Abstract
Bcl10 (B-cell lymphoma 10) is an adaptor protein comprised of an N-terminal caspase recruitment domain and a C-terminal serine/threonine-rich domain. Bcl10 plays a critical role in antigen receptor-mediated NF-κB activation and lymphocyte development and functions. Our current study has discovered that T-cell activation induced monophosphorylation and biphosphorylation of Bcl10 and has identified S138 within Bcl10 as one of the T-cell receptor-induced phosphorylation sites. Alteration of S138 to an alanine residue impaired T-cell activation-induced ubiquitination and subsequent degradation of Bcl10, ultimately resulting in prolongation of TCR-mediated NF-κB activation and enhancement of interleukin-2 production. Taken together, our findings demonstrate that phosphorylation of Bcl10 at S138 down-regulates Bcl10 protein levels and thus negatively regulates T-cell receptor-mediated NF-κB activation.
Engagement of the T-cell receptor (TCR) or B-cell receptor (BCR) initiates the activation of a cascade of protein tyrosine kinases (PTK) and adaptor proteins, resulting in the recruitment and activation of several effector enzymes, including phospholipase Cγ (PLCγ), which hydrolyzes inositol phospholipid (PIP2) into inositol triphosphate (IP3), and diacylglycerol (DAG) (5, 20, 34). DAG activates protein kinase C (PKC), which ultimately leads to the activation of nuclear factor-κB (NF-κB). NF-κB has been shown to play a critical role in the function and development of lymphocytes (10, 17, 43). NF-κB family members include c-Rel, RelA (p65), RelB, NF-κB1 (p105/50), and NF-κB2 (p100/52) (11, 23). Normally, NF-κB is sequestered in the cytoplasm by a family of cytoplasmic inhibitory proteins, termed IκB (1), which mask the nuclear localization signal of NF-κB. Activation of PKC eventually leads to the activation of IκB kinase (IKK) (21), which is a complex composed of a regulatory subunit, IKKγ (also known as NEMO), and two catalytic subunits, IKKα and IKKβ (10, 17, 43). IKK phosphorylates the regulatory serine residues located in the N terminus of IκB molecules, triggering swift ubiquitination and proteolysis of IκBs by the proteasome complex (21). The degradation of IκBs unmasks the nuclear localization signal in NF-κB, which subsequently translocates into the nucleus and binds to the cognate κB enhancer elements upstream of its target genes (12). Studies have demonstrated that PKCθ is essential for TCR-induced, whereas PKCβ is required for BCR-induced, activation of IKK and subsequent NF-κB activation (38, 46, 47). Nonetheless, the molecular mechanism by which PKC activates IKK is not fully understood.
Recent studies have identified a three-component complex composed of CARMA1 (for “caspase recruitment domain [CARD], membrane-associated guanylate kinase, protein 1”), Bcl10, and MALT1 (mucosa-associated lymphoid tissue lymphoma translocation protein 1) that plays an essential role in coupling PKC to IKK in antigen receptor-induced activation of NF-κB (24, 48). Current models propose that CARMA1 constitutively associates with the plasma membrane via its PDZ-SH3-GUK region, whereas Bcl10 constitutively associates with the immunoglobulin-like domains of MALT1 via the region between the CARD and serine/threonine (S/T)-rich domain of Bcl10 in the cytoplasm of unstimulated lymphocytes (8, 28, 50). Stimulation of T cells through TCR engagement results in PKC activation and translocation of CARMA1 to the lipid rafts (8). Activated PKC phosphorylates CARMA1, resulting in translocation of the Bcl10/MALT1 complex to the lipid rafts from the cytoplasm, and the resulting CARMA1/Bcl10/MALT1 (CBM) complex leads to raft recruitment and activation of IKK (24, 25, 27, 44). The essential role of the CBM complex in NF-κB activation by antigen receptors has been demonstrated by recent in vivo genetic studies (7, 14, 19, 30, 35-37, 54, 60). Deficiency of any of these three genes specifically impairs TCR- or BCR-induced NF-κB activation, although it does not affect TCR- or BCR-induced overall tyrosine phosphorylation, calcium flux, or activation of ERK and AP-1 (7, 14, 19, 30, 35-37, 54, 60). Mice deficient in any of these three genes also share developmental and functional abnormalities. For example, interleukin-2 (IL-2) production is severely impaired in T cells, although T-cell development appears to be grossly normal, whereas B-cell development, antibody production, and proliferation are similarly impeded in these mice. Therefore, Bcl10, along with CARMA1 and MALT1, plays a critical role in antigen receptor-mediated signaling and is essential for the development and function of lymphocytes.
Several studies have shown that Bcl10 is phosphorylated following TCR stimulation in T cells (2, 40, 41) or phorbol 12-myristate 13-acetate (PMA) plus ionomycin stimulation in B cells (19). More recent studies have shown that Bcl10 is ubiquitinated following TCR engagement, resulting in down-regulation of Bcl10 protein levels, correlating with attenuation of NF-κB activity (18, 41). Most recently, it was reported that IKKβ phosphorylates a series of serine residues at the C terminus of Bcl10, which in turn interferes with IKKγ ubiquitination and T-cell activation (55). In the current study, we discovered that T-cell activation induced monophosphorylation and biphosphorylation of Bcl10. S138 of Bcl10 served as one of the TCR-induced phosphorylation sites. We also made the important connection between Bcl10 phosphorylation and Bcl10 ubiquitination. We demonstrated that replacement of both S134 and S138 or S138 alone with alanine residues impaired activation-induced phosphorylation of Bcl10, inhibited Bcl10 ubiquitination, and delayed degradation of Bcl10, which ultimately resulted in prolonged NF-κB activation and increased IL-2 production by cells expressing these mutants. Our studies demonstrate that phosphorylation of S138 within Bcl10 negatively regulates antigen receptor-mediated NF-κB activation by promoting Bcl10 ubiquitination and consequent Bcl10 degradation.
MATERIALS AND METHODS
Mice, cell lines, reagents, and constructs.
Bcl10-deficient mice were generated as described previously (60). Mice were maintained in the Biological Resource Center at the Medical College of Wisconsin. All animal protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Jurkat cells were maintained in RPMI 1640 (Media Tech Inc.) with 10% fetal bovine serum (FBS) (HyClone Inc). CTLL-2 cells were maintained in RPMI 1640 with 10% FBS, 50 μM 2-mercaptoethanol, and 2 U/ml IL-2.
Bcl10 or N-terminally Flag-tagged Bcl10 constructs were generated by standard PCR (QIAGEN Inc). Antibodies against Bcl10 (sc-5273 and sc-9560) and ubiquitin (sc-8017) were purchased from Santa Cruz Biotechnology. Another anti-ubiquitin antibody was provided by Arthur L. Hass (Louisiana State University Health Sciences Center School of Medicine). Anti-FLAG (F3165) antibody was obtained from Sigma. Antibodies against actin (MAB150 1R) and N-terminal Bcl10 (AB16506) were from Chemicon. Antibodies against mouse CD3 (16-0031), mouse CD28 (16-0281), human CD3 (OKT3; 16-0037), and human CD28 (CD28.2; 16-0289) and phycoerythrin-conjugated anti-B220 antibody were purchased from eBioscience. Lambda protein phosphatase (λ-PPase) was purchased from New England Biolabs.
Wild-type Bcl10 or N-terminally Flag-tagged Bcl10 constructs were generated by standard PCR (QIAGEN Inc). Flag-tagged Bcl10 was constructed by adding the eight amino acid residues of the Flag sequence to the N terminus of Bcl10 after the starting methionine. The 5′ primers used in the PCR contained EcoRI restriction sites and the 3′ primer contained an XhoI site. The sequences for the primers are the following: 5′ primer for Bcl10, GGA ATT CCC GGC CTG GGC CAT; 5′ primer for Flag-Bcl10, GGA ATT CGA CAT GGA CTA CAA GGA CGA CGA TGA CAA GGA GGC TCC CGC ACC GTC CCT CAC GG; 3′ primer for both constructs, CCC TCG AGT CAT TGG CGT GAA AGA GCC CG. The constructs were cloned into murine stem cell virus (MSCV)-internal ribosomal entry site (IRES)-green fluorescent protein (GFP) vector at EcoRI and XhoI sites.
Two-dimensional (2-D) electrophoresis.
Jurkat cells expressing Flag-tagged Bcl10 were left untreated or were stimulated with OKT3 (1 μg/ml) plus CD28.2 (5 μg/ml) for 15 min and then collected. Rehydration buffer {8 M urea, 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, 50 mM dithiothreitol (DTT), 4% Bio-Lyte 3/10 Ampholyte, 0.1 μM sodium vanadate, protease inhibitors} was added to extract total cell proteins, which were then resolved on an isoelectric focusing (IEF) strip (ReadyStrip IPG strips, pH 5 to 8; Bio-Rad) (first dimension) in a Protean IEF cell (Bio-Rad), followed by separation with 8 to 16% linear gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Criterion Tris-HCl gel; Bio-Rad) (second dimension).
Retroviral transduction.
Retroviruses expressing wild-type or mutant Bcl10 were generated as described previously (58). Briefly, the mouse wild-type or mutant Bcl10 gene was cloned into a bicistronic retrovirus MSCV-IRES-GFP vector (15). The expression of the cloned gene and GFP is under MSCV promoter control. GFP is a marker for identification of retrovirally transduced cells. The construct was cotransfected with the helper plasmid pEQPAM3 containing the required gag, pol, and env retroviral genes into 293T cells. The cell culture supernatant was collected 2 days later and was used to transduce ecotropic virus packaging cells (GP+E86) in the presence of 6 μg/ml of Polybrene. Cells exhibiting high GFP expression were sorted and subsequently expanded as virus-producing cells.
