Abstract
Listeria monocytogenes evades the antimicrobial mechanisms of macrophages by escaping from the phagosome into the cytosolic space via a unique cytolysin that targets the phagosomal membrane, listeriolysin O (LLO), encoded by hly. Gamma interferon (IFN-γ), which is known to play a pivotal role in the induction of Th1-dependent protective immunity in mice, appears to be produced, depending on the bacterial virulence factor. To determine whether the LLO molecule (the major virulence factor of L. monocytogenes) is indispensable or the escape of bacteria from the phagosome is sufficient to induce IFN-γ production, we first constructed an hly-deleted mutant of L. monocytogenes and then established isogenic L. monocytogenes mutants expressing LLO or ivanolysin O (ILO), encoded by ilo from Listeria ivanovii. LLO-expressing L. monocytogenes was highly capable of inducing IFN-γ production and Listeria-specific protective immunity, while the hly-deleted mutant was not. In contrast, the level of IFN-γ induced by ILO-expressing L. monocytogenes was significantly lower both in vitro and in vivo, despite the ability of this strain to escape the phagosome and the intracellular multiplication at a level equivalent to that of LLO-expressing L. monocytogenes. Only a negligible level of protective immunity was induced in mice against challenge with LLO- and ILO-expressing L. monocytogenes. These results clearly show that escape of the bacterium from the phagosome is a prerequisite but is not sufficient for the IFN-γ-dependent Th1 response against L. monocytogenes, and some distinct molecular nature of LLO is indispensable for the final induction of IFN-γ that is essentially required to generate a Th1-dependent immune response.
Listeria monocytogenes is a gram-positive facultative intracellular bacterium that often causes life-threatening infections in immunocompromised hosts, newborns, and the elderly (8, 11, 40). The virulence of L. monocytogenes is attributed largely to intracellular parasitism once it invades host cells. By means of the virulence factors encoded in Listeria pathogenicity island 1, L. monocytogenes can evade intraphagosomal killing and multiply inside the cytosolic space of the macrophage, a professional phagocyte. The escape from the phagosome into the cytosol is mediated mainly by a 56-kDa cytolytic protein, listeriolysin O (LLO), the most essential virulence determinant encoded by hly on Listeria pathogenicity island 1 (7, 35, 50).
In mice infected with L. monocytogenes, various cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6, are produced and contribute to the host defense at an early stage of infection. Such an innate immune response is due to the direct activation of macrophages and dendritic cells by L. monocytogenes via Toll-like receptors (TLRs) (12, 13, 53, 60). In addition to these proinflammatory cytokines, gamma interferon (IFN-γ)-inducing cytokines such as IL-12, IL-18, and IFN-γ are also produced at an early stage of infection (43, 47, 53, 61). Among these cytokines, IFN-γ is the most important one for host defense against L. monocytogenes, since antilisterial resistance was enhanced by the in vivo administration of recombinant IFN-γ (5, 51) and was abolished by the administration of an anti-IFN-γ antibody (42) or in mice that lack IFN-γ (20) or the IFN-γ receptor (9, 21). We also reported previously that IFN-γ is the most essential cytokine for the development of Th1-dependent acquired resistance against L. monocytogenes and Mycobacterium bovis BCG (67, 68). Thus, the initial production of IFN-γ is important for nonspecific innate defense and for generating L. monocytogenes-specific protective immunity against L. monocytogenes infection.
The induction of IFN-γ after stimulation of naïve spleen cells in vitro and the generation of Th1-dependent acquired immunity in vivo can be observed by using wild-type L. monocytogenes cells that can produce LLO. However, such a response is not observed if the cells are stimulated or immunized with heat-killed L. monocytogenes or live L. monocytogenes that cannot produce LLO (58, 65). Clearly, the IFN-γ response at an early stage requires stimulation by viable L. monocytogenes cells that can escape from the phagosome and is not due simply to the presence of TLR ligands on the bacterial surface. As LLO exhibits multiple functions (29), this observation raises two possibilities: (i) LLO simply disrupts the phagosomal membrane, favoring the delivery of bacterial ligands into the cytosolic space after the escape of the bacterium from the phagosome, and (ii) the LLO molecule is required in the cytosol to induce the initial response of macrophages, resulting in the production of IFN-γ-inducing cytokines.
LLO was previously reported to be the ligand for TLR4 (48), and we previously reported that both purified LLO and recombinant LLO (rLLO) are highly capable of inducing IFN-γ production from spleen cells (44, 45, 48). On the other hand, ivanolysin O (ILO) produced by Listeria ivanovii showed no such activity despite a greater than 80% homology with LLO in the amino acid sequence (16, 19). Although L. ivanovii is highly capable of escaping from the phagosome in macrophages, this species of bacterium belonging to the same genus, Listeria, could not induce IFN-γ production and protective immunity in mice in our previous study (31). As ILO actively disrupts the phagosomal membrane like LLO, ILO appeared to be a useful tool to address this point and to examine the two possibilities. In the present study, we first constructed an LLO-deficient L. monocytogenes mutant by in-frame deletion of hly and then established isogenic L. monocytogenes mutants expressing LLO or ILO. We used these isogenic L. monocytogenes mutants to analyze the precise role of LLO in the induction of the host Th1 response in vitro and in vivo.
MATERIALS AND METHODS
Mice.
Female C3H/HeN mice were purchased from Japan SLC (Shizuoka, Japan), maintained in specific-pathogen-free conditions, and used at 7 to 9 weeks of age. This strain was chosen mainly because a series of our previous studies was carried out with C3H/HeN mice. All the experimental procedures performed on mice were approved by the Animal Ethics and Research Committee of Kyoto University Graduate School of Medicine.
Bacterial strain and growth conditions.
The parental wild-type L. monocytogenes strain used in this study was Listeria monocytogenes EGD (serovar 1/2a). Bacteria were grown overnight in brain heart infusion (BHI) broth (EIKEN CHEMICAL, Tokyo, Japan) at 37°C with shaking. One volume of the culture grown overnight was added to 100 volumes of fresh BHI medium and cultured further for 5 h. Bacterial cells were washed, suspended in phosphate-buffered saline (PBS) supplemented with 10% glycerol, and stored in aliquots at −80°C. The concentration of bacteria was determined by plating 10-fold serially diluted suspensions on a tryptic soy agar (TSA) (EIKEN) plate and counting the number of colonies after cultivation for 24 h.
Construction of an hly-deleted L. monocytogenes mutant strain.
Escherichia coli DH5α was used as the host strain for all the plasmid constructions (TOYOBO, Osaka, Japan). We constructed a shuttle vector that could replicate in E. coli and thermosensitively replicate in L. monocytogenes from pHS-LV provided by Yoshihiro Asano (54) by inserting multiple cloning sites of pUC18 into LV region-deleted pHS. Cloning in E. coli was done by using the thus constructed pHS-MCS. A 2,257-bp fragment located upstream of hly was amplified from the chromosomal DNA of wild-type L. monocytogenes by PCR with the following primer set: 5′-CGATGGTACCTTAATTTAATTTTCCCCAAG-3′ and 5′-ACGCCCCGGGGGGTTTCACTCTCCTTCTAC-3′. These primers were designed to generate restriction sites for KpnI and XmaI, respectively. A 1,919-bp fragment located downstream of hly was amplified similarly, with a primer set consisting of 5′-CGATCCCGGGTTGTAAAAGTAATAAAAAATTAAGA-3′ and 5′-ACGCGTCGACATGAATTATTTTAAGAATATCACTT-3′. These primers were designed to generate restriction sites for XmaI and SalI, respectively. The two amplified fragments were ligated into pHS-MCS with Ligation High (TOYOBO). The resulting plasmid was introduced into competent cells of wild-type L. monocytogenes by electroporation (49). Transformants were selected initially at the permissive temperature (30°C) on BHI agar plates supplemented with erythromycin (5 μg/ml; Nacalai Tesque, Kyoto, Japan). Transformants were then grown on BHI agar plates with erythromycin at the nonpermissive temperature (42°C) to select only those transformants with chromosomal integration of plasmids. Cointegrates were subsequently grown in BHI broth without antibiotic selection at the permissive temperature (30°C) to facilitate the isolation of derivatives in which integrated plasmids were resolved by a second recombination event (3, 54). The in-frame deletion of hly was confirmed by a sequence analysis of the PCR product amplified from the corresponding region of genomic DNA of the erythromycin-sensitive clones.
