Abstract
Neuroborreliosis (NB) is a chronic infectious disease of the central nervous system (CNS) caused by a tick-borne spirochete, Borrelia burgdorferi. In addition to direct effects of the causative infectious agent, additional immunity-mediated mechanisms are thought to play a role in the CNS pathology of NB. In order to further understand the involvement of humoral immune mechanisms in NB, we dissected the intrathecal antibody responses down to the single-plasma-cell level. Starting with single-cell reverse transcription-PCR of fluorescence-activated cell sorter-sorted cerebrospinal fluid plasma cells from an NB patient, we identified expanded clones and resurrected the antigen specificity of their secreted antibodies through recombinant expression of the correctly paired immunoglobulin heavy- and light-chain genes as monoclonal antibodies (MAbs). As expected, we found specificity for the causative infectious agent, B. burgdorferi, among the clonally expanded plasma cell (cePC)-derived MAbs. However, from an independent cePC of the same patient, we could derive MAbs specific for human CNS myelin, without detectable cross-reactivity with B. burgdorferi antigens. While reactivity against B. burgdorferi is a known feature of humoral immune responses in NB, we show (i) that immune responses specific for self antigens may be a distinct feature of CNS infections independent of pathogen reactivity and (ii) that humoral autoimmunity in NB (since found in cePC) is the result of a truly antigen-driven immune response. Our findings indicate that in NB mechanisms may be at play that induce distinct immune responses specific for pathogen and self antigens independent from “molecular mimicry.”
Neuroborreliosis (NB) is a frequent and serious manifestation of Lyme borreliosis, caused by the tick-borne spirochete Borrelia burgdorferi (2, 5, 21). It is a complex disease including a number of different possible clinical and pathological manifestations (18), such as meningitis, polyradiculitis, cranial neuritis, and encephalomyelitis. Accordingly, diffuse white matter lesions have been demonstrated by magnetic resonance imaging in chronic disease courses (18). Studies with cerebrospinal fluid (CSF)-derived T lymphocytes demonstrated reactivity with B. burgdorferi-derived antigens and also with central nervous system (CNS) self antigens (16). Further studies found B. burgdorferi-specific T cells cross-reacting with self antigens, suggesting that molecular mimicry is responsible for autoimmune mechanisms leading to chronic disease manifestation (11, 14).
A common characteristic of NB is oligoclonal immunoglobulins (OCB) in the CSF, a feature suggesting the presence of a CSF-specific antigen-driven immune response. Analysis of immunoglobulins (Ig) in the CSF has revealed specificity for B. burgdorferi (10, 15) and also for CNS autoantigens (12). However, the mechanisms underlying B. burgdorferi-directed humoral immune defense and CNS tissue damage still remain poorly understood, and more in-depth characterization of the focused antibody response in the CSF has been hampered by both the limited availability and the oligoclonal/polyclonal composition of CSF Ig. Because clonally expanded plasma cells (cePC) are thought to be the producers of OCB, recombinant reconstruction of cePC-derived antibodies would be expected to provide valuable information on immunologic properties of antigen-driven immune responses in the CSF of patients with NB.
In order to accurately dissect the various antigen specificities produced by CSF-derived cePC, we used an experimental approach allowing reconstitution of their antigen specificities. We amplified the paired rearranged IgG heavy (H)- and light (L)-chain genes of individual cePC from CSF by single-cell reverse transcription (RT)-PCR and resurrected them as recombinant monoclonal antibodies (MAbs) in a eukaryotic expression system.
Among the MAbs derived from the cePC of a patient with NB we found a diverse pattern of specificities. On the one hand, we found, as expected, reactivity with B. burgdorferi confirmed by an enzyme-linked immunosorbent assay (ELISA), immunofluorescence, and immunoprecipitation. On the other hand, recombinant MAbs derived from a different expanded CSF plasma cell clone revealed reactivity with CNS self antigens in immunofluorescence staining of human facial nerve and white matter tissue without cross-reactivity with B. burgdorferi. These findings suggest that aside from pathogen-directed humoral immune responses, immunopathological mechanisms occurring in CNS infectious disease may also evoke autoimmunity. Additionally, our findings imply that apart from “molecular mimicry” additional mechanisms may be responsible for the induction of autoimmune reactivity.
MATERIALS AND METHODS
Patient data and hybridoma cell line.
This study was approved by the local ethics committee. CSF was obtained from a 41-year-old male patient with NB. On admission (25 July 2004), he presented with bilateral peripheral facial nerve palsy, mild clinical signs of meningism, fading erythema chronicum migrans (ECM) on the left side of the chest, and burning dysesthesias in a number of dermal segments. The patient had first noticed an ECM approximately 3 to 4 weeks earlier but did not remember a tick bite in the days before the ECM appeared. However, he reported having had a number of tick bites a few months prior to the ECM without having noticed any additional clinical symptoms. The first CSF analysis on the day of admission revealed a pleocytosis of 190 cells/μl (95% lymphocytes), elevated levels of lactic acid and total protein (glucose levels were normal), positive oligoclonal bands, and signs of intrathecal production of B. burgdorferi-specific IgM and IgG. We could not exclude the possibility that this intrathecal B. burgdorferi-specific immune response had been elicited by an infection with B. burgdorferi preceding the infection which caused the ECM. Upon diagnosis of NB (Bannwarth meningopolyneuritis) the patient was treated with intravenous ceftriaxone for 14 days. The second CSF analysis (5 August 2004), which was performed to monitor the treatment response and was also used for the experiments described below, showed decreasing pleocytosis (118 cells/μl [98% lymphocytes and 2% plasma cells]) and persisting oligoclonal bands.
