Abstract
Alveolar macrophages are the effector cells largely responsible for clearance of Pneumocystis carinii from the lungs. Binding of organisms to β-glucan and mannose receptors has been shown to stimulate phagocytosis of the organisms. To further define the mechanisms used by alveolar macrophages for clearance of P. carinii, mice deficient in the expression of scavenger receptor A (SRA) were infected with P. carinii, and clearance of organisms was monitored over time. SRA-deficient (SRAKO) mice consistently cleared P. carinii faster than did wild-type control mice. Expedited clearance corresponded to elevated numbers of activated CD4+ T cells in the alveolar spaces of SRAKO mice compared to wild-type mice. Alveolar macrophages from SRAKO mice had increased expression of CD11b on their surfaces, consistent with an activated phenotype. However, they were not more phagocytic than macrophages expressing SRA, as measured by an in vivo phagocytosis assay. SRAKO alveolar macrophages produced significantly more tumor necrosis factor alpha (TNF-α) than wild-type macrophages when stimulated with lipopolysaccharide in vitro but less TNF-α in response to P. carinii in vitro. However, upon in vivo stimulation, SRAKO mice produced significantly more TNF-α, interleukin 12 (IL-12), and IL-18 in response to P. carinii infection than did wild-type mice. Together, these data indicate that SRA controls inflammatory cytokines produced by alveolar macrophages in the context of P. carinii infection.
Pneumocystis carinii is an opportunistic fungal pathogen that causes pneumonia in immunodeficient patients. Extensive studies using animal models have confirmed that alveolar macrophages are critical for host defense against P. carinii (28, 31, 38, 44). Alveolar macrophages bind to the major surface glycoprotein on P. carinii, resulting in organism attachment and uptake (12, 35). However, mannose receptors are not critical for clearance of P. carinii, as evidenced by the resistance of mannose receptor-deficient mice to P. carinii pneumonia (45). Alveolar macrophages also bind P. carinii cell wall β-glucans via cognate receptors such as dectin-1, resulting in the production of tumor necrosis factor alpha (TNF-α) and MIP-2 and uptake and killing of the organisms (44, 47). Finally, it has been recently found that P. carinii stimulates NF-κB activation in alveolar macrophages by an MyD88-mediated signaling event (30), presumably through Toll-like receptor 2 (TLR2) (49).
In spite of the fact that unopsonized organisms seem to bind alveolar macrophages efficiently in vitro, P. carinii is quite adept at evading immune surveillance in the lungs when growing at low levels (17). Stimulation of inflammation in vivo is likely due to growth of the organisms to a critical level that results in ligation of enough receptors on macrophages, epithelial cells, and/or dendritic cells to trigger a signaling event, resulting in activation and production of proinflammatory cytokines. Prior to this, it is likely that lung inflammation is controlled by anti-inflammatory elements present in the lungs, including constitutive interleukin 10 (IL-10) production by alveolar epithelial cells (13). It is unclear whether the first signaling events that stimulate inflammation in response to P. carinii in vivo are a result of ligation of mannose receptors, β-glucan receptors, TLR2, or a combination of these. In this regard it has been shown that TLR2 may work in concert with dectin-1 to stimulate macrophage activation in response to fungal antigens (14). However, it is also possible that other pattern recognition receptors are involved in stimulation of alveolar macrophages in response to P. carinii.
Scavenger receptor A (SRA) belongs to a class of pattern recognition receptors found on macrophages that have been shown to bind an array of polyanionic molecules, including modified (oxidized or acylated) low-density lipoprotein (LDL), as well as bacterial products, including lipopolysaccharide, polyribonucleic acids, lipoteichoic acid, and polysaccharides (e.g., dextran sulfate) (46). SRA has two functionally expressed isoforms, both of which have a collagenous domain that is thought to be the binding domain for polyanionic molecules including lipid A (46). Recent studies have shown that SRA binds a number of bacteria, resulting in nonopsonic uptake, including Neisseria meningiditis, Escherichia coli, Streptococcus pneumoniae, Brucella abortus, and Staphylococcus aureus (1, 18, 25, 37). Interestingly, there is also some evidence that SRA is important for limiting proinflammatory cytokine responses to mycobacteria (22, 36). Together, these data imply that SRA acts to induce uptake of bacteria without inducing an overly exuberant inflammatory response. Notably, to date the only pathogens that have been shown to interact with SRA are bacterial, while nothing is known about the interactions of SRA with fungal pathogens.
The goal of this project was to determine whether SRA expression on alveolar macrophages is important for phagocytosis and inflammation in the lungs in response to the opportunistic fungal pathogen P. carinii. We found that SRA was not involved in uptake of fungi, though it was important for controlling the inflammatory response to P. carinii.
MATERIALS AND METHODS
Mice.
129x1/SvJ mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred in the Lexington Veteran's Administration Veterinary Medical Unit. C57BL/6 mice were purchased from Taconic (Germantown, NY). SRA I/II-deficient mice (SRAKO) on a 129x1/SvJ or a C57BL/6 background and CB.17scid/scid mice (originally from Taconic) were bred in the Veteran's Administration Veterinary Medical Unit. Mice were maintained on acidified water and sterile rodent chow in sterile microisolator caging. Experiments with adult animals were performed with either all-male or all-female cohorts when mice were between 8 and 16 weeks of age. Neonatal mice were used at 24 to 48 h of age.
Infection with and enumeration of Pneumocystis carinii f. sp. muris.