For retroviral transduction of Bcl10-deficient bone marrow (BM)-derived large pre-B cells in vitro, BM cells derived from Bcl10-deficient mice were cultured in RPMI 1640 supplemented with 10% FBS and 2 ng/ml recombinant IL-7 for 5 days. The cells were then cocultured with the irradiated (1,500 rads) retrovirus-producing GP+E86 cells in the presence of 6 μg/ml Polybrene. Two days later, GFP+ B220+ cells were sorted by a fluorescence-activated cell sorter (FACS) and used for biochemical studies. For retroviral transduction of primary Bcl10-deficient splenic B cells in vitro, splenocytes from Bcl10-deficient mice were cultured in primary lymphocyte culture medium (RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, 10 mM nonessential amino acid, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol) with 10 μg/ml lipopolysaccharide (LPS) for 1 day. Activated cells were then cocultured with irradiated (1,500 rads) retrovirus-producing GP+E86 cells in the presence of 6 μg/ml Polybrene. Two days later, GFP+ B220+ cells were sorted by FACS and rested for 4 h before restimulation for biochemical studies. For retroviral transduction of primary Bcl10-deficient splenic T cells in vitro, splenic T cells from Bcl10-deficient mice were cultured in primary lymphocyte culture medium in the presence of PMA (10 ng/ml) plus ionomycin (500 ng/ml) for 1 day. The cells were then cocultured with irradiated (1,500 rads) retrovirus-producing GP+E86 cells in the presence of 6 μg/ml Polybrene. Two days later, GFP+ cells were sorted by FACS and rested overnight before restimulation for biochemical studies.
For retroviral transduction of Bcl10-deficient T cells in vivo via BM transplantation, 8- to 12-week-old Bcl10-deficient donor mice were injected intraperitoneally with 5-fluorouracil (150 mg/kg of body weight) 2 days before BM harvest. BM cells were isolated and cultured for 2 days in the presence of 20 ng/ml of IL-3, 50 ng/ml of IL-6, and 50 ng/ml of stem cell factor (Peptech, Ltd.). The BM cells were then cocultured with the irradiated (1,500 rads) retrovirus-producing GP+E86 cells in the presence of 6 μg/ml Polybrene. Two days later, 1 × 106 to 2 × 106 nucleated BM cells were retroorbitally injected into 8- to 16-week-old sublethally irradiated (300 rads) Jak3-deficient recipient mice. Two months after BM transplantation, T cells purified from the recipients were analyzed.
[32P]orthophosphate labeling experiments.
Parental Jurkat cells or Jurkat cells (1 × 107/ml) expressing Flag-tagged wild-type or mutant Bcl10 were cultured in a phosphate-free medium (Gibco, Invitrogen) for 60 min and then cultured with the addition of [32P]orthophosphate (0.2 mCi/ml) for 15 min. The labeled cells (1 × 107/sample) were cultured in the presence or absence of OKT3 (1 μg/ml) plus CD28.2 (5 μg/ml) for 15 min. Subsequently, Bcl10 was immunoprecipitated with anti-Bcl10 antibody plus G protein-conjugated agarose beads or anti-FLAG-conjugated agarose beads (Sigma) from the cell lysates. The immunoprecipitates were subjected to SDS-PAGE and transferred to membranes for autoradiography and Western blot analysis.
Ubiquitination assay.
Parental Jurkat cells or Jurkat cells (4 × 107) expressing wild-type Flag-Bcl10 were stimulated with OKT3 (1 μg/ml) plus CD28.2 (5 μg/ml) or PMA (10 ng/ml) plus ionomycin (500 ng/ml), and cells were lysed in buffer (10 mM Tris-HCl, pH 7.6, 50 mM NaCl, 1% Triton X-100, 5 mM EDTA, 0.1 mM Na3VO4, 50 mM NaF, 30 mM Na4P2O7, 1 mM DTT, 0.25 mM Nα-p-tosyl-l-lysine chloromethyl ketone [TLCK], 0.25 mM N-tosyl-l-phenylalanine chloromethyl ketone [TPCK], 5 mM N-ethylmaleimide [NME]) in the presence of a mixture of protease inhibitors. The cell lysates were separated into two aliquots, immunoprecipitated with anti-Bcl10 or anti-Flag antibody, and resolved by SDS-PAGE, followed by Western blot analysis for ubiquitin and Bcl10. Alternatively, Jurkat cells expressing wild-type Bcl10, Bcl10(S134A/S138A), or Bcl10(S138A) were treated with 0.4 μM MG132 for 1 h prior to being stimulated with OKT3 (1 μg/ml) plus CD28.2 (5 μg/ml). The cells were lysed in buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.25 mM TLCK, 0.25 mM TPCK, 5 mM NME) that contained 1% SDS in the presence of protease inhibitors, and the cell lysates were boiled to efficiently disrupt protein-protein interactions. The cell lysates were then diluted with 9 volumes of the lysis buffer without 1% SDS. After sonication, the supernatants were immunoprecipitated with anti-Flag antibody for Western blot analysis with rabbit polyclonal anti-ubiquitin antibody (a gift from Arthur L. Hass) (4).
IL-2 production.
The murine T-cell line CTLL, which is dependent on IL-2 for growth, was used for assay of IL-2. Cell culture supernatants were collected from Jurkat cells expressing wild-type Flag-Bcl10, Flag-Bcl10(S134A/S138A), and Flag-Bcl10(S138A) after stimulation with OKT3 (5 μg/ml) plus CD28.2 (5 μg/ml) for 8 h; from Bcl10-deficient T cells reconstituted with wild-type Bcl10 or Bcl10(S134A/S138A) after stimulation with plate-bound anti-mouse CD3 (5 μg/ml) plus anti-mouse CD28 (2 μg/ml) for 18 h; and from FACS-sorted GFP+ Thy1.2+ splenic T cells reconstituted with wild-type Bcl10 or Bcl10(S134A/S138A) in vivo following a BM transplantation procedure after stimulation with plate-bound anti-mouse CD3 plus anti-mouse CD28 for 18 h. CTLL cells were seeded at 2 × 105/ml for two consecutive days, washed three times with PBS, and resuspended in medium without IL-2 at 1 × 105 cells/ml. CTLL cells (50 μl) were added to 96-well round-bottom tissue culture plates containing 50 μl of diluted cell culture supernatant or IL-2 standards. IL-2 standards were generated by a serial twofold dilution of recombinant IL-2 from 10 U/ml to 0.0195 U/ml. Twenty hours later, [3H]thymidine was added to the culture and pulsed for 12 h. Cells were harvested by a Micro96 Harvester (Skatron Instruments), and [3H]thymidine incorporation was measured by a MicroBeta TriLux and MicroBeta JET liquid scintillation and luminescence counter (Pelkin Elmer). The concentration of IL-2 in cell culture supernatant was calculated based on a standard curve.
RESULTS
T-cell activation induces monophosphorylated and biphosphorylated forms of Bcl10.
The small phosphate group often does not resolve phosphoproteins from their unphosphorylated counterparts on SDS-PAGE, which separates proteins according to their molecular weight. Thus, it is difficult to detect phosphorylation of Bcl10 upon TCR engagement by SDS-PAGE in combination with Western blot analysis, although some studies with this method indicate that Bcl10 is phosphorylated (2, 40, 41). To further investigate whether Bcl10 is phosphorylated upon T-cell activation, we performed [32P]orthophosphate labeling experiments. Jurkat cells expressing Flag-tagged Bcl10 were metabolically labeled with [32P]orthophosphate followed by incubation in medium alone or in the presence of anti-CD3 plus anti-CD28. Cell lysates were immunoprecipitated with anti-Flag antibodies and subjected to SDS-PAGE. Subsequent autoradiography clearly demonstrated incorporation of [32P]orthophosphate into Bcl10 molecules upon TCR engagement in Jurkat T cells (Fig. 1A). Thus, Bcl10 is phosphorylated upon T-cell activation by TCR engagement.
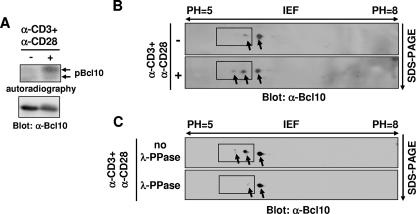
FIG. 1.
Bcl10 is monophosphorylated and biphosphorylated following TCR stimulation. (A) Bcl10 is phosphorylated following TCR engagement in Jurkat cells. Jurkat cells were labeled with [32P]orthophosphate followed by incubation in medium alone (−) or in the presence (+) of anti-CD3 (α-CD3) plus anti-CD28 (α-CD28) for 15 min. Cell lysates were immunoprecipitated with anti-Bcl10 (α-Bcl10) antibodies and then subjected to SDS-PAGE, followed by autoradiography or Western blot analysis with α-Bcl10 antibodies. (B) TCR engagement induces two phosphorylated isoforms of Bcl10. Jurkat cells were untreated (−) or stimulated (+) with α-CD3 plus α-CD28 for 15 min. Cell lysates were subjected to 2-D electrophoresis with IEF (pH 5 to 8) in one dimension and gradient SDS-PAGE in the second dimension, followed by Western blot analysis with α-Bcl10 antibodies. (C) The major Bcl10 isoform existing in T cells is a nonphosphorylated form. Jurkat cells were stimulated with α-CD3 plus α-CD28. Cell lysates were untreated or were treated with λ-PPase and subjected to 2-D electrophoresis followed by Western blot analysis with α-Bcl10 antibodies. Arrows point to Bcl10 isoforms, and phosphorylated Bcl10 isoforms are enclosed by rectangles.
