Abstract
Three signal transduction pathways, the two-component systems CpxRA and BaeSR and the alternative sigma factor σE, respond to extracytoplasmic stress that facilitates bacterial adaptation to changing environments. At least the CpxRA and σE pathways control the production of protein-folding and degradation factors that counter the effects of protein misfolding in the periplasm. This function also influences the biogenesis of multicomponent extracellular appendages that span the bacterial envelope, such as various forms of pili. Herein, we investigated whether any of these regulatory pathways in the enteropathogen Yersinia pseudotuberculosis affect the functionality of the Ysc-Yop type III secretion system. This is a multicomponent molecular syringe spanning the bacterial envelope used to inject effector proteins directly into eukaryotic cells. Disruption of individual components revealed that the Cpx and σE pathways are important for Y. pseudotuberculosis type III secretion of Yops (Yersinia outer proteins). In particular, a loss of CpxA, a sensor kinase, reduced levels of structural Ysc (Yersinia secretion) components in bacterial membranes, suggesting that these mutant bacteria are less able to assemble a functional secretion apparatus. Moreover, these bacteria were no longer capable of localizing Yops into the eukaryotic cell interior. In addition, a cpxA lcrQ double mutant engineered to overproduce and secrete Yops was still impaired in intoxicating cells. Thus, the Cpx pathway might mediate multiple influences on bacterium-target cell contact that modulate Yersinia type III secretion-dependent host cell cytotoxicity.
Type III secretion (T3S) is an extensively studied virulence mechanism used by many gram-negative bacterial pathogens of both animals and plants. Resembling a syringe composed of ∼25 protein components that span the bacterial envelope (52, 56), T3S systems (T3SSs) efficiently secrete and deliver toxic effectors into target cells during intimate contact with a host (27, 30). This enables bacteria to evade host immune responses and facilitate often lethal infections.
Pathogenic bacteria occupy a diverse array of infection niches inside a host. Each niche boasts a unique combination of environmental signals that serve as a detailed grid reference, allowing individual bacteria to monitor their precise location (4, 9). However, bacterial infections are dynamic processes, so bacteria must constantly recognize and respond to different sets of environmental cues depending on the stage of infection. This active spatial and temporal environmental fingerprint is interpreted by multiple and overlapping control factors that coordinately regulate bacterial virulence gene expression, ensuring that each virulence factor is expressed only when needed (4, 9).
The bacterial cell envelope is a critical data-processing center. It contains multiple relay pathways that transmit external signals into the bacterial cytoplasm. These activate subsets of DNA binding proteins that cooperate with RNA polymerase to specifically stimulate gene transcription (4, 9). At least three of these pathways respond to extracytoplasmic stress (ECS): the two-component systems CpxRA and BaeSR and the alternative sigma factor σE (RpoE) (1, 22, 79). Growth in adverse environments triggers the CpxRA- and σE-responsive elements to induce the production of protein-folding and degradation factors designed to counter protein misfolding in the bacterial periplasm. It is less clear what is induced by the BaeSR system, although an Escherichia coli transcriptome analysis indicated a regulon that includes genes related to carbohydrate and multidrug transporters, chemotaxis responses, and flagellum biosynthesis (69). Another group of proteins, collectively functioning in the phage shock response, may also aid in tolerance to periplasmic stress (61). Interestingly, these pathways are now also linked to bacterial pathogenesis (16, 79, 85). They ensure the accurate assembly of multicomponent virulence determinants spanning the bacterial envelope, such as P pili (41, 45), type IV bundle-forming pili (68), and curli (47).
Human-pathogenic Yersinia spp. all harbor a large virulence plasmid encoding the Ysc-Yop T3SS (11). Use of this system to translocate several toxic effectors into the target cell interior enables Yersinia to resist phagocytosis and proliferate extracellularly (24, 91). Yop synthesis and secretion are controlled by temperature and calcium in laboratory media or target cell contact (76, 84). While the virulence plasmid encodes several built-in regulatory elements (55), only a few additional chromosomally encoded regulatory factors have been described: YmoA (12) and its regulated proteolysis via the ClpXP and Lon proteases (44), adenylate cyclase (Cya) and the cyclic AMP receptor (Crp) (75), the SmpB-SsrA translational control system (70), DNA adenine methylase overproduction (48), and the ttsA allele of unknown function (17). Although the mechanism by which most of these components modulate T3S remains obscure, it illustrates how the inherent complexity of T3SSs requires fine-tuning by the activities of multiple regulatory factors.
We propose that Ysc-Yop biogenesis in the bacterial envelope also engages one or more of the ECS response pathways. Herein, we describe the systematic disruption of components comprising the three ECS-responsive pathways. Supporting our hypothesis, disruption of the σE and Cpx pathways affected the functionality of the Ysc-Yop T3SS in vitro. Moreover, the CpxA sensor kinase was required for T3S-dependent cellular cytotoxicity. Thus, ECS response pathways contribute to the pathogenicity of Yersinia pseudotuberculosis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids are listed in Table 1. Bacteria were routinely cultivated in Luria-Bertani (LB) agar or broth at either 26°C (Y. pseudotuberculosis) or 37°C (E. coli) with aeration. To examine T3S, we used Y. pseudotuberculosis YPIII/pIB102 (serotype III), in which pIB102 is the virulence plasmid encoding the Ysc-Yop T3SS. This plasmid is a derivative of pIB1 (7, 29), distinguished only by a kanamycin resistance cartridge inserted into the yadA gene. This disruption does not attenuate the virulence of YPIII/pIB102 in the mouse model of infection (8). Yersinia cells were grown at 26°C overnight in brain heart infusion (BHI) broth supplemented with either 5 mM EGTA [ethylene glycol-bis(β-aminoethyl ether)-N′,N′,N′,N′-tetra-acetic acid] and 20 mM MgCl2 (medium without Ca2+) or 2.5 mM CaCl2 (medium with Ca2+). Induction of secretion followed after a growth shift from 26°C to 37°C. Where required, antibiotics were added at the final concentrations of 100 μg per ml carbenicillin (Cb), 50 μg per ml kanamycin (Km), 20 μg per ml spectinomycin (Sp), and 25 μg per ml chloramphenicol (Cm).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5 | F−recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1 | 33 |
| S17-1λpir | recA thi pro hsdR M+ Smr <RP4:2-Tc:Mu:Ku:Tn7> Tpr | 86 |
| Y. pseudotuberculosis | ||
| YPIII/pIB102 | yadA::Tn5 Kmr (wild type) | 8 |
| YPIII07/pIB102 | YPIII, cpxA in-frame deletion of codons 41 to 449, Kmr | This study |
| YPIII08/pIB102 | YPIII, cpxR in-frame deletion of codons 11 to 193, Kmr | This study |
| YPIII09/pIB102 | YPIII, rpoE in-frame deletion of codons 3 to 181, Kmr | This study |
| YPIII40/pIB102 | YPIII, baeS in-frame deletion of codons 24 to 437, Kmr | This study |
| YPIII29/pIB102 | YPIII, baeR in-frame deletion of codons 22 to 227, Kmr | This study |
| YPIII28/pIB102 | YPIII, rseA in-frame deletion of codons 14 to 194, Kmr | This study |
| YPIII42/pIB102 | YPIII, rseB in-frame deletion of codons 22 to 290, Kmr | This study |
| YPIII/pIB26 | pIB102, exchange of lcrQ with a streptomycin-spectinomycin resistance cassette, Kmr Smr Spr | 76 |
| YPIII07/pIB26 | YPIII/pIB26, cpxA in-frame deletion of codons 41 to 449, Kmr Smr | This study |
| YPIII/pIB202 | pIB102, yscF in-frame deletion, Kmr | 23 |
| Plasmids | ||
| pCR4-TOPO | TA cloning vector, Kmr Cbr | Invitrogen |
| pDM4 | Suicide plasmid carrying sacBR, Cmr | 58 |
| pPJE006 | ∼925-bp XhoI/XbaI PCR fragment of ΔcpxA(41-449) in pDM4, Cmr | This study |
| pMF586 | ∼485-bp XhoI/XbaI PCR fragment of ΔcpxR(11-193) in pDM4, Cmr | This study |
| pMF587 | ∼510-bp XhoI/XbaI PCR fragment of ΔrpoE(3-181) in pDM4, Cmr | This study |
| pMF682 | ∼410-bp XhoI/XbaI PCR fragment of ΔbaeS(24-437) in pDM4, Cmr | This study |
| pKEC009 | ∼445-bp XhoI/XbaI PCR fragment of ΔbaeR(22-227) n pDM4, Cmr | This study |
| pKEC010 | ∼420-bp XhoI/XbaI PCR fragment of ΔrseA(14-194) in pDM4, Cmr | This study |
| pMF680 | ∼460-bp XhoI/XbaI PCR fragment of ΔrseB(22-290) in pDM4, Cmr | This study |
| pJF006 | ∼1,020-bp XhoI/XbaI PCR fragment of cpxR encoding the mutation D51A in pDM4, Cmr | This study |
| pMMB208 | Expression vector, Cmr | 62 |
| pKEC021 | 700-bp XbaI/KpnI PCR fragment of cpxR in pMMB208, Cmr | This study |
| pJF015 | 700-bp XbaI/KpnI PCR fragment of cpxR encoding the mutation D51A in pMMB208, Cmr | This study |
| pET22b(+) | Expression vector, Ampr | Novagen |
| pMF581 | 1,381-bp NdeI/EagI PCR fragment of cpxA in pET22b(+), Cbr | This study |
Mutant and plasmid construction.
Amplified DNA fragments used for constructing individual in-frame deletions were generated by overlap PCR (38). The primer combinations used to create each mutation are listed in Table 2 and were synthesized by either DNA Technology A/S (Aarhus, Denmark) or TAG Copenhagen A/S (Copenhagen, Denmark). All amplified fragments were first cloned into pCR4-TOPO TA (Invitrogen AB, Stockholm, Sweden) and then sequenced commercially (MWG Biotech AG, Ebersberg, Germany). Confirmed fragments were then subcloned into the XhoI/XbaI-digested suicide mutagenesis vector pDM4 (58). Conjugal mating experiments with Y. pseudotuberculosis and selection for the appropriate allelic exchange events used conventional methodology with one exception: standard LB agar with appropriate antibiotics was used to selectively cultivate Yersinia after conjugation (28, 58).
