Abstract
Clostridium difficile toxins A and B (TcdA and TcdB) are the causative agents of antibiotic-associated pseudomembranous colitis. Mucosal mast cells play a crucial role in the inflammatory processes underlying this disease. We studied the direct effects of TcdA and TcdB on the human mast cell line HMC-1 with respect to degranulation, cytokine release, and the activation of proinflammatory signal pathways. TcdA and TcdB inactivate Rho GTPases, the master regulators of the actin cytoskeleton. The inactivation of Rho GTPases induced a reorganization of the actin cytoskeleton accompanied by morphological changes of cells. The TcdB-induced reorganization of the actin cytoskeleton in HMC-1 cells reduced the number of electron-dense mast cell-specific granules. Accordingly, TcdB induced the release of hexosaminidase, a marker for degranulation, in HMC-1 cells. The actin rearrangement was found to be responsible for degranulation since latrunculin B induced a comparable hexosaminidase release. In addition, TcdB as well as latrunculin B induced the activation of p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase 1/2 and also resulted in a p38 MAPK-dependent increased formation of prostaglandins D2 and E2. The autocrine stimulation of HMC-1 cells by prostaglandins partially contributed to the degranulation. Interestingly, TcdB-treated HMC-1 cells, but not latrunculin B-treated HMC-1 cells, showed a strong p38 MAPK-dependent increase in interleukin-8 release. Differences in the mast cell responses to TcdB and latrunculin B are probably due to the presence of functionally inactive Rho GTPases in toxin-treated cells. Thus, the HMC-1 cell line is a promising model for studying the direct effects of C. difficile toxins on mast cells independently of the tissue context.
The anaerobic, gram-positive bacterium Clostridium difficile is the causative pathogen of nosocomial C. difficile-associated diarrhea and pseudomembranous colitis, the latter being characterized by profound colonic inflammation (10, 29). Pseudomembranous colitis is an economic problem, as an estimated 1 to 3% of all hospitalized patients under antibiotic treatment become infected with C. difficile (4). Furthermore, the incidence and severity of C. difficile-associated diarrhea seem to be increasing (34). The major virulence factors are two exotoxins, toxin A (TcdA) and toxin B (TcdB).
Because TcdA is capable of inducing all symptoms of pseudomembranous colitis in an animal model, studies of the pathomechanism focus solely on the toxins. Epithelial cells, neurons of the enteric nervous system, and mast cells are the major players in inflammation. All cells affected by these toxins release mediators that govern the inflammatory process of the intestine. The intestinal epithelium, the initial target of the toxins, responds by secreting interleukin-8 (IL-8) (15) and other C-X-C or C-C chemokines, like GROα, MCP-1, and MIP-2, as shown in HT29 cells (24). It is assumed that the cytokines secreted from the epithelium contribute to the activation of the sensory neurons of the enteric nervous system (7). Neunlist and coworkers described the IL-1β-dependent activation of the transcription factor c-Fos of submucosal vasoactive intestinal peptide-positive neurons of human colon segments after the application of TcdB (32). Furthermore, cells of the enteric nervous system respond to TcdB by IL-1β-mediated IL-8 secretion involving p38 mitogen-activated protein kinase (p38 MAPK) and extracellular signal-regulated kinase 1/2 (ERK1/2) (40).
In addition, studies of the involvement of mast cells in the inflammatory response to TcdA demonstrated the central role of this cell population. The depletion of mast cells significantly diminished intestinal inflammation in a KitW/KitW−v mast cell-deficient mouse model (44). The reconstitution of mast cells resulted in a TcdA-induced fluid secretion comparable to that of wild-type mice. In a second study, Pothoulakis and coworkers stabilized mucosal mast cells by ketotifen, thereby reducing the severity of secretory diarrhea and inflammation (36). Ketotifen inhibited the TcdA-induced release of mast cell mediators such as the leukotrienes B4 and C4 and rat mast cell protease II. However, the role of intestinal mast cells in C. difficile-induced inflammation is still unclear (36, 44). So far, rat basophilic leukemia (RBL) cells and murine peritoneal mast cells have been used to characterize the direct effects of the toxins on isolated mast cells. In both models, TcdB inhibits FcɛR- or calcium-mediated degranulation and histamine release, as has been shown by a hexosaminidase assay (8, 37, 45). In addition, TcdB also hampered phagocytosis in RBL cells (28). These studies implicate an inhibition rather than a stimulation of mast cell functions by TcdB. This is in line with the underlying molecular mechanism: TcdA and TcdB are glucosyltransferases that transfer a glucose moiety onto the Rho family of GTPases (Rho, Rac, and Cdc42) (20, 22). Monoglucosylation at Thr-37 of Rho (Thr-35 of Rac and Cdc42) causes functional inactivation and thus blocks downstream signaling. At least Rac1 and Cdc42 were reported to be positively involved in degranulation events in RBL mast cells (16, 17). The most prominent effect of the TcdB-catalyzed inactivation of Rho GTPases is the reorganization of the actin cytoskeleton, leading to a rounding of cells (19). Since FcɛR clustering and endocytosis-dependent receptor recycling are disturbed by the breakdown of the actin filaments, this effect should be expected to contribute to mast cell silencing. Besides organizing the actin cytoskeleton, Rho family GTPases rule other signaling pathways that are also involved in proinflammatory processes. The mitogen-activated protein kinases p38 MAPK, ERK1/2, and the c-Jun N-terminal protein kinase (JNK) activate transcription factors that are implemented in the expression of genes for inflammatory mediators (27). Rac GTPases are upstream of these MAPKs, and dominant negative forms of Rac are able to block signal transduction towards p38 MAPK and ERK1/2 (3, 27, 43, 46). As a result of the inhibition of Rho subfamily GTPases, anti-inflammatory effects of TcdA and TcdB should be expected (48).
The following study focuses on the immature mast cell line HMC-1 because of its human origin, and despite its limitations, it should be more appropriate as a human model than a rat-derived cell line (5). By applying TcdA and TcdB directly to these cells, we observed stimulatory effects, such as IL-8 secretion, prostaglandin synthesis, and degranulation events. These results underline the important role of mast cells in the pathogenesis of TcdA- or TcdB-induced colitis. Furthermore, the study characterizes an alternative model system for the investigation of the molecular mechanisms underlying the inflammatory processes of pseudomembranous colitis.
MATERIALS AND METHODS
Materials.
All cell culture media were from Biochrom, Germany. The cell culture plasticware was from Nunc, Germany. The water-soluble tetrazolium salt test kit was obtained from Roche, Germany. We also obtained glutaraldehyde (Polysciences, PA); sodium cacodylate (Merck, Germany); osmium tetroxide (Polysciences); ethanol (Baker, The Netherlands); 1,2-propylene oxide (Merck, Germany); latrunculin B, nocodazol, phorbol myristic acid (PMA), A23187, and SB202190 (Calbiochem, Germany); prostaglandin E2 (PGE2) and indomethacin (Sigma, Germany); p-nitrophenyl-N-acetyl-β-d-glucosaminide (ICN, Germany); monoclonal anti-phospho-p38 MAPK (Cell Signaling, Germany); monoclonal anti-phospho ERK1/2 (clone MAPK-YT) and monoclonal anti-β-actin (clone AC-15) (Sigma, Germany); anti-mouse peroxidase conjugate and anti-rabbit peroxidase conjugate (Rockland); and rhodamine-labeled phalloidin (Molecular Probes, Germany).