Next, it is important to determine the number of TCR-induced phosphorylation sites in Bcl10. To this end, we employed a 2-D electrophoresis method with IEF in one dimension, which separates proteins by their isoelectric points (pIs), and gradient SDS-PAGE in the second dimension, which separates proteins by their molecular weights. Although a phosphate group might not markedly change the molecular weight of Bcl10, its negative charge might be expected to alter its pI. Jurkat cell lysates prepared before and after stimulation were subjected to 2-D electrophoresis followed by Western blot analysis for Bcl10. In resting Jurkat cells, endogenous Bcl10 existed as one major isoform with a high pI and one minor isoform with a slightly lower pI (Fig. 1B). After stimulation of Jurkat cells with anti-CD3 plus anti-CD28, an additional Bcl10 isoform with an even lower pI was induced, and the amount of Bcl10 with an intermediate pI increased (Fig. 1B). Treatment of the lysates from activated Jurkat cells with λ-PPase returned the low- and the intermediate-pI isoforms of Bcl10 to the major, high-pI isoform (Fig. 1C), indicating that this major, high-pI form is the nonphosphorylated Bcl10 molecule. Moreover, pIs of Bcl10 with zero, one, or two phosphate groups were theoretically calculated as 6.16, 5.91, or 5.71, respectively (according to pI calculation at the web site http://scansite.mit.edu). The pH gradient is linearly distributed in the IEF dimension; thus, based on the distance between the isoforms of Bcl10 separated by the IEF electrophoresis, we deduced that the two TCR activation-induced isoforms of Bcl10 corresponded to Bcl10 with one and two phosphate groups, respectively. Taken together, these data demonstrate that TCR activation induces monophosphorylated and biphosphorylated Bcl10.
S138 of Bcl10 contains a TCR-induced phosphorylation site.
We next tried to identify the TCR-induced phosphorylation sites within Bcl10 using truncation and site-directed mutagenesis analysis. Bcl10 contains an N-terminal CARD domain and a C-terminal S/T-rich domain, which consists of about 20% S/T residues (6, 22, 45, 49, 61). Previous studies of fibroblasts suggested that the phosphorylation site(s) of Bcl10 is likely to be in its C-terminal S/T-rich domain (22). To narrow down the region within which Bcl10 is phosphorylated, we generated a series of Bcl10 truncation mutants that terminate at amino acid residues 106 [Bcl10(1-106)], 130 [Bcl10(1-130)], 157 [Bcl10(1-157)], 168 [Bcl10(1-168)], 188 [Bcl10(1-188)], or 208 [Bcl10(1-208)]. In order to conveniently deliver genes in later studies, these Bcl10 deletion mutants were cloned into the MSCV-IRES-GFP retrovirus vector (15). Subsequently, the constructs were employed to generate retrovirus-producing cell lines (GP+E86) that stably express individual Bcl10 deletion mutants. Given the fact that overexpression of wild-type Bcl10 molecules in fibroblasts results in Bcl10 phosphorylation, as detected by Western blot analysis (9, 22, 45), we reasoned that phosphorylation of Bcl10 mutants, if it occurs, could be detected when these mutants were overexpressed in GP+E86 cells. Thus, the phosphorylation status of individual Bcl10 truncation mutants expressed in GP+E86 cells was examined. Whereas truncation mutants that terminated before amino acid residue 130 migrated as a single isoform, the Bcl10 truncation mutant that terminated after amino acid residue 157 and full-length Bcl10 migrated as a fast and slow form (Fig. 2A and data not shown). Treatment of the cell lysates with λ-PPase caused the slow migrating form of wild-type Bcl10 and Bcl10(1-157) to migrate with the fast form (Fig. 2A). Therefore, when overexpressed in GP+E86 cells, truncation mutant Bcl10(1-130) failed to be phosphorylated, whereas Bcl10(1-157), like wild-type Bcl10, could be phosphorylated. These data indicate that the suspected phosphorylation site(s) within Bcl10 is between amino acid residues 130 and 157. To determine whether the phosphorylation site(s) within Bcl10 in T cells is also located between amino acid residues 130 and 157, we introduced Bcl10(1-130) and Bcl10(1-157) into Jurkat cells. As shown in Fig. 2B, Bcl10(1-157) displayed phosphorylated forms both before and after anti-CD3 plus anti-CD28 stimulation, whereas only one form of Bcl10(1-130) was observed. Therefore, amino acid residues between 130 and 157 of Bcl10 contain a phosphorylation site(s) in T cells.
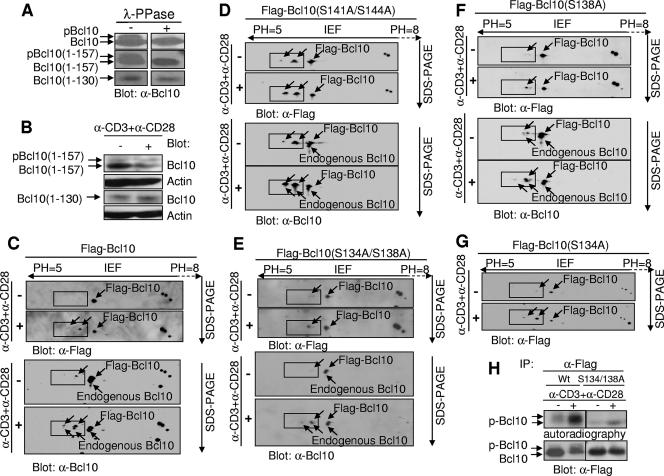
FIG. 2.
S138 is one of the activation-dependent phosphorylation sites. (A) Bcl10 expressed in GP+E86 cells contains a phosphorylation site(s) between residues 130 and 157. Cell lysates (20 μg) from the retrovirus packaging cells GP+E86 that express wild-type Bcl10, truncation mutant Bcl10(1-157), or truncation mutant Bcl10(1-130) were treated with (+) λ-PPase or were left untreated (−), followed by SDS-PAGE and Western blot analysis with anti-Bcl10 (α-Bcl10) antibodies. (B) Bcl10 expressed in Jurkat cells contains a phosphorylation site(s) between residues 130 and 157. Jurkat cells expressing Bcl10(1-157) or Bcl10(1-130) were untreated or were stimulated with α-CD3 plus α-CD28 for 15 min. The cells were then collected, and cell lysates were resolved on SDS-PAGE followed by Western blot analysis with the indicated antibodies. (C) Flag-tagged wild-type Bcl10 has two TCR-induced phosphorylated isoforms, the same as endogenous Bcl10. Jurkat cells expressing wild-type Flag-tagged Bcl10 (Flag-Bcl10) were untreated (−) or were stimulated (+) with α-CD3 plus α-CD28 for 15 min. Cell lysates were subjected to 2-D electrophoresis, followed by Western blot analysis, first with α-Flag antibodies and later with α-Bcl10 antibodies. (D) Replacement of S141/S144 with alanine does not affect the two TCR-induced phosphorylated isoforms of Bcl10. Jurkat cells expressing Flag-Bcl10(S141A/S144A) were treated and analyzed as described for panel C. (E) Replacement of S134/S138 with alanine abolishes one of the two TCR-induced phosphorylated isoforms of Bcl10. Jurkat cells expressing Flag-Bcl10(S134A/S138A) were treated and analyzed as described for panel C. (F) Replacement of S138 with alanine abolishes one of the two TCR-induced phosphorylated isoforms of Bcl10. Jurkat cells expressing Flag-Bcl10(S138A) were treated and analyzed as described for panel C. (G) Replacement of S134 with alanine does not affect the two TCR-induced phosphorylated isoforms of Bcl10. Jurkat cells expressing Flag-Bcl10(S134A) were treated and analyzed as described for panel C. (H) S138 is an activation-dependent phosphorylation site in Bcl10, as demonstrated by a [32P]orthophosphate labeling experiment. Jurkat cells expressing Flag-Bcl10 or Flag-Bcl10(S134A/S138A) were labeled with [32P]orthophosphate, followed by incubation in medium alone (−) or in the presence (+) of α-CD3 plus α-CD28 for 15 min. Cell lysates were immunoprecipitated (IP) with α-Bcl10 antibodies and then subjected to SDS-PAGE, followed by autoradiography or Western blot analysis with α-Bcl10 antibodies. (C to G) Arrows point to Bcl10 isoforms, and phosphorylated Bcl10 isoforms are enclosed by rectangles.
