Abstract
The myristoylated calcium sensor SOS3 and its interacting protein kinase, SOS2, play critical regulatory roles in salt tolerance. Mutations in either of these proteins render Arabidopsis thaliana plants hypersensitive to salt stress. We report here the isolation and characterization of a mutant called enh1-1 that enhances the salt sensitivity of sos3-1 and also causes increased salt sensitivity by itself. ENH1 encodes a chloroplast-localized protein with a PDZ domain at the N-terminal region and a rubredoxin domain in the C-terminal part. Rubredoxins are known to be involved in the reduction of superoxide in some anaerobic bacteria. The enh1-1 mutation causes enhanced accumulation of reactive oxygen species (ROS), particularly under salt stress. ROS also accumulate to higher levels in sos2-1 but not in sos3-1 mutants. The enh1-1 mutation does not enhance sos2-1 phenotypes. Also, enh1-1 and sos2-1 mutants, but not sos3-1 mutants, show increased sensitivity to oxidative stress. These results indicate that ENH1 functions in the detoxification of reactive oxygen species resulting from salt stress by participating in a new salt tolerance pathway that may involve SOS2 but not SOS3.
High salinity is a major adverse environmental factor affecting plant growth and development, leading to large global yield losses in crops. In most saline soils, Na+ is the major toxic ion. Excess salts are harmful to plants because of ion toxicity and osmotic stress (6). Salt stress causes disrupted ion homeostasis in plants, leading to excess accumulation of toxic Na+ in the cytoplasm and a deficiency of essential ions, such as K+ (22, 33).
It appears that several ion transporters act to facilitate Na+ entry into and exit out of plant cells. Na+ can also be compartmentalized in the vacuole through tonoplast-localized Na+/H+ antiporters (4). The uptake of Na+ into plant cells appears to occur at least partly through the transporter HKT1 (30, 40, 51) and through nonselective cation channels (2). In Arabidopsis thaliana, Na+ efflux is mediated by the plasma membrane Na+/H+ antiporter encoded by the SOS1 gene (47, 48, 52, 53) aided by plasma membrane H+-ATPases, which generate the proton gradient needed to drive Na+ transport. SOS1 facilitates Na+ efflux into the root medium, thereby delaying Na+ accumulation in the cytoplasm to allow time for Na+ sequestration in the vacuole. SOS1 appears also to control long-distance Na+ transport between roots and leaves by mediating loading and unloading of Na+ in the xylem and phloem. Overexpression of the SOS1 gene appears to confer increased salt tolerance, at least in part, in transgenic Arabidopsis plants by these processes (54).
Activation of the Na+/H+ antiport activity of SOS1 by salt stress is controlled by the SOS3 and SOS2 proteins (47, 48). SOS3 is a myristoylated calcium-binding protein that is capable of sensing cytosolic calcium changes elicited by salt stress (24, 34). SOS2 is a serine/threonine protein kinase with a unique regulatory domain at the carboxyl terminus and an amino-terminal catalytic domain (35). The carboxyl-terminal regulatory domain of SOS2 interacts with SOS3 through the FISL motif (19). In the presence of calcium, SOS3 activates the substrate phosphorylation activity of SOS2 (21). Recent data demonstrate that the SOS3-SOS2 protein kinase complex activates SOS1 via phosphorylation (47, 48).
Although failure to reestablish ion homeostasis is a major cause of salt-induced injury, the etiology of salt toxicity in plants is more complex (60) and includes oxidative damage caused by reactive oxygen species (ROS) elicited by high levels of salt. ROS such as superoxide radicals (O2·−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH·) are produced in aerobic cellular processes such as mitochondrial and chloroplast electron transport or oxidation of several metabolites, such as glycolate (photorespiration), xanthine, and glucose. Abiotic stresses, including salinity, significantly increase the rate of ROS production (8, 17, 23, 56).
To minimize the damaging effects of ROS, organisms have evolved detoxification mechanisms. Plants utilize nonenzymatic antioxidants, such as ascorbate, glutathione, flavonoids, alkaloids, and carotenoids, to scavenge ROS (3). Detoxifying enzymes employed in plants include superoxide dismutase, catalase, and enzymes of the ascorbate-glutathione cycle (3, 12). The importance of oxidative protection in salt tolerance is indicated by the characterization of the Arabidopsis pst1 (for photoautotrophic salt tolerance 1) mutant, which is more tolerant to both salt and direct oxidative stresses than the wild type. The pst1 mutant also has significantly higher activities of superoxide dismutase and ascorbate peroxidase than wild-type Arabidopsis when treated with high levels of NaCl (57). Overexpression of genes involved in ROS scavenging, such as NtGST/GPX (glutathione S-transferase [GST]/glutathione peroxidase) also improve salt and chilling stress tolerance by reducing ROS-mediated membrane damage (49, 50).
Emerging evidence indicates that ROS also can act as an important signal controlling salt adaptive responses mediated through mitogen-activated protein kinases (MAPKs). An Arabidopsis MAPK kinase kinase, ANP1, is also activated by H2O2. After activation by ROS, ANP1 initiates a phosphorylation cascade involving two MAPKs, AtMAP4 and AtMAP6 (14, 58). Transgenic tobacco plants expressing a constitutively active ANP1 ortholog, NPK1, show improved tolerance to multiple environmental stress conditions including salinity. An Arabidopsis nucleoside diphosphate (NDPK2) may also positively regulate H2O2-mediated MAPK signaling in plants (42). So far, the SOS pathway, which controls ion homeostasis, has not been linked to ROS detoxification or ROS-mediated signaling initiated by salt stress.
Here, we describe a second site mutation, enh1-1 (enhancer 1-1), in the sos3-1 mutant background of Arabidopsis ecotype Columbia. The enh1-1 lesion causes salt sensitivity on its own and enhances the salt sensitivity of sos3-1. This indicates that ENH1 plays a role in salt tolerance in a pathway separate from SOS3. Further, genetic analysis revealed that the enh1-1 mutation did not enhance the salt sensitivity of sos2-1, indicating that SOS2 and ENH1 may function in the same pathway. ROS hyperaccumulate in enh1-1, sos3-1 enh1-1, sos2-1 enh1-1, and sos2-1 mutant plants but not in sos3-1 plants. The enh1 mutant thus reveals a new pathway involved in salt tolerance that appears to connect the ion homeostasis function of the SOS pathway via SOS2 with components of oxidative stress suppression signaling. The ENH1-mediated pathway may also be under the control of SOS1.
MATERIALS AND METHODS
Isolation of the enh1-1 mutant.
Arabidopsis (ecotype Col gl1) salt overly sensitive 3 (sos3-1) (33) mutant plants were mutagenized with Agrobacterium tumefaciens-mediated transferred DNA (T-DNA) transformation (strain GV3101) with the activation tagging vector pSKI015 (61). Seeds from T2 plants were screened for mutants that exhibited increased sensitivity to 75 mM NaCl. Four-day-old seedlings grown on cellophane membrane (Bio-Rad, Hercules, CA) placed over germination medium (1× Murashige and Skoog [MS] salts [43], 2% sucrose, 1.2% agar, pH 5.7) were transferred to MS medium supplemented with 75 mM NaCl. When appropriate, seedlings were transferred to soil and grown to maturity (61).
Ion content measurements.