TABLE 2.
Oligonucleotides used in this study
| Purpose | Oligonucleotide pair(s) (sequence)a |
|---|---|
| Mutagenesis (plasmid) | |
| ΔbaeS (pMF682) | pbaeSa (5′-CTC GAG CAC AGC GCG TTC TTA TAC AG-3′) (XhoI) and pbaeSb (5′-TAT CAA TAC CAA CAT GCA AGT G-3′) |
| pbaeSc (5′-TGC ATG TTG GTA TTG ATA ATC AGC GCT AAA CAT TCA CCT-3′) and pbaeSd (5′-TCT AGA GTC AGC CAC TGG GTG CGG T-3′) (XbaI) | |
| ΔbaeR (pKEC009) | pbaeRa (5′-CTC GAG ACG GAA AGT TCA CGT AAT C-3′) (XhoI) and pbaeRb (5′-GGG TTC ATC TTC AAC GAT C-3′) |
| pbaeRc (5′-ATC GTT GAA GAT GAA CCC GGT ATC GCT GGG AAG CAG AGA-3′) and pbaeRd (5′-TCT AGA GTC AGC CAC TGG GTG CGG T-3′) (XbaI) | |
| ΔcpxR (pMF586) | pcpxRa1 (5′-ACG CTC GAG AAC GAA CAT TGA CGC CAT-3′) (XhoI) and pcpxRb (5′-GTC ATC ATC AAC TAA TAG GA-3′) |
| pcpxRc (5′-TTA GTT GAT GAT GAC GAC CGC GCG ATT GAT A-3′) and pcpxRd1 (5′-ACG TCT AGA ACA GTG AGT TGA CGC GA-3′) (XbaI) | |
| cpxR(D51A) (pJF006) | pcpxRa1 and pcpxR-D51Ab (5′-CAT AAT AGC AAG CAA TAA CAA GTC G-3′) |
| pcpxR-D51Ac (5′-TTA TTG CTT GCT ATT ATG ATG CCG C-3′) and pcpxRd1 | |
| ΔcpxA (pPJE006) | pcpxAa (5′-CCG CCG CTC GAG TTA ATG ATC GCG AGC TGG-3′) (XhoI) and pcpxAb (5′-AAC AGT GAG TTG ACG CGA-3′) |
| pcpxAc (5′-CGT CAA CTC ACT GTT TGG CTA CCG CTG CAT-3′) and pcpxAd (5′-TAC TAG TCT AGA AGT CGG CGG TGA TGC-3′) (XbaI) | |
| ΔrpoE (pMF587) | prpoEa (5′-AGC TCG AGT CGT ATG ATG TGT GCA-3′) (XhoI) and prpoEb (5′-GCT CAT CCG AGG TGA ACT CT-3′) |
| prpoEc (5′-AGT TCA CCT CGG ATG AGC GAT AAC AAA GTT CAG CCG CT-3′) and prpoEd (5′-ACG TCT AGA CAC GAC TAG CAA TAT CCA GAT-3′) (XbaI) | |
| ΔrseA (pKEC010) | prseAa (5′-CTC GAG CAC ATT AAG GGA GCT AGA TGG-3′) (XhoI) and prseAb (5′-TTC TCC ATC CAT CAA AGC GG-3′) |
| prseAc1 (5′-GCT TTG ATG GAT GGA GAA CAG CAG CCT TCA GAT TCA ACG-3′) and prseAd (5′-TCT AGA AGC TCA TAA TTG AGC GAC-3′) (XbaI) | |
| ΔrseB (pMF680) | prseBa (5′-CTC GAG AAT CAG GTT CAA GAG CAG-3′) (XhoI) and prseBb (5′-AGC AAT GTT TGC CAT GAA CAG-3′) |
| prseBc (5′-TTC ATG GCA AAC ATT GCT AAC GTC GAG ATA ACG GTA GT-3′) and prseBd (5′-TCT AGA TTG ATG GTC GGT CTC AGG-3′) (XbaI) | |
| Complementation | |
| pCpxA+ (pMF581) | pcpxA-for(ET) (5′-TAC ATA TGA TAA ACA GTT TAA CGA CGC-3′) (NdeI) and pcpxA-rev(ET) (5′-ATC GGC CGT TAA GAT TTA AGC GGA TGC A-3′) (EagI) |
| pCpxR+ (pKEC021) | pcpxR-Nde(ET) (5′-CAT ATG CAT AAA ATC CTA TTA GTT GAT G-3′) (NdeI) and pcpxR-Xho(ET) (5′-CTC GAG ATC ATG TTT CTG ATA CCA TCA-3′) (XhoI) |
| pCpxRD51A+ (pJF015) | pcpxR-ETrbs (5′-CCT CTA GAA ATA ATT TTG TTT AAC TTT AAG AAG GAG ATA TAC ATA TGC ATA AAA TCC TAT TAG TTG ATG-3′) (XbaI) and pcpxR-208rev (5′-ACT GGT ACC TCA TGT TTC TGA TAC CAT CA-3′) (KpnI) |
| Transcriptional analysis by RT-PCR (size) | |
| rpoA (330 bp) | prpoAa (5′-GTT CGA CGC ACG CCA AGG TGA-3′) and prpoAb (5′-ACG TCC TGC GGC TTG ACG AT-3′) |
| yegB (260 bp) | pyegBfor (5′-ATT GTC TCG TAT GTC CTG ACC-3′) and pyegBrev (5′-CAT TGC CGC CAT ATA TTG CTC-3′) |
| yepP (270 bp) | pyepPfor (5′-ATC TGA AGG CCA GTA ATG GTG-3′) and pyepPrev (5′-TCT TTG ATA ACC GTA GAC AGG-3′) |
| YPTB0068 (260 bp) | p0068for (5′-GCT GGT TGG CAC AGT TAA GAG-3′) and p0068rev (5′-CTG CCA GTT CAA TCA ACC GAC-3′) |
| baeS (245 bp) | pbaeSfor (5′-ATC GCC ATG CTA AGT CAT GCG-3′) and pbaeSrev (5′-TTA TGG CAC TGC GGC TAG CTT CT-3′) |
| baeR (265 bp) | pbaeRfor (5′-ATC TTG CTG GAT CTC ATG CTG-3′) and pbaeRrev (5′-TTC ATT GAT CAG CAG TGG TGC-3′) |
| cpxR (390 bp) | pcpxRfor (5′-GTG AAC TGA CGT CGC TGT TGA-3′) and pcpxRrev (5′-TTG CAG GCA ATC AAC TTC CAG-3′) |
| cpxA (295 bp) | pcpxAsac (5′-ACG TAC GAG CTC TTC ACC ATG ACG GCG-3′) and pcpxAkpn (5′-ACG TAC GGT ACC ATC AGT GCA CCG CTG-3′) |
| cpxP (220 bp) | pcpxPfor (5′-GTT ATG GCG TCA ATG TTC GTT C-3′) and pcpxPrev (5′-CAA GAT CAA GGC GCG GCT GAC-3′) |
| degP (410 bp) | pdegPsac (5′- ACG TAC GAG CTC CAA CCT TCA TGG CTT-3′) and pdegPkpn (5′-ACG TAC GGT ACC ATC TGA CTG CGA TTA-3′) |
| ppiA (210 bp) | pppiAfor (5′-CCG GGA ATA TTG AGC TGG AG-3′) and pppiArev (5′-CGC AGG CCA TTA TCT GCT TC-3′) |
| dsbA (335 bp) | pdsbAfor (5′-TGT ATG GTT AGC ACT CGT TGG-3′) and pdsbArev (5′-TCT GCA CAC CTT CAA ACA TCA-3′) |
| rpoE (280 bp) | prpoEfor (5′-CGG ATC AGA TGC TGG TTG AAC-3′) and prpoErev (5′-CCA TCC ACA TCA CTG GAT GG-3′) |
| rseA (215 bp) | prseAfor (5′-AAA CTC TCG ATA GCG AGC TGC-3′) and prseArev (5′-AAA GAT GTG GCT GTG GCT GAG-3′) |
| rseB (220 bp) | prseBfor1 (5′-GCA ACT TTG GTT TTC CGT CTG-3′) and prseBrev1 (5′-TAC GCA ACA ATT GCG CTA ACG-3′) |
| rseC (370 bp) | prseCfor (5′-TGG TAT TGC CCT ATT ACG TTG-3′) and prseCrev (5′-AAC AGG CTG ATA AGC ACT GAG-3′) |
| dsbC (290 bp) | pdsbCfor (5′-AAG GGC CGC TGT ATG ACG T-3′) and pdsbCrev (5′-CGA TCA GCC ATA CAC CAG ATA G-3′) |
| fkpA (210 bp) | pfkpAfor (5′-TGA CGC AAC CTC ACC AGT AG-3′) and pfkpArev (5′-TTC GAT CTC TTC GTC AGT CAG-3′) |
| lcrF (330 bp) | plcrFfor (5′-TGT TGA GCA TTC ACA AGA TGG-3′) and plcrFrev (5′-ATC ACT CCG TTC CAA TAT GGC-3′) |
| yscF (235 bp) | pyscFfor (5′-CGA AAG GAA CCG ATA TCG CAG-3′) and pyscFrev (5′-GAA CTT CTG TAG GAT GCC TTG-3′) |
| yscN (395 bp) | pyscNfor (5′-TCA CTC AAG TGA CAG GAA CGT-3′) and pyscNrev (5′-GGT AAG CAA ACC GTC AAT AAC-3′) |
| yopN (330 bp) | pyopNfor (5′-CGA CGC TTC ATA ACC TAT CT-3′) and pyopNrev (5′-CTG TTA CTC AAC AGA CTG AG-3′) |
| lcrG (305 bp) | plcrG1 (5′-GTA AGA ATT CAG GAG GTA GAT TAT-3′) and plcrG2 (5′-TTG TCT GCA GGC TCT AAT CAT A-3′) |
| yopD (570 bp) | pSEQ1372 (5′-AGA GTA GCG AGG CAA GCT-3′) and pSEQrev3 (5′-GTC CTA ACG GCT GCG TTG-3′) |
| yerA (395 bp) | pyerA1 (5′-TAA CTA CCC GGG GAT CCT AAT GTA TTC ATT TGA ACA AGC-3′) and pyerA2 (5′-ACA ACC AGA TCT GCA GTC AAC TAA ATG ACC GTG-3′) |
| yopE (330 bp) | pyopEfor (5′-ATG TGT GTC AGG ATC TAG CAG-3′) and pyopErev (5′-GCA TCC AAG CTA TTC AAC TGC-3′) |
| yopH (500 bp) | pyopHfor (5′-GAA CGA ATG ATC CGC GTT-3′) and pyopHseq4rev (5′-TGA TCT ACC AGT GAA GCG A-3′) |
| yopK (590 bp) | pyopK1 (5′- AGT AGA ATT CGG AGT AGT AAC-3′) and pyopK2 (5′-AAC TCT GCA GTA TAG CTT CAT-3′) |
The nucleotide sequences in italics identify the incorporated XhoI, XbaI, NdeI, KpnI, or EagI restriction sites used for cloning of the amplified DNA fragments. The underlined sequence indicates the complementary overlap between respective primers in the overlap PCRs.
