Abstract
Candida albicans, a dimorphic fungus composed of yeast and mycelial forms, is the most common human fungal pathogen. Th1 cytokines such as interleukin-2 (IL-2), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α), which are induced by macrophage IL-12, are critical to resistance against systemic candidiasis, while Th2 cytokines such as IL-4 and IL-5 are less critical. Farnesol is a quorum-sensing molecule produced by C. albicans that controls the formation of mycelia but is also a virulence factor. To determine whether farnesol enhances the virulence of C. albicans by modulating the production of Th1 and Th2 cytokines, mice were pretreated with farnesol prior to intravenous infection with a sublethal dose of farnesol-producing C. albicans. Production of IL-2, IL-4, IL-5, TNF-α, IFN-γ, and IL-12 was evaluated by bead-array flow cytometry and enzyme-linked immunosorbent assay. Mice exhibited an elevation in serum TNF-α levels at 48 h and an elevation in IFN-γ and IL-12 levels at 6 to 12 h after infection with C. albicans. Pretreatment with farnesol significantly reduced the elevation of both IFN-γ and IL-12 but not TNF-α. In contrast, mice pretreated with farnesol exhibited an unexpected elevation in IL-5 levels. To determine whether farnesol has a direct effect on macrophage production of IL-12, peritoneal macrophages were pretreated with farnesol prior to stimulation with IFN-γ plus lipopolysaccharide (LPS). Farnesol inhibited production of both IL-12 p40 and p70 from IFN-γ/LPS-stimulated macrophages. Therefore, the role of farnesol in systemic candidiasis is likely due to its ability to inhibit the critical Th1 cytokines IFN-γ and IL-12 and perhaps to enhance a Th2 cytokine, IL-5.
Candida albicans is a dimorphic commensal fungus and a medically important opportunistic pathogen, particularly in immunocompromised individuals (37). Previous work showed that yeast-mycelium dimorphism in C. albicans is regulated in part by secretion of a lipophilic molecule identified as farnesol (15). Farnesol prevents mycelial development in a quorum-sensing or cell density-dependent manner, and it was the first quorum-sensing molecule (QSM) discovered in a eukaryotic organism. This work was recently reviewed by Nickerson et al. (33). However, all of the work on farnesol as a QSM has been done in vitro, leaving open the question of what role farnesol or farnesol analogs have in vivo. In this regard, Hornby et al. (15) proposed two competing hypotheses. The first was that the shift from yeast to mycelium was a critical step in pathogenesis and that exogenous farnesol could block this transition, thus acting in a therapeutic manner. The alternate hypothesis was that the lipophilic farnesol excreted during infection would interact with host cells in a manner that promoted virulence. The latter idea was supported by our work on the pathogenicity of C. albicans cells pretreated with subinhibitory concentrations (0.5 to 1 μM) of fluconazole (29). These cells secreted 10 times more farnesol than did untreated cells, and they were 4.2 to 8.5 times more lethal (P < 0.001) than untreated cells in a mouse model of intravenously disseminated candidiasis.
This indication that farnesol might be a virulence factor was explored more fully in our recent work, which showed that farnesol promoted virulence by C. albicans (30). This increased virulence was observed when mice received farnesol either intraperitoneally or orally in their drinking water. It was also observed for a C. albicans mutant with a knockout of DPP3, encoding a phosphatase that converts farnesyl pyrophosphate to farnesol. This mutant (KWN2) produced 6 times less farnesol and was 4.2 times less pathogenic to mice than its parent. The strain with DPP3 reconstituted (KWN4) regained both its farnesol production levels and pathogenicity.
These data suggest that farnesol excretion should be included among a list of virulence factors for C. albicans, but they say nothing about what the mode of action of farnesol might be. At that time we suggested four possibilities: farnesol might (i) promote invasiveness by altering the membrane fluidity of host cells; (ii) lyse red blood cells, making iron available for fungal growth; (iii) protect C. albicans from phagocytes by countering their oxidative burst (51); or (iv) interfere with aspects of host defense necessary to survive fungal infection. The present work shows that the last possibility is a correct one but possibly not the only correct one. Farnesol interferes with the normal progression of cytokine induction in mice infected with C. albicans.
The pathophysiologies of disseminated candidiasis in mice and humans are very similar. During early innate immune responses against systemic candidiasis, phagocytosis and both oxidative and nonoxidative mechanisms (8) of monocytes and macrophages (4) destroy both yeast and hyphae of C. albicans. During the adaptive immune response, cell-mediated immunity, with macrophages, T cells, and their cytokines, is required for complete resistance against disseminated candidiasis. In this regard, macrophage interleukin-12 (IL-12) induces the differentiation of CD4 T cells to a Th1 phenotype. The cytokines produced by Th1 cells, gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), signal an increase in the anticandidal activities of macrophages and reinforce expression of IL-12 (2). Therefore, IL-12 is a critical macrophage cytokine in immunity to systemic candidiasis.