Cell labeling and single-cell sorting.
CSF CD138+ plasma cells were labeled with an anti-human CD138 MAb (Serotec). Single CD138+ cells were sorted (FACS-Aria; Becton Dickinson) into individual wells of 96-well PCR plates containing RT reaction buffer.
Single-cell RT-PCR.
For the initial single-cell RT-PCR, reverse primers specific for the constant (C) regions of Ig genes (IgG-H, -κL, and -λL chains) and forward primers specific for conserved framework region 1 complementary to H- and L-chain Ig variable (V) region families were used, as described previously (1, 17). To avoid PCR cross-contamination between individual wells of the PCR plate and to rule out contamination of PCR components, we took a number of precautions: (i) several negative controls, including wells of the PCR plate in which no plasma cells were deposited, were used; (ii) the areas in which PCR setup, PCR analysis by gel electrophoresis, and cloning of PCR products were performed were spaces which were physically separated and equipped with designated sets of pipetters and filter tips; and (iii) fresh aliquots of PCR components were used for this experiment. PCR products were sequenced (Synergene), and V regions were analyzed for Ig gene usage (http://imgt.cines.fr/textes/vquest/) to determine the closest V-region germ line segments and degree of homology for the H- and L-chain sequences expressed by individual plasma cells. For cloning, nested PCR was performed for Ig genes that had been amplified from more than one plasma cell with primers containing restriction sites as described previously (22). Ig H- and L-chain PCR products were cloned into vectors containing the C region of human IgG1, Ig(κ), or Ig(λ) (kindly provided by H. Wardemann), as described previously (22). V regions of 8-18c5 H- and L-chain genes were amplified from mRNA of 8-18c5 hybridoma cells (kindly provided by C. Linington) by RT-PCR using mouse Ig-specific primers (4) and cloned into the expression vectors described above.
Antibody production and purification.
293-T human embryonic kidney fibroblasts were cotransfected with Ig H- and L-chain-encoding plasmid DNA as described previously (7). Transfected cells were cultured in serum-free media, and IgG was purified from supernatants using protein G columns (Amersham). Three MAbs (RA17, RA20, and RA77), originally produced by CSF cePC of patient RA, and chimeric 8-18c5 (hu8-18c5) were recombinantly produced with this system. Murine hybridoma-derived 8-18c5 antibody (m8-18c5; specific for myelin oligodendrocyte glycoprotein [MOG]) was a kind gift of C. Linington. When necessary, purified antibodies were biotinylated by following the manufacturer's instructions (Sigma).
ELISA.
Maxisorp ELISA plates (Nunc) were coated overnight at 4°C with 50 μg/ml B. burgdorferi protein lysate (kindly provided by O. Péter), 50 μg/ml Escherichia coli protein lysate, or 2.5 μg/ml purified recombinant Borrelia afzelii p41 antigen (PKo) (Mikrogen). The plates were blocked with 1% bovine serum albumin (BSA) (Sigma, St. Louis, MO). After washing, 10 μg/ml recombinant human antibody or 1 μg/ml goat polyclonal anti-E. coli antibody (abcam) in phosphate-buffered saline (PBS)-1% BSA was incubated for 1 h at 37°C. Bound antibody was detected by horseradish peroxidase-conjugated goat anti-human IgG+IgM (1:100,000; Jackson ImmunoResearch), peroxidase-conjugated rabbit anti-goat IgG (1:10,000; abcam), or streptavidin-peroxidase conjugate (SA-HRP) (1:500; Sigma). Enzymatic activity was revealed by 3,3,5′,5′-tetramethylbenzidine (TMB) (Pierce) and quantified at 450 nm using a microplate reader (Bio-Rad).
Competition assays.
Initially, saturation experiments were conducted to obtain the half-maximal binding capacity of biotinylated RA77 for 5 μg B. burgdorferi lysate and 0.25 μg recombinant p41, as well as the half-maximal binding capacity of biotinylated 8-18c5. ELISA plates were coated with 5 μg/well B. burgdorferi lysate, 0.25 μg/well recombinant p41, or 1 μg/well recombinant MOG and incubated with increasing concentrations of biotinylated RA77 or both hybridoma-derived 8-18c5 (m8-18c5) and recombinant chimeric 8-18c5 (hu8-18c5). Bound biotinylated antibodies were detected with SA-HRP and TMB as described above. Competition experiments with biotinylated and nonbiotinylated antibodies were conducted to obtain the affinity of RA77 for B. burgdorferi lysate or recombinant p41 and the affinity of m8-18c5 or hu8-18c5 for recombinant MOG. Increasing concentrations of nonbiotinylated antibodies were added to ELISA wells coated with the appropriate antigens in the presence of the previously determined 50% saturation concentration of the corresponding biotinylated antibody. After 1 h of incubation at 37°C, the wells were washed, and bound biotinylated antibodies were detected by SA-HRP and TMB. The affinity (Ki) was calculated as described by Cheng and Prusoff (8).
