UTIs, E. COLI ABUs, AND UPEC
Mucosal surfaces in the intestinal, respiratory, and urogenital tracts are populated by a highly diverse bacterial flora. Most host-dwelling bacteria are commensals and maintain a symbiotic relationship with the host; however, a minority are pathogens that attack the mucosa, causing tissue damage and disease symptoms. Bacterial pathogens differ from commensals by the expression of specific virulence factors, such as those that mediate tissue assault. Commensals, in contrast, have generally been regarded as bacteria lacking such virulence factors or other specific mechanisms for interaction with host tissues. Asymptomatic carriage of bacteria may also result after a primary symptomatic infection. However, this type of carrier state differs from commensalism in that variants of pathogenic strains persist without evoking a host response. This review is focused on the molecular basis for attenuation of virulence and adaptation to commensalism, using the prototypic asymptomatic bacteriuria strain Escherichia coli 83972 as a model.
Most E. coli strains coexist peacefully with their human host, while others cause disease. E. coli is a versatile pathogen causing a spectrum of diseases, including urinary tract infections (UTIs), diarrhea, sepsis, and meningitis (28). UTIs are among the most common infectious diseases of humans and are a major cause of morbidity. It is estimated that 40 to 50% of healthy adult women have experienced at least one UTI episode (15). Acute pyelonephritis and asymptomatic bacteriuria (ABU) represent the two extremes of UTI. Escherichia coli is responsible for >80% of UTIs. Acute pyelonephritis is a severe acute systemic infection caused by uropathogenic E. coli (UPEC) clones with virulence genes clustered on “pathogenicity islands” (13, 16, 25, 46, 67, 72). ABU, on the other hand, is an asymptomatic carrier state that resembles commensalism. Paradoxically, a large proportion of UTIs are caused by ABU E. coli. Individuals infected with ABU group E. coli may carry high urine titers of a single E. coli strain for months or years without provoking a host response. According to conventional notions, pathogens can develop from commensals by the acquisition of virulence-associated genes located on, for example, pathogenicity islands or plasmids. The flip side of this evolutionary force is the equally important adaptation of the newly minted pathogen to its new host niche, where genes are inactivated by either point mutation, insertion, or deletion (39). Here we illustrate such an example, where the commensal-to-pathogen shift in E. coli is bidirectional.
STRAIN 83972 AS A PROPHYLACTIC AGENT
E. coli 83972 is a prototype ABU strain and is probably the best-characterized ABU-type E. coli strain to date. It is a clinical isolate capable of long-term bladder colonization. The strain lacks defined O and K surface antigens and is nonmotile (2). Also, electron microscopy of 83972 did not reveal the presence of flagella or a capsule (30, 56). Strain 83972 was originally isolated from a young Swedish girl with ABU who had carried it for at least 3 years without symptoms (2, 37). It is well adapted for growth in the urinary tract (UT), where it establishes long-term bacteriuria (2, 23, 75).
A number of studies have compared the outcomes of treated versus untreated cases of ABU (20, 36, 66). These studies concluded that not only do patients with ABU not profit from antibiotic treatment, but also, if left untreated, they actually have a reduced risk of kidney infection. Treatment of ABU is generally recommended only for pregnant women, in whom complications might arise (44). These observations and the nonvirulent attributes of strain 83972 inspired a number of studies in which 83972 was used for prophylactic purposes in patients with recurrent UTI. In these studies, the bladders of patients were deliberately colonized with strain 83972 in order to prevent the establishment of UPEC and other uropathogens. Strain 83972 has been used successfully in several such studies and has been shown to establish bacteriuria without jeopardizing the health of the patient. In one study, women with chronic symptomatic UTI were treated for eradication of their infection and then deliberately colonized with E. coli 83972. Stable bacteriuria was established for more than 30 days in 7 of the 12 individuals (2). In another study, a group of patients with recurrent symptomatic UTI had their bladders deliberately colonized with E. coli 83972. Successful long-term colonization (5 months to 3 years) was achieved in 6/12 patients with neurogenic bladder disorder (75). Deliberate colonization with E. coli 83972 has also been shown to reduce the frequency of UTI in patients with a neurogenic bladder secondary to spinal cord injury (23, 68), and the strain can prevent catheter colonization by bacterial and fungal uropathogens (10, 70, 71). Taken together, these studies have convincingly indicated the potential of 83972 as a probiotic. Although not yet approved by the U.S. FDA or a similar European authority as a routine therapy, it has a safe and well-established track record as an alternative treatment for patients with recalcitrant infections in cases where conventional therapy has shown limited success.
STRAIN 83972 DOES NOT TRIGGER HOST DEFENSE MECHANISMS AND LACKS FUNCTIONAL FORMS OF THE PRIMARY UTI-ASSOCIATED ADHESINS
ABU patients may carry high urine titers of a single E. coli strain for months or years without provoking a host response. In early studies of ABU strains, it was suggested that this was due to a lack of virulence genes, as the majority of ABU-associated E. coli strains are nonhemolytic and nonadherent and lack hemagglutination ability (13, 27, 37). Molecular epidemiology revealed, however, that >60% of ABU strains carry virulence genes, even though they fail to express the corresponding phenotype (49, 50, 55).
