Abstract
The bacterial response to stress is controlled by two proteins, RelA and SpoT. RelA generates the alarmone (p)ppGpp under amino acid starvation, whereas SpoT is responsible for (p)ppGpp hydrolysis and for synthesis of (p)ppGpp under a variety of cellular stress conditions. It is widely accepted that RelA is associated with translating ribosomes. The cellular location of SpoT, however, has been controversial. SpoT physically interacts with the ribosome-associated GTPase CgtA, and we show here that, under an optimized salt condition, SpoT is also associated with a pre-50S particle. Analysis of spoT and cgtA mutants and strains overexpressing CgtA suggests that the ribosome associations of SpoT and CgtA are mutually independent. The steady-state level of (p)ppGpp is increased in a cgtA mutant, but the accumulation of (p)ppGpp during amino acid starvation is not affected, providing strong evidence that CgtA regulates the (p)ppGpp level during exponential growth but not during the stringent response. We show that CgtA is not associated with pre-50S particles during amino acid starvation, indicating that under these conditions in which (p)ppGpp accumulates, CgtA is not bound either to the pre-50S particle or to SpoT. We propose that, in addition to its role as a 50S assembly factor, CgtA promotes SpoT (p)ppGpp degradation activity on the ribosome and that the loss of CgtA from the ribosome is necessary for maximal (p)ppGpp accumulation under stress conditions. Intriguingly, we found that in the absence of spoT and relA, cgtA is still an essential gene in Escherichia coli.
A pleiotropic physiological response termed the “stringent response,” which involves a rapid down regulation of rRNA biosynthesis and ribosome production, occurs when bacterial cells encounter nutritional stresses such as amino acid starvation (5). Accompanying this response is the accumulation of ppGpp (guanosine 3′,5′-bispyrophosphate) and pppGpp (guanosine 3′-diphosphate,5′-triphosphate), collectively called (p)ppGpp (5). (p)ppGpp is a global regulator that accumulates in response to a variety of nutritional and growth arrest stress conditions. The regulatory function is exerted mainly at the transcriptional level through binding to the RNA polymerase core enzyme (33). (p)ppGpp nucleotides are synthesized by transfer of the pyrophosphate group from ATP to either GDP or GTP (5, 8).
In Escherichia coli, the (p)ppGpp level is maintained by two proteins, RelA and SpoT (5, 8), although most bacteria have only one RelA/SpoT protein. RelA is a (p)ppGpp synthetase and a well-established ribosome-associated protein (15, 40, 55). The (p)ppGpp synthetase activity of RelA is activated by uncharged tRNA at the ribosome A site (19) and is partially dependent on the large ribosomal protein L11 (55, 58). SpoT is a bifunctional enzyme that possesses both (p)ppGpp synthetase and hydrolase activity (ppGpp is hydrolyzed to 5′-GDP and PPi and pppGpp is hydrolyzed to 5′-GTP and PPi) (21, 22, 27, 57). The cellular localization of SpoT, however, is unclear. SpoT enzymatic activity was reported in ribosome-containing fractions but not with ribosomes washed with 0.5 M NH4Cl, suggesting that it associates weakly with ribosomes (20, 48). Moreover, the hydrolase activity of SpoT is inhibited in the presence of uncharged tRNA and more severely inhibited in the presence of ribosomes (42), suggesting that the activity of SpoT may be controlled by tRNA on the ribosome, as seen with RelA. SpoT was not, however, found associated with the ribosomal particles by sucrose density centrifugation (15).
The E. coli GTPase CgtA (also called ObgE, YhbZ, or CgtAE) is important for late 50S ribosome assembly (25, 43). The Obg/CgtA proteins have also been implicated in a variety of other cellular processes including DNA replication, sporulation, morphological differentiation, and stress responses (3, 11, 13, 29, 39, 46, 53, 56). The relationship between the role of Obg/CgtA proteins in ribosome assembly and these other functions is not well characterized.
Several lines of evidence suggest that Obg/CgtA proteins are related to cellular stress responses. First, in Bacillus subtilis, Obg cocrystallized with ppGpp (3). In vitro, higher concentrations of ppGpp inhibit the GTP hydrolysis activity of Obg, whereas lower concentrations of ppGpp stimulate hydrolysis (3). Second, B. subtilis Obg interacts with several regulators (RsbT, RsbW, and RsbX) necessary for the stress activation of σB, the global controller of the stress regulon in B. subtilis (46). It has been shown that RelA is also important for σB activation independent of (p)ppGpp synthesis, providing a functional relationship between Obg and RelA in B. subtilis. Third, the Saccharomyces cerevisiae Obg/CgtA protein Rbg1p interacts with Gir2p, which, in turn, interacts with Gcn1p, a protein participating in the stress response pathway in yeast (P. K. Wout and J. R. Maddock, unpublished data). Rbg1p, Gir2p, and Gcn1p all associate with translating polyribosomes (44; Wout and Maddock, unpublished), indicating that they may belong to the same complex on the polysomes. Fourth, the E. coli CgtA specifically interacts with SpoT (56). Yeast two-hybrid studies demonstrated that CgtA interacts with both the N-terminal catalytic and the C-terminal regulatory (ACT) domain of SpoT (56).
In this report we provide evidence that CgtA regulates the activity of SpoT on pre-50S particles. We describe the ribosome association of SpoT and a further examination of the physical and functional relationships among CgtA, SpoT, and the ribosome. We show that SpoT is also ribosome associated and that the positions of SpoT and CgtA in sucrose gradients overlap. Overexpression and loss-of-function studies show that the ribosome associations of SpoT and CgtA are mutually independent. Interestingly, CgtA is not associated with the ribosomes under conditions in which (p)ppGpp is vastly accumulated in the cell. In the steady state, the level of (p)ppGpp is increased in a cgtA mutant. In E. coli, the essential nature of CgtA is not entirely due to its control of SpoT. We propose that, on the pre-50S particle, CgtA regulates the hydrolysis activity of SpoT during steady-state growth. Moreover, we propose that the mechanism to prevent the regulation of SpoT by CgtA during stringent response involves the relocalization of CgtA.
MATERIALS AND METHODS
E. coli strains, culture conditions, and growth measurements.
E. coli strains used in this study are described in Table 1. The cgtAG80ED85N mutant (hereafter called the cgtA mutant) is lethal at 42°C, grows slowly at 30°C, and has been described previously (25, 28). To create a markerless ΔrelA strain, JM4873 (BW25113 ΔrelA::kan) (1) was transformed with pCP20, (9), screened for Kans, and subsequently screened for the loss of pCP20, thus generating JM4962 (BW25113 ΔrelA). The ΔspoT::cat deletion from strain CF1693 (22) was introduced into JM4962 by P1 transduction, resulting in JM4977. The deletion of spoT and relA in JM4977 was confirmed by PCR and by immunoblotting using anti-SpoT and anti-RelA antibodies (generous gifts from James Hernandez). To create a repressible cgtA helper plasmid, full-length cgtA was PCR amplified and cloned into PstI/HindIII sites of pJM4867 (a lab derivative of pJN105 [38] with an expanded multiple cloning site [S. L. Bardy and J. R. Maddock, unpublished data]). pJM4867 was transformed into JM4977 to create JM4980. The ΔcgtA::kan deletion from GN5002 (28) was introduced into JM4980 by P1 transduction, generating JM4981. The chromosomal deletion of cgtA in JM4981 was confirmed by PCR. JM3867 was created by transforming MG1655 with a plasmid containing cgtA under an arabinose-inducible promoter (28).
