aspx?id=36); and P. angustum S14 (http://www.moore.org/microgenome/detail.aspx?id=8). Annotation of open reading frames (ORFs) for P. angustum SKA34 and S14 genome sequences in GenBank as of April 2007, including gene identifying number, cluster of orthologous groups (COG) number, and COG inferred protein group, are as follows: SKA34_07688, VAS14_5429, and COG1566 for orf1 (hypothetical multidrug resistance efflux pump); SKA34_07693, VAS14_15434, and COG3158 for orf2 (hypothetical potassium ion transporter) (COG identification applies to SKA34 only); SKA34_07678 for orf5 (found in SKA34 only) (hypothetical protein); and SKA34_07683, VAS14_15424, and COG2207 for orf6 (hypothetical transcriptional regulator, AraC/XylS family protein). orf3 and orf4 are hypothetical bacterial transposases and are not homologous to any sequences proximal to the lux-rib regions of any other strains examined here.
RESULTS
Multiple luxA genes in P. leiognathi.
The sequencing chromatograms of luxA PCR amplicons of some P. leiognathi strains contained multiple discrete peaks. Exhaustive attempts to eliminate these anomalies through attempts to identify possible sources of DNA contamination were unsuccessful, which suggested that some strains carry multiple luxA genes. To test this possibility, we cloned the luxA PCR amplicon from lnuch.13.1, a strain that showed multiple chromatogram peaks. Individual recovered clones were found to carry one or the other of two different luxA sequences, which demonstrated the presence of two luxA genes in lnuch.13.1.
Identification of two complete luxCDABEG-ribEBHA operons.
PCR and sequence analysis of lnuch.13.1 genomic DNA revealed that other lux genes were associated with each luxA gene, which suggested that some or all of the lux operon luxCDABEG might be present in two copies. Despite a concerted effort based on PCR and sequence analysis, however, we were unable to find a close physical linkage between the two putative lux operons, as might have resulted from a tandem duplication of all or part of the lux operon. This observation suggested that if two operons were present, they were not closely associated. To test this possibility and to define the gene content, organization, and sequence of each putative lux operon, we constructed a fosmid library of lnuch.13.1 genomic DNA. Sequence-based screening of the recovered fosmid clones identified two clones, B7-25 and C30-24, with different lux gene sequences. For both clones, the region containing and immediately flanking the lux operon was sequenced (see http://www-personal.umich.edu/∼pvdunlap/supplementalinfo.html for PCR primers and sequencing strategy).
Both clones contained a complete luxCDABEG operon (Fig. 1). Furthermore, luxC was preceded in both clones by the lumQ gene. A short region with homology to part of lumP also was identified in B7-25 but was absent from C30-24. The ribEBHA genes were also present downstream of luxG in both clones. The individual lux and rib genes were of the same lengths in both clones, but the lengths of the individual intergenic spacer regions varied, especially in the AT-rich region between lumQ and luxC (Fig. 1). These results establish the presence of two distinct luxCDABEG-ribEBHA operons in P. leiognathi lnuch.13.1, designated here lux-rib1 (from fosmid B7-25) and lux-rib2 (from fosmid C30-24). Fosmid maps with additional details are presented in the supplemental material. In addition to having the same gene content and organization, the two operons are of similar lengths, approximately 11 and 11.5 kb, respectively; their lux and rib gene coding regions are approximately 90% identical in nucleotide sequence overall, and the genes of both operons are complete and translatable. This is the first example of multiple lux-rib operons (or multiple lux genes or multiple rib genes) in a luminous bacterium.
Chromosomal locations of lux-rib1 and lux-rib2.
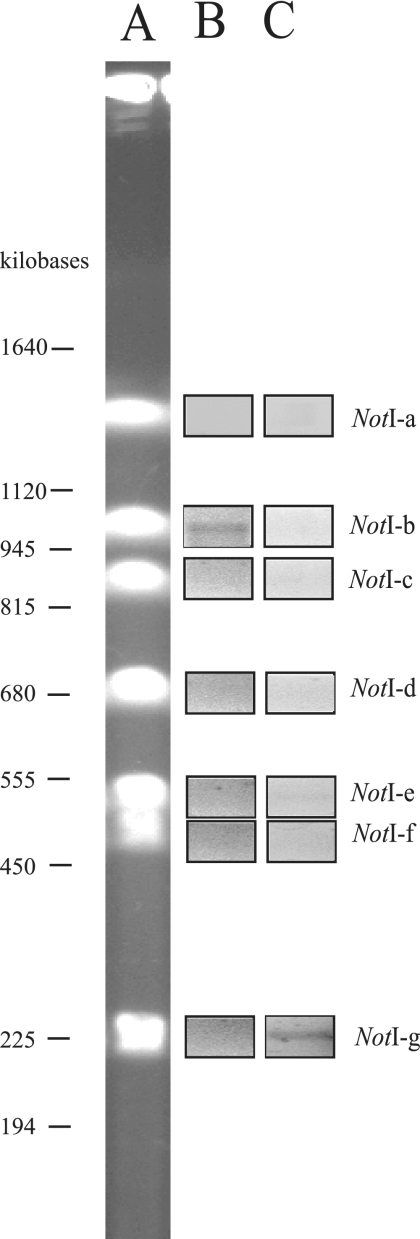
The absence of a plasmid large enough to account for either fosmid clone B7-25 or C30-24 (Materials and Methods) in lnuch.13.1 suggested that both lux-rib operons were likely to be chromosomal. To test this possibility, we examined genomic DNA of lnuch.13.1 using PFGE and PCR amplification. Analysis of undigested genomic DNA demonstrated the presence of two apparently circular chromosomes in lnuch.13.1, one larger than the other (data not shown), as reported previously for P. leiognathi and other examined members of the Vibrionaceae (40). Digestion of lnuch.13.1 genomic DNA with restriction endonuclease NotI yielded seven fragments, which ranged in size from approximately 1,500 kb to 220 kb (Fig. 2). The genome size of lnuch.13.1 was therefore estimated to be approximately 5.3 megabases. The seven NotI digestion fragments were purified and used as templates for PCR amplification of each of the two lux-rib operons, with primers for a luxD-luxA region that is specific in sequence to each operon. The lux-rib1-specific primers amplified a product only from fragment NotI-g, whereas the lux-rib2-specific primers amplified a product only from fragment NotI-b (Fig. 2). Sequence analysis confirmed that the two amplification products were luxD-luxA of lux-rib1 and of lux-rib2, respectively. These results demonstrate that the two lux-rib operons of lnuch.13.1 are located on different NotI chromosomal digestion fragments. Given the sizes of the fosmid clones B7-25 and C30-24 and the lack of DNA in common to them, the two lux-rib operons are separated at least by 9 kb of DNA.
