Abstract
Cj0859c, or FspA, is a small, acidic protein of Campylobacter jejuni that is expressed by a σ28 promoter. Analysis of the fspA gene in 41 isolates of C. jejuni revealed two overall variants of the predicted protein, FspA1 and FspA2. Secretion of FspA occurs in broth-grown bacteria and requires a minimum flagellar structure. The addition of recombinant FspA2, but not FspA1, to INT407 cells in vitro resulted in a rapid induction of apoptosis. These data define a novel C. jejuni virulence factor, and the observed heterogeneity among fspA alleles suggests alternate virulence potential among different strains.
Campylobacter jejuni is among the leading causes of bacterial diarrhea worldwide (12, 40). Campylobacter enteritis can present with a range of clinical symptoms, and this variability has been attributed to either differences in the host or differences in virulence among strains. The molecular pathogenesis of C. jejuni has proven particularly refractory to analysis. This may be due, in part, to the absence of virulence factors in the genome that are analogous to those of better-characterized pathogens. Thus, the only virulence factor in C. jejuni that is shared with other pathogens is cytolethal distending toxin (CDT). There are no genes encoding other known toxins or specialized secretion systems common to all C. jejuni strains.
Flagella are considered to be virulence factors for C. jejuni for numerous reasons. The motility imparted by the locomotory organelle is critical to intestinal colonization of both animals and humans (3, 31). Motility and chemotaxis also modulate the invasion of intestinal epithelial cells in vitro (7, 20, 47-49). The flagellin subunits in the Campylobacter flagellar filament are heavily glycosylated with pseudaminic acid and legionaminic acid (29, 44). This glycosylation is required for filament assembly (15), and changes in glycan composition can affect both the immunogenicity of flagellin and microcolony formation (17, 28). Campylobacter flagella also appear to function as a type III secretion system (T3SS) in the absence of a specialized T3SS in this pathogen. Thus, strains of C. jejuni secrete the Cia (Campylobacter invasion antigen) proteins (24, 25, 39) and FlaC (41) through the filament.
There is limited information about the flagellar regulon of C. jejuni. However, it appears that most genes encoding the structural components of the basal body, hook, and minor flagellin, FlaB, are regulated by σ54 promoters, and the major flagellin, FlaA, is regulated by a σ28 promoter (16) and possibly a σ70 promoter as well (21). Carrillo et al. previously performed expression profiling studies on two variants of NCTC 11168 with different virulence levels (6). Those workers observed that a number of nonflagellar genes that were controlled by σ54 or σ28 promoters displayed distinct levels of expression, suggesting that some of these flagellum-coregulated genes may contribute to virulence (6). We have recently shown that mutation of one of these σ28-regulated genes, Cj0977, affected the virulence of C. jejuni 81-176 in vitro and in vivo (14). Another of these putative σ28-regulated genes, Cj0859c, which encodes a protein with no homology to known proteins, maps in a region of the chromosome that has been reported to be variable among strains in a recent microarray study (33). Analysis of this region in the C. jejuni genomes deposited in GenBank indicated that the putative σ28 promoter was conserved but that there was variation in the Cj0859c gene. Here, we demonstrate that the predicted proteins encoded by the Cj0859c genes in isolates of C. jejuni from different geographical areas show considerable allelic variation. We have characterized two variants of the protein and demonstrate that both forms of the protein are secreted into the supernatant in a process that requires a minimum flagellar structure. Moreover, we demonstrate significant biological differences in two variants of the protein.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. jejuni strain 81-176 and Campylobacter coli strain VC167 have been described previously (3, 19). Table 1 summarizes mutants of 81-176 used in this study. All of the flagellar mutants, except PG2157 (flgI) and PG2158 (flgK), were previously described (14). PG2157 and PG2158 were generated by transposon mutagenesis of clones of the flgI and flgK genes in Escherichia coli, respectively, from 81-176 using Tn5-based in vitro transposition (15, 18), followed by electroporation into 81-176. The insertion into flgI occurred at bp 363 of the 1,047-bp open reading frame; the insertion into flgK occurred at bp 113 of the 1,827-bp open reading frame. Both mutants were nonmotile and lacked all flagellar structure by negative-stain electron microscopy. Other strains of wild-type C. jejuni are shown in Table 2. Campylobacter strains were grown routinely on Mueller-Hinton (MH) agar supplemented with 50 μg/ml kanamycin and/or 15 μg/ml chloramphenicol as needed.
TABLE 1.
C. jejuni 81-176 mutants used in this study
| Strain | Gene mutated | Parent strain | Genotype | Reference or source |
|---|---|---|---|---|
| PG2572 | Cj0859c | 81-176 | fspA81-176::cat | This study |
| DRH311 | Cj0061 | 81-176 | ΔfliA | 19 |
| PG2662 | None | 81-176 | astA::fspA8486aphA3 | This study |
| PG2573 | Cj0859c | PG2572 | fspA81-176::cat (pCPE2575) | This study |
| PG2663 | Cj0859c | PG2572 | fspA81-176::cat astA::fspA8486aphA3 | This study |
| PG2453 | Cj1729c | 81-176 | flgE::cat | 13 |
| PG2139 | Cj0548 | 81-176 | fliD::cat | 13 |
| PG2157 | Cj1462 | 81-176 | flgI::cat | This study |
| PG2158 | Cj1466 | 81-176 | flgK::cat | This study |
TABLE 2.
Strains used in the characterization of Cj0859c variant frequency in C. jejuni
| C. jejuni strain | Alternative strain name | Origin | Penner serotype | GenBank accession no. |
|---|---|---|---|---|
| NCTC 11168a | United Kingdom | 2 | NC_002163.1 | |
| 84-25b | United States | ZP_01100726 | ||
| RM1221b | United States | 53 | NC_003912.7 | |
| 81-176b | United States | 23/36 | ZP_01087888 | |
| HB93-13b | China | 19 | ZP_01071420 | |
| CF93-6b | Japan | ZP_01068377 | ||
| 260.94b | South Africa | 41 | ZP_01070269 | |
| MK19 | Kuwait | EF058200 | ||
| MK50 | Kuwait | EF058201 | ||
| MK52 | Kuwait | EF058202 | ||
| MK53 | Kuwait | EF058203 | ||
| MK59 | Kuwait | EF058204 | ||
| ATCC 43429 | MSC 57360 | Canada | 1 | EF058205 |
| ATCC 43430 | PC 72 | Canada | 2 | EF058206 |
| ATCC 43432 | MK 7 | Canada | 4 | EF058207 |
| ATCC 43438 | Canada | 10 | EF058208 | |
| ATCC 43446 | MK 104 | Canada | 19 | EF058209 |
| ATCC 43449 | MK 198 | Canada | 23 | EF058210 |
| ATCC 43456 | MK 290 | Canada | 36 | EF058212 |
| ATCC 43431 | TGH 9011 | Canada | 3 | EF058211 |
| LAN749 | Egypt | EF058213 | ||
| HS000128 | Egypt | EF058214 | ||
| HS001578 | Egypt | EF058215 | ||
| HS008516 | Egypt | EF058216 | ||
| OH 4384 | Japan | 19 | EF058217 | |
| PG836 | Puerto Rico | 10 | EF058218 | |
| CG-99-8013 | Thailand | EF058219 | ||
| CG-99-8023 | Thailand | EF058220 | ||
| CG-99-8071 | Thailand | EF058221 | ||
| CG-99-8087 | Thailand | EF058222 | ||
| CG-99-8109 | Thailand | 4/13/64/66 | EF058223 | |
| CG-99-8131 | Thailand | EF058224 | ||
| CG-99-8153 | Thailand | 4/10/13/16/64/66 | EF058225 | |
| CG-99-8245 | Thailand | EF058226 | ||
| CG-99-8261 | Thailand | 13 | EF058227 | |
| CG-99-8265 | Thailand | 13 | EF058228 | |
| CG-99-8289 | Thailand | EF058229 | ||
| CG-99-8431 | Thailand | EF058230 | ||
| CG-99-8437 | Thailand | EF058231 | ||
| CG-99-8486 | Thailand | 4/13/64/66 | EF058232 | |
| HC37 | United States | 27 | EF058233 |
See reference 34.