TABLE 1.
Escherichia coli strains used in this study
| Strain | Relevant genotype | Reference |
|---|---|---|
| MG1655 | rph-1 | 2 |
| GN5002 | ΔcgtA::kan plus PBAD-cgtA | 28 |
| DH5α | Δ(lab)U169 φ80 Δ(lacZ)M15 hsdR17 endA1 gyrA96 recA1 supE44 thi-1 | 17 |
| CF1693 | MG1655 ΔrelA251::kan ΔspoT207::cat | 57 |
| JM3867 | MG1655 plus PBAD-cgtA | This work |
| JM3903 | MG1655 ΔcgtA::kan plus PcgtA-cgtA | 25 |
| JM3907 | MG1655 ΔcgtA::kan plus PcgtA-cgtAG80ED85N | 25 |
| JM4867 | DH5α plus PBAD-cgtA | This work |
| JM4873 | BW25113 ΔrelA::kan | 1 |
| JM4962 | BW25113 ΔrelA | This work |
| JM4977 | BW25113 ΔrelA ΔspoT::cat | This work |
| JM4980 | BW25113 ΔrelA ΔspoT::cat plus PBAD-cgtA | This work |
| JM4981 | BW25113 ΔrelA ΔspoT::cat cgtA::kan plus PBAD-cgtA | This work |
For amino acid starvation and carbon starvation, polysome analysis, and (p)ppGpp analysis, cells were grown in MOPS (potassium morpholinopropanesulfonate) medium (37) supplemented with 0.1% glucose and 20 μg/ml of all 20 amino acids, except in amino acid starvation conditions, when serine was omitted. For the amino acid starvation experiment, serine hydroxamate (SH) was added to the culture at a final concentration of 1 mg/ml at an optical density at 600 nm (OD600) of 0.4 to 0.5 and cells were harvested 20 min after the addition. For carbon starvation, α-methylglucoside was added to a final concentration of 2.6% at an OD600 of 0.4 to 0.5 and cells were incubated for a further 2 min before harvest. For the stationary-phase experiment, a saturated overnight culture was diluted 1:100 and cells were grown in EP medium (medium E with 0.5% glucose and 2% peptone) (24) for 24 h before harvest. For all other purposes, cells were grown in Luria-Bertani (LB) broth (10 g tryptone, 5 g yeast extract, 10 g NaCl per liter) or on LB agar plates (LB broth plus 1.5% agar). MG1655 and JM3867 were grown at 37°C whereas CF1693, JM3903, JM 3907, JM4977, and JM4981 were grown at 30°C. Culture growth was monitored by measuring the absorption at 600 nm. Antibiotics were used at the following concentrations: 100 μg/ml ampicillin, 30 μg/ml kanamycin, and 20 μg/ml chloramphenicol.
Preparation of cell lysates for ribosome profiles, polysome fractionation, and immunoblotting.
Cell lysate preparations for polysome analysis, fractionation of the lysates, and the subsequent immunoblotting were performed as previously described (25), except for the stationary-phase experiment in which 5 to 20% sucrose gradients [in 100 mM CH3COONH4, 15 mM (CH3COO)2Mg, 20 mM Tris-HCl, pH 7.6] were used to separate the 100S from the remainder of the ribosomal particles. For immunoblotting, the following antibody concentrations were used: anti-CgtA, 1:2,000; anti-SpoT (preabsorbed with acetone powder generated from CF1693, as described previously [10]), 1:2,000; anti-S4, 1:4,000; anti-L3, 1:4,000; goat anti-rabbit immunoglobulin G-horseradish peroxidase (Pierce), 1:20,000.
(p)ppGpp measurements.
Saturated cultures grown in the presence of 2 mM phosphate (K2HPO4) were diluted at least 100-fold in MOPS medium containing 0.4 mM phosphate. [32P]H3PO4 was added to a final concentration of 100 μCi/ml when the OD600 reached 0.05, and incubation was continued for a minimum of two generations before the first sample was taken (OD600 of 0.2 to 0.25). At time zero, SH was added to a final concentration of 1 mg/ml to induce amino acid starvation. Samples were collected at indicated time points, and 100-μl samples were mixed with an equal volume of 13 M formic acid and chilled on dry ice. The mixtures were then subjected to two rounds of freezing and thawing and centrifuged at 14,000 rpm for 2 min to remove debris. Supernatants were spotted onto 20- × 20-cm polyethylenemine-cellulose plates (EMD Chemicals) and separated by thin-layer chromatography in 1.5 M KH2PO4 (pH 3.4) for ∼1 h. Following chromatography, labeled nucleotides were visualized by autoradiography and quantified with a Molecular Imager FX PhosphorImager and Quantity One software (Bio-Rad). Unlabeled ATP and GTP were spotted on the plates as markers and visualized after chromatography by UV light-induced fluorescence. The identities of the labeled (p)ppGpp were inferred from their positions in the chromatograph relative to the origin and GTP. (p)ppGpp levels are normalized to levels of GTP observed in the same sample.
RESULTS
SpoT is ribosome associated.
Intrigued by the controversial results regarding the ribosome association of SpoT (15, 20, 48), we decided to reinvestigate the localization of SpoT by using sucrose density centrifugation of ribosomal particles. Several experimental details were taken into consideration. First, it has been demonstrated previously that buffer conditions, especially salt conditions, are critical for the detection of weakly associated proteins, such as the GTPase CgtA, on ribosomal particles separated by sucrose gradients (28, 32, 56). Different salt concentrations may influence protein-nucleic acid (e.g., rRNA) interactions and change the association pattern of certain proteins. Second, the nature of the lysis procedure is critical to obtaining clear immunoblot results. Using the common freeze-thaw method (28, 56), significant smearing rather than sharp SpoT bands was often observed by immunoblotting (data not shown). By changing the lysis method to a glass bead-based method (25), we obtained much cleaner immunoblots (Fig. 1). Finally, we optimized the conditions for separating RelA and SpoT by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and included control lysates (ΔrelA ΔspoT and ΔrelA) to verify the specific detection of SpoT and not RelA (SpoT and RelA share 31% sequence identity [35]).
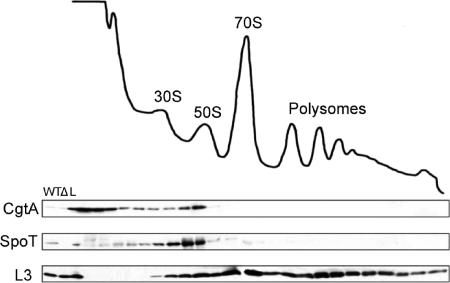
FIG. 1.