P. carinii was maintained in a colony of severe combined immunodeficient mice. Lungs from heavily infected donors were pushed through wire mesh screens in Hank's buffered saline solution (HBSS) and spun at 100 × g for 3 min to pellet lung debris. Organisms in the supernatant were spun onto glass slides, fixed in methanol, and stained with DiffQuik (Dade International, FL). Organisms were enumerated microscopically by counting nuclei in either cyst or trophic life forms as previously described (15). Adult mice were given intratracheal inoculations of 107 nuclei, and neonatal mice were given intranasal inoculations of 106 nuclei per gram of body weight under light halothane anesthesia. P. carinii lung burdens in experimental mice were determined microscopically on DiffQuik-stained lung aliquots as previously described (32). The P. carinii burden in lungs is expressed as log10 P. carinii organisms per right lung lobes, and the limit of detection was 3.23.
Isolation of cells from alveolar spaces, lungs, and lymph nodes.
Mice were killed by exsanguination under deep halothane anesthesia. The lungs were subjected to lavage with HBSS containing 3 mM EDTA. Lungs were minced and digested for 1 h with 1 mg/ml collagenase A (Sigma, St. Louis, MO) and 50 U/ml DNase (Sigma) and then dispersed in single-cell suspensions by pushing through 70-μm nylon mesh screens. Tracheobronchial lymph nodes (TBLN) were pushed through nylon mesh screens to obtain single-cell suspensions. Red blood cells were removed from cell suspensions by treatment with a hypotonic lysis buffer. Leukocytes were enumerated prior to use in flow cytometry assays.
Flow-cytometric analysis of lung and TBLN cells.
Bronchoalveolar lavage fluids (BALF), lung digests, and TBLN cells were washed in phosphate-buffered saline containing 0.1% bovine serum albumin and 0.02% NaN3 and stained with appropriate concentrations of fluorochrome-conjugated antibodies specific for CD4, CD8, CD62L, CD44, CD11c, CD11b, and major histocompatibility complex class II. Antibodies were purchased from BD Biosciences or eBioscience. Expression of molecules on the surfaces of leukocytes was determined by multiparameter flow cytometry using a FACSCalibur cytofluorimeter (BD Biosciences). We routinely acquired 50,000 events for analysis.
Quantitation of lung cytokines.
The first wash of BALF was set aside for cytokine analysis. IL-12 and IL-18 were quantitated utilizing commercially available enzyme-linked immunosorbent assay kits (R&D Systems) per the manufacturer's instructions. TNF-α was quantitated with cytokine bead array kits (BD Bioscience) and flow cytometry. Data were obtained from individual mice.
In vivo phagocytosis assay.
P. carinii organisms were purified by differential low- and high-speed spin and hypotonic lysis of lung cells as previously described (15). To disperse clumps, organisms were maintained in HBSS containing 0.5% glutathione during purification and pushed through a 22-gauge and then a 26-gauge needle. Organisms were incubated with the fluorescent membrane-permeant dye DiO (Molecular Probes) and washed extensively. P. carinii organisms (5 × 106 nuclei) were inoculated intratracheally into adult mice as described above. Four hours later, lungs were subjected to lavage, and cells were collected for analysis by flow cytometry. Cells were stained with fluorochrome-conjugated anti-CD11c and anti-F4/80 and analyzed with a FACSCalibur cytofluorimeter. Macrophages that fluoresced above background in the green channel were considered positive for having phagocytosed P. carinii. Data are expressed as the percent positive macrophages and as the mean fluorescence intensity (MFI) as a relative measure of the number of organisms taken up by the macrophages.
In vitro analysis of alveolar macrophage cytokine production.
Alveolar macrophages were isolated from wild-type and SRAKO mice by lavage as described above. Aliquots of cells were spun onto glass slides, fixed in methanol, and stained with DiffQuik. Cells were examined microscopically for macrophage morphology. Samples that were not >95% macrophages were not used for experiments. Dendritic cell contamination was not a significant problem, since in uninfected mice dendritic cells generally obtain samples of the alveolar space by sending in dendrites from the parenchyma and so are not a part of the population of cells subjected to lavage from the alveoli (24). Macrophages were placed in 96-well plates at 2 × 105 macrophages per well in RPMI containing 5% heat-inactivated fetal bovine serum, l-glutamine, and 2-β-mercaptoethanol and rested overnight at 37°C under an atmosphere of 5% CO2 in room air. Macrophages were stimulated with 5 μg/ml lipopolysaccharide (LPS), P. carinii sonicate, or whole P. carinii organisms (20 nuclei per macrophage) for 24 h, and supernatants were collected for subsequent cytokine quantitation by cytokine bead array as described. Triplicate wells were used per culture condition, and data were analyzed for individual wells and expressed as means.
Statistical analysis.
In vivo time course experiments were analyzed using two-way analysis of variance followed by Student Newman Kuel's post-hoc test, where appropriate. Data sets were considered significantly different when the P value was less than 0.05. Statistical analysis was not performed on data generated from in vitro assays, since data represent replicate wells of pooled cells and so n is 1. However, experiments were repeated two to four times to confirm results.
RESULTS
SRA has minor effects on clearance of P. carinii from the lungs in neonatal and adult mice.