On the other hand, IL-4 plays the pivotal role in development of an another subset of CD4+ T cells, Th2, which secrete IL-4, IL-5, IL-10, and IL-13 (28, 45). These cytokines are necessary for antibody responses but antagonize the development and activity of Th1 cells. Therefore, cytokines from Th1 cells have been associated with resistance to C. albicans infection, while Th2 appears to increase susceptibility to infection.
This hypothesis is supported by a study which showed that patients with chronic mucocutaneous candidiasis exhibit Th2 cytokine production during the immune response to C. albicans (20). Furthermore, TNF-α, released by both hematopoietic and nonhematopoietic cells, has been shown to play a vital role in host defense in systemic candidiasis (5). Therefore, TNF-α, IFN-γ, and IL-12 are associated with effective resistance to C. albicans infection in mice. A comparative study of C. glabrata and C. albicans revealed that delayed induction or absence of these cytokines during early infection increases susceptibility to C. albicans (5). Therefore, it is likely that if innate macrophage and adaptive cell-mediated immune responses are compromised, then the containment of commensal C. albicans at mucosal surfaces is impaired, leading to systemic candidiasis (2, 39).
C. albicans may have a number of virulence factors that delay T-cell and macrophage cytokine expression. We have shown that C. albicans produces farnesol, which acts as a QSM to control mycelial development in vitro. In this study, we examined a possible role of farnesol in modulating resistance against systemic candidiasis, as well as in modulating T-cell and macrophage cytokine expression. The results show that farnesol increases the susceptibility of mice to systemic candidiasis. In addition, farnesol decreases expression of the Th1 cytokine IFN-γ and the Th1-inducing cytokine IL-12 and increases expression of the Th2 cytokine IL-5.
MATERIALS AND METHODS
Preparation of farnesol.
Commercially available E,E-farnesol (Sigma, St. Louis, MO) was used in all experiments. Tween 80 (0.5%) in sterile nonpyrogenic normal saline was used to dilute the farnesol to the required concentrations. The solubility of farnesol in water is 1.2 mM; therefore, 0.5% Tween 80 was used to increase the concentrations at which farnesol could be resuspended. Farnesol in 0.5% Tween 80 or Tween 80 alone (control) was delivered by intraperitoneal (i.p.) injection immediately before inoculation.
C. albicans and animal inoculation.
Stock cultures of C. albicans A72 cells were kept at 4°C and passaged once a month to maintain viability. Before each experiment, C. albicans A72 cells were grown for 24 h in 50 ml of modified glucose salts biotin medium (15) at 30°C with aeration. Cells were harvested by centrifugation at 4,750 × g for 10 min. Cells were washed three times with 50 ml of sterile nonpyrogenic saline, counted with a hemacytometer, and resuspended in saline to the required concentrations. Mice were inoculated intravenously (i.v.) in the lateral tail vein. For those mice receiving both C. albicans (i.v.) and farnesol/Tween 80 or Tween 80 alone, i.p. treatment occurred within 5 min of the i.v. inoculation.
Mice.
Female CF-1 mice (9 to 11 weeks old, 20 to 25 g) (Charles River Laboratories, Wilmington, MA) were used for all experiments. Mice were housed at five animals per cage and cared for according to approved protocols of the Institutional Animal Care and Use Committee of the University of Nebraska. The institutional standards are in accordance with all federal, state, and university rules and regulations. Forty-eight mice were inoculated with 1.3 × 106 C. albicans cells. Of those, 24 animals were also given farnesol i.p. Other control groups consisted of 24 animals given farnesol administered i.p. and 24 animals given 0.1% Tween 80 (the solvent for the farnesol stock) administered i.p. These control groups received no fungal challenge. Therefore, the experimental and control groups were as follows: C. albicans infected and also given an i.p. injection of Tween 80, C albicans infected and also given an i.p. injection of farnesol in Tween 80, uninfected with an i.p. injection of farnesol in Tween 80, and uninfected with an i.p. injection of Tween 80. Three animals from each group were sacrificed at 1, 3, 6, 12, 18, 24, and 48 h postinoculation (p.i.). Twelve control animals, i.e., untreated and uninfected, were sacrificed, and the serum was collected. The mean results for these 12 control animals are shown as time zero values in Fig. 1 through 3. Sera separated from the blood collected from individual mice were stored at −80°C until the day of analysis. The same experiment was repeated to examine the IL-12 response by enzyme-linked immunosorbent assay (ELISA) but did not include the 18-h p.i. groups.
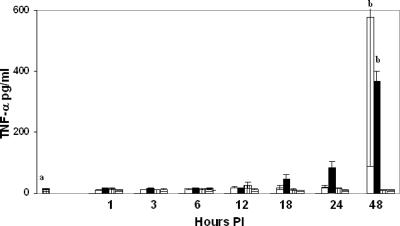
FIG. 1.