SpoT associates with ribosomes in E. coli. Cell lysates from MG1655 were sedimented through 7 to 47% sucrose gradients, and the samples were monitored by UV at 254 nm. The subsequent fractions were analyzed by immunoblotting using anti-SpoT, anti-CgtA, or anti-L3, as indicated. The positions of the 30S and 50S subunits, the 70S monosome, and the polysomes are labeled. WT, MG1655 cell extract; Δ, ΔspoT ΔrelA cell extract; L, 1/100 of the total sample loaded onto the gradient.
Here we show that SpoT is a bona fide ribosome-associated protein (Fig. 1). SpoT was seen predominantly in the fractions migrating slightly slower than the 50S fractions. This migration pattern of SpoT is similar to that of the helicases SrmB and CsdA or the pseudouridylases RluB and RluC, which predominantly bind to a ∼40S particle (6, 7, 26). The trailing of SpoT into earlier fractions (Fig. 1) may result from dissociation of SpoT from ribosomes during centrifugation and suggests a somewhat weak ribosome association. Very little free SpoT is found at the top of the gradient (Fig. 1). Thus, the majority of SpoT, like RelA, is associated with ribosomes.
We have previously shown that SpoT and the GTPase CgtA physically interact and that CgtA associates with late 50S particles (56) and is required for 50S assembly (25). We show here that the ribosome association of CgtA and that of SpoT overlap to a great extent, indicating that, in vivo, significant amounts of these two proteins associate with the same particles.
Ribosome association of CgtA and SpoT is mutually independent.
Since CgtA and SpoT associate with pre-50S particles (Fig. 1) and these two proteins physically interact (56), one possibility is that SpoT may be important for the association of CgtA with the ribosome, or vice versa. To examine whether SpoT affects the ribosome association of CgtA, the location of CgtA was determined in a ΔrelA ΔspoT double mutant (Fig. 2A). The spoT gene is essential in the relA+ background due to the necessity to degrade (p)ppGpp that would otherwise accumulate in the absence of SpoT (p)ppGpp hydrolase activity. The spoT gene, however, is not essential in a ΔrelA background (57). The ΔrelA ΔspoT mutant displays a normal polysome profile (Fig. 2A), indicating that, although SpoT appears to bind to a pre-50S particle (Fig. 1), SpoT is not required for ribosome assembly. In the ΔrelA ΔspoT mutant, a significant amount of CgtA binds to the 50S particle, indicating that neither SpoT nor RelA is critical for the ribosome association of CgtA (Fig. 2A). Moreover, the ΔrelA ΔspoT mutant is a (p)ppGpp0 strain, lacking all detectable (p)ppGpp under any conditions (22, 57), and therefore, (p)ppGpp is also not required for CgtA to associate with the ribosomes.
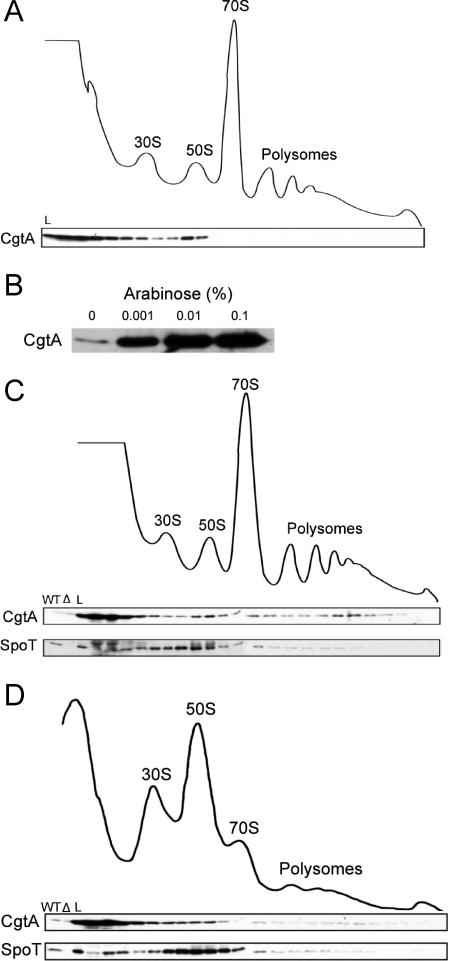
FIG. 2.
Ribosome association of SpoT and CgtA is mutually independent. (A, C, and D) Cell lysates from CF1693 (ΔspoT::cat ΔrelA::kan) (A), JM3867 (MG1655 plus PBAD-cgtA) with CgtA expression induced with 0.001% arabinose (C), and JM3907 (ΔcgtA::kan plus PcgtA-cgtAG80ED85N) (D) were sedimented through 7 to 47% sucrose gradients, and the subsequent fractions were subjected to immunoblotting using anti-CgtA and anti-SpoT antibodies, as indicated. The positions of the 30S and 50S subunits, the 70S monosome, and the polysomes are labeled. WT, MG1655 cell extract; Δ, ΔspoT ΔrelA cell extract; L, 1/100 of the total sample loaded onto the gradient. (B) Immunoblot showing the level of CgtA expression from JM3867 (MG1655 plus PBAD-cgtA) with various levels of arabinose, as indicated.
We next addressed the question of whether CgtA is a docking factor for SpoT. As cgtA is an essential gene, the association of SpoT with the ribosome cannot be directly assayed in a ΔcgtA strain. Two other approaches were employed to answer this question. First, cgtA was expressed, from a PBAD promoter, to high levels by varying the concentrations of arabinose in the medium (Fig. 2B). CgtA was vastly overexpressed, even in the presence of only 0.001% arabinose (Fig. 2B), and overexpression of CgtA led to a considerable accumulation of free CgtA at the top of the gradient, as well as some CgtA migrating with translating 70S and polysomes (Fig. 2C). Since CgtA and SpoT can interact directly, we asked whether SpoT would migrate with the free CgtA under these conditions. We found that the ribosome association of SpoT was similar to that seen in cells expressing native levels of CgtA (compare Fig. 1 and 2C) and conclude that excess free CgtA does not sequester SpoT away from the pre-50S particle. One caveat to this conclusion is that it is possible that only ribosome-bound CgtA is capable of interacting with SpoT.
To further examine whether SpoT requires CgtA for its ribosome association, we examined the ribosome association of SpoT in a cgtA mutant strain (Fig. 2D). We previously reported that this cgtA mutant accumulates a pre-50S particle and concluded that CgtA is required for late ribosome assembly (25). In this mutant, the majority of the CgtA protein is found at the top of the gradient, indicating a defect in ribosome association of the mutant CgtA protein (Fig. 2D). SpoT, however, remained associated with ribosomes (Fig. 2D), although the distribution of SpoT was somewhat broader than that seen in gradients from wild-type extracts (Fig. 1), perhaps due to the accumulation of pre-50S particles that accumulate in this mutant (25). Taken together, we conclude that the ribosome associations of SpoT and CgtA are mutually independent.