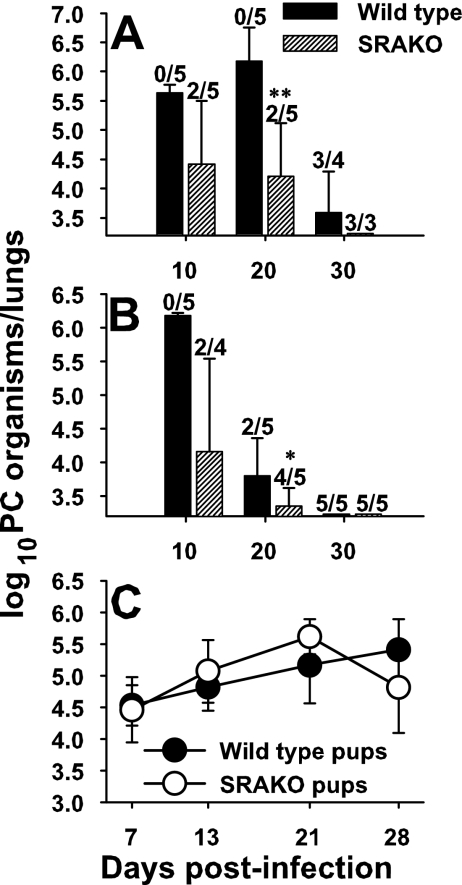
SRAKO mice have been reported to be more susceptible to bacterial lung infection than wild-type control mice (1). This is likely due to the ability of SRA to bind bacterial cell wall components, such as lipopolysaccharide, lipoteichoic acid, and lipoproteins, and to facilitate phagocytosis (18, 37, 39). To determine whether SRA is also important for defense against a fungal pathogen, wild-type and SRAKO mice were infected with P. carinii, and lung organism burden was evaluated over time. Interestingly, SRAKO mice were fully competent to clear P. carinii and in some experiments were able to do so more efficiently than wild-type mice. As shown in Fig. 1A and B, some adult SRAKO mice were able to clear P. carinii infection as early as day 10 postinfection. SRAKO mice cleared P. carinii equally well whether they were on C57BL/6 (Fig. 1A) or 129x1/SvJ (Fig. 1B) backgrounds. Because we made the interesting observation that SRAKO mice may clear P. carinii faster than wild-type mice, we also examined clearance in neonatal SRAKO mice. We previously reported that neonatal mice clear P. carinii much more slowly than adult mice and were interested in whether the clearance could be expedited in the absence of SRA (10, 15, 16). As shown in Fig. 1C, the kinetics of clearance of P. carinii in SRAKO mice infected at 48 h of age was not significantly different from that in infant wild-type mice. Together, these data indicated that SRA is not critical for clearance of this fungal pathogen. However, the subtle differences in the kinetics of clearance of P. carinii in SRAKO mice led to an examination of the inflammatory response to P. carinii in these mice.
FIG. 1.
Clearance kinetics of Pneumocystis infection are relatively unchanged in SRAKO mice. Adult (A and B) or neonatal (C) wild-type and SRAKO mice were given lung inoculations of P. carinii, and lung burden was determined at various times postinfection. (A) C57BL/6 background; (B and C) 129/SvJ background. Data are means ± standard deviations for three to five mice per group. The numbers above the bars are numbers of mice per group that had a P. carinii burden below the limit of detection (log10 3.23). **, P < 0.001 for wild-type mice compared to SRAKO mice; *, P < 0.05 compared to wild-type controls at the same time point.
SRA expression dampens the lung lymphocyte response to P. carinii.
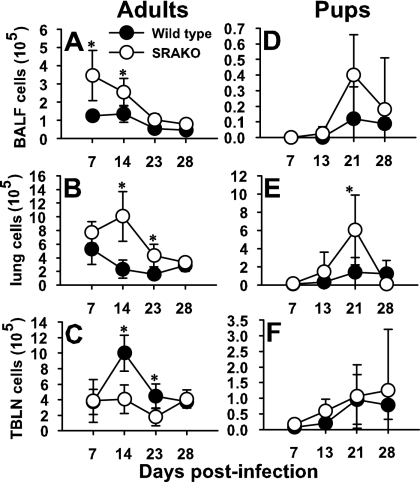
Clearance of P. carinii is dependent on multiple immune cell populations, including CD4+ T cells (20, 41), macrophages (31), and B cells (33, 34). Interestingly, in every experiment, SRAKO mice had elevated numbers of CD4+ T cells in the alveoli and lung interstitium. As shown in Fig. 2, the number of CD4+ T cells expressing an activated phenotype was significantly greater in the BALF of SRAKO than in that of wild-type mice at day 7 postinfection. These numbers started to decrease by day 14 and were similar to those for the wild type by day 23 postinfection. Similar trends were seen in the lung interstitium, where SRAKO mice had greater numbers of activated CD4+ T cells than wild-type mice at day 14 postinfection (Fig. 2B). However, we found reduced numbers of activated CD4+ T cells in the draining lymph nodes of SRAKO mice (Fig. 2C), possibly due to a more rapid exit to the site of infection. Interestingly, we also found that SRAKO mice infected as neonates had elevated numbers of activated CD4+ T cells in the alveoli at day 21 postinfection and in the lung interstitium as early as day 13 postinfection (Fig. 2D and E). There were no differences in activated CD4+ T cells in the draining lymph nodes (Fig. 2F). These data suggest that SRA may play an indirect role in controlling expansion of CD4+-T-cell populations. In contrast, we did not see any differences in B-cell numbers in the lungs or draining lymph nodes or in P. carinii-specific-antibody levels in serum in SRAKO compared to wild-type mice infected with P. carinii, regardless of the age at which the mice were challenged (data not shown).
FIG. 2.
Elevated numbers of activated CD4+ T cells migrate into the lungs of adult or neonatal SRAKO mice in response to Pneumocystis infection. CD4+ cells with an activated phenotype (CD44hi CD62Llo) were enumerated from the BALF (A and D), lung digests (B and E), or draining lymph nodes (C and F) from mice infected with P. carinii as adults (A to C) or neonates (D to F) using flow cytometry. Data are means ± standard deviations for three to five mice per group. *, P < 0.05 compared to wild-type controls at the same time point.
Alveolar macrophage responses to P. carinii in SRAKO mice are comparable to or better than those in wild-type mice.
