Abstract
The pathogenicity of Mycobacterium ulcerans, the agent of Buruli ulcer, depends on the cytotoxic exotoxin mycolactone. Little is known about the immune response to this pathogen. Following the demonstration of an intracellular growth phase in the life cycle of M. ulcerans, we investigated the production of tumor necrosis factor (TNF) induced by intramacrophage bacilli of diverse toxigenesis/virulence, as well as the biological relevance of TNF during M. ulcerans experimental infections. Our data show that murine bone marrow-derived macrophages infected with mycolactone-negative strains of M. ulcerans (nonvirulent) produce high amounts of TNF, while macrophages infected with mycolactone-positive strains of intermediate or high virulence produce intermediate or low amounts of TNF, respectively. These results are in accordance with the finding that TNF receptor P55-deficient (TNF-P55 KO) mice are not more susceptible than wild-type mice to infection by the highly virulent strains but are more susceptible to nonvirulent and intermediately virulent strains, demonstrating that TNF is required to control the proliferation of these strains in animals experimentally infected by M. ulcerans. We also show that mycolactone produced by intramacrophage M. ulcerans bacilli inhibits, in a dose-dependent manner, but does not abrogate, the production of macrophage inflammatory protein 2, which is consistent with the persistent inflammatory responses observed in experimentally infected mice.
Mycobacterium ulcerans is the etiological agent of Buruli ulcer (BU), a devastating, necrotizing skin disease that has increased dramatically over the past decade and has become the third most common mycobacterial infection in humans, after tuberculosis and leprosy (15, 85). In some African tropical areas, the number of BU cases may even exceed those of tuberculosis and leprosy (16, 86). BU has a huge socioeconomic impact in the affected populations and represents an important public health issue in terms of morbidity, treatment, and functional disabilities (3). In addition to the increase in the actual number of cases, there has also been an increasing geographical spread of BU within some tropical countries (86).
Mycobacteria have developed several mechanisms to avoid the harmful effects of the host immune response, including the secretion of soluble factors that have cytotoxic activity against the host cells, the impairment of the bactericidal activity of macrophages, or the modulation of the immune response. Mycobacterium tuberculosis, a mycobacterium closely related to M. ulcerans (83), secretes culture filtrate protein 10, early secretory antigen target 6, and Man-LAM that exhibit cytotoxic activities against the infected cells or inhibit the production of macrophage-activating cytokines, such as tumor necrosis factor (TNF) (29, 45, 51, 77, 81). Furthermore, it has been suggested that M. tuberculosis induces an increased production of interleukin-10 (IL-10), a cytokine with immunosuppressive activity (34). M. ulcerans also exhibits cytotoxic activity (32, 50, 56, 67). However, unlike M. tuberculosis, M. ulcerans cytotoxicity is primarily associated with the production of a unique polyketide lipid exotoxin, mycolactone (32, 33). The genetic basis for mycolactone production was elucidated with the discovery of a giant plasmid that carries all the genes required for mycolactone production (80). It is also known that M. ulcerans isolates from different geographical origins produce diverse types of mycolactones (56). The African strains produce mainly mycolactones A/B, whereas Australian strains produce mainly mycolactone C (56). These mycolactones are very similar, with the exception that mycolactone C has one less hydroxyl group on C-12 of the fatty acid side chain (46, 79). Despite this sole difference between mycolactones A/B and C, the latter has been shown to be less cytopathic (46, 56). The different profiles in mycolactone production might contribute to the lower severity of BU disease observed in Australia compared to that in Africa (46). However, it cannot be excluded that production of different amounts of mycolactone may also account for the different degrees of virulence among M. ulcerans strains.
In the mouse fibroblast L929 cell line, mycolactone has a cytopathic effect characterized by cell cycle arrest, cell rounding, detachment from the monolayer and, ultimately, apoptosis (31-33, 56). Injection of mycolactone in the guinea pig skin or infection with wild-type (WT) mycolactone-producing M. ulcerans induces the formation of ulcers, while infection with a mycolactone-negative mutant strain fails to induce ulceration in this animal model (32). The pathology of M. ulcerans infections is therefore closely associated with the secretion of this exotoxin, which is primarily responsible for the extensive necrosis that characterizes BU. Additionally, it has been shown that mycolactone inhibits TNF production (60).
TNF is an effector cytokine produced mainly by macrophages and has an autocrine effect on the activation of the macrophage's microbicidal activity against intracellular parasites, including Listeria monocytogenes (39), M. tuberculosis (17, 26), Mycobacterium bovis BCG (48), and some strains of Mycobacterium avium (69). TNF is, therefore, critical for both the antibacterial and the inflammatory host responses against mycobacteria (25). TNF and other cytokines and chemokines produced by macrophages, such as IL-6, IL-1, and macrophage inflammatory protein 2 (MIP-2) (the murine homologue of human IL-8), have an impact on the recruitment of inflammatory cells (5, 18), as well as on the activation and phenotype of T cells (61, 73).
It has been recently shown that M. ulcerans has an intramacrophage growth phase (84), which is in accordance with the occurrence of cell-mediated immunity and delayed-type hypersensitivity responses in BU patients (20, 35, 36, 40, 41, 49, 53, 54, 58, 64, 66, 76, 78, 82, 86, 87). The correlation between the amounts of TNF produced by macrophages infected with different strains of M. ulcerans and the mycolactones produced by these strains, as well as the biological relevance of TNF in the progression of infection by M. ulcerans, is currently unknown. A previous report showed that a lipidic fraction of M. ulcerans culture filtrates containing mycolactone suppressed in vitro the production of TNF by human monocytes (60). On the other hand, the expression of TNF is detected in high concentrations in the lesions of BU patients (66). Furthermore, TNF is known to participate in the formation of granulomas (25), which in BU has been shown to occur during the healing phase of the disease (1, 40, 41, 78, 86).
Taking these data into consideration, it is important to study the effects of infection with different strains of M. ulcerans on the cytokine production by macrophages, as well as to assess the role of TNF in M. ulcerans infection in vivo. A panel of strains of M. ulcerans that produce different types of mycolactone or that are mycolactone negative was used in the present work in order to characterize the production of both the proinflammatory cytokine TNF and the chemokine MIP-2 by infected bone marrow-derived macrophages (BMDM). In addition, the biological relevance of TNF production in vivo was assessed by using TNF receptor P55-deficient (TNF-P55 KO) mice infected with different strains of M. ulcerans. To further dissect the role of mycolactone on the production of TNF by M. ulcerans-infected macrophages, we compared WT M. ulcerans with isogenic mutants in which the mycolactone genes were deleted or interrupted by transposon insertion.
We found that BMDM infected with highly virulent but not with intermediately virulent or nonvirulent strains of M. ulcerans produced low levels of TNF. In addition, we show that mycolactone inhibits TNF production, in a dose-dependent manner. Interestingly, MIP-2 production was decreased but not abrogated, even in macrophages infected with highly virulent strains. Finally, our data show that TNF induced during M. ulcerans infection plays a protective role.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The M. ulcerans strains used in this study were selected based on their geographical origin and on the type of mycolactone produced (Table 1). Strain 97-1116 is an isolate from Benin that produces a mycolactone A/B that is characteristic of African strains (57). Strain 98-912 was isolated from a Chinese patient and produces a mycolactone A/B slightly different from those in African strains (44). Strain 94-1327 is an isolate from Australia and produces the characteristic mycolactone C (56); its plasmid lacks the gene MUP053 encoding a P450 hydroxylase required to hydroxylate the mycolactone side chain at C-12 to produce mycolactone A/B (79). Strain 94-1331 is an isolate from Papua New Guinea that is similar to the mycolactone C-producing Australian strains in the sense that its plasmid also lacks the gene MUP053 that is necessary for mycolactone A/B production (79), although it was suggested that this strain can also produce mycolactone A/B (43). Strain 5114 is an isolate from Mexico and does not produce mycolactone (56) due to the loss of key genes involved in the synthesis of this macrolide (79). Mycobacterium marinum 00-1026, obtained from a patient living in France, is closely related genetically to M. ulcerans and was used as a negative control for mycolactone production (8, 89). Finally, the mycolactone-defective M. ulcerans 1615A, a spontaneous mutant, was isolated from nonpigmented colonies of M. ulcerans 1615 (32), a strain from Malaysia that produces mycolactone A/B (56). All strains used in this work, except for strain 1615, are from the collection of the Institute of Tropical Medicine, Antwerp, Belgium.