Neither CgtA nor SpoT associates with 100S during stationary phase.
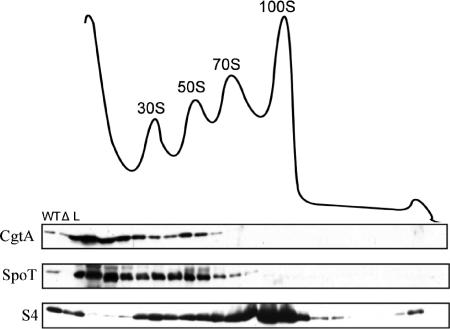
We next examined the ribosome association of SpoT and CgtA in stationary-phase cells, a condition known to accumulate (p)ppGpp, which might be from altered SpoT activity (30). During the transition from exponential growth to the stationary phase, the 70S ribosome is dimerized into 100S, which has no translational activity (54). This conversion is directed by a protein called ribosome modulation factor, whose transcription is dependent on (p)ppGpp (24), the level of which increases as cells enter stationary phase (30). To assay CgtA and SpoT ribosome association in the presence of increased (p)ppGpp levels, stationary-phase wild-type cell lysates were fractionated by sucrose gradients and the ribosome associations of SpoT and CgtA were examined by immunoblotting. Neither SpoT nor CgtA displayed an association with the 100S ribosomal particles (Fig. 3), consistent with the near absence of these two proteins on the 70S (Fig. 3) from which the 100S is derived. Interestingly, however, a partial loss of both proteins from the ribosome is seen and a significant amount of both proteins is found in the lightest fractions (Fig. 3). The physiological basis for this change in location is unknown.
FIG. 3.
Neither CgtA nor SpoT associates with the 100S ribosomal particle that accumulates in stationary phase. Overnight-grown E. coli MG1655 cells were diluted 1:100, grown in EP medium for 24 h, lysed, and fractionated on sucrose gradients. The ribosome profile is shown with the positions of the 30S, 50S, 70S, and 100S particles indicated. Fractions were analyzed by immunoblotting with anti-SpoT, anti-CgtA, or anti-S4, as indicated. WT, MG1655 cell extract; Δ, ΔspoT ΔrelA cell extract; L, 1/100 of the total sample loaded onto the gradients.
CgtA is not bound to the pre-50S particle during amino acid starvation.
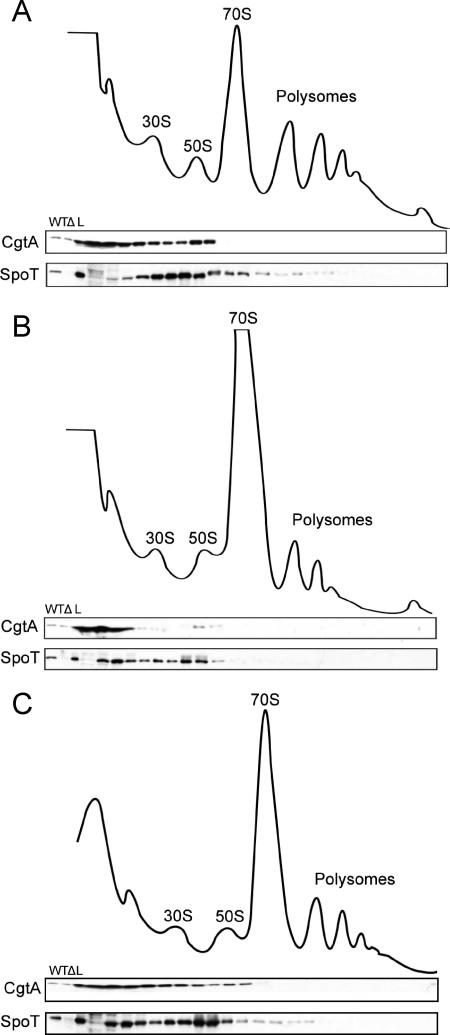
To further examine whether ribosome association influences the functions of CgtA and SpoT, their ribosome associations were examined under two conditions in which SpoT function is altered and (p)ppGpp rapidly accumulates. When cells are treated with SH, an inhibitor of seryl-tRNA synthetase, amino acid starvation is induced (49). RelA is quickly activated by uncharged tRNA at the ribosome A site (19), and at the same time, SpoT (p)ppGpp hydrolase activity is inhibited (36), leading to the swift accumulation of high levels of (p)ppGpp. When the cells are grown in MOPS minimal medium, the ribosome association of the two proteins (Fig. 4A) is similar to that seen in LB medium (Fig. 1), indicating that the richness of the growth medium does not significantly affect the ribosome localization of either CgtA or SpoT. When cells are treated with SH, however, there is a dramatic alteration of the association pattern of CgtA and, to a lesser degree, SpoT (Fig. 4B). The majority of the CgtA is found at the top of the gradient, and very little CgtA is detected associated with the 50S particle. The ribosome association of SpoT also changed, albeit not as dramatically as that of CgtA. Under these conditions, approximately half of the SpoT is associated with the pre-50S particles. In addition, a significant amount of SpoT migrates in fractions below the 30S subunit but not at the very top of the gradient, indicating that this SpoT is in a smaller complex. Although we do not know the nature of the complex, it does not appear to contain CgtA, as CgtA is predominantly found in the very early fractions.
FIG. 4.
Ribosome association of CgtA and SpoT under amino acid and carbon starvation conditions. Cell lysates from E. coli MG1655 cells grown in MOPS minimal medium (A), MOPS medium treated with SH (1 mg/ml) for 20 min before harvest (B), and MOPS medium treated with α-methylglucoside (2.6%) for 2 min before harvest (C) were fractionated over 7 to 47% sucrose gradients. The subsequent fractions were analyzed by immunoblotting with anti-SpoT or anti-CgtA (1/2,000), as indicated. The positions of the 30S, 50S, and 70S particles and the polysomes are labeled. WT, MG1655 cell extract; Δ, ΔspoT ΔrelA cell extract; L, 1/100 of the total sample loaded onto the gradients.
(p)ppGpp levels also increase when cells are starved for carbon. In this case, the accumulation of (p)ppGpp is not as dramatic as that during amino acid starvation (36) and is believed to mainly result from the inhibition of the (p)ppGpp hydrolysis function of SpoT but not from the activation of its synthesis activity (16, 36). We induced carbon starvation by the addition of α-methylglucoside, an inhibitor of glucose uptake, and harvested the cells at the time point when ppGpp is known to accumulate the most (18). Under these conditions, we observed a modest alteration in the ribosome association of both CgtA and SpoT, consistent with a less dramatic response (Fig. 4C).
The steady-state level of (p)ppGpp is increased in a cgtA mutant.
