Abstract
Melioidosis is caused by the soil saprophyte Burkholderia pseudomallei and is endemic in Southeast Asia. The pathogenesis of melioidosis is still largely unknown, although gamma interferon (IFN-γ) seems to play an obligatory role in host defense. Previously, we have shown that IFN-γ production in melioidosis is controlled in part by interleukin-18 (IL-18). The aim of the present study was to determine the role of IL-18 in the immune response to B. pseudomallei. For this the following investigations were performed. (i) Plasma IL-18 and blood monocyte IL-18 mRNA levels were elevated in 34 patients with culture-proven melioidosis compared to the levels in 32 local healthy controls; in addition, IL-18 binding protein levels were markedly elevated in patients, strongly correlating with mortality. (ii) IL-18 gene-deficient (IL-18 knockout [KO]) mice showed accelerated mortality after intranasal infection with a lethal dose of B. pseudomallei, which was accompanied by enhanced bacterial growth in their lungs, livers, spleens, kidneys, and blood at 24 and 48 h postinfection, compared to wild-type mice. In addition, IL-18 KO mice displayed evidence of enhanced hepatocellular injury and renal insufficiency. Together, these data indicate that the enhanced production of IL-18 in melioidosis is an essential part of a protective immune response to this severe infection.
Melioidosis is caused by the aerobic gram-negative soil-dwelling bacillus Burkholderia pseudomallei and is an important cause of severe sepsis in Southeast Asia and northern Australia (3, 29). Humans acquire melioidosis by inoculation through skin abrasions or inhalation. More than one-half of patients with melioidosis present with pneumonia associated with bacterial dissemination to distant organs. The mortality due to melioidosis is high, varying from 50% in northeast Thailand to 20% in the higher-technology setting of northern Australia. Interest in the disease pathogenesis of melioidosis has increased following its classification as category B disease/agent of bioterrorism by the U.S. Centers for Disease Control and Prevention.
B. pseudomallei is an intracellular pathogen that multiplies within macrophages. Although more is becoming known about the pathogenesis of melioidosis, host-pathogen interactions are still ill defined. Several investigations have implicated gamma interferon (IFN-γ) as an important mediator in protective immunity against melioidosis. In mice infected with B. pseudomallei intraperitoneally, inhibition of IFN-γ lowered the 50% lethal dose from >5 × 105 to ∼2 CFU and was associated with 8,500- and 4,400-fold increases in the bacterial loads in the liver and spleen, respectively (22). Similarly, IFN-γ-deficient mice displayed early mortality after intraperitoneal infection with B. pseudomallei together with strongly increased bacterial burdens in their spleens (8). Inhibition of interleukin-12 (IL-12) or IL-18, the predominant endogenous inducers of IFN-γ production, resulted in increased mortality in the same model (8, 22).
IL-18 is produced mainly by activated macrophages and is first synthesized as a precursor protein (pro-IL-18; 24 kDa), which requires splicing by caspase-1 to liberate the 18-kDa mature active protein. The biological activity of IL-18 is further regulated by IL-18 binding protein (IL-18BP), which binds IL-18, thereby preventing the interaction with its cell-associated receptor. Besides its IFN-γ-inducing effect, IL-18 has many proinflammatory effects on T and natural killer cells, enhancing proliferation and cytotoxicity, activating nuclear factor-κB, and stimulating the production of cytokines, including tumor necrosis factor alpha (TNF-α), IL-1, IL-2, and IL-6 (5). Thus far, only one investigation has directly addressed the role of endogenous IL-18 in experimental melioidosis (8). In that study administration of a blocking anti-IL-18 receptor antibody increased the early mortality after intraperitoneal injection of B. pseudomallei; the impact of IL-18 inhibition on antibacterial defense was not examined (8). The aim of the present study was to determine the role of IL-18 in the immune response to melioidosis. For this we measured the expression of IL-18 and IL-18BP in 34 Thai patients with culture-proven melioidosis and assessed the function of endogenous IL-18 using IL-18 knockout (KO) mice and a model of intranasal infection with B. pseudomallei that mimics melioidosis with severe pneumonia and bacterial dissemination to distant body sites.
MATERIALS AND METHODS
Patients in the study.
Thirty-four patients with melioidosis (mean age, 52 years; age range, 18 to 86 years; 50% male) were recruited prospectively at Sapprasithiprasong Hospital, Ubon Ratchathani, northeast Thailand, in 2004. Sepsis due to melioidosis was defined as culture positivity for B. pseudomallei from any clinical sample plus systemic inflammatory response syndrome (12). To meet the systemic inflammatory response syndrome criteria, patients had to meet at least three of the following four criteria: a core temperature of ≥38°C or ≤36°C; a heart rate of ≥90 beats/min; a respiratory rate of ≥20 breaths/min, an arterial CO2 pressure of ≥32 mm Hg, or the use of mechanical ventilation for an acute respiratory process; and a white cell count of ≥12 × 109 cells/liter or ≤4 × 109 cells/liter or a differential count showing >10% immature neutrophils. These definitions have been used in large clinical trials and were modified according to the latest revisions (1, 7). The exclusion criteria were the use of dialysis and/or immunosuppressive therapy, known coagulation disorders, and concomitant infection with human immunodeficiency virus. Blood samples were drawn within 36 h of the start of appropriate antimicrobial therapy. Thirty-two healthy blood donors (mean age, 41 years; age range, 21 to 59 years; 71% male) recruited from the Sapprasithiprasong Hospital blood bank served as a control population. The study was approved by both the Ministry of Public Health, Royal Government of Thailand, and the Oxford Tropical Research Ethics Committee, University of Oxford, United Kingdom, and written informed consent was obtained from all study subjects.