Full-length cpxA and cpxR were amplified by PCR using the primer combinations listed in Table 2. The cpxA fragment was cloned into pET22b (Novagen, Madison, WI) using NdeI/EagI restriction to develop pMF581. The cpxR fragment went through a series of cloning events: it was first cloned into pET22b using NdeI/XhoI-compatible ends to give pKEC016 and was then excised using XbaI/XhoI restriction and subcloned into pBBR1MCS-3 (51) to create plasmid pMF687 before a final round of cleavage from pMF687 to yield an XbaI/KpnI fragment that was subcloned into the low-copy-number expression vector pMMB208 (62) to give pKEC021. In addition, cpxR(D51A), encoding an alanine substitution of the phosphorylatable aspartate residue at position 51, was amplified from the mutagenesis vector pJF006. This fragment was cloned via XbaI/KpnI restriction into pMMB208, yielding pJF015. Gene expression is under the control of IPTG (isopropyl-β-d-thiogalactopyranoside).
Analysis of gene transcription by RT-PCR.
Duplicate cultures were grown overnight in BHI broth minus Ca2+ or in BHI broth plus Ca2+. Where appropriate, the growth temperature was either 26°C or 37°C. To mimic conditions conducive to T3S, cultures grown overnight at 26°C were subcultured into the same fresh medium. Different growth rates were compensated for by subculturing 0.08 volumes of cultures grown in BHI broth minus Ca2+ into fresh medium, while 0.04 volumes of bacteria grown in BHI broth plus Ca2+ were added. Cultures were incubated at 26°C for 30 min before they were transferred to 37°C and grown for approximately 1.5 h to an optical density at 600 nm of 0.4 to 0.6. RNA was immediately stabilized by the addition of the RNAprotect bacterial reagent (QIAGEN Nordic, West Sussex, United Kingdom) and then extracted using the NucleoSpin RNA II method (Macherey Nagel, Düren, Germany) including an on-column DNase treatment. To generate cDNA, 0.2 μg of total RNA was reversed transcribed with the ImProm-II reverse transcription (RT) system (Promega, Madison, WI). To confirm the absence of contaminating DNA in the RNA preparations, a duplicate reaction lacking the RT enzyme was always performed in parallel. Transcripts derived from genes of the T3S and belonging to ECS regulons were detected by PCR with the specific internal primer combinations listed in Table 2. The conditions for DNA amplification in a 20-μl reaction mixture volume included 0.5 μl template and the Easy-A high-fidelity PCR cloning enzyme (Stratagene). Fractionated DNA fragments were visualized by using a Fluor-S MultiImager (Bio-Rad), and the density of each fragment was analyzed with Quantity One software (version 4.2.3; Bio-Rad Laboratories, Sundbyberg, Sweden). Cycling conditions used for cDNA amplification were an initial denaturation step at 94°C for 3 min; denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 30 s (5 cycles); and then denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension for 72°C for 30 s (25 cycles), before a final extension step at 72°C for 5 min in a GeneAmp PCR system.
Analysis of Yop synthesis and secretion.
Routine analysis of Yop synthesis and secretion was performed by using established methods (25, 26, 28, 71). Protein fractions separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to immunoblotting were identified with rabbit polyclonal antisera raised against a total pool of secreted Yops or the nonsecreted YerA chaperone.
Whole-membrane preparations.
Yersinia membranes were enriched by a modification of a method described previously by Kumar et al. (53). Bacterial cultures were grown to the mid-logarithmic phase under T3S-permissive and -restrictive conditions. Pelleted bacteria were washed in Tris-EDTA buffer and resuspended in 4 ml of the same buffer prior to pulsing by ultrasonication for 2 min. The solution was clarified by low-speed centrifugation at 6,000 × g for 10 min before the supernatant was subjected to ultracentrifugation at 100,000 × g for 1 h. The total membrane fraction contained in the pellet was resuspended in sample buffer and heat inactivated at 95°C for 10 min. Protein fractions were separated by 15% Tris-tricine SDS-PAGE, transferred onto a nitrocellulose membrane, and then blotted with polyclonal antibodies recognizing the T3SS components LcrV, YscF, YscJ, YscP, YscU, and YopH. For control purposes, antibodies recognizing the E. coli outer membrane protein TolC (89) and the inner membrane protein FtsH (90) were also used.
Cultivation and infection of HeLa cells.
Cultivation and infection of HeLa cells for cytotoxicity assays were performed by using standard methods (28). At 30-min intervals, the extent of the morphological change was visualized by light microscopy and recorded on a sliding scale whereby that induced by wild-type Y. pseudotuberculosis (YPIII/pIB102) defined the upper limit, while the lower limit was defined by a ΔyscF null mutant (YPIII/pIB202), which is defective for needle assembly and T3S (23).
RESULTS
Identification of Yersinia ECS-responsive pathways.
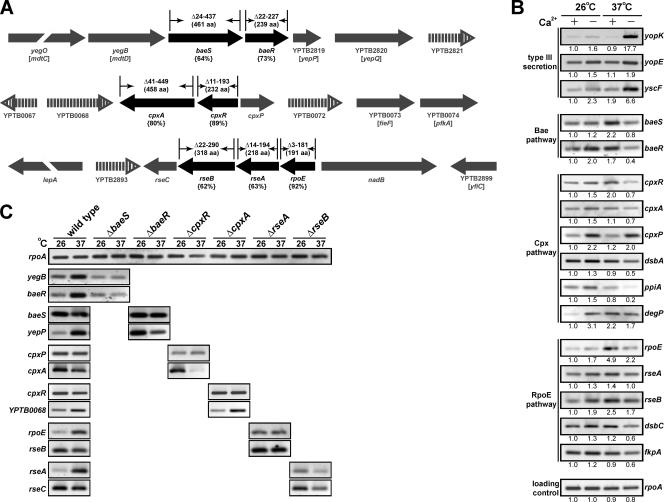
T3S requires the assembly of ∼25 components, which together span the bacterial envelope. Coordinated assembly of these building blocks might require periplasmic quality control systems to deal with misfolded or misassembled proteins. Known quality control systems of the bacterial envelope that respond to ECS include a pair of two-component phosphorelay systems, the sensor kinases BaeS and CpxA and their respective cognate response regulators BaeR and CpxR, and the alternate sigma factor RpoE (1, 22, 79). To investigate if the biogenesis of the Ysc-Yop T3S of Y. pseudotuberculosis engages one or more of the ECS-responsive pathways, we used the genome sequence generated from Y. pseudotuberculosis IP32953 serotype I (10) to locate the baeS, baeR, cpxR, cpxA, and rpoE genes. The operons and their genetic neighborhood are shown in Fig. 1A. The gene arrangement surrounding the three operons bears some similarity to that found for E. coli K-12, particularly with those genes flanking the baeSR operon (6). However, alleles unique to Yersinia with no known function disrupt this gene order. This is most evident for the genes in the neighborhood of the cpxRA operon (Fig. 1A). Despite these differences, identity to the equivalent proteins of E. coli is high for BaeS (64% amino acid identity to BaeS of E. coli), BaeR (73%), CpxA (80%), CpxR (89%), and RpoE (92%).
FIG. 1.
Analysis of the ECS-responsive pathways of pathogenic Yersinia. (A) Shown in black is a schematic representation of the operons encoding the ECS-responsive pathways that encompass the two-component phosphorelay systems BaeSR and CpxRA and the alternate sigma factor RpoE. Below, in parentheses, the amino acid identity of the gene products to those characterized in E. coli is given. The extent of the coding region deleted from each ECS gene is shown above each respective gene along with the precise size of the full-length product (also in parentheses). The direction of transcription is represented by the arrows. The gene order within the Y. pseudotuberculosis IP32953 (10) and E. coli K-12 (6) genomes was determined with the aid of the Graph display setting at http://www.ncbi.nlm.nih.gov/entrez/. This genetic neighborhood is indicated in gray. Filled arrows represent a conserved genetic order between the two species, while the open vertical lined arrows indicate genes unique to Yersinia. B and C are RT-PCR assays of mRNA isolated from Y. pseudotuberculosis. In B, RNA was isolated from log-phase wild-type bacterial cultures grown at 26°C and 37°C in BHI medium with (+) and without (−) Ca2+. In C, RNA was isolated from stationary-phase wild-type and the respective ECS mutant cultures grown at 26°C and 37°C. Samples were subjected to RT and then amplified by PCR using primers specific for the genes indicated at the side of each panel. Analysis of rpoA expression levels was used to control for the usage of equivalent amounts of mRNA in each reaction. Strains analyzed were as follows: YPIII/pIB102 (wild type), YPIII40/pIB102 (baeS null mutant), YPIII29/pIB102 (baeR null mutant), YPIII08/pIB102 (cpxR null mutant), YPIII07/pIB102 (cpxA null mutant), YPIII28/pIB102 (rseA null mutant), and YPIII42/pIB102 (rseB null mutant). Images were first acquired using a Fluor-S MultiImager (Bio-Rad). Those images shown represent one complete data set. Where appropriate, the intensity of each band after image inversion was quantified using the Quantity One quantitation software, version 4.2.3 (Bio-Rad). Given below each image is the relative intensity of each fragment standardized to gene transcription during growth at 26°C with Ca2+.