TIGR Campylobacter Genome Projects (D. E. Fouts and K. E. Nelson, unpublished data).
RT-PCR analysis.
To confirm the regulation of fspA by σ28, the relative expression of this gene was determined by real-time PCR (RT-PCR) in wild-type 81-176 and DRH311, which is a σ28 deletion of 81-176 (ΔfliA) (21). RNAs were extracted from mid-log-phase cultures of C. jejuni 81-176 and the ΔfliA strain grown in biphasic MH medium. Synthesis of cDNA was performed using an iScript cDNA synthesis kit (Bio-Rad, La Jolla, CA). RT-PCR was performed with the ABI Prism 7000 DNA analyzer (Applied Biosystems, Foster City, CA) using a QuantiTect SYBR green RT-PCR kit (QIAGEN, Valencia, CA) according to the manufacturer's protocol. Primer sequences are given in Table 3. RT-PCR was followed by a melting curve analysis in order to confirm amplification of specific PCR products. The expression of fspA in each strain was normalized to 16S and 23S RNA. The relative fspA expression level was obtained using the ΔΔCT method as recommended in the Applied Biosystems manual. Results are based on at least three independent experiments.
TABLE 3.
Primer pairs used in RT-PCR studies
| Locus | Primer | Primer sequence (5′-3′) |
|---|---|---|
| fspA1 | RT-01 | AAAACGCTTTAGCGCAAGAT |
| RT-02 | TTGTTGCGTGTTGTTTTGTG | |
| fspA2 | RT-03 | TGTTCATAGCGACAATGTGGT |
| RT-04 | AAGCCTCTAATGCGGGATTT | |
| flaA | RT-05 | TCGTATTAACACCAATGTTGCAG |
| RT-06 | GCTTGAGATCTTAAACTATCTGCTATC | |
| ilvC | RT-07 | AAAAAGTAGCAATTATAGGCTTTGG |
| RT-08 | TTGCTTTAACAGCACTTACACTACC | |
| Cj0977 | RT-09 | ACCACAAGCGAAATGGTAGC |
| RT-10 | TCGTCAAAAAGTGCATGAGC | |
| 23S RNA | RT-11 | GGTAGGAGAGCGTTCTATTTGC |
| RT-12 | CGACTTAGGACCCGACTAACC | |
| 16S RNA | RT-13 | GGGAAAGTTTTTCGGTGTAGG |
| RT-14 | GTTGCTGCGTCAGGGTTT |
PCR amplification and sequence analysis of different Cj0859c alleles.
The Cj0859c-Cj0860 region of the chromosome of C. jejuni strains was PCR amplified using primers pg06.14 (5′-CCTATTTATGGATTGCAATTTCACCCCG-3′) and pg06.15 (5′-CTTGAAACGATCAAGGGTAGGGCAGC-3′). Primer pg06.14 bound to the pabA gene (Cj0861), and pg06.15 bound to murA (Cj0858c). Amplicons were subjected to DNA sequence analysis using BigDye chemistry on an Applied Biosystems model 3100 DNA sequencer, and the sequences were assembled using Sequencher 4.5 (Gene Codes). Each consensus sequence was manually inspected for quality assessment of the assembly. The Cj0859c gene sequence was identified for each consensus using Aretemis software (http://www.sanger.ac.uk/Software/Artemis/). Biological sequences were compared using the SIM alignment tool (http://ca.expasy.org/tools/sim-prot.html). The phylogenetic tree was made using CLC Free Workbench 3 software (http://www.clcbio.com/index.php?id=28).
Expression of Cj0859c in E. coli.
The fspA81-176 gene was amplified from 81-176 by PCR using Easy-A High Fidelity PCR master mix (Stratagene, La Jolla, CA). The template was DNA from strain 81-176, and the primers used were pg05.53 (5′-GACGACGACAAGATGCAAATTAACAATTCCTTAAATAGC-3′) and pg05.54 (5′-GAGGAGAAGCCCGGTTCAAGCTTGTTGGCTTTGGAGTTC-3′). These primers included sequences for cloning into pET41-EK LIC, a vector that allows fusion to both a glutathione S-transferase (GST) tag and a hexahistidine tag (EMD Bioscience, Madison, WI). The 429-bp amplicon was purified using QIAquick PCR purification columns (QIAGEN, Valencia, CA), ligated into pET41-EK LIC as directed by the manufacturer, and transformed into NovaBlue GigaSingles cells (EMD Biosciences). Several resulting clones were sequenced, and one clone, which showed the predicted sequence, was selected for protein purification. The GST-His-Cj0859c protein was purified by nickel chromatography (QIAGEN).
The FspA protein from 81-176 was also expressed as a histidine-tagged protein in E. coli for biological experiments as follows. The gene was PCR amplified from 81-176 using primers DR101 (5′-CCATATGCAAATTAACAATTCCTTAAATAGC-3′) and DR102 (5′-GGGATCCTCAAGCTTGTTGGCTTTGGAGTTC-3′) and HF2 DNA polymerase (Clontech). The resulting amplicon was digested with NdeI and BamHI and cloned into NdeI-BamHI-digested pET-19b (Novagen, San Diego, CA) in E. coli DH5α. Following confirmation of the correct construction by DNA sequencing, one resulting clone was transformed into BL21(DE3), and the protein was overexpressed and purified on Ni-nitrilotriacetic acid resin as recommended by the supplier (QIAGEN).
FspA from CG8486 was expressed as a histidine-tagged protein in pET-19b using primers DR103 (5′-CCATATGAAAATAGATACTTTGACAAAAAATTTTAGC-3′) and DR102 (shown above), as described above for 81-176.
Antibodies against different forms of FspA.
Rabbit polyclonal antiserum against the GST-His-FspA from 81-176 and from the His-tagged FspA protein from CG8486 described above were generated by Harlan Biosciences (Madison, WI).
SDS-PAGE and immunoblotting.
Proteins were separated on 12.5% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gels and detected with Gel Code Blue (Pierce, Rockford, IL) or transferred onto nitrocellulose and immunoblotted using the indicated rabbit polyclonal antisera. Proteins were immunodetected with either anti-GST-His-FspA81-176 or anti-His-FspA8486 polyclonal rabbit antiserum at a final dilution of 1:5,000. For assays to measure the binding of both recombinant proteins to INT407 cells, a mouse monoclonal antibody (Novagen) that recognizes the histidine tag was used at a final dilution of 1:5,000. The secondary antibody was horseradish peroxidase-labeled goat anti-rabbit immunoglobulin G or goat anti-mouse antibody (Immunopure). The reactions were detected using Supersignal West Pico detection kits (Pierce, Rockford, IL). Chemiluminescence was detected using a Kodak (Rochester, NY) Image Station 2000R.