One-week-old seedlings (n = 40 to 60) grown on germination medium were transferred to a 250-ml flask containing 60 ml half-strength MS salts and 2% sucrose (pH 5.7). The flasks were shaken at 100 rpm under cool fluorescent light (16-h light, 8-h dark) at 22 ± 1°C. After 1 week, NaCl was added to give the desired final NaCl concentrations, and the seedlings were allowed to grow for an additional 2 days. The seedlings were then harvested, rinsed with an excess amount of deionized H2O, dried in an oven at 65°C for 48 h, and weighed. Ground tissues were then extracted with 0.1 M nitric acid for 4 h and filtered through Whatman no. 1 filter paper. Na+ or K+ content was determined by use of an atomic absorption spectrometer (model SpectrAA-10; Varian, Springvale, Australia).
Cloning of the ENH1 gene.
Genomic DNA flanking the left border of the inserted T-DNA in sos3-1 enh1-1 plants was isolated by thermal asymmetric interlaced PCR (TAIL-PCR) and subcloned into cloning vector pBluescript SK(+) as described elsewhere (61). The entire isolated fragment was sequenced. The primers used in the TAIL-PCR were as described previously (61). We also designed the following primer pair to examine whether the T-DNA insert is homozygous or heterozygous on chromosome 5 of plants that are homozygous for the sos3-1 enh1-1 mutant: forward, 5′-ACTCACACATTTTCGTCAATTGG-3′; reverse, 5′-GAAGTAGTGTGATGTGTGCGTTAG-3′. We have used the primers enh1-2LP, 5′-TTTTTAACTCTTGCTGCTGCAC-3′, and enh1-2RP, 5′-TTTCCTTTGAATCCTGTGCAC-3′, to identify the mutation in the enh1-2 (SALK_018190) allele.
RT-PCR and complementation of the sos3-1 enh1-1 mutant.
Total RNA was extracted from different wild-type (Col-0) plant tissues with the RNeasy plant mini kit (QIAGEN, Valencia, CA), and 3 μg of total RNA was used for the first-strand cDNA synthesis with the thermoscript reverse transcription-PCR (RT-PCR) system (Invitrogen, Carlsbad, CA). Gene-specific primers for ENH1 were 5′-CAAAGCTTCTTCGTTCGATCACACTAG-3′ and 5′-GGTTTGCAATTACAAAGCGGACTTG-3′. The RT-PCR product amplified from nontreated wild-type plant tissues was subcloned into the pGEM-T Easy vector (Promega, Madison, WI), resulting in the clone ENH1-4, and the identity of the insert was confirmed by sequencing.
The coding region of ENH1 was amplified by PCR using ENH1-4 as a template with the primers 5′-AGCTGAGCTCATGGCAACGTCTGGTGCG-3′ and 5′-AGTCTCTAGATTATTGAAGACCATAGACAAGC-3′. The PCR fragment was cloned into the binary vector 99-1 (62) under the control of the CAM 35S promoter, and the identity of the cloned insert was confirmed by sequencing. The construct was introduced into sos3-1 enh1-1 mutant plants through Agrobacterium tumefaciens-mediated (strain GV3101) T-DNA transformation.
ENH1 promoter::GUS construct.
A genomic fragment promoter including 2,014 bp 5′ to the ATG start codon of the ENH1 gene was amplified by PCR with the wild-type genomic DNA with the primer pair 5′-AGTCGGATCCGTCTCCAAAACCACACCATTGTC-3′ and 5′-AGTCCTGCAGCTTTCTTTCTAGTGTGATCGAACG-3′. The PCR fragment was cloned into the binary vector pCAMBIA1381Z between the BamHI and PstI sites, and the identity of the clone insert was confirmed by sequencing. The construct was introduced into Arabidopsis wild-type plants (ecotype Columbia) through an Agrobacterium tumefaciens-mediated (strain GV3101) T-DNA transformation. β-Glucuronidase (GUS) staining was performed on tissues or organs from transgenic plants in 1 mM 5-bromo-4-chloro-3-indolyl-β-d-glucuronide (Rose Scientific Ltd., Cincinnati, OH) in 0.1 M potassium phosphate buffer (pH 7.5) with 0.1% Triton X-100 (25). Chlorophyll was removed with 70% ethanol.
ENH1-GFP construct.
The ENH1-coding region was amplified by PCR with the primers 5′-AGTCTCTAGAATGGCAACGTCTGGTGCG-3′ and 5′-AGTCGAATCCGTTGAAGACCATAGACAAGC-3′, with ENH1-4 used as a template. The PCR product was cloned into vector 326-SGFP between XbaI and BamHI sites, and the entire insert and the conjunction regions were sequenced. The resulting construct was then transformed into Arabidopsis protoplasts, and expression of the fusion constructs was monitored as described elsewhere (44).
GST-ENH1 recombinant protein construct.
To produce Escherichia coli-expressed GST-ENH1 recombinant protein, the coding region of ENH1 was cloned in frame between the BamHI and XhoI sites of the expression vector pGEX-4T-1. The resulting construct was transformed into E. coli BL21(DE3) cells to obtain the GST-ENH1 fusion protein. Rubredoxin activity assays were performed as described previously (26).
ROS detection.
One-week-old seedlings were used to determine the generation of ROS in the root as described elsewhere (55). For salt treatment, the seedlings were transferred to medium containing 100 mM NaCl and allowed to grow for 12 h. The untreated and treated seedlings were incubated with 50 μM 5- (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA; Molecular Probes) for 30 min. All fluorescence images were obtained with a Leica SP2 confocal microscope (Mannheim, Germany).
Quantitative measurement of H2O2 production was performed with 1-week-old seedlings with an Amplex red hydrogen/peroxidase assay kit (Molecular Probes) as described elsewhere (9). For salt treatment, the seedlings were transferred to medium containing 100 mM NaCl and allowed to grow for an additional 12 h.
The detection of superoxide was performed essentially as described previously (31) with modifications. One-week-old seedlings germinated on germination medium were transferred to medium containing 0 mM or 80 mM NaCl. These seedlings were allowed to grow for one additional day and vacuum infiltrated with 0.1 mg/ml nitroblue tetrazolium (Sigma) in 25 mM HEPES buffer (pH 7.6). In a control treatment, 10 mM MnCl2 and 10 units/ml superoxide dismutase were added to the buffer. Samples were incubated at room temperature in darkness for 2 h. Chlorophyll was removed with 70% ethanol.
Quantification of chlorophyll.
Frozen seedlings were ground and extracted with 80% (vol/vol) acetone. Chlorophyll was quantified photometrically as described elsewhere (32).
Northern hybridization.
Two-week-old seedlings grown on germination medium (1× MS salts, 2% sucrose, 0.6% agar, pH 5.7) were untreated or treated with NaCl (200 mM; 12 h). Total RNA was extracted from whole seedlings with TRIzol reagent (Invitrogen) following the manufacturer's instructions. Total RNA was then separated on a formaldehyde-containing agarose (1.2% [wt/vol]) gel, transferred to a nylon transfer membrane (Schleicher & Schuell, Keene, NH) overnight and cross-linked. Blots were hybridized overnight (0.5 M phosphate buffer [pH 7.2], 7% [wt/vol] sodium dodecyl sulfate [SDS], 1 mM EDTA, 2 mM bovine serum albumin) with 32P-labeled probe at 60°C. Washes, 20 min each at 60°C, were performed first in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% SDS, then in 1× SSC, 0.1% SDS, and finally in 0.5× SSC, 0.1% SDS. The 26S rRNA gene was used as a loading control.
RESULTS
Identification of enh1-1 mutant.
