Abstract
We have previously shown that Bacillus anthracis lethal toxin represses glucocorticoid receptor (GR) transactivation. We now report that repression of GR activity also occurs with the large clostridial toxins produced by Clostridium sordellii and C. difficile. This was demonstrated using a transient transfection assay system for GR transactivation. We also report that C. sordellii lethal toxin inhibited GR function in an ex vivo assay, where toxin reduced the dexamethasone suppression of the proinflammatory cytokine tumor necrosis factor alpha (TNF-α). Furthermore, the glucocorticoid antagonist RU-486 in combination with C. sordellii lethal toxin additively prevented glucocorticoid suppression of TNF-α. These findings corroborate the fact that GR is a target for the toxin and suggest a physiological role for toxin-associated GR repression in inflammation. Finally, we show that this repression is associated with toxins that inactivate p38 mitogen-activated protein kinase (MAPK).
Bacterial infection in mammals initiates an acute-phase response in which the release of acute-phase proteins such as proinflammatory cytokines activates the hypothalamic-pituitary-adrenal axis, resulting in the release of glucocorticoids. Since glucocorticoids are generally anti-inflammatory, these hormones play a crucial role in regulating the immune/inflammatory response to infection and restoring homeostasis. Glucocorticoids exert their effects by binding to the glucocorticoid receptor (GR), which is located in the cytoplasm. Upon ligand binding, the hormone-receptor complex translocates to the nucleus, where it interacts with promoter elements or other transcription factors to regulate gene expression. If the function of the GR is inhibited, severe inflammatory disease can result, leading to the development of shock and eventually death (3, 21).
Previously, our laboratory has shown that lethal factor (LF) from Bacillus anthracis, in combination with protective antigen (PA), represses the transactivation of GR (24, 26). Whether this occurs with other bacterial toxins associated with toxic shock is unknown, and the molecular mechanisms underlying this effect are unclear. We therefore undertook the current study to identify other bacterial toxins that interfere with GR function and to determine whether this effect is mediated by a common mechanism among the different toxins.
To address the mechanism, we examined the possible involvement of the p38 mitogen-activated protein kinase (MAPK) pathway, as LF is known to cleave the MAPK kinases (MKK1 to 4, 6, and 7), enzymes that activate Jun N-terminal protein kinase, p38 MAPK, and extracellular signal-regulated kinases 1 and 2 (22). Until recently, these kinases were not associated with GR activation; however, a recent report from Miller and coworkers (13) showed that p38 MAPK phosphorylates GR, leading to an increase in activity. This is further supported by our earlier studies showing that GR transactivation is specifically repressed by p38 inhibitors to a level equal to that seen for LeTx (24). This evidence led us to hypothesize that other bacterial toxins that target the p38 MAPK pathway might also repress GR transactivation.
Other toxins known to target the p38 MAPK pathway include the large clostridial toxins, which through inactivation of the small GTPases such as Rho and Ras (5, 18) can also inactivate p38 (6, 27). These toxins include lethal toxin (TcsL) from Clostridium sordellii, toxin A (TcdA) from C. difficile, and toxin B (TcdB) from C. difficile. C. sordellii is an emerging bacterial infection associated with toxic shock and has been associated with the deaths of five women in North America who have taken the medical abortion drug mifepristone (RU-486) (4, 12, 20). The closely related bacterium C. difficile is also of clinical concern, with increased reports of more-virulent infections with toxin-positive strains (1, 11). We also investigated toxins that target other cellular pathways, including edema factor (EF) from B. anthracis and diphtheria toxin (DT) from Corynebacterium diphtheriae. EF in combination with PA acts as a calcium- and calmodulin-dependent adenylate cyclase which increases cellular cyclic AMP (cAMP) levels (9, 10). DT is an ADP ribosylation enzyme that inactivates elongation factor 2, which is essential for protein synthesis and cell survival (2, 7).
To determine the effect of each toxin on GR function, we measured changes in GR transactivation in a transient transfection system where recombinant GR was coexpressed with a glucocorticoid-responsive luciferase gene in a GR-deficient cell line. We also examined toxin effects on GR transrepression by measuring cytokine secretion in an ex vivo splenocyte assay. Our results clearly show that only bacterial toxins that targeted the p38 MAPK pathway repressed GR function.
MATERIALS AND METHODS
Materials.