IL-18 and IL-18BP measurements.
Human IL-18 and IL-18BPa were measured by an enzyme-linked immunosorbent assay (ELISA) (R&D, Minneapolis, MN). In addition, IL-18 mRNA levels were measured as follows. Heparin blood samples were drawn from the antecubital vein and immediately put on ice. Leukocytes were isolated using erylysis buffer. Monocyte- and granulocyte-enriched populations were isolated using Polymorphprep (Axis-Shield, Dundee, United Kingdom). Monocyte and granulocyte fractions were >98% pure as determined by their scatter patterns on flow cytometry. After isolation leukocytes, monocytes, and granulocytes were dissolved in Trizol and stored at −80°C until they were used for RNA isolation. RNA was isolated and analyzed by multiplex ligation-dependent probe amplification as described previously (25), using an inflammation-specific kit developed in collaboration with MRC-Holland (Amsterdam, The Netherlands). All samples were tested with the same batch of reagents. The levels of mRNA were expressed as a normalized ratio of the peak area divided by the peak area of the β2-microglobulin gene, resulting in the relative abundance of mRNAs of the genes of interest (25). Transcription of the β2-microglobulin gene was not affected by B. pseudomallei infection.
Mouse infection.
The Animal Care and Use of Committee of the University of Amsterdam approved all experiments. Pathogen-free 8- to 10-week-old wild-type (WT) C57BL/6 mice were purchased from Harlan Sprague Dawley Inc. (Horst, The Netherlands). IL-18 KO mice (backcrossed six times to a C57BL/6 background) were generated previously as described by Takeda et al. (26) and were generously provided by Shizuo Akira (Osaka University, Japan). Age- and sex-matched animals were used in all experiments. For each time point one separate experiment was performed. The survival experiment was performed once.
For preparation of the inoculum, B. pseudomallei strain 1026b (kindly provided by Don Woods, University of Calgary, Canada) was pipetted from frozen aliquots into 50 ml Luria broth (Difco, Detroit, MI) and incubated overnight at 37°C in a shaking incubator. Thereafter, a 1-ml portion was transferred to fresh Luria broth and grown for ∼5 h to the mid-logarithmic phase. Bacteria were harvested by centrifugation at 1,500 × g for 15 min, washed, and resuspended in sterile isotonic saline at a concentration of 5 × 102 CFU/50 μl, as determined by plating serial 10-fold dilutions on blood agar plates. Pneumonia was induced by intranasal inoculation of 50 μl (5 × 102 CFU) of a bacterial suspension. For this procedure mice were lightly anesthetized by inhalation of isoflurane (Upjohn, Ede, The Netherlands).
Determination of bacterial outgrowth.
Twenty-four and 48 h after infection, mice were anesthetized with Hypnorm (active ingredients, fentanyl citrate and fluanisone; Janssen Pharmaceutica, Beerse, Belgium) and midazolam (Roche, Mijdrecht, The Netherlands) and sacrificed by bleeding from the vena cava inferior. The lungs, spleen, liver, kidneys, and brain were harvested and homogenized at 4°C in 4 volumes of sterile saline using a tissue homogenizer (Biospec Products, Bartlesville, OK). Numbers of CFU were determined from serial dilutions of organ homogenates and blood; organisms were plated on blood agar plates and incubated at 37°C in the presence of 5% CO2 for 16 h before colonies were counted.
Preparation of lung tissue for cytokine measurements.
For cytokine measurements, lung homogenates were diluted 1:2 in lysis buffer containing 300 mM NaCl, 30 mM Tris, 2 mM MgCl2, 2 mM CaCl2, 1% Triton X-100, 8 μg/ml 4-(2-aminoethyl)benzenesulfonyl fluoride, 100 μg/ml Na2-EDTA, 20 μg/ml pepstatin, and 20 μg/ml leupeptin (pH 7.4) and incubated at 4°C for 30 min. Homogenates were centrifuged at 1,500 × g at 4°C for 15 min, and supernatants were stored at −20°C until assays were performed.
Assays.
Mouse IL-18 was measured by an ELISA (MBL International, Woburn, MA). Mouse TNF-α, IFN-γ, monocyte chemoattractant protein 1 (MCP-1), and IL-6 were measured by a cytometric bead array multiplex assay (BD Biosciences, San Jose, CA) in accordance with the manufacturer's recommendations. Aspartate aminotransferase (ASAT), creatinine, and urea were measured with commercially available kits (Sigma-Aldrich), using a Hitachi analyzer (Roche) according to the manufacturer's instructions.