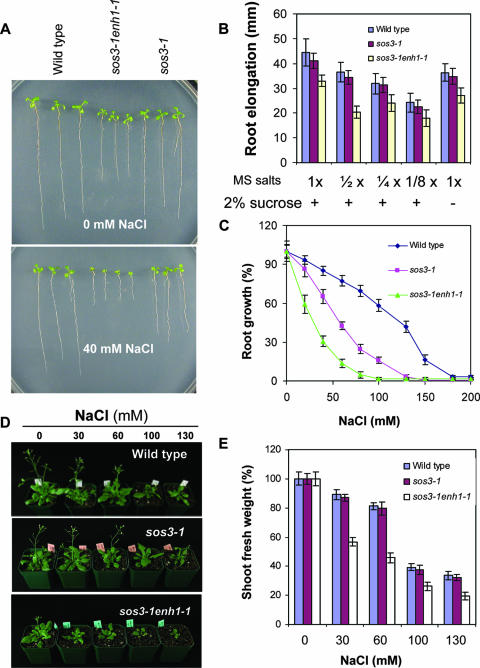
T2 seedlings from 65,000 individual T-DNA insertion lines in the sos3-1 genetic background were screened for suppressors and enhancers of sos3-1 hypersensitivity to NaCl. The enh1-1 mutant was identified as conferring increased sensitivity of sos3-1 to as little as 40 mM NaCl (Fig. 1A to C). The sos3-1 enh1-1 double mutant grows slightly slower than sos3-1 in medium without added NaCl (Fig. 1A). The severely sensitive phenotype of the sos3-1 enh1-1 mutant to NaCl indicates that it even may be sensitive to salts or other aspects of the normal growth environment that affect ROS production. However, lowering the concentrations of MS salts or sucrose in the medium did not affect the growth of sos3-1 enh1-1 relative to the other genotypes (Fig. 1B).
FIG. 1.
The enh1-1 mutation confers hypersensitivity of sos3-1 to NaCl. (A) Root elongation of wild-type, sos3-1, and sos3-1 enh1-1 plants with or without 40 mM NaCl. (B) Growth of plants shown in panel A in different strengths of MS medium or full-strength MS medium without sucrose. (C) Dose response of plants shown in panel A to NaCl. The root growth was presented as a proportion of growth compared to growth without NaCl (100%). (D) The sos3-1 enh1-1 plants grown in soil are hypersensitive to salt treatment. Photographs were taken 18 days after the salt treatments. (E) Shoot fresh weight at the end of each treatment as shown in panel D. Shoot fresh weight data are shown as a percentage of shoot fresh weight in the absence of NaCl. Error bars in panels B, C, and E represent standard deviations (n = 18).
The sos3-1 enh1-1 mutant plants were backcrossed with sos3-1 plants. All resulting F1 plants (21 of 21) exhibited a sos3-1-like phenotype in the presence of 60 mM NaCl. F2 progeny from the self-crossed F1 segregated 391:128 (sos3-1:sos3-1 enh1-1), which indicates that enh1-1 is a monogenic recessive mutation in a nuclear gene.
The sos3-1 enh1-1 mutant plants also displayed significantly increased sensitivity to NaCl treatment in soil compared to sos3-1 (Fig. 1D). The double mutant gained much less fresh weight during 18 days of NaCl treatment compared to sos3-1 plants (Fig. 1E). The salt-hypersensitive phenotype of sos3-1 was not evident until more severe stress was applied (data not shown).
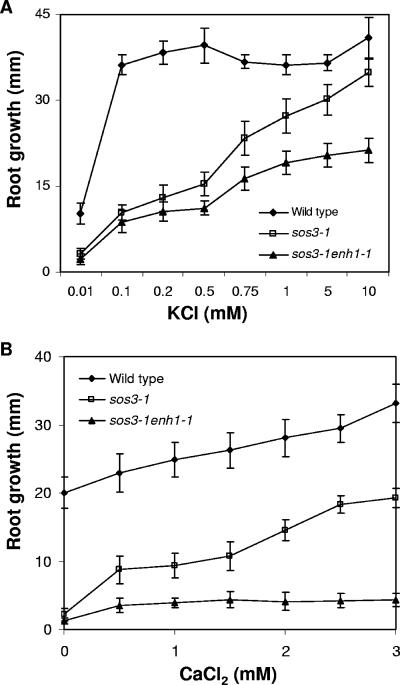
The enh1-1 mutation enhances the K+-deficient phenotype of the sos3-1 mutant.
Seedlings of the sos3-1 mutant have impaired growth on medium containing low levels of K+ (33) and require 10 mM K+ for optimal root growth (Fig. 2A). Root elongation of sos3-1 enh1-1 mutant seedlings was substantially less than that of sos3-1 in medium with low K+. The enhancement of the K+ deficiency phenotype of sos3-1 by the enh1-1 mutation indicates that ENH1 is involved in controlling K+ nutrition separately from SOS3 (Fig. 2A) and appears to function in a separate signal pathway that mediates stress tolerance.
FIG. 2.
Responses of sos3-1 enh1-1 mutant plants to different levels of K+ or Ca2+. (A) Growth (over a period of 8 days) of sos3-1 enh1-1 in medium supplemented with different levels of KCl. (B) Response (over a period of 8 days) of the sos3-1 enh1-1 mutant to various levels of external Ca2+ in the presence of 50 mM NaCl. Error bars represent standard deviations (n = 18).
External Ca2+ fails to improve the salt tolerance of the sos3-1 enh1-1 mutant.
High levels of external Ca2+can ameliorate sos3-1 sensitivity to NaCl stress, at least in part through improved K+/Na+ selectivity (33). Without added Ca2+, the root growth of both sos3-1 and sos3-1 enh1-1 plants is arrested at 50 mM NaCl. Increasing the Ca2+ concentration to 0.5 mM or higher greatly improves the salt tolerance of sos3-1 plants, but not the tolerance of sos3-1 enh1-1 plants (Fig. 2B). These results further substantiate that ENH1 mediates salt tolerance by a new pathway, independent of SOS3.
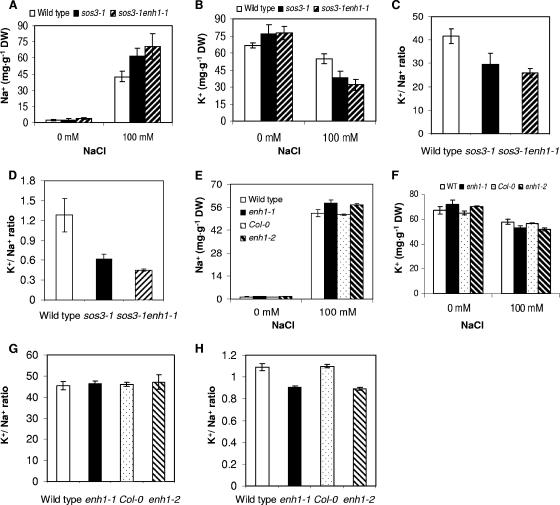
The increased salt sensitivity of sos3-1 enh1-1 involves imbalanced ion homeostasis.