Construction of isogenic hly-deleted mutants complemented with hly or ilo in the chromosome.
Each cloned gene encoding LLO or ILO, hly or ilo, respectively, was inserted into the hly-deleted position on the chromosomal DNA of the L. monocytogenes Δhly strain. An antisense primer for PCR amplification of the upstream fragment for hly was 5′-CGCCCGGGGGATATCCTTTGCTTCAGTTTG-3′. This primer was designed to generate restriction sites for EcoRV and XmaI and to contain the signal sequence for hly. Other primers, a sense primer for upstream fragment and sense/antisense primers for the downstream fragment of hly, were the same as those used to construct the L. monocytogenes Δhly strain. Amplification of hly was done with the primers 5′-CGATTGCGCATCTGCTTCAATAAAG-3′ and 5′-ATCCCGGGTTATTCGATTGGATTATCTAC-3′, which were designed to generate restriction sites for FspI and XmaI, respectively. ilo was amplified with primers 5′-phosphate-GCCTCAGTATATAGTTAC-3′ and 5′-ATCCCGGGTTACTTAATTGGATTATCTAC-3′. The antisense primer was designed to generate restriction sites for XmaI. Either the hly or the ilo gene and the genes flanking the upstream/downstream region were then ligated into pHS-MCS, and transformants were generated by using the homologous recombination method. The erythromycin-sensitive clones were examined for the insertion site of hly and ilo by a sequence analysis of the PCR products amplified from the genomic DNA. Stable mutants with a confirmed back insertion of hly or ilo into the hly-deleted site on the chromosome were used for the present study.
Detection of cytolysin by immunoblotting and cytolytic activity.
Cytolysin production from bacteria was determined according to methods described previously (15, 30), with some modifications. Bacteria were grown overnight in BHI broth at 37°C with shaking. A 0.1-ml portion of the culture grown overnight was inoculated into 10 ml of fresh BHI medium, and bacteria were cultured further for 3 h. Bacteria were harvested by centrifugation and transferred into 1 ml of RPMI 1640 medium (Gibco-BRL, Life Technologies, Rockville, MD). After cultivation at 37°C for 3 h, the supernatant was collected. Aliquots of the supernatant samples were treated in 2% sodium dodecyl sulfate (SDS) sample buffer at 94°C for 2 min. The samples were subjected to SDS-polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene difluoride membrane by electroblotting. LLO and ILO were identified by immunoblotting with rabbit polyclonal antibodies prepared by hyperimmunization with rLLO and recombinant ILO (rILO) emulsified in Freund's complete adjuvant. The hemolytic activity of bacterial culture supernatants was measured as follows. Sheep erythrocytes (SRBCs) washed three times with PBS were suspended at 2% (vol/vol) in PBS. Fifty microliters of the SRBC suspension was added to an equal volume of serially diluted bacterial culture supernatants and incubated at 37°C for 30 min. Hemolytic activity was estimated by the amount of hemoglobin released into the supernatant as measured by the absorbance at 415 nm. One hundred percent hemolysis was defined as the level of hemoglobin release when SRBCs were treated with 2% Triton X-100.
In vitro infection and immunofluorescence analysis.
Peritoneal exudate cells (PECs) of mice were obtained 3 days after an intraperitoneal injection of 2 ml of thioglycolate medium (EIKEN). PECs were seeded into a 24-well plate at 3 × 105 cells/well and then infected with bacteria at a multiplicity of infection (MOI) of 10 for 30 min at 37°C. Cells were washed three times and cultured for 2 h at 37°C in the presence of 5 μg/ml gentamicin (Wako Pure Chemical Industries, Osaka, Japan). After several washings, the cells were fixed in 3% paraformaldehyde and incubated overnight at 4°C with blocking solution, which is PBS containing 10% Blocking One (Nacalai) and 0.1% saponin (Nacalai). F-actin formation was visualized by staining infected cells with Alexa 488-phalloidin (Invitrogen, Carlsbad, CA), and the bacterial cell was stained by treatment with goat anti-Listeria polyclonal antibody (Kirkegaard & Perry Laboratories, Gaithersburg, MD) in blocking solution at room temperature for 2 h and then with Alexa 546-anti-goat immunoglobulin G antibody (Invitrogen) at room temperature for 2 h in a darkroom. Cells were examined 1.5 and 2 h after infection under a fluorescent microscope for bacterial escape from the phagosome into the cytosol. Three hundred bacterial cells were examined for the presence or absence of associating F-actin (actin clouds and tails), and the percent escape was expressed as the percentage of F-actin-positive bacteria.
Bacterial growth in vitro.
Thioglycolate-induced whole PECs were seeded into a 96-well plate at 1.5 × 105 cells/well and then infected with each strain of L. monocytogenes at an MOI of 0.1 for 1 h. The cells were washed three times and then cultured for 1 to 12 h at 37°C in the presence of 5 μg/ml gentamicin. The amount of gentamicin used was based on our previous finding of the appropriate concentration that blocks the extracellular growth without affecting the bacterial growth inside the cells (46). After extensive washing, the cells were lysed with PBS containing 0.05% Triton X-100. Cell lysates were 10-fold serially diluted and plated onto TSA plates to count the viable bacteria inside cells. The intracellular number of viable bacteria was expressed as log CFU per well.
Bacterial growth in vivo.
Mice were infected intravenously with 1 × 103 CFU of wild-type and mutant L. monocytogenes strains. On days 1 and 2, the spleens were removed and homogenized in 5 ml of PBS. Homogenates were 10-fold serially diluted, and colony counting was done on TSA plates.
Survival of mice immunized with L. monocytogenes strains after challenge infection with wild-type L. monocytogenes or the L. monocytogenes Δhly::ilo strain.
Mice were immunized by an intravenous infection of 1 × 103 CFU of wild-type and mutant L. monocytogenes strains. To examine the level of Listeria-specific protection, mice were challenged intravenously with wild-type L. monocytogenes (50% lethal dose of 6 × 104 CFU) or the L. monocytogenes Δhly::ilo strain (50% lethal dose of 1 × 106 CFU) on day 7 and day 28 after immunization. The survival of a group of five mice was monitored for 7 days.
Measurement of cytokine response.
Thioglycolate-induced whole PECs were seeded into a 96-well plate at 1.5 × 105 cells/well without removing nonadherent cells to determine the infection-induced production of TNF-α, IL-12p40, and IFN-γ as reported previously (18). Bacteria were added to the whole PECs at an MOI of 0.1 for 1 h, and the cells were then cultured for 24 h at 37°C in the presence of 5 μg/ml gentamicin. Culture supernatants were saved, and the cytokine titer was determined by two-site sandwich enzyme-linked immunosorbent assay (ELISA). ELISA kits for TNF-α and IL-12p40 were purchased from eBiosciences (San Diego, CA). For the titration of IFN-γ, wells of an Immunoplate (Nunc, Roskilde, Denmark) were precoated with rat anti-mouse IFN-γ monoclonal antibody (Endogen, Woburn, MA) in carbonate-bicarbonate buffer (pH 9.6). A 50-μl sample and biotin-conjugated anti-mouse IFN-γ antibody (Endogen) were added sequentially to each well at 1-h intervals. Wells were washed, and horseradish peroxidase-conjugated streptavidin (Endogen) was added. After incubation for 30 min, IFN-γ was detected by adding 3,3′,5,5′-tetramethylbenzidine dihydrochloride dehydrate (Nacalai) solution (50 μg/ml) containing 0.01% H2O2, and the absorbance was measured at 450 nm. The IFN-γ titer was calculated according to the absorbance of an authentic IFN-γ sample.
