Abstract
To investigate in vivo roles of a murine hypothalamic homeobox gene, Bsx, we generated and analyzed two mutant alleles, BsxΔHD and BsxlacZ. BsxΔHD lacks the homeodomain, and BsxlacZ is an insertion of a lacZ reporter gene. Bsx-lacZ expression was detected in the hypothalamus and pineal gland and reiterates Bsx expression. Bsx homozygous mutant mice were born at the expected Mendelian ratio, but their growth was impaired. Offspring from Bsx homozygous mutant females exhibited a low survival rate due to a nursing defect. Mammary glands of the mutant females developed normally during pregnancy; however, they involuted quickly after parturition. These results demonstrate that Bsx is required for postnatal growth and maintenance of lactating mammary glands. Thus, mouse Bsx is likely involved in systemic control of suppression of apoptosis of postpartum mammary epithelial cells.
Homeobox genes encode a specific class of helix-turn-helix transcription factors characterized by a DNA-binding motif called the homeodomain, which consists of approximately 60 amino acid residues (14). The expression of homeobox genes is controlled in a temporally and spatially restricted manner, and homeoproteins function as a crucial factor in specifying and maintaining cell types. Numerous homeobox genes are expressed in developing and mature brains, including in the hypothalamus and pituitary gland (38). Pituitary homeoproteins have been intensively characterized in terms of pituitary development; however, relatively little is known about the function of hypothalamic transcription factors (8, 22, 32, 37, 42, 45).
The hypothalamus organizes two major sets of neurons: the magnocellular and the parvocellular neurons (34, 35, 37). The cell bodies of magnocellular neurons are located in the paraventricular and supraoptic nuclei of the hypothalamus and project axons to the posterior pituitary gland, where vasopressin (AVP) and oxytocin (OT) are released. The parvocellular neurosecretory system regulates the release of anterior pituitary hormones, such as growth hormone (GH), thyrotropin, adrenocorticotropin, gonadotropins, and prolactin (PRL), through paracrine systems of releasing/inhibitory factors, including GH-releasing hormone (GHRH), somatostatin (SS), thyrotropin-releasing hormone (TRH), corticotropin-releasing hormone (CRH), and gonadotropin-releasing hormone. While it is known that dopamine inhibits PRL secretion from the anterior pituitary gland, PRL-releasing factors are unclear. Five transcription factors that are involved in the development of the hypothalamus have been identified and have subsequently been mutated in mice. Brn-2 is essential for the production of AVP, OT, and CRH (24, 30). Sim1, Arnt-2, and Otp are required for the generation of AVP, OT, CRH, TRH, and SS-producing neurons (2, 12, 15, 21, 41). Gsh-1 is necessary for GHRH synthesis (18). The hypothalamus-pituitary gland axis is a major component of the neuroendocrine system that controls the homeostasis of energy balance and reproduction by secreting hormones into the systemic circulation. Therefore, dysfunction of these homeoproteins could affect not only the hypothalamus but also distant target organs, including the mammary glands. However, the postnatal lethality of mutant mice obscures the roles of these hypothalamic transcription factors in reproduction.
Lobuloalveolar development in the mammary gland during pregnancy and its remodeling during involution are controlled by extrinsic factors, PRL and leukemia inhibitory factor (LIF). PRL and LIF signals are mediated by intrinsic factors, Stat5 and Stat3, respectively. PRL and PRL receptor-deficient mice fail to develop normal mammary glands (4, 11), and inactivation of Stat5 results in the failure of mammary gland development and function (20). Stat5 and Jak2 are also involved in the maintenance of differentiated secretory mammary epithelium (7, 33, 39). LIF induces Stat3-mediated apoptosis of mammary epithelial cells (17, 29, 43). Stat3 conditional knockout mice display suppression of mammary gland apoptosis and delayed involution (5). It was recently reported that Stat3 induces apoptosis by suppressing Akt-mediated cell survival signaling (1). Transgenic mice overexpressing active Akt in the mammary gland exhibit a delay in involution (31). Similarly, forced activation of overexpressed Stat5 in the mammary gland delays postlactational apoptosis (13). While PRL is released from the pituitary gland, LIF is produced in the mammary gland and is considered a local factor. Therefore, in contrast to lactational control, it is not clear how involution is regulated by the neuroendocrine system.
Recently, a novel hypothalamic homeobox gene, Bsx (for “brain-specific homeobox”), was identified from vertebrates including humans, mice, chickens, zebrafish, and frogs (6). Bsx is expressed in a broad region of the developing and postnatal hypothalamus; however, its molecular functions are unknown. In this study, we generated Bsx mutant mice to investigate in vivo roles of Bsx. Differing from the previously reported mutant mice of the hypothalamic homeobox genes, Bsx mutant mice are viable and fertile and Bsx homozygous mutant mice exhibit a nursing defect. Examination of Bsx mutant mice may shed new light on the interaction between the hypothalamus and mammary glands.
MATERIALS AND METHODS
Generation of Bsx mutant mice.