Without stress, both sos3-1 enh1-1 and sos3-1 plants accumulated slightly higher levels of Na+ and K+ compared to wild-type plants (Fig. 3A and B). When treated with 100 mM NaCl, sos3-1 enh1-1 plants accumulated the highest amount of Na+ but the lowest K+ among the genotypes tested (Fig. 3A and B). Consequently, the ratio of K+ to Na+ in the sos3-1 enh1-1 mutant plants is the lowest, with or without NaCl stress (Fig. 3C and D). We isolated the enh1-1 single mutant from the F2 plants from a cross between the wild type and the sos3-1 enh1-1 double mutant. The levels of Na+ in enh1 single mutants under NaCl stress were elevated compared to wild-type plants, whereas enh1 mutants accumulated less K+ than the wild type (Fig. 3E and F). Under normal conditions, the levels of K+ and Na+ accumulation as well as the ratios of K+ to Na+ in the enh1 mutants are essentially the same as wild type. However, when treated with NaCl, the ratios of K+ to Na+ in the enh1 mutants are significantly lower than wild type (Fig. 3H).
FIG. 3.
Ion content in sos3-1 enh1-1 mutant plants. (A) Na+ content in sos3-1 enh1-1 seedlings with or without NaCl in the medium. The mean values between genotypes are statistically significantly different (P < 0.05, paired t test) under 100 mM NaCl. (B) K+ content in sos3-1 enh1-1 seedlings with or without NaCl in the medium. The mean values between genotypes are statistically significantly different (P < 0.05, paired t test) under 100 mM NaCl. (C) Ratio of K+ and Na+ accumulation in the sos3-1 enh1-1 seedlings without NaCl. The mean values between genotypes are statistically significantly different (P < 0.05, paired t test). (D) Ratio of K+ and Na+ accumulation in sos3-1 enh1-1 seedlings treated with NaCl. The mean values between genotypes are statistically significantly different (P < 0.05, paired t test). (E) Na+ content in enh1 mutant seedlings with or without NaCl in the medium. The mean values between genotypes are statistically significantly different (P < 0.05, paired t test) under 100 mM NaCl. (F) K+ content in enh1 mutant seedlings with or without NaCl in the medium. The mean values between genotypes are statistically significantly different (P < 0.05, paired t test) under 100 mM NaCl. (G) Ratio of K+ and Na+ accumulation in enh1 mutant seedlings without NaCl. The mean values between genotypes are not statistically significantly different (P > 0.05, paired t test). (H) Ratio of K+ and Na+ accumulation in enh1 mutant seedlings treated with 100 mM NaCl. The mean values between genotypes are statistically significantly different (P < 0.05, paired t test). Error bars are standard deviations (n = 7). DW, dry weight.
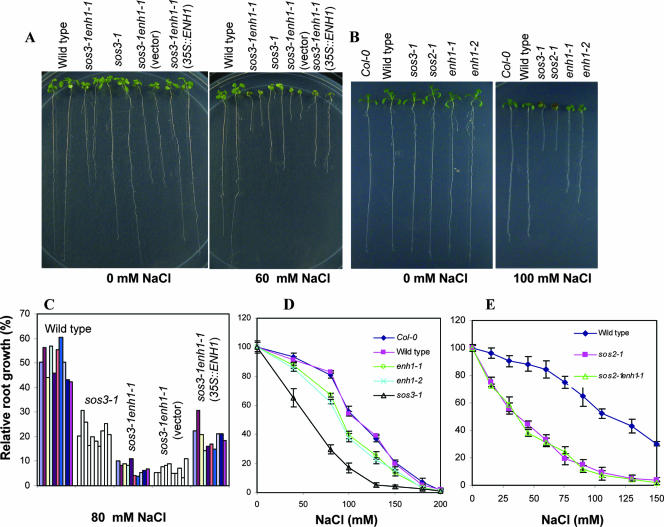
The enh1-1 mutation does not enhance the salt sensitivity of sos2-1 mutant plants.
In the absence of NaCl, the growth of sos2-1 enh1-1 is similar to that of sos2-1 (data not shown). The growth response of sos2-1 enh1-1 to NaCl is similar to that of sos2-1 over various concentrations of NaCl (Fig. 4E). The inability of the enh1-1 mutation to affect the salt sensitivity of sos2-1 plants indicates that SOS2 and ENH1 may function in a common pathway controlling salt tolerance.
FIG. 4.
Complementation of sos3-1 enh1-1 mutant by constitutive expression of ENH1 and enh1 single mutants are more sensitive to NaCl than wild type. (A) Constitutive expression of ENH1 complemented sos3-1 enh1-1 mutant. (B) enh1-1 and enh1-2 plants are more sensitive to NaCl than wild type. (C) Quantification of root growth data of genotypes shown in (A). Each bar represents root growth of one individual line. (D) Growth of Col-0, wild type, sos3-1, enh1-1 and enh1-2 in media containing various levels of NaCl. (E) Response of sos2-1 enh1-1 mutant plants to different concentrations of NaCl. Error bars are standard deviation (n = 18).
ENH1 encodes a PDZ domain containing rubredoxin-like protein.
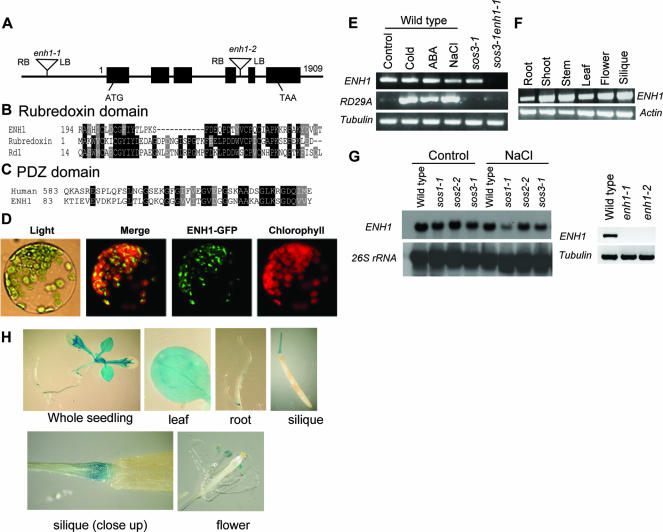
We isolated the genomic DNA fragment flanking the left border of a T-DNA insertion in sos3-1 enh1-1 by TAIL-PCR. Sequence analysis indicated that the T-DNA was located 636 bp upstream of the start codon of an open reading frame for At5g17170/MKP11.2 (Fig. 5A). Constitutive expression of the ENH1 coding sequence under control of the CaMV 35S promoter in sos3-1 enh1-1 mutant plants reversed the effect of the enh1-1 mutation (Fig. 4A and C).
FIG. 5.
ENH1 encodes a PDZ and rubredoxin domain-containing protein. (A) Structure of the ENH1 gene. Positions are relative to the translation start codon. Filled boxes represent the exons, and lines between filled boxes represent introns. (B) Alignment of ENH1 and other proteins in the rubredoxin domain. Proteins compared are ENH1 (accession number AAK59624) from Arabidopsis thaliana; rubredoxin (AAF03228), rubredoxin from Pyrococcus furiosus; and Rd1 (P58992), rubredoxin from Chlorobaculum tepidum. (C) Comparison of ENH1 with its homolog in the PDZ domain. Human (NP_057424), PDZ domain-containing guanine nucleotide exchange factor I human (Homo sapiens). (D) Subcellular localization of ENH1 protein. (E) The ENH1 gene is constitutively expressed. Control, no treatment; cold, 0°C for 24 h; ABA, 100 μM for 3 h; NaCl, 300 mM for 5 h. (F) Transcript abundance of ENH1 in different plant tissues. (G) ENH1 expression in different genotypes by Northern analysis (left panel) and RT-PCR analysis (right panel). (H) ENH1 promoter::GUS expression in transgenic Arabidopsis plants.