Generation of IFN-γ-producing T cells after immunization with L. monocytogenes strains.
Seven days after the immunizing infection of mice with 1 × 103 CFU of each strain, the spleens were removed, and a single-cell suspension was prepared. Cells were separated into CD90+ (Thy1.2) T cells and cells depleted for CD90+, CD4+ (L3T4), and CD8+ (Ly-2) T cells with a magnetic cell sorting system (Miltenyi Biladbach, Germany) according to the manufacturer's instructions. These cells were stimulated in the presence of bone marrow-derived macrophages (BMDMs) pulsed in advance with wild-type L. monocytogenes or the L. monocytogenes Δhly::ilo strain for 24 h at 37°C. The IFN-γ level in the culture supernatants was determined as mentioned above.
Statistical analysis.
The statistical significance of the data was determined by a Student's t test; a P value of <0.01 was regarded as being significant.
RESULTS
Construction and characterization of mutant L. monocytogenes strains.
We constructed isogenic L. monocytogenes strains that produced either LLO or ILO to study the role of listerial cytolysin in IFN-γ induction in L. monocytogenes infection. We first constructed an in-frame deletion mutant of hly and then established two recombinant strains that complemented either hly or ilo into the chromosomes of the L. monocytogenes Δhly, Δhly::hly, and Δhly::ilo strains. We confirmed the stability of these three mutant L. monocytogenes strains and found no significant difference in the pattern of in vitro growth in BHI broth compared with that of wild-type L. monocytogenes (data not shown).
We examined the secretion of cytolysin into the culture supernatant of each recombinant strain by immunoblotting with anti-LLO or anti-ILO polyclonal antibodies. We confirmed that LLO was not produced in the L. monocytogenes Δhly strain and that LLO and ILO were produced in the L. monocytogenes Δhly::hly and Δhly::ilo strains, respectively. There was no significant difference in the amount of cytolysin produced between wild-type L. monocytogenes, the L. monocytogenes Δhly::hly strain, and the L. monocytogenes Δhly::ilo strain as judged by the intensity of the bands (Fig. 1A). We also measured the hemolytic activity in the bacterial culture supernatant and detected no hemolytic activity in the culture supernatant of the L. monocytogenes Δhly strain. In contrast, the culture supernatants of the L. monocytogenes Δhly::hly and Δhly::ilo strains exhibited a high level of hemolytic activity that was comparable with that of wild-type L. monocytogenes (Fig. 1B). These results, along with the sequence analysis of the site of the hly and ilo insertions in the complemented strains (data not shown), indicated that the L. monocytogenes Δhly, Δhly::hly, and Δhly::ilo strains were successfully constructed and functionally active as designed.
FIG. 1.
Production of cytolysins by L. monocytogenes mutants. Wild-type and mutant L. monocytogenes strains were cultured first in BHI medium and then transferred into RPMI 1640 medium. Three hours after cultivation, the supernatant was collected by centrifugation. (A) An aliquot of supernatant was applied to SDS-polyacrylamide gels and transferred onto a polyvinylidene difluoride membrane. Immunoblotting was performed with anti-LLO polyclonal antibody (left) or anti-ILO polyclonal antibody (right). (B) Culture supernatants were twofold serially diluted with PBS and mixed with an equal volume of a 2% suspension of SRBCs. After incubation for 30 min at 37°C, the hemolytic activity was measured by the amount of hemoglobin released into the supernatant. One hundred percent hemolysis was defined as the level of hemoglobin released after treatment with 2% Triton X-100.
Escape from the phagosome and intracellular multiplication of mutant L. monocytogenes strains inside macrophages.
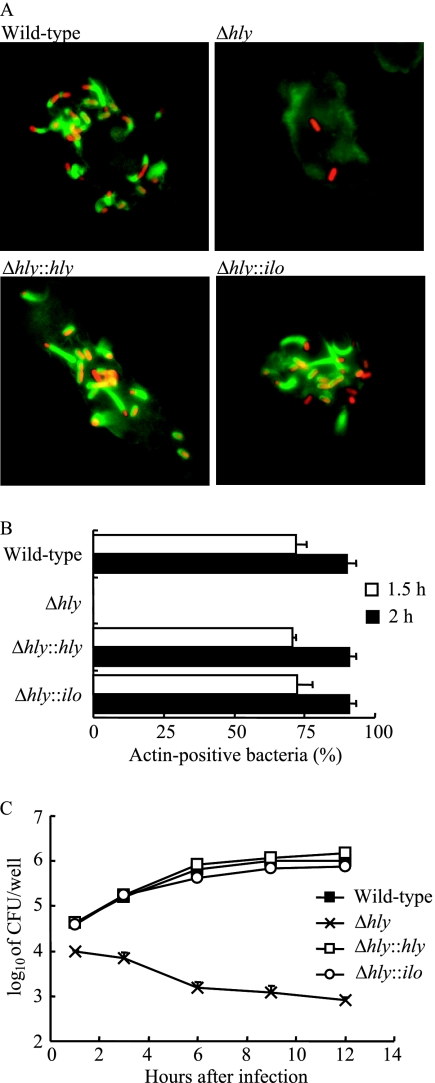
Escape of the bacterium from the phagosome is indispensable for the intracellular growth of L. monocytogenes and is important for the expression of virulence in vivo, as the cytolysin-deleted mutant cannot grow inside macrophages or exhibit virulence in mice (15). PECs of mice were infected in vitro, and the ability of each mutant to escape the phagosome was assessed by immunofluorescence staining of bacterial cells and F-actin, which is indicative of bacteria in the cytoplasmic space (32). At 2 h after infection, none of the cells of the L. monocytogenes Δhly strain was associated with F-actin (Fig. 2A). In macrophages infected with wild-type L. monocytogenes or the Δhly::hly or Δhly::ilo strain, a number of bacteria were positive for F-actin, and there was no apparent difference between them (Fig. 2A). In a quantitative assessment of the percentage of F-actin-positive bacterial cells, there was no apparent difference between these three strains at 1.5 and 2 h postinfection (Fig. 2B). We confirmed that the L. monocytogenes Δhly::hly and Δhly::ilo strains have an ability to escape from the phagosome that is similar to that of wild-type L. monocytogenes.
FIG. 2.
Intracellular escape and multiplication of L. monocytogenes mutants inside macrophages. (A) Whole PECs were infected with each L. monocytogenes strain at an MOI of 10 for 30 min. After washing with RPMI 1640 medium, cells were cultured for 2 h in the presence of gentamicin (5 μg/ml). Bacteria were labeled with goat anti-Listeria antibody and stained with Alexa 546-anti-goat immunoglobulin G antibody (red). F-actin was visualized by staining with Alexa 488-phalloidin (green). (B) After staining, 300 bacteria were counted. The percentage of bacteria positive for associating F-actin was calculated for each strain at 1.5 and 2 h postinfection. The column represents the mean of three independent wells, and the bar indicates the standard deviation. (C) Whole PECs were infected with each L. monocytogenes strain at an MOI of 0.1 for 1 h. After washing with RPMI 1640 medium, cells were cultured for 1 to 12 h in the presence of gentamicin (5 μg/ml). At the indicated time points, the cells were lysed, diluted, and plated onto TSA plates to count the viable bacteria. Each point represents the mean of three wells, and the bar indicates the standard deviation. Similar results were obtained in two independent experiments.
We next examined the intracellular growth of each L. monocytogenes strain in vitro. Whole PECs were infected with each strain, and the number of viable intracellular bacteria was assessed at various time points after gentamicin was added. Wild-type L. monocytogenes multiplied gradually for the initial 6 h and then reached a plateau, while the L. monocytogenes Δhly strain could not grow inside PECs and decreased gradually. Both the L. monocytogenes Δhly::hly and Δhly::ilo strains could grow inside PECs in a pattern similar to that of wild-type L. monocytogenes (Fig. 2C). These data confirmed that the complementation of the L. monocytogenes Δhly strain with hly or ilo resulted in the recovery of abilities for escape from the phagosome and intracellular multiplication in macrophages to a level comparable to that of wild-type L. monocytogenes.