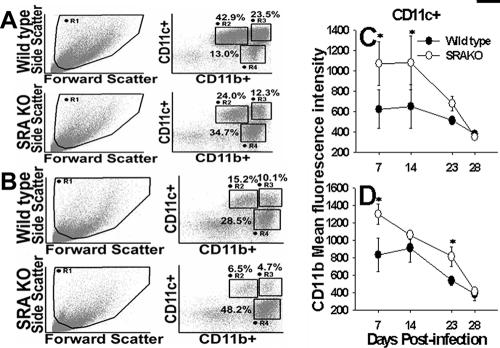
Since SRA is expressed almost exclusively on myeloid-lineage cells, we examined whether alveolar macrophage function in response to P. carinii was altered in the absence of SRA. Interestingly, there was a significant influx in neutrophils into the alveolar spaces of SRAKO mice by day 7 postinfection; however, by day 14, neutrophil numbers were not different from those in wild-type mice (data not shown). We also found that expression of CD11b, an integrin and β-glucan receptor, on alveolar macrophages from SRAKO mice infected with P. carinii was higher than in wild-type mice (Fig. 3A, C). There was a decrease in the proportion of nonlymphoid cells that were CD11chi CD11bint and an increase in CD11cint CD11bhi cells in the SRAKO BALF and lung digests compared to the wild type (Fig. 3). This may correspond to either activation of the alveolar macrophages or infiltration of monocytes. We and others have found that alveolar macrophages upregulate CD11b during inflammatory states (10, 29). However, we did not see differences in expression of major histocompatibility complex class II in alveolar macrophages from SRAKO compared to wild-type mice (data not shown). We found no difference between the absolute number of alveolar macrophages in the lungs or BALF of SRAKO mice and that in wild-type mice (data not shown). In addition, no differences were found in the expression levels of dectin-1, the β-glucan receptor critical for binding and phagocytosis of P. carinii by macrophages (data not shown) (43, 44). These data suggest that SRAKO mice have more CD11b+ alveolar macrophages early after infection with P. carinii, which may account for the slight reductions in P. carinii burdens we observed in some experiments. In contrast, the proportion of CD11chi CD11bhi cells at day 7 postinfection in the lung digests of SRAKO mice was about twofold lower than that in wild-type mice (Fig. 3B). This may be due to migration of dendritic cells into the draining lymph nodes.
FIG. 3.
Alveolar macrophages from SRAKO mice express elevated levels of CD11b. Mice were infected with P. carinii, and BALF (A and C) and lung digests (B and D) were collected at the indicated times. Flow cytometry was used to compare CD11b expression on CD11c+ macrophages. Dot plots from individual mice are representative of four mice per group at day 7 postinfection. The proportions of nonlymphocytes within gates R2, R3, and R4 are indicated. Data in panels C and D were gated on CD11c+ cells and include cells in gates R2 + R3. Data are means ± standard deviations of the MFI of cells from four mice per group. *, P < 0.05 compared to wild-type controls at the same time point.
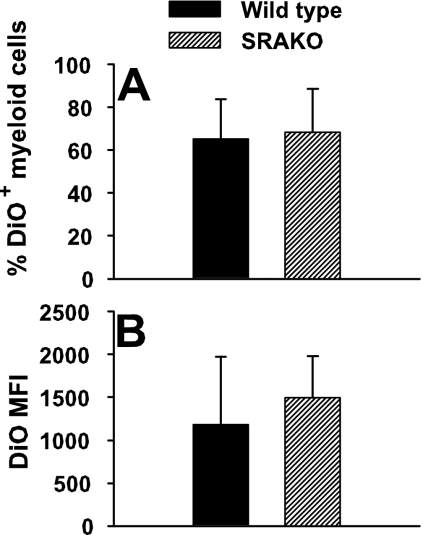
We next examined whether there were functional differences between alveolar macrophages from wild-type mice and those from SRAKO mice. P. carinii organisms were fluorescently labeled with DiO, a stable lipophilic dye, and injected intratracheally into mice. Twenty-four hours later, lungs were subjected to lavage, and the proportion of alveolar macrophages that had phagocytosed organisms was determined by flow cytometry. Figure 4A shows that the proportion of P. carinii-containing macrophages was not different between wild-type and SRAKO cells. Moreover, the MFI, a measure of the number of organisms ingested per cell, was also similar between SRAKO and wild-type macrophages (Fig. 4B).
FIG. 4.
The functional capacity of alveolar macrophages from SRAKO mice differs from wild-type macrophages. To quantitate the phagocytic capacity of alveolar macrophages, P. carinii organisms were labeled with DiO and inoculated into the lungs of wild-type or SRAKO mice. At 24 h, BALF cells were obtained and the proportion of DiO-associated cells determined by flow cytometry. The percent DiO+ cells (A) and MFI of DiO on individual macrophages (B) were not statistically different between wild-type and SRAKO cells.
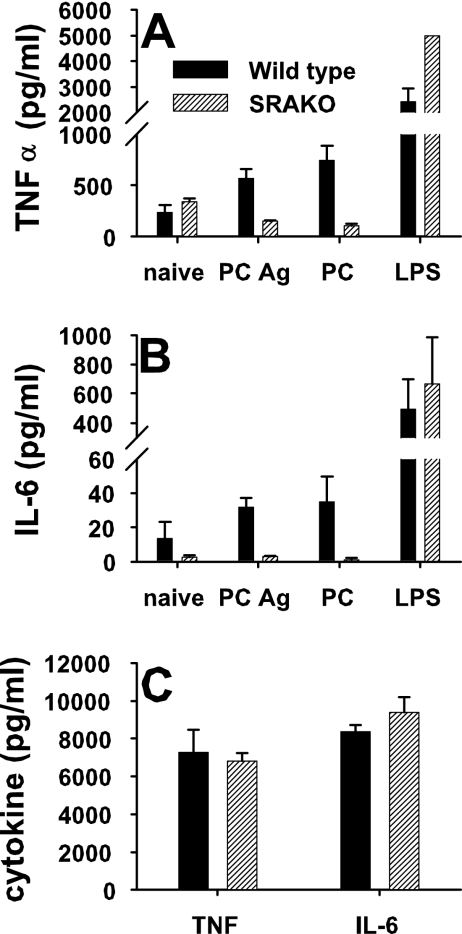
In vitro stimulation of alveolar macrophages was performed to measure cytokine production in response to LPS or P. carinii. After 24 h of stimulation, SRAKO alveolar macrophages elaborated significantly more TNF-α than did wild-type macrophages (Fig. 5A). However, there was a decrease in TNF-α production by SRAKO alveolar macrophages in response to either whole P. carinii organisms or P. carinii antigens compared to that by unstimulated cells (Fig. 5A). In contrast, wild-type alveolar macrophages produced increased TNF-α in response to P. carinii. Production of IL-6 followed similar trends, in that LPS stimulated IL-6 production from SRAKO alveolar macrophages, while P. carinii did not stimulate IL-6 production (Fig. 5B). In contrast, stimulation of alveolar macrophages with zymosan, a β-glucan-rich yeast cell wall extract, resulted in increased production of TNF-α and IL-6 by both wild-type and SRAKO cells (Fig. 5C). Together, these data suggest that SRA is a negative regulator of LPS-induced proinflammatory cytokines but may be important for secretion of cytokines in response to P. carinii.