Immunoprecipitation.
Protein G Sepharose (Amersham) was incubated with RA77 or human IgG1 (Fitzgerald) in PBS for 3 h at 4°C. The Sepharose-antibody complex was washed once and resuspended in IP buffer (20 mM HEPES, 5 mM MgCl, 0.05% NP-40, 120 mM NaCl, protease inhibitors [Roche]), and B. burgdorferi lysate was added. The reaction mixtures were incubated overnight at 4°C, centrifuged at 500 × g, and resuspended in IP buffer. The resuspended Sepharose mixtures were added to preequilibrated empty columns (Amersham) and washed with IP buffer. Immunoprecipitated proteins were eluted with 0.1 M glycine buffer at pH 3 and separated by reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were visualized by silver staining (Pierce). Bands for matrix-assisted laser desorption ionization (MALDI) analysis were excised from duplicate gels stained with Coomassie blue (Pierce).
MALDI-time of flight (TOF) analysis.
Gel bands were cut into small pieces. In-gel digestion was performed as described by Schrimpf et al. (20) by rehydrating the washed and dried gel pieces with 20 μl of a trypsin solution (modified trypsin; Promega). After digestion overnight at 37°C the supernatant was removed, and the gel pieces were extracted with 20 μl of 5% (vol/vol) formic acid. One microliter of the combined supernatants was mixed with 1 μl of a saturated solution of alpha-cyano-4-hydroxycinnamic acid (Fluka) in 0.1% (vol/vol) trifluoroacetic acid-acetonitrile (2:1), and 1 μl was applied onto the MALDI target. Mass spectra were recorded with a Biflex III (Bruker Daltonics). The resulting list of peptide masses was searched against the MSDB protein database using Mascot (http://www.matrixscience.com).
Immunofluorescence staining.
B. burgdorferi and Treponema pallidum spirochetes attached to microscope slides (Euroimmun) were stained with RA77-biotin and either B. burgdorferi-specific anti-p41 antibody (MAb 1 C11; kindly provided by V. Fingerle, Munich, Germany) or anti-T. pallidum positive human control serum (Euroimmun). Immunofluorescence staining of frozen human tissue was performed with biotinylated RA17, RA20, RA77, and human IgG1 (Fitzgerald) using sections of postmortem white matter, facial nerve, colon, lung, and kidney tissue (kindly provided by the NICHD Brain and Tissue Bank for Developmental Disorders) with 10 μg/ml per MAb. Sections were fixed with formaldehyde, permeabilized with Triton X-100, treated with an avidin-biotin blocking protocol (Vectorlabs), and blocked with 10% normal goat serum in PBS supplemented with 0.2% Triton X-100. Subsequent incubation with individual biotinylated MAbs took place overnight at 4°C, if necessary, in the presence of antibodies for simultaneous staining of CNS resident cell types (mouse anti-neurofilament 200 [Sigma], mouse anti-MOG 8-18c5-Ab, myelin). After washes in PBS, bound MAbs were detected with streptavidin-Alexa 546, streptavidin-Alexa 488, anti-mouse IgG-Alexa 488, anti-mouse IgG-Alexa 592, and anti-human IgG-Alexa 546 conjugates (1:700; Molecular Probes) as secondary antibodies. Nuclei were counterstained with Hoechst 55432, and tissue sections were mounted with an aqueous mounting medium.
RESULTS
Recombinant expression of MAbs preserves both antigen specificity and affinity.
In pilot experiments we evaluated the fidelity of the eukaryotic expression system used in our study. We generated a chimeric antibody (hu8-18c5) consisting of murine V and human C regions from the well-characterized murine hybridoma 8-18c5, which is specific for MOG. Experiments comparing the resulting recombinant antibody hu8-18c5 and the original murine antibody (m8-18c5) confirmed that our methodology preserves the antigen specificity and affinity of the original antibody (not shown).
RT-PCR analysis of individual fluorescence-activated cell sorter-sorted plasma cells from NB CSF demonstrates clonal expansion.
