Abstract
Human polymorphonuclear neutrophils (PMN) chemotax to a foreign entity. When the chemoattractants’ origins are reached, specific receptors bind to the invader's surface, initiating phagocytosis, phagosome formation, and fusion with granule membranes, generating the bactericidal oxidative burst, and releasing lytic enzymes, specific peptides, and proteins. We explored the initial signaling involved in these functions by observing naïve, unprimed PMN in suspension using fluorescent indicators of cytoplasmic signals (Δ[Ca2+]i and ΔpHi) and of bactericidal entities (oxidative species and elastase) exposed to N-formyl-methionyl-leucyl-phenylalanine (fMLP) and/or multivalent immune complexes (IC). fMLP and IC each initiate a rapid transient rise in [Ca2+]i, mostly from intracellular stores, simultaneously with a drop in pHi; these are followed by a drop in [Ca2+]i and a rise in pHi, with the latter being due to a Na+/H+ antiport. The impact of a second stimulation depends on the order in which stimuli are applied, on their dose, and on their nature. Provided that [Ca2+]i is restored, 10−7 M fMLP, previously shown to elicit maximal Δ[Ca2+]i but no bactericidal functions, did not prevent the cells’ responses with Δ[Ca2+]i to a subsequent high dose of fMLP or IC; conversely, cells first exposed to 120 μg/ml IC, previously shown to elicit maximal Δ[Ca2+]i and bactericidal functions, exhibited no subsequent Δ[Ca2+]i or ΔpHi to either stimulus. While exposure to 10−7 M fMLP, which saturates the PMN high-affinity receptor, did not elicit bactericidal release from these naïve unprimed PMN in suspension, 10−5 M fMLP did, presumably via the low-affinity receptor, using a different Ca2+ source.
Human polymorphonuclear neutrophil (PMN) activation is triggered by the liganding of the stimulus to its specific receptors (4, 8, 29, 30, 40-43). Chemoattractants released by an organism or by the opsonins that coat it, such as N-formyl-methionyl-leucyl-phenylalanine (fMLP), are recognized by their PMN surface receptors. Naïve PMN have been shown to possess two classes of fMLP receptors (fPR1 and fPR2, of high and low affinity, respectively) (25, 40-43), which, when liganded with up to 100 nM peptide, mediate the activation of signals, including a rapid transient rise in cytoplasmic Ca2+ ([Ca2+]i), due largely to release from intracellular stores, and a simultaneous drop in cytoplasmic pH (pHi) (14, 19, 26, 27, 37); when the fMLP concentrations are above 1 μM, the lower-affinity fMLP receptor also initiates some release of bactericidal entities (7, 25, 42, 43, 49). The initial rapid cytoplasmic signals initiated by fPR1 have been studied extensively; they are followed within less than 1 min by a redistribution such that a final [Ca2+]i up to 100 nM above the resting [Ca2+]i ([Ca2+]i°) of naïve PMN as well as a pHi 0.2 to 0.3 units higher than its resting pHi (pH0) of 7.05 to 7.07 are attained (2, 15, 37). These changes, in turn, cause the PMN, after cytoskeletal rearrangement (15, 33, 37) and expression of adhesion proteins on their surface, to move (chemotax) up the chemoattractant gradient to its source via multiple contacts between a given PMN and higher fMLP concentrations (32), a motion that has been captured in real time (23).
Many investigations have shown that at or below 100 nM, liganding with fMLP does not induce the activation of the bactericidal functions of human PMN (e.g., oxidative burst and lytic enzyme and inhibitory protein release) unless the PMN have been primed (3, 6, 13, 15, 39, 48, 50). When the PMN reach the maximal chemoattractant concentration and hence the entity from which it originated, a different set of receptors, such as FcγR, C3R, CD14, and Toll-like receptor (specific receptors for the Fc ends of immunoglobulin G, complement components, lipopolysaccharides, and Toll-like ligands, respectively), which mediates phagocytosis and degranulation, is liganded by the entity itself or its coating opsonins, and the bactericidal functions of the PMN are initiated (30).