FIG. 2.
PFGE analysis of the chromosomal locations of lux-rib1 and lux-rib2. (A) NotI digestion of lnuch.13.1 genomic DNA. (B) PCR amplification using lux-rib1-specific primers (luxDfor1 and luxArevsec#3) (see http://www-personal.umich.edu/∼pvdunlap/supplementalinfo.html) with genomic fragments NotI-a through NotI-g as templates. (C) PCR amplification using lux-rib2-specific primers (luxDfor1 and luxArevprim#3) (see http://www-personal.umich.edu/∼pvdunlap/supplementalinfo.html) and genomic fragments NotI-a through NotI-g as templates. Genomic DNA of lnuch.13.1 was used as a positive control (data not shown).
Consistent with these observations, DNA flanking lux-rib1 in lnuch.13.1 was distinct from that flanking lux-rib2 (Fig. 1). For lux-rib1, the putA gene was present upstream of lumQ, and a gene for a hypothetical multidrug resistance efflux pump (orf1) was present downstream of ribA. In P. angustum strains SKA34 and S14, which lack lux genes, putA and the gene for orf1 are present, and they are near each other, separated by less than 2 kb of DNA; this DNA codes for two unidentified ORFs that are unrelated to the lux or rib genes (Fig. 1). As in P. leiognathi, the lux-rib genes in P. mandapamensis are flanked by lumP, lumQ, and putA and by the gene for orf1 (Fig. 1).
In contrast, the DNA flanking lux-rib2 of lnuch.13.1 contained neither putA upstream of luxC nor the gene for orf1 downstream of ribA. Instead, homologs of putative bacterial transposase genes flanked lux-rib2. Specifically, the region upstream of luxC in lux-rib2 contained a sequence homologous to a putative transposase gene identified from the genome of Photobacterium profundum SS9, and the region downstream of ribA in lux-rib2 contained a homolog to bacterial transposase genes of the IS66 family (Fig. 1). These results establish that different, nonhomologous DNA flanks lux-rib1 and lux-rib2.
Presence and chromosomal locations of multiple lux-rib operons in other strains of P. leiognathi.
Using the information from lnuch.13.1, we next asked if other strains of P. leiognathi carry the two lux-rib operons and if the chromosomal locations of the operons were the same as those in lnuch.13.1. We examined in detail two strains, lelon.2.1 and lnuch.21.1, that, like lnuch.13.1, showed multiple luxA chromatogram peaks, and one strain, ATCC 25521T, the sequenced luxA amplicon of which consistently exhibited only single peaks. Primers based on lnuch.13.1 lux-rib1 and lux-rib2 were used for the amplification of lux-rib sequences from genomic DNA of these strains. Two different luxA sequences were identified in lelon.2.1 and lnuch.21.1, indicating the presence of two lux-rib operons, whereas only a single luxA sequence was identified in ATCC 25521T. Complete sequencing of the lux-rib operons from lelon.2.1 and lnuch.21.1 (see http://www-personal.umich.edu/∼pvdunlap/supplementalinfo.html for PCR primers and sequencing strategy) demonstrated the presence of two distinct luxCDABEG-ribEBHA operons in each of these strains (Fig. 1). As in lnuch.13.1, the genes of both operons in lelon.2.1 and lnuch.21.1 are, with one exception, intact and translatable. The exception is the luxC gene in lux-rib2 of lelon.2.1, which has a single nucleotide deletion; the deletion results in a translational stop codon approximately midway into the gene (Fig. 1). From DNA of ATCC 25521T, however, no amplicon was generated by any primers based on sequences of lux-rib2 of lnuch.13.1. These PCR and sequencing chromatogram results demonstrate that both lux-rib1 and lux-rib2 are present in some strains of P. leiognathi, e.g., lnuch.13.1, lelon.2.1, and lnuch.21.1, whereas other strains, e.g., ATCC 25521T, apparently have only lux-rib1. These results also establish that the presence of multiple luxA chromatogram peaks, versus single luxA chromatogram peaks, provides a reliable indication of the presence of multiple versus single lux-rib operons in P. leiognathi.