Dexamethasone, RU-486, lipopolysaccharide (LPS), anisomycin, and SB 203580 were obtained from Sigma-Aldrich (St Louis, MO). The B. anthracis toxins LF and PA were a kind gift from S. H. Leppla (NIAID, Bethesda, MD). Lethal toxin from C. sordellii was purified from strain IP82 as previously described (17). The B. anthracis EF, C. difficile toxin A and toxin B, and C. diphtheriae DT were purchased from List Biological Laboratories (Campbell, CA).
Plasmids.
The human GR expression plasmid (pSV-GR) was donated by Jan Carlstedt-Duke (Karolinska Institute, Huddinge, Sweden), and the mouse mammary tumor virus-firefly luciferase reporter construct (pLTR-Luc) was donated by Gordon Hager (NCI, Bethesda, MD). Transfection efficiency was determined by cotransfection with the Renilla luciferase plasmid phRG-TK (Promega, Madison, WI), and normalization of the amount of DNA used per transfection was achieved using herring sperm DNA (Promega). All plasmids were ampicillin resistant and were purified for transfection using a QiaFilter Plasmid Maxi kit (QIAGEN, Valencia, CA) as described by the manufacturer.
Cell culture.
Cos-7 cells (ATCC, Manassas, VA) were cultured in Dulbecco's modified Eagle's medium (Mediatech Inc., Herndon, VA) supplemented with 10% (vol/vol) fetal calf serum (Mediatech) and 2 mM l-glutamine (Gibco, Carlsbad, CA) at 37°C in an atmosphere of 5% CO2. Cytotoxicity assays were performed with alamar blue (Biosource, Carlsbad, CA), and cell integrity was confirmed by trypan blue exclusion.
Splenocyte culture.
Spleens were removed from 12 female Fischer rats (ages, 8 to 10 weeks), minced, and passed through a 70 μM cell strainer (BD Falcon, Bedford, MA). After complete lysis of red blood cells in lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.2 to 7.4), cells were seeded at 1.2 × 106 cells/well into a 48-well plate in RPMI 1640 supplemented with 10% (vol/vol) charcoal-stripped fetal bovine serum (Biomeda Corporation, Foster City, CA), 2 mM l-glutamine, and penicillin-streptomycin. To induce cytokine secretion, 1 μg/ml LPS was used with or without dexamethasone (1 to 1,000 nM) to repress cytokine production. Toxin was added to untreated and treated wells, which were incubated for 24 h before supernatants were collected for enzyme-linked immunosorbent assay (ELISA). Experiments were performed in triplicate for each animal. The estrus cycle stage was determined for each animal by histological examination of cervical swabs.
Measuring cytokine expression.
The proinflammatory cytokine TNF-α was measured using an OptEIA kit for rat TNF (BD Biosciences, San Diego, CA) as described by the manufacturer.
Transient transfections.
Cos-7 cells were seeded into 48-well plates at 5 × 104 cells per well in Dulbecco's modified Eagle's medium containing 10% (vol/vol) charcoal-stripped serum and incubated overnight. The transfection mix was prepared in serum-free medium. Per well, this mix contained a total of 100 ng plasmid DNA and 0.3 μl FuGene6 (Roche Applied Science, Indianapolis, IN) in a final volume of 10 μl. The plasmid breakdown was as follows: 10 ng receptor plasmid, 50 ng luciferase reporter plasmid, 10 ng phRG-TK, and 30 ng herring sperm DNA. The mix was incubated for 15 min and then laid on the cells for 6 h. Appropriate dilutions of hormones and toxins were then added to duplicate cells and incubated for 24 h. Cells were then lysed in passive lysis buffer, and both firefly and Renilla luciferase activities were measured using a dual luciferase reporter assay system (Promega).
cAMP assay.
To identify which toxins induced an increase in intracellular cAMP, Cos-7 cells were seeded into six-well plates and treated with toxin and, as a positive control, with forskolin (25 μM) for 24 h. Cells were lysed in 300 μl 0.1 N HCl for 20 min, and the amount of cAMP in the lysates was measured using a direct cAMP enzyme immunoassay kit from Assay Designs (Ann Arbor, MI) as described by the manufacturer.
p38 assay.