Using RT-PCR, we first analyzed the expression of these genes in Yersinia cells grown at low (26°C) and high (37°C) temperatures in BHI medium that is restrictive (plus Ca2+) or inductive (minus Ca2+) for Yop synthesis and secretion. Although not mimicking ysc- or yop-inducible expression, Yersinia clearly reacted to growth under these conditions. Without exception, Ca2+ depletion elevated the three ECS regulons at low growth temperatures (Fig. 1B). However, this same medium restricted gene expression within these regulons at high growth temperatures. Moreover, baeS, cpxR, and rpoE were maximally expressed at elevated temperatures in the presence of Ca2+. Intriguingly, therefore, it appears that under conditions where T3S gene expression is maximal, ECS responsiveness is actually reduced. We interpret this seemingly inverse relationship to indicate cross talk between T3S and the ECS-responsive pathways in Yersinia. However, it might not be as straightforward as ECS-responsive pathways overseeing the T3SS assembly process.
Functional Ysc-Yop type III secretion requires an intact Cpx pathway.
As a consequence of these findings, we generated in-frame, near-full-length deletions of baeS, baeR, cpxA, cpxR, and rpoE in Y. pseudotuberculosis YPIII/pIB102 serotype III (Fig. 1A). We confirmed by RT-PCR that none of these deletions generated a polar effect on the transcription of genes flanking each deletion (Fig. 1C and data not shown). The null mutants were given the following designations: YPIII40/pIB102 (ΔbaeS), YPIII29/pIB102 (ΔbaeR), YPIII08/pIB102 (ΔcpxR), YPIII07/pIB102 (ΔcpxA), and YPIII09/pIB102 (ΔrpoE).
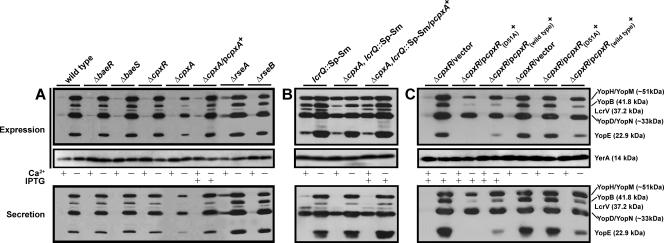
Yersiniae induce the secretion of Yops through the Ysc-Yop system when grown at elevated temperatures in the absence of Ca2+ ions. We used these growth conditions to examine Yop secretion by mutant bacteria deficient in a particular ECS-responsive pathway. Mutants no longer producing BaeS, BaeR, or CpxR still maintained the production and secretion of Yops, occurring only in the absence of Ca2+ in a pattern akin to that of wild-type bacteria (Fig. 2A). In contrast, the ΔcpxA null mutant displayed a marked reduction in Yop production and secretion. This phenotype is due to the loss of the CpxA sensor kinase, because functional secretion could be restored in this mutant when ectopically producing a wild-type copy of CpxA (Fig. 2A). It should be noted that the ΔcpxA null mutant, but not the ΔcpxR equivalent, has a slight defect in growth during routine culturing (data not shown). This minor defect is not alone expected to cause the loss of T3S, because even wild-type Y. pseudotuberculosis cells cease to grow when producing and secreting Yops.
FIG. 2.
Analysis of Yop synthesis and secretion from Y. pseudotuberculosis strains grown either with (+) or without (−) Ca2+. Total Yops associated with bacteria or secreted free into the growth medium (expression fraction) (top) or contained solely within the cleared culture medium (secreted fraction) (bottom) were separated by SDS-PAGE and identified by immunoblot analysis using polyclonal rabbit antisera recognizing all secreted Yops or the cytoplasmic YerA chaperone. Where indicated (+), IPTG was added at a final concentration of 0.4 mM upon a temperature shift. (A) Wild-type strain YPIII/pIB102, baeS null mutant strain YPIII40/pIB102, baeR null mutant strain YPIII29/pIB102, cpxR null mutant strain YPIII08/pIB102, cpxA null mutant strain YPIII07/pIB102, complemented ΔcpxA/pcpxA+ strain YPIII07/pIB102, pMF581, rseA null mutant strain YPIII28/pIB102, and rseB null mutant strain YPIII42/pIB102 are shown. (B) lcrQ::Sp-Sm mutant strain YPIII/pIB26, lcrQ::Sp-Sm ΔcpxA double mutant strain YPIII07/pIB26, and complemented lcrQ::Sp-Sm ΔcpxA/pcpxA+ strain YPIII07/pIB26, pMF581 are shown. (C) Noncomplemented ΔcpxR/vector alone (YPIII08/pIB102, pMMB208), complemented ΔcpxR with nonphosphorylatable CpxR(D51A) (YPIII08/pIB102, pJF015), and complemented ΔcpxR/pcpxR+ (YPIII08/pIB102, pKEC021) are shown. Molecular masses shown in parentheses are deduced from the primary sequence.
We also observed that the ΔrpoE null mutant was defective for Ysc-Yop T3S (data not shown). However, this mutant was apparently unstable and/or acquired second-site suppressor mutations that rapidly restored wild-type-like levels of Yop production and secretion. These isolates have not been further characterized. Given that rpoE is an essential gene in many bacteria, we mutated rseA and rseB, giving rise to strains YPIII28/pIB102 (ΔrseA) and YPIII42/pIB102 (ΔrseB), respectively (Fig. 1). These genes in E. coli encode proteins that modulate the σE response; RseA possesses anti-σE activity, and RseB exerts its negative effect by binding to RseA (19, 59). Their function in Yersinia would appear to be similar to that observed in E. coli, since amino acid identity (63% for RseA and 62% for RseB) is high and the gene arrangement within the operon is essentially conserved (Fig. 1). Interrogation of these mutants revealed that a loss of rseA consistently resulted in a modest elevation of Yop synthesis and secretion levels, with small amounts visible even under Yop-restrictive conditions (Fig. 2A). This is consistent with the loss of rpoE negatively affecting bacterial Ysc-Yop T3S (data not shown): the absence of rseA would be expected to activate the σE-dependent ECS response (2, 49). Thus, both the Cpx and σE ECS-responsive pathways affect Yersinia Ysc-Yop T3S.
The Cpx pathway affects type III secretion system biogenesis.
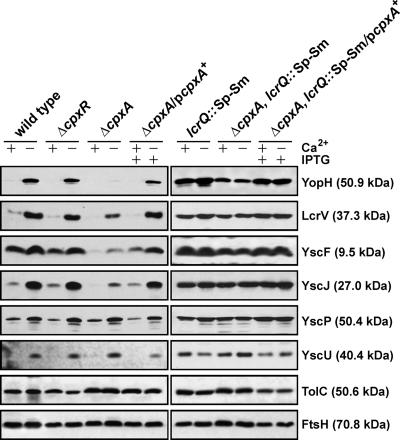
Functional CpxA is necessary for the full production and secretion of Yops into laboratory media. We wondered whether this secretion defect was due to an inability to assemble fully functional secretion complexes. T3SSs are thought to be assembled in an ordered fashion whereby an appropriate incorporation of a preceding component serves as the platform for the subsequent assembly of additional components (50, 88). We therefore inspected whether the loss of CpxA induced a general assembly defect or if just one or at most a few specific components of the Ysc-Yop T3SS were affected. To begin to address this, we purified total membrane preparations of Y. pseudotuberculosis grown under both T3S-permissive and -restrictive conditions. We analyzed protein content by immunoblot using available rabbit antibodies raised against the core components YscU (54), YscJ (95), and YscP (23, 46); the needle components YscF and LcrV (37, 46, 63); and the secreted effector YopH (74). To control for membrane isolation, we also utilized antibodies that recognized the outer membrane protein TolC (89) and the inner membrane protein FtsH (90) in the blotting procedure. Interestingly, only levels of needle-associated YscJ, YscF, and LcrV and the effector YopH were reduced in the absence of CpxA (Fig. 3). YscU and YscP levels remained consistent regardless of the strain analyzed. Based on these findings, we suspect that the Cpx pathway might exert an activity at a specific step beyond the integration of YscU and YscP into the growing structure. This point would represent a Cpx-dependent T3S assembly checkpoint. Alternatively, it could indicate that the assembly of YscU and YscP does not involve the periplasm, thereby avoiding any involvement of the Cpx pathway.
FIG. 3.
Analysis of total membrane fractions of Yersinia. Bacteria were grown at 37°C in BHI medium either with (+) or without (−) Ca2+. The bacteria were washed and harvested by centrifugation, and the resuspended pellets were sonicated to recover soluble material. The clarified lysate was subjected to ultracentrifugation to pellet total membrane material. Proteins contained within this material were fractionated by SDS-PAGE and immunoblotted using a rabbit polyclonal antiserum recognizing YopH, LcrV, YscF, YscJ, YscP, and YscU. The experiment was controlled using antibodies recognizing the E. coli outer membrane protein TolC and the inner membrane protein FtsH. Lanes: wild type, YPIII/pIB102; ΔcpxR null mutant, YPIII08/pIB102; ΔcpxA null mutant, YPIII07/pIB102; complemented ΔcpxA/pcpxA+, YPIII07/pIB102, pMF581; lcrQ::Sp-Sm mutant, YPIII/pIB26; lcrQ::Sp-Sm ΔcpxA double mutant, YPIII07/pIB26; complemented lcrQ::Sp-Sm ΔcpxA/pcpxA+, YPIII07/pIB26, pMF581. Molecular masses shown in parentheses are deduced from the primary sequence.
Analysis of T3S gene transcription.