Pathology.
The lungs, spleen, liver, kidneys, and brain of each mouse were harvested after infection, fixed in 10% formalin, and embedded in paraffin. Four-micrometer sections were stained with hematoxylin and eosin and analyzed by a pathologist who was blinded for groups. To score lung inflammation and damage, the entire lung surface was analyzed with respect to the following parameters: percentage of the surface with pneumonia, necrosis/abscess formation, interstitial inflammation, endothelialitis, bronchitis, edema, thrombus formation, and pleuritis. Liver and spleen sections were scored for inflammation, necrosis/abscess formation, and thrombus formation. Each parameter was graded on a scale of 0 to 4, as follows: 0, absent; 1, mild; 2, moderate; 3, severe; and 4, very severe.
Statistical analysis.
Values are expressed as means ± standard errors of the means (SEM) unless indicated otherwise. Differences between groups were analyzed by the Mann-Whitney U test. Correlations were calculated using Spearman's rho test. For survival analysis, Kaplan-Meier analysis followed by a log rank test was performed. These analyses were performed using GraphPad Prism, version 4.00 (GraphPad Software, San Diego, CA). P values of <0.05 were considered statistically significant.
RESULTS
Elevated plasma IL-18 and IL-18BP and blood leukocyte IL-18 mRNA levels in patients with culture-proven melioidosis.
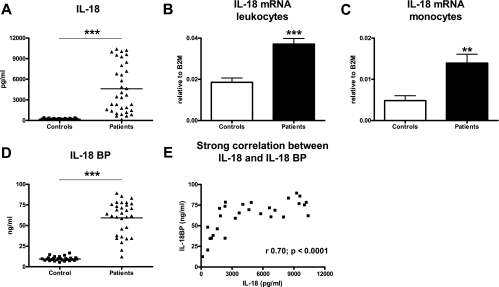
To obtain insight into IL-18 and IL-18BP expression during septic melioidosis, we measured these cytokines in the plasma from patients with septic melioidosis and healthy controls. In addition, we quantified IL-18 mRNA in the blood leukocytes (Fig. 1). We first confirmed our earlier data on increased serum IL-18 concentrations in patients with melioidosis (11) in the current study population; relative to healthy controls (195 ± 13 pg/ml), the patients displayed strongly elevated plasma IL-18 levels (4,616 ± 601 pg/ml; P < 0.001) (Fig. 1A). We extended this finding by showing elevated plasma IL-18 mRNA levels in whole-blood (unfractionated) leukocytes of patients with septic melioidosis (Fig. 1B) (P < 0.001 for a comparison with controls). Monocytes were at least in part responsible for the enhanced IL-18 mRNA production since patients had significantly elevated IL-18 mRNA levels in monocytes-enriched cell fractions (Fig. 1C) (P < 0.01 for a comparison with controls). IL-18 mRNA was not detectable in granulocyte-enriched cell fractions (data not shown). Of note, the difference in circulating IL-18 levels between patients and controls was much larger than the difference in IL-18 mRNA expression in peripheral blood leukocytes, suggesting that cell types not present in peripheral blood (e.g., tissue macrophages) contribute to circulating IL-18 levels and/or that more mature IL-18 is generated from pre-IL-18 in patients. Plasma IL-18BP levels were strongly elevated in patients (59 ± 3.7 ng/ml) relative to healthy controls (10 ± 0.5 ng/ml) (P < 0.001) (Fig. 1D). In melioidosis patients, plasma IL-18 and IL-18BP levels showed a strong positive correlation (r = 0.70; P < 0.0001) (Fig. 1E).
FIG. 1.
Plasma IL-18 and IL-18BP and blood leukocyte IL-18 mRNA levels in patients with culture-proven melioidosis: increased levels of IL-18 in plasma (A), IL-18 mRNA in peripheral blood leukocytes (B), and IL-18 mRNA in peripheral blood monocytes (C) of patients (n = 34) with septic melioidosis compared to healthy controls (n = 32). The strongly increased levels of IL-18BP (D) correlated with IL-18 levels (E). Two asterisks, P < 0.01; three asterisks, P < 0.001. B2M, β2-microglobulin.
Plasma IL-18 and IL-18BP levels correlate with mortality.
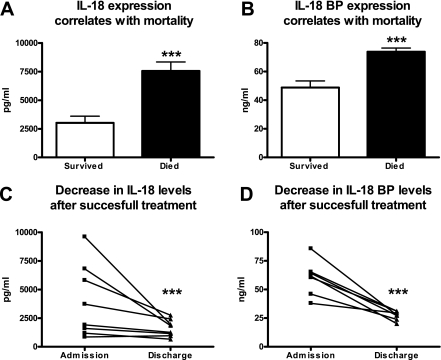
High plasma levels of both IL-18 and IL-18BP on admission correlated with an adverse outcome. The patients who died during hospital admission had higher IL-18 concentrations (7,552 ± 797 pg/ml versus 3,014 ± 591 pg/ml) and higher IL-18BP concentrations (73 ± 2.6 ng/ml versus 48 ± 4.7 ng/ml) than the patients who survived to discharge (P < 0.001 for the differences between groups) (Fig. 2A and B). Further proof for a correlation between the plasma levels of IL-18 and IL-18BP and disease severity was obtained with eight patients who survived and from whom a second blood sample was drawn after successful therapy. In these patients strongly significant decreases in plasma IL-18 and IL-18BP concentrations were detected (P < 0.001 for both) (Fig. 2C and D).