To isolate Bsx genomic DNA, a mouse 129/SvEv strain genomic library (Stratagene) was screened with a Bsx probe, as described previously (25). The Bsx probe was obtained by PCR using primers 5′-AGC ATC AAC ACA GAG GCC-3′ and 5′-CCT GAG GTG GTC AGG AAA-3′. To generate the BsxΔHD and BsxlacZ alleles, we used ∼2.5-kb SacII-SmaI and ∼4.3-kb SmaI-SacI genomic DNA fragments as 5′ and 3′ homologous arms for a targeting vector, respectively. The targeting vector replaces a ∼1.7-kb SmaI-SmaI genomic sequence encoding the entire homeodomain and adjacent exon/intron sequences by a floxed Neo or internal ribosomal entry sequence (IRES)-lacZ-floxed Neo cassette (Fig. 1). A herpes simplex virus thymidine kinase expression cassette was inserted in the 3′ arms of homology of both targeting vectors for negative selection. Embryonic stem (ES) cell targeting and generation of chimeric mice were performed as described previously (25). When protamine-Cre (PC3) ES cells differentiate into spermatids in male chimeras, Cre recombinase is expressed and catalyzes the removal of the floxed Neo cassettes (Fig. 1). To obtain 129 inbred Bsx mutant mice, the chimeric mice were crossed with 129 females. Phenotypic analyses were performed on B6,129 mixed, and 129 inbred mice. We have not observed any effects of genetic background. A ∼0.7-kb SacII fragment was used as a probe for Southern analysis. PCR primers used for genotyping were as follows: a, 5′-CAT CCT CAT GTC TCA GCC-3′; b, 5′-ATG GGC GCT GCC TGG ATC-3′; c, 5′-TGT TCC CGC ACC CGC AGC-3′; d, 5′-AGT TGA GTG CGT TCG TCG-3′; e, 5′-GCA TCG AGC TGG GTA ATA AGC G-3′; and f, 5′-GAC ACC AGA CCA ACT GGT AAT GG-3′. PCR conditions were 94°C for 3 min, followed by 35 cycles of 94°C for 50 s, 55°C for 50 s, and 72°C for 1 min, and a final extension of 72°C for 3 min.
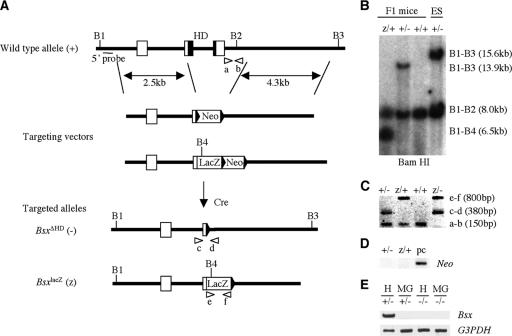
FIG. 1.
Generation of Bsx mutant mice. (A) Strategies of generating BsxΔHD (−) and BsxlacZ (z) mice are shown. Bsx consists of three coding exons (square boxes). To generate the BsxΔHD and BsxlacZ alleles, part of the second and the entire third exons were replaced by a floxed Neo cassette and an IRES-lacZ-floxed Neo cassette, respectively. As a result, the entire homeodomain (black squares) was deleted in both BsxΔHD and BsxlacZ. The length of the 5′ and 3′ homology arms are ∼2.5 and ∼4.3 kb, respectively. Essential diagnostic BamHI enzyme sites are indicated by vertical lines (B1 to B4). Positions of a probe for Southern analysis and PCR primers are indicated by a horizontal line (5′ probe) and arrowheads (a to f), respectively. HD, homeodomain; Cre, Cre recombinase. (B) Genotyping of mice by Southern analysis. (C) Genotyping of mice by PCR analysis. (D) Confirmation of removal of the floxed Neo cassettes by PCR. pc, positive control. (E) Bsx expression by RT-PCR. H, hypothalamus; MG, postpartum mammary glands.
β-Galactosidase staining and histological analysis.
β-Galactosidase staining and histological analyses were performed as described previously (26). Primary antibodies used for immunohistochemical analysis were anti-α-smooth muscle actin (1:400) (Dako) and anti-NeuN (1:1,000) (Chemicon). Immunological reactions were visualized with a Vector ABC kit or by peroxidase-DAB reaction. For immunofluorescence, anti-Stat5 (1:100) (Santa Cruz Biotechnology) and anti-E-cadherin (1:200) (BD Biosciences) were used as primary antibodies. After 1 h of incubation at room temperature with fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (1:100) (Sigma) or Texas red-conjugated anti-rabbit immunoglobulin G (1:200) (Jackson ImmunoResearch) secondary antibodies in the dark, slides were mounted with DAPI (4′,6′-diamidino-2-phenylindole)-containing Vectashield (Vector Laboratories).
Cell apoptosis and proliferation assays.
To detect cell death, the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) In Situ Cell Death kit (Roche) was used according to the manufacturer's instructions. Active caspase 3 was detected by immunohistochemistry using anti-cleaved caspase 3 antibody (1:200) (Cell Signaling). For 5-bromo-2′-deoxyuridine (BrdU) labeling, mice were intraperitoneally injected with 50 mg/kg of BrdU 3 h prior to sacrifice. Following rehydration through a graded ethanol series, the samples were incubated first in 3% H2O2-10% methanol in phosphate-buffered saline for 15 min at room temperature, second in 0.2 mg/ml pepsin in 0.1 N HCl for 20 min at 37°C, third in 2 N HCl for 45 min at room temperature, and fourth in Mouse to Mouse block (ScyTek) for 1 h at room temperature. The immunohistochemistry protocol was then followed.
RNA extraction, Northern analysis, and RT-PCR.
Total RNA extraction was performed with TRIzol reagent (Invitrogen). Northern analysis was performed by a standard procedure. A C/EBPδ probe for Northern analysis was obtained by reverse transcription (RT)-PCR using primers 5′-ACC AGG AGA TGC AGC AGA AGC-3′ and 5′-GTA GAG GCA ACG AGG AAT CAA-3′. PCR conditions were 95°C for 2 min, followed by 35 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, and a final extension of 72°C for 2 min. RT-PCR analyses were performed as described previously (25).
Western analysis.
The fourth inguinal mammary gland was harvested and homogenized in a lysis buffer containing proteinase inhibitors. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis, proteins were transferred to a Hybond-ECL membrane (Amersham). Membranes were blocked in 5% powdered milk for 1 h at room temperature and incubated with 1:1,000 primary antibodies (pStat3, Stat3, pAkt, Akt, and C/EBPδ from Cell Signaling; β-actin from Sigma) at 4°C overnight, and then the immunoreactive proteins were visualized with the ECL Western Blotting System Analysis kit (Amersham) and BioMax XAR film (Kodak).
RESULTS
Generation of Bsx mutant mice.