Cell culture.
Human mast cells from the cell line HMC-1 were grown in Iscove's basal medium supplemented with 100 μM penicillin, 100 μg/ml streptomycin, and 0.01% (vol/vol) monothioglycerol. The medium was changed every three days. Before the experiments were started, the cells were serum starved overnight. For the semiadherent culture of HMC-1, cells were seeded on fibronectin-coated cell culture dishes 16 h prior to the experiments. The cells were washed twice with prewarmed (37°C) Iscove's basal medium without phenol red and resuspended to a concentration of 2 × 106 cells per ml prior to the toxin treatment. Aliquots of 0.2 × 106 cells were used for hexosaminidase assays and an IL-8 enzyme-linked immunosorbent assay (ELISA).
RBL cells were grown in minimum essential medium-Earle salts supplemented with 100 μM penicillin and 100 μg/ml streptomycin. The cells were passaged twice a week and seeded onto 24-well dishes for experiments. If not indicated otherwise, HMC-1 cells were treated with 3 nM (1 μg/ml) TcdA or TcdB, and RBL cells were treated with 1 nM (330 ng/ml) TcdA or TcdB. Toxin treatment was performed in 1 ml Iscove's basal medium without phenol red for 5 h or as indicated. The viability of HMC-1 and RBL cells was not affected by toxin treatment for 5 hours as verified by a water-soluble tetrazolium salt test and trypan blue exclusion.
Expression and purification of toxins.
TcdA and TcdB from the culture supernatant of C. difficile were purified by ion-exchange chromatography using a MonoQ column (Amersham Bioscience, Germany) as described by Just et al. (21). The TcdA fraction of the eluate was further purified by affinity chromatography on immobilized thyroglobulin as described by Krivan and Wilkins (26). The preparation of the glutathione S-transferase fusion protein of Clostridium botulinum exoenzyme C3 was performed as described in detail by Ahnert-Hilger et al. (2). The fusion toxin was purified by glutathione-Sepharose (Amersham Biosciences) according to a standard protocol.
Electron microscopy.
Semiadherent HMC-1 cells were treated with TcdB under conditions where cells remained attached to the substratum (3 nM, for only 90 min). Cells were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate, pH 7.3. After postfixation in 2% osmium tetroxide in the same buffer, the cells were dehydrated with increasing concentrations of ethanol. The cells were detached from the culture dishes with 1,2-propylene oxide and immediately embedded in epoxy resin (Serva, Heidelberg, Germany). Thin sections were stained with uranyl acetate and lead citrate and analyzed using the Philips 301 electron microscope at a ×71,000 magnification for quantitative evaluation. The numbers of mast cell-specific granules per cell were counted in 40 control cells and in 40 TcdB-treated cells sectioned through the cell center.
Hexosaminidase assay.
Cells were incubated with latrunculin B, nocodazol, PMA, A23187, SB202190, PGE2, indomethacin (the last two were from Sigma, Germany), or TcdA and TcdB as indicated in Fig. 3. The hexosaminidase assay was performed as described by Djouder et al. (8). In brief, cells (0.2 × 106) were stimulated in Tyrode solution and harvested by centrifugation, and 30-μl aliquots of each supernatant were incubated with 50 μl substrate solution (1.3 mg/ml p-nitrophenyl-N-acetyl-β-d-glucosaminide in 100 mM sodium citrate, pH 4.5) in a microtiter plate for 1 h at 37°C. The reaction was stopped by the addition of 50 μl of 400 mM glycine, pH 10.7. The absorbance was measured with an ELISA reader at 405 nm.
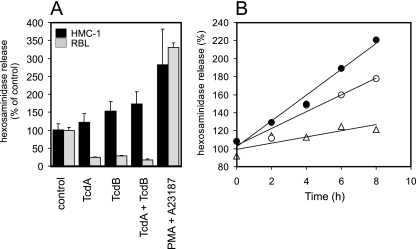
FIG. 3.
Hexosaminidase release of TcdA- and TcdB-treated HMC-1 and RBL cells. (A) TcdA and TcdB have divergent effects on HMC-1 and RBL cells. The release of hexosaminidase was increased in HMC-1 cells after treatment for 5 h with 6 nM TcdA (122% ± 25%), 6 nM TcdB (154% ± 26%), and a combination of TcdA and TcdB, each at 6 nM (174% ± 33%), compared to the release in controls (100% ± 18%). As a positive control, the release induced with PMA and A23187 was used (281% ± 100%). In contrast to what occurred in HMC-1 cells, TcdA (1 nM) and TcdB (1 nM) decreased the hexosaminidase release in RBL cells to 24% ± 2% and 28% ± 3%, respectively. The combination of both toxins also reduced the release to 18% ± 8%. As a positive control, PMA-A23187 induced 331% ± 12% of the hexosaminidase release of the controls (35 experiments with HMC-1 cells and 6 experiments with RBL cells; all values show significant changes from values for the controls). (B) Time-dependent hexosaminidase release of untreated HMC-1 cells (open triangles) or cells treated with TcdB (6 nM; open circles) or latrunculin B (Lat B; 2 μg/ml; filled circles).
ADP ribosylation of cell lysates.
Toxin-treated human mast cells (0.5 × 106) were harvested by centrifugation. The supernatant was removed, and cells were lysed by sonication in 50 μl ribosylation buffer (50 mM HEPES [pH 7.4], 10 mM thymidine, 5 mM MgCl2, 2.5 mM dithiothreitol, and 2.5 mM NAD). To start ADP ribosylation, 0.5 μCi [32P]NAD (3,000 μCi/mmol) (NEN Life Science, Germany) and 100 ng glutathione S-transferase-C3 exoenzyme were added. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The gel was dried and exposed on a screen for filmless autoradiography.
Immunoblots.
Cells were harvested by centrifugation at 1,000 × g for 3 min. The pellets were resuspended in 50 μl phosphate-buffered saline (PBS) containing 0.5% Triton X-100, 1 mM Na-orthovanadate, 100 μM phenylmethylsulfonyl fluoride, and 10 μg/ml leupeptin. After 5 min of incubation on ice, the cells were centrifuged at 14,000 × g for 10 min. Ten microliters of 5× Laemmli sample buffer was added to the supernatant, which was boiled for 3 min at 95°C and subjected to sodium dodecyl sulfate-gel electrophoresis. Western blotting was generally performed by incubating nitrocellulose with primary antibody overnight at 4°C. The detection of peroxidase-conjugated secondary antibody was performed using enhanced chemiluminescence substrate (SuperSignal West Femto; Pierce, Germany).
F-actin staining.