FIG. 2.
Correlation of IL-18 and IL-18BP levels with outcome in patients with severe melioidosis. Both IL-18 and IL-18BP levels strongly correlated with outcome. Both IL-18 (A) and IL-18BP (B) were strongly upregulated in patients who died in comparison to the patients who survived. Patients (n = 8) who survived after 2 weeks of intensive treatment showed near normalization of IL-18 (C) and IL-18BP (D) levels. Three asterisks, P < 0.001.
IL-18 KO mice show accelerated mortality during experimental melioidosis.
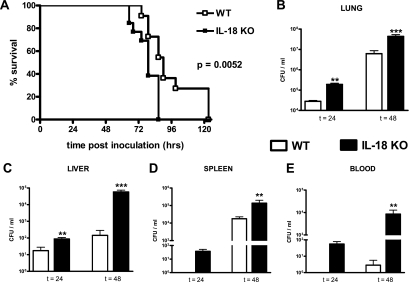
Having established that IL-18 is upregulated in patients with severe melioidosis and is correlated with mortality, we next investigated the involvement of IL-18 in the host response to B. pseudomallei infection in a murine model of melioidosis. WT and IL-18 KO mice were intranasally infected with B. pseudomallei. Inoculation with B. pseudomallei resulted in an increase in the IL-18 concentrations in lung homogenates (from 44 ± 27 pg/ml to 269 ± 44 pg/ml; P = 0.0025) but not in plasma of WT mice at 48 h after infection; in IL-18 KO mice IL-18 remained undetectable throughout the study. As a first experiment, WT and IL-18 KO mice were intranasally infected with a lethal dose of B. pseudomallei and monitored until death. IL-18 deficiency had a negative effect on survival. Whereas all WT mice were dead after 123 h (median survival time, 90 h), all IL-18 KO mice died within 87 h (median survival time, 79 h; P = 0.0052 for the difference between groups) (Fig. 3A).
FIG. 3.
Survival and bacterial outgrowth in IL-18 KO mice infected with B. pseudomallei compared to WT mice. (A) Survival of WT and IL-18 KO mice after intranasal inoculation with B. pseudomallei. Mortality was assessed every 4 h (12 mice per group; the P value indicates the significance of the difference between the groups). (B to D) IL-18 KO mice display strongly increased bacterial loads at 24 and 48 h after infection with B. pseudomallei in the lungs (B) and liver (C) in addition to greater bacterial outgrowth in the spleen (D) and blood (E). The data are means ± SEM (eight mice per group). Two asterisks, P < 0.01; three asterisks, P < 0.001. t, time (in hours).
IL-18 KO mice have an enhanced B. pseudomallei bacterial load.
To obtain insight into the mechanisms underlying the accelerated mortality of IL-18 KO mice during experimental melioidosis, we infected WT and IL-18 KO mice with B. pseudomallei and sacrificed them after 24 and 48 h (i.e., before the first deaths occurred) to determine the bacterial loads in the lungs (the primary site of the infection), spleen, kidneys, brain, and blood in order to evaluate bacterial loads and dissemination to distant body sites (Fig. 3B to E). Relative to WT mice, IL-18 KO mice displayed strongly increased bacterial loads in the lungs and liver at 24 and 48 h after infection, as well as in the blood, kidneys (data not shown), and spleen at 48 h (Fig. 3B to E). There was no bacterial growth in the brains of either WT or IL-18 KO mice (data not shown).
Lung histology and distant organ injury.
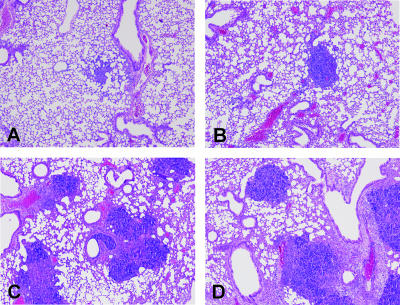
To further evaluate the role of IL-18 in the early antibacterial defense against B. pseudomallei, histological samples of the lungs, spleen, liver, kidneys, and brain obtained 24 and 48 h after infection were semiquantitatively scored for the extent of inflammation. Pulmonary inflammation was characterized by significant inflammation, pleuritis, peribronchial inflammation, edema, and endothelialitis in both WT and IL-18 KO mice (Fig. 4). The lung inflammation scores were similar at both 24 and 48 h after infection in the two mouse strains (data not shown). In contrast, the livers of IL-18 KO mice showed significantly more inflammation after 48 h than the livers of WT mice after inoculation with B. pseudomallei (Fig. 5A to C). Consistent with these pathology data, the plasma levels of ASAT were higher in IL-18 KO mice 48 h postinfection, reflecting increased hepatocellular injury in these animals (P < 0.05 for a comparison with WT mice) (Fig. 5D). In addition, IL-18 KO mice showed evidence of renal failure, as indicated by elevated plasma concentrations of urea (P < 0.05 for a comparison with WT mice) (Fig. 5F), whereas the increase in the plasma creatinine level was not significant (Fig. 5H). On pathological examination, renal histology was unremarkable in both IL-18 KO and WT mice. Spleen histology showed mild inflammation that was equally distributed in both mouse strains. Moreover, histological examination of the harvested brains revealed no signs of inflammation.