Bsx is a newly identified homeobox gene that has restricted expression in the central nervous system. Mouse Bsx is encoded by three coding exons on chromosome 9. To investigate in vivo functions of Bsx, we generated two mutant alleles of Bsx by gene targeting in mouse ES cells. In the BsxΔHD and BsxlacZ alleles, part of the second and the entire third coding exons and their flanking intronic sequences have been replaced by a loxP sequence and a lacZ expression cassette, respectively, which removes the homeobox and the C-terminal regions, including a transcriptional activation domain (Fig. 1). Thus, both alleles are expected to be functionally deficient. Bsx expression was not detected by RT-PCR (Fig. 1E). Removal of the floxed Neo cassettes was confirmed by Southern and PCR analyses (Fig. 1B and D). Mice heterozygous for the BsxΔHD and BsxlacZ alleles appeared normal and were fertile. Bsx homozygous mutant mice were obtained by intercrossing Bsx heterozygotes. No phenotypic difference was observed between the BsxΔHD and BsxlacZ homozygous mutant mice except for the LacZ expression of BsxlacZ.
Expression of Bsx-lacZ in Bsx mutant mice.
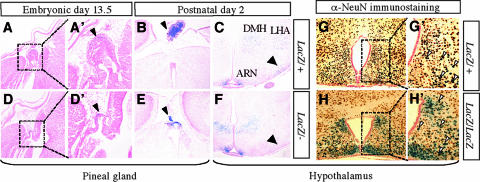
We examined Bsx-lacZ expression by using BsxlacZ heterozygous mutant mice. At embryonic day 13.5 (E13.5), lacZ expression was detected as a spot at the midline region of the developing brain that seems to correspond to the pineal gland (Fig. 2). In the postnatal central nervous system, lacZ expression was specifically detected in the pineal gland and hypothalamus at postnatal day 2 (P2). At P21, lacZ expression was detected in the caudal region of the hypothalamus. Strong expression was observed in the area surrounding the third ventricle, where hypothalamic hormone-producing neurons are located. Histological examination revealed that Bsx-lacZ was expressed in a broad region of the developing and postnatal hypothalamus, including the arcuate nuclei (ARN), the dorsomedial nuclei (DMH), and the lateral hypothalamic area (LHA), as well as the pineal gland (Fig. 3). The expression pattern of Bsx-lacZ is similar to the previously reported Bsx expression (6). Thus, BsxlacZ appears to be a faithful reporter of Bsx expression. Bsx-lacZ-expressing cells express a neuronal marker, NeuN, indicating that Bsx-expressing cells are neurons.
FIG. 2.
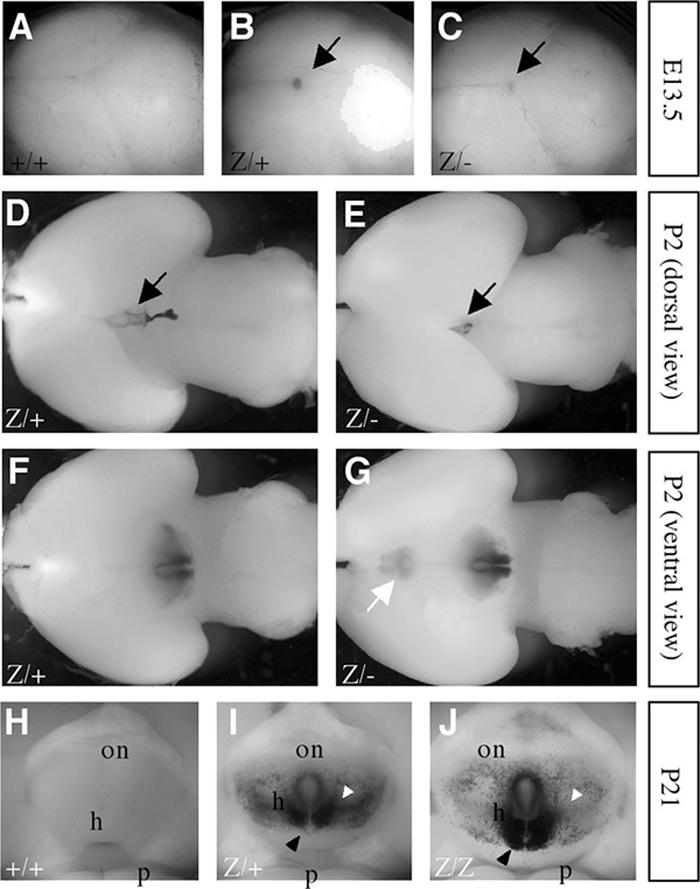
Bsx-lacZ expression in the embryonic and postnatal brain. (A to C) Bsx-lacZ expression was detected as a blue spot in the temporal region of E13.5 mouse brains (black arrows). (D to G) Bsx-lacZ expression was detected in the pineal gland (black arrows) and the hypothalamus (ventral view) of P2 mouse brains. Ectopic Bsx-lacZ expression was detected in the ventral preoptic region of Bsx homozygous mutant mice (white arrow). (H to J) Bsx-lacZ expression was detected in the caudal region of the hypothalamus at P21. The expression around the third ventricle was extended caudally in homozygous mutants compared to heterozygous mutants (black arrowheads). The expression in the lateral region was reduced in homozygous mutants (white arrowheads). on, optic nerve; h, hypothalamus; p, pons; +/+, wild-type; z/+, BsxlacZ heterozygote; z/z, BsxlacZ homozygote; z/−, BsxlacZ/BsxΔHD.
FIG. 3.
Histological analysis of Bsx-lacZ positive cells. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)-stained postnatal embryos and brains were paraffin embedded, sectioned, and counterstained with eosin Y (A and D) or Nuclear Fast Red (B, C, E, and F). (A, B, D, and E) Pineal gland. The pineal gland of BsxlacZ/ΔHD is hypoplastic compared to that of BsxlacZ/+ (arrowheads). (C and F) Hypothalamus. Bsx-lacZ-positive cells are detected in the ARN, the DMH, and the LHA. The number of Bsx-lacZ-positive cells in a region adjacent to the ARN is reduced in BsxlacZ/ΔHD (arrows), which is consistent with Fig. 2. (A, B, and C) BsxlacZ/+. (D, E, and F) BsxlacZ/ΔHD. A′ and D′ are higher magnifications of A and D, respectively. (G and H) X-Gal-stained postnatal brains were paraffin embedded, sectioned, and subjected to immunohistochemical analysis using anti-NeuN antibody, which marks neuronal cells (brown). Sections were counterstained by Nuclear Fast Red (pink). Most X-Gal-positive cells are also positive for NeuN (arrowheads), indicating that Bsx is expressed in neurons. More LacZ-positive cells were observed to cluster around the third ventricle in homozygous mutants. (G) BsxlacZ heterozygote (LacZ/+). (H) BsxlacZ homozygote (LacZ/LacZ). G′ and H′ are higher magnifications of G and H, respectively.