The region spanning amino acid residues 130 to 157 of mouse Bcl10 contains five serine residues, one threonine residue, and one tyrosine residue, each of which is a potential phosphorylation site. We excluded the possibility of phosphorylation on tyrosine by Western blot analysis using an anti-phosphotyrosine antibody (data not shown). To determine which of the remaining serine and threonine residues served as phosphorylation sites, two N-terminal Flag-tagged Bcl10 mutants with S134/S138 or S141/S144 replaced with alanine residues [Flag-Bcl10(S134A/S138A) and Flag-Bcl10(S141A/S144A)] were generated and introduced into Jurkat cells. As a control, N-terminal Flag-tagged wild-type Bcl10 (Flag-Bcl10) was also introduced into Jurkat cells. Following anti-CD3 plus anti-CD28 stimulation, biphosphorylated and monophosphorylated isoforms of Flag-tagged wild-type Bcl10 were observed on a 2-D gel, similar to that of endogenous Bcl10 (Fig. 2C). Whereas Flag-Bcl10(S141A/S144A) displayed a phosphorylation pattern similar to that of wild-type Bcl10 both before and after TCR stimulation (Fig. 2D), Flag-Bcl10(S134A/S138A) exhibited undetectable biphosphorylated and reduced monophosphorylated isoforms of Bcl10 following TCR activation (Fig. 2E). These data demonstrate that TCR engagement induces phosphorylation of one of the serine residues at S134/S138 and excludes S141/S144 as phosphorylation sites within Bcl10. To further discriminate between S134 and S138 as the actual activation-dependent phosphorylation site in Bcl10, we made Flag-Bcl10(S134A) and Flag-Bcl10(S138A) and introduced these mutants into Jurkat cells. Whereas replacement of S138 with alanine abolished one of the activation-dependent phosphorylation sites in Bcl10 (Fig. 2F), replacement of S134 with alanine did not affect the phosphorylation pattern of Bcl10 (Fig. 2G). In addition, [32P]orthophosphate labeling experiments also confirmed that replacement of S134/S138 with alanine residues abolished at least one of the activation-dependent phosphorylated forms of Bcl10 (Fig. 2H). Together, these data demonstrate that S138 of Bcl10 is an activation-dependent phosphorylation site.
Phosphorylation at S138 results in down-regulation of Bcl10 protein levels.
Down-regulation of Bcl10 protein levels following TCR engagement was observed previously (41). Consistent with the previous study, the protein levels of wild-type Flag-Bcl10 in Jurkat cells declined following stimulation with anti-CD3 plus anti-CD28 (Fig. 3A). Treatment of the cells with MG132, a proteasome inhibitor, only slightly affected the reduction of Bcl10 protein, although the degradation of phosphorylated IκBα was substantially blocked (Fig. 3B). These data demonstrated that Bcl10 degradation is not primarily dependent on proteasome, consistent with a previous study (41). In contrast to wild-type Bcl10, the protein levels of both Flag-Bcl10(S134A/S138A) and Flag-Bcl10(S138A) were only marginally reduced after cell activation by anti-CD3 plus anti-CD28 (Fig. 3C). Thus, TCR-induced reduction of Bcl10 protein was impaired by replacement of S134/S138 or S138 of Flag-Bcl10 with alanine residues, demonstrating that phosphorylation on S138 is the signal for Bcl10 degradation after cell activation.
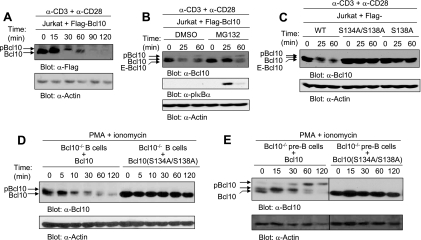
FIG. 3.
Replacement of S134/S138 or S138 alone with alanine impairs activation-induced degradation of Bcl10 in lymphocytes. (A) The Bcl10 protein level is down-regulated after TCR stimulation. Jurkat cells expressing Flag-Bcl10 were stimulated with anti-CD3 (α-CD3) plus α-CD28 for the indicated times. Cell lysates were subjected to SDS-PAGE and Western blot analysis with α-Flag or α-actin antibodies. (B) Bcl10 degradation is insensitive to proteasome inhibitor treatment. Jurkat cells expressing Flag-Bcl10 were left untreated or were treated with MG132 for 40 min. The cells were then stimulated with α-CD3 plus α-CD28 for the indicated times, followed by Western blot analysis with the indicated antibodies. (C) Replacement of S134/S138 or S138 alone with alanine impairs TCR-induced Bcl10 degradation in Jurkat cells. The cells were then stimulated with α-CD3 plus α-CD28 for the indicated times, followed by Western blot analysis with the indicated antibodies. (D) Replacement of S134/S138 with alanine impairs activation-induced Bcl10 degradation in primary splenic B cells. LPS-activated Bcl10-deficient splenic B cells were reconstituted with wild-type (WT) Bcl10 or Bcl10(S134A/S138A) by retrovirus transduction. Subsequently, the retrovirus-transduced GFP+ cells were sorted and stimulated with PMA plus ionomycin for the indicated times. Cell lysates were subjected to SDS-PAGE and Western blot analysis with α-Bcl10 or α-actin antibodies. (E) Replacement of S134/S138 with alanine impairs activation-induced Bcl10 degradation in primary large pre-B cells. BM culture-derived Bcl10-deficient large pre-B cells were reconstituted with wild-type Bcl10 or Bcl10(S134A/S138A) by retrovirus transduction. Subsequently, the retrovirus-transduced GFP+ cells were sorted and stimulated with PMA plus ionomycin for the indicated times. Cell lysates were subjected to SDS-PAGE and Western blot analysis with α-Bcl10 or α-actin antibodies. E-Bcl10, endogenous Bcl10. DMSO, dimethyl sulfoxide.
The impairment of activation-induced reduction of Flag-Bcl10(S134A/S138A) was also confirmed in primary B cells. First, Bcl10-deficient splenic B cells were induced to enter the cell cycle by treatment with LPS, after which they were reconstituted with wild-type Bcl10 or Bcl10(S134A/S138A) (without Flag tag) by transduction with a retrovirus to express the relevant form of Bcl10 along with GFP. Successfully reconstituted cells were sorted based on GFP expression and rested in LPS-free medium. Following stimulation with PMA plus ionomycin, the protein levels of wild-type Bcl10 decreased in activated splenic B cells, whereas levels of Bcl10(S134A/S138A) largely remained the same (Fig. 3D), a result consistent with what we had observed in Jurkat cells. Second, the BM-derived Bcl10-deficient large pre-B cells were reconstituted with wild-type Bcl10 or Bcl10(S134A/S138A) by retrovirus transduction. Consistent with our observations in splenic B cells, protein levels of wild-type Bcl10 declined, whereas the protein levels of Bcl10(S134A/S138A) remained constant following activation by PMA plus ionomycin (Fig. 3D). Of note, wild-type Bcl10 overexpressed in the large pre-B cells displayed two phosphorylated forms on SDS-PAGE, reminiscent of what has been previously observed in fibroblasts and, occasionally, activated T cells (9, 13, 22, 40, 45, 53). Mutation of S134/S138 to alanine abolished at least the slowest-migrating phosphorylated form of Bcl10 in stimulated large pre-B cells and apparently blocked the degradation of Bcl10 following stimulation as well (Fig. 3E). Taken together, these data further support the notion that phosphorylation at S138 is the signal for Bcl10 degradation after cell activation.
Phosphorylation of Bcl10 at S138 induces Bcl10 ubiquitination.
It has been shown in Jurkat cells that ubiquitination of Bcl10 precedes its activation-induced degradation (18, 41). If phosphorylation of Bcl10 at S138 signals degradation of Bcl10, it ought to be the cue that induces the ubiquitination of Bcl10. Thus, we investigated the effect of replacing S134/S138 or S138 alone with alanine residues on activation-induced ubiquitination of Bcl10.
We first examined ubiquitination of endogenous Bcl10 following T-cell activation. Bcl10 was immunoprecipitated from duplicate sets of Jurkat cells, which had been stimulated with PMA plus ionomycin in the presence of N-ethylmaleimide, an inhibitor of deubiquitination, for the indicated times. The immunoprecipitates were resolved in parallel by SDS-PAGE. Subsequently, one set of samples was subjected to Western blot analysis with antiubiquitin antibodies, whereas the other set was subjected to Western blot analysis with anti-Bcl10 antibodies. We observed that total ubiquitinated proteins, including Bcl10-associated ubiquitinated proteins as well as ubiquitinated Bcl10 (Fig. 4 A, left panel), and higher-molecular-weight Bcl10, specifically representing ubiquitinated Bcl10 (Fig. 4A, right panel), were both detectable after cell stimulation. These data demonstrated that endogenous Bcl10 was ubiquitinated following activation of Jurkat cells (Fig. 4A). Studies of Jurkat cells expressing Flag-Bcl10 displayed that Flag-Bcl10 was also ubiquitinated following stimulation with anti-CD3 plus anti-CD28 (Fig. 4B). The anti-Flag antibody was not able to immunoprecipitate ubiquitinated proteins from parental Jurkat cells, excluding the possibility that the detected ubiquitination signals were derived from proteins nonspecifically associated with the anti-Flag antibody (Fig. 4B). Next, we compared the activation-induced ubiquitination levels of Flag-tagged wild-type Bcl10, Bcl10(S134A/S138A), and Bcl10(S138A) in Jurkat cells. It is possible that the ubiquitination signal observed in Fig. 4A and B could be derived from Bcl10-associated proteins that are coimmunoprecipitated with Bcl10 by the anti-Flag antibody. To exclude this possibility, in comparing the ubiquitination levels of the wild-type and the mutant Bcl10, we lysed the cells in the presence of 1% SDS and boiled the cell lysates to disrupt protein-protein interactions. The cell lysates were then diluted 10-fold with the lysis buffer without SDS followed by immunoprecipitation and Western analysis with antiubiquitin antibody. Consistent with the earlier results, we could still observe significant amounts of ubiquitination signals of wild-type Bcl10. Importantly, Bcl10(S134A/S138A) and Bcl10(S138A) displayed highly reduced levels of ubiquitination signals compared to wild-type Bcl10 (Fig. 4C). These data demonstrate that obstruction of phosphorylation at S138 prevents activation-induced ubiquitination of Bcl10. Therefore, phosphorylation of Bcl10 at S138 signals ubiquitination of Bcl10 for its subsequent degradation following TCR activation.