FIG. 4.
Pulmonary inflammation in WT and IL-18 KO mice infected with B. pseudomallei: representative histopathology slides of lungs of WT (A and C) and IL-18 KO (B and D) mice infected with 5 × 102 CFU B. pseudomallei at 24 h (A and B) and 48 h (C and D) after infection. The preparations were stained with hematoxylin and eosin. Original magnification, ×4.
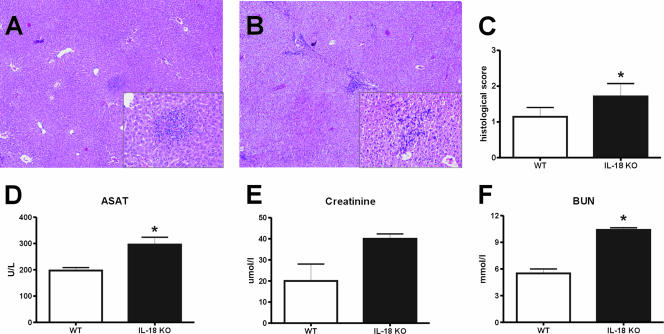
FIG. 5.
Liver inflammation in IL-18 KO mice. Livers of IL-18 KO mice showed more inflammation after 48 h than livers of WT mice after inoculation with B. pseudomallei. (A and B) Representative hematoxylin- and eosin-stained liver histology slides for WT (A) and IL-18 KO (B) mice at 48 h after inoculation with 5 × 102 CFU B. pseudomallei. Original magnification, ×4; original magnification for insets, ×20. (C) Pathology scores determined 48 h after inoculation (means ± standard errors) as described in Materials and Methods. (D to F) At 48 h after inoculation IL-18 KO mice also showed enhanced hepatic injury, as reflected by the plasma concentrations of ASAT (D), and more renal failure, as reflected by plasma creatinine (E) and blood urea nitrogen (BUN) (F) levels. The data are the means ± SEM for eight WT mice and eight IL-18 KO mice. U/L, units per liter. An asterisk indicates that the P value is <0.05 for a comparison with the WT control.
Enhanced inflammatory cytokine profile of IL-18 KO mice infected with B. pseudomallei.
To further examine the impact of IL-18 deficiency on the host response to melioidosis, we measured the concentrations of IFN-γ, TNF-α, IL-6, and MCP-1 in lungs and plasma of IL-18 KO and WT mice 24 and 48 h after infection with B. pseudomallei (Table 1). IFN-γ remained undetectable in lung homogenates of either IL-18 KO or WT mice; however, WT mice, but not IL-18 KO mice, exhibited elevated IFN-γ levels in plasma. The TNF-α concentrations did not differ in the two mouse strains. The IL-6 and MCP-1 levels were higher in IL-18 KO mice at 48 h but not at 24 h after infection (the difference was not significant for IL-6 in plasma).
TABLE 1.
Cytokine profile during experimental melioidosis in IL-18 KO mice
| Location | Cytokine | Concn (pg/ml) ata:
|
||||
|---|---|---|---|---|---|---|
| 0 h (WT mice) | 24 h
|
48 h
|
||||
| WT mice | IL-18 KO mice | WT mice | IL-18 KO mice | |||
| Lungs | IFN-γ | NDb | ND | ND | ND | ND |
| TNF-α | ND | ND | ND | 75 ± 24 | 43 ± 25 | |
| IL-6 | 51 ± 11 | 373 ± 27 | 376 ± 65 | 981 ± 151 | 2,012 ± 369c | |
| MCP-1 | 300 ± 47 | 3,278 ± 1,084 | 1,881 ± 894 | 5,537 ± 1,306 | 1,362 ± 1,305c | |
| Plasma | IFN-γ | ND | 30 ± 5c | ND | 1,516 ± 335c | ND |
| TNF-α | ND | 9 ± 1 | 7 ± 1 | 31 ± 12 | 31 ± 11 | |
| IL-6 | ND | 216 ± 14 | 154 ± 41 | 477 ± 134 | 1,383 ± 539 | |
| MCP-1 | 17 ± 2 | 572 ± 74 | 610 ± 79 | 683 ± 204 | 2,451 ± 697c | |
The data are means ± SEM for eight mice per group per time point.