TABLE 1.
Mycobacterial strains
| Species | Strain | BU clinical form | Geographical origin | Yr of isolation | Type of mycolactone |
|---|---|---|---|---|---|
| M. ulcerans | 98-912 | Ulcer | China | 1997 | A/Ba |
| M. ulcerans | 97-1116 | Plaque | Benin | 1997 | A/B |
| M. ulcerans | 1615 | Ulcer | Malaysia | 1964 | A/B |
| M. ulcerans | 94-1331 | NDc | Papua New Guinea | 1994 | A/Bb |
| M. ulcerans | 94-1327 | Ulcer | Australia | 1994 | C |
| M. ulcerans | 5114 | Ulcer | Mexico | 1953 | − |
| M. marinum | 00-1026 | −d | France | 2000 | − |
Mycolactone A/B slightly different from those in African strains (44).
Like mycolactone C-producing Australian strains, this strain lacks the plasmid gene MUP053 that is involved in mycolactone A/B production (79), although it has been suggested that it also produces mycolactone A/B (43).
ND, not determined.
−, negative result.
The isolates were grown on Löwenstein-Jensen medium at 32°C for approximately 1 month, recovered from slants, diluted in phosphate-buffered saline to a final concentration of 1 mg/ml, and vortexed using 2-mm glass beads. The number of acid-fast bacilli (AFB) in the inocula was determined according to the method described by Shepard and McRae (72), using Ziehl-Neelsen staining (Merck, Darmstadt, Germany). The final suspensions revealed more than 90% viable cells as assessed with a LIVE/DEAD Baclight kit (Molecular Probes, Leiden, The Netherlands).
Animals.
Eight-week-old female BALB/c, C57BL/6, and TNF-P55 KO mice were obtained from Charles River (Barcelona, Spain) and were housed under specific-pathogen-free conditions with food and water ad libitum.
Footpad model of infection.
Mice were infected in the left hind footpad with 0.03 ml of M. ulcerans suspension containing 5 log10 AFB. The right hind footpad was used as a control.
Culture of murine BMDM.
Macrophages were derived from the bone marrow as follows: mice were sacrificed with CO2 and femurs removed under aseptic conditions. Bones were flushed with 5 ml cold Hanks' balanced salt solution (HBSS; Gibco, Paisley, United Kingdom). The resulting cell suspension was centrifuged at 500 × g and resuspended in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10 mM HEPES (Sigma, St. Louis, MO), 1 mM sodium pyruvate (Gibco), 10 mM glutamine (Gibco), 10% heat-inactivated fetal bovine serum (Sigma), and 10% L929 cell conditioned medium (complete DMEM [cDMEM]). To remove fibroblasts or differentiated macrophages, cells were cultured for a period of 4 h on cell culture dishes (Nunc, Naperville, IL) with cDMEM. Nonadherent cells were collected with warm HBSS, centrifuged at 500 × g, distributed in 24-well plates at a density of 5 × 105 cells/well, and incubated at 37°C in a 5% CO2 atmosphere. On day 4 after seeding, 0.1 ml of L929 cell conditioned medium was added, and medium was renewed on the seventh day. After 10 days in culture, cells were completely differentiated into macrophages. Twelve hours before infection, macrophages were incubated at 32°C in a 5% CO2 atmosphere and maintained until the end of the experimental infection as described elsewhere (59).
Macrophage infectivity assays.
Bacterial suspensions were prepared as described above and further diluted in cDMEM before infecting macrophage monolayers. M. ulcerans suspensions (0.2 ml) were diluted in cDMEM and added to each well in order to obtain the multiplicity of infection (MOI) indicated for each experiment (bacterium/macrophage ratio). Cells were incubated for 4 h at 32°C in a 5% CO2 atmosphere and then washed four times with warm HBSS to remove noninternalized bacteria and reincubated in cDMEM for a maximum period of 6 days. To confirm the MOI, counting of AFB in infected macrophages was performed at the beginning of experimental infection as described previously (72).
Mycolactone purification.
After purification of mycolactone as described elsewhere (32, 56), the toxin was dissolved in ethanol (100%) and stored at 4°C. The toxin was added to cultured cells by diluting the original preparation in culture medium (DMEM) to a maximum concentration of 0.001% of ethanol. In addition, the wells with control, noninfected macrophages were kept with the same amount of diluted ethanol that was found to be noncytotoxic for macrophages at this residual concentration.
Cytokine analysis by ELISA.
At selected time points postinfection, macrophage cultures were centrifuged and the supernatants removed and stored frozen until cytokine analysis by enzyme-linked immunosorbent assay (ELISA). TNF and MIP-2 were measured in the culture supernatant using commercial kits (R&D Systems, Minneapolis, MN) according to the manufacturer's specifications.
Statistical analysis.
Statistical significance of values was determined using the Student t test.
RESULTS
Different strains of M. ulcerans exhibit diverse cytotoxicity in vitro that correlates with mycolactone production and with virulence for mice.
It was previously shown that mycolactone A/B-producing M. ulcerans bacilli exhibit cytotoxic activity against infected macrophages (59), following an intramacrophage phase of proliferation (84). These same strains induced progressive infections following experimental inoculation of mouse footpads (59). On the other hand, a mycolactone-negative strain was shown to be noncytotoxic and unable to induce lesions in infected footpads (59). In the present work, we expanded our observations by evaluating the cytotoxic activities of a panel of M. ulcerans strains that produce diverse types of mycolactone or are nonproducers of mycolactone, using a model of BMDM infection at 32°C. Additionally, we investigated the association between cytotoxicity and virulence for experimentally infected mice.
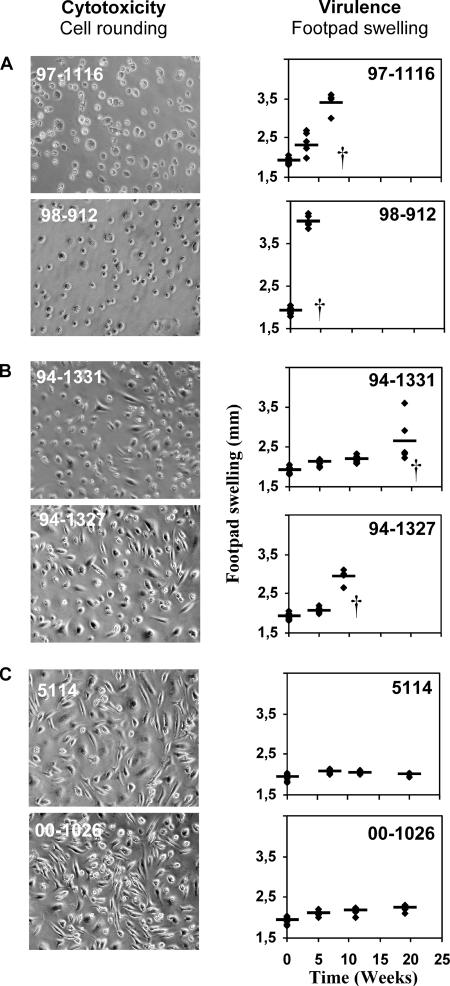
Macrophages were infected at a 1:1 MOI with the mycolactone-negative strain 5114 or with mycolactone-producing strain 94-1331, 94-1327, 97-1116, or 98-912. As a control, macrophages were infected with M. marinum 00-1026, which does not produce mycolactone. The cytotoxicity of M. ulcerans was assessed by the occurrence of the typical cytopathic changes induced in cultured cells either by isolated mycolactone (31-33) or by intracellular infection with mycolactone-producing M. ulcerans bacilli (59, 84). Those cytopathic changes include cell rounding and detachment from the monolayer followed by cell death (31-33). The evaluation of virulence was performed by measuring over time of the swelling of footpads infected with 5.3 log10 AFB of M. ulcerans (59).