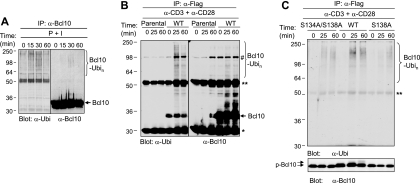
FIG. 4.
Replacement of S134/S138 or S138 alone with alanine impairs activation-induced ubiquitination of Bcl10 in Jurkat cells. (A) Endogenous Bcl10 is ubiquitinated following activation in Jurkat cells. Jurkat cells were stimulated with PMA plus ionomycin (P + I) for the indicated times. Duplicate cell samples were collected, and cell lysates were immunoprecipitated (IP) with anti-Bcl10 (α-Bcl10) antibody (sc-9560). Subsequently, one set of samples was subjected to Western blot analysis with α-ubiquitin (α-Ubi) (sc-8017) antibody, whereas the other set was subjected to Western blot analysis with α-Bcl10 (sc-5273) antibody. (B). Anti-Flag antibody does not nonspecifically associate with ubiquitinated Bcl10. Parental Jurkat cells or Jurkat cells expressing Flag-Bcl10 were stimulated with α-CD3 plus α-CD28 for the indicated times. Duplicate cell samples were collected, and cell lysates were immunoprecipitated with α-Flag antibodies. Subsequently, one set of samples was subjected to Western blot analysis with α-ubiquitin antibody (sc-8017), whereas the other set was subjected to Western blot analysis with α-Bcl10 antibodies. (C) Replacement of S134/S138 or S138 with alanine directly reduces ubiquitination of Bcl10 but not Bcl10-associated protein. Jurkat cells expressing Flag-Bcl10, Flag-Bcl10(S134A/S138A), or Flag-Bcl10(S138A) were treated with 0.4 μM MG132 for 1 h and then were stimulated with α-CD3 plus α-CD28 for the indicated times. The samples were collected and lysed in the presence of 1% SDS and boiled for 5 min. The cell lysates were diluted 10-fold with the lysis buffer in the absence of SDS before being immunoprecipitated with α-Flag antibodies. Subsequently, the immunoprecipitates were subjected to Western blot analysis with α-ubiquitin antibodies (a gift from Arthur L. Hass). The data shown are representative of two independent experiments. *, immunoglobulin light chain; **, immunoglobulin heavy chain; Ubin, polyubiquitin; #, nonspecific band; WT, wild type.
Phosphorylation of Bcl10 at S138 negatively regulates TCR-mediated NF-κB activation and IL-2 production.
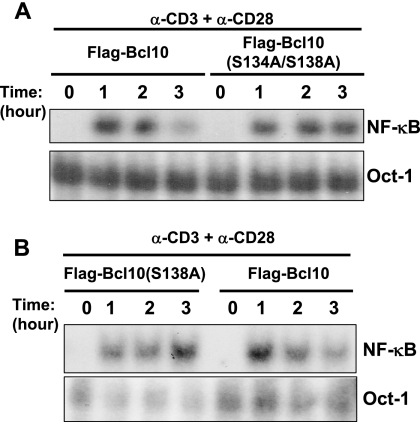
Down-regulation of Bcl10 protein levels following TCR engagement could be one important way to properly turn off T-cell activation (41). Given the fact that Bcl10 is essential for TCR-mediated NF-κB activation and IL-2 production (36, 60), we investigated the role of phosphorylation of Bcl10 at S138 in TCR-mediated signaling and function. We first stimulated Jurkat cells expressing Flag-Bcl10 or Flag-Bcl10(S134A/S138A) with anti-CD3 plus anti-CD28 and then examined the kinetics of NF-κB activation in these cells by gel shift analysis. TCR-induced activation of NF-κB was high initially but was dramatically reduced by 3 h after TCR engagement in Jurkat cells expressing wild-type Flag-Bcl10 (Fig. 5A). In contrast, NF-κB activation remained high throughout the 3-h experimental period in Jurkat cells expressing Flag-Bcl10(S134A/S138A). Consistently, Jurkat cells expressing Flag-Bcl10(S138A) also displayed prolonged NF-κB activation compared to those expressing wild-type Flag-Bcl10 (Fig. 5B). These data demonstrate that expression of Bcl10(S134A/S138A) or Bcl10(S138A) in Jurkat cells leads to prolonged NF-κB activation following TCR engagement. We conclude from these observations that phosphorylation of Bcl10 at S138 normally negatively regulates TCR-induced NF-κB activation.
FIG. 5.
Replacement of S134/S138 or S138 alone with alanine prolongs TCR-induced NF-κB activation. Jurkat cells expressing Flag-Bcl10, Flag-Bcl10(S134A/S138A) (A), or Flag-Bcl10(S138A) (B) were stimulated with anti-CD3 (α-CD3) plus α-CD28 for the indicated times. Subsequently, the cells were subjected to gel shift assays for NF-κB activity with the 32P-labeled oligonucleotide containing the NF-κB binding site in the IL-2 gene promoter as a probe. Gel shift for Oct1 binding was used as a loading control. The data shown are representative of at least two independent experiments.
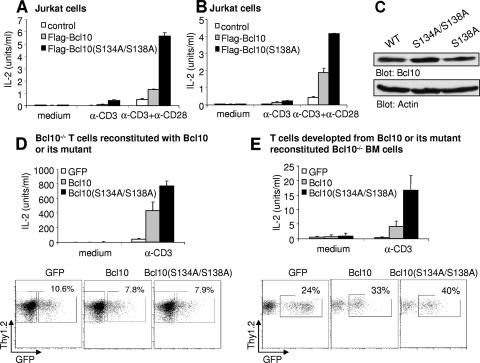
IL-2 production is the key biological outcome of T-cell activation and is dependent on Bcl10 and NF-κB activation (36). We thus examined IL-2 production in Jurkat cells expressing wild-type Flag-Bcl10, Flag-Bcl10(S134A/S138A), or Flag-Bcl10(S138A) after stimulation with anti-CD3 or anti-CD3 plus anti-CD28. Consistent with prolonged NF-κB activation, IL-2 production was also enhanced in Jurkat cells expressing Flag-Bcl10(S134A/S138A) or Flag-Bcl10(S138A) relative to control parental Jurkat cells or those expressing wild-type Flag-Bcl10 (Fig. 6A and B). This is not due to differences in protein expression levels, for Flag-Bcl10(S134A/S138A) and Flag-Bcl10(S138A) were expressed at levels similar to those of Flag-Bcl10 (Fig. 6C). We also examined the effect of a serine-to-alanine alteration at S134/S138 within Bcl10 on IL-2 production in primary T cells. Bcl10-deficient T cells were induced to enter the cell cycle by stimulation with PMA plus ionomycin and subsequently reconstituted with GFP alone, wild-type Bcl10/GFP, or Bcl10(S134A/S138A)/GFP by transduction with MSCV-IRES-GFP retroviruses expressing corresponding Bcl10 constructs. Successfully reconstituted cells were sorted by FACS based on GFP expression and were rested. Following restimulation with anti-CD3, IL-2 production from these cells was measured. As expected, upon TCR activation, wild-type Bcl10 restored IL-2 production in Bcl10-deficient primary T cells, whereas GFP-transduced Bcl10-deficient primary T cells barely produced any IL-2 (Fig. 6D, upper panel). Interestingly, Bcl10(S134A/S138A) expression further markedly enhanced TCR-induced IL-2 production relative to wild-type Bcl10 (Fig. 6D, upper panel). This result was not due to higher levels of expression of Bcl10(S134A/S138A) relative to Bcl10, since the GFP fluorescence intensities were equivalent in Bcl10-deficient cells reconstituted with Bcl10(S134A/S138A) or Bcl10, indicating that these two proteins were expressed at similar levels (Fig. 6D, lower panel). Moreover, we confirmed the enhancement of TCR-induced IL-2 production by replacing S134/S138 with alanine within Bcl10 in a more physiologically relevant setting. Bcl10-deficient BM cells were transduced with MSCV-IRES-GFP, MSCV-Bcl10-IRES-GFP, or MSCV-Bcl10(S134A/S138A)-IRES-GFP retroviruses and subsequently transplanted into T-cell-deficient Jak3−/− recipient mice as previously described (56-58). Two months after BM transplantation, the repopulated GFP+ Thy1.2+ T cells were FACS sorted from recipient splenocytes and then stimulated with anti-CD3. IL-2 secretion from these cells was measured. Again, reconstitution of wild-type Bcl10 in Bcl10-deficient primary T cells in vivo restored TCR-mediated IL-2 production, whereas in vivo GFP-transduced Bcl10-deficient primary T cells failed to produce IL-2 (Fig. 6E, upper panel). Importantly, reconstitution of Bcl10(S134A/S138A) in Bcl10-deficient primary T cells in vivo dramatically enhanced TCR-induced IL-2 production relative to that of wild-type Bcl10 (Fig. 6E, upper panel). This result was not due to higher levels of expression of Bcl10(S134A/S138A) relative to Bcl10, since the GFP fluorescence intensities in cells reconstituted with Bcl10(S134A/S138A) or wild-type Bcl10 were not significantly different, suggesting that these proteins were expressed at similar levels in the reconstituted T cells (Fig. 6E, lower panel). Taken together, these data demonstrate that failure of S138 within Bcl10 to become phosphorylated prolongs TCR-mediated NF-κB activation in Jurkat cells and enhances TCR-induced IL-2 production in Jurkat cells and primary T cells. Therefore, phosphorylation of Bcl10 at S138 negatively regulates TCR-mediated NF-κB activation and subsequent IL-2 production.