Eighty-eight CD138+ plasma cells were sorted into wells of a 96-well PCR plate at a ratio of one cell/well. RT-PCR of Ig genes yielded Ig H-chain amplicons and corresponding sequence information from 54 of the 88 (61.4%) individual plasma cells. By assembly of expressed Ig H-chain V-region genes in groups of sequences sharing identical H-chain CDR3 regions, we were able to discern three independent cePC (RA17, RA20, and RA77) (Table 1). Matching Ig L-chain gene amplicons were obtained for each of the three cePC. The H- and L-chain V-region genes expressed by all three cePC showed some deviations from the most homologous germ line segments (Table 1). In addition, H-chain V-region genes of the RA17 group of sequences obtained from four individual plasma cells contained nucleotide mutations (not shown); no mutations were found in the RA20 and RA77 groups of sequences. As a number of precautions had been taken to avoid cross-contamination between wells of PCR plates (see Materials and Methods) and we also obtained several additional “unique” sequences from other plasma cells analyzed in the same PCR experiment, we expected sequences with 100% homology, as observed in the RA20 and RA77 groups of sequences, to not be the product of contamination. Table 1 summarizes the frequencies of the expanded plasma cells and the characteristics of the corresponding VH and VL sequences.
TABLE 1.
Characteristics of VH and VL region sequences of recombinant antibodies derived from expanded CD138+ CSF plasma cells (NB patient RA)a
| Clone | Frequency | Chain | Closest germ line
|
CDR3 amino acid sequence | |||
|---|---|---|---|---|---|---|---|
| V segment | % Homology | J segment | D segment | ||||
| RA77 | 4/54 | H | IGHV5-a | 97.6 | IGHJ5 | IGHD2-15 | AGEAAGTRIDRWFDP |
| L | IGLV9-49 | 99.3 | IGLJ3 | NAb | GADHGSGSNFVWV | ||
| RA20 | 3/54 | H | IGHV4-59 | 98.2 | IGHJ6 | IGHD5-24 | ARVPGERDGYHYMAYAMDV |
| L | IGLV3-1 | 97.1 | IGLJ3 | NA | QAWDSSTLGV | ||
| RA17 | 4/54 | H | IGHV5-a | 97.8 | IGHJ32 | IGHD6-6 | ASLAARGAFDI |
| L | IGLV3-25 | 97.8 | IGLJ3 | NA | QSADSSGSYRV | ||
The frequency indicates the number of the H-chain sequences containing the indicated H-chain CDR3 region among all plasma cells analyzed by PCR. The most homologous V, D, and J germ line segments, the degree of homology to the closest germ line segment for the H- and L-chain V region, and the corresponding CDR3 amino acid sequence are shown.
NA, not applicable.
Recombinant antibody RA77 is specific for Borrelia flagellum protein p41.
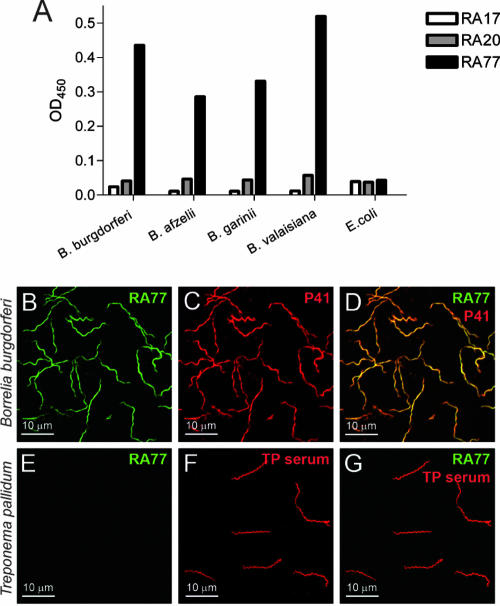
Correctly paired Ig H- and L-chain amplicons of each of the three cePC were cloned into eukaryotic expression vectors and expressed as recombinant human MAbs with an IgG1 backbone (RA77, RA20, and RA17). In ELISA experiments RA77 showed clear reactivity with extracts of four different strains of Borrelia (B. burgdorferi sensu stricto, B. afzelii, B. garinii, and B. valaisiana), while—in contrast to a commercial anti-E. coli positive control antibody (data not shown)—no binding to E. coli could be detected (Fig. 1A). No significant binding to any Borrelia strain could be detected for RA17 and RA20 (Fig. 1A). To further investigate the Borrelia specificity of RA77 and to rule out possible cross-reactivity with other spirochetes, we performed immunofluorescence experiments with B. burgdorferi and T. pallidum attached to microscope slides. While specific binding of RA77 to B. burgdorferi could be confirmed, no binding to T. pallidum could be detected (Fig. 1B to G).
FIG. 1.
Specificity of RA77 for B. burgdorferi without cross-reactivity with E. coli and T. pallidum. (A) ELISA using plates coated with 5 μg/well of either lysates of different Borrelia strains or E. coli lysate. Recombinant MAbs RA17, RA20, and RA77 were used at a concentration of 10 μg/ml. OD450, optical density at 450 nm. (B and C) Staining of B. burgdorferi attached to polycarbonate slides with RA77 (B) and anti-p41 antibody 1C11 (C). (D) Overlay of panels B and C. (E and F) Staining of T. pallidum attached to polycarbonate slides with RA77 (E) and anti-T. pallidum serum (F). (G) Overlay of panels E and F.