Confirming our previous observations (59), we found that the M. ulcerans strains 98-912 and 97-1116, which produce mycolactone A/B, were highly cytotoxic, inducing cell rounding, shrinkage, and detachment of more than 90% of cultured macrophages at 6 days postinfection (Fig. 1A), and highly virulent, as shown by the occurrence of footpad swelling between the second and fourth weeks following infection (Fig. 1A). In contrast, no significant alterations were found in monolayers infected with the noncytotoxic, mycolactone-negative strain 5114 or with M. marinum 00-1026 (Fig. 1C), which also did not induce measurable footpad swelling up to 25 weeks postinfection (Fig. 1C). The Australian mycolactone C-producing strain, M. ulcerans 94-1327, showed intermediate cytotoxic activity in vitro and intermediate virulence for mice (Fig. 1B). Interestingly, strain 94-1331 from Papua New Guinea, which, like Australian strains, lacks the gene MUP053 that encodes the P450 hydroxylase necessary to hydroxylate C-12 to form mycolactone A/B, also showed an intermediate pattern of in vitro cytotoxicity and virulence (Fig. 1B).
FIG. 1.
Cytotoxic activity and virulence of different strains of M. ulcerans. (Left) BMDM were infected with different strains of M. ulcerans at an MOI (bacilli/macrophages) of 1:1. Macrophages were photographed by phase-contrast microscopy 6 days after infection. M. ulcerans cytotoxicity was determined based on the microscopic observation of cell rounding and detachment from the monolayer. (Right) Mice were infected in the left hind footpad with 5.3 log10 AFB of different M. ulcerans strains. Virulence was determined by measuring footpad swelling. For ethical reasons mice were sacrificed after the emergence of ulceration. Results are from one representative experiment out of three independent experiments.
These results confirm the importance of mycolactone as the key pathogenic factor of M. ulcerans, showing a correlation between mycolactone production and cytotoxic activity for infected cells and virulence for the host.
BMDM infected with strains of M. ulcerans of diverse cytotoxicity/virulence produce different amounts of TNF.
Macrophages are key elements in the immune response against intracellular parasites. It is known that among other factors, the autocrine activity of TNF on infected macrophages is critical to control the proliferation of the intracellular mycobacterium M. tuberculosis (17, 26) and M. avium (69). It was recently shown that M. ulcerans has a phase of intramacrophage residence and proliferation before it induces the mycolactone-dependent lysis of infected host cells (84). It is therefore important to study the production of TNF by macrophages infected with different clinical isolates of M. ulcerans.
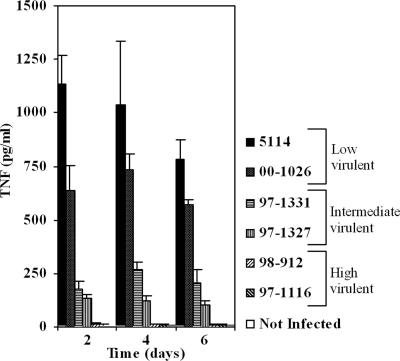
Following the characterization of cytotoxicity/virulence of the panel of M. ulcerans strains, we found that infection of BMDM at a 1:1 MOI with those strains led to the production of different amounts of TNF. TNF production was highest in BMDM infected with the mycolactone-negative, nonvirulent strain 5114 or M. marinum 00-1026. In cells infected with highly cytotoxic/virulent strains that produce mycolactone type A/B, TNF levels were lower than 100 pg/ml (Fig. 2). Accordingly, TNF production by strains of M. ulcerans of intermediate cytotoxicity/virulence was intermediate (Fig. 2). To investigate if the reduced production of TNF by macrophages infected with mycolactone A/B-producing M. ulcerans strains was associated with a decreased production of other macrophage-derived proinflammatory cytokines, we evaluated the expression of IL-1 and IL-6. Expression of high amounts of both cytokines occurred in BMDM infected with the mycolactone-negative strain 5114 but not with the mycolactone A/B-producing strain 98-912 (data not shown).
FIG. 2.
TNF production by BMDM infected with strains of M. ulcerans that produce different amounts and types of mycolactone. Strains of M. ulcerans from different origins were used to infect BMDM at an MOI of 1:1. At days 2, 4, and 6 postinfection the supernatants of three independent wells were removed for each strain and frozen until cytokine measurement by ELISA. Low amounts of TNF were produced by macrophages infected with high cytotoxic/virulent strains (98-912 and 97-1116), whereas high amounts of TNF were produced in the case of the low cytotoxicity/low virulence strain 5114 or M. marinum 00-1026. Intermediate levels of TNF are produced with strains of intermediate cytotoxicity/virulence (94-1327 and 94-1331). Error bars indicate standard deviations. Results are from one representative experiment out of three independent experiments.
These results show that the production of proinflammatory cytokines such as TNF by BMDM infected with M. ulcerans is dependent on the strain's mycolactone-associated toxicity.
The reduced production of TNF by BMDM infected with mycolactone A/B-producing strains is not related to an early cytotoxic effect or to cell death.
It has been previously shown that at high MOI, mycolactone-producing M. ulcerans strains exhibit an early cytotoxic effect on cultured cells (13, 84). However, the damage to monolayers was found to be prevented when the experimental infection was performed at a low MOI (84).
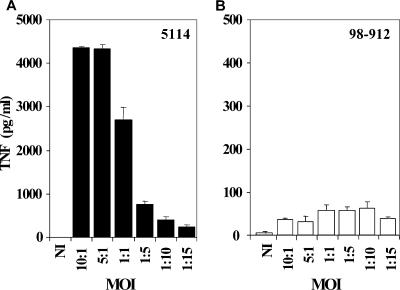
To evaluate if the inhibition of TNF production by the mycolactone A/B-producing strains was due to an early cytotoxic effect on BMDM, monolayers were infected with different MOIs of M. ulcerans 98-912 or M. ulcerans 5114, as a control. As shown in Fig. 3A, production of high amounts of TNF was found at high MOIs in macrophages infected with M. ulcerans 5114, which diminished as the mycobacterium/macrophage ratio decreased. In contrast, infection with strain 98-912 was not associated with high amounts of TNF production by macrophages, even at a very low MOI (Fig. 3B), for which cytotoxic activity was not observed (data not shown). Additionally, we did not find significant levels of apoptosis in infected macrophages 24 h after infection and macrophages were metabolically active, since they could phagocytose latex beads (data not shown). These results suggest that the very low production of TNF by BMDM infected with the highly virulent strain M. ulcerans 98-912 is not associated with an early death of macrophages but possibly to an inhibitory effect of mycolactone.
FIG. 3.
TNF production by BMDM infected at different MOIs with the mycolactone A/B-producing strain 98-912 or the mycolactone-negative strain 5114. BMDM were infected at MOIs ranging from 10:1 to 1:15 with the M. ulcerans strains 5114 (A) or 98-912 (B). Twenty-four hours postinfection, the supernatant from three independent wells was removed for each strain and stored frozen until cytokine measurement by ELISA. BMDM infected with M. ulcerans 5114 produce high amounts of TNF at high MOIs, and TNF production decreases as the MOI decreases. With M. ulcerans 98-912, production of TNF is low, independent of the MOI. Results are from one representative experiment out of three independent experiments.
Addition of mycolactone A/B to BMDM infected with a mycolactone-defective strain inhibits or completely suppresses the production of TNF in a dose-dependent manner.
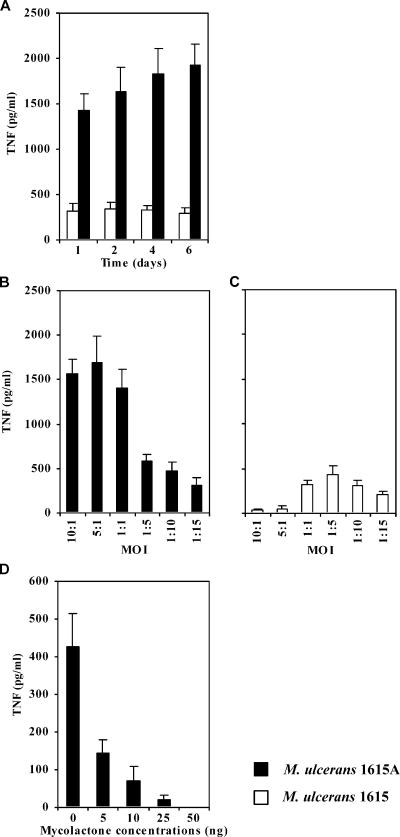
To address the contribution of mycolactone to the inhibition of TNF production by M. ulcerans-infected BMDM, we performed experiments using isogenic strains: the WT, mycolactone A/B-producing strain M. ulcerans 1615, which is virulent for mice (13), and the isogenic mycolactone-deficient, nonvirulent strain, M. ulcerans 1615A (32).