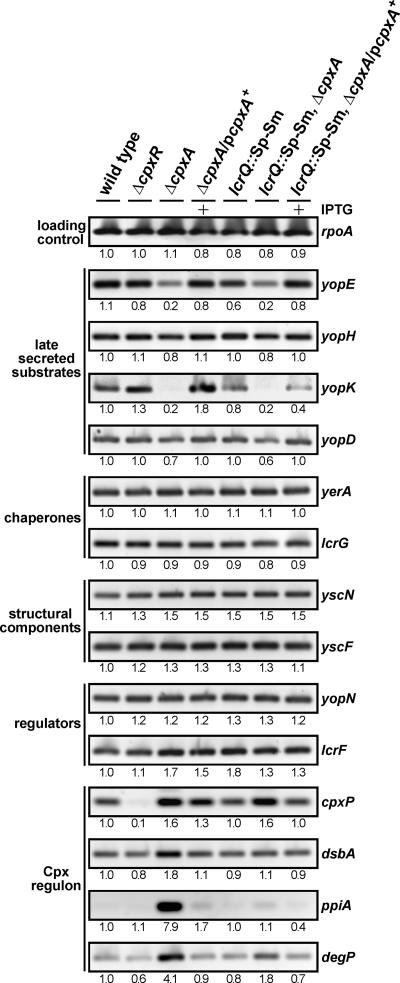
It is also possible that CpxA may influence the activity of a regulator that in turn affects transcription from promoters lying upstream of specific T3S genes. However, one of the difficulties with pinpointing the precise function of CpxA during T3S is that the Ysc-Yop system is regulated by a phenomenon known as feedback inhibition (11). If secretion is compromised, the negative regulatory element LcrQ (YscM1 and YscM2 in Yersinia enterocolitica) remains trapped inside the bacteria, where it still exerts its repressive effect, so that both Yops production and secretion remain low (76). To analyze ysc-yop transcription in the Y. pseudotuberculosis wild type, the ΔcpxR null mutant, and the ΔcpxA null mutant, we employed the RT-PCR technique in which the template was cDNA derived from mRNA isolated from exponentially grown bacteria cultured under T3S-permissive conditions (BHI broth depleted of Ca2+ ions). PCRs used specific primer pairs recognizing the genes encoding late-secreted products encompassing effectors (yopE, yopH, and yopK) and translocators (yopD), cytoplasmic chaperones (yerA and lcrG), structural proteins (yscF and yscN), and regulatory proteins (yopN and lcrF). To control for the responsiveness of the Cpx regulon, four confirmed CpxRA-regulated genes, at least in E. coli, were included in the analysis: cpxP (encoding a negative regulatory element of the Cpx signaling cascade) (13, 80), dsbA (disulfide bond catalyst) (14, 77), ppiA (peptidyl-prolyl cis-trans isomerase) (77), and degP (serine-threonine protease) (15, 77). Like their counterparts in E. coli, we confirmed that the expression of these four genes was modulated by the functional status of the Cpx pathway: it was reduced in the absence of CpxR but elevated in the absence of CpxA (Fig. 4). When T3SS gene transcription was analyzed, yopE, yopH, yopK, and yopD were the only ones displaying reduced transcription in the ΔcpxA null mutant (Fig. 4). This low expression could essentially be complemented by providing back a wild-type copy of inducible cpxA in trans. Significantly, these genes all encoded products that are among the last to be secreted. Generally, the transcription of all other tested T3SS genes was apparently unaffected by the status of the Cpx pathway.
FIG. 4.
RT-PCR of mRNA isolated from Y. pseudotuberculosis. RNA was isolated from log-phase bacterial cultures grown at 37°C in BHI medium without Ca2+. Samples were subjected to RT-PCR using primers specific for rpoA, members of the CpxR-P regulon (cpxP, dsbA, ppiA, and degP), and various T3SS genes grouped depending on the function of their encoded product. Lanes: wild type, YPIII/pIB102; ΔcpxR null mutant, YPIII08/pIB102; ΔcpxA null mutant, YPIII07/pIB102; complemented ΔcpxA/pcpxA+, YPIII07/pIB102, pMF581; lcrQ::Sp-Sm mutant, YPIII/pIB26; lcrQ::Sp-Sm ΔcpxA double mutant, YPIII07/pIB26; complemented lcrQ::Sp-Sm ΔcpxA/pcpxA+, YPIII07/pIB26, pMF581. Data were quantified as described in the legend of Fig. 1. The relative intensity of each fragment standardized to the transcription from wild-type bacteria when grown without Ca2+ is given below each image.
To examine if reduced yopE, yopH, yopK, and yopD transcription was due to the effect of feedback inhibition in a ΔcpxA null mutant, we short-circuited this phenomenon by introducing a mutated lcrQ allele into the ΔcpxA null mutant background (76). Significantly, in this ΔcpxA lcrQ::Sp-Sm double mutant, transcription from these four gene promoters under T3S-permissive conditions was still notably lower (Fig. 4). This is evidence that the reduced transcription seen in CpxA-defective bacteria is more likely due to a direct effect of an altered Cpx pathway and not necessarily due to feedback inhibition caused by the loss of a competent T3SS. This suggests some action of the Cpx pathway on control regions of a specific subset of T3S genes. Moreover, it is interesting that the transcription of all other T3S genes assayed in the ΔcpxA null mutant was not visibly altered (Fig. 4). In particular, this occurred for yscF even though a significant loss of YscF production was visualized (Fig. 3). It is possible, therefore, that CpxA might also influence the levels of another subset of T3S components by an undisclosed posttranscriptional mechanism.
Disruption of the intrinsic negative regulatory loop restores T3S.
It was a surprise to find that the reduced transcription seen in the ΔcpxA null mutant was also observed in an isogenic double mutant lacking the negative repressive element LcrQ. Since a loss of LcrQ elevates Yop synthesis and secretion even by bacteria grown under conditions that are not normally T3S permissive, we utilized the ΔcpxA lcrQ::Sp-Sm double mutant to analyze levels of secreted Yops. Significantly, secretion levels were restored in the ΔlcrQ cpxA double mutant similarly to the ΔlcrQ mutant parental strain (Fig. 2B). These observations are in accordance with the increased presence of the YscJ, YscF, and LcrV structural components, all found to be reduced in the single mutant, in the bacterial envelope (Fig. 3). This suggests that the secretion defect observed in the ΔcpxA null mutant can be suppressed in bacteria lacking the negative regulatory loop that in turn permits consistent Yop synthesis. Presumably, these higher protein production levels increase the odds of assembling at least a few T3SSs that are functional, even when CpxA is lacking. An understanding of how this occurs, particularly when the transcription of a subset of T3S genes remains low, requires further investigation.
Removal of CpxA inhibits Yop effector translocation into eukaryotic cells.
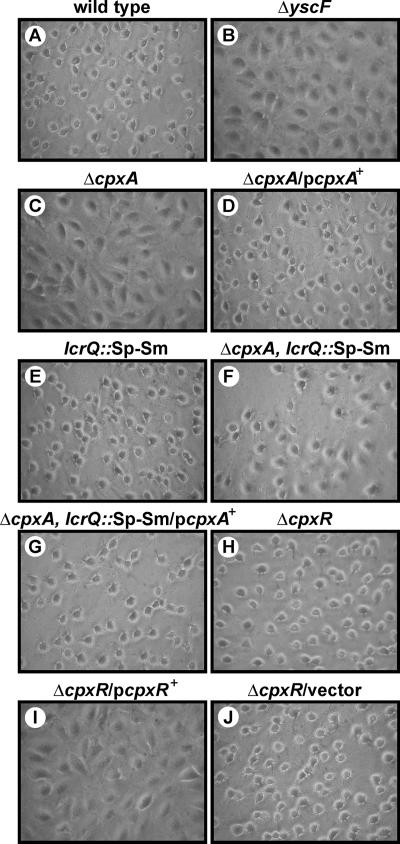
T3SSs are designed to translocate antihost effectors into infected eukaryotic cells. We wanted to determine if the reduced secretion of Yops into laboratory medium by the ΔcpxA null mutant also equated to a deficiency in Yop translocation. We performed a HeLa cell cytotoxicity assay with the various Y. pseudotuberculosis strains. This well-established assay qualitatively scores YopE cytotoxin translocation by extracellular bacteria via the monitoring of cell monolayer morphology (83). YopE specifically induces cellular cytotoxicity via its GTPase-activating activity towards small G proteins involved in regulating actin-nucleating dynamics (5, 92). Despite secreting low levels of Yops in vitro, the ΔcpxA null mutant failed to induce any cytotoxicity towards target cells (Fig. 5). This was distinct from the complemented mutant, which induced a degree of cellular cytotoxicity similar to those seen for wild-type and ΔcpxR null mutant bacteria. Significantly, the ΔbaeS, ΔbaeR, ΔrseA, and ΔrseB null mutants could still translocate the YopE cytotoxin normally (data not shown).
FIG. 5.
Infection of HeLa cells by Y. pseudotuberculosis. Strains were allowed to infect a monolayer of growing HeLa cells. At ∼1 h postinfection, the effect of the bacteria on the HeLa cells was recorded by phase-contrast microscopy. Shown are phase-contrast images of the wild type (YPIII/pIB102) (A), the ΔyscF null mutant (YPIII/pIB202) (B), the ΔcpxA null mutant (YPIII07/pIB102) (C), complemented ΔcpxA/pcpxA+ (YPIII07/pIB102, pMF581) (D), the lcrQ::Sp-Sm mutant (YPIII/pIB26) (E), the lcrQ::Sp-Sm ΔcpxA double mutant (YPIII07/pIB26) (F), complemented lcrQ::Sp-Sm ΔcpxA/pcpxA+ (YPIII07/pIB26, pMF581) (G), the ΔcpxR null mutant (YPIII08/pIB102) (H), noncomplemented ΔcpxR/pcpxR+ (YPIII08/pIB102, pKEC021) (I), and ΔcpxR/vector alone (YPIII08/pIB102, pMMB208) (J).
We considered that the ΔcpxA null mutant did not translocate YopE because it was poorly secreted (see Fig. 2A). This proved not to be the case, however, because a ΔlcrQ cpxA double mutant, which secretes Yops abundantly (Fig. 2B), also poorly translocated YopE. This was due solely to a defect in CpxA, since ΔcpxA lcrQ::Sp-Sm ectopically expressing CpxA efficiently restored full cytotoxicity (Fig. 5). Hence, it appears that CpxA may have at least two roles, one required to ensure the efficiency of Ysc-Yop T3S of proteins originating from inside bacteria and another that could modulate the timely translocation of this secreted protein cargo into the eukaryotic cell interior.