Concentrations of TNF-α in mouse sera at 1 to 48 h p.i. Values at time zero are mean values for control sera from 12 mice, open bars represent mean values for mice administered i.v. C. albicans and i.p. Tween 80, closed bars represent mean values for mice administered i.v. C. albicans and i.p. farnesol in Tween 80, vertical-lined bars represent mean values for mice administered i.p. farnesol in Tween 80, and horizontal-lined bars represent mice administered i.p. Tween 80. Data are means ± standard deviations for 3 mice except where “a” indicates 12 mice per group; “b” indicates means that are significantly different from control values.
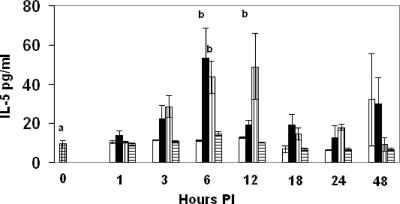
FIG. 3.
Expression of IL-5 in mouse sera at 1 to 48 h p.i. Experimental groups are represented as described in the legend to Fig. 1. Data are means ± standard deviations for 3 mice except where “a” indicates 12 mice per group; “b” indicates means that are significantly different from control values.
Macrophages.
Macrophages were harvested from the peritoneal cavity 3 days after injection of 3% thioglycolate medium (Difco, Detroit, MI). Macrophages were maintained in Dulbecco modified Eagle medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS) (Invitrogen) and 0.05 mg/ml gentamicin (Invitrogen) and incubated at 37°C with 5% CO2. Peritoneal macrophages were cultured overnight, nonadherent cells were removed, and adherent cells were either left untreated or were pretreated with 100 μM farnesol and then were either left unstimulated or were stimulated with 10 ng/ml recombinant mouse IFN-γ (BD Pharmingen, San Diego, CA) plus 500 ng/ml Escherichia coli O111:B4 lipopolysaccharide (LPS) (Invivogen, San Diego, CA). After 24 h, supernatants were collected for IL-12 ELISAs.
Cytokine assays. (i) Bead array.
Murine serum was collected from sacrificed mice at various time points following infection with C. albicans with and without farnesol pretreatment. The BD Cytometric Bead Array (CBA), the mouse Th1/Th2 cytokine kit (catalog no. 551287) of BD Biosciences, San Diego, CA, was used for the detection IL-2, IL-4, IL-5, TNF-α, and IFN-γ, according to the manufacturer's specifications. Briefly, microbead populations with distinct fluorescence intensities were coated with capture antibody specific for each cytokine. The capture beads, phycoerythrin-conjugated detection antibodies, and recombinant standards or test samples were incubated at room temperature. The samples are then washed with 1 ml of wash buffer and recovered by centrifugation (200 × g for 5 min). After careful aspiration of the supernatant, the beads were resuspended in 300 μl of wash buffer, vortexed briefly, and analyzed with a BD FACScan and BD CBA analysis software. Each assay had a sensitivity range of 20 to 5,000 pg/ml.
(ii) ELISA.
Ninety-six-well polyvinylchloride plates were coated with 2.0 μg/ml purified unconjugated anti-IL-12 p70 (clone 9A5) or anti-IL-12 p40 (clone C15.6) monoclonal antibody (BD Pharmingen, San Diego, CA) in bicarbonate buffer (pH 9.6) at 4°C overnight. After three washes with phosphate-buffered saline (PBS)-0.05% Tween 20 (Sigma, St. Louis, MO), each plate was blocked with PBS-10% FBS. For standard curves, serial dilutions of recombinant IL-12 p70 and IL-12 p40 (BD Pharmingen) were added to additional wells coated with antibody. The plates were incubated at room temperature for 2 h. After four washes with PBS-Tween 20, each plate was incubated with 2.5 μg/ml biotinylated anti-mouse IL-12 p40/p70 (clone C17.8) in PBS-10% FBS at room temperature for 30 min. After five washes with PBS-Tween 20, the plates were incubated with diluted (1:1,000) avidin-peroxidase (BD Pharmingen) at room temperature for 30 min. The plates were washed five times with PBS-Tween 20 and incubated with 3,3′,5,5′-tetramethylbenzidine substrate/hydrogen peroxide solution (BD Pharmingen). IL-12 p40 and p70 were measured by determining the optical densities at 450 nm with an ELISA plate reader.
Statistics.
The mixed procedure of the SAS system was used to analyze serum cytokine expression patterns among all treatment groups at various time points. Time trends of treatments were compared for significant differences by estimating and comparing means of each triplicate reading at various time points.
RESULTS
Farnesol reduces serum IFN-γ levels but increases serum IL-5 levels during systemic candidiasis.