In Fig. 4A, we show that, as previously found for the mycolactone-negative strain 5114 (Fig. 2 and 3), high levels of TNF were produced by macrophages infected with the mycolactone-defective strain 1615A compared to WT, mycolactone-positive M. ulcerans. The high levels of TNF produced by macrophages infected with the mutant strain were particularly significant for MOIs of 1:1 or higher (Fig. 4B). On the contrary, much lower amounts of TNF were produced by macrophages infected with WT M. ulcerans 1615 at high MOI (Fig. 4C). In fact, for a 1:1 MOI, the reduction of the amount of TNF produced was 4.5-fold, but at an MOI of 5:1 the reduction was 33-fold, comparing the WT with the mutant strain. These results strongly suggest that mycolactone plays a major role in the inhibition of TNF production by M. ulcerans-infected BMDM in a dose-dependent manner. To confirm if this inhibitory effect was in fact mycolactone dependent, we infected BMDM with M. ulcerans 1615A in the presence of different amounts of mycolactone A/B. Figure 4D shows that the addition of mycolactone inhibited the TNF production induced by the M. ulcerans mutant strain in a dose-dependent manner. The addition of 5 ng of mycolactone, the minimal dose tested, reduced significantly the production of TNF, and the highest dose tested (50 ng) abrogated the production of this cytokine (Fig. 4D).
FIG. 4.
TNF production by BMDM infected with WT M. ulcerans 1615 or with the mycolactone-defective mutant 1615A: kinetics and effects of MOI and supplementation with mycolactone. BMDM were infected with a 1:1 MOI (A and D) or at different MOIs ranging from 10:1 to 1:15 (B and C) with M. ulcerans 1615A or 1615 WT, in the presence (D) or absence (A to C) of mycolactone. For cytokine measurement by ELISA, supernatants of three independent wells were collected from each experimental group. (A) Higher amounts of TNF are produced with infection by M. ulcerans 1615A in comparison to M. ulcerans 1615. (B and C) BMDM infected with M. ulcerans 1615A produce large amounts of TNF at high MOIs, and that production declines as the MOI is decreased. M. ulcerans 1615 induces the production of smaller amounts of TNF peaking at low MOI. (D) Addition of mycolactone to M. ulcerans 1615A-infected macrophages diminishes or abrogates the production of TNF. TNF was not detected in the presence of 50 ng mycolactone. Results are from one representative experiment out of two independent experiments.
Overall, these results demonstrate that mycolactone plays a major role in the inhibition of TNF production by BMDM infected with virulent M. ulcerans.
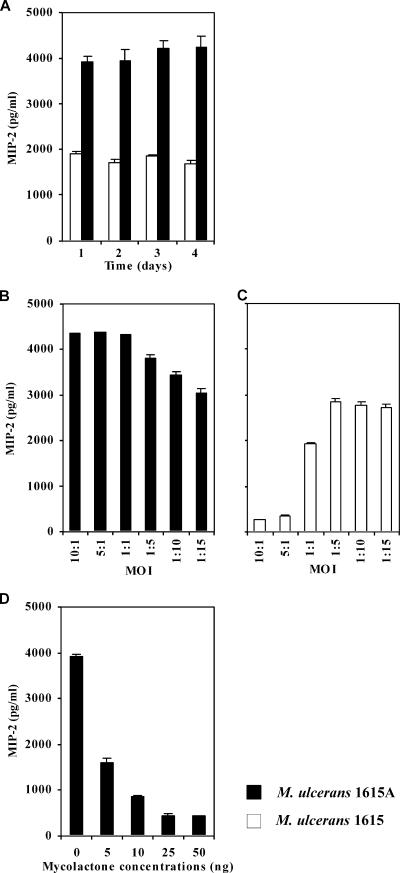
Addition of mycolactone A/B to BMDM infected with a mycolactone-defective strain moderates the production of MIP-2 in a dose-dependent manner but does not abrogate its production.
In contrast with previous descriptions, recent results described inflammatory responses with infiltration of neutrophils and mononuclear cells in a high percentage of cases of M. ulcerans infection in humans (38, 84). In addition, it was shown in the mouse model that M. ulcerans infection induces a persistent inflammatory response, with the recruitment of neutrophils and macrophages to the infection focus (59). We hypothesized that the recruitment of inflammatory cells in response to infections by M. ulcerans strains associated with low levels of TNF production could be mediated by macrophage-derived chemokines, such as MIP-2. MIP-2 is a chemokine associated with the recruitment of neutrophils and is produced mainly by macrophages (88).
To address this point, we measured the production of MIP-2 following the infection of BMDM with M. ulcerans 1615 or 1615A. As shown in Fig. 5A, we found a relevant production of MIP-2 by BMDM infected at a 1:1 MOI, with either strain, although with higher values for the mycolactone-defective strain. For low MOIs, both M. ulcerans 1615 as well as the mutant strain defective in mycolactone induced the production of high levels of MIP-2 (Fig. 5B and C). In BMDM infected with the WT strain, low levels of MIP-2 production were only found at high MOIs (Fig. 5C). In fact, at 1:1 and 1:5 MOIs, the reduction of MIP-2 production by BMDM infected with M. ulcerans 1615 was 2.2- and 1.3-fold, respectively, compared to the strain defective in mycolactone. Accordingly, the addition of increasing amounts of mycolactone to BMDM cultures only induced a moderate decrease in the production of MIP-2 (Fig. 5D), compared to the effect reported above for TNF production (Fig. 4C). Moreover, this chemokine was still produced in relevant amounts after the addition of the maximal dose of mycolactone tested.
FIG. 5.
MIP-2 production by BMDM infected with WT M. ulcerans 1615 or with the mycolactone-defective mutant 1615A: kinetics and effects of MOI and supplementation with mycolactone. BMDM were infected with a 1:1 MOI (A and D) or with MOIs ranging from 10:1 to 1:15 (B and C) of M. ulcerans strain 1615A or 1615, in the presence (D) or absence (A to C) of mycolactone. For cytokine measurement by ELISA, supernatants of three independent wells were collected from each experimental group. (A) Although M. ulcerans 1615A induces larger amounts of MIP-2 in comparison with M. ulcerans 1615, significant amounts of the cytokine are produced with the mycolactone-positive strain. (B and C) BMDM infected with M. ulcerans 1615A produce large amounts of MIP-2 irrespective of MOI. M. ulcerans 1615 induces the production of small but important amounts of MIP-2 at low MOIs. (D) Addition of mycolactone to M. ulcerans 1615A-infected macrophages diminishes but does not abrogate the production of MIP-2, even at high concentrations. Results are from one representative experiment out of two independent experiments.
Overall, these results show that the infection of macrophages with a virulent strain of M. ulcerans induces the production of important amounts of MIP-2, even at a high MOI, which is in accordance with the occurrence of the inflammatory responses observed in BU patients and experimentally infected mice.
TNF plays a role in the control of infections by M. ulcerans.
Studies in the mouse model have shown that TNF is a critical cytokine for protective immune responses against M. tuberculosis (25). This cytokine has been reported to be produced in BU lesions (64, 66); however, its biological relevance following M. ulcerans infection is not known.
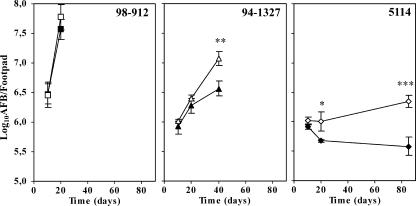
Following the characterization of TNF production by macrophages infected with M. ulcerans, we tested the biological role of this cytokine in an experimental model of BU. WT or TNF-P55 KO mice were infected with different strains of M. ulcerans. The proliferation of mycobacteria in the infected footpads was monitored for a period of 3 months. The growth of strain 5114, which was found to be a strong inducer of TNF, was hindered in vivo by WT mice but not by TNF-P55 KO mice (Fig. 6). For this M. ulcerans strain, the increase in the bacterial proliferation between TNF-P55 KO and WT mice was of 0.8 log10 over an 85-day period. In contrast, M. ulcerans 98-912, which is not associated with the production of relevant amounts of TNF in vitro, showed similar patterns of mycobacterial proliferation in both mouse strains, before the emergence of ulceration (Fig. 6). Interestingly, we found an intermediate increase of 0.5 log10 in bacterial counts, at day 40 postinfection, between TNF-P55 KO and WT mice infected with strain 94-1327 (Fig. 6), for which an intermediate production of TNF was seen in vitro, as shown above (Fig. 2).