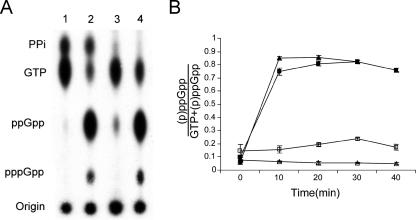
SpoT and CgtA interact (56), but the functional significance of this interaction is unknown. Because CgtA is not associated with ribosomes under amino acid starvation, a condition in which the hydrolysis of SpoT is inhibited, we predicted that CgtA might, on the assembling 50S particle, promote (p)ppGpp hydrolysis by SpoT. If true, then the steady-state levels of (p)ppGpp should be elevated in the absence of CgtA. To determine whether this is the case, we directly assayed the (p)ppGpp levels in a cgtA mutant and its isogenic wild-type strain under steady-state and amino acid starvation conditions (Fig. 5). Under normal growth conditions, the cellular (p)ppGpp level is relatively low in the wild-type strain but increases promptly upon SH treatment (Fig. 5A, lanes 1 and 2). In the cgtA mutant, however, there is a noticeable increase in the steady-state level of (p)ppGpp compared to that of the isogenic control (Fig. 5A, lane 3 versus lane 1). Moreover, the spots that represent PPi [the hydrolysis product of (p)ppGpp] are greatly reduced in the mutant (Fig. 5A). Upon amino acid starvation, a quick accumulation of (p)ppGpp also occurs in the mutant, the level of which is comparable to that seen in the wild type (Fig. 5A, lanes 2 and 4). These data indicate that although the steady-state levels of (p)ppGpp require functional CgtA, the rapid accumulation of (p)ppGpp during amino acid starvation does not. We verified that this was the case by quantifying the accumulation of (p)ppGpp levels in a time course study and showed that (i) the steady-state level of (p)ppGpp is consistently two- to threefold higher in the cgtA mutant and (ii) the accumulation of (p)ppGpp during amino acid starvation does not differ between the wild type and the mutant strains (Fig. 5B). Thus, CgtA is important for maintaining the steady-state levels of (p)ppGpp but not for accumulation of (p)ppGpp during the stringent response.
FIG. 5.
(p)ppGpp steady-state level is increased in a cgtA mutant. (A) JM3903 (ΔcgtA::kan plus PcgtA-cgtA) and JM3907 (ΔcgtA::kan plus PcgtA-cgtAG80ED85N) cells were grown at 30°C in low-phosphate medium and uniformly 32Pi labeled (100 μCi/ml) before being treated with SH (1 mg/ml). Samples were taken immediately before the addition and at 10-min intervals thereafter, as indicated. The formic acid-extracted nucleotides were resolved by one-dimensional thin-layer chromatography on polyethyleneimine-cellulose sheets, autoradiographed, and quantified with a phosphorimager. Unlabeled ATP and GTP were spotted on the plates as markers. (A) Autoradiograms of polyethyleneimine thin-layer chromatography plates with or without SH treatment (20-min time point). Lanes 1 and 2 are JM3903 without and with SH, respectively; lanes 3 and 4 are JM3907 without and with SH, respectively. (B) (p)ppGpp levels normalized according to the GTP levels. Open and closed symbols are nontreated and SH-treated JM3903 (triangles) and JM3907 (squares) samples, respectively. Error bars are standard deviations from triplicate experiments.
cgtA is an essential gene in a relA spoT mutant background.
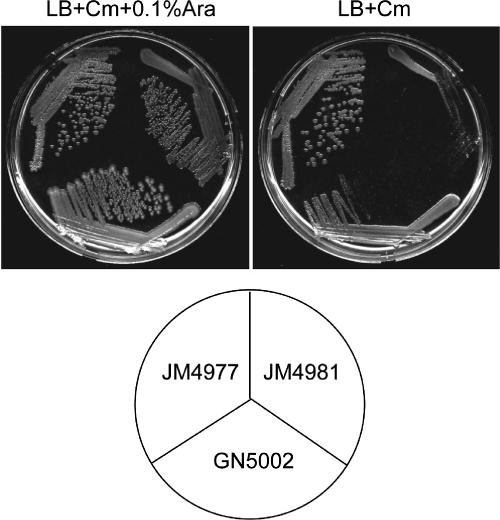
spoT is an essential gene because it is required to hydrolyze (p)ppGpp that would otherwise accumulate in its absence. Since we propose that CgtA is involved in promoting the (p)ppGpp hydrolysis of SpoT, one possibility is that the essential nature of CgtA is through its control of SpoT. If true, a relA spoT cgtA triple mutant would be viable. To test this, we generated a ΔrelA ΔspoT::cat cgtA::kan triple mutant harboring a plasmid expressing cgtA from the inducible/repressible PBAD promoter (PBAD-cgtA). Growth of cgtA::kan mutants is complemented by the PBAD-cgtA plasmid in the presence of arabinose, but cells grow very slowly on plates without arabinose (Fig. 6). The slow growth is likely due to the leaky expression from this promoter in the absence of arabinose. A ΔrelA ΔspoT::cat mutant grows well independently of arabinose. In contrast, the ΔrelA ΔspoT::cat cgtA::kan triple mutant harboring PBAD-cgtA grows very slowly on plates without arabinose, comparably to the cgtA::kan mutant plus PBAD-cgtA (Fig. 6). We conclude that, in E. coli, cgtA is essential even in the absence of spoT and relA.
FIG. 6.
A cgtA relA spoT triple mutant is not viable. GN5002 (ΔcgtA::kan plus PBAD-cgtA), JM4977 (ΔrelA ΔspoT::cat), and JM4981 (ΔrelA ΔspoT::cat cgtA::kan plus PBAD-cgtA) were streaked on LB plates containing chloramphenicol (Cm) with or without 0.1% arabinose (Ara) at 30°C, as indicated.
DISCUSSION
We demonstrate that SpoT is associated with the pre-50S ribosomal particle (Fig. 1). This observation is entirely consistent with earlier studies that showed that the (p)ppGpp hydrolase activity of SpoT cofractionates with ribosome and is inhibited by uncharged tRNA (20, 42, 48). Moreover, we had previously shown that SpoT copurified with the pre-50S-associated protein, CgtA (56), also suggesting that SpoT is ribosome associated. It is likely that previous attempts to detect a direct association of SpoT with ribosomes failed due to less-than-optimal conditions used (15), a problem that was also seen for the CgtA protein (28).
SpoT migrates on sucrose gradients over a number of pre-50S fractions, peaking at a ∼40S position. A similar migration profile is seen with other pre-50S-associated proteins such as the pseudouridine synthetases RluB and RluC (26) and the helicases CsdA (6) and SrmB (7). SpoT also copurified with these proteins in a large-scale affinity purification project (4), further supporting that these proteins are on the same pre-50S intermediate particles.