Semiadherent HMC-1 cells were treated as indicated in the legend to Fig. 3B, and incubation was stopped by rinsing the cells with ice-cold PBS. Cells were fixed with 3% (wt/vol) paraformaldehyde and permeabilized with 0.1% Triton X-100 in PBS for 10 min at room temperature. Subsequently, cells were incubated with 3 U/ml rhodamine-labeled phalloidin for 30 min at room temperature. The cells were then subjected to fluorescence microscopy (Zeiss, Germany).
Quantification of prostaglandins.
Frozen cell suspensions (1 ml) were treated with an ethanolic solution (400 μl) of the tetradeuterated internal standards (each, 500 pg) [3,3′,4,4′-2H4]PGE2 and [3,3′,4,4′-2H4]PGD2 (each, >98% 2H; Cayman Chemical, Ann Arbor, MI). Samples were thawed, mixed, and evaporated to dryness; residues were reconstituted with 0.1 M formic acid (1 ml); and prostaglandins were immediately extracted with ethyl acetate (2 ml) by vortex mixing for 1 min. The organic phase was dried over anhydrous sodium sulfate, and ethyl acetate was evaporated. Subsequently, samples were processed and analyzed by gas chromatography-tandem mass spectrometry (GC-tandem MS) as described elsewhere (42). Briefly, endogenous prostaglandins and their stable-isotope-labeled analogs were converted to their pentafluorobenzyl methoxime trimethylsilyl ether derivatives. GC-tandem MS analyses were carried out using a Thermoquest triple-stage quadrupole mass spectrometer, model TSQ 7000, interfaced with a Thermoquest gas chromatograph, model Trace 2000 (Egelsbach, Germany). A fused-silica capillary column (model VF 17, 30-m by 0.25-mm inside diameter, 0.25-μm film thickness) from Varian Deutschland (Darmstadt, Germany) was used. Quantification was performed by selected-reaction monitoring of the product ions at m/z 268 for the endogenous compounds and m/z 272 for the tetradeuterated analogs, which were generated by the collision-induced dissociation of the parent [M-pentafluorobenzyl]− ions at m/z 524 and m/z 528, respectively. Isomeric PGE2 and PGD2 possess the same parent and product ions, but their derivatives were completely separated by GC.
Flow cytometry.
Flow cytometry (fluorescence-activated cell sorter [FACS] analysis) was performed to estimate the number of apoptotic and necrotic cells that show less DNA content than cells within the G1 phase. HMC-1 cells were treated as indicated in the legend to Fig. 4. Cells (5 × 105) were fixed in ice-cold ethanol (70%) for 30 min. After being washed once with 1% bovine serum albumin in PBS, the DNA was stained using the fluorescent nucleotide acid dye propidium iodide (150 μg/ml in Tris-HCl, pH 7.4, containing 1% bovine serum albumin and 1% Triton X-100). RNA was removed by incubating the cells with 0.5% RNase for 30 min. Subsequently, the cells were subjected to FACS analysis. A fluorescence area of 400 was set to correlate with a 2n set of chromosomes within the G1 phase. Cells found in the sub-G1 phase were considered apoptotic and necrotic due to the decrease in DNA content.
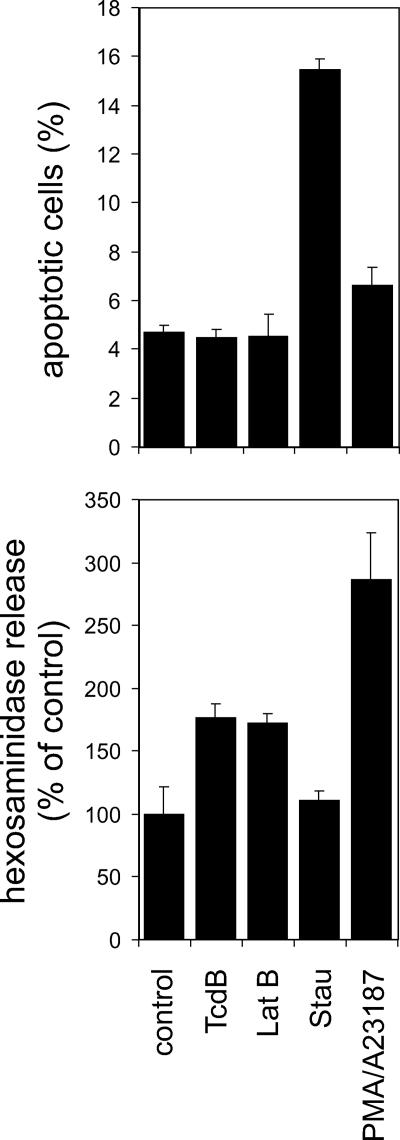
FIG. 4.
TcdB-induced hexosaminidase release does not correlate with cell death. The percentages of apoptotic cells among control cells and among cells treated for 5 h with TcdB (6 nM), latrunculin B (Lat Β, 2 μg/ml), staurosporin (Stau, 1 mg/ml), or PMA (100 nM)-ionophore A23187 (10 μM) were estimated by FACS analysis (upper panel). The corresponding hexosaminidase release is shown in the lower panel.
IL-8 ELISA.
For the measurement of IL-8 secretion, 100-μl supernatants of 106 cells were used for an IL-8-specific ELISA (PromoKine, Heidelberg, Germany). The ELISA was performed according to the supplied protocol.
Statistics.
All values are expressed as means ± standard deviations. For the statistical evaluation, Student's t test was performed, and P values of <0.05 were considered significant.
RESULTS
Glucosylation of Rho GTPases caused morphological changes of HMC-1 cells.
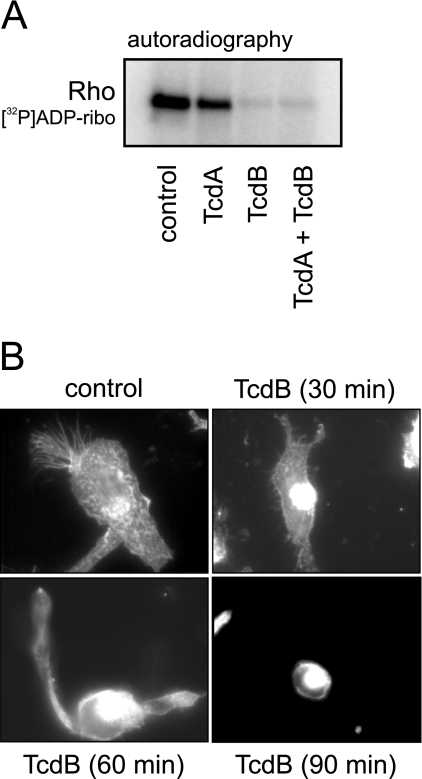
The biological activities of TcdA and TcdB are mediated by their inherent glucosyltransferase activity. Intracellular toxin activity can be checked by means of the glucosylation state of target Rho GTPases by applying sequential ADP ribosylation. The rationale of sequential ADP ribosylation is that cellular RhoA glucosylated by TcdA/B is not a substrate for subsequent exoenzyme C3-catalyzed [32P]ADP ribosylation. An alternative approach is to detect the functional outcome of Rho glucosylation, i.e., the rounding of cells and the reorganization of the actin cytoskeleton. The treatment of nonadherent HMC-1 cells with TcdA or TcdB (both 6 nM) for 5 h caused a 50% or 90%, respectively, decrease in RhoA [32P]ADP ribosylation, reflecting the extent of intracellular Rho glucosylation (Fig. 1A). Furthermore, this experiment pointed out the different cytopathic potencies of TcdA and TcdB. The cytopathic effect of TcdB was also checked on semiadherent HMC-1 cells that were seeded onto fibronectin-coated cell culture dishes (Fig. 1B). The treatment of cells with 3 nM TcdB for 90 min resulted in the rounding of cells accompanied by the development of retraction fibers. The rhodamine-phalloidin staining showed stress fibers as well as granulated actin accumulations homogeneously distributed within the control cells. One brush-like structure of actin filaments per cell is typical and indicative of the polarization of the cell. TcdB caused the disappearance of the brush-like structure and the condensation of actin in the perinuclear region. Complete cell rounding was observed after 90 min.