When Bsx-lacZ expression between BsxlacZ heterozygous and homozygous mutant embryos was compared, the expression area in the pineal gland narrowed and only faint staining was observed (Fig. 2C). This is likely due to the pineal gland hypoplasticity in Bsx homozygous mutant mice (Fig. 2E; Fig. 3D and E). In the Bsx homozygous mutants, the hypothalamic expression extended more caudally and less laterally, and more Bsx-lacZ-positive cells were observed around the third ventricle. (Fig. 2G and J). Ectopic Bsx-lacZ expression was detected in the ventral preoptic region of the Bsx homozygous mutant brain (Fig. 2G). Histological analysis confirmed this distinct expression pattern of Bsx-lacZ in the homozygotes. These expression pattern changes were observed in both males and females and are not due to a copy number effect of the lacZ gene, because similar results were obtained with BsxlacZ/BsxΔHD mice, which have a single copy of the lacZ gene. The aberrant expression of Bsx-lacZ in the brain was detected in neonates and maintained into adulthood regardless of pregnancy status. These observations suggest that Bsx regulates its own expression and/or is involved in neuronal cell development/migration. Consistently with RT-PCR results (Fig. 1E), we did not detect Bsx-lacZ expression in the pregnant or postpartum mammary epithelia of either BsxlacZ heterozygous or homozygous mutant mice (our unpublished results).
Growth retardation of Bsx homozygous mutant mice.
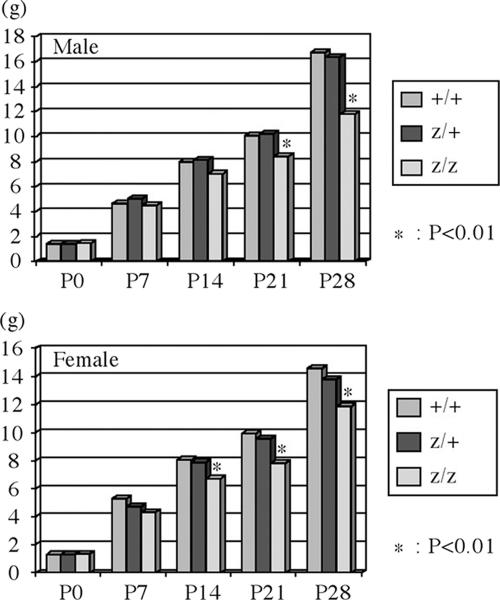
Although Bsx homozygous mutant mice were obtained at nearly the expected Mendelian ratios from intercrossings of Bsx heterozygous mutant mice, they demonstrated lower body weight after 2 weeks of age in both males and females than did wild-type and Bsx heterozygous mutant mice (Fig. 4). Bsx homozygous mutant mice were proportionally smaller than their wild-type littermates. Heterozygotes were indistinguishable from their wild-type littermates (not shown).
FIG. 4.
Growth defect of Bsx mutant mice. (A) Body weight of wild-type (+/+), heterozygous (z/+), and homozygous (z/z) mutant mice at P0, P7, P14, P21, and P28. The upper and lower panels show body weights of male and female mice, respectively. Statistical analyses were scored by t test. The pups were nursed by Bsx heterozygous mutant dams. The penetrance of the growth defect is 100%, and Bsx homozygous mutant mice can be distinguished from their wild-type or heterozygous littermates by observation and/or weight.
Nursing defect of Bsx mutant mice.
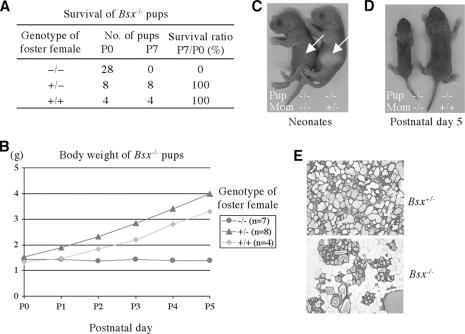
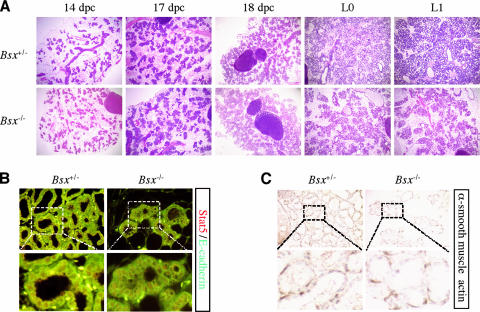
Both male and female Bsx homozygous mutant mice are fertile. However, while breeding Bsx homozygous mutant mice, we noticed that none of the offspring from Bsx homozygous mutant females survived (Fig. 5). Both heterozygous and homozygous mutant offspring from Bsx homozygous mutant females exhibited postnatal lethality and poor growth. However, Bsx homozygous mutant pups from Bsx heterozygous mutant females or Bsx homozygous mutant pups fostered onto wild-type females gained weight and survived to adulthood, suggesting that the lethality and growth defect was not related to the genotype of the pups and that Bsx homozygous mutant females have a nursing defect. Accordingly, when wild-type pups were fostered onto Bsx homozygous mutant females, the pups did not gain weight and died. Maternal behaviors, such as nest building and pup retrieval, seemed normal in Bsx mutant females, and they did not abandon their pups. It appears that the lethality and poor growth are caused by malnutrition, since the affected pups often had little or no milk in their stomachs (Fig. 5C) and they died without gaining weight. To test this hypothesis, we examined postpartum lactating day 1 mammary glands and noticed less alveoli in Bsx homozygous mutant females (Fig. 5E). To identify the onset of the abnormal lobuloalveolar structure, we next examined the mammary glands of pregnant females. Notably, the mammary gland of Bsx homozygous mutant females was similar to that of Bsx heterozygous mutant females up to 18.5 days postcoitum (Fig. 6A), suggesting that mammary gland development occurs normally during pregnancy even in the absence Bsx. Correspondingly, active nuclear Stat5 was detected by immunohistochemistry through pregnancy and after birth, indicating that Bsx mutant females retain PRL-Stat5 signaling (Fig. 6B). Furthermore, E-cadherin and α-smooth muscle actin expression was detected (Fig. 6B and C), supporting the hypothesis that the mutant mammary glands develop normally. Collectively, Bsx homozygous mutant females manifest an abnormality in their mammary gland epithelium soon after parturition.