For lelon.2.1, lnuch.21.1, and ATCC 25521T, the order and orientation of genes flanking lux-rib1 were found to be the same as those for lnuch.13.1. Specifically, the DNA upstream of luxC contained a short region with some homology to lumP, followed by lumQ and putA, and the region downstream of ribA contained the gene for orf1 (Fig. 1). These results demonstrate positional homology of lux-rib1 in the four strains of P. leiognathi tested. In contrast, positional homology of the lux-rib2 operons in lnuch.13.1, lelon.2.1, and lnuch.21.1 could not be demonstrated. Attempts to amplify the regions upstream of luxC and downstream of ribA in lelon.2.1 and lnuch.21.1 using primers based on the sequences from fosmid clone C30-24 (lnuch.13.1; lux-rib2) failed to produce the predicted products. Therefore, either the chromosomal locations of lux-rib2 or the sequences flanking lux-rib2 differ in these three strains.
Evolutionary origin of lux-rib2.
With the complete sequences of lux-rib1 from lnuch.13.1, lelon.2.1, lnuch.21.1, and ATCC 25521T and of lux-rib2 from lnuch.13.1, lelon.2.1, and lnuch.21.1, we were in a position to define the extent of phylogenetic divergence between genes of the two operons. We hypothesized that lux-rib2, which is flanked by putative bacterial transposase genes in lnuch.13.1, was acquired by lateral gene transfer from another species of luminous bacteria.
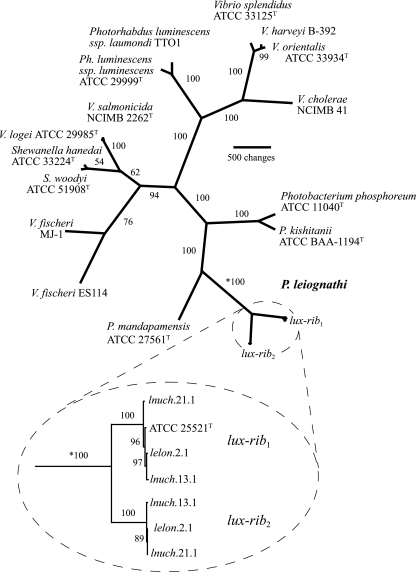
To test this hypothesis and gain insight into the evolutionary origin of lux-rib2, we carried out a parsimony analysis of lux-rib1, lux-rib2, and lux and rib genes of other species of luminous bacteria in Vibrionaceae, Shewanellaceae, and Enterobacteriaceae. Instead of a close relationship between lux-rib2 and the lux and rib genes of one of these other species of bacteria, we found that lux-rib2 was most closely related to lux-rib1 of P. leiognathi (Fig. 3). These results rule out lateral transfer from another bacterial species as the origin of lux-rib2 in P. leiognathi. Instead, the results indicate that lux-rib2 originated from within P. leiognathi. Separate phylogenetic analysis of each lux and rib gene resolved the same relationships as those shown in Fig. 1 (see the supplemental material).
FIG. 3.
Phylogenetic hypothesis of relationships among luminous bacteria based on lux and rib gene sequences. The tree is unrooted because bacterial lux genes are present only in members of the Vibrionaceae, Shewanellaceae, and Enterobacteriaceae; no outgroup bearing the lux genes is known. The total number of aligned nucleotide positions in the data set is 11,021; exclusion of noncoding spacer regions and parsimony-uninformative characters resulted in 4,745 nucleotides for analysis. The single most parsimonious hypothesis is shown (length, 13,739; consistency index, 0.615; retention index, 0.712). Numbers at nodes are jackknife resampling values. The two lux-rib operons from P. leiognathi strains lnuch.13.1, lelon.2.1, and lnuch.21.1 (circled with dashed line) have distinct sequences but are each other's closest relatives (circled inset below the main figure; the asterisk indicates that the jackknife value is for the same branch on each part of the figure). The primary lux-rib operon (lux-rib1) is proximal to putA in all strains, whereas the secondary lux-rib operon (lux-rib2) is flanked by putative bacterial transposases in strain lnuch.13.1. Phylogenetic analysis based on housekeeping genes (i.e., gapA, gyrB, recA, rpoA, and rpoD) of Photobacterium species yielded trees consistent with the lux-rib hypothesis shown here.
This analysis also revealed that the lux-rib2 operons of lnuch.13.1, lelon.2.1, and lnuch.21.1 are very closely related to each other, as are the lux-rib1 operons of these strains and of ATCC 25521T (Fig. 3). Therefore, although lux-rib1 and lux-rib2 are phylogenetically distinct from each other, each has diverged very little among the different strains carrying them. It should be noted in this regard that the merodiploid strains were collected from different geographic locations and as much as 20 years apart (Table 1).
Recombination between lux-rib1 and lux-rib2.