Purification of supernatants from C. jejuni.
Bacteria were grown in MH biphasic flasks (50 ml of MH broth over 20 ml MH agar in T75 tissue culture flasks) for 18 h. Growth from two flasks (or 100 ml of MH broth) was centrifuged, and the supernatants were filtered through 0.2-μm polyvinylidene difluoride membrane filters (Millipore, Billerica, CA). Trichloroacetic acid was added to a final concentration of 10%, and the sample was iced for 45 min. The samples were centrifuged in a Sorvall RC5C refrigerated centrifuge at 10,000 × g for 15 min, and the pellets were washed twice in 20 ml acetone. The pellets were air dried and resuspended in 64 μl of phosphate-buffered saline per 56 ml of culture. An equal volume of 2× solubilization buffer (1 M Tris [pH 6.8], 0.001% bromophenol blue, 5% glycerol, 3% SDS, 5% β-mercaptoethanol) was added, and the samples were boiled for 10 min prior to electrophoresis. Equal loading of samples was confirmed by silver staining of SDS-PAGE gels.
Generation of an fspA mutant of 81-176.
A mutant in fspA81-176 was constructed using Tn5-based in vitro transposition (Epicenter, Madison, WI) with a cat cassette as previously described (18). The in vitro reaction was performed according to the manufacturer's instructions with a clone from a partially Sau3AI-digested ordered genomic library of 81-176 (L. Holder and P. Guerry, unpublished data), called pLCH8-63, as the target DNA. This clone contained the region of the 81-176 chromosome that corresponded to genes Cj0856c to Cj0860. The reaction products were transformed into E. coli DH5α, and plasmid DNAs from individual transformants were sequenced using primers that read out from within the cat cassette to determine the insertion point and the orientation of the transposon within the gene. A plasmid in which the cat cassette had inserted 259 bp from the translational start of fspA in the same orientation as fspA was selected. This plasmid was used to electroporate C. jejuni 81-176 with selection on MH agar supplemented with chloramphenicol (15 μg/ml). The successful mutation of fspA was verified by PCR with primers bracketing the Cmr insertion point to confirm that the DNA had undergone a double crossover. This mutant was called PG2572.
Complementation of the Cj0859c mutant in trans.
The fspA gene and its σ28 promoter were PCR amplified from 81-176 using the following primers: pg05.133 (5′-CGGGATCCCACCGCTAATAGCCCAAAAAATACCTCCC-3′) and pg05.134 (5′-GGAATTCCGCAAGTATACTTGAAACGATCAAGGGTAGGG-3′). These primers added BamHI and EcoRI sites, respectively. The resulting 744-bp amplicon was digested with BamHI and EcoRI (New England Biolabs, Beverly, MA) and cloned into similarly digested pRY107 (50). After confirmation that the clone was correct by DNA sequence analysis, the plasmid was transformed into DH5α cells carrying the conjugative plasmid RK212.2. The resulting cells were used as donors to conjugatively transfer the complementing plasmid, pCE2575, into PG2166, the Cj0859c mutant, with selection on kanamycin and chloramphenicol, as previously described (17, 18).
Insertion of fspA8486 into the astA gene of 81-176 and selected mutants.
The fspA8486 gene and its σ28 promoter were PCR amplified from CG8486 using primers C360.07 F (5′-TGCGGATCCCCGAAGCGGTTTTAACTCAA-3′) and C360.07 R (5′-ATGCGAATTCAAGGGTAGGGCAGCATTTTT-3′), which included BamHI and EcoRI restriction sites. The resulting amplicon was digested with these restriction enzymes and cloned into pBluescript. A SmaI-ended aphA3 cassette was cloned into the EcoRV site of the resulting clone. DNA sequence analysis indicated that the aphA3 gene was inserted 3′ to fspA8486 and was in the same orientation as fspA8486. This plasmid was digested with BamHI and XhoI, which released fspA8486 and the adjacent aphA3 gene. This fragment was blunted with Klenow polymerase (New England Biolabs, Beverly, MA) and cloned into the EcoRV site of plasmid pYG660 containing the astA (arylsulfatase) gene of 81-176 (51). This plasmid was used to electroporate 81-176 and selected flagellar mutants to kanamycin resistance, as shown in Table 1. The resulting Kmr colonies were screened for a loss of arylsulfatase activity using MH agar supplemented with a chromogenic substrate (X-S; Sigma, St. Louis, MO), as previously described (17, 51).
Adherence and invasion assays.
Adherence and invasion assays were done as previously described (2, 32, 49). Briefly, about 2 × 106 bacteria were added to a monolayer of about 3 × 105 INT407 cells. After centrifugation at 200 × g for 5 min, the assay mixtures were incubated at 37°C for 2 h. For the determination of adherence, the cells were washed four times with Hanks’ balanced salt solution (HBSS) for 1 min before lysing the monolayer with 0.01% Triton X-100 and enumerating the total bacteria by plate counting on MH agar. For the determination of invasion, the monolayer was washed twice with HBSS, and fresh prewarmed modified Eagle's medium (MEM) supplemented with 100 μg/ml gentamicin was added to wells for an additional 2 h to kill extracellular bacteria. The monolayer was washed four times in HBSS and lysed with 0.01% Triton X-100 for 30 min. Released intracellular bacteria were enumerated by plate count. Invasion was expressed as the percentage of the inoculum surviving the gentamicin treatment, and adherent bacteria were expressed as the total number of bacteria enumerated without antibiotic treatment.
Binding of recombinant histidine-tagged C. jejuni proteins to INT407 cells.
INT407 cells were seeded into 24-well tissue culture plates at about 5 × 105 cells per well in MEM plus 10% fetal bovine serum. Following incubation at 37°C for about 19 h, culture medium was removed and replaced with fresh MEM plus 10% fetal bovine serum prewarmed at 37°C. Aliquots of 5, 10, 25, and 50 μg of histidine-tagged FspA8486 and 50 μg of histidine-tagged FspA81-176 were added, and the cells were incubated for 2 h at 37°C. The monolayer was washed five times with phosphate-buffered saline and lysed in 200 μl of gel loading buffer (10% glycerol, 3% SDS, 0.01% bromophenol blue, 5% β-mercaptoethanol, 1 M Tris, and Halt protease inhibitor cocktail, EDTA free, from Pierce [Rockford, IL]). The samples were boiled for 10 min, and 10-μl aliquots were loaded onto a 12.5% SDS-PAGE gel for each sample. The gel was transferred onto nitrocellulose and immunodetected with a mouse anti-His-tag monoclonal antibody, as described above.
Apoptosis assays.
Monolayers of INT407 cells in 24-well flat-bottom tissue culture dishes were treated with increasing amounts of recombinant FspA8486 or 50 μg of FspA81-176 and incubated for 4 h. Control wells were treated with 10 μg of membrane proteins from 81-176 containing CDT (8, 13, 26) or 10 μg of membrane proteins from DS104, an isogenic cdtA mutant of 81-176 (22, 23). Cells were harvested and stained using the Guava Nexin assay kit according to the manufacturer's instructions (Guava Technologies, Hayward, CA). Basically, the cells were stained with annexin V-phycoerythrin and Nexin 7-amino-actinomycin D (7-AAD) in cold 1× Nexin buffer in a 50-μl reaction mixture and analyzed in a Guava Technologies personal cytometer. Cells that stained positive with annexin V-phycoerythrin but negative with Nexin 7-AAD were scored as being early apoptotic.