In contrast to exponentially growing cells in which a significant amount of CgtA associates with a pre-50S particle, during amino acid starvation, CgtA is not associated with ribosomes. We envision two possible mechanisms by which amino acid starvation could result in a loss of CgtA from the pre-50S particle: either (i) free CgtA does not bind to the ribosomal particles under these conditions or (ii) ribosome-bound CgtA dissociates from the ribosome. We suggest that both of these mechanisms may be responsible for the change in ribosome association of CgtA during amino acid starvation. First, CgtA is involved in late ribosome assembly and is not significantly associated with translating ribosomes (25) and therefore dissociates from the 50S prior to generation of the 70S particle. Thus, CgtA is normally cycling on and off of the ribosomal particle. Since CgtA proteins have modest affinity for guanine nucleotides and rapid guanine nucleotide exchange rates (31, 56), it was proposed that their guanine nucleotide occupancy would be determined by the relative levels of GTP/GDP in the cell (31, 39). In vitro, the GTP-bound CgtA but not the GDP-bound form has a higher affinity for ribosomes (25) and rRNA (43). During amino acid starvation there is a 40% drop in the GTP pool (14). Thus, under these conditions, the cellular balance of GTP/GDP is altered and may result in more free GDP-bound CgtA. Second, CgtA may be lost from ribosomes due to the accumulation of (p)ppGpp. The B. subtilis Obg protein was crystallized as an asymmetric unit with one monomer in its apo state and the other bound to ppGpp (3). In vitro, low levels of ppGpp accelerate the hydrolysis of GTP by the B. subtilis Obg (3). Thus, the increase in (p)ppGpp that accompanies amino acid starvation may also result in an increase in GTP hydrolysis and therefore an increase in GDP-bound CgtA. Alternatively, it could be that (p)ppGpp-bound CgtA is not ribosome associated independently of GTP hydrolysis.
We demonstrate that CgtA is required for maintaining the proper level of (p)ppGpp in the cell. In the cgtA mutant, the steady-state levels of (p)ppGpp are elevated by three- to fourfold (Fig. 5). The steady-state level of (p)ppGpp in exponentially growing cells depends on the balance of SpoT-dependent (p)ppGpp synthesis and (p)ppGpp hydrolysis (5). An increase in the levels of (p)ppGpp, therefore, could be due to an increase in the (p)ppGpp synthesis or a decrease in the (p)ppGpp hydrolysis rates of SpoT. Thus, there are two mechanisms by which CgtA could control SpoT function, either by promoting (p)ppGpp hydrolysis or by inhibiting (p)ppGpp synthesis. CgtA is not, however, required for the high levels of (p)ppGpp that accumulate during amino acid starvation (Fig. 5B). During amino acid starvation, the accumulation of (p)ppGpp is regulated predominantly by the level of (p)ppGpp degradation (36). Moreover, the rate of (p)ppGpp degradation abruptly decreases, indicating that the mechanism for controlling degradation works relatively rapidly (36). Consistently, a decrease in (p)ppGpp hydrolysis seen during amino acid starvation is independent of protein synthesis (36), indicating that neither the translating ribosome nor the newly synthesized protein is involved in controlling SpoT hydrolase activity. We show that during amino acid starvation, CgtA does not colocalize with SpoT and, therefore, under these conditions is not involved in controlling SpoT. We suggest that it is the loss of CgtA from the assembling pre-50S particle (and, therefore, also a lack of interaction between SpoT and CgtA) that is responsible for the rapid inhibition of SpoT (p)ppGpp hydrolysis.
It is of interest that the pre-50S intermediates to which CgtA and SpoT predominantly bind are not identical. There are, however, a subset of intermediates to which both proteins bind, indicated by their overlapping migration on sucrose gradients. Their colocalization was also confirmed by the copurification of both CsdA and SpoT with affinity-purified CgtA (56). A long-standing question has been how the cells maintain two populations of SpoT protein, those that synthesize (p)ppGpp and those that degrade (p)ppGpp. According to the structural analysis, SpoT/RelA protein exists in either a (p)ppGpp-hydrolase-OFF/(p)ppGpp-synthetase-ON or a hydrolase-ON/synthetase-OFF state (23). One possibility is that the different active states of SpoT may reflect whether SpoT is on the same assembling 50S particles as CgtA or not. If that is true, we predict that the form of SpoT on the 40S particles would be that of a (p)ppGpp synthetase and the form on particles with CgtA would be a (p)ppGpp hydrolase. In this manner, CgtA would control only a subset of SpoT proteins in the cell at any given time.
While CgtA is required for late assembly of the 50S subunit (25, 43), SpoT does not seem to play a role in the assembly process as judged by the normal polysome profile of the relA spoT double mutant (Fig. 2A). We have recently identified a number of additional ribosome-associated proteins that, like SpoT, are also not critical for ribosome assembly (26). One possibility is that association with the maturing ribosomal particle allows for coupling ribosome assembly with the protein activity. Doing so would allow for a coordination between critical cellular functions with ribosome assembly, as previously proposed (52).
We have shown that CgtA has two distinct cellular functions and is required for the last stages of 50S assembly (25) and in the control of SpoT (this study). We predicted that the essential nature of cgtA would not be due to its role in ribosome assembly, as the majority of the assembly factors are not essential. We show here, however, that cgtA is essential in mutants also lacking spoT and relA. Thus, the essential function of the E. coli CgtA protein remains unknown.
A role for CgtA in controlling cellular (p)ppGpp levels may explain some of the many pleiotropic phenotypes that have been assigned to CgtA. We suggest that at least some of the cgtA mutant phenotypes are due to indirect consequences of altering (p)ppGpp levels. For instance, it was recently reported that CgtA promotes replication fork stability (13). These studies focused on two types of cgtA mutations, CgtAP168V and a Tn insertion that resulted in loss of the C-terminal 9 amino acids and addition of 68 amino acids (13). We have shown that the Caulobacter crescentus CgtAP168V mutant protein does not have a defect in GTP hydrolysis but does have a reduced affinity for GDP and therefore, in vivo, may be predominantly bound to GTP (11). If that is also true in E. coli, the GTP-bound CgtAP168V may result in increased (p)ppGpp hydrolysis by SpoT and, therefore, result in lower levels of (p)ppGpp in the cells. A role for (p)ppGpp levels in controlling replication fork progression has been previously reported (34, 50). It has also been proposed by several groups that CgtA controls the initiation of replication initiation (12, 13, 51). The initiation of DNA replication, however, is under stringent control (45, 59), and therefore, the role of CgtA may be indirect. Support for this premise comes from a recent report showing that the growth of the cgtAG80ED85N mutant was partially suppressed by ectopic expression of dnaA (47). Clearly a reinvestigation of the phenotypes associated with cgtA mutants in the context of (p)ppGpp levels is warranted.
Since the submission of the manuscript, Raskin, Judson, and Mekalanos (41) have published a very nice paper verifying, in Vibrio cholerae, many of the findings that we report here for E. coli. Taking a very different approach, they posit that CgtA is involved in control of SpoT and show that depletion of V. cholerae CgtA results in an increase in ppGpp levels. In contrast to our findings in E. coli, however, they show that in V. cholerae CgtA is not essential in the absence of RelA. Clearly, further studies are necessary to sort out the differences in the essential nature of this conserved GTPase.