FIG. 5.
Nursing defect of Bsx mutant mice. (A and B) All pups (heterozygotes and homozygotes) from Bsx homozygous mutant females died in the neonatal period without gaining weight. The affected pups had no or little milk in their stomachs (white arrows in panel C) and were severely growth retarded, with malnutrition (D). However, when Bsx homozygous mutant pups were cross-fostered on wild-type females, they survived and gained weight. Note that the cross-fostered pups showed delayed weight gain due to a lack of milk supply from Bsx homozygous mutant dams on the day of birth. (E) Hematoxylin-eosin Y staining of postpartum mammary glands. +/+, wild-type; +/−, Bsx heterozygote; −/−, Bsx homozygote.
FIG. 6.
Normal pregnancy-induced mammary gland development. (A) Histological analysis of hematoxylin-eosin Y-stained mammary glands at several time points: 14, 17, and 18 days postcoitum (dpc), lactating day 0 (L0), and L1. (B) Stat5 (red) and E-cadherin (green) immunostaining of postpartum mammary glands. Stat5 and DAPI signals overlapped, indicating that Stat5 is located in the nucleus (not shown). (C) α-Smooth muscle actin immunostaining. Bsx+/−, Bsx heterozygous mutant female; Bsx−/−, Bsx homozygous mutant female.
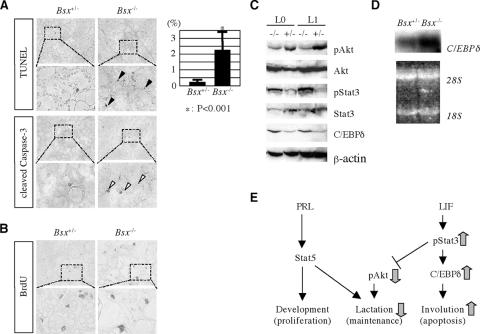
Next, we examined proliferation and cell death in the postpartum mammary gland. More apoptotic mammary epithelial cells were observed in Bsx homozygous mutant females than in Bsx heterozygous mutant females, but cell proliferation appeared similar (Fig. 7A and B). Active phosphorylated Stat3 (pStat3) and C/EBPδ, which are markers for involution (28, 36), were up-regulated in the mutant mammary gland (Fig. 7C and E). The increased amount of C/EBPδ protein in the Bsx homozygotes is regulated at least in part by transcriptional control, as we detected a higher expression level of C/EBPδ mRNA (Fig. 7D). In contrast, phosphorylated Akt (pAkt), a marker for lactating mammary gland, was down-regulated, indicating the activation of a Stat3-induced cell apoptotic signal. Taken together, these data demonstrate that the nursing defect of Bsx mutant females is caused by precocious involution of the lactating mammary gland and that Bsx is required for maintaining lactation and preventing involution in the postpartum period.
FIG. 7.
Premature involution of Bsx homozygous mutant mammary glands. (A) Cell apoptosis analyses of postpartum mammary glands of Bsx heterozygous (Bsx+/−) and homozygous (Bsx−/−) mutant females. Sections were counterstained with methyl green. TUNEL-positive cells per visual field at 40-fold magnification were counted in at least five visual fields per mouse. Four Bsx heterozygous and four homozygous mice were counted. Statistical significance was examined by t test. Black arrowheads, TUNEL-positive cells; white arrowheads, cleaved caspase 3-positive cells. (B) Cell proliferation analyses of postpartum mammary glands. BrdU-positive cells per visual field at 40-fold magnification were counted in at least five visual fields per mouse. Two Bsx heterozygous and three homozygous mice were counted. No statistical significance was observed by t test (P = 0.49). (C) Western blot analyses of lactating mammary glands. L0, lactating day 0; L1, lactating day 1; +/−, BsxΔHD heterozygote; −/−, BsxΔHD homozygote. (D) Northern blot analyses. Total RNAs were isolated from postpartum mammary glands and hybridized with C/EBPδ-radiolabeled probes. The lower panel shows an ethidium bromide-stained gel to show the amount of loaded RNAs. 28S, 28S rRNA band; 18S, 18S rRNA band. (E) PRL-Stat5 and LIF-Stat3 pathways regulate mammary gland development and involution, respectively. Mammary gland development during pregnancy and lactation is controlled by reciprocal actions of the PRL-Stat5 and LIF-Stat3 pathways. The former regulates lactoalveolar structure development and its function, whereas the latter induces involution by inhibiting a cell survival factor, Akt, and activating a cell apoptotic factor, C/EBPδ. In Bsx homozygous mutant mice, lactation and involution signals are down- and up-regulated, respectively (gray arrows).
DISCUSSION
In this study, we investigated the in vivo functions of a recently identified homeoprotein, BSX, by generating and analyzing two mutant alleles of mouse Bsx, BsxΔHD and BsxlacZ. We have shown that mouse BSX is essential for normal postnatal growth and nursing. This is the first report of in vivo roles for BSX. Bsx is conserved among nonmammalian vertebrates, suggesting that mammalian BSX acquired the novel capability of regulating mammary gland function during evolution.
Transcriptional activity of BSX.