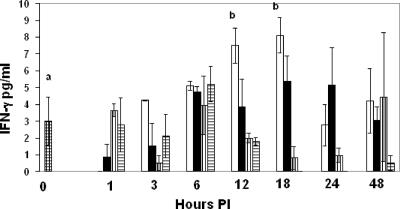
IFN-γ and TNF-α are critical for immunity against systemic candidiasis. To determine whether farnesol modulates these cytokines during systemic candidiasis, the following experimental design was employed. Sera from 12 control mice were compared with a 1- to 48-h time sequence for four categories of three test mice each: C. albicans infected and also given an i.p. injection of Tween 80, C. albicans infected and also given an i.p. injection of farnesol in Tween 80, uninfected with an i.p. injection of farnesol in Tween 80, and uninfected with an i.p. injection of Tween 80. Serum TNF-α, IFN-γ, IL-5, and IL-12 concentrations are shown in Fig. 1 through 4, respectively. For convenience, the control sera (uninfected and untreated) are shown at time zero. C. albicans infection significantly elevated TNF-α levels at 48 h p.i. (P < 0.01), but farnesol alone did not stimulate TNF-α (Fig. 1) nor did it modulate TNF-α production during systemic candidiasis. Similarly, the serum IL-2 and IL-4 levels were present and detectable but not affected by C. albicans infection or by farnesol administration either by itself or in combination with C. albicans infection (data not shown). However, C. albicans infection significantly increased IFN-γ levels after 12 and 18 h (P, <0.03 and 0.01, respectively), and, in contrast to its effect on TNF-α, farnesol significantly decreased IFN-γ levels (Fig. 2) following C. albicans infection at 12 h (P < 0.04).
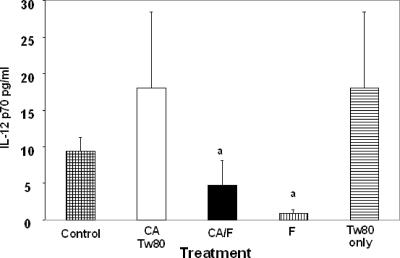
FIG. 4.
Serum IL-12 levels at 12 h p.i. Treatments were C. albicans (CA)/Tween 80 (Tw80), C. albicans plus farnesol (CA/F), farnesol (F), and Tween 80 (Tw80) only. Data are means ± standard deviations for three mice. “a” indicates means that are significantly lower than those for the C. albicans only and Tween 80 only groups.
FIG. 2.
Concentrations of IFN-γ in mouse sera at 1 to 48 h p.i. Experimental groups are represented as described in the legend to Fig. 1. Data are means ± standard deviations for 3 mice except where “a” indicates 12 mice per group; “b” indicates means that are significantly different from control values.
Unlike Th1 cytokines, Th2 cytokines may antagonize effective immunity against systemic candidiasis. C. albicans infection alone did not increase serum IL-5 levels compared to control mice. However, farnesol significantly elevated serum IL-5 levels in C. albicans-infected mice at 3 and 6 h p.i. (P, <0.05 and 0.0001, respectively). In addition, farnesol itself elevated IL-5 levels at 3, 6, and 12 h p.i. (P, 0.03, 0.0001, and <0.0001, respectively). As another control, the Tween 80 used to dissolve farnesol did not significantly affect serum IL-5 levels (Fig. 3).
Farnesol reduces serum IL-12 levels during systemic candidiasis.
IL-12 is the determining macrophage cytokine that stimulates both development of the Th1 subset and IFN-γ production from CD4 T cells. Therefore, we examined serum IL-12 levels in untreated or farnesol-treated mice infected with C. albicans. Significantly different levels of IL-12 were observed only at 12 h after C. albicans infection. At this time, farnesol pretreatment significantly reduced the levels of serum IL-12 (P < 0.04) in mice infected with C. albicans (Fig. 4).
Farnesol reduces in vitro macrophage production of IL-12 p70 and p40 expressed in response to IFN-γ plus LPS.
The data so far indicate that farnesol suppresses IFN-γ production in response to C. albicans infection through its impact on macrophage IL-12 p70, a cytokine composed of p35 and p40 subunits. In addition, p40 can form homodimers that have antagonistic and agonistic effects. However, IL-12 p70-induced IFN-γ feeds back and reinforces IL-12 production from macrophages, while p40 homodimers antagonize this effect (22). Macrophages produce the maximum amount of IL-12 p70 in response to IFN-γ plus LPS (49). Thus, to determine whether farnesol directly suppresses IL-12 p70 production compared with IL-12 p40, primary peritoneal macrophages were pretreated with 100 μM farnesol prior to stimulation with IFN-γ plus LPS. This concentration of farnesol is considered relevant for cells cultured in medium with 10% FBS (27). The data indicate that the combination of IFN-γ and LPS potently stimulates production of both IL-12 p40 and p70, 2,100- and 10-fold, respectively (Table 1), while farnesol significantly suppresses production of IL-12 p40 (1,900-fold) and p70 (5-fold) from IFN-γ/LPS-stimulated macrophages. Therefore, farnesol suppression of immunity against systemic candidiasis likely occurs through suppression of both IFN-γ production by T cells and IL-12 production by macrophages.
TABLE 1.
Effects of farnesol on IL-12 expression in macrophages
| Treatment | Mean IL-12 production ± SD (pg/ml)
|
|
|---|---|---|
| p40 | p70 | |
| None | 1.5 ± 2 | 4.25 ± 8 |
| Farnesola | 0.33 ± 0.5 | 2 ± 3.4 |
| IFN-γ and LPSb | 3,215 ± 2,300 | 40.8 ± 24 |
| Farnesol/IFN-γ and LPSc | 1.7 ± 1.4 | 7.1 ± 14 |
Macrophages pretreated with 100 μM farnesol.