The ENH1 gene encodes a polypeptide containing two interesting domains, a putative PDZ domain in the N-terminal region that is potentially involved in protein-protein interactions and a domain at the C-terminal part resembling rubredoxin. ENH1 shows highest sequence similarity in the rubredoxin domain with rubredoxin proteins from Pyrococcus furiosus (26) and Chlorobaculum tepidum (15) (Fig. 5B). ENH1 also shares high sequence similarity in the PDZ domain with the human PDZ domain-containing guanine nucleotide exchange factor I (Fig. 5C).
Expression of ENH1 and localization of ENH1-GFP.
ENH1 is constitutively expressed in wild-type plants, and its expression is abolished in sos3-1 enh1-1, enh1-1, and enh1-2 mutant plants (Fig. 5E and G). The transcript of ENH1 is more abundant in the shoot than root (Fig. 5F). Interestingly, we observed that ENH1 expression was also reduced in the sos1-1 mutant under salt stress (Fig. 5G), indicating that SOS1 is a positive regulator of ENH1 transcript accumulation. We also produced Arabidopsis transgenic plants expressing an ENH1 promoter-GUS fusion gene. The expression of ENH1::GUS was observed in all tissues of seedlings, with weaker expression in the root tips (Fig. 5H). Strong GUS expression was also detected in the anther (Fig. 5H). The subcellular localization of ENH1 was determined by a transient expression assay of the ENH1-GFP fusion protein, which revealed that ENH1 accumulates in the chloroplast (Fig. 5D).
enh1 single mutations also confer reduced tolerance to salt.
The enh1-1 single mutant seedlings are more sensitive to NaCl than the wild type (Fig. 4B and D). This result indicates that ENH1 is required for normal salt tolerance. A T-DNA insertion line (SALK_018190, referred to as enh1-2 hereafter), which has a T-DNA insertion in the At5g17170 gene (Fig. 5A), is more sensitive to NaCl than its wild-type background (Col-0) (Fig. 4B and D). Consistent with their allelic nature, F1 plants (18 of 18) from a cross between enh1-1 and enh1-2 showed a level of sensitivity to 100 mM NaCl that was similar to the parental plants. Both enh1-1 and enh1-2 seedlings grew nearly normally compared to their wild types in control medium without stress (Fig. 4B).
ENH1 and SOS2 are involved in the control of ROS detoxification.
Rubredoxins are known to act as electron carriers for various enzymes. Reports have described the involvement of rubredoxins in oxidative stress protection in Desulfovibrio gigas, Desulfovibrio vulgaris, and Treponema pallidum (5, 11, 13). ENH1 encodes a chloroplast protein with a putative rubredoxin domain that may also function as an electron carrier. We assayed ENH1 recombinant protein for possible rubredoxin activity and found that it does not appear to act as an electron donor or acceptor in a superoxide reductase reaction system from Pyrococcus furiosus (26) (data not shown).
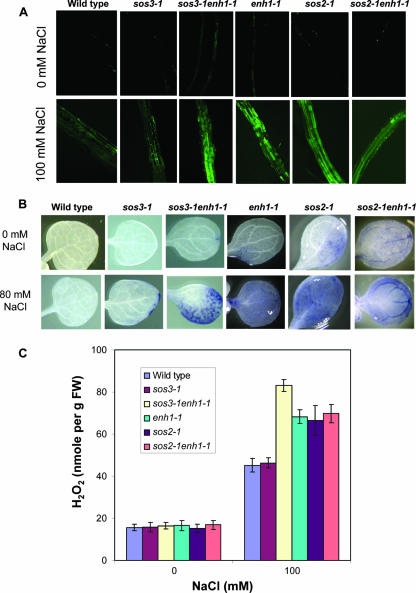
Nevertheless, an analysis of the ROS levels in sos3-1 enh1-1 and enh1-1 plants revealed that the mutants accumulate higher amounts of ROS as detected by CM-H2DCFDA staining followed by confocal imaging (Fig. 6A). Stress treatment with 100 mM NaCl for 12 h stimulated the accumulation of ROS in wild type, sos3-1, sos3-1 enh1-1, and enh1-1 plants. However, sos3-1 enh1-1 and enh1-1 plants accumulated ROS to much higher levels (Fig. 6A). Staining with nitroblue tetrazolium specifically detects superoxide radicals. As shown in Fig. 6B, under normal growth conditions, sos3-1 enh1-1 and enh1-1 plants have slightly more superoxide accumulation than sos3-1 or wild-type plants. However, after treatment with 80 mM NaCl for 1 day, sos3-1 enh1-1 and enh1-1 plants accumulated much more superoxide than sos3-1 plants or wild-type plants, indicating that hyperaccumulation of superoxide resulting from the enh1-1 mutation is independent of sos3-1 (Fig. 6B). Without stress, enh1 plants displayed a low level of H2O2 similar to that in the wild type (Fig. 6C). When treated with 100 mM NaCl for 12 h, sos3-1 enh1-1 and enh1-1 plants all accumulated substantially higher levels of H2O2 than sos3-1 or wild-type plants (Fig. 6C). Interestingly sos2-1 mutant plants also accumulated more ROS than wild-type or sos3-1 plants with or without NaCl stress (Fig. 6). However, the enh1-1 mutation has no additive effect with the sos2-1 mutation (Fig. 6), which indicates that genetically, ENH1 and SOS2 may function in a common pathway controlling ROS detoxification in response to NaCl stress.
FIG. 6.
Detection of ROS. (A) ROS levels in roots in different mutants after CM-H2DCFDA staining. (B) Nitroblue tetrazolium staining for superoxide in unstressed or salt-treated (80 mM NaCl) seedlings. (C) H2O2 production under salt treatment. Error bars represent standard deviations (n = 6). FW, fresh weight.
SOS2 and ENH1 mediate tolerance to general oxidative stress.
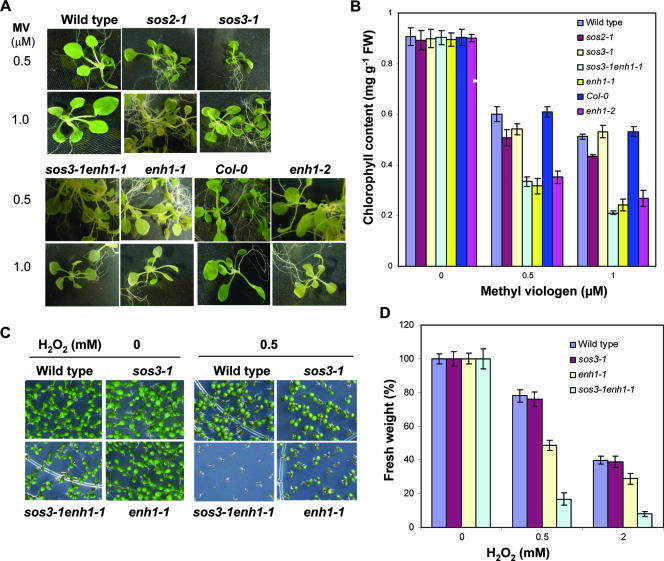
We examined the tolerance of enh1 mutants to oxidative stress caused by conditions other than NaCl stress. Wild-type, Col-0, and sos3-1 plants can tolerate 0.5 and 1.0 μM methyl viologen treatment as indicated by their abilities to maintain chlorophyll in leaves (Fig. 7A and B). The enh1-1 mutation enhances the sensitivity of sos3-1 to both 0.5 and 1.0 μM methyl viologen (Fig. 7A and B). The enh1-1, enh1-2, and sos2-1 mutant plants all showed a hypersensitive response to these stress conditions (Fig. 7A and B). In addition, the germination and postgermination growth and development of sos3-1 enh1-1 and enh1-1 mutant plants are more inhibited by H2O2 compared to sos3-1 or wild-type control plants (Fig. 7C and D).