Acknowledgments
We are grateful to James Hernandez for his ongoing support in providing strains, antibodies, and advice. We also thank Daniel Smith for technical assistance.
This work was supported, in part, by NSF grant MCB-0316357.
Footnotes
Published ahead of print on 6 July 2007.
REFERENCES
- 1.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 3.Buglino, J., V. Shen, P. Hakimian, and C. D. Lima. 2002. Structural and biochemical analysis of the Obg GTP binding protein. Structure 10:1581-1592. [DOI] [PubMed] [Google Scholar]
- 4.Butland, G., J. M. Peregrin-Alvarez, J. Li, W. Yang, X. Yang, V. Canadien, A. Starostine, D. Richards, B. Beattie, N. Krogan, M. Davey, J. Parkinson, J. Greenblatt, and A. Emili. 2005. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature 433:531-537. [DOI] [PubMed] [Google Scholar]
- 5.Cashel, M., D. Gentry, V. J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1488-1496. In F. C. Neidhardt, R. Curtis, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 6.Charollais, J., M. Dreyfus, and I. Iost. 2004. CsdA, a cold-shock RNA helicase from Escherichia coli, is involved in the biogenesis of 50S ribosomal subunit. Nucleic Acids Res. 32:2751-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charollais, J., D. Pflieger, J. Vinh, M. Dreyfus, and I. Iost. 2003. The DEAD-box RNA helicase SrmB is involved in the assembly of 50S ribosomal subunits in Escherichia coli. Mol. Microbiol. 48:1253-1265. [DOI] [PubMed] [Google Scholar]
- 8.Chatterji, D., and A. K. Ojha. 2001. Revisiting the stringent response, ppGpp and starvation signaling. Curr. Opin. Microbiol. 4:160-165. [DOI] [PubMed] [Google Scholar]
- 9.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datta, K., J. L. Fuentes, and J. R. Maddock. 2005. The yeast GTPase Mtg2p is required for mitochondrial translation and partially suppresses an rRNA methyltransferase mutant, mrm2. Mol. Biol. Cell 16:954-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta, K., J. M. Skidmore, K. Pu, and J. R. Maddock. 2004. The Caulobacter crescentus GTPase CgtAC is required for progression through the cell cycle and for maintaining 50S ribosomal subunit levels. Mol. Microbiol. 54:1379-1392. [DOI] [PubMed] [Google Scholar]
- 12.Dutkiewicz, R., M. Slominska, G. Wegrzyn, and A. Czyz. 2002. Overexpression of the cgtA (yhbZ, obgE) gene, coding for an essential GTP-binding protein, impairs the regulation of chromosomal functions in Escherichia coli. Curr. Microbiol. 45:440-445. [DOI] [PubMed] [Google Scholar]
- 13.Foti, J. J., J. Scheienda, V. A. J. Sutera, and S. T. Lovett. 2005. A bacterial G protein-mediated response to replication arrest. Mol. Cell 17:549-560. [DOI] [PubMed] [Google Scholar]
- 14.Gallant, J., and B. Harada. 1969. The control of ribonucleic acid synthesis in Escherichia coli. 3. The functional relationship between purine ribonucleoside triphosphate pool sizes and the rate of ribonucleic acid accumulation. J. Biol. Chem. 244:3125-3132. [PubMed] [Google Scholar]
- 15.Gentry, D., and M. Cashel. 1995. Cellular location of the Escherichia coli SpoT protein. J. Bacteriol. 177:3890-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentry, D. R., and M. Cashel. 1996. Mutational analysis of the Escherichia coli spoT gene identifies distinct but overlapping regions involved in ppGpp synthesis and degradation. Mol. Microbiol. 19:1373-1384. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 18.Hansen, M. T., M. L. Pato, S. Molin, N. P. Fill, and K. von Meyenburg. 1975. Simple downshift and resulting lack of correlation between ppGpp pool size and ribonucleic acid accumulation. J. Bacteriol. 122:585-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haseltine, W. A., and R. Bock. 1973. Synthesis of guanosine tetra and pentaphosphate requires the presence of a codon-specific uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc. Natl. Acad. Sci. USA 70:1564-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinemeyer, E.-A., and D. Richter. 1977. In vitro degradation of guanosine tetraphosphate (ppGpp) by an enzyme associated with the ribosomal fraction from Escherichia coli. FEBS Lett. 84:357-361. [DOI] [PubMed] [Google Scholar]
- 21.Heinemeyer, E. A., M. Geis, and D. Richter. 1978. Degradation of guanosine 3′-diphosphate 5′-diphosphate in vitro by the spoT gene product of Escherichia coli. Eur. J. Biochem. 89:125-131. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez, V. J., and H. Bremer. 1991. Escherichia coli ppGpp synthetase II activity requires spoT. J. Biol. Chem. 266:5991-5999. [PubMed] [Google Scholar]
- 23.Hogg, T., U. Mechold, H. Malke, M. Cashel, and R. Hilgenfeld. 2004. Conformational antagonism between opposing active sites in a bifunctional RelA/SpoT homolog modulates (p) ppGpp metabolism during the stringent response. Cell 117:57-68. [DOI] [PubMed] [Google Scholar]
- 24.Izutsu, K., A. Wada, and C. Wada. 2001. Expression of ribosome modulation factor (RMF) in Escherichia coli requires ppGpp. Genes Cells 6:665-676. [DOI] [PubMed] [Google Scholar]
- 25.Jiang, M., K. Datta, A. Walker, J. Strahler, P. Bagamasbad, P. C. Andrews, and J. R. Maddock. 2006. The Escherichia coli GTPase CgtAE is involved in late steps of large ribosome assembly. J. Bacteriol. 188:6757-6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang, M., S. M. Sullivan, A. K. Walker, J. R. Strahler, P. C. Andrews, and J. R. Maddock. 2007. Identification of novel Escherichia coli ribosome-associated proteins using isobaric tags and multidimensional protein identification techniques. J. Bacteriol. 189:3434-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson, G. S., C. R. Adler, J. J. Collins, and D. Court. 1979. Role of the spoT gene product and manganese ion in the metabolism of guanosine 5′-diphosphate 3′-diphosphate in Escherichia coli. J. Biol. Chem. 254:5483-5487. [PubMed] [Google Scholar]
- 28.Kobayashi, G., S. Moriya, and C. Wada. 2001. Deficiency of essential GTP-binding protein ObgE in Escherichia coli inhibits chromosome partition. Mol. Microbiol. 41:1037-1051. [DOI] [PubMed] [Google Scholar]
- 29.Kok, J., K. A. Trach, and J. A. Hoch. 1994. Effects on Bacillus subtilis of a conditional lethal mutation in the essential GTP-binding protein Obg. J. Bacteriol. 176:7155-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer, M., E. Kecskes, and I. Horvath. 1981. Guanosine polyphosphate production of Escherichia coli stringent and relaxed strains in the stationary phase of growth. Acta Microbiol. Acad. Sci. Hung. 28:165-170. [PubMed] [Google Scholar]