The ability of UPEC to cause symptomatic UTIs is enhanced by adhesins (31, 45). Adherence to the UT epithelium enables the bacterium to resist the hydrodynamic forces of urine flow and to establish infection. The three primary fimbrial adhesive organelles associated with urovirulence are P, type 1, and F1C fimbriae. These three UPEC class fimbriae are all 7-nm-wide and approximately 1-μm-long surface polymers. The bulk of a UPEC class fimbria is made up of about 1,000 subunits of a major building element, viz, the PapA, FimA, or FocA protein, for P, type 1, and F1C fimbriae, respectively. Additionally, a few copies of minor components, for example, FimH in the case of type 1 fimbriae, are integral parts of the fimbriae and are responsible for their adhesive properties (33). The ability of uropathogens to synthesize P fimbriae shows a very strong correlation with clinical disease, and these adherence factors have been shown to enhance the establishment of bacteriuria and to activate the innate immune response in animal models and in the human UT (5, 21, 52, 53). Type 1 fimbriae enhance colonization and host response induction in the murine UTI model and promote biofilm formation and invasion (8, 32, 43). Type 1 fimbriae confer binding to α-d-mannosylated proteins, such as uroplakins, which are abundant in the bladder (8, 73), while P fimbriae recognize the α-d-galactopyranosyl-(1-4)-β-d-galactopyranoside receptor epitope in the globoseries of glycolipids (35). Both P and type 1 fimbriae recognize their receptor targets by virtue of organelle tip-located adhesins, namely, PapG and FimH, respectively (12, 63). The host response includes cytokine production, inflammation, and exfoliation of infected epithelial cells (22, 43, 58, 74). F1C fimbriae are expressed by a large proportion of UTI E. coli strains; depending on the study, 14 to 30% of UTI strains have been reported to be able to express these fimbriae (47, 60). F1C fimbriae specifically recognize galactosylceramide targets present on epithelial cells in the kidneys, ureters, and bladder, as well as globotriaosylceramide, present only in the kidneys (4, 29). Recently, it was shown that human renal epithelial cells specifically produce the proinflammatory cytokine interleukin-8 in response to F1C fimbria-mediated attachment (4).
The ability of E. coli 83972 to establish efficient long-term colonization of the human bladder without evoking countermeasures from the host defense system is not fully understood. The strain has been reported to carry genes of the pap, fim, and foc gene clusters, encoding P, type 1, and F1C fimbriae, respectively (24). However, the 83972 strain does not express detectable fimbrial phenotypes when recovered from the UT or after in vitro subculture and has never been reported to adhere to any relevant cell targets (54). In an attempt to resolve this discrepancy, we recently investigated the genetic and molecular status of the three UPEC class fimbriae in strain 83972 (30, 56). From these studies, it transpired that the strain is unable to express any of these adhesins in a functional form. It is interesting that the type 1, F1C, and P fimbrial systems of E. coli 83972 have been incapacitated in quite different ways (Fig. 1). In all cases, mutational events have inactivated the fimbrial systems, but the underlying genetic events are quite different, as follows. The type 1 system has been inactivated by a major deletion encompassing 4.25 kb of the fim gene cluster, affecting all genes except those encoding the minor components, viz, fimF, fimG, and fimH. The F1C system has been inactivated by point mutations in the fimbrial transport system, affecting the focD gene, encoding the usher protein (56). Meanwhile, the foc genes are still transcribed, and the major structural protein, FocA, is produced, although it is unable to reach the cell surface. This suggests that the inactivation of FocD might be a recent evolutionary event. Interestingly, strain 83972 still has the capacity to produce P fimbriae; however, these are unable to bind to their cognate receptor since point mutations in the papG gene have rendered the PapG adhesin nonfunctional (30). This also suggests that inactivation of P fimbria functionality might be a recent evolutionary event since, as in the case of the F1C system, strain 83972 is unable to produce functional organelles but still wastes resources by churning out fimbrial proteins.
FIG. 1.
The E. coli ABU strain 83972 carries the fim, foc, and pap clusters, encoding type 1, F1C, and P fimbriae, respectively. However, all three gene clusters have accrued mutations that destroy their function, rendering 83972 incapable of expressing functional fimbriae on the surface of the cell (30, 56). The three fimbrial systems have been incapacitated in quite different ways (as indicated in gray). (A) The type 1 system has been inactivated by a major deletion encompassing 4.25 kb of the fim gene cluster. (B) The F1C system has been inactivated by point mutations in the fimbrial transport system, affecting the focD gene encoding the usher protein, making it impossible for the fimbrial components to reach the cell surface. (C) The P system has been made nonfunctional by the introduction of point mutations in the papG gene: 83972 can produce P fimbriae, but these are unable to bind to their cognate receptor.
STRAIN 83972 WAS ONCE A PATHOGEN
ABU patients may carry a single strain for months or years, creating a condition that resembles commensalism, but with a strain that may have evolved from a pathogenic ancestor. Several lines of evidence support the notion that the ancestor of strain 83972 was a pyelonephritic UPEC strain. First, multilocus sequence typing of 83972 shows that it belongs to the B2 clonal group (http://www.mlst.net/). E. coli strains belonging to group B2 are associated with pyelonephritis and other extraintestinal invasive clinical syndromes, such as bacteremia, prostatitis, and meningitis. Second, the strain contains gene clusters encoding the three primary UPEC class fimbriae in various stages of erosion. Arguably, the ancestor of strain 83972 was once able to express functional F1C, type 1, and P fimbriae. Third, despite the fact that the fim and pap gene clusters of the strain contain mutations that destroy their function, they contain sequence information suggestive of a pathogenic past. Thus, the fimH allele of 83972 encodes minor amino acid variations (compared with the K-12 version) that are consistent with those of previously characterized pyelonephritis isolates (62-64). Finally, the strain has a copy of the F14 papA variant that has been associated with other virulence factors, including hemolysin and cytotoxic necrotizing factor 1, from E. coli strains in the B2 phylogenetic group (26).