FIG. 6.
Proliferation of the mycolactone A/B-producing strain 98-912, mycolactone C-producing strain 94-1327, or mycolactone-negative strain 5114 in wild-type and TNF receptor-deficient mice. Mice (WT [closed symbols] or TNF-P55 KO [open symbols]) were infected subcutaneously in the left hind footpad with 5.3 log10 AFB of M. ulcerans 5114 (diamonds), 94-1327 (triangles), or 98-912 (squares). The number of AFB in homogenates from four footpads in each infected group was counted at the indicated time points. WT mice control the proliferation of M. ulcerans 5114 but do not eliminate the infection, whereas TNF-P55 KO mice are more susceptible to this strain. An increase in bacterial counts was also found in the footpads of TNF-P55 KO mice infected with strain 94-1327 compared with the WT mice. No significant differences were found for mice infected with M. ulcerans 98-912. For ethical reasons, mice were sacrificed after the emergence of ulceration. Statistical differences were determined by comparing TNF-P55 KO mice with WT mice. Calculations were performed using Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Results are from one representative experiment out of two independent experiments.
Overall, these results show that TNF plays a protective role in M. ulcerans infection.
DISCUSSION
The histopathology of BU lesions in patients infected with M. ulcerans has been characterized by a description of minimal or absent cellular inflammation (1, 9, 11, 12, 22, 31-33, 35-37, 40, 41, 56, 65, 75), in contrast with what is known to occur in response to infections by other pathogenic mycobacteria (6, 10, 14, 19, 47, 55, 62, 70, 71, 74, 85). Such a histopathological hallmark has been associated with the M. ulcerans toxin mycolactone that, among other effects, inhibits in vitro the production by activated macrophages of the proinflammatory cytokine TNF (13, 60). However, recent publications have demonstrated that M. ulcerans induces cellular inflammatory responses in specific areas of infection foci, both in BU patients (38) and in experimentally infected mice (59), with persistence of recruited phagocytes at the periphery of mycolactone-induced necrotic acellular areas (59, 84). These data suggest that infection with viable M. ulcerans organisms induces the production of cytokines and/or chemokines responsible for the recruitment and, possibly, activation of inflammatory cells.
In the present work we show that the levels of TNF production by BMDM infected with different strains of M. ulcerans are dependent on the cytotoxicity/virulence of the strain. Production of TNF by macrophages was low, intermediate, or high when they were infected with high, intermediate, or noncytotoxic/nonvirulent strains, respectively. The decreased production of TNF by BMDM infected with highly cytotoxic/virulent strains of M. ulcerans is not associated with a premature death of macrophages in culture. Indeed, that production was quite scant from the early time points of infection with these strains, as well as in macrophage cultures infected with low MOIs that did not induce cytotoxicity. Macrophages infected with the highly virulent strains can, however, produce relevant amounts of MIP-2, and the addition of mycolactone to cell cultures infected with a mycolactone-defective strain does not abrogate MIP-2 production. These results indicate that the decreased production of TNF during intracellular infection with M. ulcerans is not due to a general toxic effect of mycolactone.
MIP-2 is the murine homologue of human IL-8 and plays a central role in the recruitment of neutrophils in mice (27, 88). This chemokine was previously shown to be induced in mice infected with M. tuberculosis (68). Recently, the expression of IL-8 was also reported in the lesions of patients infected with M. ulcerans (64). The expression of this chemokine in BU lesions is in accordance with the results from Guarner et al. (38) and our own results (84) describing inflammatory infiltrates containing neutrophils in the majority of the BU specimens studied. Coutanceau and coworkers recently showed that a mycolactone-producing strain of M. ulcerans induces expression of MIP-2 in infected macrophages (13). These and our present results explain the occurrence of persistent inflammatory infiltrates containing neutrophils in mice experimentally infected with virulent strains of M. ulcerans (13, 59). In addition, it cannot be excluded that dead M. ulcerans, or bacilli not producing mycolactone, may stimulate MIP-2 production and contribute to the inflammatory response.
Our findings on the levels of virulence for mice, as well as on the cytotoxic activity for infected macrophages, among M. ulcerans organisms that are mycolactone A/B or mycolactone C producers are in accordance with previous reports showing a higher cytotoxic effect of purified mycolactone A/B compared to mycolactone C (56). It is clear from our data that the outcome of infection by a particular M. ulcerans strain depends on the type of mycolactone secreted by the pathogen. However, other factors contributing to the mycolactone-dependent virulence of M. ulcerans cannot be discarded, namely, differences in the amount of toxin produced by each strain and even the possible regulation of toxin production that could be switched on/off during defined periods of the pathogen's life cycle in the host, as previously discussed (84). In addition, the possible existence of other M. ulcerans virulence factors cannot be discarded. Further work is therefore necessary to elucidate the causes for the variability in virulence found among M. ulcerans strains.
As discussed above, the association between the mycolactone profile and virulence in mice correlates inversely with the capacity of M. ulcerans-infected BMDM to produce TNF. Decreased production of TNF in response to virulent mycobacteria has been previously reported (28, 69). It is known, for instance, that virulent M. tuberculosis H37Rv inhibits the production of TNF by infected macrophages to a higher extent than the nonvirulent strain H37Ra (23). However, the mechanisms for the decreased TNF production by these mycobacterial species are still unknown.
M. ulcerans is the only Mycobacterium known to produce a cytotoxic exotoxin capable of inhibiting IL-2 and TNF production from phorbol myristate acetate-ionomycin-activated T-cell lines and lipopolysaccharide-activated human monocytes, respectively (60). In the present work, we expand these observations by using a model of infection of primary macrophages with live bacteria from a panel of M. ulcerans strains that produce different amounts and types of mycolactone and that exhibit diverse degrees of virulence for mice. The capacity of virulent, mycolactone-producing M. ulcerans strains to downregulate the production of TNF might have consequences for the outcome of infection, given that it has recently been showed that M. ulcerans has a phase of intramacrophage residence and proliferation (84). In fact, mice deficient in TNF or in its P55 receptor are very susceptible to M. tuberculosis infections (4, 26). From studies in the mouse model, it is known that TNF is required to upregulate the macrophage bactericidal mechanisms, particularly in concert with IFN-γ (7, 24, 52). Our present data demonstrate that in experimental M. ulcerans infection, the production of TNF also plays a protective role. By inhibiting the production of the macrophage-activating cytokine TNF, virulent M. ulcerans strains would be protected from the microbicidal mechanisms of macrophages, allowing an extended time of intracellular proliferation until a significant bacterial load is achieved.
The present and previous results (13, 60) show that mycolactone has an inhibitory activity on cytokine production in vitro. It is still not clear whether this is an early manifestation of impending cell death or a specific suppression of cytokine production. Adusumilli and coworkers recently showed that very low concentrations of mycolactone induce apoptosis in a culture cell line (2). However, more studies are required to address this question in vivo. The presence of TNF in the lesions of BU patients has been inferred by the detection of mRNA for this cytokine in samples of nonulcerative and ulcerative lesions (63, 64, 66), although a high variability in TNF message has been detected in different patients (64, 66) as well as in different areas within the same lesion (63).
These results show that production of TNF in BU lesions can occur despite the presence of mycolactone, which may be associated with bacilli or free in specific areas of the lesions. In this context it is relevant to take into consideration that the spatial relationship of TNF with the cytological pattern in specific areas of a BU lesion, namely, the presence of macrophages and M. ulcerans bacilli, is still not clear (63). We can hypothesize that TNF would be produced in BU lesions by (i) macrophages infected with M. ulcerans bacilli in which mycolactone production would be temporarily switched off, (ii) macrophages or other cell types distant from mycolactone-producing bacilli in areas not reached by the toxin but in contact with mycobacterial molecules, and (iii) macrophages or other cell types activated by proinflammatory molecules derived from the process of tissue necrosis, regardless of the contact with M. ulcerans or M. ulcerans-derived molecules.