In immunoprecipitation experiments using RA77 together with B. burgdorferi lysate, an approximately 40-kDa protein band was detectable in the precipitate. In the control immunoprecipitation with human IgG1 (hIgG1) no band in addition to the expected Ig H- and L-chain bands was detected (Fig. 2A). MALDI-TOF analysis of the 40-kDa band revealed peptide masses matching theoretically derived masses from trypsin-digested flagellin protein p41 of B. burgdorferi. ELISA experiments with purified recombinant p41 flagellum protein confirmed the results from mass spectrometry (Fig. 2B). Competition experiments using RA77 and native B. burgdorferi lysate demonstrated that there was a high-affinity interaction (Ki = 1 × 10−9). Interestingly, the interaction between RA77 and recombinant p41 yielded 1,000-fold-lower affinity (Ki = 1 × 10−6) (Fig. 2C).
FIG. 2.
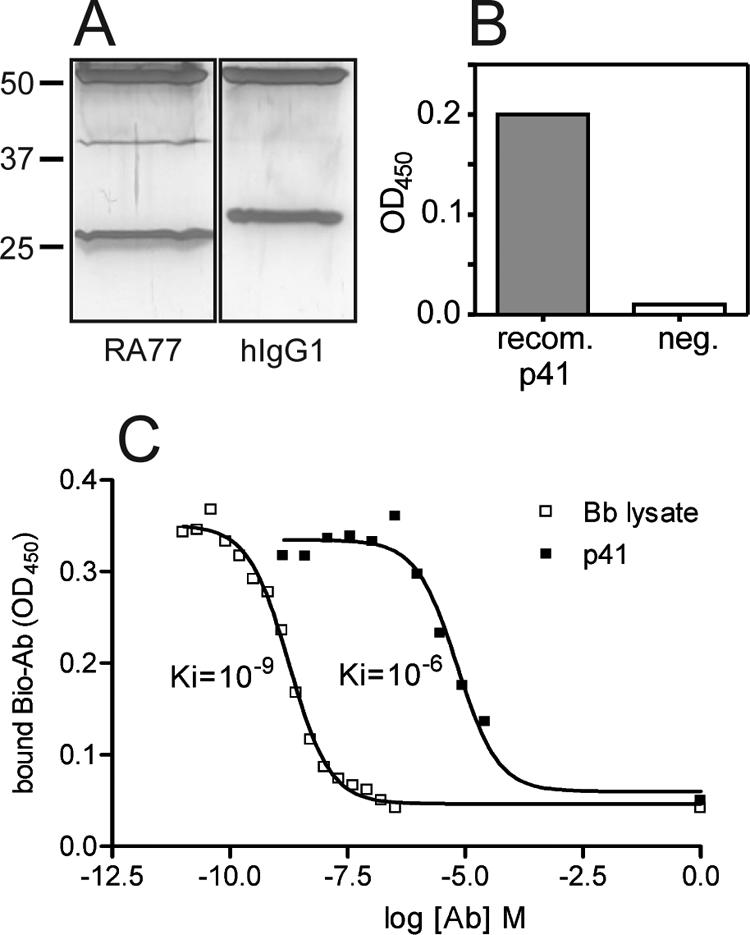
B. burgdorferi-specific MAb RA77 recognizes p41 flagellin protein. (A) Immunoprecipitation of p41 flagellin protein from B. burgdorferi lysate by RA77. The ∼40-kDa band corresponds to the p41 flagellin protein, as verified by MALDI-TOF analysis. No such band was detected after immunoprecipitation with hIgG1. The protein bands around 50 kDa and 25 kDa correspond to the Ig H- and L-chains of the RA77 and hIgG1 control antibody. The positions of molecular mass standards are indicated on the left. (B) Verification of the specificity of RA77 for p41. The data are the results of an ELISA experiment with RA77 binding to recombinant p41 (recom. P41) and BSA (negative control [neg.]). OD450, optical density at 450 nm. (C) Different binding affinities of RA77 to native B. burgdorferi lysate and recombinant p41. The data are the results of competition ELISAs of biotinylated and nonbiotinylated RA77 for binding to B. burgdorferi lysate (Bb lysate) (□) (Ki = 10−9) and recombinant p41 (p41) (▪) (Ki = 10−6). Bio-Ab, biotinylated antibody; Ab, antibody.
Recombinant antibody RA17 is specific for autoantigens in CNS myelin.