FIG. 7.
enh1 mutations cause hypersensitivity to oxidative stress. (A)Three-week-old seedlings grown in germination medium were treated with 0.5 or 1.0 μM methyl viologen under light for 4 days. Damaged seedlings exhibited a loss of chlorophyll content. (B) Quantification of chlorophyll levels of each genotype shown in (A). Error bars represent standard errors (n = 6). FW, fresh weight. (C) Tolerance of enh1 mutant plants to H2O2. Seeds were directly sown in media containing 0.5 mM H2O2. (D) Fresh weight of 30 seedlings per genotype shown in (C). Error bars represent standard error (n = 6).
DISCUSSION
Several observations here indicate that ENH1 participates in a new pathway involved in salt tolerance that is at least partially separate from the SOS pathway. ENH1-mediated salt tolerance appears to involve maintaining Na+ and K+ ion homeostasis, since the sos3-1 enh1-1 mutant has a decreased ability to control ion homeostasis (Fig. 3). The enh1-1 mutation does not enhance the salt sensitivity of sos2-1 mutant plants (Fig. 4E). This result, together with the evidence for ROS accumulation and oxidative stress sensitivity phenotypes of sos2-1 plants (Fig. 6 and 7), suggests that the ENH1 pathway is connected with SOS2 and that ENH1 may be controlled directly or indirectly by SOS2.
Although the salt hypersensitivity of sos3-1 can be ameliorated by an external calcium supply (33), the rescuing effect of external calcium could not be observed with the sos3-1 enh1-1 (Fig. 2B) mutant or sos2 mutants. Therefore, both SOS2 and ENH1 are required for the pathway mediating the amelioration effect of external calcium. Yeast two-hybrid interaction studies have identified several SOS3-like proteins that interact with SOS2 (20). It is possible that the ENH1 pathway is important for the rescuing effect of external calcium, and it involves not only ENH1 but also SOS2 and another member of the family of SOS3-like calcium-binding proteins that may interact and activate SOS2.
The ENH1 gene encodes a chloroplast-localized rubredoxin-like protein that shows greatest sequence similarity to rubredoxin proteins from Pyrococcus furiosus and Nostoc sp. strain PCC 7120 (Fig. 5). Rubredoxins are known as nonheme iron proteins in which a single Fe atom is covalently attached by four S atoms from cysteine residues (10). The suggested biochemical role for rubredoxin proteins is to act as a mediator of electron transfer for various enzymes (41). The rubredoxin from Pseudomonas oleovorans has been reported to be involved in the electron transfer pathway for the ω-hydroxylation of alkanes and fatty acids in the presence of NADH and O2 (46), whereas the rubredoxin from Desulfovibrio gigas functions as an electron donor to a favohemoprotein, rubredoxin-oxygen oxidoreductase (11).
Recently, an alternative pathway for superoxide reduction has been proposed for anaerobic microorganisms, whereby the reduction of superoxide to H2O2 is carried out by the catalytic action of a mononuclear iron-containing enzyme, superoxide reductase (SOR) (5, 13, 26, 27, 36-38). H2O2, the only product of the SOR reaction, is then likely reduced to H2O by peroxidases or other enzymes (1, 26). This role is in sharp contrast to the role of superoxide dismutase from aerobic organisms, which converts superoxide to H2O2 and O2. Rubredoxins from various anaerobes, including Pyrococcus furiosus and Desulfovibrio vulgaris, appear to function as an electron donor to SOR to avoid the production of O2 in superoxide detoxification (7, 13, 26, 38). Rubredoxin is then reduced by the enzyme NADPH:rubredoxin oxidoreductase, with NADPH as the electron source (18, 39).
No rubredoxin activity could be detected using recombinant ENH1 protein in vitro with the SOR reaction system (26) from Pyrococcus furiosus (data not shown). However, we observed that the enh1 mutant plants accumulated much higher levels of ROS than their wild-type parental plants (Fig. 6). This finding indicates that ENH1 somehow plays an important role in ROS detoxification that is most likely related to chloroplast function where ENH1 is localized.
From our results, it is clear that the ion homeostasis pathway is connected to a pathway that controls salt stress-elicited ROS detoxification. This connection involves not only ENH1 and SOS2 but also SOS1, because SOS1 appears to control ENH1 expression under salt stress (Fig. 5G). Besides being an ion transporter regulated by SOS2, some evidence suggests that SOS1 may also have a regulatory role. SOS1 interacts with RCD1, a regulator of oxidative stress (28), and sos1 mutant plants are also more sensitive to H2O2. How SOS1 regulates ENH1 expression under salt stress is not known at the present time, although this regulation may involve RCD1.
The ENH1 pathway may also have a role in ion homeostasis regulation, since the enh1 mutation enhances the ion homeostasis defect of sos3-1 and blocks the rescuing effect of external calcium (Fig. 2 and 3). How ENH1 and salt stress-induced ROS regulate ion transport is unclear. However, the function of ROS in signal transduction has been extensively studied (3), and ROS signaling targets several mediators of ion transport (16, 29, 45, 55). The activity of ROS to control K+ channels could explain both the hypersensitivity of enh1 mutants to K+ deficiency, and possibly even to excess Na+, by interfering with K+/Na+ transport selectivity. The role of ROS in ion transport control of stomatal behavior has been particularly examined in detail also, and the function of stomata in enh1 mutants needs to be examined more closely. In addition, ROS have been shown to affect cytoplasmic pH (59), which is crucial to the cell response to excessive Na+. Entry of Na+ increases cytoplasmic pH, which must be readjusted. This crucial adjustment in response to excess Na+ may be substantially affected by excess ROS production. Although ENH1 may affect ROS and subsequently Na+ and K+ responses in one or more of these general manners, it may also involve a more specific function through RCD1, since RCD1 interacts with SOS1. Regardless of the exact mechanisms, the discovery of the ENH1 ROS detoxification pathway and of the cross talk between the two salt tolerance pathways opens up new approaches of research that are important for a full understanding of salt tolerance in plants. The connection between SOS1 and both ENH1 and RCD1 suggests that SOS1 may have multiple roles in stress adaptation besides its role in ion transport. The apparent multiple roles of SOS1 and SOS2 in both ion homeostasis and ROS regulation emphasize the central role of the SOS pathway in salt stress tolerance.
Acknowledgments
This work was supported by National Science Foundation grant DBI9813360, and a gift from Futragene Inc. West Lafayette of Indiana (to P.M.H. and R.A.B.), National Institutes of Health grant R01GM0707501 (to J.-K. Zhu), and in part by grants from the Plant Diversity Research Center of the 21st Century Frontier Research Program of MOST (PF0330401-00) (to D.-J. Yun) and the Environmental Biotechnology National Core Research Center Project of KOSEF (R15-2003-012-01002-00) (to D.-J. Yun).
Footnotes
Published ahead of print on 7 May 2007.