Cytokine-inducing ability of mutant L. monocytogenes strains in vitro.
The ability of mutant L. monocytogenes strains to induce various cytokines was determined in vitro. In our in vitro system, TNF-α and IL-12p40 were produced at levels that were significantly higher than the background level after stimulation with any type of bacteria (Fig. 3A and B). Although there were some variations between groups, the production of these two cytokines may have been induced by bacterial cells irrespective of their virulence. In contrast, the IFN-γ-inducing ability of wild-type L. monocytogenes was lost by the deletion of hly (the L. monocytogenes Δhly strain). This ability was restored by complementation of the L. monocytogenes Δhly strain with hly (the L. monocytogenes Δhly::hly strain). Interestingly, such a significant level of restoration was not observed in the L. monocytogenes Δhly strain complemented with ilo (the L. monocytogenes Δhly::ilo strain) (Fig. 3C). Fukasawa et al. previously reported that IFN-γ production by whole PECs in response to bacterial stimulation comes mainly from NK cells (18). In the same culture supernatants, we also determined the level of IL-12p70 and the mature form of IL-18, two major cytokines that induce the production of IFN-γ from NK cells. However, the level of these two cytokines was below the detection limit of our ELISA system (data not shown).
FIG. 3.
Production of cytokines in PECs after stimulation with each L. monocytogenes strain in vitro. Whole PECs were infected with each L. monocytogenes strain at an MOI of 0.1 for 1 h. Cells were cultured for 24 h in the presence of gentamicin (5 μg/ml), and the culture supernatant was then collected. The amounts of (A) TNF-α, (B) IL-12p40, and (C) IFN-γ were determined by using an ELISA specific for each cytokine. Data represent the means of triplicate assays and standard deviations. Similar results were obtained in three independent experiments. *, P < 0.005.
Growth of mutant L. monocytogenes strains in vivo.
After we confirmed the in vitro virulence of mutant L. monocytogenes strains constructed in this study, we monitored the growth of each L. monocytogenes strain in vivo during primary infection. Bacterial growth during the early stage of infection is not yet under the influence of specific T-cell-dependent immunity that is induced by an immunizing infection (39). In addition, the magnitude of the Listeria-specific T-cell response depends mainly on the bacterial burden during the first 24 h of infection (6, 36). Based on these reports, we compared the in vivo growth of each L. monocytogenes strain by enumerating the bacterial burden in the spleens on days 1 and 2 after infection to rule out any possible effect of Listeria-specific T cells to be generated after day 3 of infection. Wild-type L. monocytogenes multiplied significantly in the spleens during the early stage of infection, and such an in vivo growth was lost in the L. monocytogenes Δhly strain (Fig. 4). There was no significant difference in the magnitude of bacterial growth between wild-type L. monocytogenes and the L. monocytogenes Δhly::hly and Δhly::ilo strains, indicating that gene complementation with hly or ilo effectively restored the in vivo growth of the L. monocytogenes Δhly strain.
FIG. 4.
Growth of L. monocytogenes strains in vivo. Groups of mice were infected intravenously with 103 CFU of each strain. The spleen was removed on days 1 (A) and 2 (B) for CFU counting by plating the serially diluted homogenates onto a TSA plate, and the viable bacteria per spleen were enumerated. Circles represent the bacterial number per each spleen from a group of five mice, and the bar indicates the mean of five. The detectable limit of the bacterial count was 50 CFU/ml. Similar results were obtained in two independent experiments.
Generation of acquired protective immunity against challenge infection in mice immunized with mutant L. monocytogenes strains.
Listeria-specific effector or memory T cells induced by immunization play protective roles in the convalescent phase of primary or secondary infection, respectively (70). Since a significant level of acquired protective immunity is generated as early as 6 to 7 days after mice are immunized with a nonlethal dose of wild-type L. monocytogenes (67), we immunized groups of mice with 103 CFU of each strain and then compared levels of protective immunity on day 7 against challenge with wild-type L. monocytogenes or the L. monocytogenes Δhly::ilo strain at doses that are lethal to nonimmune mice. The lethal activity of the L. monocytogenes Δhly::ilo strain was lower than that of wild-type L. monocytogenes, so we used a 10-fold-higher dose of the L. monocytogenes Δhly::ilo strain for the challenge infection. Immunization with the L. monocytogenes Δhly strain induced no protective immunity, but all the mice from groups immunized with wild-type or complemented strains survived the challenge infection with wild-type L. monocytogenes (Fig. 5A, left). A similar pattern of survival was observed after challenge with the L. monocytogenes Δhly::ilo strain (Fig. 5A, right). However, when mice were challenged with a 10-fold-higher dose of each strain, all the mice immunized with the L. monocytogenes Δhly::ilo strain succumbed, and 100% of the mice immunized with the L. monocytogenes Δhly::hly strain survived (Fig. 5B). In repeated experiments, mice immunized with the L. monocytogenes Δhly::hly strain survived the challenge infection with as high as 108 CFU. The level of protective immunity was examined on day 28 after immunization, as the contribution of non-specifically-activated effector cells other than antigen-specific T cells could not be completely ruled out on day 7 postimmunization (70). Protective immunity was observed against both wild-type L. monocytogenes and the L. monocytogenes Δhly::ilo strain in mice immunized with wild-type L. monocytogenes or the L. monocytogenes Δhly::hly strain but not in the group immunized with the L. monocytogenes Δhly::ilo strain (Fig. 5C). These results clearly showed a significant difference in the ability to induce protective immunity of mice between the L. monocytogenes Δhly::hly and Δhly::ilo strains. Compared to nonimmune and L. monocytogenes Δhly-immune mice, some level of protective immunity could be induced by the L. monocytogenes Δhly::ilo strain, but the protection was not at the level induced by wild-type L. monocytogenes or the L. monocytogenes Δhly::hly strain. Thus, the L. monocytogenes Δhly::ilo strain might be deficient in the ability to induce a full level of protective immunity. We can rule out the possibility that the low level of acquired protection induced by the L. monocytogenes Δhly::ilo strain against the challenge with wild-type L. monocytogenes is due to the ineffectiveness of ILO-specific T cells against the LLO-producing strain because the protective level was the same as that of the challenge with the L. monocytogenes Δhly::ilo strain. The ability of each strain to induce protective immunity was consistent with the profile of the IFN-γ-inducing ability of each strain in vitro (Fig. 3).
FIG. 5.
Survival of mice immunized with L. monocytogenes strains after challenge infection. Groups of mice were intravenously immunized with 103 CFU of each L. monocytogenes strain. On day 7 (A and B) or day 28 (C) after immunization, mice were challenged intravenously with wild-type L. monocytogenes (left) or the L. monocytogenes Δhly::ilo strain (right). (A and C) Mice were challenged with 1 × 106 CFU of wild-type L. monocytogenes or 1 × 107 CFU of the L. monocytogenes Δhly::ilo strain. (B) Mice were challenged with 1 × 107 CFU of wild-type L. monocytogenes or 1 × 108 CFU of the L. monocytogenes Δhly::ilo strain. Survival was monitored for 7 days. Each experimental group consisted of five mice. Similar results were obtained in two independent experiments.
Induction of endogenous IFN-γ after infection in vivo.
To address whether the difference in the induction of acquired immunity between the L. monocytogenes Δhly::hly and Δhly::ilo strains reflects the IFN-γ-inducing ability in vivo, we determined the level of endogenous IFN-γ on days 1 and 2 after immunization. The IFN-γ detected after immunization with the L. monocytogenes Δhly strain was below the background level. In mice immunized with wild-type L. monocytogenes, we observed a high level of endogenous IFN-γ production in the spleen and serum on day 2 and saw a comparable level of IFN-γ production with a kinetic similar to that in mice immunized with the L. monocytogenes Δhly::hly strain (Fig. 6). In contrast, the IFN-γ level was significantly lower in mice immunized with the L. monocytogenes Δhly::ilo strain. These findings and the similarity in the in vivo growth patterns of the L. monocytogenes Δhly::hly and Δhly::ilo strains (Fig. 4) clearly indicated the exclusive importance of the LLO molecule in the induction of endogenous IFN-γ, which is critical for generating acquired protective immunity in mice infected with L. monocytogenes (2, 67).