Statistical analyses.
Statistical analyses of the RT-PCR results were calculated with a Wilcoxon two-group test. Statistical analyses of adherence, invasion, and apoptosis assays were done with two-tailed t tests.
RESULTS
Cj0859c is expressed by a σ28 promoter in 81-176.
Carrillo et al. previously demonstrated reduced expression of Cj0859c in a fliA mutant of NCTC 11168 by microarray, suggesting that this gene is σ28 regulated, and the putative promoter sequence is consistent with a σ28 recognition sequence (6). Table 4 shows the results of quantitative RT-PCR analysis of Cj0859c expression in 81-176 and DRH311 (ΔfliA) (Table 1) (21). The data indicate that there is a >30-fold reduction in the expression of Cj0859c in DRH311 compared to that of wild-type 81-176. This reduction is comparable to that seen for two other σ28-regulated genes, flaA and Cj0977. In contrast, there was no significant change in the expression of the σ70-regulated ilvC (Cj0632) in DRH311 (ΔfliA) compared to that of 81-176.
TABLE 4.
Confirmation of σ28 regulation of Cj0859c by RT-PCRa
| Gene | Avg fold down-regulation in ΔfliA ± SD | P value |
|---|---|---|
| Cj0859c | 31.00 ± 17.90 | ≤0.028 |
| Cj0977 | 28.64 ± 8.62 | ≤0.028 |
| flaA | 32.20 ± 14.73 | ≤0.028 |
| ilvC | 0.77 ± 1.40 | ≤0.88 |
The average down-regulation (n-fold) of each gene in DRH311 (ΔfliA) compared to 81-176 is shown. Statistical significance (P value) was calculated using a Wilcoxon two-group test.
Characterization of the Cj0859c locus in multiple strains of C. jejuni.
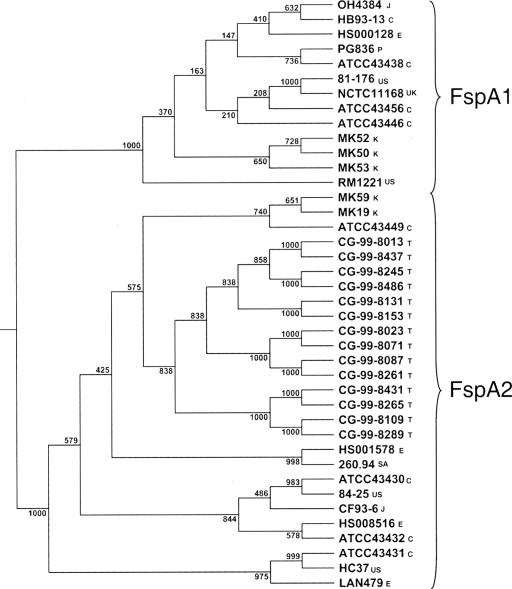
Analysis of the predicted proteins encoded by the homologs of Cj0859c in three C. jejuni genomes deposited in GenBank, 260.94, CF93-6, and 84-25, revealed that the predicted proteins, which are highly homologous to each other, show considerable divergence with the Cj0859c protein encoded by both NCTC 11168 and 81-176. The gene corresponding to Cj0859c has been called fspA (flagellar secreted protein) for reasons explained below. We studied the variation of fspA by DNA sequence analysis of PCR products generated from a collection of isolates from diverse geographical locations, as shown in Table 2. The results, summarized in Fig. 1, revealed that there were two overall clusters of Cj0859c alleles. The FspA1 cluster, which included 81-176, NCTC 11168, and RM1221, comprised 32% of the total strains (13/41). The FspA2 cluster comprised 68% of the total strains (28/41). The FspA2 cluster includes TGH9011, which was originally reported to lack Cj0859c based on a microarray analysis using the NCTC 11168 allele as a probe (36). The allele found in TGH9011, however, is truncated and is not detected by immunoblot (see below). The FspA1 proteins share a minimum of 81.7% identity within the group, and the FspA2 proteins share a minimum of 95.1% identity. There appeared to be no association of allele type and geographical isolation, although all of the Thai isolates examined contained the FspA2 allele.
FIG. 1.
Phylogenetic tree based on protein sequences encoded by alleles of the fspA gene. The tree is based on 1,000 bootstraps; scores are shown for all nodes. The tree clearly separates the two versions of Cj0859c, FspA1 and FspA2. The letters following the strain name refer to the country of isolation. C, Canada; CH, China; E, Egypt; J, Japan; K, Kuwait; PR, Puerto Rico; SA, South Africa; T, Thailand; UK, United Kingdom; US, United States. All the strains, with the exception of C. jejuni RM1221, which was isolated from a chicken carcass (30), were clinical isolates.
The PCR primers used to amplify Cj0859c from these strains mapped in homologs of Cj0858 and Cj0861 (see Materials and Methods). Curiously, it appeared that the FspA2 allele is always associated with the absence of the Cj0860 gene, which encodes a putative integral membrane protein. Thus, the PCR product obtained from all strains in the FspA1 cluster was 1,626 bp, and that obtained from all the strains in FspA2 cluster was 799 bp. Although adjacent on the chromosome, Cj0859c and Cj0860 are on opposite strands and are controlled by distinct promoters.
We further characterized two of the variant forms of Cj0859c. The Cj0859c gene from 81-176 (GenBank accession no. NZAANY01000003.1; locus tag CJJ81176_0875) encodes a predicted soluble, cytoplasmic protein of 15.5 kDa (pI 4.4). The Cj0859c gene from CG8486, a clinical isolate from Thailand (38), encodes a predicted soluble, cytoplasmic protein of 16.0 kDa (pI 5.62). These two variants are 41.6% identical at the protein level (Fig. 2) and 58% identical at the DNA level. Both share identical σ28 promoters (data not shown). The Cj0859c gene from 81-176 (from the FspA1 group) will be called fspA81-176, and that from GC8486 (from the FspA2 group) will be called fspA8486.
FIG. 2.
ClustalW alignment (45) of FspA81-176 (FspA1) and FspA8486 (FspA2). *, identical amino acids; :, high-similarity amino acids; ., low-similarity amino acids.
FspA81-176 is secreted into the supernatant.