STRAIN 83972 STAYS IN THE HUMAN BLADDER BY VIRTUE OF A LACK OF HOST DEFENSE ACTIVATION, FAST GROWTH, AND POSSIBLY BIOFILM FORMATION
Strain 83972 was carried by a young girl without any symptoms for 3 years. Later long-term carriage studies with 83972 have confirmed that the strain does not trigger a mucosal response in patients and does not trigger host defense mechanisms. It seems that a major reason for the failure of 83972 to trigger host defenses and to keep a low profile vis-à-vis the human host is its inability to express functional UPEC class fimbriae (30, 56). Thus, it avoids the activation of aggressive host countermeasures, such as the production of cytokines, inflammation, and exfoliation of infected bladder epithelial cells, and can continue to occupy its privileged environmental niche.
Meanwhile, the lack of UPEC class fimbriae in strain 83972 raises the issue of how it is able to persist in a high-flow environment like the human UT. Healthy adult humans normally produce 1 to 2 liters of urine per day, which must pass through the bladder and corresponds to an average flow rate of 40 to 80 ml per hour. An adult human bladder has a holding capacity of 200 to 400 ml, and micturition causes the release of roughly the same volume of urine; the volume of residual urine is about 1 milliliter (59). These would seem to be conditions that are hardly compatible with colonization by a nonadherent microbe. The contribution of bladder hydrodynamics to the elimination of bacteria has been recognized for several decades (6, 38), and it has even been suggested that without adhesin-assisted attachment to the bladder surface, E. coli would not be able to become established in the UT (3, 69). Implicit in this suggestion is the notion that the growth rate of E. coli in urine is too low to cope with the losses incurred by micturition. Meanwhile, mathematical modeling indicates that provided that the growth rate of a strain is high enough, it will be able to become established in the bladder in an adhesion-independent manner, and growth rates of E. coli isolated from UTIs have been shown to be sufficient to overcome the losses due to micturition (17). Indeed, strain 83972 grows extremely well in human urine in vitro, with a doubling time of less than 45 min (57). Furthermore, we recently showed that the capacity to grow fast in urine is not a unique property of 83972, since several other ABU isolates also possess similar growth characteristics (55). This suggests that fast growth in human urine can account to a large degree for the ability of E. coli 83972 to establish itself in the human bladder.
Bacterial biofilms can be established on virtually any solid surface of organic or inorganic nature, spanning a wide spectrum of environments. More than 50% of all microbial infections have now been associated with the formation of biofilms (9). Biofilm formation is generally considered to be important for long-term bacterial establishment on surfaces subjected to hydrodynamic flow, such as the human UT, since bacterial biofilms are highly resistant to removal by liquid flow forces. Indeed, previous studies have advocated the importance of bacterial biofilm formation in UTIs, notably in chronic cystitis and infections associated with catheters (42, 51). Recent studies of UTIs not associated with catheters support the notion that the ability of UTI E. coli to form biofilms correlates with persistence and recurrence (1, 34, 65). We recently studied biofilm formation of strain 83972 on various surfaces in human urine and found that it was an excellent biofilm former under these conditions. In fact, our data suggested that biofilm formation is associated with many ABU E. coli strains, but not with UPEC strains, and may be an important strategy for persistence in the high-flow environment of the UT (18, 19). In addition, global gene expression profiling of biofilm formation of strain 83972 in human urine revealed similarities with the expression profile in patients, indicating that biofilm formation might play a role in efficient colonization of the human UT (19). Taken together, a picture of how ABU strain 83972 manages to colonize the human UT is forming; the two primary strategies seem to be (i) fast growth and (ii) possible biofilm formation.
HOW DOES 83972 KEEP UROPATHOGENS OUT?
The human bladder seems to be an attractive environmental niche for a microorganism like strain 83972, with a steady supply of nutrients, constant temperature, etc. Arguably, it might be an advantage to keep competitors out. As previously mentioned, strain 83972 has been used successfully for prophylactic purposes in patients with recurrent UTIs, where it was shown to prevent the establishment of uropathogens (10, 11, 23, 71). However, the underlying mechanisms for this phenomenon were not clear. The excellent growth characteristics of 83972 in urine prompted us to compete it with a range of well-characterized UPEC strains (57). Indeed, it transpired that when E. coli 83972 was pitted against the UPEC strains in pairwise competition experiments, it outcompeted all of them to a significant degree; competition against UPEC strain CFT073, using a starting ratio of 1:1, resulted in a population of cells that comprised 96% 83972 and 4% CFT073 after 17 h of growth. Furthermore, in experiments using a starting ratio of 1 (83972) to 20 (NU14), strain 83972 managed to totally outcompete NU14 such that it comprised 93% of the final population. Strain 83972 also grew faster than any of the UPEC strains and displayed superior growth characteristics in that it reached a higher maximum cell density and exhibited a shorter lag phase in human urine. These results were confirmed in mouse experiments; the 83972 strain was almost totally dominant in the urine of mice 24 h after they received a mixed challenge consisting of 83972 and the UPEC isolate NU14 (1:1). The prophylactic properties of 83972 against UPEC strains therefore seem to be associated with its excellent growth properties in human urine.