Macrophage IL-12 induced with 10 ng/ml IFN-γ and 500 ng/ml LPS.
Macrophages pretreated with 100 μM farnesol; IL-12 induced with 10 ng/ml IFN-γ and 500 ng/ml LPS.
DISCUSSION
The results of the present investigation show that farnesol, a QSM of C. albicans, suppresses production of IFN-γ and IL-12, but not TNF-α, induced during the host response to C. albicans. It is clear that TNF-α, IFN-γ, and IL-12 are required for resistance against systemic candidiasis. TNF-α is required for resistance to a number of pathogens, including Mycobacterium tuberculosis (42), Listeria monocytogenes (19), and Neisseria meningitidis (50). It is reported to be secreted by hematopoietic and nonhematopoietic cells during fungal infections (21). Even though low levels of TNF-α play a protective role by inducing macrophages to produce microbicidal reactive intermediates (11) and natural killer cells to produce IFN-γ (23), high levels of TNF-α are associated with organ failure and septic shock in experimental lethal infections (24). Our findings showing increased TNF-α at 48 h p.i. in C. albicans-infected mice agree with previous findings (18, 36, 39, 41). TNF-α is produced by a number of cell types following exposure to C. albicans. This cytokine recruits inflammatory cells to the site of infection, promotes growth and differentiation of T cells, and activates endothelia (2). Therefore, increased TNF-α has been associated with Candida infection.
It was somewhat surprising that serum IL-2 and IL-4 were present but not elevated at 48 h p.i. with C. albicans. However, Romani et al. (40) similarly reported that C. albicans-infected mice did not exhibit increased IL-2 and IL-4 levels at 2 days p.i. but that they were elevated at 3 days p.i. Therefore, time after C. albicans infection is likely critical to IL-2 and IL-4 expression. Interestingly, Scaringi et al. (43) reported that as early as 2 h after i.p. infection with C. albicans, and persisting for 5 days, IL-2 expression was detected in cells of the peritoneal cavity, while TNF-α and IL-5 expression was not detected. In contrast, we clearly showed that after i.v. inoculation with C. albicans, TNF-α and IL-5 were detected in serum. This suggests that the route of C. albicans inoculation may dictate the T-cell cytokine phenotype.
In view of the robust expression of IFN-γ by 48 h p.i., and since IL-4 expression is repressed by IFN-γ, it was not surprising that IL-4 expression was not induced further at 48 h p.i. It was, however, somewhat surprising that we detected IL-5, another Th2 cytokine, in response to farnesol. Since IL-4 is the prototypical Th2 cytokine, IL-5 is secreted by activated Th2 following IL-4-induced differentiation (45, 46). While it is possible that farnesol directly stimulates expression of IL-5 from CD4 T cells, the source of IL-5 is more likely to be mast cells (35) or eosinophils (9) within the time frame that it was induced in the present study. IL-5, together with IL-4 and IL-2, contributes to activation and proliferation of B cells, while IL-5 by itself is also the terminal eosinophil differentiation factor (53). Therefore, it is likely that farnesol-treated mice exhibit a surge in eosinophil production. IL-5 is also a significant contributing cytokine to the development of asthma (47), due to its stimulation of eosinophil development (12). Others have reported IL-5 expression during C. albicans infection (6), and it is thought that C. albicans may play a role in the development of certain allergies, including asthma (26, 34). Therefore, for the first time, we show the mechanism by which C. albicans induction of IL-5 could contribute to this crucial event during the development of asthma (10, 17, 31, 38). It remains to be determined whether the elevated levels of IL-5 produced in response to farnesol play a significant role in inhibiting Th1 cytokine expression or in suppressing immunity to systemic candidiasis.
In contrast to IL-2, we found that serum IFN-γ levels increased in mice infected with C. albicans. Numerous studies with a mouse model noted significant IFN-γ responses to C. albicans infection (2, 40). However, in the present study, experimental groups administered farnesol and then challenged with C. albicans exhibited a significant decrease in IFN-γ levels compared with groups challenged with C. albicans but not administered farnesol. Therefore, our finding of depressed elevation of IFN-γ levels in Candida-infected mice treated with farnesol may explain why farnesol decreases resistance to systemic C. albicans infection (30). It is not surprising that farnesol had no effect on IFN-γ levels after 12 h, since our recent report (30) shows that administered farnesol is cleared from the system after 12 h. Similar to IL-5, the source of IFN-γ during C. albicans infection at 12 h is not likely to be CD4 T cells of the adaptive immune response. It is more likely that NK cells are a source of early IFN-γ secretion (1). However, systemic candidiasis usually involves chronic infection of the kidney. Spellburg et al. (44) reported that the basal concentration of IFN-γ is markedly higher in the kidneys than in spleen. In addition, this group describes cells other than CD4+ lymphocytes in the kidney that produce IFN-γ. Therefore, C. albicans may have a virulence factor, such as farnesol, that suppresses IFN-γ production in the kidney. It is likely that the significant source of serum IFN-γ is the kidney, and we propose that farnesol produced locally in the kidney by C. albicans has a direct effect on IFN-γ production there.