- 31.Lin, B., K. L. Covalle, and J. R. Maddock. 1999. The Caulobacter crescentus CgtA protein displays unusual guanine nucleotide binding and exchange properties. J. Bacteriol. 181:5825-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin, B., D. A. Thayer, and J. R. Maddock. 2004. The Caulobacter crescentus CgtAC protein cosediments with the free 50S ribosomal subunit. J. Bacteriol. 186:481-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magnusson, L. U., A. Farewell, and T. Nystrom. 2005. ppGpp: a global regulator in Escherichia coli. Trends Microbiol. 13:236-242. [DOI] [PubMed] [Google Scholar]
- 34.McGlynn, P., and R. G. Lloyd. 2000. Modulation of RNA polymerase by (p)ppGpp reveals a RecG-dependent mechanism for replication fork progression. Cell 101:35-45. [DOI] [PubMed] [Google Scholar]
- 35.Metzger, S., G. Schreiber, E. Aizenman, M. Cashel, and G. Glaser. 1989. Characterization of the relA1 mutation and a comparison of relA1 with new relA null alleles in Escherichia coli. J. Biol. Chem. 264:21146-21152. [PubMed] [Google Scholar]
- 36.Murray, K. D., and H. Bremer. 1996. Control of spoT-dependent ppGpp synthesis and degradation in Escherichia coli. J. Mol. Biol. 259:41-57. [DOI] [PubMed] [Google Scholar]
- 37.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman, J. R., and C. Fuqua. 1999. Broad-host-range expression vectors that carry the L-arabinose-inducible Escherichia coli araBAD promoter and the araC regulator. Gene 227:197-203. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto, S., and K. Ochi. 1998. An essential GTP-binding protein functions as a regulator of differentiation in Streptomyces coelicolor. Mol. Microbiol. 30:107-119. [DOI] [PubMed] [Google Scholar]
- 40.Ramagopal, S., and B. D. Davis. 1974. Localization of the stringent protein of Escherichia coli on the 50S ribosomal subunit. Proc. Natl. Acad. Sci. USA 71:820-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raskin, D. M., N. Judson, and J. J. Mekalanos. 2007. Regulation of the stringent response is the essential function of the conserved bacterial G protein CgtA in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 104:4636-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richter, D. 1980. Uncharged tRNA inhibits guanosine 3′,5′-bis (diphosphate) 3′-pyrophosphohydrolase [ppGppase], the spoT gene product, from Escherichia coli. Mol. Gen. Genet. 178:325-327. [DOI] [PubMed] [Google Scholar]
- 43.Sato, A., G. Kobayashi, H. Hayashi, H. Yoshida, A. Wada, M. Maeda, S. Hiraga, K. Takeyasu, and C. Wada. 2005. The GTP binding protein Obg homolog ObgE is involved in ribosome maturation. Genes Cells 10:393-408. [DOI] [PubMed] [Google Scholar]
- 44.Sattlegger, E., and A. G. Hinnebusch. 2000. Separate domains in GCN1 for binding protein kinase GCN2 and ribosomes are required for GCN2 activation in amino acid-starved cells. EMBO J. 23:6622-6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schreiber, G., E. Z. Ron, and G. Glaser. 1995. ppGpp-mediated regulation of DNA replication and cell division in Escherichia coli. Curr. Microbiol. 30:27-32. [DOI] [PubMed] [Google Scholar]
- 46.Scott, J. M., and W. G. Haldenwang. 1999. Obg, an essential GTP binding protein of Bacillus subtilis, is necessary for stress activation of transcription factor σB. J. Bacteriol. 181:4653-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sikora, A. E., R. Zielke, A. Wegrzyn, and G. Wegrzyn. 2006. DNA replication defect in the Escherichia coli cgtA(ts) mutant arising from reduced DnaA levels. Arch. Microbiol. 185:340-347. [DOI] [PubMed] [Google Scholar]
- 48.Sy, J. 1977. In vitro degradation of guanosine 5′-diphosphate, 3′-diphosphate. Proc. Natl. Acad. Sci. USA 74:5529-5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tosa, T., and L. I. Pizer. 1971. Effect of serine hydroxamate on the growth of Escherichia coli. J. Bacteriol. 106:966-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trautinger, B. W., R. P. Jaktaji, E. Rusakova, and R. G. Lloyd. 2005. RNA polymerase modulators and DNA repair activities resolve conflicts between DNA replication and transcription. Mol. Cell 19:247-258. [DOI] [PubMed] [Google Scholar]
- 51.Ulanowska, K., A. Sikora, G. Wegrzyn, and A. Czyz. 2003. Role of the cgtA gene function in DNA replication of extrachromosomal elements in Escherichia coli. Plasmid 50:45-52. [DOI] [PubMed] [Google Scholar]
- 52.VanBogelen, R. A., and F. C. Neidhardt. 1990. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc. Natl. Acad. Sci. USA 87:5589-5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vidwans, S. J., K. Ireton, and A. D. Grossman. 1995. Possible role for the essential GTP-binding protein Obg in regulating the initiation of sporulation in Bacillus subtilis. J. Bacteriol. 177:3308-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wada, A., K. Igarashi, S. Yoshimura, S. Aimoto, and A. Ishihama. 1995. Ribosome modulation factor: stationary growth phase-specific inhibitor of ribosome functions from Escherichia coli. Biochem. Biophys. Res. Commun. 214:410-417. [DOI] [PubMed] [Google Scholar]
- 55.Wendrich, T. M., G. Blaha, D. N. Wilson, M. A. Marahiel, and K. H. Nierhaus. 2002. Dissection of the mechanism for the stringent factor RelA. Mol. Cell 10:779-788. [DOI] [PubMed] [Google Scholar]
- 56.Wout, P., K. Pu, S. M. Sullivan, V. Reese, S. Zhou, B. Lin, and J. R. Maddock. 2004. The Escherichia coli GTPase, CgtAE, cofractionates with the 50S ribosomal subunit and interacts with SpoT, a ppGpp synthetase/hydrolase. J. Bacteriol. 186:5249-5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao, H., M. Kalman, K. Ikehara, S. Zemel, G. Glaser, and M. Cashel. 1991. Residual guanosine 3′,5′-bispyrophosphate synthesis activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 266:5980-5990. [PubMed] [Google Scholar]
- 58.Yang, X., and E. E. Ishiguro. 2001. Involvement of the N terminus of ribosomal protein L11 in regulation of the RelA protein of Escherichia coli. J. Bacteriol. 183:6532-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zyskind, J. W., and D. W. Smith. 1992. DNA replication, the bacterial cell cycle, and cell growth. Cell 69:5-8. [DOI] [PubMed] [Google Scholar]