FIG. 1.
Effects of TcdA and TcdB on Rho GTPases and on the actin cytoskeleton. (A) Autoradiography of sequential [32P]ADP ribosylation ([32P]ADP-ribo) of cell lysates from control HMC-1 cells and cells treated with TcdA and TcdB for 5 h. [32P]ADP ribosylation of lysates from toxin-treated cells indicates the amount of nonglucosylated Rho GTPases. (B) Rhodamine-phalloidin staining of the actin cytoskeleton. TcdB induced a time-dependent shrinkage of cells and a reorganization of the actin cytoskeleton.
TcdA and TcdB induced the degranulation of HMC-1 cells.
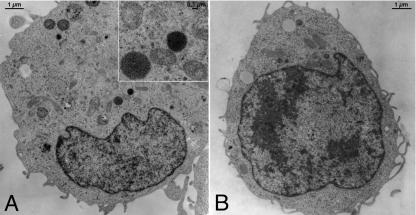
Electron microscopy studies showed that the semiadherent HMC-1 cells contained mast cell-specific granules (Fig. 2A). Since semiadherent cells detach after treatment with 6 nM TcdB for 60 min, cells were treated with only 3 nM TcdB for 90 min before the performance of electron microscopy of attached cells. After treatment with TcdB, the cells became rounded and displayed slender microvilli. Signs of necrosis or apoptosis were not detected. In comparison to that of control cells, there was no change in the ultrastructure of the organelles of the treated cells. Uniquely, the mast cell-specific electron-dense granules were lost (Fig. 2B). The quantitative analysis of mast cells, sectioned through the middle axis, confirmed these significant differences in the cellular contents of specific granules. Whereas in controls, six ± three specific granules per cell (n = 40) were counted, only one ± one mast cell-specific granule per cell (n = 40) remained after TcdB treatment (P < 0.001). Although no exocytotic fusion process could directly be visualized by electron microscopy, the presented data suggest that the toxin induces the exocytosis of mast cell-specific granules.
FIG. 2.
Electron microscopy of a semiadherent HMC-1 cell without (A) and with (B) treatment with TcdB (3 nM) for 90 min. Typical mast cell-specific granules (granules filled with electron-dense [black] material; inset in panel A) are present in these cells, which were presumably exocytosed after stimulation by TcdB.
The degranulation of HMC-1 cells was studied by analyzing the effects of TcdA and TcdB on hexosaminidase release. In general, hexosaminidase is an accepted marker for histamine release and, therefore, provides a convenient means to estimate mast cell degranulation (39). The treatment of HMC-1 cells with TcdA or TcdB, 6 nM each, resulted in increases in the release of hexosaminidase of 122% ± 25% (TcdA) and 154% ± 26% (TcdB) after 5 h (Fig. 3A). Simultaneous treatment with both toxins further increased hexosaminidase release up to 174% ± 33% of that of controls. The maximum level of physiological release was estimated by the treatment of cells with 100 ng/ml PMA and a 10 μM concentration of the calcium ionophore A23187, resulting in a hexosaminidase release level 281% ± 100% of that of controls. This amount reflected approximately 30% of the total hexosaminidase content of these cells as measured after lysis by Triton X-100 (data not shown). Since a toxin-induced degranulation event was inconsistent with published data based on the mast cell model RBL cells (9), we also studied hexosaminidase release from adherent RBL cells. As these cells are an order of magnitude more sensitive to TcdB than HMC-1 cells, one should expect an even higher level of release of hexosaminidase. However, the degranulation of these cells was strongly reduced by both toxins down to values that were 24% ± 2% (TcdA, 1 nM) and 28% ± 3% (TcdB, 1 nM) of control values. The PMA-A23187-induced hexosaminidase release, however, was comparable to that of HMC-1 cells, with values of 331% ± 12%. Thus, HMC-1 cells in fact behave differently from RBL cells. We next compared the TcdB-induced effects with those caused by latrunculin B, a sponge-derived potent agent directly inducing the depolymerization of F-actin. Figure 3B shows representative time courses of hexosaminidase release induced by TcdB or latrunculin B. Both agents induced an increase in hexosaminidase release with a rate of 9% per hour (TcdB) and 14% per hour (latrunculin B) compared to 3.5% per hour for nontreated controls. The level of hexosaminidase release continuously increased over the observed time period of 8 h, and thus, degranulation was not a single event but a continuous process. The disintegration of the microtubular system did not affect degranulation as tested with nocodazole (data not shown).
A correlation of hexosaminidase release and apoptosis was studied by flow cytometry (FACS). FACS analysis revealed that HMC-1 cells showed no signs of apoptosis or necrosis within 5 h of treatment (Fig. 4, upper panel). The relative amount of apoptotic cells did not increase by treatment either with 6 nM TcdB (4.5% ± 0.4%) or with 2 μg/ml latrunculin B (4.5% ± 0.9%) in comparison to the amount in control cells (4.7% ± 0.3%). The positive-control staurosporin (1 μg/ml) induced apoptosis in 15.5% ± 0.5% of cells, whereas the combination of PMA (100 ng/ml) and A23187 (10 μM) induced apoptosis in 6.6% ± 0.7% of cells. The supernatants of cells used for FACS analysis were further tested for hexosaminidase (Fig. 4, lower panel). Compared to the level of induction in control cells (100% ± 22%), TcdB and latrunculin B induced the release of 177% ± 11% and 171% ± 9% as much hexosaminidase, respectively. Staurosporin induced the release of 111% ± 8%, whereas and PMA-A23187 induced 266% ± 38% of the hexosaminidase release of the control cells. Thus, the TcdB- or latrunculin B-induced release of hexosamidase is not based on the leakage of HMC-1 cells due to apoptosis or necrosis.
p38 MAPK contributed to degranulation induced by cytoskeletal rearrangement.