Meanwhile, in recent studies, we found that biofilm formation in human urine seems to be inversely related to urovirulence (14, 18). Indeed, the biofilm-forming capacity of strain 83972 (and other selected ABU strains) was markedly superior to that of the UPEC group. It is interesting that in our hands, strain 83972, which has been shown to inhibit UT colonization by uropathogens (70, 71), was able to outperform the UPEC strain CFT073 completely with respect to biofilm formation in a flow cell system (14, 18, 19). Furthermore, it was also demonstrated that strain 83972 was able to exclude UPEC strains during biofilm formation in human urine (14). The excellent biofilm-forming faculty of strain 83972 might also contribute to its ability to outcompete other microbes and to inhibit UT infection and catheter colonization by uropathogens.
GLOBAL GENE EXPRESSION PROFILES OF 83972 IN URINE AND IN HUMAN HOSTS
The bacterial transcriptome is a dynamic entity that reflects the organism's immediate, ongoing response to its environment. DNA microarray-assisted functional genomics provides the global expression profile of a genome, disclosing how the bacterium adapts to an environmental niche. Global gene expression profiles of strain 83972 in human urine compared with minimal medium revealed that the strain has adapted well to growth in this iron-limiting environment. It does so by up-regulation of its numerous iron uptake systems (57). Furthermore, the array data revealed high expression levels of genes involved in transportation and degradation pathways of sugar acids and carbohydrates (e.g., galacturonate, glucuronate, galactonate, arabinose, sorbitol, galactose, maltose, xylose, and mannose), indicating how E. coli 83972 is able to reach high growth rates in human urine by efficiently utilizing the nutrients available in this growth medium.
Global gene expression profiling of strain 83972 in the bladders of three individuals revealed a number of interesting facets about the strain in vivo (54). Not only is it unable to express functional forms of type 1, F1C, and P fimbriae because of various genetic lesions, but also most of the fimbria-encoding genes that are actually present turned out to be shut down in human hosts. Furthermore, several other known virulence factors were also down-regulated in the bladder, such as genes involved in hemolysin, lipopolysaccharide (LPS), and capsule synthesis. This lends further support to the notion that 83972 is a benign strain that does not harm the host and cannot trigger host defense mechanisms.
It is interesting that the majority of the most highly expressed genes of E. coli 83972 in humans encode products that are involved in protein synthesis, such as ribosome components. The high expression of ribosomal genes in E. coli 83972 suggests a high growth rate in the human UT and supports our hypothesis that the strain's optimized growth properties in human urine explain its ability to successfully colonize the human UT in the absence of functional fimbriae. The data also revealed that the strain has an impressive array of iron acquisition systems and that all of these are active in the human bladder. These characteristics might also contribute to its ability to grow fast in human urine and to outcompete UPEC strains and other pathogens. The results further revealed an in vivo lifestyle of microaerobic growth, with respiration of nitrate coupled to degradation of sugar acids and amino acids. However, the use of sugar acids, amino acids, and carbohydrates varied among the three patients (presumably due to urine composition), and very few metabolic pathways were up-regulated in all patients. One of the few commonly used nutrients was d-serine: the two genes encoding d-serine deaminase and permease, dsdA and dsdX, respectively, were up-regulated up to 8.3-fold compared with their levels in minimal lab medium. Interestingly, the expression profile for the strain in the human UT showed partial overlap with the biofilm expression profile, indicating a partial biofilm-like life of strain 83972 in the human UT (19).
COMPARISON OF TWO PROTOTYPIC STRAINS
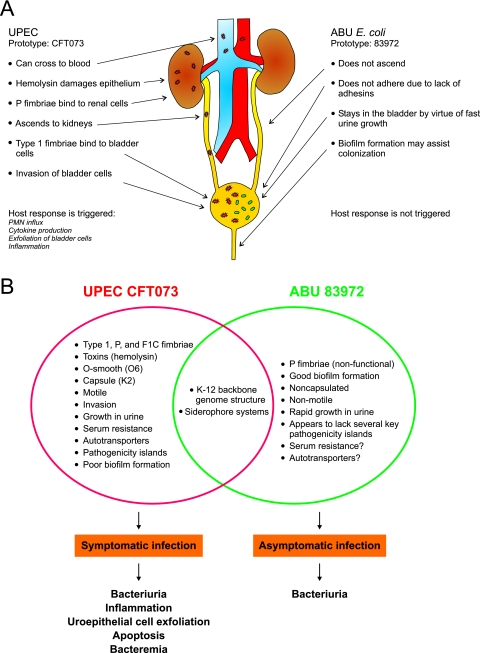
The UTI E. coli strains 83972 and CFT073 are prototype strains representing the two extremes of UTI strains and their interaction modes vis-à-vis the human host. One, 83972, is a harmless commensal-like isolate causing asymptomatic infections, whereas the other, CFT073, a highly virulent pyelonephritis isolate, is a bona fide virulent UPEC strain that causes severe symptoms in the host. The prototypic strain CFT073 was originally isolated from the blood of a female patient with a severe symptomatic UTI (40). CFT073 is able to express an impressive armamentarium of virulence factors, including the UPEC class fimbriae, hemolysin, the siderophore aerobactin, flagella, the K2 capsule, and LPS. E. coli 83972, on the other hand, is defunct in all three UTI-associated fimbrial clusters, has never been reported to adhere to UT cells, is nonhemolytic, and lacks flagella and defined O and K antigens. However, despite the fact that the two strains show very different phenotypes and interactions with the host, both strains belong to the same E. coli reference group, i.e., the B2 clonal group associated with highly virulent strains. This suggests that the ancestor of 83972 was quite similar to CFT073.