It has been reported that the process of healing of necrotic ulcers is associated with a granulomatous histopathology (1, 40, 41, 78, 86) and, in contrast, that disseminated disease and osteomyelitis are associated with defects in granuloma formation (42, 78). It is well known that TNF has an important role in the development and maintenance of the granuloma, a key structure that prevents mycobacterial dissemination (4, 21, 26, 30, 48). The recently reported positive correlation between the expression of proinflammatory cytokines, including TNF, and the formation of granulomas in BU lesions (64) supports our interpretation that TNF plays a protective role in M. ulcerans infections and that a mycolactone-associated decreased production of that cytokine would occur in the active phase of the infection.
In conclusion, the data presented here show that macrophages infected with virulent, mycolactone-positive strains of M. ulcerans produce amounts of the macrophage-activating cytokine TNF that depend on the strain's toxigenesis and that this cytokine contributes to the protection of the infected host against experimental M. ulcerans infection.
Acknowledgments
This work was supported by a grant from the Health Services of Fundação Calouste Gulbenkian and by FCT Fellowships Praxis SFRH/BD/9757/2003 and SFRH/BD/15911/2005 to E. Torrado and A. G. Fraga, respectively.
We thank Manuel T. Silva for helpful discussions and Françoise Portaels for helpful discussions and for providing the ITM M. ulcerans strains.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Abalos, F. M., J. Aguiar, Sr., A. Guedenon, F. Portaels, and W. M. Meyers. 2000. Mycobacterium ulcerans infection (Buruli ulcer): a case report of the disseminated nonulcerative form. Ann. Diagn. Pathol. 4:386-390. [DOI] [PubMed] [Google Scholar]
- 2.Adusumilli, S., A. Mve-Obiang, T. Sparer, W. Meyers, J. Hayman, and P. L. Small. 2005. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M. ulcerans in vitro and in vivo. Cell. Microbiol. 7:1295-1304. [DOI] [PubMed] [Google Scholar]
- 3.Asiedu, K., and S. Etuaful. 1998. Socioeconomic implications of Buruli ulcer in Ghana: a three-year review. Am. J. Trop. Med. Hyg. 59:1015-1022. [DOI] [PubMed] [Google Scholar]
- 4.Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. [PubMed] [Google Scholar]
- 5.Bendtzen, K. 1988. Interleukin 1, interleukin 6 and tumor necrosis factor in infection, inflammation and immunity. Immunol. Lett. 19:183-191. [DOI] [PubMed] [Google Scholar]
- 6.Busam, K. J., T. E. Kiehn, S. P. Salob, and P. L. Myskowski. 1999. Histologic reactions to cutaneous infections by Mycobacterium haemophilum. Am. J. Surg. Pathol. 23:1379-1385. [DOI] [PubMed] [Google Scholar]
- 7.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chemlal, K., G. Huys, F. Laval, V. Vincent, C. Savage, C. Gutierrez, M. A. Laneelle, J. Swings, W. M. Meyers, M. Daffe, and F. Portaels. 2002. Characterization of an unusual Mycobacterium: a possible missing link between Mycobacterium marinum and Mycobacterium ulcerans. J. Clin. Microbiol. 40:2370-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clancey, J. K., O. G. Dodge, H. F. Lunn, and M. L. Oduori. 1961. Mycobacterial skin ulcers in Uganda. Lancet 278:951-954. [DOI] [PubMed] [Google Scholar]
- 10.Clark, R. B., H. Spector, D. M. Friedman, K. J. Oldrati, C. L. Young, and S. C. Nelson. 1990. Osteomyelitis and synovitis produced by Mycobacterium marinum in a fisherman. J. Clin. Microbiol. 28:2570-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor, D. H., and H. F. Lunn. 1965. Mycobacterium ulcerans infection (with comments on pathogenesis). Int. J. Lepr. 33:698-709. [PubMed] [Google Scholar]
- 12.Connor, D. H., and H. F. Lunn. 1966. Buruli ulceration. A clinicopathologic study of 38 Ugandans with Mycobacterium ulcerans ulceration. Arch. Pathol. 81:183-199. [Google Scholar]
- 13.Coutanceau, E., L. Marsollier, R. Brosch, E. Perret, P. Goossens, M. Tanguy, S. T. Cole, P. L. Small, and C. Demangel. 2005. Modulation of the host immune response by a transient intracellular stage of Mycobacterium ulcerans: the contribution of endogenous mycolactone toxin. Cell. Microbiol. 7:1187-1196. [DOI] [PubMed] [Google Scholar]
- 14.Dannenberg, A. M., Jr. 1994. Pathogenesis of pulmonary tuberculosis: an interplay of time-damaging and macrophage activating immune responses. Dual mechanisms that control bacilary multiplication, p. 459-483. In B. R. Bloom (ed.), Tuberculosis: protection, pathogenesis, and control. American Society for Microbiology, Washington, DC.
- 15.Debacker, M., J. Aguiar, C. Steunou, C. Zinsou, M. W. Meyers, A. Guedenom, J. T. Scott, M. Dramaix, and F. Portaels. 2004. Mycobacterium ulcerans disease (Buruli ulcer) in rural hospital, southern Benin, 1997-2001. Emerg. Infect. Dis. 10:1391-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debacker, M., J. Aguiar, C. Steunou, C. Zinsou, W. M. Meyers, J. T. Scott, M. Dramaix, and F. Portaels. 2004. Mycobacterium ulcerans disease: role of age and gender in incidence and morbidity. Trop. Med. Int. Health 9:1297-1304. [DOI] [PubMed] [Google Scholar]
- 17.Denis, M. 1991. Involvement of cytokines in determining resistance and acquired immunity in murine tuberculosis. J. Leukoc. Biol. 50:495-501. [DOI] [PubMed] [Google Scholar]
- 18.Dinarello, C. A. 1992. The role of interleukin-1 in host responses to infectious diseases. Infect. Agents Dis. 1:227-236. [PubMed] [Google Scholar]
- 19.Dobos, K. M., F. D. Quinn, D. A. Ashford, C. R. Horsburgh, and C. H. King. 1999. Emergence of a unique group of necrotizing mycobacterial diseases. Emerg. Infect. Dis. 5:367-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobos, K. M., E. A. Spotts, B. J. Marston, C. R. Horsburgh, Jr., and C. H. King. 2000. Serologic response to culture filtrate antigens of Mycobacterium ulcerans during Buruli ulcer disease. Emerg. Infect. Dis. 6:158-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehlers, S., J. Benini, S. Kutsch, R. Endres, E. T. Rietschel, and K. Pfeffer. 1999. Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infect. Immun. 67:3571-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans, M. R., H. S. Thangaraj, and M. H. Wansbrough-Jones. 2000. Buruli ulcer. Curr. Opin. Infect. Dis. 13:109-112. [DOI] [PubMed] [Google Scholar]
- 23.Falcone, V., E. B. Bassey, A. Toniolo, P. G. Conaldi, and F. M. Collins. 1994. Differential release of tumor necrosis factor-alpha from murine peritoneal macrophages stimulated with virulent and avirulent species of mycobacteria. FEMS Immunol. Med. Microbiol. 8:225-232. [DOI] [PubMed] [Google Scholar]
- 24.Flesch, I. E., and S. H. Kaufmann. 1990. Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect. Immun. 58:2675-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 26.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 27.Fulton, S. A., S. M. Reba, T. D. Martin, and W. H. Boom. 2002. Neutrophil-mediated mycobacteriocidal immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice. Infect. Immun. 70:5322-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furney, S. K., P. S. Skinner, A. D. Roberts, R. Appelberg, and I. M. Orme. 1992. Capacity of Mycobacterium avium isolates to grow well or poorly in murine macrophages resides in their ability to induce secretion of tumor necrosis factor. Infect. Immun. 60:4410-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao, L. Y., S. Guo, B. McLaughlin, H. Morisaki, J. N. Engel, and E. J. Brown. 2004. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol. Microbiol. 53:1677-1693. [DOI] [PubMed] [Google Scholar]