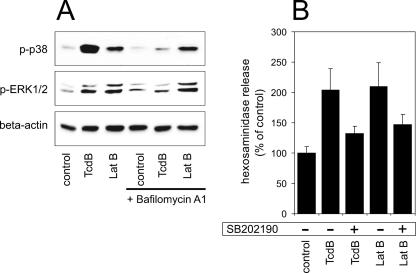
The TcdB-induced activation of p38 MAPK as well as of another member of MAPKs, ERK1/2, was detected by Western blotting against the doubly phosphorylated forms of these kinases. Both TcdB (6 nM) and latrunculin B (2 μg/ml) led to an activation of p38 MAPK and ERK1/2 after 1 h (Fig. 5A). To exclude the possibility of a direct receptor-mediated ligand effect of TcdB on the activation of p38 MAPK, cells were treated in the presence of bafilomycin A1. Bafilomycin inhibits the acidification of the endosomal vesicular lumen, which is required for the translocation of TcdB into the cytosol. Bafilomycin completely abolished the TcdB-induced activation of p38 MAPK and ERK1/2, excluding a receptor-mediated effect. The latrunculin B-induced activation of p38 MAPK and ERK1/2 was not altered by bafilomycin, since latrunculin B is membrane permeable and cell entry is not mediated by endocytosis. The contribution of the p38 MAPK signaling pathway to TcdB- and latrunculin B-induced degranulation was determined by applying the p38 inhibitor SB202190. TcdB- and latrunculin-induced hexosaminidase release was reduced a great extent by coincubating the cells with SB202190 (Fig. 5B).
FIG. 5.
(A) Activation of p38 MAPK and ERK1/2 in response to the reorganization of the actin cytoskeleton. Cells were treated with TcdB (6 nM) and latrunculin B (Lat B; 2 μg/ml) for 1 h in the absence or presence of 3 μM bafilomycin A1. Immunoblot analysis shows the phosphorylation of p38 MAPK (p-p38) (upper panel) and ERK1/2 (p-ERK1/2) (middle panel). Beta-actin, shown in the lower panel, serves as a control for identical protein loads. (B) Effect of p38 MAPK inhibition by SB202190 (10 μM) on hexosaminidase release induced by TcdB (6 nM) or latrunculin B (2 μg/ml). In comparison to what occurred in control cells (100% ± 34%), the TcdB-induced (288% ± 8%) and the latrunculin B-induced (196% ± 23%) release of hexosaminidase was partially reduced by the p38 MAPK inhibitor SB202190 (TcdB, 236% ± 39%; latrunculin B, 133% ± 11%). Shown are mean values ± standard deviations from three separate experiments.
IL-8 secretion and PGD2/PGE2 synthesis were p38 MAPK dependent.
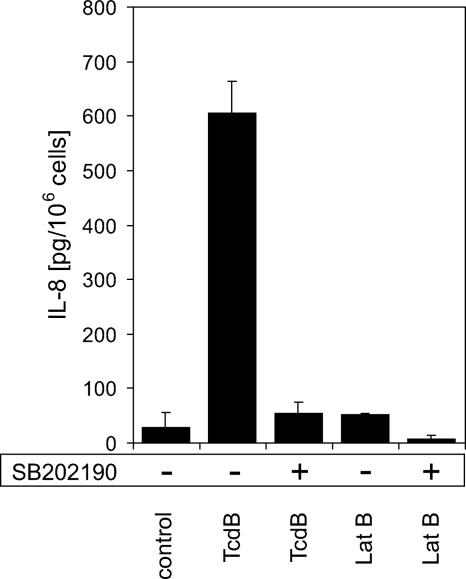
We next analyzed IL-8 secretion by HMC-1 cells in response to TcdB (6 nM) and latrunculin B (2 μg/ml), respectively. The constitutive IL-8 secretion was 15 ± 14 pg/106 cells in nonstimulated cells within 5 h, whereas TcdB treatment led to a 45-fold increase (678 ± 60 pg/106 cells) (Fig. 6). Surprisingly, depolymerization of the actin cytoskeleton by latrunculin B did not induce a strong increase in IL-8 secretion (52 ± 2 pg/106 cells). IL-8 secretion strongly depended on the activation of p38 MAPK, since the specific inhibitor SB202190 reduced the TcdB- and latrunculin B-induced IL-8 response almost to control levels (55 ± 21 and 8 ± 2 pg/106 cells, respectively).
FIG. 6.
TcdB, but not latrunculin B, induced IL-8 secretion in HMC-1 cells. TcdB (6 nM) induced the secretion of 605 ± 58 ng per 106 cells over 5 h of treatment, whereas controls showed a constitutive secretion of 29 ± 28 ng per 106 cells. IL-8 secretion induced by TcdB was prevented in the presence of the p38 MAPK inhibitor SB202190 (58 ± 21 ng per 106 cells). In contrast to TcdB, latrunculin B (Lat B; 2 μg/ml) did not induce a strong increase in IL-8 secretion compared to that in controls (52 ± 2 ng per 106 cells). Shown are mean values ± standard deviations from six separate experiments.
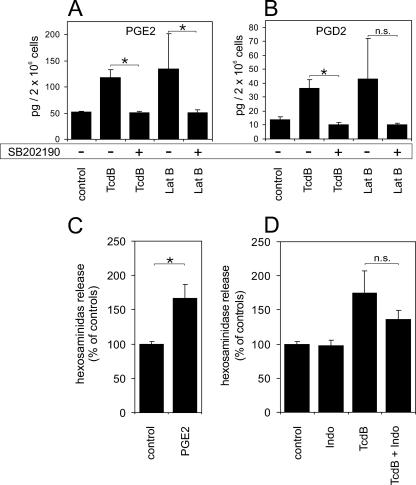
PGD2 and PGE2, further well-known mast cell mediators, were also studied. TcdB (6 nM) as well as latrunculin B (2 μg/ml) induced an increase in the levels of both prostaglandins (Fig. 7). PGE2 synthesis increased from 51 ± 2 pg/2 × 106 cells in control cells to 118 ± 2 pg/2 × 106 cells with TcdB and to 135 ± 66 pg/2 × 106 cells with latrunculin B (Fig. 7A). Accordingly, PGD2 synthesis increased from the control values of 14 ± 2 pg/2 × 106 cells up to 36 ± 6 pg/2 × 106 cells after TcdB treatment and to 43 ± 29 pg/2 × 106 after latrunculin B treatment (Fig. 7B). The increase in PGE2/PGD2 synthesis was completely abolished (51 ± 3 pg/2 × 106 cells and 51 ± 5 pg/2 × 106 cells with PGE2; 10 ± 1 pg/2 × 106 cells and 10 ± 1 pg/2 × 106 cells with PGD2) by the inhibition of p38 MAPK by 10 μM SB202190. However, due to the high standard deviation of latrunculin B-induced PGD2 synthesis, the inhibitory effect of SB202190 was not considered significant. That PGE2 contributes to degranulation was proven by applying 1 ng/ml PGE2 (a concentration 10 times that induced by TcdB) on HMC-1 cells. PGE2 significantly increased hexosaminidase release to 173% ± 12% of that of controls (100% ± 5%) (Fig. 7C). Specific inhibition of the cyclooxygenase by 10 μM indomethacin partially reduced the TcdB-induced hexosaminidase release from 170% ± 37% to 136% ± 13% of that of controls (Fig. 7D); however, this was not a significant effect. Thus, prostaglandins are not solely causative of TcdB-induced hexosaminidase release but contribute to a minor extent in an autocrine manner.