FIG. 5.
Alveolar macrophage production of proinflammatory cytokines in response to P. carinii is decreased in SRAKO cells compared to wild type. To assess cytokine production by alveolar macrophages, cells were isolated from the BALF of uninfected mice and cultured for 24 h with LPS, sonicated P. carinii antigens (PC Ag), P. carinii organisms (PC), medium alone (naïve) (A and B), or zymosan (C). TNF-α (A and C) and IL-6 (B and C) were quantitated from culture supernatants with a cytokine bead array. Data are representative of replicate wells and three separate experiments.
Lung proinflammatory cytokine levels are increased in SRAKO mice in response to P. carinii.
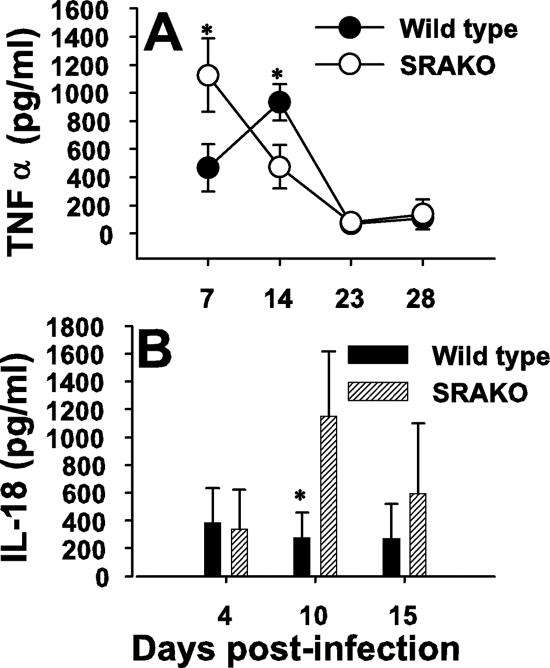
Previous studies have shown that proinflammatory cytokines such as TNF-α are readily made in the lungs of immunocompetent mice exposed to P. carinii (5-7). Moreover, TNF-α production, perhaps in conjunction with gamma interferon, is necessary for host defense against P. carinii (6, 27, 42). Since we and others have shown that alveolar macrophages elaborate TNF-α in response to P. carinii (23, 26, 38), we examined whether TNF-α production was altered in P. carinii-infected SRAKO mice. As shown in Fig. 6A, at day 7 postinfection TNF-α concentrations were significantly greater in the alveolar spaces of P. carinii-infected SRAKO mice than in those of wild-type mice. The peak of TNF-α in the lungs of wild-type mice occurred at day 14 postinfection, whereas TNF-α concentrations were reduced by this time in SRAKO mice. Elevated TNF-α levels corresponded to the elevated CD4+ T cells and neutrophils in the lungs of SRAKO mice at day 7 postinfection. Interestingly, we also found that there was a significant increase in IL-18 concentrations in the BALF of SRAKO mice compared to those of wild-type mice at day 10 postinfection (Fig. 6B). IL-12 concentrations tended to be higher in the BALF of SRAKO mice, but differences were not statistically significant (data not shown). Together, these data indicate that SRA plays a role in controlling inflammation during P. carinii infection.
FIG. 6.
P. carinii infection in SRAKO mice results in elevated proinflammatory cytokines in the lungs. Mice were infected with P. carinii, and BALF was analyzed for TNF-α (A) and IL-18 (B) by enzyme-linked immunosorbent assay. Data are means plus standard deviations for three to five mice per group and are representative of at least two separate experiments. *, P < 0.05 compared to wild-type controls at the same time point.
DISCUSSION
The data presented here identify SRA as an important pattern recognition receptor for controlling the inflammatory response to the opportunistic fungal pathogen P. carinii. We have demonstrated that in the absence of SRA there is an exacerbated CD4+-T-cell, neutrophil, and proinflammatory cytokine response to P. carinii in the lungs. Interestingly, this is in contrast to the direct response to P. carinii we observed in vitro. Alveolar macrophages from SRAKO mice produced reduced levels of TNF-α and IL-6 in response to P. carinii but elevated cytokines in response to LPS. Together, these data suggest that SRA protects mice against elevated proinflammatory cytokines in response to P. carinii; however, other cells, such as dendritic cells, and not macrophages may be responsible for the in vivo observations.
Studies with SRAKO mice have shown that ligation of SRA is important for phagocytosis of bacterial pathogens such as pneumococcus and Francisella and at the same time controls inflammation (1, 22, 37, 39). In our study, we found that SRA had no effect on phagocytosis of P. carinii. This is consistent with current literature, which indicates that β-glucan and mannose receptors are critical for unopsonized phagocytosis of P. carinii (4, 11, 12, 19, 23, 30, 35, 44, 47). However, as has been shown for bacterial pathogens, we also found that P. carinii infection induced elevated proinflammatory cytokines in the lungs of SRAKO mice (1, 22). These data provide added evidence that SRA-dependent phagocytosis and inhibition of cytokine production are controlled independently.