The presence of two complete lux-rib operons with a high level of sequence identity in strains of P. leiognathi would presumably provide opportunities for recombination. We therefore tested for recombination between lux-rib1 and lux-rib2 by carrying out a partition congruence analysis on the sequences of the aligned lux-rib1 and lux-rib2 coding regions of lnuch.13.1, lelon.2.1, and lnuch.21.1. Analysis of the partition trees revealed that nearly all of them, 166 of 172, were topologically congruent, indicating no detectable recombination between the two operons. Six partitions did exhibit incongruence, but for five of these partitions, the incongruence could be ascribed to a lack of character change rather than recombination. One partition, however, for the luxB1 and luxB2 genes in strain lnuch.21.1 showed evidence consistent with recombination, with a portion of luxB1 apparently exchanged for luxB2 sequence (Fig. 4). These results indicate that despite the extensive homology, recombination between genes of the lux-rib1 and lux-rib2 operons of merodiploid strains of P. leiognathi apparently occurs only rarely.
FIG. 4.
Alignment of a region of luxB from lux-rib1 and lux-rib2 of P. leiognathi lnuch.21.1. (A) Nucleotide alignment. (B) Amino acid alignment. Asterisks highlight differences between the sequences; variable nucleotides are shown in boldface type.
Nonrandom geographic distribution of merodiploid P. leiognathi strains.
We noted that the collection locations of lux-rib merodiploid strains, lnuch.13.1, lelon.2.1, and lnuch.21.1, all from Honshu, Japan, differed from the collection location, the Gulf of Thailand, of ATCC 25521T, which carries only lux-rib1. This difference suggested that strains bearing multiple lux-rib operons might have a nonrandom geographic distribution. To test this possibility, we examined 170 additional strains of P. leiognathi collected from various locations from Honshu, Shikoku, and Okinawa, Japan; Taiwan the Philippine Islands; and the Gulf of Thailand (Fig. 5; see also the supplemental material). These strains were tested for single or multiple lux-rib operons using the primers that revealed multiple luxA chromatogram peaks for lnuch.13.1, lelon.2.1, and lnuch.21.1. One hundred five of the additional strains were found to carry a single lux-rib operon, whereas 65 of the additional strains carried multiple lux-rib operons. Strains bearing a single lux-rib operon were obtained throughout the geographic sampling range, but strains bearing multiple lux-rib operons were found only in coastal waters of central Honshu (Fig. 5). Thus, despite extensive sampling, we were unable to find P. leiognathi strains bearing multiple lux-rib operons south of Honshu, Japan. Therefore, lux-rib merodiploid strains have a geographic distribution that apparently is limited to coastal waters of Honshu. This is the first indication that a natural merodiploid state in a bacterial species can correlate with geography.
DISCUSSION
Certain strains of P. leiognathi, a coastal marine luminous bacterium, carry two intact and apparently functional luxCDABEG-ribEBHA operons. Merodiploidy extends to an 11th gene, lumQ, which is closely associated with but transcribed divergently from the lux-rib operons. The two lux-rib operons are distinct in sequence and chromosomal location. One operon, lux-rib1, is flanked by putA and the gene for a hypothetical multidrug resistance efflux pump (orf1). Positional homologies of lux-rib1 in strains of P. leiognathi, of the lux-rib operon of P. mandapamensis, and of flanking DNA in P. angustum suggest that the site between putA and the orf1 gene is the ancestral chromosomal location of the lux-rib operon in P. leiognathi and P. mandapamensis. The second operon, lux-rib2, is present in many but not all strains of P. leiognathi, and it is flanked by genes specifying transposases but not by putA and the orf1 gene.
The lux-rib merodiploidy reported here is the first example of multiple copies of lux or rib genes in a luminous bacterium. Natural merodiploidy is not uncommon in bacteria, but it usually involves individual genes or small genetic regions and is thought to arise typically by tandem duplication (see, e.g., references 1, 19, 45, and 54). The actinomycete Actinomadura sp., for example, carries two rpoB genes that are 93% identical on the same 3-kb genomic DNA fragment; both copies are stably inherited without recombination (50). It is also known that many bacteria carry multiple copies of the rrn operon, with the number of operons apparently corresponding to the rate of response to resource availability (24). The situation in P. leiognathi, however, differs markedly and apparently is unique: the two lux-rib operons are well separated, each operon contains 11 genes, and merodiploidy apparently did not arise by a tandem duplication of one of the operons. Furthermore, lux-rib merodiploidy is common in P. leiognathi, having been detected in 68 of 174 tested strains, but the merodiploid state is not found in all strains of this species.