FIG. 7.
Release of prostaglandins. Treatment of HMC-1 cells with either TcdB (6 nM) or latrunculin B (Lat B; 2 μg/ml) for 5 h induced the synthesis of PGE2 (A) and PGD2 (B). The formation of prostaglandins was completely abolished by the preincubation of cells with the p38 MAPK inhibitor SB202190 (10 μM). Due to the high standard deviation of PGD2 synthesis induced by latrunculin B, the effect of SB202190 was not considered significant (n.s.). (C) Effect of PGE2 (1 ng/ml) on the hexosaminidase release of HMC-1 cells. (D) Inhibition of the cyclooxygenase by 10 μM indomethacin (Indo) only partially reduced TcdB-induced hexosaminidase release. All values are means ± standard deviations from three separate experiments. Significant effects (P < 0.05) as determined by the unpaired Student t test are indicated by asterisks.
DISCUSSION
Mast cells play a crucial role in the intestinal defense mechanisms against pathogens (1, 13). The involvement of mast cells in C. difficile-induced pseudomembranous colitis is well documented (44). However, a direct effect of TcdA and TcdB on mast cells was shown only for RBL cells (9, 45) and murine peritoneal mast cells (6). In those studies, TcdB inhibited FcɛR-mediated mast cell activation, and therefore, the studies indicate a mast cell silencing effect of both toxins. In contrast to that, our data show a direct activation of human HMC-1 cells by TcdA and TcdB and that TcdB was more potent than TcdA. This reflects the well-known difference between the enterotoxin TcdA, which acts primarily on enterocytes, and the cytotoxin TcdB, which is about 100-fold more potent on most other cell types. Different cell receptors are responsible for the uptake of toxins into cells and therefore account for different potencies (20). Since the toxins have identical substrate specificities, the difference in potency is not a result of different Rho GTPases being inactivated. Based on the reported positive involvement of mast cells in TcdA/TcdB-induced inflammation of the colon, these new data introduce an alternative novel model system for studying the underlying mast cell-mediated processes.
The observed effects on HMC-1 cells can be summarized as follows: HMC-1 cells are sensitive to TcdA as well as to TcdB, and intracellular glucosylation of Rho GTPases results in the rearrangement of the actin cytoskeleton. This rearrangement supports degranulation and induces the formation of prostaglandins. Furthermore, cytoskeletal breakdown also causes the activation of p38 MAPK and ERK1/2. All these effects are comparably induced by latrunculin B, supporting the hypothesis that the processes are actin mediated. Latrunculin B is a cell-permeable agent which sequesters G-actin, thereby directly inducing actin depolymerization. The finding that a rearrangement of the actin cytoskeleton leads to an increased synthesis of PGE2 and PGD2 was also shown to be true for colonocytes (23) and renal mesangial cells (35). PGE2/PGD2 contributes to degranulation in an autocrine manner. However, the inhibition of prostaglandin formation by indomethacin reduces TcdB-induced degranulation only by about 50%. This is in accordance with the results of SB202190 experiments where inhibition of the p38 MAPK completely inhibited prostaglandin synthesis. Since SB202190 did not completely inhibit hexosaminidase release, other mediators supposedly contribute to the TcdB-induced degranulation of mast cells.
The activation of p38 MAPK has been reported to be a glucosyltransferase-independent effect of TcdA, possibly mediated by reactive oxygen species (23). We checked this hypothesis by applying bafilomycin, which inhibits the acidification of endosomes. Acidification is essential for toxin translocation from the endosomes to the cytoplasm, and bafilomycin entraps the toxin in the endosomes. Since bafilomycin completely inhibited p38 MAPK activation, this experiment clearly demonstrates that p38 MAPK activation is based on an intracellular activity of the toxin and is not induced merely by receptor binding.
The biological effects of TcdB and latrunculin B in this study differ only in IL-8 release. Whereas TcdB causes a strong IL-8 release, latrunculin B does not significantly change the basal IL-8 formation. Two hypotheses may explain this difference. (i) Strong IL-8 release requires the simultaneous activation of two parallel signal pathways, e.g., NF-κB and p38 MAPK, which might be driven by TcdB but not by latrunculin B. The inhibition of either of the pathways would strongly reduce IL-8 synthesis. As was reported by Kim and coworkers, NF-κB is also involved in C. difficile toxin-induced IL-8 secretion (25). (ii) IL-8 restricts its own synthesis via an autocrine mechanism or by the induction of further mediators. This feedback loop is hypothetically Rho GTPase dependent, and restriction cannot take place in TcdB-treated cells in which Rho GTPases were inactivated by glucosylation. A verification of either of these hypotheses would require a more detailed study and is beyond the aim of this study. A mechanism by which two simultaneous pathways converge to result in the activation of a strong IL-8 synthesis was reported for the Bacteroides fragilis enterotoxin (47). However, Németh and coworkers also described a p38 MAPK and NF-κB-dependent induction of IL-8 secretion by cytochalasin D and latrunculin B in HT-29 and Caco-2 cells (31). In our study, we detected only a marginal increase in IL-8 secretion caused by latrunculin B (80%) compared to that caused by TcdB (2,000%). IL-8 secretion in response to latrunculin B treatment as described by Németh et al. (31) was measured after 18 h of incubation in contrast to the 5 h used in our study, and the values determined in that study were merely 200% of the values for control cells.
The most prominent finding of the present study was the increased hexosaminidase release by HMC-1 cells induced with TcdA and TcdB. This release was neither a strong nor a single degranulation event that resembles an anaphylactic reaction. Instead, TcdB-treated cells showed a moderate, continuous, and constantly increasing release over the observed time period of 9 h. The hypothesis that the disruption of the actin cytoskeleton is the basis for an increased exocytosis was supported by the latrunculin experiment in which identical kinetics were observed. Thus, we suggest that the disruption of the cortical actin meshwork by either TcdA/TcdB or latrunculin B reduces the mechanical barrier for vesicle fusion with the plasma membrane, thereby facilitating exocytosis. The actin cytoskeleton is crucial for the receptor-mediated activation of mast cells, as shown by previous studies of immunoglobulin E-mediated degranulation (9, 33, 37). On the other hand, F-actin also sets the threshold for degranulation, and diminished F-actin sensitizes mast cells (12, 41). Corresponding findings were described by the group of Gutierrez et al., who showed the influence of the F-actin cytoskeleton on the slow and rapid release of vesicles in bovine chromaffine cells (14, 30). These desensitizing and sensitizing effects of the actin cytoskeleton have been summarized by Eitzen (11). However, the contrary results regarding hexosaminidase release in RBL and HMC-1 cells shown in this study cannot be explained by mere cytoskeletal effects. Downstream signal pathways have to be involved and must be decisive for degranulation events. It can be supposed that the contributions of various signaling pathways, such as ERK and p38 MAPK, account for the different effects of TcdA/TcdB on various cell types. Additional mediators, such as tumor necrosis factor alpha and gamma interferon, are released in response to TcdB and contribute to the symptoms of C. difficile-induced colitis (18, 38). Nevertheless, the differentiation state of HMC-1 cells is most probably of minor relevance, since the toxin-induced effects are not mediated by cell surface proteins, as the bafilomycin experiment showed. Additionally, important aspects of the present study, e.g., prostaglandin synthesis and IL-8 secretion, were also shown for differentiated cells (15, 24, 35).