FIG. 6.
IFN-γ production in the spleen and serum of infected mice. Mice were intravenously infected with 103 CFU of each L. monocytogenes strain. Sera were collected daily from the uninfected and infected mice on days 1 and 2 after infection. Spleens were removed and homogenized in PBS. The level of IFN-γ production was determined by ELISA. Data represent the means of five mice and standard deviations. Similar results were obtained in two independent experiments. *, P < 0.005.
Generation of IFN-γ-producing T cells in mice immunized with mutant L. monocytogenes strains.
We prepared CD90+ splenic T cells 7 days after immunization and stimulated them in vitro with BMDMs prepulsed with viable wild-type L. monocytogenes to confirm that the difference in the levels of protective immunity between groups immunized with the L. monocytogenes Δhly::hly and Δhly::ilo strains reflected antigen-specific T-cell generation in vivo. A significant level of L. monocytogenes-specific T-cell response resulting in a high level of IFN-γ production was observed in CD90+ cells obtained from mice immunized with either wild-type L. monocytogenes or the L. monocytogenes Δhly::hly strain but not in similarly prepared cells from mice immunized with the L. monocytogenes Δhly::ilo strain (Fig. 7A). There was no significant change in the very low level of T-cell response of the group immunized with the L. monocytogenes Δhly::ilo strain even by stimulation with BMDMs prepulsed with the L. monocytogenes Δhly::ilo strain, suggesting that the observed difference was not due simply to some differences in T-cell epitopes between LLO and ILO (Fig. 7B). The cell depletion study revealed that CD4+ T cells and CD8+ T cells were responsible for the antigen-specific IFN-γ response that was observed at a high level in both groups immunized with wild-type L. monocytogenes and the Δhly::hly strain (Fig. 7C).
FIG. 7.
Generation of IFN-γ-producing T cells in mice immunized with L. monocytogenes strains. Mice were intravenously infected with 103 CFU of each L. monocytogenes (LM) strain. Seven days after immunization, CD90+ T cells were prepared from the spleens and stimulated for 24 h with BMDMs pulsed with (A) wild-type L. monocytogenes or (B) the L. monocytogenes Δhly::ilo strain. (C) Whole spleen cells from mice immunized with each L. monocytogenes strain were depleted for CD90+, CD4+, or CD8+ cells, and the cells of each fraction were stimulated with BMDMs pulsed with wild-type L. monocytogenes for 24 h. The level of IFN-γ production in the supernatant was determined by ELISA. Data represent the means of triplicate assays and standard deviations.
DISCUSSION
The antigen-specific T-cell response is indispensable for resolving primary infection with L. monocytogenes and for expressing acquired protective immunity against a challenge with a lethal dose. Protective T cells are generated only when mice are immunized with a nonlethal dose of a viable and virulent L. monocytogenes strain but not after immunization with a killed or nonvirulent strain of L. monocytogenes (62). This suggests that the virulence factor of L. monocytogenes may help induce protective immunity. On the basis of this finding, we have been studying the contribution of LLO, a cytolysin essential for the expression of bacterial virulence, to the induction of protective immunity in mice. In previous studies, we found that the LLO-deficient L. monocytogenes strain is less virulent and less capable of inducing specific protective immunity (58, 64). Moreover, we showed that in contrast to L. monocytogenes, antigen-specific protective immunity was hardly induced with L. ivanovii (31). Endogenous IFN-γ production at the initial stage of immunization is important for generating protective Th1 cells. In our previous study using recombinant cytolysins derived from L. monocytogenes and L. ivanovii, there was a significant difference in the ability to induce IFN-γ between LLO from L. monocytogenes and ILO from L. ivanovii. Cytolysin-dependent IFN-γ production may be a limiting factor for the generation of acquired immunity in mice. The present study was undertaken to elucidate the role of LLO in the induction of acquired immunity against L. monocytogenes, with special reference to its ability to induce IFN-γ.
In the present study, we constructed LLO- and ILO-secreting isogenic mutants by complementing an in-frame deletion mutant for hly with a gene that encodes the mature secreted protein of LLO or ILO. The construction of a similar ILO-producing mutant isogenic with LLO-producing L. monocytogenes is reported. Frehel et al. previously employed a pAT28-based expression vector, but the constructed pAT28-ilo mutant exhibited a reduced ability for ILO secretion and virulence compared with the pAT28-hly mutant (15). The inefficient secretion of ILO could be ascribed to the signal sequence. Based on this assumption, we did not use a plasmid-harboring mutant but did use complementation in the chromosomal DNA. The mutant L. monocytogenes Δhly::ilo strain constructed in the present study was designed so that the sequence of hly encoding the signal sequence of LLO was ligated into the ilo sequence that encodes the mature secreted protein of ILO to ensure a similar level of ILO secretion.
Both the L. monocytogenes Δhly::hly and Δhly::ilo strains thus constructed produced each cytolysin to a level comparable to that of wild-type L. monocytogenes, and the cytolytic activity was almost the same (Fig. 1). This was consistent with the strong hemolytic activity observed in rLLO and rILO in our previous studies (31, 63). As expected, the deletion of the LLO-producing ability (the L. monocytogenes Δhly strain) resulted in a complete loss of escape of the bacterium from the phagosome and subsequent intracellular multiplication inside macrophages. Complementing the LLO-deficient mutant with the hly or ilo gene could completely restore its ability to escape from the phagosome and multiply in the cytoplasm (Fig. 2).
The most critical finding in the present study was a significant reduction of the IFN-γ-inducing ability in the L. monocytogenes Δhly::ilo strain despite its similarity with the L. monocytogenes Δhly::hly strain in in vitro bacterial growth and the ability to induce TNF-α and IL-12p40 (Fig. 3). The production of these two cytokines was induced even by stimulation with a nonvirulent mutant L. monocytogenes Δhly strain, suggesting that such an innate response is directed mainly against common bacterial cell wall TLR ligands like lipoteichoic acid or peptidoglycan (52, 53). The loss of the IFN-γ-inducing ability by the deletion of hly and the full restoration by gene complementation with hly indicated that LLO is indispensable for the activation of the cellular response resulting in IFN-γ production as an innate immune response to wild-type L. monocytogenes. These findings definitely showed that bacterial escape from the phagosome and the subsequent delivery of bacterial cell wall ligands into the cytosolic space are not sufficient to induce the IFN-γ response of the host. Instead, bacterial escape is the prerequisite, and the LLO molecule is indispensable for the cellular response that results in robust IFN-γ production.
In our previous study using recombinant cytolysin, we showed that the cytokine-inducing ability of rLLO was very high, but that of rILO was not (31). The IFN-γ-inducing ability was attributed to the region of LLO at domains 1 to 3 (33). Although the experimental conditions were different between the stimulation by exogenously added recombinant cytolysin and the stimulation by infection with cytolysin-producing bacteria, some structural difference between LLO and ILO likely determined the level of the IFN-γ-inducing ability after escape into the cytosolic space in Listeria-infected macrophages.