We do not know which ligand for SRA is expressed by P. carinii. There are limited previous observations that carboxymethylated β-1,3-glucan binds SRA and may protect against endotoxic shock (9, 48). β-Glucans are a component of the P. carinii cyst cell wall (4, 11, 19, 23, 30, 35, 47). However, Dushkin et al. (9) reported that unmodified β-glucans had only slight effects on acylated-LDL metabolism, suggesting that only the modified carboxymethylated β-glucans may bind SRA (9, 48), and there is no indication from the literature that P. carinii β-glucans are modified in any way. Recently, protein ligands for SRA have been identified from intact Neisseria meningitidis; one of them, NMB1220, induced endocytosis (37). These results may suggest that other microorganisms express surface proteins that can act as ligands for SRA.
Interestingly, zymosan, a β-glucan- and mannan-rich yeast wall extract, but not P. carinii, caused increased TNF-α production in alveolar macrophages from SRAKO mice in vitro. We and others have previously found that zymosan is a more potent stimulator of macrophage proinflammatory cytokine production than P. carinii (reference 43 and our unpublished observations). These results were accentuated in SRAKO macrophages that produced very high levels of TNF-α in response to both LPS and zymosan but reduced TNF-α in response to P. carinii (Fig. 5). Our in vitro stimulation results seem contradictory to what we found in SRAKO mice when we challenged them with P. carinii. Direct stimulation of alveolar macrophages with P. carinii in vitro resulted in reduced levels of TNF-α in SRAKO cells compared to wild-type cells. In contrast, when we infected SRAKO mice with P. carinii, we found elevated levels of TNF-α in the lungs at day 7 postinfection. This corresponded to increased numbers of lung macrophages with elevated CD11b expression. It is possible that the elevated TNF-α levels were a result of increased T-cell infiltration into the lungs. We have found that a significant proportion of lung-infiltrating CD4+ T cells produce TNF-α during P. carinii pneumonia (unpublished observations). If this was the case, it may imply that the effects of SRA on inflammation during P. carinii infection are a result of ligation of SRA not on macrophages but on another cell type. It has been recently shown that SRA is a functional pattern recognition receptor on dendritic cells and may affect T cell function, particularly by cross-presentation of antigen (2, 3, 21). We are quite interested in this possibility and are pursuing this experimentally.
Since adult SRAKO mice tended to clear P. carinii faster than wild-type mice and certainly exhibited elevated inflammation, we used a model of neonatal immunity to determine whether neonatal SRAKO mice were capable of expedited clearance of P. carinii. We have extensive data demonstrating that there is a significant delay in the ability of neonatal mice to clear P. carinii compared to adult mice, and this delay corresponds to delayed activation of alveolar macrophages, delayed entry of CD4+ cells into the lungs, and delayed specific antibody responses to P. carinii (10, 15, 40). Consistent with our observations in adults, neonatally infected SRAKO mice had elevated numbers of CD4+ cells in the lungs and draining lymph nodes compared to wild-type pups. Moreover, there were increased numbers of activated macrophages in the lungs of neonatally infected SRAKO mice. However, this did not equate to faster clearance of P. carinii organisms, possibly because the kinetics of cellular infiltration was not significantly altered, only the intensity of the response. These data suggest that SRA also exerts control of inflammation in neonatal lungs but does not alter the kinetics of the response to a great degree. We have previously shown that the neonatal lung environment is largely immunosuppressive (reference 16 and unpublished observations), and the data presented here suggest that ligation of SRA on lung myeloid cells does not significantly alter the environment.
Our data provide the first evidence that SRA is involved in control of inflammation in the lungs of mice infected with a fungal pathogen. To date, there have only been reports of SRA involvement in recognition of bacterial ligands, modified LDL, polyanions, and environmental particles (1, 18, 37). Cunha-Rodrigues et al. (8) found no role for SRA in defense against the parasitic pathogen Plasmodium berghei, an etiological agent of malaria; however, that study was limited to examination of parasite burden and not inflammation (8). Further study is necessary to identify potential SRA ligands expressed by P. carinii. Since the pathogenesis of P. carinii is intimately related to inflammation, understanding how SRA dampens TNF-α production could have important ramifications for treatment of the fungal pathogen.
Acknowledgments
This work was supported in its entirety by Public Health Service grant HL062053 from the National Heart, Lung, and Blood Institute.
We appreciate the technical assistance of Kevin Schuer, Suzanne Humphreys, and Jessica Tzou.
Editor: A. Casadevall
Footnotes
Published ahead of print on 4 June 2007.