β-Galactosidase analyses demonstrated distinct expression patterns of Bsx-lacZ in Bsx homozygous mutant mice, suggesting that Bsx regulates its expression and/or is involved in neuron development/migration. Our unpublished results indicate that BSX functions as a transcriptional activator in vitro and that candidate cis-regulatory elements of Bsx contain several potential BSX-binding sequences. Therefore, it is likely that BSX autoactivates its own expression, especially in the LHA. However, this does not account for the extended or ectopic Bsx-lacZ expression observed in Bsx homozygous mutant mice. Thus, more-complicated mechanisms are probably employed to regulate BSX expression and function in vivo. BSX may negatively control its own expression directly or indirectly in the ARN. Alternatively, BSX is involved in Bsx-expressing cell migration in the hypothalamus. The fact that Bsx is expressed in developing and mature brains suggests that BSX is involved in both brain development and function. The pineal gland hypoplasia seen in Bsx mutant mice demonstrates the requirement of BSX for normal brain development. Bsx-lacZ expressing cells retain a neuronal marker, NeuN, in the Bsx homozygous mutant hypothalamus, indicating that BSX is dispensable for neuronal cell fate determination of Bsx-expressing cells. Rather, BSX-dependent transcriptional control may play important roles in neuronal activity.
Growth retardation caused by Bsx deficiency.
Bsx homozygous mutant mice manifested growth retardation after 2 weeks of age. The onset of growth retardation corresponds to that of dwarfisms caused by pituitary deficiencies, in which growth defects are usually observed within a couple of weeks after birth (2, 16, 27, 44). Therefore, the growth defects of Bsx mutant mice may be caused by dysfunction of the hypothalamus-pituitary gland axis. Accordingly, the serum GH level is slightly reduced in Bsx homozygous mutant mice compared to wild-type and Bsx heterozygous mutant mice at 3 to 5 weeks of age (our unpublished results). Because Bsx is expressed in the ARN, where GHRH is produced, and Bsx expression is not detected in the pituitary gland, Bsx mutants may have a defect in GHRH production and/or secretion. Given that Bsx and Gsh-1 share a DNA-binding sequence and that rat Gsh-1 regulates the GHRH promoter (23; also, our unpublished results), Bsx may be involved in the regulation of GHRH expression.
Premature involution in Bsx mutant females.
The lactation and involution of mammary glands are regulated by reciprocal activation of Stat5 and Stat3. It is believed that Stat5 is activated by systemic hormonal changes in PRL levels and that local factors such as LIF control the phosphorylation state of Stat3 (17, 20, 29). The mammary glands of Bsx mutant mice involuted quickly after parturition, and mammary epithelial cells underwent apoptosis, although they maintained nuclear Stat5. These results show that Bsx is important for preventing programmed cell death of epithelial cells after giving birth and for maintaining lactating mammary glands. Our observations suggest that the failure to inhibit apoptotic signals overrides the PRL-Stat5 pathway. This is consistent with previous reports that PRL replacement is ineffective to inhibit involution (9) and that the mammary gland-derived involution signal is dominant over the presence of systemic lactating signals (19). Several studies concluded that local involution signals suffice to induce apoptosis in lactating mammary epithelia (10, 19). However, these studies do not exclude the possibility that systemic hormonal regulation contributes to the inhibition of involution in lactating mammary glands. Our results indicate that Bsx is required for antiapoptotic signals in the postpartum mammary gland. It should be noted that Bsx expression was not detected by RT-PCR and Bsx-lacZ expression was not detected in the pregnant or postpartum mammary epithelia. Thus, our data suggest that there is a link between local involution factors in the mammary glands and systemic neuroendocrine regulation, although this hypothesis needs to be verified in future studies.
OT is produced in magnocellular neurons in the hypothalamus and is required for milk removal. OT-deficient mice are unable to eject milk, and the mammary glands undergo involution due to milk stasis. We investigated the possibility that Bsx mutant mice are impaired in OT production/secretion. We detected expression of OT in the paraventricular neurons of Bsx mutant mice by in situ hybridization, and OT replacement did not restore the nursing defect (our unpublished results). Moreover, mammary epithelial cell proliferation was observed in Bsx mutant females after parturition, while no proliferation was detected in the mammary gland of OT-deficient dams (40). Therefore, we conclude that the nursing defect of Bsx mutant mice is not likely a result of OT deficiency. The mammary gland of Bsx mutant females exhibited a dramatic change shortly after parturition. The onset resembles that of E-cadherin mutant mice, whose mammary glands develop normally during pregnancy but show dysfunction around parturition (3). It was reported that E-cadherin is required for survival of the lactating mammary gland and that E-cadherin mutant mice cannot nurse their offspring. The phenotype is similar to that of the Bsx mutant female; however, the Bsx mutant mammary glands express E-cadherin. Moreover, Bsx mutant mice retain nuclear Stat5, while E-cadherin mutant mammary glands show weak or no nuclear Stat5, indicating that the premature involution is caused by distinct mechanisms in E-cadherin and Bsx mutant mice. Further research on Bsx may help clarify the currently unknown steps in the neuroendocrine regulation of involution in mammals.
Acknowledgments
We thank Hui-Yi Chu and James DeWille for Northern blotting and discussion.
Footnotes
Published ahead of print on 7 May 2007.