Cpx responsiveness is inhibitory to T3S.
Our data imply that CpxA can function independently from the cognate response regulator CpxR. Perhaps CpxA can cross talk with another unidentified response regulator(s). At least in E. coli, however, no experimental support for this exists (94). A more plausible explanation concerns the levels of accumulated phosphorylated CpxR (CpxR-P). Normally, the phosphatase activity of CpxA keeps the level of CpxR-P low (81). However, in the absence of CpxA, CpxR can be phosphorylated by low-molecular-weight phosphodonors such as acetyl phosphate (13, 15). In this context, the presence of high levels of CpxR-P may attenuate Y. pseudotuberculosis T3S. To test this, we examined the T3S profile of the ΔcpxR null mutant expressing an IPTG-inducible wild-type copy of cpxR in trans. We compared this profile to that obtained from the parental strain and two control strains in which the ΔcpxR null mutant harbored only the empty vector or a derivative producing a mutant form, CpxR(D51A), which is unable to be phosphorylated. After IPTG induction, elevated levels of both wild-type CpxR and CpxR(D51A) notably reduced both the production and secretion of Yops even under T3S-permissive growth conditions (Fig. 2C). Furthermore, the overexpression of CpxR, but not the CpxR(D51A) variant, prevented the translocation of the YopE cytotoxin into HeLa cell monolayers (data not shown) (Fig. 5I). Because excessive copies of CpxR are toxic to bacteria (20), we also performed this analysis in the absence of IPTG induction. Significantly, low-level production of the CpxR(D51A) nonphosphorylated variant maintained a normal T3S profile, but similarly produced wild-type CpxR still had a negative impact on Yop synthesis and secretion (Fig. 2C). While the latter strain does exhibit a moderate growth defect during routine culturing (data not shown), it is comparable to the ΔcpxA null mutant that is also thought to produce high levels of phosphorylated CpxR (13, 15). Thus, a tantalizing interpretation of these data is that it is the uncontrolled activation of the Cpx response leading to high levels of CpxR-P, and not its removal, that compromises the pathogenicity of Y. pseudotuberculosis.
DISCUSSION
In this study, we have established that the Cpx two-component signal transduction pathway is important for T3S by Y. pseudotuberculosis, implicating it as an important regulatory circuit in Yersinia pathogenicity. This further underscores previous studies that described a role for CpxRA in the phase-variable expression and/or assembly of several adhesion organelles in pathogenic strains of E. coli (21, 34, 41, 45, 47, 68, 72) and for the cellular attachment, invasion, and intracellular survival of Salmonella enterica serovar Typhimurium (40). Interestingly, the latter effect might be mediated by influences on T3S by this bacterium (65), which has also been observed for Shigella sonnei (60, 66, 67).
It was anticipated that the Cpx pathway would serve a quality control function, overseeing the assembly of working T3SSs. This is based on members of the CpxR-P regulon functioning in the periplasm to assist in protein folding and degradation. These include peptidyl prolyl cis-trans isomerases (PPIases) (32), the disulfide bond catalyst DsbA (96), and the serine protease and chaperone DegP (HtrA) (73). In particular, DsbA is already known to help fold the T3S secretins in Yersinia pestis (43), Shigella flexneri (93), and S. enterica serovar Typhimurium (57) and for T3S gene expression in Pseudomonas aeruginosa (31). However, we are unable to demonstrate a role for DsbA in Y. pseudotuberculosis T3S (K. E. Carlsson, unpublished data). This is also the case for the various periplasmic PPIases (M. S. Francis, unpublished data). On the other hand, Y. pseudotuberculosis lacking DegP is affected in T3S, but this mutant is highly unstable (Carlsson, unpublished). Interestingly, DegP is responsible for degrading misfolded proteins in the periplasm during P-pilus assembly in E. coli (42, 45). Perhaps DegP assumes a similar role during T3S biogenesis in Yersinia. Nevertheless, our data herein indicate that Cpx pathway activation, resulting in accumulated CpxR-P, actually negatively impacts Yersinia T3S. These observations create a conundrum: why would an ECS-sensing pathway controlling the production of useful protein-folding and degradation factors be deleterious to the assembly of a complex multicomponent T3SS spanning the bacterial envelope? We will endeavor to address this intriguing connection in the future, initially by focusing on the effect of CpxR-P accumulation in Yersinia cytoplasm. For example, it would be beneficial to examine the effect of CpxR-P on T3S when the signal transduction cascade is still intact. This might be possible by overexpressing nlpE, encoding an outer membrane lipoprotein from E. coli (87).
It is interesting that the ΔcpxA null mutant exhibits two distinct regulatory effects. In a subset of genes (such as yopE, yopH, yopK, and yopD) that encode late-secreted substrates, both transcription and translation are reduced. Apparently, this effect is not a consequence of feedback inhibition (a functional T3SS is required for the expression of genes encoding secretion substrates) (11) because this same phenotype is still observed in an isogenic double mutant lacking the LcrQ-dependent negative regulatory loop that relieves feedback inhibition. However, another phenotype was observed for yscF, encoding the needle component, where transcription remained normal even though protein levels were significantly reduced. This indicates that at least YscF could also be controlled posttranscriptionally. The mechanism(s) underpinning these two distinct regulatory pathways remains unclear, although an undisclosed CpxA-dependent posttranscription regulatory pathway is an implicated feature of T3S control in S. sonnei (60).
In all likelihood, elevated CpxR-P levels negatively regulate T3S by Yersinia. In E. coli, CpxR-P is thought to recognize a putative consensus sequence, 5′-GTAAA(N)5GTAAA-3′, located in target gene promoters (20). However, scanning the promoters of Yersinia T3S genes failed to reveal any obvious CpxR-P recognition motifs. Therefore, maybe CpxR-P does not directly engage T3S gene promoters. In E. coli, the CpxR-P regulon boasts over 100 different members. In this sense, CpxR-P might directly control the production of another factor(s) associated with the regulation of T3S genes. Apart from the above-mentioned periplasmic quality control factors (DsbA, DegP, and the PPIases), the CpxR-P regulon also affects ribosome assembly, the production of tRNAs, mRNA processing, small regulatory RNAs, RNA binding proteins, and chaperones (20). Thus, there is a plethora of factors that could potentially differentially influence individual components of the Ysc-Yop T3SS. To find out why gene subsets are regulated differently, determining their regulatory mechanisms and determining how they converge together to coordinate T3S are our future goals. It is also worth considering that the partial overlap between the Cpx and RpoE (σE) ECS-responsive pathways can mean that the loss of CpxA may alter the expression of σE -regulated genes (22). This might also have an impact on T3S in Y. pseudotuberculosis, given that σE is probably required.
The alternative sigma factor σE is proposed to modulate a core regulon responsible for the maintenance of lipopolysaccharide and porin biosynthesis conserved across a number of bacteria. In addition, another variable regulon apparently performs pathogenesis-related functions necessitated by the unique environmental niches inhabited by individual bacteria (82). The finding that ΔrpoE and ΔrseA null mutants were affected in T3S supports a pathogenicity role for σE in Y. pseudotuberculosis. In contrast, a loss of RseB was not seen to alter T3S. This is not surprising, judging from studies of E. coli (19). The ΔrseB null mutant still probably possesses RseA activity, suggesting that RseA has the ability to modulate σE-dependent effects on T3S independently of RseB. Given that rpoE is an essential gene in several bacteria (18, 35), which was reflected in the instability of our mutant described here, the ΔrseA null mutant is important since it will provide opportunities to dig deeper into the connections between σE and T3S. In this sense, it is also significant that survival of our ΔrpoE mutant likely occurs through the acquisition of second-site suppressor mutations (18). Interestingly, these mutations restore T3S, suggesting that they position in genes whose products also modulate T3S.
We were unable to assign a function for the BaeSR two-component phosphorelay system in Yersinia T3S in vitro or in the intoxication of target cells. This system has not yet been implicated in bacterial pathogenesis (79, 85), but a genome-wide analysis revealed that the overproduction of BaeR affected the expression of E. coli gene clusters associated mainly with chemotaxis, flagellar biosynthesis, maltose transport, and multidrug efflux (69). Furthermore, genetic studies of E. coli and S. enterica indicate that BaeSR is important for resistance to antimicrobial compounds (3, 36, 39, 64) and chemical-induced ECS (78). It remains to be seen what physiological functions the BaeSR pathway modulates in Yersinia and whether this impacts bacterial survival in the environment or during a host infection.
Finally, by removing the LcrQ negative regulatory element, we could create an isogenic Y. pseudotuberculosis ΔcpxA null mutant to consistently produce and secrete Yops. However, this strain was still significantly impaired in its ability to intoxicate infected cell monolayers with Yops, a defect that was complementable by the ectopic expression of CpxA. This indicates one of two possibilities: either T3S occurs differently in the presence of cells, such that the ΔcpxA null mutant has a different effect in vivo, or the Cpx pathway affects additional processes required for T3S-dependent cytotoxicity. It is possible that the CpxR-P regulon of Yersinia includes other loci that enable eukaryotic cell contact. In fact, Yersinia strains lacking CpxA do associate poorly with cells, whereas contact by CpxR-defective bacteria is enhanced (our unpublished data). Furthermore, the modulation of cell contact in these strains is dependent on levels of the predominant Yersinia adhesion, invasin (our unpublished data). These collective findings provide additional support for CpxRA responsiveness being an important elicitor of bacterial pathogenesis.
Acknowledgments
This work, performed within the framework of the Umeå Centre for Microbial Research, is supported by grants from the Carl Tryggers Foundation for Scientific Research (M.S.F.), the Umeå University Basic Science-Oriented Biotechnology Research Fund (M.S.F.), the Swedish Research Council (M.S.F.), the Foundation for Medical Research at Umeå University (M.S.F.), and the J. C. Kempes Memorial Fund (K.E.C. and P.J.E.). J.L. is sponsored by a Carl Tryggers postdoctoral fellowship.