REFERENCES
- 1.Adams, M. W. W., F. E. Jenney, Jr., M. D. Clay, and M. K. Johnson. 2002. Superoxide reductase: fact or fiction? J. Biol. Inorg. Chem. 7:647-652. [DOI] [PubMed] [Google Scholar]
- 2.Amtmann, A., and D. Sanders. 1999. Mechanisms of Na+ uptake by plant cells. Adv. Bot. Res. 29:75-122. [Google Scholar]
- 3.Apel, K., and H. Hirt. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55:373-399. [DOI] [PubMed] [Google Scholar]
- 4.Apse, M. P., G. S. Aharon, W. A. Snedden, and E. Blumward. 1999. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiporter in Arabidopsis. Science 285:1256-1258. [DOI] [PubMed] [Google Scholar]
- 5.Auchère, F., R. Sikkink, C. Cordas, P. Raleiras, P. Tavares, I. Moura, and J. J. Moura. 2004. Overexpression and purification of Treponema pallidum rubredoxin; kinetic evidence for a superoxide-mediated electron transfer with the superoxide reductase neelaredoxin. J. Biol. Inorg. Chem. 9:839-849. [DOI] [PubMed] [Google Scholar]
- 6.Binzel, M. L., and M. Reuveni. 1994. Cellular mechanisms of salt tolerance in plant cells. Hort. Rev. 16:33-69. [Google Scholar]
- 7.Blake, P. R., J. B. Park, F. O. Bryant, S. Aono, J. K. Magnuson, E. Eccleston, J. B. Howard, M. F. Summeres, and M. W. W. Adams. 1991. Determinants of protein hyperthermostability: purification and amino acid sequence of rubredoxin from the hyperthermophilic archaebacterium Pyrococcus furiosus and secondary structure of the zinc adduct by NMR. Biochemistry 30:10885-10891. [DOI] [PubMed] [Google Scholar]
- 8.Borsani, O., V. Valpuesta, and M. A. Botella. 2001. Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol. 126:1024-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borsani, O., J. Zhu, P. E. Verslues, R. Sunkar, and J.-K. Zhu. 2005. Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in Arabidopsis. Cell 123:1279-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, H., and J. L. Markley. 1995. NMR spectroscopic studies of paramagnetic proteins: iron-sulfur proteins. Annu. Rev. Biophys. Biomol. Struct. 24:209-237. [DOI] [PubMed] [Google Scholar]
- 11.Chen, L., M.-Y. Liu, J. LeGall, P. Fareleira, H. Santos, and A. V. Xavier. 1993. Rubredoxin oxidase, a new flavo-hemo-protein, is the site of oxygen reduction to water by the “strict anaerobe” Desulfovibrio gigas. Biochem. Biophys. Res. Commun. 193:100-105. [DOI] [PubMed] [Google Scholar]
- 12.Chinnusamy, V., and J.-K. Zhu. 2003. Plant salt tolerance. Top. Curr. Genet. 4:241-270. [Google Scholar]
- 13.Coulter, E. D., and D. M. Kurtz, Jr. 2001. A role for rubredoxin in oxidative stress protection in Desulfovibrio vulgaris: catalytic electron transfer to rubrerythrin and two-iron superoxide reductase. Arch. Biochem. Biophys. 394:76-86. [DOI] [PubMed] [Google Scholar]
- 14.Desikan, R., J. T. Hancock, K. Ichimura, K. Shinozaki, and S. J. Neill. 2001. Harpin induces activation of the Arabidopsis mitogen-activated protein kinases AtMPK4 and AtMPK6. Plant Physiol. 126:1579-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisen, J. A., K. E. Nelson, I. T. Paulsen, J. F. Heidelberg, M. Wu, R. J. Dodson, R. Deboy, M. L. Gwinn, W. C. Nelson, D. H. Haft, E. K. Hickey, J. D. Peterson, A. S. Durkin, J. L. Kolonay, F. Yang, I. Holt, L. A. Umayam, T. Mason, M. Brenner, T. P. Shea, D. Parksey, W. C. Nierman, T. V. Feldblyum, C. L. Hansen, M. B. Craven, D. Radune, J. Vamathevan, H. Khouri, O. White, T. M. Gruber, K. A. Ketchum, J. C. Venter, H. Tettelin, D. A. Bryant, and C. M. Fraser. 2002. The complete genome sequence of Chlorobium tepidum TLS, a photosynthetic, anaerobic, green-sulfur bacterium. Proc. Natl. Acad. Sci. USA 99:9509-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foreman, J., V. Demidchik, J. H. Bothwell, P. Mylona, H. Miedema, M. A. Torres, P. Linstead, S. Costa, C. Brownlee, J. D. Jones, J. M. Davies, and L. Dolan. 2003. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442-446. [DOI] [PubMed] [Google Scholar]
- 17.Gomez, J. M., J. A. Hernandez, A. Jimenez, L. A. del Rio, and F. Sevilla. 1999. Differential response of antioxidative enzymes of chloroplast and mitochondria to long term NaCl stress of pea plants. Free Radic. Res. 31:S11-S18. [DOI] [PubMed] [Google Scholar]
- 18.Guedon, E., and H. Petitdemange. 2001. Identification of the gene encoding NADH-rubredoxin oxidoreductase in Clostridium acetobutylicum. Biochem. Biophys. Res. Commun. 285:496-502. [DOI] [PubMed] [Google Scholar]
- 19.Guo, Y., U. Halfter, M. Ishitani, and J.-K. Zhu. 2001. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 13:1383-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo, Y., L. Xiong, C.-P. Song, D. Gong, U. Halfter, and J.-K. Zhu. 2002. A calcium sensor and its interacting protein kinase are global regulators of abscisic acid signaling in Arabidopsis. Dev. Cell 3:233-244. [DOI] [PubMed] [Google Scholar]
- 21.Halfter, U., M. Ishitani, and J.-K. Zhu. 2000. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA 97:3735-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa, P. M., R. A. Bressan, J.-K. Zhu, and H. J. Bohnert. 2000. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51:463-499. [DOI] [PubMed] [Google Scholar]
- 23.Hernández, J. A., M. A. Ferrer, A. Jiménez, A. R. Barceló, and F. Sevilla. 2001. Antioxidant systems and O2.−/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 127:817-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishitani, M., J. Liu, U. Halfter, C.-S. Kim, W. Shi, and J.-K. Zhu. 2000. SOS3 function in plant salt tolerance requires N-myristoylation and calcium-binding. Plant Cell 12:1667-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jefferson, R. A., T. A. Kavanagh, and M. W. Bevan. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6:3901-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenney, F. E., Jr., M. F. J. M. Verhagen, X. Cui, and M. W. W. Adams. 1999. Anaerobic microbes: oxygen detoxification without superoxide dismutase. Science 286:306-309. [DOI] [PubMed] [Google Scholar]
- 27.Jovanovic, T., C. Ascenso, K. R. Hazlett, R. Sikkink, C. Krebs, R. Litwiller, L. M. Benson, J. J. Moura, J. D. Radolf, B. H. Huynh, S. Naylor, and F. Rusnak. 2000. Neelaredoxin, an iron-binding protein from the syphilis spirochete, Treponema pallidum, is a superoxide reductase. J. Biol. Chem. 275:28439-28448. [DOI] [PubMed] [Google Scholar]
- 28.Katiyar-Agarwal, S., J. Zhu, K. Kim, M. Agarwal, X. Fu, A. Huang, and J.-K. Zhu. 2006. The plasma membrane Na+/H+ antiporter SOS1 interacts with RCD1 and functions in oxidative stress tolerance in Arabdiopsis. Proc. Natl. Acad. Sci. USA 103:18816-18821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohler, B., A. Hills, and M. R. Blatt. 2003. Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathway. Plant Physiol. 131:385-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurie, S., K. A. Feeney, F. J. M. Maathuis, P. J. Heard, S. J. Brown, and R. A. Leigh. 2002. A role for HKT1 in sodium uptake by wheat roots. Plant J. 32:139-149. [DOI] [PubMed] [Google Scholar]