To identify which toxins interfered with p38 activation, Cos-7 cells were cultured in duplicate 96-well plates. They were serum starved for 16 h and treated with toxin in triplicate for 16 h before the stimulation of p38 with 10 μg/ml anisomycin with or without 10 μM SB 203580 (p38 inhibitor) for 15 min. Cells were fixed with 4% formaldehyde, and both total p38 (plate 1) and phosphorylated p38 (plate 2) were measured using a direct-cell p38 ELISA kit from Active Motif (Carlsbad, CA).
Statistics.
All results are expressed as the means ± standard errors of the means. Statistical significance was determined by using the Student t test. A P value of <0.05 was considered significant.
RESULTS
Toxin effects on GR transactivation.
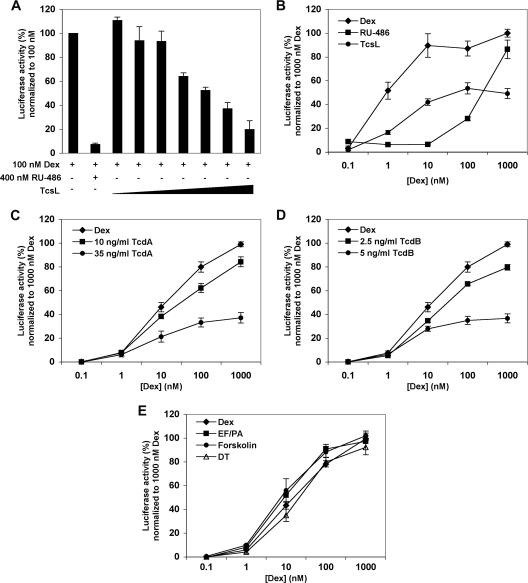
To determine if bacterial toxins other than B. anthracis lethal toxin interfere with GR transactivation, we coincubated Cos-7 cells transfected with GR with 100 nM dexamethasone and increasing concentrations of toxin. TcsL (0 to 20 ng/ml) caused a dose-dependent repression in dexamethasone-induced luciferase activity (Fig. 1A). We also treated the transfectants with increasing concentrations of dexamethasone (0 to 1,000 nM) in the presence of either the competitive GR antagonist RU-486 (400 nM) or 2.5 ng/ml TcsL. This concentration of toxin was calculated as the 50% inhibitory concentration for GR repression. Figure 1B shows that TcsL consistently repressed dexamethasone-induced luciferase activity by 40 to 60% in a noncompetitive manner. No repression was observed when cells were treated with heat-denatured TcsL (data not shown). Similar results were seen with the GR transfectants in the presence of 10 and 35 ng/ml TcdA (Fig. 1C) and 1 and 5 ng/ml TcdB (Fig. 1D), indicating that the C. difficile toxins also repressed GR in a dose-dependent and noncompetitive manner. No effect was seen on GR transactivation when the transfectants were treated with denatured TcdA or TcdB (data not shown).
FIG. 1.
Toxin effects on GR transactivation. Cos-7 cells transfected with human GR were treated for 24 h with 100 nM dexamethasone (Dex) with or without 0.25, 0.5, 1, 2.5, 5, 10, and 20 ng/ml TcsL or 400 nM RU-486 (A); 0 to 1,000 nM dexamethasone and either 400 nM RU-486 or 2.5 ng/ml TcsL (B); 0 to 1,000 nM dexamethasone with or without 10 or 35 ng/ml TcdA (C), 0 to 1,000 nM dexamethasone with or without 1 or 5 ng/ml TcdB (D); or 0 to 1,000 nM dexamethasone with or without either 0.1 ng/ml DT, 50 ng/ml EF plus 200 ng/ml PA, or 25 μM forskolin (E). Each treatment was performed in duplicate, and the results shown are from three to nine separate experiments.
However, when GR transfectants were treated with either 100 pg/ml DT, 50 ng/ml EF plus 200 ng/ml PA, or 25 μM forskolin, there was no effect on dexamethasone-induced luciferase activity, indicating that these toxins did not repress GR transactivation (Fig. 1E).
Ex vivo splenocyte assay.