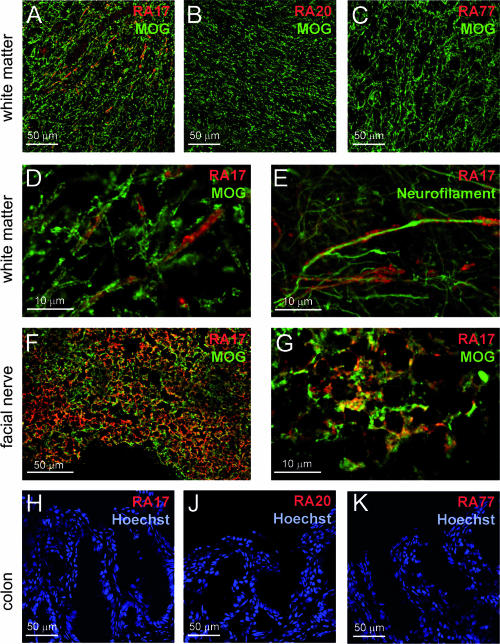
Immunofluorescence staining of cryostat sections of frozen human facial nerve tissue showed the reactivity of RA17 with the myelin component of the nerve, as identified by costaining with the myelin-specific antibody 8-18c5 (Fig. 3F and G). Further immunofluorescence staining of human white matter tissue revealed the specificity of RA17 for structures also stained with the myelin antibody 8-18c5 (Fig. 3A and D). The structures stained by RA17 enfold neurons, as demonstrated by double-immunofluorescence staining with a neurofilament-specific antibody, thus indirectly confirming the myelin reactivity of RA17 (Fig. 3E). Neither Borrelia-specific RA77 antibody, antibody RA20, nor the human IgG1 isotype control showed any reactivity with human CNS tissue (Fig. 3B and C). None of the antibodies examined (RA17, RA20, and RA77) showed staining greater than that of controls with human colon (Fig. 3H to K), kidney, and lung tissues (not shown).
FIG. 3.
CNS reactivity of RA17: micrographs of immunofluorescence staining of human white matter (A to E), facial nerve (F and G), and colon (H to K) tissue. White matter staining was performed with myelin-specific antibody 8-18c5 (A to D), RA17 (A), RA20 (B), and RA77 (C). (D) Greater magnification of panel A. (E) Staining of white matter tissue with neurofilament-specific antibody and RA17. (F) Staining of human facial nerve tissue with myelin-specific antibody 8-18c5 and RA17. (G) Greater magnification of panel F. (H to K) Staining of human colon tissue with RA17 (H), RA20 (J), and RA77 (K). Nuclei were stained with Hoechst 55432 (blue).
DISCUSSION
In order to investigate characteristics of the humoral immune response in the CSF of a patient with a typical course of NB, we sought to resurrect the antigen specificities of antibodies from CSF-derived cePC by recombinant reconstruction of their secreted IgG. Instead of analyzing the clonally diverse CSF Ig itself, we dissected the antigen-driven immune responses in the CSF down to the clonal level, which made it possible to discern the antigen specificity of individual expanded CSF plasma cell clones. We validated our system by recombinant expression of a well-characterized MAb (8-18c5), demonstrating that our methodology preserves both the specificity and the affinity of the antibodies secreted by the “original” antibody-producing cells.
By means of assembly of Ig H-chain sequences with identical CDR3 regions, we were able to identify three expanded plasma cell clones in the CSF of a patient with NB (RA17, RA20, and RA77). The VH sequences (n = 4) contained in the RA17 group had a number of nucleotide mutations, suggesting that these sequences were the result of somatic hypermutation during affinity maturation. No mutations were found in the RA20 and RA77 groups of sequences. However, a comparison with germ line V-region segments revealed mutations in the H- and L-chain V-region sequences in all groups (Table 1), thus providing additional evidence that the antibodies had undergone affinity maturation.
Of the recombinant human MAbs derived from the groups containing identical H-chain CDR3 regions, one (RA77) specifically reacted with B. burgdorferi, the causative infectious agent of NB, as determined by ELISA and immunofluorescence staining of B. burgdorferi with RA77. No cross-reactivity of RA77 with T. pallidum spirochetes or E. coli could be detected, indicating that RA77 is specific for B. burgdorferi. This finding of a B. burgdorferi-specific antibody produced by a cePC is consistent with previous studies showing that OCB in the CSF of NB patients contain antibodies directed against B. burgdorferi (10, 15), particularly in view of recent data from our laboratory supporting the conclusion that cePC are in fact the producers of OCB (unpublished data). To date, the only other inflammatory disease for which a link between CNS-derived plasma cells and the causative agent has been demonstrated is subacute sclerosing panencephalitis. Burgoon et al. obtained measles virus-specific antibodies from individual plasma cells excised from postmortem brain tissue of a patient with subacute sclerosing panencephalitis by laser capture microdissection (6). Our findings, together with those of Burgoon et al., suggest that such an experimental setup may be helpful in identifying disease-relevant target antigens in neuroinflammatory diseases of unknown origin.
To further analyze the exact antigen specificity of the B. burgdorferi-reactive RA77 MAb, we performed immunoprecipitation and subsequent MALDI-TOF analyses. In this way, we were able to identify the cognate antigen of RA77, namely, the B. burgdorferi flagellum protein p41. While we found a high-affinity interaction between antibody RA77 and native B. burgdorferi lysate, the affinity to recombinant p41 protein was significantly lower. This finding suggests that the interaction between RA77 and native p41 may depend not only on the amino acid sequence and resulting conformation but also on potential posttranslational modifications. Such modifications have been demonstrated in flagellum proteins of other spirochetes (3, 13, 23). Another explanation for the lower affinity of antibody RA77 for the recombinant p41 protein than for the native protein may be partial misfolding of the recombinant protein. Nevertheless, the high-affinity interaction between RA77 and native B. burgdorferi lysate suggests that RA77 is the result of an affinity-matured antibody response.