ND, not detectable or below the detection limit.
P < 0.05.
DISCUSSION
In this study we sought to determine the role of IL-18 in the immune response to melioidosis. We demonstrated that the plasma concentrations of IL-18 and IL-18BP are elevated in patients with severe melioidosis and that high IL-18 and IL-18BP levels on admission are correlated with death. In experimental melioidosis induced by intranasal infection with a lethal dose of B. pseudomallei, IL-18 KO mice displayed enhanced bacterial growth in the lungs, blood, and distant organs, which was accompanied by increased hepatocellular injury and renal insufficiency and was associated with accelerated mortality. Together, these data indicate that the enhanced production of IL-18 in melioidosis is an essential part of a protective immune response to this severe infection.
The current study builds on an earlier investigation by our group which reported elevated circulating levels of IL-18 in patients with melioidosis (11). Here we confirm these findings and in addition show that blood monocytes are a source of IL-18. IL-18 mRNA was also detectable in monocyte-enriched cell fractions obtained from healthy controls, albeit at lower levels, which is consistent with a previous study showing constitutive IL-18 production by human monocytes in vitro (19). Circulating IL-18 levels showed a strong positive correlation with IL-18BP. This naturally occurring secreted protein has a high affinity for binding to IL-18 (16). IL-18BP is not a soluble receptor for IL-18 and is only distantly related to the cell-associated IL-18 receptor (5). IL-18BP exists as four isotypes (IL-18BPa, -b, -c, and -d), only two of which, IL-18BPa (measured in the present study) and IL-18BPc, are able to neutralize IL-18 (5). Both IL-18 and IL-18BP levels were higher in patients who eventually died and were decreased in survivors after successful treatment. Our data are in line with other studies on IL-18 and IL-18BP performed with septic patients. In a heterogeneous group of septic patients IL-18 and IL-18BPa levels were both significantly elevated (17). At the observed high levels, most IL-18 was bound to IL-18BP; however, the amount of remaining free IL-18 was still greater than the amount in healthy individuals (17). Furthermore, patients with septic shock who did not survive displayed higher IL-18 levels than patients who survived (18). In addition, in a small cohort of 13 patients with sepsis IL-18 levels correlated significantly with APACHE II scores (6).
One previous investigation studied the role of IL-18 in experimental melioidosis. In a recent study by Haque et al. focusing on the role of T cells in the immune response against B. pseudomallei, blockade of the IL-18 receptor in a mouse model resulted in decreased survival after intraperitoneal injection of B. pseudomallei; the effect of IL-18 inhibition on bacterial growth and the host inflammatory response was not investigated (8). Humans usually acquire melioidosis by inoculation through skin abrasions or inhalation. Pneumonia with bacterial dissemination to distant body sites is a common presentation of melioidosis (4, 29). We therefore used a model of melioidosis in which mice were infected with B. pseudomallei via the airways. In this model we found a strong protective role for IL-18: IL-18 KO mice were unable to control the infection, which resulted in increased distant organ injury and early mortality. The bacterial loads in particular increased in the livers of IL-18 KO mice between 24 and 48 h after infection, which was accompanied by significant pathology in this organ. The increased hepatocellular injury observed in IL-18 KO mice is in line with an earlier study performed in our laboratory showing a similar response of these animals during abdominal sepsis caused by Escherichia coli (28). Of note, IL-18 KO mice had biochemical evidence of renal insufficiency, in particular elevated plasma urea concentrations, without histological changes in their kidneys, suggesting that there was a prerenal cause of the diminished kidney function.
IFN-γ release into the circulation was completely abrogated in IL-18 KO mice, indicating that IL-18 is an important factor in IFN-γ production induced by B. pseudomallei. In accordance with this, our group previously showed that addition of anti-IL-18 to whole blood stimulated with heat-killed B. pseudomallei reduced IFN-γ levels (11). Moreover, treatment with anti-IL-18 receptor antibody reduced the number of IFN-γ-producing T and natural killer cells in mice injected with B. pseudomallei intraperitoneally (8). The protective role of IL-18 can be explained at least in part by reduced IFN-γ production, considering that elimination of IFN-γ rendered mice more susceptible to intraperitoneal infection with B. pseudomallei (8, 22).