- 30.Garcia, I., Y. Miyazaki, G. Marchal, W. Lesslauer, and P. Vassalli. 1997. High sensitivity of transgenic mice expressing soluble TNFR1 fusion protein to mycobacterial infections: synergistic action of TNF and IFN-gamma in the differentiation of protective granulomas. Eur. J. Immunol. 27:3182-3190. [DOI] [PubMed] [Google Scholar]
- 31.George, K. M., L. P. Barker, D. M. Welty, and P. L. Small. 1998. Partial purification and characterization of biological effects of a lipid toxin produced by Mycobacterium ulcerans. Infect. Immun. 66:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George, K. M., D. Chatterjee, G. Gunawardana, D. Welty, J. Hayman, R. Lee, and P. L. Small. 1999. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283:854-857. [DOI] [PubMed] [Google Scholar]
- 33.George, K. M., L. Pascopella, D. M. Welty, and P. L. Small. 2000. A Mycobacterium ulcerans toxin, mycolactone, causes apoptosis in guinea pig ulcers and tissue culture cells. Infect. Immun. 68:877-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giacomini, E., E. Iona, L. Ferroni, M. Miettinen, L. Fattorini, G. Orefici, I. Julkunen, and E. M. Coccia. 2001. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J. Immunol. 166:7033-7041. [DOI] [PubMed] [Google Scholar]
- 35.Gooding, T. M., P. D. Johnson, D. E. Campbell, J. A. Hayman, E. L. Hartland, A. S. Kemp, and R. M. Robins-Browne. 2001. Immune response to infection with Mycobacterium ulcerans. Infect. Immun. 69:1704-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gooding, T. M., P. D. Johnson, M. Smith, A. S. Kemp, and R. M. Robins-Browne. 2002. Cytokine profiles of patients infected with Mycobacterium ulcerans and unaffected household contacts. Infect. Immun. 70:5562-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gooding, T. M., A. S. Kemp, R. M. Robins-Browne, M. Smith, and P. D. Johnson. 2003. Acquired T-helper 1 lymphocyte anergy following infection with Mycobacterium ulcerans. Clin. Infect. Dis. 36:1076-1077. [DOI] [PubMed] [Google Scholar]
- 38.Guarner, J., J. Bartlett, E. A. Whitney, P. L. Raghunathan, Y. Stienstra, K. Asamoa, S. Etuaful, E. Klutse, E. Quarshie, T. S. van der Werf, W. T. van der Graaf, C. H. King, and D. A. Ashford. 2003. Histopathologic features of Mycobacterium ulcerans infection. Emerg. Infect. Dis. 9:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Havell, E. A. 1992. Role of TNF in resistance to bacteria. Immunol. Ser. 56:341-363. [PubMed] [Google Scholar]
- 40.Hayman, J. 1993. Out of Africa: observations on the histopathology of Mycobacterium ulcerans infection. J. Clin. Pathol. 46:5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayman, J., and A. McQueen. 1985. The pathology of Mycobacterium ulcerans infection. Pathology 17:594-600. [DOI] [PubMed] [Google Scholar]
- 42.Hofer, M., B. Hirschel, P. Kirschner, M. Beghetti, A. Kaelin, C. A. Siegrist, S. Suter, A. Teske, and E. C. Bottger. 1993. Brief report: disseminated osteomyelitis from Mycobacterium ulcerans after a snakebite. N. Engl. J. Med. 328:1007-1009. [DOI] [PubMed] [Google Scholar]
- 43.Hong, H., P. J. Gates, J. Staunton, T. Stinear, S. T. Cole, P. F. Leadlay, and J. B. Spencer. 2003. Identification using LC-MSn of co-metabolites in the biosynthesis of the polyketide toxin mycolactone by a clinical isolate of Mycobacterium ulcerans. Chem. Commun. (Cambridge) 22:2822-2823. [DOI] [PubMed] [Google Scholar]
- 44.Hong, H., J. B. Spencer, J. L. Porter, P. F. Leadlay, and T. Stinear. 2005. A novel mycolactone from a clinical isolate of Mycobacterium ulcerans provides evidence for additional toxin heterogeneity as a result of specific changes in the modular polyketide synthase. Chembiochem 6:1-5. [DOI] [PubMed] [Google Scholar]
- 45.Hsu, T., S. M. Hingley-Wilson, B. Chen, M. Chen, A. Z. Dai, P. M. Morin, C. B. Marks, J. Padiyar, C. Goulding, M. Gingery, D. Eisenberg, R. G. Russell, S. C. Derrick, F. M. Collins, S. L. Morris, C. H. King, and W. R. Jacobs, Jr. 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. USA 100:12420-12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Judd, T. C., A. Bischoff, Y. Kishi, S. Adusumilli, and P. L. C. Small. 2004. Structure determination of mycolactone C via total synthesis. Org. Lett. 6:4901-4904. [DOI] [PubMed] [Google Scholar]
- 47.Kaufmann, S. H. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11:129-163. [DOI] [PubMed] [Google Scholar]
- 48.Kindler, V., A. P. Sappino, G. E. Grau, P. F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56:731-740. [DOI] [PubMed] [Google Scholar]
- 49.Kiszewski, A. E., E. Becerril, L. D. Aguilar, I. T. Kader, W. Myers, F. Portaels, and P. R. Hernandez. 2006. The local immune response in ulcerative lesions of Buruli disease. Clin. Exp. Immunol. 143:445-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krieg, R. E., W. T. Hockmeyer, and D. H. Connor. 1974. Toxin of Mycobacterium ulcerans. Production and effects in guinea pig skin. Arch. Dermatol. 110:783-788. [DOI] [PubMed] [Google Scholar]
- 51.Lewis, K. N., R. Liao, K. M. Guinn, M. J. Hickey, S. Smith, M. A. Behr, and D. R. Sherman. 2003. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guerin attenuation. J. Infect. Dis. 187:117-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liew, F. Y., Y. Li, and S. Millott. 1990. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J. Immunol. 145:4306-4310. [PubMed] [Google Scholar]
- 53.MacCallum, P., J. C. Tolhurst, G. Buckle, and H. A. Sissons. 1948. A new mycobacterial infection in man. J. Pathol. Bacteriol. 60:93-122. [PubMed] [Google Scholar]
- 54.Marston, B. J., M. O. Diallo, C. R. Horsburgh, Jr., I. Diomande, M. Z. Saki, J. M. Kanga, G. Patrice, H. B. Lipman, S. M. Ostroff, and R. C. Good. 1995. Emergence of Buruli ulcer disease in the Daloa region of Cote d'Ivoire. Am. J. Trop. Med. Hyg. 52:219-224. [DOI] [PubMed] [Google Scholar]
- 55.Mor, N., I. Lutsky, and L. Levy. 1981. Response in the hindfoot pad and popliteal lymph node of C57BL mice to infection with Mycobacterium marinum. Isr. J. Med. Sci. 17:236-244. [PubMed] [Google Scholar]
- 56.Mve-Obiang, A., R. E. Lee, F. Portaels, and P. L. Small. 2003. Heterogeneity of mycolactones produced by clinical isolates of Mycobacterium ulcerans: implications for virulence. Infect. Immun. 71:774-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mve-Obiang, A., R. E. Lee, E. S. Umstot, K. A. Trott, T. C. Grammer, J. M. Parker, B. S. Ranger, R. Grainger, E. A. Mahrous, and P. L. Small. 2005. A newly discovered mycobacterial pathogen isolated from laboratory colonies of Xenopus species with lethal infections produces a novel form of mycolactone, the Mycobacterium ulcerans macrolide toxin. Infect. Immun. 73:3307-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noeske, J., C. Kuaban, S. Rondini, P. Sorlin, L. Ciaffi, J. Mbuagbaw, F. Portaels, and G. Pluschke. 2004. Buruli ulcer disease in Cameroon rediscovered. Am. J. Trop. Med. Hyg. 70:520-526. [PubMed] [Google Scholar]
- 59.Oliveira, M. S., A. G. Fraga, E. Torrado, A. G. Castro, J. P. Pereira, A. L. Filho, F. Milanezi, F. C. Schmitt, W. M. Meyers, F. Portaels, M. T. Silva, and J. Pedrosa. 2005. Infection with Mycobacterium ulcerans induces persistent inflammatory responses in mice. Infect. Immun. 73:6299-6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pahlevan, A. A., D. J. Wright, C. Andrews, K. M. George, P. L. Small, and B. M. Foxwell. 1999. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T cell cytokine production and NF-kappa B function. J. Immunol. 163:3928-3935. [PubMed] [Google Scholar]