While the mechanism by which farnesol induces IL-5 is unclear, the data presented here suggest that farnesol likely reduces expression of IFN-γ through a concomitant reduction in IL-12 expression. Macrophage lineage cells produce IL-12 during innate and adaptive immune responses. In addition to Th1 development, IL-12 stimulates proliferation of NK cells and activation of CD8+ cytolytic T cells (3, 7, 16, 48). IL-12 is a heterodimer composed of p35 and p40 subunits (52). Deficiencies in IL-12 result in low IFN-γ production and impaired Th1 immune responses (13, 14, 25). However, a recent report shows that IL-5 plays a significant role in repression of both IL-12 and IFN-γ production (32). Thus, an understanding of the molecular mechanisms by which farnesol decreases IL-12 p35 and p40 expression will suggest strategies for therapeutically influencing IL-12 production during systemic candidiasis.
Our next goal is to examine what C. albicans genetically programmed to produce more farnesol would do to cytokine expression in disseminated candidiasis. We recently found that fluconazole-pretreated C. albicans is more virulent (29). We hypothesize that a possible overproduction of farnesol during antifungal therapy might cause greater virulence during disseminated candidiasis.
Acknowledgments
This work was supported by the University of Nebraska Tobacco Settlement Biomedical Research Enhancement Fund, the John C. and Nettie V. David Memorial Trust Fund, and the Farnesol and Candida albicans Research Fund of the University of Nebraska Foundation.
Editor: A. Casadevall
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Algarra, I., E. Ortega, M. J. Serrano, G. Alvarez de Cienfuegos, and J. J. Gaforio. 2002. Suppression of splenic macrophage Candida albicans phagocytosis following in vivo depletion of natural killer cells in immunocompetent BALB/c mice and T-cell-deficient nude mice. FEMS Immunol. Med. Microbiol. 33:159-163. [DOI] [PubMed] [Google Scholar]
- 2.Ashman, R. B., and J. M. Papadimitriou. 1995. Production and function of cytokines in natural and acquired immunity to Candida albicans infection. Microbiol. Rev. 59:646-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 4.Blasi, E., L. Pitzurra, A. Bartoli, M. Puliti, and F. Bistoni. 1994. Tumor necrosis factor as an autocrine and paracrine signal controlling the macrophage secretory response to Candida albicans. Infect. Immun. 62:1199-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brieland, J., D. Essig, C. Jackson, D. Frank, D. Loebenberg, F. Menzel, B. Arnold, B. DiDomenico, and R. Hare. 2001. Comparison of pathogenesis and host immune responses to Candida glabrata and Candida albicans in systemically infected immunocompetent mice. Infect. Immun. 69:5046-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cenci, E., A. Mencacci, R. Spaccapelo, L. Tonnetti, P. Mosci, K. H. Enssle, P. Puccetti, L. Romani, and F. Bistoni. 1995. T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J. Infect. Dis. 171:1279-1288. [DOI] [PubMed] [Google Scholar]
- 7.D'Andrea, A., M. Rengaraju, N. M. Valiante, J. Chehimi, M. Kubin, M. Aste, S. H. Chan, M. Kobayashi, D. Young, E. Nickbarg, et al. 1992. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J. Exp. Med. 176:1387-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domer, J. E., L. G. Human, G. B. Andersen, J. A. Rudbach, and G. L. Asherson. 1993. Abrogation of suppression of delayed hypersensitivity induced by Candida albicans-derived mannan by treatment with monophosphoryl lipid A. Infect. Immun. 61:2122-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubucquoi, S., P. Desreumaux, A. Janin, O. Klein, M. Goldman, J. Tavernier, A. Capron, and M. Capron. 1994. Interleukin 5 synthesis by eosinophils: association with granules and immunoglobulin-dependent secretion. J. Exp. Med. 179:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faergemann, J. 2002. Atopic dermatitis and fungi. Clin. Microbiol. Rev. 15:545-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farah, C. S., Y. Hu, S. Riminton, and R. B. Ashman. 2006. Distinct roles for interleukin-12p40 and tumour necrosis factor in resistance to oral candidiasis defined by gene-targeting. Oral Microbiol. Immunol. 21:252-255. [DOI] [PubMed] [Google Scholar]
- 12.Frigas, E., and G. J. Gleich. 1986. The eosinophil and the pathophysiology of asthma. J. Allergy Clin. Immunol. 77:527-537. [DOI] [PubMed] [Google Scholar]
- 13.Goriely, S., B. Vincart, P. Stordeur, J. Vekemans, F. Willems, M. Goldman, and D. De Wit. 2001. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166:2141-2146. [DOI] [PubMed] [Google Scholar]