The presence of transposase genes flanking lux-rib2 indicates a potential for this operon to transfer among P. leiognathi strains and to other species. Despite the potential for mobility, lux-rib2 apparently was not acquired by P. leiognathi by lateral transfer from another species of luminous bacteria (Fig. 3). The two operons are phylogenetically distinct but are more closely related to each other than either one is to the lux or rib genes of any other known species of luminous bacteria. The evolutionary divergence between lux-rib1 and lux-rib2, which is evident both at the level of the whole operon and at the level of individual genes (Fig. 3; also see the supplemental material), also demonstrates that lux-rib2 is not likely to have arisen by a recent duplication of lux-rib1. Furthermore, the merodiploid state appears to be stable; more than 20 years separate the collection of merodiploid strains of P. leiognathi from the environment (Table 1; also see the supplemental material), with little sequence divergence for either operon over that time. We also found that an extended period of growth of lnuch.13.1 in continuous culture (approximately 400 generations) did not lead to a loss or altered chromosomal location of lux-rib2 (data not shown). It is possible, therefore, that lux-rib2 arose in the distant past within, and was acquired by transposon-mediated transfer from, a lineage of P. leiognathi that either has not yet been sampled or has gone extinct. If borne out by future studies, this scenario would represent the first documented instance of intraspecific transfer of the lux-rib genes in luminous bacteria. With respect to phylogenetic analysis based on lux and rib genes, the presence of two distinct lux-rib operons in certain strains of P. leiognathi adds complexity to analyses based on these genes, but it does not prevent the discrimination of this species from other luminous bacteria.
The putative transposases flanking lux-rib2 show similarity to insertion sequence elements characteristic of some plasmids (10) and pathogenic islands (20) in the Vibrionaceae. In this regard, Rajanna et al. (42) previously proposed that the Vibrio cholerae pathogenicity island is excised from the chromosome and transferred between cells as an extrachromosomal circular molecule, thereby spreading V. cholerae pathogenicity island-encoded genes among strains. One possibility, therefore, is that lux-rib2 was transferred among some strains of P. leiognathi, possibly during light organ symbiosis with fishes and squids, where bacterial population densities are high, by a mechanism similar to that involved in the transfer of pathogenic islands. However, determining the frequency, mechanism, and conditions under which lux-rib2 is transferred will require much additional work. Given the potential for mobility of lux-rib2, we also cannot exclude the possibility that some strains of P. leiognathi carry more than two lux-rib operons.
The presence of multiple copies of the lux-rib operon in a cell presumably provides opportunities for recombination and for the accumulation of nonsense mutations in the duplicate genes. These events could presumably lead to changes in the gene content and gene order of the lux operon of P. leiognathi and possibly lead to the evolution of novel functions in the duplicate genes. Recombination between the genes of lux-rib1 and lux-rib2, however, is apparently rare. Only one apparent instance of possible recombination, between the luxB1 and luxB2 genes in strain lnuch.21.1, was identified (Fig. 5). Similarly, mutations leading to a loss of function in the genes of either lux-rib operon are remarkably infrequent. With a single exception, all the genes of both operons in lnuch.13.1, lelon.2.1, and lnuch.21.1 are intact and translatable. This retention of functionality suggests the possibility of selection for the physiological function of the genes of both lux-rib operons. In this regard, it is known that selection can protect both copies of duplicated genes under conditions in which expression benefits the cell (25) and that selection for increased levels of gene products can lead to the retention of duplicated genes (see, e.g., reference 54). The number of genes involved and the substantial energetic costs associated with lux gene expression and the activity of Lux and Rib proteins (see, e.g., reference 13) argue for this selection hypothesis.
Increases in gene copy number would be expected to increase the cellular levels of the protein products of those genes (45). The presence of two functional lux-rib operons in merodiploid P. leiognathi would therefore presumably result in higher cellular levels of the Lux and Rib proteins than in strains carrying a single operon. Possibly reflecting lux-rib merodiploidy, two different luciferases, one soluble and the other apparently associated with the cytoplasmic membrane, were reported many years ago for P. leiognathi strain S-1 (5, 7). Whether retention of the lux-rib merodiploid state is subject to selection, however, and what the possible physiological benefits may be for cells producing higher levels of Lux and Rib proteins are not obvious at this time. The limited geographic distribution of lux-rib merodiploid strains (see below) is intriguing in this regard and suggests a possible connection with environmental conditions in coastal waters of Honshu, Japan, possibly the cold winter water temperatures there (49), compared to warmer coastal areas south of Honshu. Molecular genetic and biochemical analyses will be needed to determine whether both operons are actually expressed, whether their expression may be regulated differently, and what the effects on cellular levels of Lux and Rib proteins may be. At this time, essentially nothing is known about the regulation of luminescence in P. leiognathi except that the strains tested exhibit a population density-responsive induction of luminescence and luciferase synthesis that does not appear to be dependent on acyl-homoserine lactones (11). The demonstration here of multiple lux-rib operons in certain strains of P. leiognathi provides the genetic foundation for an analysis of these issues.
The nonrandom geographic distribution of merodiploid P. leiognathi strains (Fig. 5) is unexpected. Bacterial distributions in nature are widely considered to be cosmopolitan, although nonrandom geographic patterns, especially in the presence of physical barriers to dispersal, have been identified (see, e.g., references 41, 44, 46, 51, and 52). In the case of P. leiognathi, which exists in the fluid marine environment, no physical barrier to dispersal is evident, yet our extensive sampling has found merodiploid strains only in coastal waters of central Honshu. One explanation for the nonrandom distribution of merodiploid strains is simple historical contingency (36); the merodiploid state, assuming that it arose relatively recently in cells in coastal waters of central Honshu, may not have had sufficient time to disperse to the south. Northward flow of the Kuroshio Current (49) might delay or prevent the southerly dispersal of merodipliod strains. Alternatively, strains carrying multiple lux-rib operons might have a physiological advantage in coastal waters of Honshu, as mentioned above, thereby allowing them to persist despite the presence of strains that carry only lux-rib1. Much additional work will be needed to test these notions and determine the possible environmental conditions involved in the maintenance and limited geographic distribution of lux-rib merodiploid strains of P. leiognathi.