We thank Daniel Nygren for valuable technical assistance and Hans Wolf-Watz for the gift of antibodies recognizing components of the Ysc-Yop T3SS. Teru Ogura (anti-FtsH) and Vassillis Koronakis (anti-TolC) are also acknowledged for their generous gift of antibodies.
Editor: F. C. Fang
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Alba, B. M., and C. A. Gross. 2004. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol. Microbiol. 52:613-619. [DOI] [PubMed] [Google Scholar]
- 2.Alba, B. M., J. A. Leeds, C. Onufryk, C. Z. Lu, and C. A. Gross. 2002. DegS and YaeL participate sequentially in the cleavage of RseA to activate the sigma(E)-dependent extracytoplasmic stress response. Genes Dev. 16:2156-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baranova, N., and H. Nikaido. 2002. The baeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. J. Bacteriol. 184:4168-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beier, D., and R. Gross. 2006. Regulation of bacterial virulence by two-component systems. Curr. Opin. Microbiol. 9:143-152. [DOI] [PubMed] [Google Scholar]
- 5.Black, D. S., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515-527. [DOI] [PubMed] [Google Scholar]
- 6.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 7.Bölin, I., L. Norlander, and H. Wolf-Watz. 1982. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect. Immun. 37:506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bölin, I., and H. Wolf-Watz. 1984. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect. Immun. 43:72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calva, E., and R. Oropeza. 2006. Two-component signal transduction systems, environmental signals, and virulence. Microb. Ecol. 51:166-176. [DOI] [PubMed] [Google Scholar]
- 10.Chain, P. S., E. Carniel, F. W. Larimer, J. Lamerdin, P. O. Stoutland, W. M. Regala, A. M. Georgescu, L. M. Vergez, M. L. Land, V. L. Motin, R. R. Brubaker, J. Fowler, J. Hinnebusch, M. Marceau, C. Medigue, M. Simonet, V. Chenal-Francisque, B. Souza, D. Dacheux, J. M. Elliott, A. Derbise, L. J. Hauser, and E. Garcia. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 101:13826-13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelis, G. R., C. Sluiters, I. Delor, D. Geib, K. Kaniga, C. L. de Rouvroit, M. P. Sory, J. C. Vanooteghem, and T. Michiels. 1991. ymoA, a Yersinia enterocolitica chromosomal gene modulating the expression of virulence functions. Mol. Microbiol. 5:1023-1034. [DOI] [PubMed] [Google Scholar]
- 13.Danese, P. N., and T. J. Silhavy. 1998. CpxP, a stress-combative member of the Cpx regulon. J. Bacteriol. 180:831-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danese, P. N., and T. J. Silhavy. 1997. The sigma(E) and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 11:1183-1193. [DOI] [PubMed] [Google Scholar]
- 15.Danese, P. N., W. B. Snyder, C. L. Cosma, L. J. Davis, and T. J. Silhavy. 1995. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 9:387-398. [DOI] [PubMed] [Google Scholar]
- 16.Darwin, A. J. 2005. The phage-shock-protein response. Mol. Microbiol. 57:621-628. [DOI] [PubMed] [Google Scholar]
- 17.DeBord, K. L., N. S. Galanopoulos, and O. Schneewind. 2003. The ttsA gene is required for low-calcium-induced type III secretion of Yop proteins and virulence of Yersinia enterocolitica W22703. J. Bacteriol. 185:3499-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Las Penas, A., L. Connolly, and C. A. Gross. 1997. σE is an essential sigma factor in Escherichia coli. J. Bacteriol. 179:6862-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Las Penas, A., L. Connolly, and C. A. Gross. 1997. The sigmaE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of sigmaE. Mol. Microbiol. 24:373-385. [DOI] [PubMed] [Google Scholar]
- 20.De Wulf, P., A. M. McGuire, X. Liu, and E. C. Lin. 2002. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 277:26652-26661. [DOI] [PubMed] [Google Scholar]
- 21.Dorel, C., O. Vidal, C. Prigent-Combaret, I. Vallet, and P. Lejeune. 1999. Involvement of the Cpx signal transduction pathway of E. coli in biofilm formation. FEMS Microbiol. Lett. 178:169-175. [DOI] [PubMed] [Google Scholar]
- 22.Duguay, A. R., and T. J. Silhavy. 2004. Quality control in the bacterial periplasm. Biochim. Biophys. Acta 1694:121-134. [DOI] [PubMed] [Google Scholar]
- 23.Edqvist, P. J., J. Olsson, M. Lavander, L. Sundberg, Å. Forsberg, H. Wolf-Watz, and S. A. Lloyd. 2003. YscP and YscU regulate substrate specificity of the Yersinia type III secretion system. J. Bacteriol. 185:2259-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fällman, M., F. Deleuil, and K. McGee. 2002. Resistance to phagocytosis by Yersinia. Int. J. Med. Microbiol. 291:501-509. [DOI] [PubMed] [Google Scholar]
- 25.Francis, M. S., M. Aili, M. L. Wiklund, and H. Wolf-Watz. 2000. A study of the YopD-LcrH interaction from Yersinia pseudotuberculosis reveals a role for hydrophobic residues within the amphipathic domain of YopD. Mol. Microbiol. 38:85-102. [DOI] [PubMed] [Google Scholar]
- 26.Francis, M. S., S. A. Lloyd, and H. Wolf-Watz. 2001. The type III secretion chaperone LcrH co-operates with YopD to establish a negative, regulatory loop for control of Yop synthesis in Yersinia pseudotuberculosis. Mol. Microbiol. 42:1075-1093. [DOI] [PubMed] [Google Scholar]
- 27.Francis, M. S., K. Schesser, Å. Forsberg, and H. Wolf-Watz. 2004. Type III secretion systems in animal- and plant-interacting bacteria., p. 361-392. In P. Cossart, P. Boquet, S. Normark, and R. Rappuoli (ed.), Cellular microbiology, 2nd ed. ASM Press, Washington, DC.
- 28.Francis, M. S., and H. Wolf-Watz. 1998. YopD of Yersinia pseudotuberculosis is translocated into the cytosol of HeLa epithelial cells: evidence of a structural domain necessary for translocation. Mol. Microbiol. 29:799-813. [DOI] [PubMed] [Google Scholar]
- 29.Gemski, P., J. R. Lazere, T. Casey, and J. A. Wohlhieter. 1980. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect. Immun. 28:1044-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh, P. 2004. Process of protein transport by the type III secretion system. Microbiol. Mol. Biol. Rev. 68:771-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ha, U. H., Y. Wang, and S. Jin. 2003. DsbA of Pseudomonas aeruginosa is essential for multiple virulence factors. Infect. Immun. 71:1590-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hacker, J., and G. Fischer. 1993. Immunophilins: structure-function relationship and possible role in microbial pathogenicity. Mol. Microbiol. 10:445-456. [DOI] [PubMed] [Google Scholar]
- 33.Hanahan, D. 1985. Techniques for transformation of E. coli, p. 109-136. In D. M. Glover (ed.), DNA cloning. A practical approach, vol. 1. IRL Press Ltd., Oxford, United Kingdom. [Google Scholar]
- 34.Hernday, A. D., B. A. Braaten, G. Broitman-Maduro, P. Engelberts, and D. A. Low. 2004. Regulation of the pap epigenetic switch by CpxAR: phosphorylated CpxR inhibits transition to the phase ON state by competition with Lrp. Mol. Cell 16:537-547. [DOI] [PubMed] [Google Scholar]
- 35.Heusipp, G., M. A. Schmidt, and V. L. Miller. 2003. Identification of rpoE and nadB as host responsive elements of Yersinia enterocolitica. FEMS Microbiol. Lett. 226:291-298. [DOI] [PubMed] [Google Scholar]
- 36.Hirakawa, H., K. Nishino, J. Yamada, T. Hirata, and A. Yamaguchi. 2003. Beta-lactam resistance modulated by the overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J. Antimicrob. Chemother. 52:576-582. [DOI] [PubMed] [Google Scholar]
- 37.Hoiczyk, E., and G. Blobel. 2001. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc. Natl. Acad. Sci. USA 98:4669-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horton, R. M., and L. R. Pease. 1991. Recombination and mutagenesis of DNA sequences using PCR, p. 217-247. In M. J. McPherson (ed.), Directed mutagenesis: a practical approach. Oxford University Press, New York, NY.