- 14.Hilliard, B. A., N. Mason, L. Xu, J. Sun, S. E. Lamhamedi-Cherradi, H. C. Liou, C. Hunter, and Y. H. Chen. 2002. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J. Clin. Investig. 110:843-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh, C. S., S. E. Macatonia, C. S. Tripp, S. F. Wolf, A. O'Garra, and K. M. Murphy. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260:547-549. [DOI] [PubMed] [Google Scholar]
- 17.Ishiguro, A., M. Homma, S. Torii, and K. Tanaka. 1992. Identification of Candida albicans antigens reactive with immunoglobulin E antibody of human sera. Infect. Immun. 60:1550-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob, C. O. 1992. Studies on the role of tumor necrosis factor in murine and human autoimmunity. J. Autoimmun. 5(Suppl. A):133-143. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann, S. H. E. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11:129-163. [DOI] [PubMed] [Google Scholar]
- 20.Lilic, D., I. Gravenor, N. Robson, D. A. Lammas, P. Drysdale, J. E. Calvert, A. J. Cant, and M. Abinun. 2003. Deregulated production of protective cytokines in response to Candida albicans infection in patients with chronic mucocutaneous candidiasis. Infect. Immun. 71:5690-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louie, A., A. L. Baltch, R. P. Smith, M. A. Franke, W. J. Ritz, J. K. Singh, and M. A. Gordon. 1994. Tumor necrosis factor alpha has a protective role in a murine model of systemic candidiasis. Infect. Immun. 62:2761-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma, X., J. M. Chow, G. Gri, G. Carra, F. Gerosa, S. F. Wolf, R. Dzialo, and G. Trinchieri. 1996. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J. Exp. Med. 183:147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall, J. D., D. S. Heeke, C. Abbate, P. Yee, and G. Van Nest. 2006. Induction of interferon-γ from natural killer cells by immunostimulatory CpG DNA is mediated through plasmacytoid-dendritic-cell-produced interferon-α and tumour necrosis factor-α. Immunology 117:38-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mencacci, A., E. Cenci, G. Del Sero, C. Fe d'Ostiani, P. Mosci, C. Montagnoli, A. Bacci, F. Bistoni, V. F. Quesniaux, B. Ryffel, and L. Romani. 1998. Defective co-stimulation and impaired Th1 development in tumor necrosis factor/lymphotoxin-alpha double-deficient mice infected with Candida albicans. Int. Immunol. 10:37-48. [DOI] [PubMed] [Google Scholar]
- 25.Mirani, M., I. Elenkov, S. Volpi, N. Hiroi, G. P. Chrousos, and T. Kino. 2002. HIV-1 protein Vpr suppresses IL-12 production from human monocytes by enhancing glucocorticoid action: potential implications of Vpr coactivator activity for the innate and cellular immunity deficits observed in HIV-1 infection. J. Immunol. 169:6361-6368. [DOI] [PubMed] [Google Scholar]
- 26.Mori, A., Y. Ikeda, M. Taniguchi, C. Aoyama, Y. Maeda, M. Hasegawa, N. Kobayashi, and K. Akiyama. 2001. IL-5 production by peripheral blood Th cells of adult asthma patients in response to Candida albicans allergen. Int. Arch. Allergy Immunol. 125(Suppl. 1):48-50. [DOI] [PubMed] [Google Scholar]
- 27.Mosel, D. D., R. Dumitru, J. M. Hornby, A. L. Atkin, and K. W. Nickerson. 2005. Farnesol concentrations required to block germ tube formation in Candida albicans in the presence and absence of serum. Appl. Environ. Microbiol. 71:4938-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348-2357. [PubMed] [Google Scholar]
- 29.Navarathna, D. H., J. M. Hornby, N. Hoerrmann, A. M. Parkhurst, G. E. Duhamel, and K. W. Nickerson. 2005. Enhanced pathogenicity of Candida albicans pre-treated with subinhibitory concentrations of fluconazole in a mouse model of disseminated candidiasis. J. Antimicrob. Chemother. 56:1156-1159. [DOI] [PubMed] [Google Scholar]
- 30.Navarathna, D. H., J. M. Hornby, N. Krishnan, A. Parkhurst, G. E. Duhamel, and K. W. Nickerson. 2007. Effect of farnesol on a mouse model of systemic candidiasis, determined by use of a DPP3 knockout mutant of Candida albicans. Infect. Immun. 75:1609-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nermes, M., J. Savolainen, K. Kalimo, K. Lammintausta, and M. Viander. 1994. Determination of IgE antibodies to Candida albicans mannan with nitrocellulose-RAST in patients with atopic diseases. Clin. Exp. Allergy 24:318-323. [DOI] [PubMed] [Google Scholar]