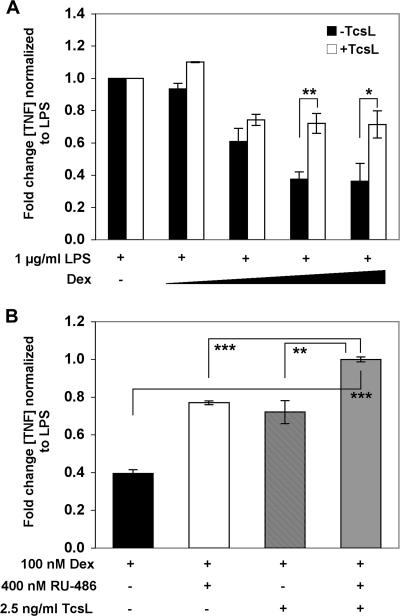
To examine the functional significance of toxin-mediated repression, we used a physiologically relevant ex vivo splenocyte model to measure the effect of TscL on the dexamethasone-mediated inhibition of TNF-α secretion. As expected, dexamethasone maximally suppressed LPS-induced TNF-α secretion by ∼70%, and this was partially reversed in the presence of 2.5 ng/ml TcsL (Fig. 2A). This partial blockage of dexamethasone-mediated suppression was completely reversed when we combined TscL with 400 ng/ml RU-486 (Fig. 2B). This concentration of RU-486 alone reversed the dexamethasone-mediated suppression of TNF-α to levels similar to those seen for TcsL alone. Interestingly, this additive effect was observed only for animals (n = 6) matched for the diestrus phase of the estrus cycle.
FIG. 2.
TcsL reverses dexamethasone-mediated suppression of TNF-α secretion. (A) Splenocytes from female Fischer rats (n = 12) were treated with 1 μg/ml LPS for 24 h in the presence of 0, 0.1, 10, 100 and 1,000 nM dexamethasone (Dex), and the level of secreted TNF-α was determined by ELISA. Cells were also cotreated with 2.5 ng/ml TcsL over the range of dexamethasone levels. (B) Analysis of splenocytes collected from animals in the diestrus phase of the estrus cycle (n = 6) treated with 100 nM dexamethasone and either 400 nM RU-486, 2.5 ng/ml TcsL, or 2.5 ng/ml TcsL plus 400 nM RU-486. Each treatment was performed in triplicate. *, P < 0.05; **, P < 0.005; ***, P < 0.001.
Potential mechanism for shock-associated toxin repression of GR.
The toxins examined in this study have different primary molecular targets in cells. In order to understand why the B. anthracis LeTx previously described (24, 26) and the clostridial toxins from this study repressed GR whereas EF and DT did not, we examined each toxin in a variety of functional assays to identify common pathways. We examined the p38 pathway, as LF is known to prevent p38 phosphorylation and we have already shown that the inactivation of p38 reduces GR transactivation (24). By use of a direct-cell ELISA that measured the level of phosphorylated p38 compared to that of total p38 and normalizing these values to cell number, Cos-7 cells were treated with LeTx (2.5 to 20 ng/ml plus 200 ng/ml PA), TscL (2.5 to 20 ng/ml), TcdA (10 to 100 ng/ml), and TcdB (2 to 20 ng/ml) for 16 h prior to the induction of p38 with anisomycin. With each toxin, there was a decrease in the amount of phosphorylated p38 (Fig. 3A). This decrease was not observed with 100 pg/ml DT or 50 ng/ml EF plus 200 ng/ml PA (data not shown). The specificity of the assay was confirmed using the p38 MAPK inhibitor SB 203850, which completely blocked anisomycin-induced p38 activation (Fig. 3B).
FIG. 3.
Toxin activity on p38 phosphorylation. (A) Cos-7 cells were serum starved and then treated overnight with either 0, 2.5, 5, 10, or 20 ng/ml LF plus 200 ng/ml PA (LeTx); 0, 2.5, 5, 10, or 20 ng/ml TcsL; 0, 2, 5, 10, or 20 ng/ml TcdB; or 0, 10, 20, 50, or 100 ng/ml TcdA. p38 MAPK was then induced for 15 min with 10 μg/ml anisomycin, and the level of phosphorylated p38 was measured by ELISA. (B) Cells induced with anisomycin were also cotreated with 10 μM SB 203580, the specific p38 inhibitor, to confirm the specificity of p38 induction. Each treatment was performed in triplicate, and the results shown are from two separate experiments. ***, P < 0.001.
EF acts as an adenylate cyclase, increasing intracellular cAMP levels. We used an ELISA to detect changes in intracellular cAMP from lysates of Cos-7 cells treated with 10 to 100 ng/ml EF plus 200 ng/ml PA or, as a positive control, with 25 μM forskolin. We observed a twofold increase in cAMP levels in the presence of forskolin (P < 0.005) and a significant dose response increase with 50 and 100 ng/ml EF plus PA (P < 0.005; data not shown). No response was seen with PA alone or with the other toxins (data not shown).