Because the patient whose cePC we used in this study suffered from facial nerve palsy, a common clinical presentation of NB, we investigated the possible specificity of RA17, RA20, and RA77 for facial nerve tissue. Interestingly, one of these antibodies, RA17, displayed reactivity with this tissue. To further evaluate the tissue components that RA17 is specific for, we performed double-immunofluorescence staining with a myelin-specific antibody (8-18c5), which strongly suggested myelin reactivity of RA17. Although the results did not overlap completely, costaining with the 8-18c5 antibody and RA17 indicated reactivity with identical structures, namely myelin. However, the myelin reactivity of RA17 was not limited to facial nerve tissue but also applied to CNS white matter myelin. Double staining with RA17 and a neurofilament-specific antibody indirectly confirmed this notion, as RA17 stained structures enfolding neurons. Immunofluorescence staining with RA17 of a number of different human control tissues (colon, kidney, and lung) yielded negative results, suggesting CNS-restricted reactivity. This autoimmune reactivity is consistent with previous studies hypothesizing that the immune responses taking place in NB may also have autoimmune features (11). The origin of autoimmunity in NB could be either cross-reactivity of Borrelia-specific antibodies with epitopes shared with human tissues (“molecular mimicry”), such as that observed in Lyme arthritis (9), or an immune response triggered by the emergence of novel epitopes during infection-mediated tissue destruction (19). Because no reactivity of RA17 with B. burgdorferi was observed, in the case of this MAb the latter hypothesis may be favored. While we could determine the specificity for the cePC RA17 and RA77, the antigens that originally drove the B-cell response into clonal expansion and final-stage maturation into plasma cells within the CNS compartment remain undetermined for RA20. Nevertheless, our data suggest that autoimmunity is a feature represented within the cePC repertoire analyzed here. Future work should provide further insight into the functional role of such autoreactive antibodies in NB.
In conclusion, by recombinant reconstruction of the antigen specificities of antibodies secreted by individual expanded CSF plasma cell clones, we were able to dissect complex repertoires of the adaptive immune system in response to CNS infection with B. burgdorferi. We provide evidence that the antigen-driven immune response in NB not only results in high-affinity pathogen-specific antibodies but can also lead to the generation of humoral autoimmunity apparently independent of mechanisms such as “molecular mimicry.”
Acknowledgments
We thank Eva Niederer (Institut fur Biomedizinische Technik, ETH Zurich) for performing the fluorescence-activated cell sorter analyses, Ingrid Briod for technical assistance, S. Chesnov and P. Hunziker (University of Zurich, Functional Genomics Center Zurich) for mass spectrometric measurements, and A. Fontana for critical discussions. Human tissue was kindly provided by the NICHD Brain and Tissue Bank for Developmental Disorders under contracts N01-HD-4-3368 and N01-HD-4-3383. Additional reagents were generously provided by V. Fingerle, C. Linington, O. Peter, and H. Wardemann, as indicated in the text.
S.K. is the recipient of a doctoral fellowship from the Betty and David Koetser Foundation for Brain Research. Funding for this study was provided by NCCR “Neural Plasticity and Repair,” Neuroscience Center Zurich, Serono, the Swiss Multiple Sclerosis Society, the Swiss National Science Foundation, and the University of Zurich.
Editor: F. C. Fang
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Andris-Widhopf, J., P. Steinberger, P. Fuller, C. Rader, and C. F. Barbas III. 2001. Generation of antibody libraries: PCR amplification and assembly of light- and heavy-chain coding sequences, p. 9.1-9.113. In C. F. Barbas III, D. R. Burtoon, J. K. Scott, and G. J. Silverman (ed.), Phage display: a laboratory manual. CSHL Press, Cold Spring Harbor, NY.