REFERENCES
- 1.Arredouani, M. S., Z. Yang, A. Imrich, Y.-Y. Ning, G. Qin, and L. Kobzik. 2006. The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am. J. Respir. Cell Mol. Biol. 35:474-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker, M., A. Cotena, S. Gordon, and N. Platt. 2006. Expression of the class A macrophage scavenger receptor on specific subpopulations of murine dendritic cells limits their endotoxin response. Eur. J. Immunol. 36:950-960. [DOI] [PubMed] [Google Scholar]
- 3.Berwin, B., J. P. Hart, S. Rice, C. Gass, S. V. Pizzo, S. R. Post, and C. V. Nicchitta. 2003. Scavenger receptor-A medicates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. EMBO J. 22:6127-6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmona, E. M., R. Vassallo, Z. Vuk-Pavlovic, J. E. Standing, T. J. Kottom, and A. H. Limper. 2006. Pneumocystis cell wall β-glucans induce dendritic cell costimulatory molecule expression and inflammatory activation through a Fas-Fas ligand mechanism. J. Immunol. 177:459-467. [DOI] [PubMed] [Google Scholar]
- 5.Chen, W., E. A. Havell, F. Gigliotti, and A. G. Harmsen. 1993. Interleukin-6 production in a murine model of Pneumocystis carinii pneumonia: relation to resistance and inflammatory response. Infect. Immun. 61:97-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, W., E. A. Havell, and A. G. Harmsen. 1992. Importance of endogenous tumor necrosis factor alpha and gamma interferon in host resistance against Pneumocystis carinii infection. Infect. Immun. 60:1279-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, W., E. A. Havell, L. L. Moldawer, K. W. McIntyre, R. A. Chizzonite, and A. G. Harmsen. 1992. Interleukin 1: an important mediator of host resistance against Pneumocystis carinii. J. Exp. Med. 176:713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha-Rodrigues, M., S. Portugal, M. Febbraio, and M. M. Mota. 2006. Infection by and protective immune responses against Plasmodium berghei ANKA are not affected in macrophage scavenger receptors A deficient mice. BMC Microbiol. 6:73-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dushkin, M. I., A. F. Safina, E. I. Vereschagin, and Y. S. Schwartz. 1996. Carboxymethylated beta-1,3-glucan inhibits the binding and degradation of acetylated low density lipoproteins in macrophages in vitro and modulates their plasma clearance in vivo. Cell Biochem. Funct. 14:209-217. [DOI] [PubMed] [Google Scholar]
- 10.Empey, K. M., M. Hollifield, K. Schuer, F. Gigliotti, and B. A. Garvy. 2004. Passive immunization of neonatal mice against Pneumocystis carinii f. sp. muris enhances control of infection without stimulating inflammation. Infect. Immun. 72:6211-6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans, S. E., P. Y. Hahn, F. McCann, T. J. Kottom, Z. V. Pavlovic, and A. H. Limper. 2005. Pneumocystis cell wall β-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor κB-dependent mechanisms. Am. J. Respir. Cell Mol. Biol. 32:490-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ezekowitz, R. A., D. J. Williams, H. Koziel, M. Y. Armstrong, A. Warner, F. F. Richards, and R. M. Rose. 1991. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature 351:155-158. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez, S., P. Jose, M. G. Avidiushko, A. M. Kaplan, and D. A. Cohen. 2004. Inhibition of IL-10 receptor function in alveolar macrophages by Toll-like receptor agonists. J. Immunol. 172:2613-2620. [DOI] [PubMed] [Google Scholar]
- 14.Gantner, B. N., R. M. Simmons, S. J. Canavera, S. Akira, and D. M. Underhill. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garvy, B. A., and A. G. Harmsen. 1996. Susceptibility to Pneumocystis carinii infection: host responses of neonatal mice from immune or naive mothers and of immune or naive adults. Infect. Immun. 64:3987-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garvy, B. A., and M. Qureshi. 2000. Delayed inflammatory response to Pneumocystis carinii infection in neonatal mice is due to an inadequate lung environment. J. Immunol. 165:6480-6486. [DOI] [PubMed] [Google Scholar]
- 17.Gigliotti, F., A. G. Harmsen, and T. W. Wright. 2003. Characterization of transmission of Pneumocystis carinii f. sp. muris through immunocompetent BALB/c mice. Infect. Immun. 71:3852-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon, S. 1999. Macrophage-restricted molecules: role in differentiation and activation. Immunol. Lett. 65:5-8. [DOI] [PubMed] [Google Scholar]
- 19.Hahn, P. Y., S. E. Evans, T. J. Kottom, J. E. Standing, R. E. Pagano, and A. H. Limper. 2003. Pneumocystis carinii cell wall β-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J. Biol. Chem. 278:2043-2050. [DOI] [PubMed] [Google Scholar]
- 20.Harmsen, A. G., and M. Stankiewicz. 1990. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J. Exp. Med. 172:937-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harshyne, L. A., M. I. Zimmer, S. C. Watkins, and S. M. Barratt-Boyes. 2003. A role for class A scavenger receptor in dendritic cell nibbling from live cells. J. Immunol. 170:2302-2309. [DOI] [PubMed] [Google Scholar]
- 22.Haworth, R., N. Platt, S. Keshav, D. Hughes, E. Darley, H. Suzuki, Y. Kurihara, T. Kodama, and S. Gordon. 1997. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J. Exp. Med. 186:1431-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman, O. A., J. E. Standing, and A. H. Limper. 1993. Pneumocystis carinii stimulates tumor necrosis factor-alpha release from alveolar macrophages through a beta-glucan-mediated mechanism. J. Immunol. 150:3932-3940. [PubMed] [Google Scholar]
- 24.Jakubzick, C., F. Tacke, J. Llodra, N. van Rooijen, and G. J. Randolph. 2006. Modulation of dendritic cell trafficking to and from the airways. J. Immunol. 176:3578-3584. [DOI] [PubMed] [Google Scholar]