As DT is known to block protein synthesis, we measured luciferase activity in the absence and presence of 10 to 1,000 pg/ml DT for Cos-7 cells transfected with the constitutively expressed Renilla luciferase reporter plasmid. The activity of luciferase should reflect the amount of protein being expressed. With 100 pg/ml DT, there was significant repression of Renilla luciferase activity (25%; P < 0.05; data not shown). At higher concentrations, there was less than 10% activity (P < 0.001) related to the inhibition of protein synthesis; no inhibition was observed with the other toxins (data not shown).
Toxin effects on cell viability and morphology.
In order to verify that the changes observed in this study were not due to cell death, cytotoxicity assays were performed. Cells were also visually examined for morphological changes, as previous studies examining clostridial toxins have reported cell rounding and membrane ruffling (17, 19) due to the action of small GTPases on the actin cytoskeleton. For TcsL, treatment for 24 h with greater than 10 ng/ml toxin resulted in ≥20% cell death, and there was obvious cell ruffling with higher concentrations of TcsL detectable at 6 h. With a 24-h exposure to TcdA, even with concentrations of toxin up to 200 ng/ml, cell death was minimal (<10%), but cell ruffling and rounding was clearly evident at concentrations of ≥50 ng/ml. A similar observation was made with cells treated with TcdB, where cell death was minimal (<10%) even with concentrations up to 40 ng/ml, but cell ruffling and rounding was evident at 24 h in cells treated with as little as 2 ng/ml. Examination of cells treated for 24 h with EF (10 to 100 ng/ml EF plus 200 ng/ml PA) or DT (0.01 to 1 ng/ml) revealed no morphological changes or cell death greater than 10%.
DISCUSSION
This study demonstrates that the large clostridial toxins from C. sordellii and C. difficile repressed GR function in a manner similar to that of anthrax lethal toxin. We have shown that this effect is associated with decreased transactivation and reduced glucocorticoid suppression of proinflammatory cytokine secretion.
We and others have previously shown that an intact glucocorticoid response is necessary for protection from mortality in otherwise inflammation-resistant rodent strains exposed to bacterial products including B. anthracis LeTx, streptococcal cell walls, and Shiga toxin (15, 25). The mechanism by which these toxins cause mortality is not fully understood, and the degree to which cytokine production in vivo contributes to this mortality is debated (14). However, we show here an association with GR repression by bacterial toxins involved with toxic shock, suggesting that toxin repression of GR may contribute to the pathological sequelae in toxic shock.
We also show that toxins that repress GR prevented the activation of p38. Although the concentration of toxin required to repress GR was lower than that required to block p38 phosphorylation, this could reflect the sensitivity of the p38 ELISA. These data indicate that the repression of GR could be related to decreased phosphorylation of p38. This interpretation is consistent with reports that activated p38, which phosphorylates GR at Ser-211, increases receptor activity, while knocking out p38 activation by blocking MKK6 activity reduces GR activity (13). Our earlier studies showing that specific p38 inhibitors repress GR transactivation to the same extent as B. anthracis lethal toxin (24) also support this hypothesis. Another example of a bacterium that targets the p38 pathway was recently reported in a study by Mukherjee and coworkers (16) that showed that the effector protein YopJ from the plague bacterium Yersinia blocks activation of MKK6, a kinase that activates p38.
Our results also show that TcdA, at the concentrations used in this study, prevented phosphorylation of p38, whereas other recent studies have shown that TcdA can activate p38 (8, 23). The main difference between these studies is the concentration of toxin being used in the cell treatments, the cell types being analyzed, and the time periods being measured. Warny and coworkers (23) showed that 100 nM toxin activates p38 within 1 to 2 min in the human monocytic cell line THP-1, and this was shown to occur prior to Rho inactivation. The same toxin at 40 nM causes 90% cell necrosis after 90 to 120 min; at higher concentrations, therefore, TcdA most likely caused cell death more quickly. A study by Kim and coworkers (8) showed a transient induction of p38 from 1 to 3 h after treatment of the human colonocytic cell line NCM460 with 3 nM TcdA which had dissipated by 6 h. They did not report cell viability or morphological changes. Our study, using the primate kidney cell line Cos-7, uses 0.03 to 3 nM TcdA (10 to 100 ng/ml), and the effect on GR and p38 was measured at 24 h after treatment. We report low levels of cell death with 200 ng/ml TcdA (6 nM), yet cell morphological changes were clearly evident at 50 ng/ml, indicating that cells were becoming stressed. The observations in the previous studies are likely related to cell death and apoptosis, which could occur with high concentrations of toxin, whereas our results were recorded at a much earlier point prior to cell death. Possibly, the repression of GR is an early event occurring with a low concentration of toxin, with necrosis being the end point, as has been reported in postmortem studies of toxic shock (4).