- 39.Hu, W. S., P. C. Li, and C. Y. Cheng. 2005. Correlation between ceftriaxone resistance of Salmonella enterica serovar Typhimurium and expression of outer membrane proteins OmpW and Ail/OmpX-like protein, which are regulated by BaeR of a two-component system. Antimicrob. Agents Chemother. 49:3955-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Humphreys, S., G. Rowley, A. Stevenson, M. F. Anjum, M. J. Woodward, S. Gilbert, J. Kormanec, and M. Roberts. 2004. Role of the two-component regulator CpxAR in the virulence of Salmonella enterica serotype Typhimurium. Infect. Immun. 72:4654-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hung, D. L., T. L. Raivio, C. H. Jones, T. J. Silhavy, and S. J. Hultgren. 2001. Cpx signaling pathway monitors biogenesis and affects assembly and expression of P pili. EMBO J. 20:1508-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isaac, D. D., J. S. Pinkner, S. J. Hultgren, and T. J. Silhavy. 2005. The extracytoplasmic adaptor protein CpxP is degraded with substrate by DegP. Proc. Natl. Acad. Sci. USA 102:17775-17779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackson, M. W., and G. V. Plano. 1999. DsbA is required for stable expression of outer membrane protein YscC and for efficient Yop secretion in Yersinia pestis. J. Bacteriol. 181:5126-5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson, M. W., E. Silva-Herzog, and G. V. Plano. 2004. The ATP-dependent ClpXP and Lon proteases regulate expression of the Yersinia pestis type III secretion system via regulated proteolysis of YmoA, a small histone-like protein. Mol. Microbiol. 54:1364-1378. [DOI] [PubMed] [Google Scholar]
- 45.Jones, C. H., P. N. Danese, J. S. Pinkner, T. J. Silhavy, and S. J. Hultgren. 1997. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 16:6394-6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Journet, L., C. Agrain, P. Broz, and G. R. Cornelis. 2003. The needle length of bacterial injectisomes is determined by a molecular ruler. Science 302:1757-1760. [DOI] [PubMed] [Google Scholar]
- 47.Jubelin, G., A. Vianney, C. Beloin, J. M. Ghigo, J. C. Lazzaroni, P. Lejeune, and C. Dorel. 2005. CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J. Bacteriol. 187:2038-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Julio, S. M., D. M. Heithoff, R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 2002. DNA adenine methylase overproduction in Yersinia pseudotuberculosis alters YopE expression and secretion and host immune responses to infection. Infect. Immun. 70:1006-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanehara, K., K. Ito, and Y. Akiyama. 2002. YaeL (EcfE) activates the sigma(E) pathway of stress response through a site-2 cleavage of anti-sigma(E), RseA. Genes Dev. 16:2147-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimbrough, T. G., and S. I. Miller. 2000. Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc. Natl. Acad. Sci. USA 97:11008-11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 52.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galán, and S. I. Aizawa. 1998. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280:602-605. [DOI] [PubMed] [Google Scholar]
- 53.Kumar, S. S., K. Sankaran, R. Haigh, P. H. Williams, and A. Balakrishnan. 2001. Cytopathic effects of outer-membrane preparations of enteropathogenic Escherichia coli and co-expression of maltoporin with secretory virulence factor, EspB. J. Med. Microbiol. 50:602-612. [DOI] [PubMed] [Google Scholar]
- 54.Lavander, M., L. Sundberg, P. J. Edqvist, S. A. Lloyd, H. Wolf-Watz, and Å. Forsberg. 2002. Proteolytic cleavage of the FlhB homologue YscU of Yersinia pseudotuberculosis is essential for bacterial survival but not for type III secretion. J. Bacteriol. 184:4500-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marceau, M. 2005. Transcriptional regulation in Yersinia: an update. Curr. Issues Mol. Biol. 7:151-177. [PubMed] [Google Scholar]
- 56.Marlovits, T. C., T. Kubori, A. Sukhan, D. R. Thomas, J. E. Galan, and V. M. Unger. 2004. Structural insights into the assembly of the type III secretion needle complex. Science 306:1040-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miki, T., N. Okada, and H. Danbara. 2004. Two periplasmic disulfide oxidoreductases, DsbA and SrgA, target outer membrane protein SpiA, a component of the Salmonella pathogenicity island 2 type III secretion system. J. Biol. Chem. 279:34631-34642. [DOI] [PubMed] [Google Scholar]
- 58.Milton, D. L., R. O'Toole, P. Horstedt, and H. Wolf-Watz. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178:1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Missiakas, D., M. P. Mayer, M. Lemaire, C. Georgopoulos, and S. Raina. 1997. Modulation of the Escherichia coli sigmaE (RpoE) heat-shock transcription-factor activity by the RseA, RseB and RseC proteins. Mol. Microbiol. 24:355-371. [DOI] [PubMed] [Google Scholar]
- 60.Mitobe, J., E. Arakawa, and H. Watanabe. 2005. A sensor of the two-component system CpxA affects expression of the type III secretion system through posttranscriptional processing of InvE. J. Bacteriol. 187:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Model, P., G. Jovanovic, and J. Dworkin. 1997. The Escherichia coli phage-shock-protein (psp) operon. Mol. Microbiol. 24:255-261. [DOI] [PubMed] [Google Scholar]
- 62.Morales, V., A. Bäckman, and M. Bagdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39-47. [DOI] [PubMed] [Google Scholar]
- 63.Mueller, C. A., P. Broz, S. A. Muller, P. Ringler, F. Erne-Brand, I. Sorg, M. Kuhn, A. Engel, and G. R. Cornelis. 2005. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310:674-676. [DOI] [PubMed] [Google Scholar]
- 64.Nagakubo, S., K. Nishino, T. Hirata, and A. Yamaguchi. 2002. The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. J. Bacteriol. 184:4161-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakayama, S., A. Kushiro, T. Asahara, R. Tanaka, L. Hu, D. J. Kopecko, and H. Watanabe. 2003. Activation of hilA expression at low pH requires the signal sensor CpxA, but not the cognate response regulator CpxR, in Salmonella enterica serovar Typhimurium. Microbiology 149:2809-2817. [DOI] [PubMed] [Google Scholar]
- 66.Nakayama, S., and H. Watanabe. 1998. Identification of cpxR as a positive regulator essential for expression of the Shigella sonnei virF gene. J. Bacteriol. 180:3522-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakayama, S., and H. Watanabe. 1995. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J. Bacteriol. 177:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nevesinjac, A. Z., and T. L. Raivio. 2005. The Cpx envelope stress response affects expression of the type IV bundle-forming pili of enteropathogenic Escherichia coli. J. Bacteriol. 187:672-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nishino, K., T. Honda, and A. Yamaguchi. 2005. Genome-wide analyses of Escherichia coli gene expression responsive to the BaeSR two-component regulatory system. J. Bacteriol. 187:1763-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okan, N. A., J. B. Bliska, and A. W. Karzai. 2006. A role for the SmpB-SsrA system in Yersinia pseudotuberculosis pathogenesis. PLoS Pathog. 2:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olsson, J., P. J. Edqvist, J. E. Bröms, Å. Forsberg, H. Wolf-Watz, and M. S. Francis. 2004. The YopD translocator of Yersinia pseudotuberculosis is a multifunctional protein comprised of discrete domains. J. Bacteriol. 186:4110-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Otto, K., and T. J. Silhavy. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl. Acad. Sci. USA 99:2287-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pallen, M. J., and B. W. Wren. 1997. The HtrA family of serine proteases. Mol. Microbiol. 26:209-221. [DOI] [PubMed] [Google Scholar]
- 74.Persson, C., R. Nordfelth, A. Holmström, S. Håkansson, R. Rosqvist, and H. Wolf-Watz. 1995. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol. Microbiol. 18:135-150. [DOI] [PubMed] [Google Scholar]
- 75.Petersen, S., and G. M. Young. 2002. Essential role for cyclic AMP and its receptor protein in Yersinia enterocolitica virulence. Infect. Immun. 70:3665-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pettersson, J., R. Nordfelth, E. Dubinina, T. Bergman, M. Gustafsson, K. E. Magnusson, and H. Wolf-Watz. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science 273:1231-1233. [DOI] [PubMed] [Google Scholar]
- 77.Pogliano, J., A. S. Lynch, D. Belin, E. C. Lin, and J. Beckwith. 1997. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 11:1169-1182. [DOI] [PubMed] [Google Scholar]
- 78.Raffa, R. G., and T. L. Raivio. 2002. A third envelope stress signal transduction pathway in Escherichia coli. Mol. Microbiol. 45:1599-1611. [DOI] [PubMed] [Google Scholar]
- 79.Raivio, T. L. 2005. Envelope stress responses and gram-negative bacterial pathogenesis. Mol. Microbiol. 56:1119-1128. [DOI] [PubMed] [Google Scholar]
- 80.Raivio, T. L., D. L. Popkin, and T. J. Silhavy. 1999. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J. Bacteriol. 181:5263-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raivio, T. L., and T. J. Silhavy. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 179:7724-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rhodius, V. A., W. C. Suh, G. Nonaka, J. West, and C. A. Gross. 2006. Conserved and variable functions of the sigmaE stress response in related genomes. PLoS Biol. 4:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosqvist, R., Å. Forsberg, and H. Wolf-Watz. 1991. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 59:4562-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosqvist, R., K. E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rowley, G., M. Spector, J. Kormanec, and M. Roberts. 2006. Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat. Rev. Microbiol. 4:383-394. [DOI] [PubMed] [Google Scholar]
- 86.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilisation system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnology 1:787-796. [Google Scholar]
- 87.Snyder, W. B., L. J. Davis, P. N. Danese, C. L. Cosma, and T. J. Silhavy. 1995. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J. Bacteriol. 177:4216-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sukhan, A., T. Kubori, J. Wilson, and J. E. Galán. 2001. Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex. J. Bacteriol. 183:1159-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thanabalu, T., E. Koronakis, C. Hughes, and V. Koronakis. 1998. Substrate-induced assembly of a contiguous channel for protein export from E. coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J. 17:6487-6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tomoyasu, T., K. Yamanaka, K. Murata, T. Suzaki, P. Bouloc, A. Kato, H. Niki, S. Hiraga, and T. Ogura. 1993. Topology and subcellular localization of FtsH protein in Escherichia coli. J. Bacteriol. 175:1352-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Viboud, G. I., and J. B. Bliska. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59:69-89. [DOI] [PubMed] [Google Scholar]
- 92.Von Pawel-Rammingen, U., M. V. Telepnev, G. Schmidt, K. Aktories, H. Wolf-Watz, and R. Rosqvist. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36:737-748. [DOI] [PubMed] [Google Scholar]
- 93.Watarai, M., T. Tobe, M. Yoshikawa, and C. Sasakawa. 1995. Disulfide oxidoreductase activity of Shigella flexneri is required for release of Ipa proteins and invasion of epithelial cells. Proc. Natl. Acad. Sci. USA 92:4927-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamamoto, K., K. Hirao, T. Oshima, H. Aiba, R. Utsumi, and A. Ishihama. 2005. Functional characterization in vitro of all two-component signal transduction systems from Escherichia coli. J. Biol. Chem. 280:1448-1456. [DOI] [PubMed] [Google Scholar]
- 95.Yip, C. K., T. G. Kimbrough, H. B. Felise, M. Vuckovic, N. A. Thomas, R. A. Pfuetzner, E. A. Frey, B. B. Finlay, S. I. Miller, and N. C. Strynadka. 2005. Structural characterization of the molecular platform for type III secretion system assembly. Nature 435:702-707. [DOI] [PubMed] [Google Scholar]
- 96.Yu, J., and J. S. Kroll. 1999. DsbA: a protein-folding catalyst contributing to bacterial virulence. Microbes Infect. 1:1221-1228. [DOI] [PubMed] [Google Scholar]