The present study adds to several previous investigations addressing the role of IL-18 in host defense against gram-negative bacterial infection in vivo. Administration of anti-IL-18 to mice intravenously infected with Salmonella enterica serovar Typhimurium resulted in enhanced bacterial growth in the liver and spleen, similar to what was found here after infection with Burkholderia (13). In line with this, anti-IL-18 treatment during Yersinia enterocolitica infection was associated with relatively enhanced growth of bacteria (2). In addition, IL-18 deficiency facilitated bacterial growth after intranasal infection with Shigella flexneri and intraperitoneal infection with E. coli (21, 28). IL-18 also contributed to an effective host defense against gram-positive infection, including systemic infection with Listeria monocytogenes and pneumonia caused by Streptococcus pneumoniae (10, 14). Remarkably, however, IL-18 deficiency was associated with enhanced clearance of Pseudomonas aeruginosa from mouse lungs and diminished dissemination of the infection (23). In this respect it should be noted that although Pseudomonas and Burkholderia have some common features, the diseases induced by intranasal infection of mice with these two pathogens markedly differ. Indeed, Pseudomonas is cleared by immunocompetent mice (23, 24), whereas Burkholderia grows exponentially. Moreover, whereas experimental Pseudomonas pneumonia is associated with acute illness and acute lung inflammation, the disease induced by Burkholderia develops more gradually, eventually resulting in disseminated abscess formation characteristic of human melioidosis. The differences are further illustrated by the fact that endogenous IFN-γ, like IL-18, impaired host defense against experimentally induced Pseudomonas pneumonia (24). In addition, Remick et al. demonstrated that inhibition of IL-18 in septic peritonitis induced by cecal ligation and puncture had a variable effect on the outcome depending on the severity of the initial inflammatory response; IL-18 inhibition decreased mortality rates in mice with an increased risk of dying but increased lethality in mice with a predicted low mortality rate (20). In acute shock produced by administration of lipopolysaccharide (LPS), IL-18 KO mice tolerated a 50%-higher LPS dose than WT mice (9), and treatment with an anti-IL-18 antiserum protected mice against the lethal effects of both E. coli and Salmonella LPS (15). Taken together, these findings are consistent with the concept that proinflammatory cytokines, like IL-18, act as double-edged swords; inhibition of their activity during exaggerated inflammation in acute overwhelming infection or shock is beneficial for the host, but their inhibition during more gradual infection facilitates bacterial growth (27). The present investigations clearly identify IL-18 as a protective mediator during melioidosis that limits replication and dissemination of B. pseudomallei.
Acknowledgments
We are grateful to the staff of Sappasitiprasong Hospital, especially Wipada Chaowagul. In addition, we thank Marieke ten Brink and Joost Daalhuisen for expert technical assistance.
Joost Wiersinga is supported by the Dutch Foundation for Tropical Research (WOTRO). Sharon Peacock is supported by the Wellcome Trust.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Bernard, G. R., J. L. Vincent, P. F. Laterre, S. P. LaRosa, J. F. Dhainaut, A. Lopez-Rodriguez, J. S. Steingrub, G. E. Garber, J. D. Helterbrand, E. W. Ely, and C. J. Fisher, Jr. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699-709. [DOI] [PubMed] [Google Scholar]
- 2.Bohn, E., A. Sing, R. Zumbihl, C. Bielfeldt, H. Okamura, M. Kurimoto, J. Heesemann, and I. B. Autenrieth. 1998. IL-18 (IFN-gamma-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J. Immunol. 160:299-307. [PubMed] [Google Scholar]
- 3.Cheng, A. C., and B. J. Currie. 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18:383-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currie, B. J. 2003. Melioidosis: an important cause of pneumonia in residents of and travellers returned from endemic regions. Eur. Respir. J. 22:542-550. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello, C. A., and G. Fantuzzi. 2003. Interleukin-18 and host defense against infection. J. Infect. Dis. 187(Suppl. 2):S370-S384. [DOI] [PubMed] [Google Scholar]
- 6.Endo, S., K. Inada, and S. Yamada. 2000. Interleukin 18 (IL-18) levels in patients with sepsis. J. Med. 31:15-20. [PubMed] [Google Scholar]
- 7.Gentili, A., E. Iannella, L. Giuntoli, and S. Baroncini. 2006. System for predicting outcome and for clinical evaluation in sepsis and septic shock: could scores and biochemical markers be of greater help in the future? Comment to: Septic shock: current pathogenic concept from a clinical perspective. Med. Sci. Monit. 12:LE11-LE12. [PubMed] [Google Scholar]
- 8.Haque, A., A. Easton, D. Smith, A. O'Garra, N. Van Rooijen, G. Lertmemongkolchai, R. W. Titball, and G. J. Bancroft. 2006. Role of T cells in innate and adaptive immunity against murine Burkholderia pseudomallei infection. J. Infect. Dis. 193:370-379. [DOI] [PubMed] [Google Scholar]