Our findings also showed that TcsL in combination with RU-486 completely reversed GR transrepression in our ex vivo model. As RU-486 is both a progesterone and glucocorticoid antagonist, this suggests a role for both progesterone and glucocorticoids, along with their receptors, in this interaction. Further studies are required to determine if this effect is synergistic or additive and to examine if this is relevant in RU-486-associated toxic shock.
In summary, we have shown GR repression by toxins that inhibit p38 MAPK activation and the absence of this effect by toxins that do not inhibit this kinase. In our previous studies using another toxin that inhibits p38 (LeTx), interruption of the glucocorticoid response resulted in greatly enhanced mortality (15, 25). This suggests that GR interruption may play a role in the pathological sequelae of exposure to such toxins. The present findings also suggest that the transient transfection assay system may be a useful method for identifying toxic-shock-associated bacteria. Finally, these data highlight the existence of a potential risk associated with interrupting GR function during infections with such toxin-producing bacteria.
Acknowledgments
This work was supported by the NIMH/NIH Intramural Research Program and an NIAID/NIH Intramural Research Program Biodefense Research Grant.
We thank Elena Belyavskaya and Cash Horn for technical assistance and Cherie Butts for assistance in the preparation of the manuscript.
The views presented in this article do not necessarily reflect those of the Food and Drug Administration.
Editor: A. D. O'Brien
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Barbut, F., B. Gariazzo, L. Bonne, V. Lalande, B. Burghoffer, R. Luiuz, and J. C. Petit. 2007. Clinical features of Clostridium difficile-associated infections and molecular characterization of strains: results of a retrospective study, 2000-2004. Infect. Control Hosp. Epidemiol. 28:131-139. [DOI] [PubMed] [Google Scholar]
- 2.Collier, R. J. 1967. Effect of diphtheria toxin on protein synthesis: inactivation of one of the transfer factors. J. Mol. Biol. 25:83-98. [DOI] [PubMed] [Google Scholar]
- 3.Fan, J., X. Q. Gong, J. Wu, Y. F. Zhang, and R. B. Xu. 1994. Effect of glucocorticoid receptor (GR) blockade on endotoxemia in rats. Circ. Shock 42:76-82. [PubMed] [Google Scholar]
- 4.Fischer, M., J. Bhatnagar, J. Guarner, S. Reagan, J. K. Hacker, S. H. Van Meter, V. Poukens, D. B. Whiteman, A. Iton, M. Cheung, D. E. Dassey, W. J. Shieh, and S. R. Zaki. 2005. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N. Engl. J. Med. 353:2352-2360. [DOI] [PubMed] [Google Scholar]
- 5.Giry, M., M. R. Popoff, C. von Eichel-Streiber, and P. Boquet. 1995. Transient expression of RhoA, -B, and -C GTPases in HeLa cells potentiates resistance to Clostridium difficile toxins A and B but not to Clostridium sordellii lethal toxin. Infect. Immun. 63:4063-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall, A. 2005. Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 33:891-895. [DOI] [PubMed] [Google Scholar]
- 7.Honjo, T., Y. Nishizuka, and O. Hayaishi. 1968. Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis. J. Biol. Chem. 243:3553-3555. [PubMed] [Google Scholar]
- 8.Kim, H., S. H. Rhee, E. Kokkotou, X. Na, T. Savidge, M. P. Moyer, C. Pothoulakis, and J. T. LaMont. 2005. Clostridium difficile toxin A regulates inducible cyclooxygenase-2 and prostaglandin E2 synthesis in colonocytes via reactive oxygen species and activation of p38 MAPK. J. Biol. Chem. 280:21237-21245. [DOI] [PubMed] [Google Scholar]
- 9.Kumar, P., N. Ahuja, and R. Bhatnagar. 2002. Anthrax edema toxin requires influx of calcium for inducing cyclic AMP toxicity in target cells. Infect. Immun. 70:4997-5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leppla, S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 79:3162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433-2441. [DOI] [PubMed] [Google Scholar]