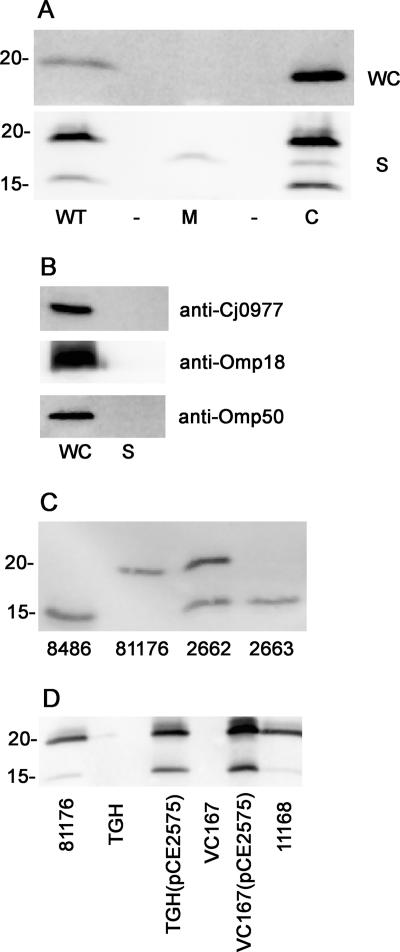
Whole cells of C. jejuni 81-176 and an isogenic insertional mutant of fspA81-176 were immunoblotted with rabbit polyclonal antiserum against a recombinant form of the protein (GST-His-FspA81-176). The results, shown in Fig. 3A, revealed the presence of a band in wild-type 81-176 with an apparent molecular mass of approximately 18 kDa, slightly larger than the predicted molecular mass of FspA81-176. A histidine-tagged version of this protein expressed in E. coli also migrated aberrantly in SDS-PAGE (data not shown). This band was missing in the fspA81-176 mutant and appeared to be overexpressed when the mutation was complemented in trans. Since C. jejuni is known to secrete multiple proteins through flagella, we examined supernatants for the presence of Cj0859c. FspA81-176 was found in supernatants of MH agar-grown 81-176 cells and the complement of the mutant but not in supernatants of the fspA mutant (Fig. 3A). Additional cross-reacting bands were also observed in some supernatant preparations and may represent processing events, degradation products, or cross-reacting proteins. To control for possible cell leakage, whole-cell and supernatant preparations of 81-176 were immunoblotted with antibodies to recombinant forms of three other proteins, as shown in Fig. 3B. These were Cj0977, another σ28-regulated cytoplasmic protein that is not secreted (14); Omp18, a lipoprotein (5); and Omp50, an outer membrane protein (4). These proteins were readily detected in whole cells but not in the supernatant preparations.
FIG. 3.
Secretion of FspA. (A) Detection of FspA81-176 in whole cells or supernatants. WT, wild-type 81-176; M, 81-176 fspA81-176::cat (strain PG2572); C, 81-176 fspA81-176::cat (pCPE2575) (strain PG2573); WC, whole cells; S, supernatants. (B) Controls for bacterial cell leakage. Whole cells and supernatants of wild-type 81-176 were detected with antibodies against recombinant forms of Cj0977, Omp18, and Omp50 (4, 5, 13). WC, whole cells; S, supernatants. (C) Detection of FspA8486 in wild-type CG8486 and strain PG2662 (wild-type 81-176 containing FspA8486 inserted into astA) and PG2663 (81-176 fspA81-176::cat containing FspA8486 inserted into astA). (D) Secretion of FspA81-176 campylobacter strains with and without plasmid pCPE2575 carrying fspA81-176. TGH, C. jejuni strain TGH9011.
Campylobacter strains that lack fspA are capable of secretion of the protein when the gene is supplied in trans.
The FspA8486 protein was also found in the supernatant of cultures of CG8486, as shown in Fig. 3C, but did not show the aberrant migration observed with FspA81-176. CG8486 is difficult to manipulate genetically (38). The fspA gene and its σ28 promoter from CG8486 were inserted into the astA gene of 81-176 to generate PG2662. The two forms of FspA can be visualized in the supernatant of this strain by immunodetection with anti-FspA8486 antiserum (Fig. 3C). Only FspA8486 can be visualized in the supernatant of strain PG2663, the fspA81-176 mutant of 81-176 containing fspA8486 inserted into astA.
Cj0859c, or fspA, has been reported to be missing in several strains of C. jejuni by microarray analysis, including ATCC 43431 (TGH9011) (see below) (33, 36, 37). The gene was also absent in sequenced strains of Campylobacter coli, Campylobacter upsaliensis, Campylobacter lari, and Helicobacter spp. (1, 11, 42, 46). Cj0859c was not detectable in whole-cell immunoblots (data not shown) or supernatants of either C. jejuni TGH9011 or a C. coli strain, VC167 T2 (Fig. 3D). However, when pCE2575 carrying fspA81-176 was transferred conjugatively into these strains, the FspA81-176 protein could readily be detected in both whole cells (data not shown) and cell supernatants (Fig. 3D), indicating that the requisite machinery was present in these other Campylobacter strains to secrete the FspA81-176 protein. Doublets of the FspA81-176 protein were also observed in supernatants from these strains, as seen in Fig. 3D.
Secretion of FspA81-176 requires a minimum flagellar structure.
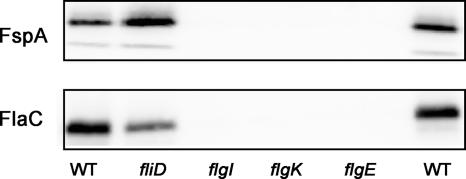
Song et al. (41) previously showed that FlaC secretion to the supernatants was not observed in C. jejuni TGH9011 mutants lacking hook (flgE) and basal body (flgF) structures. We examined the secretion of FspA81-176 from similar mutants, as shown in Fig. 4. The results indicate that neither FspA81-176 nor FlaC was secreted in 81-176 mutants in flgE, flgI, encoding the P ring subunit, or flgK, encoding the hook-filament junction protein. Both proteins were secreted from a mutant in fliD lacking the filament cap protein.
FIG. 4.
Secretion of FspA81-176 requires a minimum flagellar structure. FspA81-176 or FlaC was detected in supernatants from 81-176 and mutants. Gels were immunodetected with anti-FspA81-176 antibody. WT, wild type.
Mutation of fspA81-176 does not affect 81-176 adherence or invasion of INT407 cells.
There was no significant difference between wild-type 81-176 and an fspA::cat mutant, which was fully motile, in terms of adherence to (4.12% ± 0.74% and 4.75% ± 1.1%, respectively) (P = 0.56) or invasion of (2.30% ± 0.09% and 1.80% ± 0.92%, respectively) (P = 0.61) INT407 cells.
FspA8486, but not FspA81-176, binds to INT407 cells.
Recombinant His-tagged FspA8486 and FspA81-176 proteins were added to a monolayer of INT407 cells, incubated for 2 h, and washed extensively. The monolayer was lysed, and the proteins were separated in 12% SDS-PAGE gels and immunodetected. The results shown in Fig. 5 indicated that FspA8486 remained associated with the monolayer, while FspA81-176 could not be detected in the lysate.
FIG. 5.
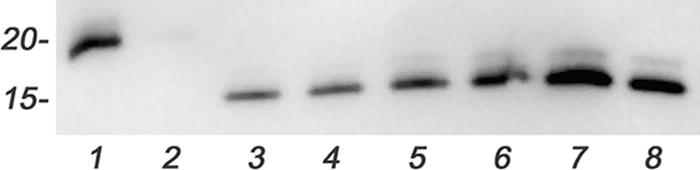
Interactions of FspA proteins with INT407 cells. Hexahistidine-tagged recombinant FspA81-176 and FspA8486 proteins were added to a monolayer of INT407 cells, and the cells were incubated for 4 h at 37°C. The monolayer was washed five times and lysed as described in Materials and Methods, and an aliquot (10 μl, or about 750 ng protein) of each lysate was electrophoresed on a 12.5% SDS-PAGE gel, transferred onto a nitrocellulose membrane, and probed with anti-histidine-tagged mouse monoclonal antibody (Novagen). Lane 1, recombinant FspA81-176 (40 ng); lane 2, INT407 cells plus 50 μg of FspA81-176; lane 3, INT407 cells plus 5 μg FspA8486; lane 4, INT407 cells plus 10 μg FspA8486; lane 5, INT407 cells plus 25 μg FspA8486; lane 6 INT407 cells plus 50 μg FspA8486; lane 7, recombinant FspA8486 (80 ng); lane 8, recombinant FspA8486 (40 ng).