Strains 83972 and CFT073 have radically different strategies for their interaction with the human host (Fig. 2). ABU strain 83972 avoids triggering host defense mechanisms, lives a commensal-like existence in bladder urine, and does not ascend further than the bladder. In contrast, strain CFT073 triggers host defenses but is apparently able to fend for itself; it can ascend to the kidneys and may even cause septicemia (40). It is therefore highly interesting to compare the genetic repertoires and expression profiles of the two strains. A simple assumption would argue that gene products that are expressed under relevant conditions, viz, in patients or animal models, by CFT073, but not by 83972, are potential candidates for virulence factors. While CFT073 showed up-regulation of type 1 fimbriae, hemolysin, and microcin secretion, as well as genes involved in capsular polysaccharide and LPS synthesis, during growth in mice (61), E. coli 83972 failed to express virtually all known virulence-associated gene products in humans (54). Furthermore, the global gene expression profile comparison of 83972 in humans and CFT073 in mice revealed some similarities; both strains grow rapidly in the UT, as indicated by the up-regulation of genes involved in the translational machinery, and respond to iron limitation by the activation of genes related to iron acquisition. Meanwhile, in spite of its inability to express virulence factors in urine, strain 83972 is able to outperform strain CFT073 with respect to both growth and biofilm formation in urine (14, 18, 57).
FIG. 2.
Comparison of two prototypic UTI E. coli strains, the UPEC strain CFT073 and the ABU strain 83972. (A) Behavior of the prototype strains in the human UT. (B) Properties of the two strains. UPEC CFT073 was isolated from the blood of a patient with acute pyelonephritis, and its entire genome has been sequenced (72). The properties of E. coli 83972 were compiled from several studies (18, 30, 54, 56, 57).
CONCLUDING REMARKS
The long-term bacterial occupancy of a privileged host niche such as the human bladder must involve adaptations to the host environment. Strain 83972 had grown in the bladder of a girl with ABU for at least 3 years (2). This represents a substantial number of generations, i.e., >30,000, assuming generation times comparable to those observed by us (57). During this period, the strain must have adapted considerably to this particular environmental niche. The strain lost the ability to express functional UPEC class fimbriae, probably as an evolutionary tradeoff with the host defense. This ensured that it did not attract the attention of aggressive host defense mechanisms, such as cytokine production, inflammation, and exfoliation of infected bladder cells. However, in order to avoid being flushed out of the system, it had to adapt to a particular ecological niche, i.e., human urine as a growth medium, and to optimize its growth rate to keep pace with the flow rate in the bladder. Whether this happened during the three-plus years it was carried by the particular girl or before then in other hosts is not possible to conclude. It is interesting, however, that the girl suffered from a voiding problem and was unable to empty her bladder completely, thus leaving a considerable volume of residual urine (2, 37). Arguably, this would provide strain 83972 with an ideal training environment for optimizing its ability to grow in urine. In line with this notion, strain 83972 must have accrued genetic changes that have favored its fitness for growth in urine. Human urine is a very complex growth medium, and the composition of urine fluctuates daily. It is known, however, that iron availability is a limiting factor. The array data indicated that strain 83972 has adapted to growth in this iron-limiting environment by significantly increasing the expression of the majority of all known genes involved in iron uptake and transport. In fact, it has adapted so well to its niche that it can outcompete a wide range of aggressive uropathogens.
Bacterial genomes are under constant change. New genes are acquired by horizontal transfer, and old ones are lost by mutations. It is thought that such changes in genetic repertoire are the primary mechanisms of bacterial adaptation to new environments. Evidence for constant expansion and retraction of genomes has been observed in cases where complete genomes have been sequenced (7, 48). It is generally believed that commensal E. coli can become pathogenic through the acquisition of novel genetic information encoding virulence factors and niche adaptation factors (28). Acquired genes range from single units to large constellations of genes, such as pathogenicity islands or plasmids. The loss of genetic material has been observed to take place via mechanisms ranging from large-scale deletions to single point mutations (7, 41). In contrast to organisms that have acquired genes for pathogenesis, E. coli 83972 is an example of an organism that has adapted to commensalism through gene loss and mutations. The relationship between bacterium and host in a persistent infection is a mutual tradeoff. In this case, the bacterium has lost its primary colonization factors; however, having done so, it does not damage the host and evades immune surveillance. In effect, the strain has become domesticated to a degree where it does not cause any symptoms, i.e., it has become benign.
Acknowledgments
This work was supported by grants from the Danish Medical Research Council (271-06-0555), Lundbeckfonden (2711-45971), the University of Queensland, and the Australian National Health and Medical Research Council (401714).
Editor: J. B. Kaper
Footnotes
Published ahead of print on 14 May 2007.