REFERENCES
- 1.Abell, K., A. Bilancio, R. W. E. Clarkson, P. G. Tiffen, A. I. Altaparmakov, T. G. Burdon, T. Asano, B. Van Haesebroeck, and C. J. Watson. 2005. Stat3-induced apoptosis requires a molecular switch in PI(3)K subunit composition. Nat. Cell Biol. 7:392-398. [DOI] [PubMed] [Google Scholar]
- 2.Acampora, D., S. Mazan, F. Tuorto, V. Avantaggiato, J. L. Tremblay, D. Lazzaro, A. di Carlo, A. Mariano, P. E. Macchia, G. Corte, V. Macchia, J. Drouin, P. Brulet, and A. Simone. 1998. Transient dwarfism and hypogonadism in mice lacking Otx1 reveal prepubescent stage-specific control of pituitary levels of GH, FSH and LH. Development 125:1229-1239. [DOI] [PubMed] [Google Scholar]
- 3.Boussadia, O., S. Kutsch, A. Hierholzer, V. Delmas, and R. Kemler. 2002. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech. Dev. 115:53-62. [DOI] [PubMed] [Google Scholar]
- 4.Brisken, C., S. Kauer, T. E. Chavarria, N. Binart, R. L. Sutherland, R. A. Weinberg, P. A. Kelly, and C. J. Ormandy. 1999. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev. Biol. 210:96-106. [DOI] [PubMed] [Google Scholar]
- 5.Charpman, R. S., P. C. Lourenco, E. Tonner, D. J. Flint, S. Selbert, K. Takeda, S. Akira, A. R. Clarke, and C. J. Watson. 1999. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev. 13:2604-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cremona, M., E. Colombo, M. Andreazzoli, G. Cossu, and V. Broccoli. 2004. Bsx, an evolutionary conserved Brain Specific homeoboX gene expressed in the septum, epiphysis, mammillary bodies and arcuate nucleus. Gene Expr. Patterns 4:47-51. [DOI] [PubMed] [Google Scholar]
- 7.Cui, Y., G. Riedlinger, K. Miyoshi, W. Tang, C. Li, C. Deng, G. R. Robinson, and L. Hennighausen. 2004. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol. Cell. Biol. 24:8037-8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dattani, M. T., and I. C. Robinson. 2000. The molecular basis for developmental disorders of the pituitary gland in man. Clin. Genet. 57:337-346. [DOI] [PubMed] [Google Scholar]
- 9.Feng, A., A. Marti, B. Jehn, H. J. Altermatt, G. Chicaiza, and R. Jaggi. 1995. Glucocorticoid and progesterone inhibit involution and programmed cell death in the mouse mammary gland. J. Cell Biol. 131:1095-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gigliotti, A. P., and J. W. DeWille. 1999. Local signals induce CCAAT/enhancer binding protein-delta (C/EBP-delta) and C/EBP-beta mRNA expression in the involuting mouse mammary gland. Breast Cancer Res. Treat. 58:57-63. [DOI] [PubMed] [Google Scholar]
- 11.Horseman, N. D., W. Zhao, E. Montecino-Rodriguez, M. Tanaka, K. Nakashima, S. J. Engle, F. Smith, E. Markoff, and K. Dorshkind. 1997. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 16:6926-6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosoya, T., Y. Oda, S. Takahashi, M. Morita, S. Kawauchi, M. Ema, M. Yamamoto, and Y. Fujii-Kuriyama. 2001. Defective development of secretory neurons in the hypothalamus of Arnt2-knockout mice. Genes Cells 6:361-374. [DOI] [PubMed] [Google Scholar]
- 13.Iavnilovitch, E., B. Groner, and I. Barash. 2002. Overexpression and forced activation of Stat5 in mammary gland of transgenic mice promotes cellular proliferation, enhances differentiation, and delays postlactational apoptosis. Mol. Cancer Res. 1:32-47. [PubMed] [Google Scholar]
- 14.Kappen, C., K. Schughart, and F. H. Ruddle. 1993. Early evolutionary origin of major homeodomain sequence classes. Genomics 18:54-70. [DOI] [PubMed] [Google Scholar]
- 15.Keith, B., D. M. Adelman, and M. C. Simon. 2001. Targeted mutation of the murine arylhydrocarbon receptor nuclear translocator 2 (Arnt2) gene reveals redundancy with Arnt. Proc. Natl. Acad. Sci. USA 98:6693-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kendall, S. K., L. C. Samuelson, T. L. Saunders, R. I. Wood, and S. A. Camper. 1995. Targeted disruption of the pituitary glycoprotein hormone α-subunit produces hypogonadal and hypothyroid mice. Genes Dev. 9:2007-2019. [DOI] [PubMed] [Google Scholar]
- 17.Kritikou, E. A., A. Sharkey, K. Abell, P. J. Came, E. Anderson, R. W. E. Clarkson, and C. J. Watson. 2003. A dual, non-redundant, role for LIF as a regulator of development and STAT3-mediated cell death in mammary gland. Development 130:3459-3468. [DOI] [PubMed] [Google Scholar]
- 18.Li, H., P. S. Zeitler, M. T. Valerius, K. Small, and S. S. Potter. 1996. Gsh-1, an orphan Hox gene, is required for normal pituitary development. EMBO J. 15:714-724. [PMC free article] [PubMed] [Google Scholar]
- 19.Li, M., X. Liu, G. Robinson, U. Bar-Peled, K. Wagner, W. S. Young, L. Hennighausem, and P. A. Furth. 1997. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc. Natl. Acad. Sci. USA 94:3425-3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, X., G. W. Robinson, K. U. Wagner, L. Garrett, A. Wynshaw-Boris, and L. Hannighausen. 1997. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 11:179-186. [DOI] [PubMed] [Google Scholar]
- 21.Michaud, J. L., C. DeRossi, N. R. May, B. C. Holdener, and C. Fan. 2000. Arnt2 acts as the dimerization partner of Sim1 for the development of the hypothalamus. Mech. Dev. 90:253-261. [DOI] [PubMed] [Google Scholar]
- 22.Mullis, P. E. 2000. Transcriptional factors in pituitary gland development and their clinical impact on phenotype. Hormone Res. 54:107-119. [DOI] [PubMed] [Google Scholar]