FspA8486 but not FspA81-176 causes induction of apoptosis of INT407 cells.
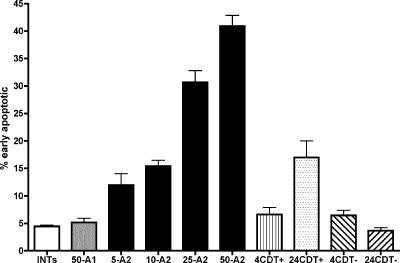
Recombinant His-tagged FspA8486, but not recombinant His-tagged FspA81-176, caused an induction of apoptosis in INT407 cells after 4 h of incubation, as shown in Fig. 6. Moreover, there was a clear dose response to the recombinant FspA8486 protein. The addition of 5 μg of recombinant FspA8486 resulted in 11.9% ± 4.7% of the cells in the early apoptotic stage (P < 0.05 compared to untreated INT407 controls). The addition of increasing amounts of FspA8486 from 10 μg to 50 μg resulted in a range of 15.4% ± 2.4% to 30.9% ± 4.8% of the cells in early apoptosis by 4 h (P < 0.001). In contrast, the addition of 50 μg of FspA81-176 to the monolayer showed no differences compared to untreated controls. The kinetics of induction of apoptosis were markedly quicker than those caused by CDT (8, 13, 23). Membrane preparations (a total of 10 μg total protein) from 81-176 (CDT positive) caused apoptosis in only 6.6% ± 3.0% of the cells in 4 h and 17% ± 8% by 24 h. Membranes from a cdtA mutant of 81-176 had no effect, as previously reported (22, 23).
FIG. 6.
Induction of apoptosis. Monolayers of INT407 cells were treated with 5 μg to 50 μg of recombinant FspA8486 or 50 μg recombinant FspA81-176 for 4 h and stained with a Guava Nexin kit, and the percentage of cells in early stages of apoptosis (annexin V positive and 7-AAD negative) was determined using a Guava Technologies personal cytometer. Bars are labeled as follows: INTs, untreated INT407 cells; 50-A1, 50 μg FspA81-176; 5-A2, 5 μg FspA8486; 10-A2, 10 μg FspA8486; 25-A2, 25 μg FspA8486; 50-A2, 50 μg FspA8486. Additional controls included 10 μg of total protein from wild-type 81-176, which has been shown to contain CDT (21, 22), and 10 μg of total protein from a cdtA mutant of 81-176. These membrane preparations were incubated for both 4 h (4 CDT+ and 4 CDT−) and 24 h (24 CDT+ and 24 CDT−). The data represent the means and standard deviations of four to seven individual experiments done in duplicate. The P value for 5 μg of FspA8486 compared to INT407 cells alone was <0.05; the P values for 10, 25, and 50 μg of FspA8486 compared to INT407 cells alone were <0.001; the P value for the CDT-positive membranes of 81-176 compared to INT407 cells at 24 h of incubation (not shown) was <0.05.
DISCUSSION
C. jejuni has proven to be remarkably recalcitrant to molecular pathogenic analysis. Genome sequencing of multiple strains has failed to identify any virulence factors shared with better-characterized pathogens other than CDT. However, the role of flagella and motility in virulence has long been recognized as critical to the pathogenesis of C. jejuni for a variety of reasons (3, 10, 17, 20, 24, 41, 47-49). C. jejuni flagella have been recognized previously to function as a T3SS to secrete both FlaC and the Cia proteins (24, 25, 39, 41). These secreted proteins modulated the invasion of some strains of C. jejuni but not 81-176 (14, 24, 25, 39, 41). Similarly, mutation of fspA in 81-176 had no effect on invasion. Despite isolation from a dysentery case, strain CG8486 is noninvasive in vitro (38), so it is unlikely that FspA8486 plays a role in invasion. Consistent with this, the transfer of fspA8486 into 81-176 (strain PG2662) or into 81-176 fspA81-176 (strain PG2663) did not affect adherence or invasion compared to the wild type (data not shown).
Carrillo et al. (6) previously compared transcription profiles of virulent and nonvirulent variants of C. jejuni NCTC 11168 and observed that several σ28- and σ54-regulated genes were among those that showed differential expression. These genes had no known role in flagellar biogenesis despite apparent regulation by the two “flagellar” promoters. This led to the hypothesis that these genes might play a role in virulence, particularly since motility and virulence are strongly associated in C. jejuni. We have previously confirmed a role for the σ28-regulated gene Cj0977 in virulence (14), and here, we have demonstrated that a second σ28-regulated gene, Cj0859c, or FspA, also appears to be a virulence determinant, at least in some strains. We have confirmed expression by a σ28 promoter for both of these genes by RT-PCR. Cj0977 and Cj0859c (FspA) proteins could not be detected by immunoblot in the ΔfliA mutant, consistent with the RT-PCR results (14) (data not shown). Moreover, no FspA protein could be detected by immunoblot when fspA8486 was transferred into DRH311 (81-176 ΔfliA) on a plasmid, indicating that both alleles are regulated by σ28 (data not shown). In contrast, there is no evidence that either flaC or ciaB is coregulated with the flagellar regulon.
The protein encoded by Cj0859c, FspA, unlike Cj0977, is secreted into the supernatant. Song et al. previously showed that FlaC was not secreted in C. jejuni mutants in either flgE or flgF (41); here, we have examined the expression of both FlaC and FspA81-176 in additional flagellar mutants, and their secretion patterns appear to be identical. Thus, neither FlaC nor FspA81-176 was secreted in mutants defective in basal body/hook structures, but both proteins were secreted in an fliD mutant. Mutants in fliD, which lack the filament cap protein, cannot assemble a filament but retain an open channel that is capable of secretion (27). However, flaC does not appear to be expressed by either a σ28 or σ54 promoter and is synthesized in an fliA mutant, unlike FspA (data not shown). The secretion of both FlaC and FspA occurs in broth-grown bacteria, unlike the Cia proteins, which require an exogenous signal from eukaryotic cells for secretion to occur (24, 25, 39).
The heterogeneity of FspA in different C. jejuni strains is striking, based on the limited numbers of strains examined. The form of FspA found in strain CG8486, but not that found in 81-176, binds tightly to eukaryotic cells and induces apoptosis. It is interesting that all of the isolates from Thailand examined, where C. jejuni is hyperendemic, contained similar forms of FspA. However, these clinical isolates all came from the same region of Thailand in 1999 and may not be representative of the entire region. In contrast, 81-176, NCTC 11168, and other strains contain an alternate form of FspA that has no observable phenotype as yet. It remains to be determined if the differences between the two proteins are in their ability to bind to epithelial cells, to induce apoptosis, or both, but binding and induction of apoptosis both show a dose dependence. The amount of FspA2 delivered during C. jejuni infection remains to be determined. However, recent observations that C. jejuni can form microcolonies on intestinal epithelial cells in vitro (17) suggest a mechanism by which the effective dose of FspA2, and perhaps other secreted proteins, could be concentrated near the cell surface. Clearly, there is considerable research remaining to understand the mechanism of action and the biological significance of FspA8486. However, the ability of FspA2 to induce apoptosis in epithelial cells suggests a mechanism by which some strains of C. jejuni could disrupt the epithelial cell barrier.