REFERENCES
- 1.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, P., I. Engberg, G. Lidin-Janson, K. Lincoln, R. Hull, S. Hull, and C. Svanborg. 1991. Persistence of Escherichia coli bacteriuria is not determined by bacterial adherence. Infect. Immun. 59:2915-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur, M., C. E. Johnson, R. H. Rubin, R. D. Arbeit, C. Campanelli, C. Kim, S. Steinbach, M. Agarwal, R. Wilkinson, and R. Goldstein. 1989. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect. Immun. 57:303-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bäckhed, F., B. Alsen, N. Roche, J. Angstrom, A. von Euler, M. E. Breimer, B. Westerlund-Wikstrom, S. Teneberg, and A. Richter-Dahlfors. 2002. Identification of target tissue glycosphingolipid receptors for uropathogenic, F1C-fimbriated Escherichia coli and its role in mucosal inflammation. J. Biol. Chem. 277:18198-18205. [DOI] [PubMed] [Google Scholar]
- 5.Bergsten, G., M. Samuelsson, B. Wullt, I. Leijonhufvud, H. Fischer, and C. Svanborg. 2004. PapG-dependent adherence breaks mucosal inertia and triggers the innate host response. J. Infect. Dis. 189:1734-1742. [DOI] [PubMed] [Google Scholar]
- 6.Boen, J. R., and D. L. Sylwester. 1965. The mathematical relationship among urinary frequency, residual urine, and bacterial growth in bladder infections. Investig. Urol. 15:468-473. [PubMed] [Google Scholar]
- 7.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 8.Connell, H., W. Agace, P. Klemm, M. Schembri, S. Marild, and C. Svanborg. 1996. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA 93:9827-9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 10.Darouiche, R. O., W. H. Donovan, M. Del Terzo, J. I. Thornby, D. C. Rudy, and R. A. Hull. 2001. Pilot trial of bacterial interference for preventing urinary tract infection. Urology 58:339-344. [DOI] [PubMed] [Google Scholar]
- 11.Darouiche, R. O., J. I. Thornby, C. Cerra-Stewart, W. H. Donovan, and R. A. Hull. 2005. Bacterial interference for prevention of urinary tract infection: a prospective, randomized, placebo-controlled, double-blind pilot trial. Clin. Infect. Dis. 41:1531-1534. [DOI] [PubMed] [Google Scholar]
- 12.Dodson, K. W., J. S. Pinkner, T. Rose, G. Magnusson, S. J. Hultgren, and G. Waksman. 2001. Structural basis of the interaction of the pyelonephritic E. coli adhesin to its human kidney receptor. Cell 105:733-743. [DOI] [PubMed] [Google Scholar]
- 13.Eden, C. S., L. A. Hanson, U. Jodal, U. Lindberg, and A. S. Akerlund. 1976. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet i:490-492. [PubMed] [Google Scholar]
- 14.Ferrières, L., V. Hancock, and P. Klemm. 2007. Biofilm exclusion of uropathogenic bacteria by selected asymptomatic bacteriuria Escherichia coli strains. Microbiology 153:1711-1719. [DOI] [PubMed] [Google Scholar]
- 15.Foxman, B. 2002. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am. J. Med. 113(Suppl. 1A):5S-13S. [DOI] [PubMed] [Google Scholar]
- 16.Funfstuck, R., H. Tschape, G. Stein, H. Kunath, M. Bergner, and G. Wessel. 1986. Virulence properties of Escherichia coli strains in patients with chronic pyelonephritis. Infection 14:145-150. [DOI] [PubMed] [Google Scholar]
- 17.Gordon, D. M., and M. A. Riley. 1992. A theoretical and experimental analysis of bacterial growth in the bladder. Mol. Microbiol. 6:555-562. [DOI] [PubMed] [Google Scholar]
- 18.Hancock, V., L. Ferrières, and P. Klemm. 2007. Biofilm formation by asymptomatic and virulent urinary tract infectious Escherichia coli strains. FEMS Microbiol. Lett. 267:30-37. [DOI] [PubMed] [Google Scholar]
- 19.Hancock, V., and P. Klemm. 2007. Global gene expression profiling of asymptomatic bacteriuria Escherichia coli during biofilm growth in human urine. Infect. Immun. 75:966-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansson, S., U. Jodal, K. Lincoln, and C. Svanborgeden. 1989. Untreated asymptomatic bacteriuria in girls. II. Effect of phenoxymethylpenicillin and erythromycin given for intercurrent infections. BMJ 298:856-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedges, S. R., W. W. Agace, and C. Svanborg. 1995. Epithelial cytokine responses and mucosal cytokine networks. Trends Microbiol. 3:266-270. [DOI] [PubMed] [Google Scholar]
- 22.Hedlund, M., R. D. Duan, A. Nilsson, M. Svensson, D. Karpman, and C. Svanborg. 2001. Fimbriae, transmembrane signaling, and cell activation. J. Infect. Dis. 183(Suppl. 1):S47-S50. [DOI] [PubMed] [Google Scholar]
- 23.Hull, R., D. Rudy, W. Donovan, C. Svanborg, I. Wieser, C. Stewart, and R. Darouiche. 2000. Urinary tract infection prophylaxis using Escherichia coli 83972 in spinal cord injured patients. J. Urol. 163:872-877. [PubMed] [Google Scholar]
- 24.Hull, R. A., D. C. Rudy, W. H. Donovan, I. E. Wieser, C. Stewart, and R. O. Darouiche. 1999. Virulence properties of Escherichia coli 83972, a prototype strain associated with asymptomatic bacteriuria. Infect. Immun. 67:429-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, J. R. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 4:80-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, J. R., P. Delavari, M. Kuskowski, and A. L. Stell. 2001. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J. Infect. Dis. 183:78-88. [DOI] [PubMed] [Google Scholar]