FIG. 6.
Replacement of S134/S138 or S138 alone with alanine enhances TCR-induced IL-2 production. (A) Replacement of S134/S138 with alanine enhances TCR-induced IL-2 production in Jurkat cells. Jurkat cells expressing Flag-Bcl10 or Flag-Bcl10(S134A/S138A) were stimulated in 96-well plates with anti-CD3 (α-CD3) or α-CD3 plus α-CD28, and then cell supernatants were harvested and subjected to assays for IL-2 biological activity. (B) Replacement of S138 with alanine enhances TCR-induced IL-2 production in Jurkat cells. Jurkat cells expressing Flag-Bcl10 or Flag-Bcl10(S138A) were stimulated, and IL-2 production was examined as described for panel A. (C) Flag-Bcl10, Flag-Bcl10(S134A/S138A), and Flag-Bcl10(S138A) are expressed at equivalent levels in Jurkat cells. (D) Replacement of S134/S138 with alanine enhances TCR-induced IL-2 production in splenic T cells. Bcl10-deficient splenic T cells were reconstituted with wild-type Bcl10 or Bcl10(S134A/S138A) by retrovirus transduction. Subsequently, the retrovirus-transduced GFP+ cells were sorted and rested overnight. Sorted cells (105) were stimulated in 96-well plates with α-CD3 for 18 h, and the supernatants were harvested and subjected to assays for IL-2 biological activity. The bottom panel shows the FACS analysis of GFP fluorescence intensities in T cells reconstituted with wild-type Bcl10 or Bcl10(S134A/S138A). (E) Replacement of S134/S138 with alanine enhances TCR-induced IL-2 production by T cells in a BM transplantation system. GFP+ Thy1.2+ T cells were sorted from the splenocytes derived from the recipients of wild-type BM transduced with retrovirus expressing Bcl10 or Bcl10(S134A/S138A). Following stimulation of these T cells in 96-well plates with α-CD3, the supernatants were harvested and subjected to assays for IL-2 biological activity. The bottom panel shows the FACS analysis of GFP fluorescence intensities in T cells reconstituted with wild-type Bcl10 or Bcl10(S134A/S138A). Bars represent mean induction and standard deviation of triplicate samples within an experiment. Data presented in panels A to E are representative of at least two independent experiments. WT, wild type.
DISCUSSION
Down-regulation of TCR signaling following engagement of the TCR is an important way to terminate sustained signaling in activated T cells, and it could be responsible for unresponsiveness and/or tolerance induction of self-reactive T cells (31, 42). Degradation of the TCR/CD3 complex, and particularly the TCRζ chain, has been demonstrated to be one mechanism by which activated T cells are switched off (3, 39, 51, 52). In addition, ubiquitin-mediated degradation of signaling molecules, including the kinase ZAP70 (32), the kinase Lck (33), the guanine nucleotide exchange factor Vav (29), the lipase PLCγ1, and the kinase PKCθ (16), have been demonstrated to be involved in turning off TCR signaling. Recent studies have discovered reduction of Bcl10 following T-cell activation (18, 41), which could be an additional important mechanism by which TCR signaling is negatively regulated. In the current report, we found that T-cell activation induced monophosphorylation and biphosphorylation of Bcl10 and that S138 of Bcl10 is one of the TCR-induced phosphorylation sites. Replacement of S138 alone or S134/S138 within Bcl10 with alanine prevented the TCR-induced ubiquitination of Bcl10 and its subsequent down-regulation, prolonged TCR-mediated NF-κB activation, and enhanced IL-2 production. Our studies provide a critical link between Bcl10 phosphorylation and down-regulation of TCR signal transduction.
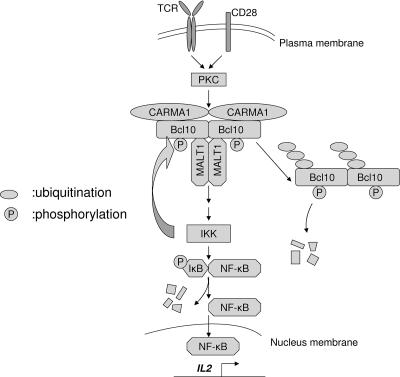
A recent study by Wegener and colleagues found that IKKβ catalyzes phosphorylation of C-terminal Bcl10, including S134/S138 (55). It is well known that IKK functions downstream of CBM in connecting PKCθ to activation of NF-κB in T cells and is required for positive activation of NF-κB (48). If IKK is also involved in Bcl10 phosphorylation, then, via functioning downstream and upstream of Bcl10, IKK can both positively and negatively control TCR-induced NF-κB activation and serve as a negative feedback modulator that keeps T-cell activation in check. Accordingly, we propose the following model: upon TCR engagement, CARMA1/Bcl10/Malt1 forms a ternary complex in the lipid raft, leading to IKK and consequent NF-κB activation. In the meantime, phosphorylation of Bcl10 at S138, possibly by IKK, leads to ubiquitination and subsequent degradation of Bcl10 and attenuation of NF-κB activation (Fig. 7).
FIG. 7.
Schematic illustration of the role of Bcl10 phosphorylation in its degradation and NF-κB activation. Engagement of TCR and CD28 results in the recruitment of IKK to the CARMA1/Bcl10/MALT complex in the lipid rafts and subsequent activation of IKK. Activated IKK phosphorylates IκB and consequently leads to NF-κB activation. In the meantime, Bcl10 is phosphorylated at S138, likely by IKK, resulting in ubiquitination and degradation of Bcl10. Reduction of Bcl10 protein causes attenuation of NF-κB activation.
The study by Wegener and colleagues identified IKKβ as the kinase for phosphorylation of the Bcl10 C terminus but did not link C-terminal Bcl10 phosphorylation to Bcl10 degradation (55). The Bcl10 mutant with the five C-terminal serine residues, including S134/S138, replaced with alanine residues undergoes TCR-induced degradation similar to wild-type Bcl10. Although the reason for the discrepancy between this study and ours is not clear, the difference may stem from mutating five serine residues of human Bcl10 in the study by Wegener et al. versus mutating two or one serine residue of mouse Bcl10 in our study. The five serine residues mutated in the studies by Wegener et al. are S134, S136, S138, S141, and S144 within human Bcl10. Mouse Bcl10 contains all of these serine residues except S136. Our 2-D electrophoresis analyses have clearly demonstrated that only S138 is the activation-dependent phosphorylation site, whereas S134, S141, and S144 within Bcl10 are not likely to be the phosphorylation sites. It is possible that the property may be further changed by replacement of an additional four serine residues on top of S138 in human Bcl10.
Our study demonstrated that activation-dependent phosphorylation at S138 initiates Bcl10 ubiquitination and subsequent degradation. Bcl10 degradation is only slightly inhibited by MG132, suggesting that the proteasome-independent mechanism may be involved in Bcl10 degradation. Our data are consistent with a previous study indicating that ubiquitination probably targets Bcl10 to traffic to the lysosome before degradation (41). Previous studies have identified Nedd4, Itch, and cIAP2 to be the E3 ligase for Bcl10 (18, 41). In one of the studies, a Bcl10 truncation mutant with only the N-terminal 1 to 116 amino acids of Bcl10 was still degraded following cell stimulation (41). However, it was not shown whether this is due to ubiquitination or to truncation-mediated Bcl10 instability. Nevertheless, given that 12 out of 14 lysine residues in the Bcl10 protein are located in the N terminus of residue 116, it is possible that the ubiquitination site is located N terminally of residue 116. Thus, phosphorylation on S138 may regulate the accessibility of Bcl10 E3 ligase to the lysine to be ubiquitinated on Bcl10 or the accessibility of the E3 binding site on Bcl10. These possibilities merit further investigation.
Increased Bcl10 protein levels have been observed in all three types of chromosomal translocation involved in MALT lymphoma development: t(1;14)(q22;q32) (59, 64), t(14;18)(q32;q21) (62), and t(11;18)(q21;q21) (18, 26, 62, 63). This suggests that increased levels of Bcl10 protein may play an important role in the pathogenesis of MALT lymphoma. In addition, Bcl10 mutation is also frequently observed in MALT lymphoma samples (59, 64). Thus, increased protein levels following inhibition of Bcl10 phosphorylation due to mutation at the phosphorylation site(s) may be pathologically relevant in MALT lymphoma development.
Acknowledgments
We thank Debra K. Newman and Lester W. Cashdollar for critical reading of the manuscript and Mary G. Holyst for excellent technical support. We also thank Arthur L. Hass (Louisiana State University Health Sciences Center School of Medicine) for the antiubiquitin antibody.
This work was supported in part by Public Health Service grant AI52327 (R.W.).
Footnotes
Published ahead of print on 14 May 2007.