- 23.Mutsuga, N., Y. Iwasaki, M. Morishita, A. Nomura, E. Yamamori, M. Yoshida, M. Asai, N. Ozaki, F. Kambe, H. Seo, Y. Oiso, and H. Saito. 2001. Homeobox protein Gsh-1-dependent regulation of the rat GHRH gene promoter. Mol. Endocrinol. 15:2149-2156. [DOI] [PubMed] [Google Scholar]
- 24.Nakai, S., H. Kawano, T. Yudate, M. Nishi, J. Kuno, A. Nagata, K. Jishage, H. Hamada, H. Fujii, K. Kawamura, K. Shiba, and T. Noda. 1995. The POU domain transcription factor Brn-2 is required for the determination of specific neuronal lineage in the hypothalamus of the mouse. Genes Dev. 9:3109-3121. [DOI] [PubMed] [Google Scholar]
- 25.Nishijima, I., and A. Ohtoshi. 2006. Characterization of a novel prospero-related homeobox gene, Prox2. Mol. Genet. Genomics 275:471-478. [DOI] [PubMed] [Google Scholar]
- 26.Ohtoshi, A., M. J. Justice, and R. R. Behringer. 2001. Isolation and characterization of Vsx1, a novel mouse CVC paired-like homeobox gene expressed during embryogenesis and retina. Biochem. Biophys. Res. Commun. 286:133-140. [DOI] [PubMed] [Google Scholar]
- 27.Pontoglio, M., J. Barra, M. Hadchouel, A. Doyen, C. Kress, J. P. Bach, C. Babinet, and M. Yaniv. 1996. Hepatocyte nuclear 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syndrome. Cell 84:575-585. [DOI] [PubMed] [Google Scholar]
- 28.Sabatakos, G., G. E. Davies, M. Grosse, A. Cryer, and D. P. Ramji. 1998. Expression of the genes encoding CCAAT-enhancer binding protein isoforms in the mouse mammary gland during lactation and involution. Biochem. J. 334:205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schere-Levy, C., V. Buggiano, A. Quaglino, A. Gattelli, M. C. Cirio, I. Piazzon, S. Vanzulli, and E. C. Kordon. 2003. Leukemia inhibitory factor induces apoptosis of the mammary epithelial cells and participates in mouse mammary gland involution. Exp. Cell Res. 282:35-47. [DOI] [PubMed] [Google Scholar]
- 30.Schonemann, M. D., A. K. Ryan, R. J. McEvilly, S. M. O'Connell, C. A. Arias, K. A. Kalla, P. Li, P. E. Sawchenko, and M. G. Rosenfeld. 1995. Development and survival of the endocrine hypothalamus and pituitary gland requires the neuronal POU domain factor Brn-2. Genes Dev. 9:3122-3135. [DOI] [PubMed] [Google Scholar]
- 31.Schwertfeger, K. L., M. M. Richert, and S. M. Anderson. 2006. Mammary gland involution is delayed by activated Akt in transgenic mice. Mol. Endocrinol. 15:867-881. [DOI] [PubMed] [Google Scholar]
- 32.Scully, K. M., and M. G. Rosenfeld. 2002. Pituitary development: regulatory codes in mammalian organogenesis. Science 295:2231-2235. [DOI] [PubMed] [Google Scholar]
- 33.Shillingford, J. M., K. Miyoshi, G. W. Robinson, S. L. Grimm, J. M. Rosen, H. Neubauer, K. Pfeffer, and L. Hennighausen. 2002. Jak2 is essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Mol. Endocrinol. 16:563-570. [DOI] [PubMed] [Google Scholar]
- 34.Silverman, A., and E. A. Zimmerman. 1983. Magnocellular neurosecretory system. Annu. Rev. Neurosci. 6:357-380. [DOI] [PubMed] [Google Scholar]
- 35.Swanson, L. W., and P. E. Sawchenko. 1983. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 6:269-324. [DOI] [PubMed] [Google Scholar]
- 36.Thangaraju, M., M. Rudelius, B. Bierie, M. Raffeld, S. Sharan, L. Hennighausen, A. Huang, and E. Sterneck. 2005. C/EBPδ is a crucial regulator of pro-apoptotic gene expression during mammary gland involution. Development 132:4675-4685. [DOI] [PubMed] [Google Scholar]
- 37.Treier, M., and M. G. Rosenfeld. 1996. The hypothalamic-pituitary axis: co-development of two organs. Curr. Opin. Cell Biol. 8:833-843. [DOI] [PubMed] [Google Scholar]
- 38.Vollmer, J. Y., and R. G. Clerc. 1998. Homeobox genes in the developmental mouse brain. J. Neurochem. 71:1-19. [DOI] [PubMed] [Google Scholar]
- 39.Wagner, K., A. Krempler, A. A. Triplett, Y. Qi, N. M. George, J. Zhu, and H. Rui. 2004. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol. Cell. Biol. 24:5510-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner, K., S. W. Young, X. Liu, E. I. Ginns, M. Li, P. A. Furth, and L. Hennighausen. 1997. Oxytocin and milk removal are required for post-partum mammary-gland development. Genes Funct. 1:233-244. [DOI] [PubMed] [Google Scholar]
- 41.Wang, W., and T. Lufkin. 2000. The murine Otp homeobox gene plays an essential role in the specification of neuronal cell lineages in the developing hypothalamus. Dev. Biol. 227:432-449. [DOI] [PubMed] [Google Scholar]
- 42.Watkins-Chow, D. E., and S. A. Camper. 1998. How many homeobox genes does it take to make a pituitary gland? Trends Genet. 14:284-290. [DOI] [PubMed] [Google Scholar]
- 43.Zhao, L., S. Hart, J. Cheng, J. Melenhorst, B. Bierie, M. Ernst, C. Stewart, F. Schaper, P. C. Heinrich, A. Ullrich, G. W. Robinson, and L. Hennighausen. 2004. Mammary gland remodeling depends on gp130 signaling through Stat3 and MAPK. J. Biol. Chem. 279:44093-44100. [DOI] [PubMed] [Google Scholar]
- 44.Zhou, Y., B. C. Xu, H. G. Maheshwari, L. He, M. Reed, M. Lozykowski, S. Okada, L. Cataldo, K. Coschigamo, T. E. Wagner, G. Baumann, and J. J. Kopchick. 1997. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc. Natl. Acad. Sci. USA 94:13215-13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu, X., C. R. Lin, G. G. Prefontaine, J. Tollkuhn, and M. G. Rosenfeld. 2005. Genetic control of pituitary development and hypopituitarism. Curr. Opin. Genet. Dev. 15:332-340. [DOI] [PubMed] [Google Scholar]