- 25.Kim, S., M. Watarai, H. Suzuki, S.-i. Makino, T. Kodama, and T. Shirahata. 2004. Lipid raft microdomains mediate class A scavenger receptor-dependent infection with Brucella abortus. Microb. Pathog. 37:11-19. [DOI] [PubMed] [Google Scholar]
- 26.Kolls, J. K., J. M. Beck, S. Nelson, W. R. Summer, and J. Shellito. 1993. Alveolar macrophage release of tumor necrosis factor during murine Pneumocystis carinii pneumonia. Am. J. Respir. Cell Mol. Biol. 8:370-376. [DOI] [PubMed] [Google Scholar]
- 27.Kolls, J. K., K. Lei, C. Vazquez, G. Odom, W. R. Summer, S. Nelson, and J. Shellito. 1997. Exacerbation of murine Pneumocystis carinii infection by adenoviral-mediated gene transfer of TNF inhibitor. Am. J. Respir. Cell Mol. Biol. 16:112-118. [DOI] [PubMed] [Google Scholar]
- 28.Koziel, H., O. R. D., A. Warner, and R. M. Rose. 1994. Alveolar macrophage interaction with Pneumocystis carinii. Immunol. Ser. 60:417-436. [PubMed] [Google Scholar]
- 29.Landsman, L., C. Varol, and S. Jung. 2007. Distinct differentiation potential of blood monocyte subsets in the lung. J. Immunol. 178:2000-2007. [DOI] [PubMed] [Google Scholar]
- 30.Lebron, F., R. Vassallo, V. Puri, and A. H. Limper. 2003. Pneumocystis carinii cell wall beta-glucans initiate macrophage inflammatory responses through NF-κB activation. J. Biol. Chem. 278:25001-25008. [DOI] [PubMed] [Google Scholar]
- 31.Limper, A. H., J. S. Hoyte, and J. E. Standing. 1997. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J. Clin. Investig. 99:2110-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lund, F. E., M. Hollifield, K. Schuer, J. L. Lines, T. D. Randall, and B. A. Garvy. 2006. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J. Immunol. 176:6147-6154. [DOI] [PubMed] [Google Scholar]
- 33.Lund, F. E., K. Schuer, M. Hollifield, T. D. Randall, and B. A. Garvy. 2003. Clearance of Pneumocystis carinii in mice is dependent on B cells but not on P. carinii-specific antibody. J. Immunol. 171:1423-1430. [DOI] [PubMed] [Google Scholar]
- 34.Marcotte, H., D. Levesque, K. Delanay, A. Bourgeault, R. de la Durantaye, S. Brochu, and M. C. Lavoie. 1996. Pneumocystis carinii infection in transgenic B cell-deficient mice. J. Infect. Dis. 173:1034-1037. [DOI] [PubMed] [Google Scholar]
- 35.O'Riordan, D., J. Standing, and A. Limper. 1995. Pneumocystis carinii glycoprotein A binds macrophage mannose receptors. Infect. Immun. 63:779-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozeki, Y., H. Tsutsui, N. Kawada, H. Suzuki, M. Kataoka, T. Kodama, I. Yano, K. Kaneda, and K. Kobayashi. 2006. Macrophage scavenger receptor down-regulates mycobacterial cord factor-induced proinflammatory cytokine production by alveolar and hepatic macrophages. Microb. Pathog. 40:171-176. [DOI] [PubMed] [Google Scholar]
- 37.Peiser, L., K. Makepeace, A. Pluddemann, S. Savino, J. C. Wright, M. Pizza, R. Rappuoli, E. R. Moxon, and S. Gordon. 2006. Identification of Neisseria meningitidis nonlipopolysaccharide ligands for class A macrophage scavenger receptor by using a novel assay. Infect. Immun. 74:5191-5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pesanti, E. L. 1991. Interaction of cytokines and alveolar cells with Pneumocystis carinii in vitro. J. Infect. Dis. 163:611-616. [DOI] [PubMed] [Google Scholar]
- 39.Pierini, L. M. 2006. Uptake of serum-opsonized Francisella tularensis by macrophages can be mediated by class A scavenger receptors. Cell Microbiol. 8:1361-1370. [DOI] [PubMed] [Google Scholar]
- 40.Qureshi, M. H., and B. A. Garvy. 2001. Neonatal T cells in an adult lung environment are competent to resolve Pneumocystis carinii pneumonia. J. Immunol. 166:5704-5711. [DOI] [PubMed] [Google Scholar]
- 41.Roths, J. B., and C. L. Sidman. 1992. Both immunity and hyperresponsiveness to Pneumocystis carinii result from transfer of CD4+ but not CD8+ T cells into severe combined immunodeficiency mice. J. Clin. Investig. 90:673-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudmann, D. G., A. M. Preston, M. W. Moore, and J. M. Beck. 1998. Susceptibility to Pneumocystis carinii in mice is dependent on simultaneous deletion of IFN-γ and type 1 and 2 TNF receptor genes. J. Immunol. 161:360-366. [PubMed] [Google Scholar]
- 43.Saijo, S., N. Fujikado, T. Furuta, S.-H. Chung, H. Kotaki, K. Seki, K. Sudo, S. Akira, Y. Adachi, N. Ohno, T. Kinjo, K. Nakamura, K. Kawakami, and Y. Iwakura. 2007. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat. Immunol. 8:39-46. [DOI] [PubMed] [Google Scholar]
- 44.Steele, C., L. Marrero, S. Swain, A. G. Harmsen, M. Zheng, G. D. Brown, S. Gordon, J. E. Shellito, and J. K. Kolls. 2003. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 β-glucan receptor. J. Exp. Med. 198:1677-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swain, S. D., S. J. Lee, M. C. Nussenzweig, and A. G. Harmsen. 2003. Absence of the macrophage mannose receptor in mice does not increase susceptibility to Pneumocystis carinii infection in vivo. Infect. Immun. 71:6213-6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor, P. R., L. Martinez-Pomares, M. Stacey, H.-H. Lin, G. D. Brown, and S. Gordon. 2005. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23:901-944. [DOI] [PubMed] [Google Scholar]
- 47.Vassallo, R., J. E. Standing, and A. H. Limper. 2000. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J. Immunol. 164:3755-3763. [DOI] [PubMed] [Google Scholar]
- 48.Vereschagin, E. I., A. A. van Lambalgen, M. I. Dushkin, Y. S. Schwartz, L. Polyakov, A. Heemskerk, E. Huisman, L. G. Thijs, and G. C. van den Bos. 1998. Soluble glucan protects against endotoxin shock in the rat: the role of scavenger receptor. Shock 9:193-198. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, C., S.-H. Wang, M. E. Lasbury, D. Tschang, C.-P. Liao, P. J. Durant, and C.-H. Lee. 2006. Toll-like receptor 2 mediates alveolar macrophage response to Pneumocystis murina. Infect. Immun. 74:1857-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]