Thale and Kiderlen previously reported that the production of IFN-γ as an innate immune response to L. monocytogenes in mice depends mainly on NK cells (59). In our present in vitro system using whole PECs for the stimulation with bacteria, IFN-γ production in response to wild-type L. monocytogenes was almost abolished if whole PECs were treated with anti-NK1.1 antibody plus complement (data not shown), suggesting that the NK cell population is also responsible for the primary IFN-γ response in vitro. NK cell-dependent production of IFN-γ in vitro in the same experimental condition stimulated with Mycobacterium tuberculosis was reported previously (18). IL-12p70 and IL-18 are believed to be the major IFN-γ-inducing cytokines targeting NK cells. Although the production of these two cytokines was not observed at detectable levels in our ELISA system, a minute amount of IL-12p70 and/or mature IL-18 may be induced by wild-type L. monocytogenes and the L. monocytogenes Δhly::hly strain but not by the L. monocytogenes Δhly::ilo strain. In our previous study on the mechanism of IFN-γ induction by rLLO in vitro, the addition of neutralizing antibodies for IL-12 and IL-18 resulted in the loss of IFN-γ production, even though IL-12p70 and IL-18 were at very low levels of detection and the cells from IL-12 knockout or IL-18 knockout mice never responded to rLLO for IFN-γ production (45). These findings support the idea that IL-12p70 or IL-18 (or both) is the central cytokine that determines the different IFN-γ response to L. monocytogenes strains.
If IL-12p70 and/or IL-18 is responsible for IFN-γ production in vitro, a question may arise about the mechanisms of different activities to induce such IFN-γ-inducing cytokines between the L. monocytogenes Δhly::hly and Δhly::ilo strains. There are several possibilities, including a difference in the activation of known TLRs on the endosomal membrane or a difference in the stimulation of an unknown cytosolic pattern recognition system after the bacteria escape into the cytosol. The former is not likely, because the production of TNF-α and IL-12p40 was observed, and there was no significant difference between the four L. monocytogenes strains (Fig. 3). Furthermore, as LLO is known as the TLR4 ligand, the IFN-γ response to both wild-type L. monocytogenes and the L. monocytogenes Δhly::hly strain should be reduced in TLR4-deficient mice. However, in our preliminary study, the cytokine responses, including IFN-γ against L. monocytogenes, showed a similar pattern in PECs from both C3H/HeN and C3H/HeJ (deficient for an intact TLR4) mouse strains (data not shown). Therefore, the most plausible interpretation is that some cytosolic pattern recognition system recognizes cytosolic LLO but not cytosolic ILO. A number of novel receptors for pattern recognition in the cytosol have recently been highlighted (38). Nucleotide-binding oligomerization domain 1 (NOD1) and NOD2 recognize the peptidoglycan-derived peptides (14, 22, 23, 55). Retinoic acid-inducible gene I and melanoma differentiation-associated gene 5 were newly identified as being the non-TLR sensing system for intracellular viral RNA (28, 37). A new member of the NOD-leucine-rich repeat protein family, cryopyrin (or Nalp3), is reportedly linked to the activation of intracellular host defense signaling pathways (4, 14, 17, 27, 34, 57, 69). As cryopyrin is essential for caspase-1 activation resulting in the maturation of IL-18 (56), future work should examine whether the L. monocytogenes Δhly::hly and Δhly::ilo strains differently activate such an intracellular pathway.
In in vivo experiments, the L. monocytogenes Δhly strain showed no growth in the spleen, as expected, and both the L. monocytogenes Δhly::hly and Δhly::ilo strains grew similarly in mice compared to wild-type L. monocytogenes during the first few days after infection (Fig. 4), which is an important period for generating T-cell-dependent acquired immunity (6, 36). Unexpectedly, the lethal activity of the L. monocytogenes Δhly::ilo strain was decreased compared with that of wild-type L. monocytogenes and the L. monocytogenes Δhly::hly mutant, and a higher dose was required in the challenge infection. The precise mechanism of this reduction in lethal activity is not yet clear and needs to be addressed in a future study. Decatur and Portnoy previously reported that a PEST-like sequence located in the N terminus of LLO is essential for listerial pathogenicity (10). As the PEST-like sequence found in LLO is absent in ILO, the lack of a PEST-like sequence may be responsible for reducing the lethal activity in the L. monocytogenes Δhly::ilo strain. However, the in vivo growth of the L. monocytogenes Δhly::ilo strain was almost the same as that of the L. monocytogenes Δhly::hly strain within 2 days after infection (Fig. 4), but significant IFN-γ production in the spleen and serum within 2 days was observed only after infection with the L. monocytogenes Δhly::hly strain (Fig. 6). Endogenous IFN-γ is also essential for innate immunity against Listeria infection (9, 41). In mice deficient for the IFN-γ receptor, a significant level of bacterial multiplication was observed (9), indicating the role of IFN-γ-dependent defense in the limitation of bacterial growth at the initial stage of infection. In an experiment using anti-IFN-γ antibody in vivo, Nakane et al. previously observed such a significant level of bacterial growth when a dose of neutralizing antibody as high as 1,000 μg was injected, but the effect of neutralization was marginal with 300 μg of antibody (41). These observations suggest that the limitation of bacterial growth at an early stage is possible with a certain level of endogenous IFN-γ. In the results of present study, the growth of the L. monocytogenes Δhly::ilo strain was almost the same as that of the L. monocytogenes Δhly::hly strain (Fig. 4), and some level of IFN-γ production was observed in mice infected with the L. monocytogenes Δhly::ilo strain despite a significant reduction in its level compared to that of the L. monocytogenes Δhly::hly strain (Fig. 6). Therefore, the small level of (not null) endogenous IFN-γ production observed after infection with the L. monocytogenes Δhly::ilo strain was likely able to control the growth of this strain at the initial stage, but this level of IFN-γ was not sufficient to develop acquired protective immunity.
High levels of protective immunity that can control the challenge with a high dose of wild-type L. monocytogenes (Fig. 5) and L. monocytogenes-specific IFN-γ-producing T cells could be generated (Fig. 7) only after immunization of mice with the L. monocytogenes Δhly::hly strain but not with the Δhly::ilo strain. These differences between the L. monocytogenes Δhly::hly and Δhly::ilo strains were also observed in challenge infection or stimulation with the L. monocytogenes Δhly::ilo strain (Fig. 5 and 7). These data indicated that the observed difference was not due simply to some differences in T-cell epitopes between LLO and ILO. As the magnitude of the Listeria-specific T-cell response induced by immunizing infection is highly dependent on the bacterial burden during the initial 24 h and not the entire duration afterward (6, 36), such a significant difference between the L. monocytogenes Δhly::hly and Δhly::ilo strains cannot be explained by the possible small difference, if any, in the total bacterial burden during the 7-day period before the challenge infection.
The findings in the present study clearly demonstrated that LLO plays an essential role not only as a T-cell antigen but also through its IFN-γ-inducing ability to establish acquired immunity. Also, the inability of L. ivanovii to induce protective immunity is probably due to the lack of a kind of adjuvant-like activity in ILO. The fact that LLO exhibits a strong adjuvant-like activity-skewing immune response to Th1 dominance was shown in our recent study by applying rLLO to ovalbumin-induced allergy in mice (66).
LLO is a representative protein belonging to a family of cholesterol-dependent cytolysins (CDCs). CDCs are produced by a variety of gram-positive bacterial species of the genera Listeria, Streptococcus, Clostridium, and others (1). In addition to L. monocytogenes and L. ivanovii, Listeria seeligeri produces seeligeriolysin O (LSO), encoded by lso. Our previous studies have shown that although L. seeligeri is not highly pathogenic and the cytolytic activity of rLSO was not so high (24), recombinant LSO induced cytokines in vitro at a magnitude higher than that of rLLO (25) in a unique profile (26). The construction of the L. monocytogenes Δhly strain complemented with genes coding for LSO or other CDCs in the future may increase our understanding of the structure-function relationship between CDC and the cytokine response in infection.
Acknowledgments
This study was supported by a grant-in-aid for scientific research on priority areas from the Ministry of Education, Science, Culture, and Sports of Japan and by a grant-in-aid for scientific research (B and C) and a grant-in-aid for young scientists (B) from the Japan Society for the Promotion of Science.