In summary, the present study describes the activation of human mast cells by Clostridium difficile TcdA/B resulting in degranulation and IL-8 release. Activation is moderate and long-lasting and is dissimilar to an anaphylactic reaction. Mast cells are crucial for the defense against microbial pathogens. Thus, the activation of mast cells in response to treatment with TcdA or TcdB fits well into the concept of mast cells being essential players in toxin-induced pseudomembranous colitis.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft SFB621 (project B5).
We thank Helma Tatge, Elke Mallon, and Gerd Preiss for excellent technical assistance. The laboratory assistance of Bibiana Beckmann and the assistance of Frank-Mathias Gutzki in GC-tandem MS analyses are also gratefully acknowledged.
Editor: D. L. Burns
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Abraham, S. N., and R. Malaviya. 1997. Mast cells in infection and immunity. Infect. Immun. 65:3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahnert-Hilger, G., M. Höltje, G. Groβe, G. Pickert, C. Mucke, B. Nixdorf-Bergweiler, P. Boquet, F. Hofmann, and I. Just. 2004. Differential effects of Rho GTPases on axonal and dendritic development in hippocampal neurons. J. Neurochem. 90:9-18. [DOI] [PubMed] [Google Scholar]
- 3.Arai, A., E. Kanda, and O. Miura. 2002. Rac is activated by erythropoietin or interleukin-3 and is involved in activation of the Erk signaling pathway. Oncogene 21:2641-2651. [DOI] [PubMed] [Google Scholar]
- 4.Beaugerie, L., A. Flahault, F. Barbut, P. Atlan, V. Lalande, P. Cousin, M. Cadilhac, and J. C. Petit. 2003. Antibiotic-associated diarrhoea and Clostridium difficile in the community. Aliment. Pharmacol. Ther. 17:905-912. [DOI] [PubMed] [Google Scholar]
- 5.Butterfield, J. H., D. Weiler, G. Dewald, and G. J. Gleich. 1988. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk. Res. 12:345-355. [DOI] [PubMed] [Google Scholar]
- 6.Calderón, G. M., J. Torres-López, T.-J. Lin, B. Chavez, M. Hernández, O. Muñoz, A. D. Befus, and J. A. Enciso. 1998. Effects of toxin A from Clostridium difficile on mast cell activation and survival. Infect. Immun. 66:2755-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castagliuolo, I., J. T. LaMont, R. Letourneau, C. P. Kelly, J. C. O′Keane, A. Jaffer, T. C. Theoharides, and C. Pothoulakis. 1994. Neuronal involvement in the intestinal effects of Clostridium difficile toxin A and Vibrio cholerae enterotoxin in rat ileum. Gastroenterology 107:657-665. [DOI] [PubMed] [Google Scholar]
- 8.Djouder, N., G. Schmidt, M. Frings, A. Cavalié, M. Thelen, and K. Aktories. 2001. Rac and phosphatidylinositol 3-kinase regulate the protein kinase B in Fc epsilon RI signaling in RBL 2H3 mast cells. J. Immunol. 166:1627-1634. [DOI] [PubMed] [Google Scholar]
- 9.Djouder, N., U. Prepens, K. Aktories, and A. Cavalié. 2000. Inhibition of calcium release-activated calcium current by Rac/Cdc42-inactivating clostridial cytotoxins in RBL cells. J. Biol. Chem. 275:18732-18738. [DOI] [PubMed] [Google Scholar]
- 10.Dobson, G., C. Hickey, and J. Trinder. 2003. Clostridium difficile colitis causing toxic megacolon, severe sepsis and multiple organ dysfunction syndrome. Intensive Care Med. 29:1030. [DOI] [PubMed] [Google Scholar]
- 11.Eitzen, G. 2003. Actin remodeling to facilitate membrane fusion. Biochim. Biophys. Acta 1641:175-181. [DOI] [PubMed] [Google Scholar]
- 12.Frigeri, L., and J. R. Apgar. 1999. The role of actin microfilaments in the down-regulation of the degranulation response in RBL-2H3 mast cells. J. Immunol. 162:2243-2250. [PubMed] [Google Scholar]
- 13.Galli, S. J., M. Maurer, and C. S. Lantz. 1999. Mast cells as sentinels of innate immunity. Curr. Opin. Immunol. 11:53-59. [DOI] [PubMed] [Google Scholar]
- 14.Gil, A., J. Rueda, S. Viniegra, and L. M. Gutierrez. 2000. The F-actin cytoskeleton modulates slow secretory components rather than readily releasable vesicle pools in bovine chromaffin cells. Neuroscience 98:605-614. [DOI] [PubMed] [Google Scholar]
- 15.He, D., S. Sougioultzis, S. Hagen, J. Liu, S. Keates, A. C. Keates, C. Pothoulakis, and J. T. LaMont. 2002. Clostridium difficile toxin A triggers human colonocyte IL-8 release via mitochondrial oxygen radical generation. Gastroenterology 122:1048-1057. [DOI] [PubMed] [Google Scholar]
- 16.Hong-Geller, E., and R. A. Cerione. 2000. Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP3/calcium pathway in RBL-2H3 mast cells. J. Cell Biol. 148:481-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong-Geller, E., D. Holowka, R. P. Siraganian, B. Baird, and R. A. Cerione. 2001. Activated Cdc42/Rac reconstitutes Fcepsilon RI-mediated Ca2+ mobilization and degranulation in mutant RBL mast cells. Proc. Natl. Acad. Sci. USA 98:1154-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishida, Y., T. Maegawa, T. Kondo, A. Kimura, Y. Iwakura, S. Nakamura, and N. Mukaida. 2004. Essential involvement of IFN-gamma in Clostridium difficile toxin A-induced enteritis. J. Immunol. 172:3018-3025. [DOI] [PubMed] [Google Scholar]
- 19.Just, I., G. Fritz, K. Aktories, M. Giry, M. R. Popoff, P. Boquet, S. Hegenbarth, and C. Von Eichel-Streiber. 1994. Clostridium difficile toxin B acts on the GTP-binding protein Rho. J. Biol. Chem. 269:10706-10712. [PubMed] [Google Scholar]
- 20.Just, I., and R. Gerhard. 2004. Large clostridial cytotoxins. Rev. Physiol. Biochem. Pharmacol. 152:23-47. [DOI] [PubMed] [Google Scholar]
- 21.Just, I., J. Selzer, F. Hofmann, and K. Aktories. 1997. Clostridium difficile toxin B as a probe for Rho GTPases, p. 159-168. In K. Aktories (ed.), Bacterial toxins: tools in cell biology and pharmacology. Chapman & Hall, Weinheim, Germany.