Supplementary Material
Acknowledgments
We thank the following individuals for assistance in field collection work: S. Kimura, Y. Tachibana, and the captain and crew of the T/S Seisui-maru, Mie University, Japan; T. Adachi, Fukui Prefectural Fisheries Experimental Station, Japan; A. Fukui, Tokai University, Japan; C. Lavilla-Pitogo, SEAFDEC, Philippine Islands; J. Ledesma, Tigbauan, Philippine Islands; T. Yoshino and H. Ishimori, University of the Ryukyus, Japan; Y. Haneda (deceased), Yokosuka City Museum, Japan; and Y. Machida and H. Endo, Kochi University, Japan. K. Davis, A. Kaeding, and A. Gorog, University of Michigan, provided technical assistance, and A. Kondrashov provided helpful advice. DNA sequencing was carried out by staff of the University of Michigan Sequencing Core.
This work was supported by grant DEB 0413441 from the National Science Foundation.
Footnotes
Published ahead of print on 22 June 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Anderson, R. P., and J. R. Roth. 1977. Tandem genetic duplications in phage and bacteria. Annu. Rev. Microbiol. 31:473-505. [DOI] [PubMed] [Google Scholar]
- 2.Ast, J. C., and P. V. Dunlap. 2004. Phylogenetic analysis of the lux operon distinguishes two evolutionarily distinct clades of Photobacterium leiognathi. Arch. Microbiol. 181:352-361. [DOI] [PubMed] [Google Scholar]
- 3.Ast, J. C., and P. V. Dunlap. 2005. Phylogenetic resolution and habitat specificity of members of the Photobacterium phosphoreum species group. Environ. Microbiol. 7:1641-1654. [DOI] [PubMed] [Google Scholar]
- 4.Ast, J. C., I. Cleenwerck, K. Engelbeen, H. Urbanczyk, F. L. Thompson, P. De Vos, and P. V. Dunlap. Photobacterium kishitanii sp. nov., a luminous marine bacterium symbiotic with deep-sea fishes. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 5.Balakrishnan, S. V., and N. Langerman. 1977. The isolation of a bacterial glycoprotein with luciferase activity. Arch. Biochem. Biophys. 181:680-682. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin, T. O., J. H. Devine, R. C. Heckel, J.-W. Lin, and G. S. Shadel. 1989. The complete nucleotide sequence of Vibrio fischeri and the luxABN region of Photobacterium leiognathi and the mechanism of control of bacterial bioluminescence. J. Biolum. Chemilum. 4:326-341. [DOI] [PubMed] [Google Scholar]
- 7.Baumann, P., and R. H. Schubert. 1984. Family II. Vibrionaceae Veron 1965, p. 516-548. In N. R. Kreig and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, 1st ed., vol. 1. Williams & Wilkins, Baltimore, MD. [Google Scholar]
- 8.Boisvert, H., R. Chatelain, and J.-M. Bassot. 1967. Étude d'un Photobacterium isolé de l'organe lumineux des poissons Leiognathidae. Ann. Inst. Pasteur (Paris) 112:520-524. [PubMed] [Google Scholar]
- 9.Chao, Y.-F., S.-F. Weng, and J.-W. Lin. 1993. Sequence of the luxD gene encoding acyltransferase of the lux operon from Photobacterium leiognathi. Gene 126:155-156. [DOI] [PubMed] [Google Scholar]
- 10.Di Lorenzo, M., M. Stork, M. E. Tolmasky, L. A. Actis, D. Farrell, T. J. Welch, L. M Crosa, A. M. Wertheimer, Q. Chen, P. Salinas, L. Waldbeser, and J. H. Crosa. 2003. Complete sequence of virulence plasmid pJM1 from the marine fish pathogen Vibrio anguillarum strain 775. J. Bacteriol. 185:5822-5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunlap, P. V. 1984. Ph.D. dissertation. University of California, Los Angeles.
- 12.Dunlap, P. V., and J. C. Ast. 2005. Genomic and phylogenetic characterization of luminous bacteria symbiotic with the deep-sea fish Chlorophthalmus albatrossis (Aulopiformes: Chlorophthalmidae). Appl. Environ. Microbiol. 71:930-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunlap, P. V., and E. P. Greenberg. 1991. Role of intercellular chemical communication in the Vibrio fischeri-monocentrid fish symbiosis, p. 219-253. In M. Dworkin (ed.), Microbial cell-cell interactions. American Society for Microbiology, Washington, DC.
- 14.Dunlap, P. V., and K. Kita-Tsukamoto. 2006. Luminous bacteria, p. 863-892. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes, a handbook on the biology of bacteria, 3rd ed., vol. 2. Ecophysiology and biochemistry. Springer, New York, NY. [Google Scholar]
- 15.Dunlap, P. V., A. Jiemjit, J. C. Ast, M. M. Pearce, R. R. Marques, and C. R. Lavilla-Pitogo. 2004. Genomic polymorphism in symbiotic populations of Photobacterium leiognathi. Environ. Microbiol. 6:145-158. [DOI] [PubMed] [Google Scholar]
- 16.Dunlap, P. V., J. C. Ast, S. Kimura, A. Fukui, T. Yoshino, and H. Endo. Phylogenetic analysis of host-symbiont specificity and codivergence in bioluminescent symbioses. Cladistics, in press.