We thank Yoshihiro Asano (University of Ehime) for providing plasmid pHS-LV.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Billington, S. J., B. H. Jost, and J. G. Songer. 2000. Thiol-activated cytolysins: structure, function and role in pathogenesis. FEMS Microbiol. Lett. 182:197-205. [DOI] [PubMed] [Google Scholar]
- 2.Buchmeier, N. A., and R. D. Schreiber. 1985. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc. Natl. Acad. Sci. USA 82:7404-7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamaillard, M., S. E. Girardin, J. Viala, and D. J. Philpott. 2003. Nods, Nalps and Naip: intracellular regulators of bacterial-induced inflammation. Cell. Microbiol. 5:581-592. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y., A. Nakane, and T. Minagawa. 1989. Recombinant murine gamma interferon induces enhanced resistance to Listeria monocytogenes infection in neonatal mice. Infect. Immun. 57:2345-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbin, G. A., and J. T. Harty. 2004. Duration of infection and antigen display have minimal influence on the kinetics of the CD4+ T cell response to Listeria monocytogenes infection. J. Immunol. 173:5679-5687. [DOI] [PubMed] [Google Scholar]
- 7.Cossart, P., M. F. Vicente, J. Mengaud, F. Baquero, J. C. Perez-Diaz, and P. Berche. 1989. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect. Immun. 57:3629-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cousens, L. P., and E. J. Wing. 2000. Innate defenses in the liver during Listeria infection. Immunol. Rev. 174:150-159. [DOI] [PubMed] [Google Scholar]
- 9.Dai, W. J., W. Bartens, G. Kohler, M. Hufnagel, M. Kopf, and F. Brombacher. 1997. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J. Immunol. 158:5297-5304. [PubMed] [Google Scholar]
- 10.Decatur, A. L., and D. A. Portnoy. 2000. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science 290:992-995. [DOI] [PubMed] [Google Scholar]
- 11.Edelson, B. T., and E. R. Unanue. 2000. Immunity to Listeria infection. Curr. Opin. Immunol. 12:425-431. [DOI] [PubMed] [Google Scholar]
- 12.Edelson, B. T., and E. R. Unanue. 2002. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 169:3869-3875. [DOI] [PubMed] [Google Scholar]
- 13.Flo, T. H., O. Halaas, E. Lien, L. Ryan, G. Teti, D. T. Golenbock, A. Sundan, and T. Espevik. 2000. Human Toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 164:2064-2069. [DOI] [PubMed] [Google Scholar]
- 14.Franchi, L., C. McDonald, T. D. Kanneganti, A. Amer, and G. Nunez. 2006. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J. Immunol. 177:3507-3513. [DOI] [PubMed] [Google Scholar]
- 15.Frehel, C., M. A. Lety, N. Autret, J. L. Beretti, P. Berche, and A. Charbit. 2003. Capacity of ivanolysin O to replace listeriolysin O in phagosomal escape and in vivo survival of Listeria monocytogenes. Microbiology 149:611-620. [DOI] [PubMed] [Google Scholar]
- 16.Freitag, N. E., and K. E. Jacobs. 1999. Examination of Listeria monocytogenes intracellular gene expression by using the green fluorescent protein of Aequorea victoria. Infect. Immun. 67:1844-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritz, J. H., R. L. Ferrero, D. J. Philpott, and S. E. Girardin. 2006. Nod-like proteins in immunity, inflammation and disease. Nat. Immunol. 7:1250-1257. [DOI] [PubMed] [Google Scholar]
- 18.Fukasawa, Y., I. Kawamura, R. Uchiyama, K. Yamamoto, T. Kaku, T. Tominaga, T. Nomura, S. Ichiyama, T. Ezaki, and M. Mitsuyama. 2005. Streptomycin-dependent exhibition of cytokine-inducing activity in streptomycin-dependent Mycobacterium tuberculosis strain 18b. Infect. Immun. 73:7051-7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas, A., M. Dumbsky, and J. Kreft. 1992. Listeriolysin genes: complete sequence of ilo from Listeria ivanovii and of lso from Listeria seeligeri. Biochim. Biophys. Acta 1130:81-84. [DOI] [PubMed] [Google Scholar]
- 20.Harty, J. T., and M. J. Bevan. 1995. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity 3:109-117. [DOI] [PubMed] [Google Scholar]
- 21.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 22.Inohara, N., M. Chamaillard, C. McDonald, and G. Nunez. 2005. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 74:355-383. [DOI] [PubMed] [Google Scholar]
- 23.Inohara, N., and G. Nunez. 2003. NODs: intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3:371-382. [DOI] [PubMed] [Google Scholar]
- 24.Ito, Y., I. Kawamura, C. Kohda, H. Baba, T. Kimoto, I. Watanabe, T. Nomura, and M. Mitsuyama. 2001. Difference in cholesterol-binding and cytolytic activities between listeriolysin O and seeligeriolysin O: a possible role of alanine residue in tryptophan-rich undecapeptide. FEMS Microbiol. Lett. 203:185-189. [DOI] [PubMed] [Google Scholar]
- 25.Ito, Y., I. Kawamura, C. Kohda, H. Baba, T. Nomura, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2003. Seeligeriolysin O, a cholesterol-dependent cytolysin of Listeria seeligeri, induces gamma interferon from spleen cells of mice. Infect. Immun. 71:234-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito, Y., I. Kawamura, C. Kohda, K. Tsuchiya, T. Nomura, and M. Mitsuyama. 2005. Seeligeriolysin O, a protein toxin of Listeria seeligeri, stimulates macrophage cytokine production via Toll-like receptors in a profile different from that induced by other bacterial ligands. Int. Immunol. 17:1597-1606. [DOI] [PubMed] [Google Scholar]
- 27.Kanneganti, T. D., N. Ozoren, M. Body-Malapel, A. Amer, J. H. Park, L. Franchi, J. Whitfield, W. Barchet, M. Colonna, P. Vandenabeele, J. Bertin, A. Coyle, E. P. Grant, S. Akira, and G. Nunez. 2006. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440:233-236. [DOI] [PubMed] [Google Scholar]
- 28.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101-105. [DOI] [PubMed] [Google Scholar]
- 29.Kayal, S., and A. Charbit. 2006. Listeriolysin O: a key protein of Listeria monocytogenes with multiple functions. FEMS Microbiol. Rev. 30:514-529. [DOI] [PubMed] [Google Scholar]
- 30.Kayal, S., A. Lilienbaum, C. Poyart, S. Memet, A. Israel, and P. Berche. 1999. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-kappa B and upregulation of adhesion molecules and chemokines. Mol. Microbiol. 31:1709-1722. [DOI] [PubMed] [Google Scholar]
- 31.Kimoto, T., I. Kawamura, C. Kohda, T. Nomura, K. Tsuchiya, Y. Ito, I. Watanabe, T. Kaku, E. Setianingrum, and M. Mitsuyama. 2003. Differences in gamma interferon production induced by listeriolysin O and ivanolysin O result in different levels of protective immunity in mice infected with Listeria monocytogenes and Listeria ivanovii. Infect. Immun. 71:2447-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kocks, C., E. Gouin, M. Tabouret, P. Berche, H. Ohayon, and P. Cossart. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68:521-531. [DOI] [PubMed] [Google Scholar]
- 33.Kohda, C., I. Kawamura, H. Baba, T. Nomura, Y. Ito, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2002. Dissociated linkage of cytokine-inducing activity and cytotoxicity to different domains of listeriolysin O from Listeria monocytogenes. Infect. Immun. 70:1334-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mariathasan, S., D. S. Weiss, K. Newton, J. McBride, K. O'Rourke, M. Roose-Girma, W. P. Lee, Y. Weinrauch, D. M. Monack, and V. M. Dixit. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228-232. [DOI] [PubMed] [Google Scholar]
- 35.Marquis, H., H. G. Bouwer, D. J. Hinrichs, and D. A. Portnoy. 1993. Intracytoplasmic growth and virulence of Listeria monocytogenes auxotrophic mutants. Infect. Immun. 61:3756-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercado, R., S. Vijh, S. E. Allen, K. Kerksiek, I. M. Pilip, and E. G. Pamer. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol. 165:6833-6839. [DOI] [PubMed] [Google Scholar]
- 37.Meylan, E., and J. Tschopp. 2006. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol. Cell 22:561-569. [DOI] [PubMed] [Google Scholar]