- 27.Kaijser, B., and S. Ahlstedt. 1977. Protective capacity of antibodies against Escherichia coli and K antigens. Infect. Immun. 17:286-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 29.Khan, A. S., B. Kniep, T. A. Oelschlaeger, I. Van Die, T. Korhonen, and J. Hacker. 2000. Receptor structure for F1C fimbriae of uropathogenic Escherichia coli. Infect. Immun. 68:3541-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klemm, P., V. Roos, G. C. Ulett, C. Svanborg, and M. A. Schembri. 2006. Molecular characterization of the Escherichia coli asymptomatic bacteriuria strain 83972: the taming of a pathogen. Infect. Immun. 74:781-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klemm, P., and M. A. Schembri. 2000. Bacterial adhesins: function and structure. Int. J. Med. Microbiol. 290:27-35. [DOI] [PubMed] [Google Scholar]
- 32.Klemm, P., and M. A. Schembri. 6 July 2004, posting date. Chapter 8.3.2.6, Type 1 fimbriae, curli, and antigen 43: adhesion, colonization, and biofilm formation. In R. Curtiss III et al. (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. http://www.ecosal.org. [DOI] [PubMed]
- 33.Krogfelt, K. A., H. Bergmans, and P. Klemm. 1990. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect. Immun. 58:1995-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumon, H. 2000. Management of biofilm infections in the urinary tract. World J. Surg. 24:1193-1196. [DOI] [PubMed] [Google Scholar]
- 35.Leffler, H., and C. Svanborg-Eden. 1981. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect. Immun. 34:920-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindberg, U., I. Claesson, L. A. Hanson, and U. Jodal. 1978. Asymptomatic bacteriuria in schoolgirls. VIII. Clinical course during a 3-year follow-up. J. Pediatr. 92:194-199. [DOI] [PubMed] [Google Scholar]
- 37.Lindberg, U., L. A. Hanson, U. Jodal, G. Lidin-Janson, K. Lincoln, and S. Olling. 1975. Asymptomatic bacteriuria in schoolgirls. II. Differences in Escherichia coli causing asymptomatic bacteriuria. Acta Paediatr. Scand. 64:432-436. [DOI] [PubMed] [Google Scholar]
- 38.Mackintosh, I. P., B. J. Hammond, B. W. Watson, and F. O'Grady. 1975. Theory of hydrokinetic clearance of bacteria from the urinary bladder. I. Effect of variations in bacterial growth rate. Investig. Urol. 12:468-472. [PubMed] [Google Scholar]
- 39.Maurelli, A. T. 2007. Black holes, antivirulence genes, and gene inactivation in the evolution of bacterial pathogens. FEMS Microbiol. Lett. 267:1-8. [DOI] [PubMed] [Google Scholar]
- 40.Mobley, H. L. T., D. M. Green, A. L. Trifillis, D. E. Johnson, G. R. Chippendale, C. V. Lockatell, B. D. Jones, and J. W. Warren. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58:1281-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moran, N. A. 2002. Microbial minimalism: genome reduction in bacterial pathogens. Cell 108:583-586. [DOI] [PubMed] [Google Scholar]
- 42.Morris, N. S., D. J. Stickler, and R. J. C. McLean. 1999. The development of bacterial biofilms on indwelling urethral catheters. World J. Urol. 17:345-350. [DOI] [PubMed] [Google Scholar]
- 43.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494-1497. [DOI] [PubMed] [Google Scholar]
- 44.Nicolle, L. E. 2006. Asymptomatic bacteriuria: review and discussion of the IDSA guidelines. Int. J. Antimicrob. Agents 28S:S42-S48. [DOI] [PubMed] [Google Scholar]
- 45.Oelschlaeger, T. A., U. Dobrindt, and J. Hacker. 2002. Virulence factors of uropathogens. Curr. Opin. Urol. 12:33-38. [DOI] [PubMed] [Google Scholar]
- 46.Orskov, I., C. Svanborg Eden, and F. Orskov. 1988. Aerobactin production of serotyped Escherichia coli from urinary tract infections. Med. Microbiol. Immunol. (Berlin) 177:9-14. [DOI] [PubMed] [Google Scholar]
- 47.Pere, A., B. Nowicki, H. Saxen, A. Siitonen, and T. K. Korhonen. 1987. Expression of P, type 1, and type 1C fimbriae of Escherichia coli in the urine of patients with acute urinary tract infection. J. Infect. Dis. 156:567-574. [DOI] [PubMed] [Google Scholar]
- 48.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 49.Plos, K., T. Carter, S. Hull, R. Hull, and C. Svanborg Eden. 1990. Frequency and organization of pap homologous DNA in relation to clinical origin of uropathogenic Escherichia coli. J. Infect. Dis. 161:518-524. [DOI] [PubMed] [Google Scholar]
- 50.Plos, K., H. Connell, U. Jodal, B. I. Marklund, S. Marild, B. Wettergren, and C. Svanborg. 1995. Intestinal carriage of P fimbriated Escherichia coli and the susceptibility to urinary tract infection in young children. J. Infect. Dis. 171:625-631. [DOI] [PubMed] [Google Scholar]
- 51.Reid, G., J. D. Denstedt, Y. S. Kang, D. Lam, and C. Nause. 1992. Microbial adhesion and biofilm formation on ureteral stents in vitro and in vivo. J. Urol. 148:1592-1594. [DOI] [PubMed] [Google Scholar]
- 52.Roberts, J. A., M. B. Kaack, G. Baskin, M. R. Chapman, D. A. Hunstad, J. S. Pinkner, and S. J. Hultgren. 2004. Antibody responses and protection from pyelonephritis following vaccination with purified Escherichia coli PapDG protein. J. Urol. 171:1682-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts, J. A., B. I. Marklund, D. Ilver, D. Haslam, M. B. Kaack, G. Baskin, M. Louis, R. Mollby, J. Winberg, and S. Normark. 1994. The Gal(alpha 1-4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc. Natl. Acad. Sci. USA 91:11889-11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roos, V., and P. Klemm. 2006. Global gene expression profiling of the asymptomatic bacteriuria Escherichia coli strain 83972 in the human urinary tract. Infect. Immun. 74:3565-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roos, V., E. M. Nielsen, and P. Klemm. 2006. Asymptomatic bacteriuria Escherichia coli strains: adhesins, growth and competition. FEMS Microbiol. Lett. 262:22-30. [DOI] [PubMed] [Google Scholar]