The clinical spectrum of C. jejuni disease can range from a mild, watery diarrhea to a dysentery-like disease. This is likely due, in part, to the immune status of the host, although it has been speculated that genomic differences among strains may also contribute. Complete genome analysis and comparative microarrays have identified gross differences among strains, largely in surface carbohydrates (9, 33, 34-37, 43). A recent microarray study has also reported heterogeneity in the Cj0859c-Cj0860 region, consistent with our data (33). The data reported here indicate that changes among FspA proteins have major effects on toxicity for epithelial cells in vitro. Clearly, there are virulent strains of C. jejuni that contain both fspA1 and fspA2 alleles, reflecting the multifactorial nature of pathogenicity. However, the observed heterogeneity among fspA alleles may modulate C. jejuni virulence. The in vitro and in vivo roles of the different forms of FspA in pathogenesis are under investigation.
Acknowledgments
We thank Daniel Scott, Shahida Baqar, David Tribble, and our colleagues at AFRIMS, Bangkok, Thailand, and NAMRU3, Cairo, Egypt, for strains; David Hendrixson and Vic DiRita for DRH311; Eva Nielsen of the Danish Veterinary Laboratory and Helen Tabor and Lawrence Price of the Canadian Science Centre for Human and Animal Health for Penner serotyping; Rob Williams for electron microscopy; and Chad Porter for statistical advice.
This work was funded by NIAID grant R01 AI043559 and the Military Infectious Disease Research Program (work unit 6000.RAD1.DA3.A0308).
Editor: A. Camilli
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Alm, R. A., L.-S. L. Ling, D. Moir, B. L. King, E. D. Brown, P. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. de Jonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jian, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:160-189. [DOI] [PubMed] [Google Scholar]
- 2.Bacon, D. J., C. M. Szymanski, D. H. Burr, R. P. Silver, R. A. Alm, and P. Guerry. 2001. A phase variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol. Microbiol. 40:769-777. [DOI] [PubMed] [Google Scholar]
- 3.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 4.Bolla, J. M., E. De, A. Dorez, and J. M. Pages. 2000. Purification, characterization and sequence analysis of Omp50, a new porin isolated from Campylobacter jejuni. Biochem. J. 352:637-643. [PMC free article] [PubMed] [Google Scholar]
- 5.Burnens, A., U. Stucki, J. Nicolet, and J. Frey. 1995. Identification and characterization of an immunogenic outer membrane protein of Campylobacter jejuni. J. Clin. Microbiol. 33:2826-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrillo, C. D., E. Taboada, J. H. Nash, P. H. Lanthier, J. Kelly, P. C. Lau, R. Verhulp, O. Mykytczuk, J. Sy, W. A. Findlay, K. Amoako, S. Gomis, P. Willson, J. W. Austin, A. Potters, L. Babiuk, B. Allan, and C. M. Szymanski. 2004. Genome-wide expression analyses of Campylobacter jejuni reveals coordinate regulation of motility and virulence by flhA. J. Biol. Chem. 279:20327-23008. [DOI] [PubMed] [Google Scholar]
- 7.Chang, C., and J. F. Miller. 2006. Campylobacter jejuni colonization of mice with limited enteric flora. Infect. Immun. 74:5261-5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortes-Bratti, X., C. Karlsson, T. Lagergard, M. Thelestam, and T. Frisan. 2001. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via DNA damage checkpoint pathways. Infect. Immun. 276:5296-5302. [DOI] [PubMed] [Google Scholar]
- 9.Dorrell, N., J. A. Mangan, K. G. Laing, J. Hinds, D. Linton, H. Al-Ghusein, B. G. Barrell, J. Parkhill, N. G. Stoker, A. V. Karylshev, P. D. Butcher, and B. W. Wren. 2001. Whole genome comparison of Campylobacter jejuni isolates using a low-cost microarray reveals extensive genetic diversity. Genome Res. 11:1706-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrero, R. L., and A. Lee. 1988. Motility of Campylobacter jejuni in a viscous environment: comparison with conventional rod shaped bacteria. J. Gen. Microbiol. 134:53-59. [DOI] [PubMed] [Google Scholar]
- 11.Fouts, D. E., E. F. Mongodin, R. E. Mandrell, W. G. Miller, D. A. Rasko, J. Ravel, L. M. Brinkac, R. T. DeBoy, C. T. Parker, S. C. Dougherty, R. J. Dodson, A. S. Durkin, R. Madupu, S. A. Sullivan, J. U. Shetty, M. A. Ayodeji, A. Shvartsbeyn, M. C. Schatz, J. H. Badger, C. M. Fraser, and K. E. Nelson. 2005. Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLOS Biol. 3:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman, C. R., J. Neiman, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 13.Gelfanova, V., E. J. Hansen, and S. M. Spinola. 1999. Cytolethal distending toxin of Haemophilus ducreyi induces apoptotic death of Jurkat T cells. Infect. Immun. 67:6394-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goon, S., C. P. Ewing, M. Lorenzo, D. Pattarini, G. Majam, and P. Guerry. 2006. A σ28-regulated nonflagella gene contributes to virulence of Campylobacter jejuni 81-176. Infect. Immun. 74:769-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goon, S., J. F. Kelly, S. M. Logan, C. P. Ewing, and P. Guerry. 2003. Pseudaminic acid, the major modification on Campylobacter flagellin, is synthesized via the Cj1293 gene. Mol. Microbiol. 50:659-671. [DOI] [PubMed] [Google Scholar]
- 16.Guerry, P., R. A. Alm, M. E. Power, S. M. Logan, and T. J. Trust. 1991. Role of two genes in Campylobacter motility. J. Bacteriol. 173:4757-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerry, P., C. P. Ewing, M. Schirm, M. Lorenzo, J. Kelly, D. Pattarini, G. Majam, P. Thibault, and S. M. Logan. 2006. Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol. Microbiol. 60:299-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerry, P., C. P. Ewing, T. E. Hickey, M. M. Prendergast, and A. P. Moran. 2000. Sialylation of lipooligosaccharide cores affects immunogenicity and serum resistance of Campylobacter jejuni. Infect. Immun. 68:6656-6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris, L. A., S. M. Logan, P. Guerry, and T. J. Trust. 1987. Antigenic variation of Campylobacter flagellin. J. Bacteriol. 169:5066-5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendrixson, D. R., and V. J. DiRita. 2004. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol. Microbiol. 52:471-484. [DOI] [PubMed] [Google Scholar]
- 21.Hendrixson, D. R., and V. J. DiRita. 2003. Transcription of σ54 dependent but not σ28 dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagella secretory apparatus. Mol. Microbiol. 50:687-702. [DOI] [PubMed] [Google Scholar]