- 17.Farris, J. S., V. A. Albert, M. Källersjö, D. Lipscomb, and A. G. Kluge. 1996. Parsimony jackknifing outperforms neighbor-joining. Cladistics 12:99-124. [DOI] [PubMed] [Google Scholar]
- 18.Fukasawa, S., and P. V. Dunlap. 1986. Identification of luminous bacteria isolated from the light organs of the squid, Doryteuthis kensaki. Agric. Biol. Chem. 50:1645-1646. [Google Scholar]
- 19.Gevers, D., K. Vandepoele, C. Simillion, and Y. Van de Peer. 2004. Gene duplication and biased functional retention of paralogs in bacterial genomes. Trends Microbiol. 12:148-154. [DOI] [PubMed] [Google Scholar]
- 20.Hacker, J., and J. B. Kaper. 2000. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54:641-679. [DOI] [PubMed] [Google Scholar]
- 21.Hendrie, M. S., W. Hodgkiss, and J. M. Shewan. 1970. The identification, taxonomy and classification of luminous bacteria. J. Gen. Microbiol. 64:151-169. [Google Scholar]
- 22.Illarionov, B. A., V. M. Blinov, A. P. Donchenko, M. V. Protopopova, V. A. Karginov, N. P. Mertvetsov, and J. I. Gitelson. 1990. Isolation of bioluminescent functions from Photobacterium leiognathi: analysis of luxA, luxB, luxG and neighboring genes. Gene 86:89-94. [DOI] [PubMed] [Google Scholar]
- 23.Kaeding, A. J., J. C. Ast, M. M. Pearce, H. Urbanczyk, S. Kimura, H. Endo, M. Nakamura, and P. V. Dunlap. 2007. Phylogenetic specificity and diversity in the bioluminescent symbioses of Photobacterium mandapamensis. Appl. Environ. Microbiol. 73:3173-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klappenbach, J. A., J. M. Dunbar, and T. M. Schmidt. 2000. rRNA operon copy number reflects ecological strategies of bacteria. Appl. Environ. Microbiol. 66:1328-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondrashov, F. A., and A. S. Kondrashov. 2006. Role of selection in fixation of gene duplications. J. Theor. Biol. 239:141-151. [DOI] [PubMed] [Google Scholar]
- 26.Lee, C. Y., and E. A. Meighen. 1992. The lux genes in Photobacterium leiognathi are closely linked with the genes corresponding in sequence to riboflavin synthesis genes. Biochem. Biophys. Res. Commun. 186:690-697. [DOI] [PubMed] [Google Scholar]
- 27.Lee, C. Y., D. J. O'Kane, and E. A. Meighen. 1994. Riboflavin synthesis genes are linked with the lux operon of Photobacterium phosphoreum. J. Bacteriol. 176:2100-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, C. Y., R. B. Szittner, and E. A. Meighen. 1991. The lux genes of the luminous bacterial symbiont, Photobacterium leiognathi, of the ponyfish. Eur. J. Biochem. 201:161-167. [DOI] [PubMed] [Google Scholar]
- 29.Lin, J.-W., Y.-F. Chao, and S.-F. Weng. 1993. The lumazine protein-encoding gene in Photobacterium leiognathi is linked to the lux operon. Gene 126:153-154. [DOI] [PubMed] [Google Scholar]
- 30.Lin, J.-W., K.-Y. Yu, Y.-F. Chao, and S.-F. Weng. 1995. The lumQ gene is linked to the lumP gene and the lux operon in Photobacterium leiognathi. Biochem. Biophys. Res. Commun. 217:684-695. [DOI] [PubMed] [Google Scholar]
- 31.Lin, J.-W., K.-Y. Yu, Y.-F. Chao, and S.-F. Weng. 1996. Regulatory region with putA gene of proline dehydrogenase that links to the lum and lux operons in Photobacterium leiognathi. Biochem. Biophys. Res. Commun. 219:868-875. [DOI] [PubMed] [Google Scholar]
- 32.Lin, J.-W., Y.-F. Chao, and S.-F. Weng. 1996. Nucleotide sequence and functional analysis of the luxE gene encoding acyl-protein synthetase of the lux operon from Photobacterium leiognathi. Biochem. Biophys. Res. Commun. 228:764-773. [DOI] [PubMed] [Google Scholar]
- 33.Lin, J.-W., Y.-F. Chao, and S.-F. Weng. 1998. Characteristic analysis of the luxG gene encoding the probable flavin reductase that resides in the lux operon of Photobacterium leiognathi. Biochem. Biophys. Res. Commun. 246:446-452. [DOI] [PubMed] [Google Scholar]
- 34.Lin, J.-W., Y.-F. Chao, and S.-F. Weng. 2001. Riboflavin synthesis genes ribE, ribB, ribH, ribA reside in the lux operon of Photobacterium leiognathi. Biochem. Biophys. Res. Commun. 284:587-595. [DOI] [PubMed] [Google Scholar]