- 22.Just, I., J. Selzer, M. Wilm, C. Von Eichel-Streiber, M. Mann, and K. Aktories. 1995. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375:500-503. [DOI] [PubMed] [Google Scholar]
- 23.Kim, H., S. H. Rhee, E. Kokkotou, X. Na, T. Savidge, M. P. Moyer, C. Pothoulakis, and J. T. LaMont. 2005. Clostridium difficile toxin A regulates inducible COX-2 and PGE2 synthesis in colonocytes via reactive oxygen species and activation of p38 MAP kinase. J. Biol. Chem. 280:21237-21245. [DOI] [PubMed] [Google Scholar]
- 24.Kim, J. M., J. S. Kim, H. C. Jun, Y. K. Oh, I. S. Song, and C. Y. Kim. 2002. Differential expression and polarized secretion of CXC and CC chemokines by human intestinal epithelial cancer cell lines in response to Clostridium difficile toxin A. Microbiol. Immunol. 46:333-342. [DOI] [PubMed] [Google Scholar]
- 25.Kim, J. M., J. Y. Lee, Y. M. Yoon, Y. K. Oh, J. Youn, and Y. J. Kim. 2006. NF-kappa B activation pathway is essential for the chemokine expression in intestinal epithelial cells stimulated with Clostridium difficile toxin A. Scand. J. Immunol. 63:453-460. [DOI] [PubMed] [Google Scholar]
- 26.Krivan, H. C., and T. D. Wilkins. 1987. Purification of Clostridium difficile toxin A by affinity chromatography on immobilized thyroglobulin. Infect. Immun. 55:1873-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 28.Massol, P., P. Montcourrier, J.-C. Guillemot, and P. Chavrier. 1998. Fc receptor-mediated phagocytosis requires Cdc42 and Rac1. EMBO J. 17:6219-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mylonakis, E., E. T. Ryan, and S. B. Calderwood. 2001. Clostridium difficile-associated diarrhea. Arch. Intern. Med. 161:525-533. [DOI] [PubMed] [Google Scholar]
- 30.Neco, P., O. Rosetto, A. Gil, C. Montecucco, and L. M. Gutierrez. 2003. Taipoxin induces F-actin fragmentation and enhances release of catecholamines in bovine chromaffin cells. J. Neurochem. 85:329-337. [DOI] [PubMed] [Google Scholar]
- 31.Németh, Z. H., E. A. Deitch, M. T. Davidson, C. Szabo, E. S. Vizi, and G. Hasko. 2004. Disruption of the actin cytoskeleton results in nuclear factor-kappaB activation and inflammatory mediator production in cultured human intestinal epithelial cells. J. Cell Physiol. 200:71-81. [DOI] [PubMed] [Google Scholar]
- 32.Neunlist, M., J. Barouk, K. Michel, I. Just, T. Oreshkova, M. Schemann, and J. P. Galmiche. 2003. Toxin B of Clostridium difficile activates human VIP submucosal neurons, in part via an IL-1β-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G1049-G1055. [DOI] [PubMed] [Google Scholar]
- 33.Pendleton, A., B. Pope, A. Weeds, and A. Koffer. 2003. Latrunculin B or ATP depletion induces cofilin-dependent translocation of actin into nuclei of mast cells. J. Biol. Chem. 278:14394-14400. [DOI] [PubMed] [Google Scholar]
- 34.Pépin, J., L. Valiquette, M. E. Alary, P. Villemure, A. Pelletier, K. Forget, K. Pepin, and D. Chouinard. 2004. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ 171:466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petry, C., G. Fritz, J. Pfeilschifter, and A. Huwiler. 2004. Inhibition of Rho modulates cytokine-induced prostaglandin E2 formation in renal mesangial cells. Biochim. Biophys. Acta 1638:108-118. [DOI] [PubMed] [Google Scholar]
- 36.Pothoulakis, C., I. Castagliuolo, and J. T. LaMont. 1998. Nerves and intestinal mast cells modulate responses to enterotoxins. News Physiol. Sci. 13:58-63. [DOI] [PubMed] [Google Scholar]
- 37.Prepens, U., I. Just, C. Von Eichel-Streiber, and K. Aktories. 1996. Inhibition of Fc ɛRI-mediated activation of rat basophilic leukemia cells by Clostridium difficile toxin B (monoglucosyltransferase). J. Biol. Chem. 271:7324-7329. [DOI] [PubMed] [Google Scholar]
- 38.Rocha, M. F. G., M. E. T. Maia, L. R. P. S. Bezerra, D. M. Lyerly, R. L. Guerrant, R. A. Ribeiro, and A. A. M. Lima. 1997. Clostridium difficile toxin A induces the release of neutrophil chemotactic factors from rat peritoneal macrophages: role of interleukin-1β, tumor necrosis factor alpha, and leukotrienes. Infect. Immun. 65:2740-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz, L. B., C. Riedel, J. P. Caulfield, S. I. Wasserman, and K. F. Austen. 1981. Cell association of complexes of chymase, heparin proteoglycan, and protein after degranulation by rat mast cells. J. Immunol. 1226:2071-2078. [PubMed] [Google Scholar]
- 40.Tixier, E., F. Lalanne, I. Just, J. P. Galmiche, and M. Neunlist. 2005. Human mucosa/submucosa interactions during intestinal inflammation: involvement of the enteric nervous system in interleukin-8 secretion. Cell. Microbiol. 7:1798-1810. [DOI] [PubMed] [Google Scholar]
- 41.Tolarová, H., L. Draberova, P. Heneberg, and P. Draber. 2004. Involvement of filamentous actin in setting the threshold for degranulation in mast cells. Eur. J. Immunol. 34:1627-1636. [DOI] [PubMed] [Google Scholar]
- 42.Tsikas, D. 1998. Application of gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry to assess in vivo synthesis of prostaglandins, thromboxane, leukotrienes, isoprostanes and related compounds in humans. J. Chromatogr. 717:201-245. [DOI] [PubMed] [Google Scholar]
- 43.Van Aelst, L., and C. D'Souza-Schorey. 1997. Rho GTPases and signaling networks. Genes Dev. 11:2295-2322. [DOI] [PubMed] [Google Scholar]
- 44.Wershil, B. K., I. Castagliuolo, and C. Pothoulakis. 1998. Direct evidence of mast cell involvement in Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology 114:956-964. [DOI] [PubMed] [Google Scholar]
- 45.Wex, C. B. A., G. Koch, and K. Aktories. 1997. Effects of Clostridium difficile toxin B on activation of rat peritoneal mast cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 355:328-334. [DOI] [PubMed] [Google Scholar]
- 46.Woo, C. H., and J. H. Kim. 2002. Rac GTPase activity is essential for lipopolysaccharide signaling to extracellular signal-regulated kinase and p38 MAP kinase activation in rat-2 fibroblasts. Mol. Cells 13:470-475. [PubMed] [Google Scholar]
- 47.Wu, S., J. Powell, N. Mathioudakis, S. Kane, E. Fernandez, and C. L. Sears. 2004. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-κB pathway. Infect. Immun. 72:5832-5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao, D., and C. Pothoulakis. 2003. Rho GTPases as therapeutic targets for the treatment of inflammatory dieseases. Expert Opin. Ther. Targets 7:583-592. [DOI] [PubMed] [Google Scholar]