- 22.Hickey, T. E., A. L. McVeigh, D. A. Scott, R. E. Michielutti, A. Bixby, S. A. Carroll, A. L. Bourgeois, and P. Guerry. 2000. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin 8 from intestinal epithelial cells. Infect. Immun. 68:6535-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickey, T. E., G. Majam, and P. Guerry. 2005. Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytolethal distending toxin. Infect. Immun. 73:5194-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konkel, M. E., J. D. Klena, V. Rivera-Amill, M. R. Monteville, D. Biswas, B. Raphael, and J. Mickelson. 2004. Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J. Bacteriol. 186:3296-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konkel, M. E., B. J. Kim, V. Rivera-Amill, and S. G. Garvis. 1991. Bacterial secreted proteins are required for the internalization of Campylobacter jejuni into cultured mammalian cells. Mol. Microbiol. 32:691-701. [DOI] [PubMed] [Google Scholar]
- 26.Korostoff, J., J. F. Wang, I. Kieba, M. Miller, B. J. Shenker, and E. T. Lally. 1998. Actinobacillus actinomycetemcomitans leukotoxin induces apoptosis in HL-60 cells. Infect. Immun. 66:4474-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutsukake, K. 1994. Excretion of the anti-sigma factor through a flagella structure couples flagellar gene expression with flagellar assembly in Salmonella typhimurium. Mol. Gen. Genet. 243:605-612. [DOI] [PubMed] [Google Scholar]
- 28.Logan, S. M., J. F. Kelly, P. Thibault, C. P. Ewing, and P. Guerry. 2002. Structural heterogeneity of carbohydrate modifications affects serospecificity of Campylobacter flagellins. Mol. Microbiol. 46:587-597. [DOI] [PubMed] [Google Scholar]
- 29.McNally, D. J., A. J. Aubry, J. P. Hui, N. H. Khieu, D. Whitfield, C. P. Ewing, P. Guerry, J.-R. Brisson, S. M. Logan, and E. C. Soo. 2007. Targeted metabolomics analysis of Campylobacter coli VC167 reveals legionaminic acid derivatives as novel flagellar glycans. J. Biol. Chem. 282:14463-14475. [DOI] [PubMed] [Google Scholar]
- 30.Miller, W. G., A. H. Bates, S. T. Horn, M. T. Brandl, M. R. Wachtel, and R. E. Mandrell. 2000. Detection on surfaces and in Caco-2 cells of Campylobacter jejuni transformed with new gfp, yfp, and cfp marker plasmids. Appl. Environ. Microbiol. 66:5426-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morooka, T., A. Umeda, and K. Amako. 1985. Motility as an intestinal colonization factor for Campylobacter jejuni. J. Gen. Microbiol. 131:1973-1980. [DOI] [PubMed] [Google Scholar]
- 32.Oelschlaeger, T. A., P. Guerry, and D. J. Kopecko. 1993. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. USA 90:6884-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker, C. T., B. Quinones, W. G. Miller, S. T. Horn, and R. E. Mandrell. 2006. Comparative genomic analysis of Campylobacter jejuni strains reveals diversity due to genomic elements similar to those present in Campylobacter jejuni strain RM1221. J. Clin. Microbiol. 44:4125-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. M. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable tracts. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 35.Pearson, B. M., C. Pin, J. Wright, K. I'Anson, T. Humphrey, and J. M. Wells. 2004. Comparative genome analysis of Campylobacter jejuni using whole genome DNA microarrays. FEBS Lett. 554:224-230. [DOI] [PubMed] [Google Scholar]
- 36.Poly, F., D. Threadgill, and A. Stintzi. 2005. Genomic diversity in Campylobacter jejuni: identification of C. jejuni 81-176-specific genes. J. Clin. Microbiol. 43:2330-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poly, F., D. Threadgill, and A. Stintzi. 2004. Identification of Campylobacter jejuni ATCC 43431-specific genes by whole microbial genome comparisons. J. Bacteriol. 186:4781-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poly, F., T. Read, D. R. Tribble, S. Baqar, M. Lorenzo, and P. Guerry. 16 April 2007. Genome sequence of a clinical isolate of Campylobacter jejuni from Thailand. Infect. Immun. doi: 10.1128/IAI.00050-07. [DOI] [PMC free article] [PubMed]
- 39.Rivera-Amill, V., B. J. Kim, J. Keshu, and M. E. Konkel. 2001. Secretion of the virulence-associated Campylobacter invasion antigens from Campylobacter jejuni requires a stimulatory signal. J. Infect. Dis. 183:1607-1616. [DOI] [PubMed] [Google Scholar]
- 40.Samuel, M. C., D. J. Vugia, S. Shallow, R. Marcus, S. Segler, T. McGivern, H. Kassenborg, K. Reilly, M. Kennedy, F. Angulo, and R. V. Tauxe. 2004. Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996-1999. Clin. Infect. Dis. 38(Suppl. 3):S165-S174. [DOI] [PubMed] [Google Scholar]
- 41.Song, Y. C., S. Jin, H. Louie, D. Ng, R. Lau, Y. Zhang, R. Weerasekera, S. A. Rashid, L. A. Ward, S. D. Der, and V. L. Chan. 2004. FlaC, a protein of Campylobacter jejuni TGH9011 (ATCC43431) secreted through the flagellar apparatus, binds epithelial cells and influences cell invasion. Mol. Microbiol. 53:541-553. [DOI] [PubMed] [Google Scholar]
- 42.Suerbaum, S., C. Josenhans, T. Sterzenbach, B. Drescher, P. Brandt, M. Bell, M. Droge, B. Fartmann, H. P. Fischer, Z. Ge, A. Horster, R. Holland, K. Kleion, J. Koning, L. Macko, G. L. Mendz, G. Nyakatura, D. B. Schauer, Z. Shen, J. Weber, M. Frosch, and J. G. Fox. 2003. The complete genome sequence of the carcinogenic bacterium Helicobacter hepaticus. Proc. Natl. Acad. Sci. USA 100:7901-7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taboada, E. N., R. R. Acedillo, C. D. Carrillo, W. A. Findlay, D. T. Medeiros, O. L. Mykytczuk, M. J. Roberts, C. A. Valencia, J. M. Farber, and J. H. E. Nash. 2004. Large scale comparative genomics meta-analysis of Campylobacter jejuni isolates reveals low level of genome plasticity. J. Clin. Microbiol. 42:4566-4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thibault, P., S. M. Logan, J. F. Kelly, J.-R. Brisson, C. P. Ewing, T. J. Trust, and P. Guerry. 2001. Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J. Biol. Chem. 276:34862-34870. [DOI] [PubMed] [Google Scholar]
- 45.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzgerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 47.Wassenaar, T. M., N. M. C. Bleumink-Pluym, and B. A. M. van der Zeijst. 1991. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 10:2055-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao, R., D. H. Burr, and P. Guerry. 1997. CheY mediated modulation of Campylobacter jejuni virulence. Mol. Microbiol. 23:1021-1032. [DOI] [PubMed] [Google Scholar]
- 49.Yao, R., D. H. Burr, P. Dogi, T. J. Trust, H. Niu, and P. Guerry. 1994. Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: the role of motility in adherence and invasion of eukaryotic cells. Mol. Microbiol. 14:883-893. [DOI] [PubMed] [Google Scholar]
- 50.Yao, R., R. A. Alm, T. J. Trust, and P. Guerry. 1993. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 130:127-130. [DOI] [PubMed] [Google Scholar]
- 51.Yao, R., and P. Guerry. 1996. Molecular cloning and site-directed mutagenesis of a gene involved in arylsulfatase production in Campylobacter jejuni. J. Bacteriol. 178:3335-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]