- 32.Nickdel, M. B., F. Roberts, F. Brombacher, J. Alexander, and C. W. Roberts. 2001. Counter-protective role for interleukin-5 during acute Toxoplasma gondii infection. Infect. Immun. 69:1044-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nickerson, K. W., A. L. Atkin, and J. M. Hornby. 2006. Quorum sensing in dimorphic fungi: farnesol and beyond. Appl. Environ. Microbiol. 72:3805-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palma-Carlos, A. G., M. L. Palma-Carlos, and A. C. Costa. 2002. Candida and allergy. Allerg. Immunol. (Paris) 34:322-324. [PubMed] [Google Scholar]
- 35.Plaut, M., J. H. Pierce, C. J. Watson, J. Hanley-Hyde, R. P. Nordan, and W. E. Paul. 1989. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature 339:64-67. [DOI] [PubMed] [Google Scholar]
- 36.Remick, D. G., and S. L. Kunkel. 1993. Pathophysiologic alterations induced by tumor necrosis factor. Int. Rev. Exp. Pathol. B 34:7-25. [DOI] [PubMed] [Google Scholar]
- 37.Rogers, T. J., and E. Balish. 1980. Immunity to Candida albicans. Microbiol. Rev. 44:660-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roig, E., J. L. Malo, and S. Montplaisir. 1997. Anti-Candida albicans IgE and IgG subclasses in sera of patients with allergic bronchopulmonary aspergillosis (ABPA). Allergy 52:394-403. [DOI] [PubMed] [Google Scholar]
- 39.Romani, L. 1999. Cytokine modulation of specific and nonspecific immunity to Candida albicans. Mycoses 42(Suppl. 2):45-48. [PubMed] [Google Scholar]
- 40.Romani, L., S. Mocci, C. Bietta, L. Lanfaloni, P. Puccetti, and F. Bistoni. 1991. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect. Immun. 59:4647-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothe, J., W. Lesslauer, H. Lotscher, Y. Lang, P. Koebel, F. Kontgen, A. Althage, R. Zinkernagel, M. Steinmetz, and H. Bluethmann. 1993. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364:798-802. [DOI] [PubMed] [Google Scholar]
- 42.Salgame, P. 2005. Host innate and Th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr. Opin. Immunol. 17:374-380. [DOI] [PubMed] [Google Scholar]
- 43.Scaringi, L., E. Rosati, P. Cornacchione, K. Fettucciari, R. Sabatini, R. Biondi, L. Mezzasoma, M. Valiani, P. D'Errico, and P. Marconi. 1995. Local and systemic immune response to inactivated Candida albicans in mice. Nat. Immunol. 14:234-249. [PubMed] [Google Scholar]
- 44.Spellberg, B., D. Johnston, Q. T. Phan, J. E. Edwards, Jr., S. W. French, A. S. Ibrahim, and S. G. Filler. 2003. Parenchymal organ, and not splenic, immunity correlates with host survival during disseminated candidiasis. Infect. Immun. 71:5756-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swain, S. L., A. D. Weinberg, and M. English. 1990. CD4+ T cell subsets. Lymphokine secretion of memory cells and of effector cells that develop from precursors in vitro. J. Immunol. 144:1788-1799. [PubMed] [Google Scholar]
- 46.Swain, S. L., A. D. Weinberg, M. English, and G. Huston. 1990. IL-4 directs the development of Th2-like helper effectors. J. Immunol. 145:3796-3806. [PubMed] [Google Scholar]
- 47.Tillie-Leblond, I., P. Gosset, and A. B. Tonnel. 2005. Inflammatory events in severe acute asthma. Allergy 60:23-29. [DOI] [PubMed] [Google Scholar]
- 48.Trinchieri, G., S. Pflanz, and R. A. Kastelein. 2003. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19:641-644. [DOI] [PubMed] [Google Scholar]
- 49.Vaidyanathan, H., J. D. Gentry, A. Weatherman, S. D. Schwartzbach, and T. M. Petro. 2001. Differential response of the murine IL-12 p35 gene to lipopolysaccharide compared with interferon-gamma and CD40 ligation. Cytokine 16:1-9. [DOI] [PubMed] [Google Scholar]
- 50.Westendorp, R. G., J. A. Langermans, T. W. Huizinga, A. H. Elouali, C. L. Verweij, D. I. Boomsma, and J. P. Vandenbrouke. 1997. Genetic influence on cytokine production and fatal meningococcal disease. Lancet 349:170-173. [DOI] [PubMed] [Google Scholar]
- 51.Westwater, C., E. Balish, and D. A. Schofield. 2005. Candida albicans-conditioned medium protects yeast cells from oxidative stress: a possible link between quorum sensing and oxidative stress resistance. Eukaryot. Cell 4:1654-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolf, S. F., P. A. Temple, M. Kobayashi, D. Young, M. Dicig, L. Lowe, R. Dzialo, L. Fitz, C. Ferenz, R. M. Hewick, et al. 1991. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J. Immunol. 146:3074-3081. [PubMed] [Google Scholar]
- 53.Yamaguchi, Y., T. Suda, J. Suda, M. Eguchi, Y. Miura, N. Harada, A. Tominaga, and K. Takatsu. 1988. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J. Exp. Med. 167:43-56. [DOI] [PMC free article] [PubMed] [Google Scholar]