- 35.Lucangeli, C., S. Morabito, A. Caprioli, L. Achene, L. Busani, E. Mazzolioni, A. Fabris, and A. Macri. 2000. Molecular fingerprinting of strains of Yersinia ruckeri serovar O1 and Photobacterium damsela subsp. piscicida isolated in Italy. Vet. Microbiol. 76:272-281. [DOI] [PubMed] [Google Scholar]
- 36.Martiny, J. B. H., B. J. Bohannan, J. H. Brown, R. K. Colwell, J. A. Fuhrman, J. L. Green, M. C. Horner-Devine, M. Kane, J. A. Krumins, C. R. Kuske, P. J. Morin, S. Naeem, L. Øvreås, A. L. Reysenbach, V. H. Smith, and J. T. Staley. 2006. Microbial biogeography: putting microorganisms on the map. Nat. Rev. Microbiol. 4:102-112. [DOI] [PubMed] [Google Scholar]
- 37.Meighen, E. A., and P. V. Dunlap. 1993. Physiological, biochemical and genetic control of bacterial bioluminescence. Adv. Microb. Physiol. 34:1-67. [DOI] [PubMed] [Google Scholar]
- 38.Nealson, K. H. 1978. Isolation, identification and manipulation of luminous bacteria. Methods Enzymol. 57:153-166. [Google Scholar]
- 39.Nealson, K. H., and J. W. Hastings. 1977. Low oxygen is optimal for luciferase synthesis in some bacteria: ecological implications. Arch. Microbiol. 112:9-16. [DOI] [PubMed] [Google Scholar]
- 40.Okada, K., T. Iida, K. Kita-Tsukamoto, and T. Honda. 2005. Vibrios commonly possess two chromosomes. J. Bacteriol. 187:752-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papke, R. T., N. B. Ramsing, M. M. Bateson, and D. M. Ward. 2003. Geographical isolation in hot spring cyanobacteria. Environ. Microbiol. 5:650-659. [DOI] [PubMed] [Google Scholar]
- 42.Rajanna, C., J. Wang, D. Zhang, Z. Xu, A. Ali, Y.-M. Hou, and D. K. R. Karaolis. 2003. The vibrio pathogenicity island of epidemic Vibrio cholerae forms precise extrachromosomal circular excision product. J. Bacteriol. 185:6893-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reichelt, J. L., and P. Baumann. 1973. Taxonomy of the marine, luminous bacteria. Arch. Mikrobiol. 94:283-330. [DOI] [PubMed] [Google Scholar]
- 44.Roberts, M. S., and F. C. Cohan. 1995. Recombination and migrations rates in natural populations of Bacillus subtilis and Bacillus mojavensis. Evolution 49:1081-1094. [DOI] [PubMed] [Google Scholar]
- 45.Romero, D., and R. Palacios. 1997. Gene amplification and genomic plasticity in prokaryotes. Annu. Rev. Genet. 31:91-111. [DOI] [PubMed] [Google Scholar]
- 46.Silva, C., P. Vinuesa, L. E. Eguiarte, V. Souza, and E. Martinez-Romero. 2005. Evolutionary genetics and biogeographic structure of Rhizobium gallicum sensu lato, a widely distributed bacterial symbiont of diverse legumes. Mol. Evol. 14:4033-4050. [DOI] [PubMed] [Google Scholar]
- 47.Spencer, R. 1955. The taxonomy of certain luminous bacteria. J. Gen. Microbiol. 13:111-118. [DOI] [PubMed] [Google Scholar]
- 48.Swofford, D. L. 2003. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland, MA.
- 49.Tomczak, M., and J. S. Godfrey. 2003. Regional oceanography: an introduction, 2nd ed. Daya Publishing House, Delhi, India.
- 50.Vigliotta, G., S. M. Tredici, F. Damiano, M. R. Montinaro, R. Pulimeno, R. di Summa, D. R. Massardo, G. V. Gnoni, and P. Alifano. 2005. Natural merodiploidy involving duplicated rpoB alleles affects secondary metabolism in producer actinomycete. Mol. Microbiol. 55:396-412. [DOI] [PubMed] [Google Scholar]
- 51.Vinuesa, P., C. Silva, D. Werner, and E. Martinez-Romero. 2005. Population genetics and phylogenetic inference in bacterial molecular systematics: the roles of migration and recombination in Bradyrhizobium species cohesion and delineation. Mol. Phylogenet. Evol. 34:29-54. [DOI] [PubMed] [Google Scholar]
- 52.Whitaker, R. J., D. W. Grogan, and J. W. Taylor. 2003. Geographic barriers isolate endemic populations of hyperthermophilic archaea. Science 301:976-978. [DOI] [PubMed] [Google Scholar]
- 53.Yang, Y., L. P. Yeh, Y. Cao, L. Baumann, P. Bauman, J. S. Tang, and B. Beaman. 1983. Characterization of marine luminous bacteria isolated off the coast of China and description of Vibrio orientalis sp. nov. Curr. Microbiol. 8:95-100. [Google Scholar]
- 54.Zhong, S., A. Khodursky, D. E. Dykhuizen, and A. M. Dean. 2004. Evolutionary genomics of ecological specialization. Proc. Natl. Acad. Sci. USA 101:11719-11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.