- 38.Meylan, E., J. Tschopp, and M. Karin. 2006. Intracellular pattern recognition receptors in the host response. Nature 442:39-44. [DOI] [PubMed] [Google Scholar]
- 39.Mitsuyama, M., K. Takeya, K. Nomoto, and S. Shimotori. 1978. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J. Gen. Microbiol. 106:165-171. [DOI] [PubMed] [Google Scholar]
- 40.Mocci, S., S. A. Dalrymple, R. Nishinakamura, and R. Murray. 1997. The cytokine stew and innate resistance to L. monocytogenes. Immunol. Rev. 158:107-114. [DOI] [PubMed] [Google Scholar]
- 41.Nakane, A., T. Minagawa, M. Kohanawa, Y. Chen, H. Sato, M. Moriyama, and N. Tsuruoka. 1989. Interactions between endogenous gamma interferon and tumor necrosis factor in host resistance against primary and secondary Listeria monocytogenes infections. Infect. Immun. 57:3331-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakane, A., A. Numata, and T. Minagawa. 1992. Endogenous tumor necrosis factor, interleukin-6, and gamma interferon levels during Listeria monocytogenes infection in mice. Infect. Immun. 60:523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neighbors, M., X. Xu, F. J. Barrat, S. R. Ruuls, T. Churakova, R. Debets, J. F. Bazan, R. A. Kastelein, J. S. Abrams, and A. O'Garra. 2001. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on interferon gamma production. J. Exp. Med. 194:343-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishibori, T., H. Xiong, I. Kawamura, M. Arakawa, and M. Mitsuyama. 1996. Induction of cytokine gene expression by listeriolysin O and roles of macrophages and NK cells. Infect. Immun. 64:3188-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nomura, T., I. Kawamura, K. Tsuchiya, C. Kohda, H. Baba, Y. Ito, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2002. Essential role of interleukin-12 (IL-12) and IL-18 for gamma interferon production induced by listeriolysin O in mouse spleen cells. Infect. Immun. 70:1049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohya, S., H. Xiong, Y. Tanabe, M. Arakawa, and M. Mitsuyama. 1998. Killing mechanism of Listeria monocytogenes in activated macrophages as determined by an improved assay system. J. Med. Microbiol. 47:211-215. [DOI] [PubMed] [Google Scholar]
- 47.Pamer, E. G. 2004. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4:812-823. [DOI] [PubMed] [Google Scholar]
- 48.Park, J. M., V. H. Ng, S. Maeda, R. F. Rest, and M. Karin. 2004. Anthrolysin O and other gram-positive cytolysins are Toll-like receptor 4 agonists. J. Exp. Med. 200:1647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park, S. F., and G. S. Stewart. 1990. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94:129-132. [DOI] [PubMed] [Google Scholar]
- 50.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Portnoy, D. A., R. D. Schreiber, P. Connelly, and L. G. Tilney. 1989. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J. Exp. Med. 170:2141-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Remer, K. A., T. Reimer, M. Brcic, and T. W. Jungi. 2005. Evidence for involvement of peptidoglycan in the triggering of an oxidative burst by Listeria monocytogenes in phagocytes. Clin. Exp. Immunol. 140:73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seki, E., H. Tsutsui, N. M. Tsuji, N. Hayashi, K. Adachi, H. Nakano, S. Futatsugi-Yumikura, O. Takeuchi, K. Hoshino, S. Akira, J. Fujimoto, and K. Nakanishi. 2002. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 169:3863-3868. [DOI] [PubMed] [Google Scholar]
- 54.Shen, H., M. K. Slifka, M. Matloubian, E. R. Jensen, R. Ahmed, and J. F. Miller. 1995. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc. Natl. Acad. Sci. USA 92:3987-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strober, W., P. J. Murray, A. Kitani, and T. Watanabe. 2006. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 6:9-20. [DOI] [PubMed] [Google Scholar]
- 56.Sutterwala, F. S., Y. Ogura, M. Szczepanik, M. Lara-Tejero, G. S. Lichtenberger, E. P. Grant, J. Bertin, A. J. Coyle, J. E. Galan, P. W. Askenase, and R. A. Flavell. 2006. Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24:317-327. [DOI] [PubMed] [Google Scholar]
- 57.Sutterwala, F. S., Y. Ogura, D. S. Zamboni, C. R. Roy, and R. A. Flavell. 2006. NALP3: a key player in caspase-1 activation. J. Endotoxin Res. 12:251-256. [DOI] [PubMed] [Google Scholar]
- 58.Tanabe, Y., H. Xiong, T. Nomura, M. Arakawa, and M. Mitsuyama. 1999. Induction of protective T cells against Listeria monocytogenes in mice by immunization with a listeriolysin O-negative avirulent strain of bacteria and liposome-encapsulated listeriolysin O. Infect. Immun. 67:568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thale, C., and A. F. Kiderlen. 2005. Sources of interferon-gamma (IFN-gamma) in early immune response to Listeria monocytogenes. Immunobiology 210:673-683. [DOI] [PubMed] [Google Scholar]
- 60.Torres, D., M. Barrier, F. Bihl, V. J. Quesniaux, I. Maillet, S. Akira, B. Ryffel, and F. Erard. 2004. Toll-like receptor 2 is required for optimal control of Listeria monocytogenes infection. Infect. Immun. 72:2131-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsuji, N. M., H. Tsutsui, E. Seki, K. Kuida, H. Okamura, K. Nakanishi, and R. A. Flavell. 2004. Roles of caspase-1 in Listeria infection in mice. Int. Immunol. 16:335-343. [DOI] [PubMed] [Google Scholar]
- 62.Tsukada, H., I. Kawamura, M. Arakawa, K. Nomoto, and M. Mitsuyama. 1991. Dissociated development of T cells mediating delayed-type hypersensitivity and protective T cells against Listeria monocytogenes and their functional difference in lymphokine production. Infect. Immun. 59:3589-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watanabe, I., T. Nomura, T. Tominaga, K. Yamamoto, C. Kohda, I. Kawamura, and M. Mitsuyama. 2006. Dependence of the lethal effect of pore-forming haemolysins of gram-positive bacteria on cytolytic activity. J. Med. Microbiol. 55:505-510. [DOI] [PubMed] [Google Scholar]
- 64.Xiong, H., I. Kawamura, T. Nishibori, and M. Mitsuyama. 1994. Cytokine gene expression in mice at an early stage of infection with various strains of Listeria spp. differing in virulence. Infect. Immun. 62:3649-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiong, H., Y. Tanabe, S. Ohya, and M. Mitsuyama. 1998. Administration of killed bacteria together with listeriolysin O induces protective immunity against Listeria monocytogenes in mice. Immunology 94:14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamamoto, K., I. Kawamura, T. Tominaga, T. Nomura, C. Kohda, J. Ito, and M. Mitsuyama. 2005. Listeriolysin O, a cytolysin derived from Listeria monocytogenes, inhibits generation of ovalbumin-specific Th2 immune response by skewing maturation of antigen-specific T cells into Th1 cells. Clin. Exp. Immunol. 142:268-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang, J., I. Kawamura, and M. Mitsuyama. 1997. Requirement of the initial production of gamma interferon in the generation of protective immunity of mice against Listeria monocytogenes. Infect. Immun. 65:72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang, J., and M. Mitsuyama. 1997. An essential role for endogenous interferon-gamma in the generation of protective T cells against Mycobacterium bovis BCG in mice. Immunology 91:529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu, J. W., J. Wu, Z. Zhang, P. Datta, I. Ibrahimi, S. Taniguchi, J. Sagara, T. Fernandes-Alnemri, and E. S. Alnemri. 2006. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ. 13:236-249. [DOI] [PubMed] [Google Scholar]
- 70.Zhao, X., Z. Li, B. Gu, and F. R. Frankel. 2005. Pathogenicity and immunogenicity of a vaccine strain of Listeria monocytogenes that relies on a suicide plasmid to supply an essential gene product. Infect. Immun. 73:5789-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]