- 12.McGregor, J. A., D. E. Soper, G. Lovell, and J. K. Todd. 1989. Maternal deaths associated with Clostridium sordellii infection. Am. J. Obstet. Gynecol. 161:987-995. [DOI] [PubMed] [Google Scholar]
- 13.Miller, A. L., M. S. Webb, A. J. Copik, Y. Wang, B. H. Johnson, R. Kumar, and E. B. Thompson. 2005. p38 mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol. Endocrinol. 19:1569-1583. [DOI] [PubMed] [Google Scholar]
- 14.Moayeri, M., D. Haines, H. A. Young, and S. H. Leppla. 2003. Bacillus anthracis lethal toxin induces TNF-α-independent hypoxia-mediated toxicity in mice. J. Clin. Investig. 112:670-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moayeri, M., J. I. Webster, J. F. Wiggins, S. H. Leppla, and E. M. Sternberg. 2005. Endocrine perturbation increases susceptibility of mice to anthrax lethal toxin. Infect. Immun. 73:4238-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee, S., G. Keitany, Y. Li, Y. Wang, H. L. Ball, E. J. Goldsmith, and K. Orth. 2006. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312:1211-1214. [DOI] [PubMed] [Google Scholar]
- 17.Popoff, M. R. 1987. Purification and characterization of Clostridium sordellii lethal toxin and cross-reactivity with Clostridium difficile cytotoxin. Infect. Immun. 55:35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popoff, M. R., E. Chaves-Olarte, E. Lemichez, C. von Eichel-Streiber, M. Thelestam, P. Chardin, D. Cussac, B. Antonny, P. Chavrier, G. Flatau, M. Giry, J. de Gunzburg, and P. Boquet. 1996. Ras, Rap, and Rac small GTP-binding proteins are targets for Clostridium sordellii lethal toxin glucosylation. J. Biol. Chem. 271:10217-10224. [DOI] [PubMed] [Google Scholar]
- 19.Servant, G., O. D. Weiner, P. Herzmark, T. Balla, J. W. Sedat, and H. R. Bourne. 2000. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287:1037-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinave, C., G. Le Templier, D. Blouin, F. Leveille, and E. Deland. 2002. Toxic shock syndrome due to Clostridium sordellii: a dramatic postpartum and postabortion disease. Clin. Infect. Dis. 35:1441-1443. [DOI] [PubMed] [Google Scholar]
- 21.Sternberg, E. M., J. M. Hill, G. P. Chrousos, T. Kamilaris, S. J. Listwak, P. W. Gold, and R. L. Wilder. 1989. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc. Natl. Acad. Sci. USA 86:2374-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitale, G., L. Bernardi, G. Napolitani, M. Mock, and C. Montecucco. 2000. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem. J. 352:739-745. [PMC free article] [PubMed] [Google Scholar]
- 23.Warny, M., A. C. Keates, S. Keates, I. Castagliuolo, J. K. Zacks, S. Aboudola, A. Qamar, C. Pothoulakis, J. T. LaMont, and C. P. Kelly. 2000. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J. Clin. Investig. 105:1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webster, J. I., L. H. Tonelli, M. Moayeri, S. S. Simons, Jr., S. H. Leppla, and E. M. Sternberg. 2003. Anthrax lethal factor represses glucocorticoid and progesterone receptor activity. Proc. Natl. Acad. Sci. USA 100:5706-5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webster, J. I., and E. M. Sternberg. 2004. Role of the hypothalamic-pituitary-adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. J. Endocrinol. 181:207-221. [DOI] [PubMed] [Google Scholar]
- 26.Webster, J. I., and E. M. Sternberg. 2005. Anthrax lethal toxin represses glucocorticoid receptor (GR) transactivation by inhibiting GR-DNA binding in vivo. Mol. Cell. Endocrinol. 241:21-31. [DOI] [PubMed] [Google Scholar]
- 27.Wesselborg, S., M. K. Bauer, M. Vogt, M. L. Schmitz, and K. Schulze-Osthoff. 1997. Activation of transcription factor NF-kappaB and p38 mitogen-activated protein kinase is mediated by distinct and separate stress effector pathways. J. Biol. Chem. 272:12422-12429. [DOI] [PubMed] [Google Scholar]