REFERENCES
- 1.Baldwin, A. S., Jr. 1996. The NF-κ B and I κ B proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 2.Cannons, J. L., L. J. Yu, B. Hill, L. A. Mijares, D. Dombroski, K. E. Nichols, A. Antonellis, G. A. Koretzky, K. Gardner, and P. L. Schwartzberg. 2004. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-κB1. Immunity 21:693-706. [DOI] [PubMed] [Google Scholar]
- 3.Cenciarelli, C., D. Hou, K. C. Hsu, B. L. Rellahan, D. L. Wiest, H. T. Smith, V. A. Fried, and A. M. Weissman. 1992. Activation-induced ubiquitination of the T cell antigen receptor. Science 257:795-797. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Y., X. Dai, A. L. Haas, R. Wen, and D. Wang. 2006. Proteasome-dependent down-regulation of activated Stat5A in the nucleus. Blood 108:566-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements, J. L., N. J. Boerth, J. R. Lee, and G. A. Koretzky. 1999. Integration of T cell receptor-dependent signaling pathways by adapter proteins. Annu. Rev. Immunol. 17:89-108. [DOI] [PubMed] [Google Scholar]
- 6.Costanzo, A., C. Guiet, and P. Vito. 1999. c-E10 is a caspase-recruiting domain-containing protein that interacts with components of death receptors signaling pathway and activates nuclear factor-κB. J. Biol. Chem. 274:20127-20132. [DOI] [PubMed] [Google Scholar]
- 7.Egawa, T., B. Albrecht, B. Favier, M. J. Sunshine, K. Mirchandani, W. O'Brien, M. Thome, and D. R. Littman. 2003. Requirement for CARMA1 in antigen receptor-induced NF-κ B activation and lymphocyte proliferation. Curr. Biol. 13:1252-1258. [DOI] [PubMed] [Google Scholar]
- 8.Gaide, O., B. Favier, D. F. Legler, D. Bonnet, B. Brissoni, S. Valitutti, C. Bron, J. Tschopp, and M. Thome. 2002. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-κ B activation. Nat. Immunol. 3:836-843. [DOI] [PubMed] [Google Scholar]
- 9.Gaide, O., F. Martinon, O. Micheau, D. Bonnet, M. Thome, and J. Tschopp. 2001. Carma1, a CARD-containing binding partner of Bcl10, induces Bcl10 phosphorylation and NF-κB activation. FEBS Lett. 496:121-127. [DOI] [PubMed] [Google Scholar]
- 10.Gerondakis, S., R. Grumont, I. Rourke, and M. Grossmann. 1998. The regulation and roles of Rel/NF-κ B transcription factors during lymphocyte activation. Curr. Opin. Immunol. 10:353-359. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109(Suppl.):S81-S96. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κ B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 13.Hara, H., C. Bakal, T. Wada, D. Bouchard, R. Rottapel, T. Saito, and J. M. Penninger. 2004. The molecular adapter Carma1 controls entry of IκB kinase into the central immune synapse. J. Exp. Med. 200:1167-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara, H., T. Wada, C. Bakal, I. Kozieradzki, S. Suzuki, N. Suzuki, M. Nghiem, E. K. Griffiths, C. Krawczyk, B. Bauer, F. D'Acquisto, S. Ghosh, W. C. Yeh, G. Baier, R. Rottapel, and J. M. Penninger. 2003. The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity 18:763-775. [DOI] [PubMed] [Google Scholar]
- 15.Hawley, R. G., A. Z. Fong, B. F. Burns, and T. S. Hawley. 1992. Transplantable myeloproliferative disease induced in mice by an interleukin 6 retrovirus. J. Exp. Med. 176:1149-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heissmeyer, V., F. Macian, S.-H. Im, R. Varma, S. Feske, K. Venuprasad, H. Gu, Y.-C. Liu, M. L. Dustin, and A. Rao. 2004. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 5:255-265. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz, B. H., M. L. Scott, S. R. Cherry, R. T. Bronson, and D. Baltimore. 1997. Failure of lymphopoiesis after adoptive transfer of NF-κB-deficient fetal liver cells. Immunity 6:765-772. [DOI] [PubMed] [Google Scholar]
- 18.Hu, S., M. Q. Du, S. M. Park, A. Alcivar, L. Qu, S. Gupta, J. Tang, M. Baens, H. Ye, T. H. Lee, P. Marynen, J. L. Riley, and X. Yang. 2006. cIAP2 is a ubiquitin protein ligase for BCL10 and is dysregulated in mucosa-associated lymphoid tissue lymphomas. J. Clin. Investig. 116:174-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jun, J. E., L. E. Wilson, C. G. Vinuesa, S. Lesage, M. Blery, L. A. Miosge, M. C. Cook, E. M. Kucharska, H. Hara, J. M. Penninger, H. Domashenz, N. A. Hong, R. J. Glynne, K. A. Nelms, and C. C. Goodnow. 2003. Identifying the MAGUK protein Carma-1 as a central regulator of humoral immune responses and atopy by genome-wide mouse mutagenesis. Immunity 18:751-762. [DOI] [PubMed] [Google Scholar]
- 20.Kane, L. P., and A. Weiss. 2003. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol. Rev. 192:7-20. [DOI] [PubMed] [Google Scholar]
- 21.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-[κ]B activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 22.Koseki, T., N. Inohara, S. Chen, R. Carrio, J. Merino, M. O. Hottiger, G. J. Nabel, and G. Nunez. 1999. CIPER, a novel NF κB-activating protein containing a caspase recruitment domain with homology to herpesvirus-2 protein E10. J. Biol. Chem. 274:9955-9961. [DOI] [PubMed] [Google Scholar]
- 23.Li, Q., and I. M. Verma. 2002. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2:725-734. [DOI] [PubMed] [Google Scholar]
- 24.Lin, X., and D. Wang. 2004. The roles of CARMA1, Bcl10, and MALT1 in antigen receptor signaling. Semin. Immunol. 16:429-435. [DOI] [PubMed] [Google Scholar]
- 25.Lucas, P. C., M. Yonezumi, N. Inohara, L. M. McAllister-Lucas, M. E. Abazeed, F. F. Chen, S. Yamaoka, M. Seto, and G. Nunez. 2001. Bcl10 and MALT1, independent targets of chromosomal translocation in malt lymphoma, cooperate in a novel NF-κ B signaling pathway. J. Biol. Chem. 276:19012-19019. [DOI] [PubMed] [Google Scholar]
- 26.Maes, B., A. Demunter, B. Peeters, and C. De Wolf-Peeters. 2002. BCL10 mutation does not represent an important pathogenic mechanism in gastric MALT-type lymphoma, and the presence of the API2-MLT fusion is associated with aberrant nuclear BCL10 expression. Blood 99:1398-1404. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto, R., D. Wang, M. Blonska, H. Li, M. Kobayashi, B. Pappu, Y. Chen, D. Wang, and X. Lin. 2005. Phosphorylation of CARMA1 plays a critical role in T cell receptor-mediated NF-κB activation. Immunity 23:575-585. [DOI] [PubMed] [Google Scholar]
- 28.McAllister-Lucas, L. M., N. Inohara, P. C. Lucas, J. Ruland, A. Benito, Q. Li, S. Chen, F. F. Chen, S. Yamaoka, I. M. Verma, T. W. Mak, and G. Nunez. 2001. Bimp1, a MAGUK family member linking protein kinase C activation to Bcl10-mediated NF-κB induction. J. Biol. Chem. 276:30589-30597. [DOI] [PubMed] [Google Scholar]
- 29.Miura-Shimura, Y., L. Duan, N. L. Rao, A. L. Reddi, H. Shimura, R. Rottapel, B. J. Druker, A. Tsygankov, V. Band, and H. Band. 2003. Cbl-mediated ubiquitinylation and negative regulation of Vav. J. Biol. Chem. 278:38495-38504. [DOI] [PubMed] [Google Scholar]
- 30.Newton, K., and V. M. Dixit. 2003. Mice lacking the CARD of CARMA1 exhibit defective B lymphocyte development and impaired proliferation of their B and T lymphocytes. Curr. Biol. 13:1247-1251. [DOI] [PubMed] [Google Scholar]
- 31.Pantaleo, G., D. Olive, A. Poggi, T. Pozzan, L. Moretta, and A. Moretta. 1987. Antibody-induced modulation of the CD3/T cell receptor complex causes T cell refractoriness by inhibiting the early metabolic steps involved in T cell activation. J. Exp. Med. 166:619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao, N., M. L. Lupher, Jr., S. Ota, K. A. Reedquist, B. J. Druker, and H. Band. 2000. The linker phosphorylation site Tyr292 mediates the negative regulatory effect of Cbl on ZAP-70 in T cells. J. Immunol. 164:4616-4626. [DOI] [PubMed] [Google Scholar]
- 33.Rao, N., S. Miyake, A. L. Reddi, P. Douillard, A. K. Ghosh, I. L. Dodge, P. Zhou, N. D. Fernandes, and H. Band. 2002. Negative regulation of Lck by Cbl ubiquitin ligase. Proc. Natl. Acad. Sci. USA 99:3794-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudd, C. E. 1999. Adaptors and molecular scaffolds in immune cell signaling. Cell 96:5-8. [DOI] [PubMed] [Google Scholar]