- 61.Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science 299:1033-1036. [DOI] [PubMed] [Google Scholar]
- 62.Pedrosa, J., B. M. Saunders, R. Appelberg, I. M. Orme, M. T. Silva, and A. M. Cooper. 2000. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect. Immun. 68:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peduzzi, E., C. Groeper, D. Schutte, P. Zajac, S. Rondini, E. Mensah-Quainoo, G. C. Spagnoli, G. Pluschke, and C. A. Daubenberger. 2006. Local activation of the innate immune system in Buruli ulcer lesions. J. Investig. Dermatol. 127:638-645. [DOI] [PubMed] [Google Scholar]
- 64.Phillips, R., C. Horsfield, J. Mangan, K. Laing, S. Etuaful, P. Awuah, K. Nyarko, F. Osei-Sarpong, P. Butcher, S. Lucas, and M. Wansbrough-Jones. 2006. Cytokine mRNA expression in Mycobacterium ulcerans-infected human skin and correlation with local inflammatory response. Infect. Immun. 74:2917-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pimsler, M., T. A. Sponsler, and W. M. Meyers. 1988. Immunosuppressive properties of the soluble toxin from Mycobacterium ulcerans. J. Infect. Dis. 157:577-580. [DOI] [PubMed] [Google Scholar]
- 66.Prevot, G., E. Bourreau, H. Pascalis, R. Pradinaud, A. Tanghe, K. Huygen, and P. Launois. 2004. Differential production of systemic and intralesional gamma interferon and interleukin-10 in nodular and ulcerative forms of Buruli disease. Infect. Immun. 72:958-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Read, J. K., C. M. Heggie, W. M. Meyers, and D. H. Connor. 1974. Cytotoxic activity of Mycobacterium ulcerans. Infect. Immun. 9:1114-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rhoades, E. R., A. M. Cooper, and I. M. Orme. 1995. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect. Immun. 63:3871-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarmento, A. M., and R. Appelberg. 1995. Relationship between virulence of Mycobacterium avium strains and induction of tumor necrosis factor alpha production in infected mice and in in vitro-cultured mouse macrophages. Infect. Immun. 63:3759-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saunders, B. M., and C. Cheers. 1995. Inflammatory response following intranasal infection with Mycobacterium avium complex: role of T-cell subsets and gamma interferon. Infect. Immun. 63:2282-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seiler, P., P. Aichele, B. Raupach, B. Odermatt, U. Steinhoff, and S. H. Kaufmann. 2000. Rapid neutrophil response controls fast-replicating intracellular bacteria but not slow-replicating Mycobacterium tuberculosis. J. Infect. Dis. 181:671-680. [DOI] [PubMed] [Google Scholar]
- 72.Shepard, C. C., and D. H. McRae. 1968. A method for counting acid-fast bacteria. Int. J. Lepr. Other Mycobact. Dis. 36:78-82. [PubMed] [Google Scholar]
- 73.Shibuya, K., D. Robinson, F. Zonin, S. B. Hartley, S. E. Macatonia, C. Somoza, C. A. Hunter, K. M. Murphy, and A. O'Garra. 1998. IL-1 alpha and TNF-alpha are required for IL-12-induced development of Th1 cells producing high levels of IFN-gamma in BALB/c but not C57BL/6 mice. J. Immunol. 160:1708-1716. [PubMed] [Google Scholar]
- 74.Silva, M. T., M. N. Silva, and R. Appelberg. 1989. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb. Pathog. 6:369-380. [DOI] [PubMed] [Google Scholar]
- 75.Snyder, D. S., and P. L. Small. 2003. Uptake and cellular actions of mycolactone, a virulence determinant for Mycobacterium ulcerans. Microb. Pathog. 34:91-101. [DOI] [PubMed] [Google Scholar]
- 76.Stanford, J. L., W. D. Revill, W. J. Gunthorpe, and J. M. Grange. 1975. The production and preliminary investigation of Burulin, a new skin test reagent for Mycobacterium ulcerans infection. J. Hyg. (London) 74:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stanley, S. A., S. Raghavan, W. W. Hwang, and J. S. Cox. 2003. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc. Natl. Acad. Sci. USA 100:13001-13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stienstra, Y., W. T. van der Graaf, G. J. te Meerman, T. H. The, L. F. de Leij, and T. S. van der Werf. 2001. Susceptibility to development of Mycobacterium ulcerans disease: review of possible risk factors. Trop. Med. Int. Health 6:554-562. [DOI] [PubMed] [Google Scholar]
- 79.Stinear, T. P., H. Hong, W. Frigui, M. J. Pryor, R. Brosch, T. Garnier, P. F. Leadlay, and S. T. Cole. 2005. Common evolutionary origin for the unstable virulence plasmid pMUM found in geographically diverse strains of Mycobacterium ulcerans. J. Bacteriol. 187:1668-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stinear, T. P., A. Mve-Obiang, P. L. Small, W. Frigui, M. J. Pryor, R. Brosch, G. A. Jenkin, P. D. Johnson, J. K. Davies, R. E. Lee, S. Adusumilli, T. Garnier, S. F. Haydock, P. F. Leadlay, and S. T. Cole. 2004. Giant plasmid-encoded polyketide synthases produce the macrolide toxin of Mycobacterium ulcerans. Proc. Natl. Acad. Sci. USA 101:1345-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Strohmeier, G. R., and M. J. Fenton. 1999. Roles of lipoarabinomannan in the pathogenesis of tuberculosis. Microbes Infect. 1:709-717. [DOI] [PubMed] [Google Scholar]
- 82.Tanghe, A., J. Content, J. P. Van Vooren, F. Portaels, and K. Huygen. 2001. Protective efficacy of a DNA vaccine encoding antigen 85A from Mycobacterium bovis BCG against Buruli ulcer. Infect. Immun. 69:5403-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tonjum, T., D. B. Welty, E. Jantzen, and P. L. Small. 1998. Differentiation of Mycobacterium ulcerans, M. marinum, and M. haemophilum: mapping of their relationships to M. tuberculosis by fatty acid profile analysis, DNA-DNA hybridization, and 16S rRNA gene sequence analysis. J. Clin. Microbiol. 36:918-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Torrado, E., A. G. Fraga, A. G. Castro, P. Stragier, M. W. Meyers, F. Portaels, M. T. Silva, and J. Pedrosa. 2007. Evidence for an intramacrophage growth phase of Mycobacterium ulcerans. Infect. Immun. 75:977-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Travis, W. D., L. B. Travis, G. D. Roberts, D. W. Su, and L. W. Weiland. 1985. The histopathologic spectrum in Mycobacterium marinum infection. Arch. Pathol. Lab. Med. 109:1109-1113. [PubMed] [Google Scholar]
- 86.van der Werf, T. S., W. T. van der Graaf, J. W. Tappero, and K. Asiedu. 1999. Mycobacterium ulcerans infection. Lancet 354:1013-1018. [DOI] [PubMed] [Google Scholar]
- 87.Westenbrink, B. D., Y. Stienstra, M. G. Huitema, W. A. Thompson, E. O. Klutse, E. O. Ampadu, H. M. Boezen, P. C. Limburg, and T. S. van der Werf. 2005. Cytokine responses to stimulation of whole blood from patients with Buruli ulcer disease in Ghana. Clin. Diagn. Lab. Immunol. 12:125-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Widmer, U., K. R. Manogue, A. Cerami, and B. Sherry. 1993. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 alpha, and MIP-1 beta, members of the chemokine superfamily of proinflammatory cytokines. J. Immunol. 150:4996-5012. [PubMed] [Google Scholar]
- 89.Yip, M. J., J. L. Porter, J. A. Fyfe, C. J. Lavender, F. Portaels, M. Rhodes, H. Kator, A. Colorni, G. A. Jenkin, and T. Stinear. 2007. Evolution of Mycobacterium ulcerans and other mycolactone-producing mycobacteria from a common Mycobacterium marinum progenitor. J. Bacteriol. 189:2021-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]