- 9.Hochholzer, P., G. B. Lipford, H. Wagner, K. Pfeffer, and K. Heeg. 2000. Role of interleukin-18 (IL-18) during lethal shock: decreased lipopolysaccharide sensitivity but normal superantigen reaction in IL-18-deficient mice. Infect. Immun. 68:3502-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauw, F. N., J. Branger, S. Florquin, P. Speelman, S. J. van Deventer, S. Akira, and T. van der Poll. 2002. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J. Immunol. 168:372-378. [DOI] [PubMed] [Google Scholar]
- 11.Lauw, F. N., A. J. Simpson, J. M. Prins, M. D. Smith, M. Kurimoto, S. J. van Deventer, P. Speelman, W. Chaowagul, N. J. White, and T. van der Poll. 1999. Elevated plasma concentrations of interferon (IFN)-gamma and the IFN-gamma-inducing cytokines interleukin (IL)-18, IL-12, and IL-15 in severe melioidosis. J. Infect. Dis. 180:1878-1885. [DOI] [PubMed] [Google Scholar]
- 12.Levy, M. M., M. P. Fink, J. C. Marshall, E. Abraham, D. Angus, D. Cook, J. Cohen, S. M. Opal, J. L. Vincent, and G. Ramsay. 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 31:1250-1256. [DOI] [PubMed] [Google Scholar]
- 13.Mastroeni, P., S. Clare, S. Khan, J. A. Harrison, C. E. Hormaeche, H. Okamura, M. Kurimoto, and G. Dougan. 1999. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect. Immun. 67:478-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neighbors, M., X. Xu, F. J. Barrat, S. R. Ruuls, T. Churakova, R. Debets, J. F. Bazan, R. A. Kastelein, J. S. Abrams, and A. O'Garra. 2001. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on interferon gamma production. J. Exp. Med. 194:343-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Netea, M. G., G. Fantuzzi, and B. J. Kullberg. 2000. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal E. coli and Salmonella typhimurium endotoxemia. J. Immunol. 164:2644-2649. [DOI] [PubMed] [Google Scholar]
- 16.Novick, D., S. H. Kim, G. Fantuzzi, L. L. Reznikov, C. A. Dinarello, and M. Rubinstein. 1999. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity 10:127-136. [DOI] [PubMed] [Google Scholar]
- 17.Novick, D., B. Schwartsburd, R. Pinkus, D. Suissa, I. Belzer, Z. Sthoeger, W. F. Keane, Y. Chvatchko, S. H. Kim, G. Fantuzzi, C. A. Dinarello, and M. Rubinstein. 2001. A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine 14:334-342. [DOI] [PubMed] [Google Scholar]
- 18.Oberholzer, A., L. Harter, A. Feilner, U. Steckholzer, O. Trentz, and W. Ertel. 2000. Differential effect of caspase inhibition on proinflammatory cytokine release in septic patients. Shock 14:253-257. [DOI] [PubMed] [Google Scholar]
- 19.Puren, A. J., G. Fantuzzi, and C. A. Dinarello. 1999. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc. Natl. Acad. Sci. USA 96:2256-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remick, D. G., G. E. Bolgos, and J. Siddiqui. 2003. Inflammatory status in sepsis alters efficacy of interleukin-18 binding protein therapy. Crit. Care Med. 31:2096-2101. [DOI] [PubMed] [Google Scholar]
- 21.Sansonetti, P. J., A. Phalipon, J. Arondel, K. Thirumalai, S. Banerjee, S. Akira, K. Takeda, and A. Zychlinsky. 2000. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12:581-590. [DOI] [PubMed] [Google Scholar]
- 22.Santanirand, P., V. S. Harley, D. A. Dance, B. S. Drasar, and G. J. Bancroft. 1999. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei. Infect. Immun. 67:3593-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schultz, M. J., S. Knapp, S. Florquin, J. Pater, K. Takeda, S. Akira, and T. van der Poll. 2003. Interleukin-18 impairs the pulmonary host response to Pseudomonas aeruginosa. Infect. Immun. 71:1630-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultz, M. J., A. W. Rijneveld, P. Speelman, S. J. van Deventer, and T. van der Poll. 2001. Endogenous interferon-gamma impairs bacterial clearance from lungs during Pseudomonas aeruginosa pneumonia. Eur. Cytokine Netw. 12:39-44. [PubMed] [Google Scholar]
- 25.Spek, C. A., A. Verbon, H. Aberson, J. P. Pribble, C. J. McElgunn, T. Turner, T. Axtelle, J. Schouten, T. Van Der Poll, and P. H. Reitsma. 2003. Treatment with an anti-CD14 monoclonal antibody delays and inhibits lipopolysaccharide-induced gene expression in humans in vivo. J. Clin. Immunol. 23:132-140. [DOI] [PubMed] [Google Scholar]
- 26.Takeda, K., H. Tsutsui, T. Yoshimoto, O. Adachi, N. Yoshida, T. Kishimoto, H. Okamura, K. Nakanishi, and S. Akira. 1998. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8:383-390. [DOI] [PubMed] [Google Scholar]
- 27.van der Poll, T., and S. J. van Deventer. 1999. Cytokines and anticytokines in the pathogenesis of sepsis. Infect. Dis. Clin. N. Am. 13:413-426, ix. [DOI] [PubMed] [Google Scholar]
- 28.Weijer, S., M. E. Sewnath, A. F. de Vos, S. Florquin, K. van der Sluis, D. J. Gouma, K. Takeda, S. Akira, and T. van der Poll. 2003. Interleukin-18 facilitates the early antimicrobial host response to Escherichia coli peritonitis. Infect. Immun. 71:5488-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiersinga, W. J., T. van der Poll, N. J. White, N. P. Day, and S. J. Peacock. 2006. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat. Rev. Microbiol. 4:272-282. [DOI] [PubMed] [Google Scholar]