- 2.Benach, J. L., E. M. Bosler, J. P. Hanrahan, J. L. Coleman, G. S. Habicht, T. F. Bast, D. J. Cameron, J. L. Ziegler, A. G. Barbour, W. Burgdorfer, R. Edelman, and R. A. Kaslow. 1983. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 308:740-742. [DOI] [PubMed] [Google Scholar]
- 3.Brahamsha, B., and E. P. Greenberg. 1988. Biochemical and cytological analysis of the complex periplasmic flagella from Spirochaeta aurantia. J. Bacteriol. 170:4023-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breithaupt, C., A. Schubart, H. Zander, A. Skerra, R. Huber, C. Linington, and U. Jacob. 2003. Structural insights into the antigenicity of myelin oligodendrocyte glycoprotein. Proc. Natl. Acad. Sci. USA 100:9446-9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis. Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 6.Burgoon, M. P., K. M. Keays, G. P. Owens, A. M. Ritchie, P. R. Rai, C. D. Cool, and D. H. Gilden. 2005. Laser-capture microdissection of plasma cells from subacute sclerosing panencephalitis brain reveals intrathecal disease-relevant antibodies. Proc. Natl. Acad. Sci. USA 102:7245-7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, C. A., and H. Okayama. 1988. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. BioTechniques 6:632-638. [PubMed] [Google Scholar]
- 8.Cheng, Y., and W. H. Prusoff. 1973. Relationship between inhibition constant (K1) and concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22:3099-3108. [DOI] [PubMed] [Google Scholar]
- 9.Gross, D. M., T. Forsthuber, M. Tary-Lehmann, C. Etling, K. Ito, Z. A. Nagy, J. A. Field, A. C. Steere, and B. T. Huber. 1998. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 281:703-706. [DOI] [PubMed] [Google Scholar]
- 10.Hansen, K., M. Cruz, and H. Link. 1990. Oligoclonal Borrelia burgdorferi-specific IgG antibodies in cerebrospinal fluid in Lyme neuroborreliosis. J. Infect. Dis. 161:1194-1202. [DOI] [PubMed] [Google Scholar]
- 11.Hemmer, B., B. Gran, Y. Zhao, A. Marques, J. Pascal, A. Tzou, T. Kondo, I. Cortese, B. Bielekova, S. E. Straus, H. F. McFarland, R. Houghten, R. Simon, C. Pinilla, and R. Martin. 1999. Identification of candidate T-cell epitopes and molecular mimics in chronic Lyme disease. Nat. Med. 5:1375-1382. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser, R. 1995. Intrathecal immune response in patients with neuroborreliosis: specificity of antibodies for neuronal proteins. J. Neurol. 242:319-325. [DOI] [PubMed] [Google Scholar]
- 13.Li, Z., F. Dumas, D. Dubreuil, and M. Jacques. 1993. A species-specific periplasmic flagellar protein of Serpulina (Treponema) hyodysenteriae. J. Bacteriol. 175:8000-8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lunemann, J. D., H. Gelderblom, M. Sospedra, J. A. Quandt, C. Pinilla, A. Marques, and R. Martin. 2007. Cerebrospinal fluid-infiltrating CD4+ T cells recognize Borrelia burgdorferi lysine-enriched protein domains and central nervous system autoantigens in early Lyme encephalitis. Infect. Immun. 75:243-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin, R., U. Martens, V. Sticht-Groh, R. Dorries, and H. Kruger. 1988. Persistent intrathecal secretion of oligoclonal, Borrelia burgdorferi-specific IgG in chronic meningoradiculomyelitis. J. Neurol. 235:229-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin, R., J. Ortlauf, V. Sticht-Groh, U. Bogdahn, S. F. Goldmann, and H. G. Mertens. 1988. Borrelia burgdorferi-specific and autoreactive T-cell lines from cerebrospinal fluid in Lyme radiculomyelitis. Ann. Neurol. 24:509-516. [DOI] [PubMed] [Google Scholar]
- 17.Owens, G. P., A. M. Ritchie, M. P. Burgoon, R. A. Williamson, J. R. Corboy, and D. H. Gilden. 2003. Single-cell repertoire analysis demonstrates that clonal expansion is a prominent feature of the B cell response in multiple sclerosis cerebrospinal fluid. J. Immunol. 171:2725-2733. [DOI] [PubMed] [Google Scholar]
- 18.Pfister, H. W., B. Wilske, and K. Weber. 1994. Lyme borreliosis: basic science and clinical aspects. Lancet 343:1013-1016. [DOI] [PubMed] [Google Scholar]
- 19.Rosen, A., and L. Casciola-Rosen. 2001. Clearing the way to mechanisms of autoimmunity. Nat. Med. 7:664-665. [DOI] [PubMed] [Google Scholar]
- 20.Schrimpf, S. P., H. Langen, A. V. Gomes, and C. Wahlestedt. 2001. A two-dimensional protein map of Caenorhabditis elegans. Electrophoresis 22:1224-1232. [DOI] [PubMed] [Google Scholar]
- 21.Steere, A. C., R. L. Grodzicki, A. N. Kornblatt, J. E. Craft, A. G. Barbour, W. Burgdorfer, G. P. Schmid, E. Johnson, and S. E. Malawista. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733-740. [DOI] [PubMed] [Google Scholar]
- 22.Wardemann, H., S. Yurasov, A. Schaefer, J. W. Young, E. Meffre, and M. C. Nussenzweig. 2003. Predominant autoantibody production by early human B cell precursors. Science 301:1374-1377. [DOI] [PubMed] [Google Scholar]
- 23.Wyss, C. 1998. Flagellins, but not endoflagellar sheath proteins, of Treponema pallidum and of pathogen-related oral spirochetes are glycosylated. Infect. Immun. 66:5751-5754. [DOI] [PMC free article] [PubMed] [Google Scholar]