- 31.Lee, B.-H., H. Lee, L. Xiong, and J.-K. Zhu. 2002. A mitochondrial complex I defect impairs cold-regulated nuclear gene expression. Plant Cell 14:1235-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lichtenthaler, H. K. 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148:350-382. [Google Scholar]
- 33.Liu, J., and J.-K. Zhu. 1997. An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Proc. Natl. Acad. Sci. USA 94:4960-4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, J., and J.-K. Zhu. 1998. A calcium sensor homolog required for plant salt tolerance. Science 280:1943-1945. [DOI] [PubMed] [Google Scholar]
- 35.Liu, J., M. Ishitani, U. Halfter, C.-S. Kim, and J.-K. Zhu. 2000. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA 97:3730-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lombard, M., M. Fontecave, D. Touati, and V. Niviere. 2000. Reaction of the desulfoferrodoxin from Desulfoarculus baarsii with superoxide anion. Evidence for a superoxide reductase activity. J. Biol. Chem. 275:115-121. [DOI] [PubMed] [Google Scholar]
- 37.Lombard, M., D. Touati, M. Fontecave, and V. Niviere. 2000. Superoxide reductase as a unique defense system against superoxide stress in the microaerophile Treponema pallidum. J. Biol. Chem. 275:27021-27026. [DOI] [PubMed] [Google Scholar]
- 38.Lumppio, H. L., N. V. Shenvi, A. O. Summers, G. Voordouw, and D. M. Kurtz, Jr. 2001. Rubrerythrin and rubredoxin oxidoreductase in Desulfovibrio vulgaris: a novel oxidative stress protection system. J. Bacteriol. 183:101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma, K., and M. W. W. Adams. 1999. A hyperactive NAD(P)H:rubredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 181:5530-5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mäser, P., B. Eckelman, R. Vaidyanathan, T. Horie, D. J. Fairbairn, M. Kubo, M. Yamagami, K. Yamaguchi, M. Nishimura, N. Uozumi, W. Robertson, M. R. Sussman, and J. I. Schroeder. 2002. Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Lett. 531:157-161. [DOI] [PubMed] [Google Scholar]
- 41.Misaki, S., Y. Morimoto, M. Ogata, T. Yagi, Y. Higuchi, and N. Yasuoka. 1999. Structure determination of rubredoxin from Desulfovibrio vulgaris Miyazaki F in two crystal forms. Acta Crystallogr. D 55:408-413. [DOI] [PubMed] [Google Scholar]
- 42.Moon, H., B. Lee, G. Choi, D. Shin, D. T. Prasad, O. Lee, S. S. Kwak, D. H. Kim, J. Nam, J. Bahk, J. C. Hong, S. Y. Lee, M. J. Cho, C. O. Lim, and D. J. Yun. 2003. NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc. Natl. Acad. Sci. USA 100:358-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murashige, T., and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15:473-497. [Google Scholar]
- 44.Nahm, M. Y., S. W. Kim, D. J. Yun, S. Y. Lee, M. J. Cho, and J. D. Bahk. 2003. Molecular and biochemical analyses of OsRab7, a rice Rab7 homolog. Plant Cell Physiol. 44:1341-1349. [DOI] [PubMed] [Google Scholar]
- 45.Pei, Z. M., Y. Murata, G. Benning, S. Thomine, B. Klusener, G. J. Allen, E. Grill, and J. I. Schroeder. 2000. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406:731-734. [DOI] [PubMed] [Google Scholar]
- 46.Peterson, J. A., and M. J. Coon. 1968. Enzymatic ω-oxidation. III. purification and properties of rubredoxin, a component of the ω-hydroxylation system of Pseudomonas oleovorans. J. Biol. Chem. 243:329-334. [PubMed] [Google Scholar]
- 47.Qiu, Q., Y. Guo, M. A. Dietrich, K. S. Schumaker, and J.-K. Zhu. 2002. Regulation of SOS1, a plasma membrane N+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 99:8436-8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quintero, F. J., M. Ohta, H. Shi, J.-K. Zhu, and J. M. Pardo. 2002. Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc. Nat1. Acad. Sci. USA 99:9061-9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roxas, V. P., R. K. Smith, E. R. Allen, and R. D. Allen. 1997. Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nat. Biotechnol. 15:988-991. [DOI] [PubMed] [Google Scholar]
- 50.Roxas, V. P., S. A. Lodhi, D. K. Garrett, J. R. Mahan, and R. D. Allen. 2000. Stress tolerance in transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol. 41:1229-1234. [DOI] [PubMed] [Google Scholar]
- 51.Rus, A., S. Yokoi, A. Sharkhuu, M. Reddy, B.-H. Lee, T. K. Matsumoto, H. Koiwa, J.-K. Zhu, R. A. Bressan, and P. M. Hasegawa. 2001. AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc. Natl. Acad. Sci. USA 98:14150-14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi, H., M. Ishitani, C. Kim, and J.-K. Zhu. 2000. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA 97:6896-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi, H., F. J. Quintero, J. M. Pardo, and J.-K. Zhu. 2002. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na transport in Plants. Plant Cell 14:465-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi, H., S.-J. Wu, and J.-K. Zhu. 2003. Overexpression of a plasma membrane Na+/H+ antiporter improves salt tolerance in Arabidopsis. Nature Biotechnol. 21:81-85. [DOI] [PubMed] [Google Scholar]
- 55.Shin, R., and D. P. Schachtman. 2004. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc. Natl. Acad. Sci. USA 101:8827-8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smirnoff, N. 1993. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 125:27-58. [DOI] [PubMed] [Google Scholar]
- 57.Tsugane, K., K. Kobayashi, Y. Niwa, Y. Ohba, K. Wada, and H. Kobayashi. 1999. A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell 11:1195-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuasa, T., K. Ichimura, T. Mizoguchi, and K. Shinozaki. 2001. Oxidative stress activates ATMPK6, an Arabidopsis homologue of MAP kinase. Plant Cell Physiol. 42:1012-1016. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, X., F. C. Dong, J. F. Gao, and C. P. Song. 2001. Hydrogen peroxide-induced changes in intracellular pH of guard cells precede stomatal closure. Cell Res. 11:37-43. [DOI] [PubMed] [Google Scholar]
- 60.Zhu, J.-K. 2001. Plant salt tolerance. Trends Plant Sci. 6:66-71. [DOI] [PubMed] [Google Scholar]
- 61.Zhu, J., Z. Gong, C. Zhang, C.-P.Song, B. Damsz, G. Inan, H. Koiwa, J.-K. Zhu, P. M. Hasegawa, and R. A. Bressan. 2002. OSM1/SYP61: a syntaxin protein in Arabidopsis controls abscisic acid-mediated and non-abscisic acid-mediated responses to abiotic stress. Plant Cell 14:3009-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu, J., P. E. Verslues, X. Zheng, B. H. Lee, X. Zhan, Y. Manabe, Y. Zhu, C. H. Dong, J. K. Zhu, P. M. Hasegawa, and R. A. Bressan. 2005. HOS10 encodes an R2R3-type MYB transcription factor essential for cold acclimation in plants. Proc. Natl. Acad. Sci. USA 102:9966-9971. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]