- 56.Roos, V., M. A. Schembri, G. C. Ulett, and P. Klemm. 2006. Asymptomatic bacteriuria Escherichia coli strain 83972 carries mutations in the foc locus and is unable to express F1C fimbriae. Microbiology 152:1799-1806. [DOI] [PubMed] [Google Scholar]
- 57.Roos, V., G. C. Ulett, M. A. Schembri, and P. Klemm. 2006. The asymptomatic bacteriuria Escherichia coli strain 83972 outcompetes uropathogenic E. coli strains in human urine. Infect. Immun. 74:615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samuelsson, P., L. Hang, B. Wullt, H. Irjala, and C. Svanborg. 2004. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect. Immun. 72:3179-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shand, D. G., J. C. MacKenzie, W. R. Cattell, and J. Cato. 1968. Estimation of residual urine volume with 131 I-hippuran. Br. J. Urol. 40:196-201. [DOI] [PubMed] [Google Scholar]
- 60.Siitonen, A., R. Martikainen, R. Ikäheimo, J. Palmgren, and P. H. Mäkelä. 1993. Virulence-associated characteristics of Escherichia coli in urinary tract infection: a statistical analysis with special attention to type 1C fimbriation. Microb. Pathog. 15:65-75. [DOI] [PubMed] [Google Scholar]
- 61.Snyder, J. A., B. J. Haugen, E. L. Buckles, C. V. Lockatell, D. E. Johnson, M. S. Donnenberg, R. A. Welch, and H. L. Mobley. 2004. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 72:6373-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sokurenko, E. V., H. S. Courtney, J. Maslow, A. Siitonen, and D. L. Hasty. 1995. Quantitative differences in adhesiveness of type 1 fimbriated Escherichia coli due to structural differences in fimH genes. J. Bacteriol. 177:3680-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sokurenko, E. V., H. S. Courtney, D. E. Ohman, P. Klemm, and D. L. Hasty. 1994. FimH family of type 1 fimbrial adhesins: functional heterogeneity due to minor sequence variations among fimH genes. J. Bacteriol. 176:748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sokurenko, E. V., M. Feldgarden, E. Trintchina, S. J. Weissman, S. Avagyan, S. Chattopadhyay, J. R. Johnson, and D. E. Dykhuizen. 2004. Selection footprint in the FimH adhesin shows pathoadaptive niche differentiation in Escherichia coli. Mol. Biol. Evol. 21:1373-1383. [DOI] [PubMed] [Google Scholar]
- 65.Soto, S. M., A. Smithson, J. P. Horcajada, J. A. Martinez, J. P. Mensa, and J. Vila. 2006. Implication of biofilm formation in the persistence of urinary tract infection caused by uropathogenic Escherichia coli. Clin. Microbiol. Infect. 12:1034-1036. [DOI] [PubMed] [Google Scholar]
- 66.Sotolongo, J. R., and N. Koleilat. 1990. Significance of asymptomatic bacteriuria in spinal-cord injury patients on condom catheter. J. Urol. 143:979-980. [DOI] [PubMed] [Google Scholar]
- 67.Stenqvist, K., T. Sandberg, G. Lidin-Janson, F. Orskov, I. Orskov, and C. Svanborg-Eden. 1987. Virulence factors of Escherichia coli in urinary isolates from pregnant women. J. Infect. Dis. 156:870-877. [DOI] [PubMed] [Google Scholar]
- 68.Sundén, F., L. Håkansson, E. Ljunggren, and B. Wullt. 2006. Bacterial interference—is deliberate colonization with Escherichia coli 83972 an alternative treatment for patients with recurrent urinary tract infection? Int. J. Antimicrob. Agents 28S:S26-S29. [DOI] [PubMed] [Google Scholar]
- 69.Svanborg-Eden, C., B. Eriksson, L. A. Hanson, U. Jodal, B. Kaijser, G. L. Janson, U. Lindberg, and S. Olling. 1978. Adhesion to normal human uroepithelial cells of Escherichia coli from children with various forms of urinary tract infection. J. Pediatr. 93:398-403. [DOI] [PubMed] [Google Scholar]
- 70.Trautner, B. W., R. O. Darouiche, R. A. Hull, S. Hull, and J. I. Thornby. 2002. Pre-inoculation of urinary catheters with Escherichia coli 83972 inhibits catheter colonization by Enterococcus faecalis. J. Urol. 167:375-379. [PMC free article] [PubMed] [Google Scholar]
- 71.Trautner, B. W., R. A. Hull, and R. O. Darouiche. 2003. Escherichia coli 83972 inhibits catheter adherence by a broad spectrum of uropathogens. Urology 61:1059-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu, X. R., T. T. Sun, and J. J. Medina. 1996. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc. Natl. Acad. Sci. USA 93:9630-9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wullt, B., G. Bergsten, H. Fischer, G. Godaly, D. Karpman, I. Leijonhufvud, A. C. Lundstedt, P. Samuelsson, M. Samuelsson, M. L. Svensson, and C. Svanborg. 2003. The host response to urinary tract infection. Infect. Dis. Clin. N. Am. 17:279-301. [DOI] [PubMed] [Google Scholar]
- 75.Wullt, B., H. Connell, P. Rollano, W. Mansson, S. Colleen, and C. Svanborg. 1998. Urodynamic factors influence the duration of Escherichia coli bacteriuria in deliberately colonized cases. J. Urol. 159:2057-2062. [DOI] [PubMed] [Google Scholar]