- 35.Ruefli-Brasse, A. A., D. M. French, and V. M. Dixit. 2003. Regulation of NF-κB-dependent lymphocyte activation and development by paracaspase. Science 302:1581-1584. [DOI] [PubMed] [Google Scholar]
- 36.Ruland, J., G. S. Duncan, A. Elia, I. del Barco Barrantes, L. Nguyen, S. Plyte, D. G. Millar, D. Bouchard, A. Wakeham, P. S. Ohashi, and T. W. Mak. 2001. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell 104:33-42. [DOI] [PubMed] [Google Scholar]
- 37.Ruland, J., G. S. Duncan, A. Wakeham, and T. W. Mak. 2003. Differential requirement for Malt1 in T and B cell antigen receptor signaling. Immunity 19:749-758. [DOI] [PubMed] [Google Scholar]
- 38.Saijo, K., I. Mecklenbrauker, A. Santana, M. Leitger, C. Schmedt, and A. Tarakhovsky. 2002. Protein kinase C beta controls nuclear factor κB activation in B cells through selective regulation of the IκB kinase alpha. J. Exp. Med. 195:1647-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.San Jose, E., A. Borroto, F. Niedergang, A. Alcover, and B. Alarcon. 2000. Triggering the TCR complex causes the downregulation of nonengaged receptors by a signal transduction-dependent mechanism. Immunity 12:161-170. [DOI] [PubMed] [Google Scholar]
- 40.Schaefer, B. C., J. W. Kappler, A. Kupfer, and P. Marrack. 2004. Complex and dynamic redistribution of NF-κB signaling intermediates in response to T cell receptor stimulation. Proc. Natl. Acad. Sci. USA 101:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scharschmidt, E., E. Wegener, V. Heissmeyer, A. Rao, and D. Krappmann. 2004. Degradation of Bcl10 induced by T-cell activation negatively regulates NF-κB signaling. Mol. Cell. Biol. 24:3860-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schonrich, G., U. Kalinke, F. Momburg, M. Malissen, A. M. Schmitt-Verhulst, B. Malissen, G. J. Hammerling, and B. Arnold. 1991. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell 65:293-304. [DOI] [PubMed] [Google Scholar]
- 43.Siebenlist, U., K. Brown, and E. Claudio. 2005. Control of lymphocyte development by nuclear factor-κB. Nat. Rev. Immunol. 5:435-445. [DOI] [PubMed] [Google Scholar]
- 44.Sommer, K., B. Guo, J. L. Pomerantz, A. D. Bandaranayake, M. E. Moreno-Garcia, Y. L. Ovechkina, and D. J. Rawlings. 2005. Phosphorylation of the CARMA1 linker controls NF-κB activation. Immunity 23:561-574. [DOI] [PubMed] [Google Scholar]
- 45.Srinivasula, S. M., M. Ahmad, J. H. Lin, J. L. Poyet, T. Fernandes-Alnemri, P. N. Tsichlis, and E. S. Alnemri. 1999. CLAP, a novel caspase recruitment domain-containing protein in the tumor necrosis factor receptor pathway, regulates NF-κB activation and apoptosis. J. Biol. Chem. 274:17946-17954. [DOI] [PubMed] [Google Scholar]
- 46.Su, T. T., B. Guo, Y. Kawakami, K. Sommer, K. Chae, L. A. Humphries, R. M. Kato, S. Kang, L. Patrone, R. Wall, M. Teitell, M. Leitges, T. Kawakami, and D. J. Rawlings. 2002. PKC-beta controls IκB kinase lipid raft recruitment and activation in response to BCR signaling. Nat. Immunol. 3:780-786. [DOI] [PubMed] [Google Scholar]
- 47.Sun, Z., C. W. Arendt, W. Ellmeier, E. M. Schaeffer, M. J. Sunshine, L. Gandhi, J. Annes, D. Petrzilka, A. Kupfer, P. L. Schwartzberg, and D. R. Littman. 2000. PKC-theta is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature 404:402-407. [DOI] [PubMed] [Google Scholar]
- 48.Thome, M. 2004. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat. Rev. Immunol. 4:348-359. [DOI] [PubMed] [Google Scholar]
- 49.Thome, M., F. Martinon, K. Hofmann, V. Rubio, V. Steiner, P. Schneider, C. Mattmann, and J. Tschopp. 1999. Equine herpesvirus-2 E10 gene product, but not its cellular homologue, activates NF-κB transcription factor and c-Jun N-terminal kinase. J. Biol. Chem. 274:9962-9968. [DOI] [PubMed] [Google Scholar]
- 50.Uren, A. G., K. O'Rourke, L. A. Aravind, M. T. Pisabarro, S. Seshagiri, E. V. Koonin, and V. M. Dixit. 2000. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol. Cell 6:961-967. [DOI] [PubMed] [Google Scholar]
- 51.Valitutti, S., S. Muller, M. Salio, and A. Lanzavecchia. 1997. Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J. Exp. Med. 185:1859-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viola, A., and A. Lanzavecchia. 1996. T cell activation determined by T cell receptor number and tunable thresholds. Science 273:104-106. [DOI] [PubMed] [Google Scholar]
- 53.Wang, D., R. Matsumoto, Y. You, T. Che, X. Y. Lin, S. L. Gaffen, and X. Lin. 2004. CD3/CD28 costimulation-induced NF-κB activation is mediated by recruitment of protein kinase C-theta, Bcl10, and IκB kinase beta to the immunological synapse through CARMA1. Mol. Cell. Biol. 24:164-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, D., Y. You, S. M. Case, L. M. McAllister-Lucas, L. Wang, P. S. DiStefano, G. Nunez, J. Bertin, and X. Lin. 2002. A requirement for CARMA1 in TCR-induced NF-κ B activation. Nat. Immunol. 3:830-835. [DOI] [PubMed] [Google Scholar]
- 55.Wegener, E., A. Oeckinghaus, N. Papadopoulou, L. Lavitas, M. Schmidt-Supprian, U. Ferch, T. W. Mak, J. Ruland, V. Heissmeyer, and D. Krappmann. 2006. Essential Role for IκB kinase beta in remodeling Carma1-Bcl10-Malt1 complexes upon T cell activation. Mol. Cell 23:13-23. [DOI] [PubMed] [Google Scholar]
- 56.Wen, R., Y. Chen, J. Schuman, G. Fu, S. Yang, W. Zhang, D. K. Newman, and D. Wang. 2004. An important role of phospholipase Cγ1 in pre-B-cell development and allelic exclusion. EMBO J. 23:4007-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen, R., Y. Chen, L. Xue, J. Schuman, S. Yang, S. W. Morris, and D. Wang. 2003. Phospholipase Cγ2 provides survival signals via Bcl2 and A1 in different subpopulations of B cells. J. Biol. Chem. 278:43654-43662. [DOI] [PubMed] [Google Scholar]
- 58.Wen, R., D. Wang, C. McKay, K. D. Bunting, J. C. Marine, E. F. Vanin, G. P. Zambetti, S. J. Korsmeyer, J. N. Ihle, and J. L. Cleveland. 2001. Jak3 selectively regulates Bax and Bcl-2 expression to promote T-cell development. Mol. Cell. Biol. 21:678-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willis, T. G., D. M. Jadayel, M. Q. Du, H. Peng, A. R. Perry, M. Abdul-Rauf, H. Price, L. Karran, O. Majekodunmi, I. Wlodarska, L. Pan, T. Crook, R. Hamoudi, P. G. Isaacson, and M. J. Dyer. 1999. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell 96:35-45. [DOI] [PubMed] [Google Scholar]
- 60.Xue, L., S. W. Morris, C. Orihuela, E. Tuomanen, X. Cui, R. Wen, and D. Wang. 2003. Defective development and function of Bcl10-deficient follicular, marginal zone and B1 B cells. Nat. Immunol. 4:857-865. [DOI] [PubMed] [Google Scholar]
- 61.Yan, M., J. Lee, S. Schilbach, A. Goddard, and V. Dixit. 1999. mE10, a novel caspase recruitment domain-containing proapoptotic molecule. J. Biol. Chem. 274:10287-10292. [DOI] [PubMed] [Google Scholar]
- 62.Ye, H., A. Dogan, L. Karran, T. G. Willis, L. Chen, I. Wlodarska, M. J. Dyer, P. G. Isaacson, and M. Q. Du. 2000. BCL10 expression in normal and neoplastic lymphoid tissue. Nuclear localization in MALT lymphoma. Am. J. Pathol. 157:1147-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye, H., H. Liu, M. Raderer, A. Chott, A. Ruskone-Fourmestraux, A. Wotherspoon, M. J. Dyer, S. S. Chuang, A. Dogan, P. G. Isaacson, and M. Q. Du. 2003. High incidence of t(11;18)(q21;q21) in Helicobacter pylori-negative gastric MALT lymphoma. Blood 101:2547-2550. [DOI] [PubMed] [Google Scholar]
- 64.Zhang, Q., R. Siebert, M. Yan, B. Hinzmann, X. Cui, L. Xue, K. M. Rakestraw, C. W. Naeve, G. Beckmann, D. D. Weisenburger, W. G. Sanger, H. Nowotny, M. Vesely, E. Callet-Bauchu, G. Salles, V. M. Dixit, A. Rosenthal, B. Schlegelberger, and S. W. Morris. 1999. Inactivating mutations and overexpression of BCL10, a caspase recruitment domain-containing gene, in MALT lymphoma with t(1;14)(p22;q32). Nat. Genet. 22:63-68. [DOI] [PubMed] [Google Scholar]