We have previously shown for naïve PMN in suspension (26, 27) that the fMLP-induced early rapid Δ[Ca2+]i response of each individual PMN is proportional to the fMLP concentration up to an fPR1-saturating concentration of 10−7 M when all the high-affinity receptors appear to be liganded. Even though these receptors are recycled, residual fMLP at that concentration still binds only to fPR1 and does not ligand measurably to fPR2. Conversely, PMN Fc receptor (FcR)-mediated signaling as well as the subsequent oxidative product and elastase release are disproportionate. That is, in contrast to its fMLP-induced signal, the response of any single cell to high-valency immune complexes (IC), as measured by flow cytometry, is either with full Δ[Ca2+]i, ΔpHi, oxidative burst, and elastase release or with none of these (4, 5, 35, 44, 45). This means that only an IC dose-dependent fraction of the PMN population responds to the incomplete saturation of the relevant FcR of PMN. Since 100% of the PMN exhibit maximal Δ[Ca2+]i and ΔpHi in response to a saturating (120 μg/ml) or supersaturating amount of IC, the cells are all equally capable of responding, but the signal is initiated in only a portion of the PMN determined by the dose of IC; e.g., 50% respond to a half-saturating dose. These kinds of data are obtainable only when cell-by-cell observations are made, i.e., on cells in suspension by flow cytometry (4, 5, 26, 27, 35, 44, 45), with appropriate gating or on attached cells by real-time imaging (11, 23) and cannot be discerned using suspension techniques that measure average values.
Using fluorescent probes, the early events in PMN activation can be monitored kinetically via Δ[Ca2+]i (26, 37) and ΔpHi (11, 15), as can the later events of oxidative product and lytic enzyme release (14, 15, 35). Mononuclear phagocytes express all three classes of FcγR, while naïve PMN express only FcγRII and FcγRIII (17, 47). In contrast to FcγRI, FcγRII and FcγRIII bind IC possessing multivalent Fc endings with much higher affinity than monovalent complexes (5, 44, 45). We reported previously that FcγRIII plays an extensive role in phagocytic activation, while FcγRII controls only a small, slow, activation-initiated Ca2+ influx, which does not appear to be involved in the subsequent degranulation (4) but does contribute to pHi regulation via a recently discovered Ca2+/H+ exchanger (2). We have also shown that these PMN responses to high-valency IC depend upon simultaneous binding to several FcγR and are not elicited by a monovalent antibody-antigen complex (44, 45). The early rapid transient Δ[Ca2+]i, 1,000 to 1,200 nM, is faster (<10 versus 15 to 25 s) when elicited by a chemoattractant rather than by an IC-responding pathway. We have previously shown that the FcγR-mediated Ca2+ transient is not required for PMN degranulation and that only a small channel-mediated influx is necessary, permitting a final [Ca2+]i of 100 to 200 nM to be reached in activated PMN (37). Although the mechanisms by which these events occur have been studied extensively, no universal agreement has been reached; it is nevertheless probable that pHi plays a critical role in controlling degranulation (14, 15, 19, 24, 35, 37).
In order to investigate the mechanisms by which the same PMN in suspension respond first to a chemoattractant and then to the organism from which the chemoattractant originated, leading to phagocytosis and degranulation, we modeled these events by the sequential addition of chemoattractant (fMLP) and the FcγR-liganding multivalent IC to naïve, unprimed PMN in suspension. We further investigated whether Ca2+ homeostasis plays a role in these responses. As noted above, because these observations were made via fluorimetry, yielding average values over all the cells in a suspension, we chose to make these observations on naïve PMN with fPR1- and FcγR-saturating concentrations of fMLP and IC to avoid any disproportionation. Studies with subsaturating doses were not performed in this series of experiments.
MATERIALS AND METHODS
Materials.
The acetoxy methyl esters (AM) of the membrane-permeable fluorescent probes Indo-1-AM and BCECF-AM as well as the probe for the detection of oxidative products in the supernatant, H2HFF-OxyBURST-Green-BSA, were obtained from Molecular Probes (Eugene, OR). Elastase substrate V (MeO-Succ-Ala-Ala-Pro-Val-AMC) was purchased from EMD Biosciences Inc. (La Jolla, CA). The rabbit anti-bovine serum albumin (BSA) antibody was obtained from MP Biomedicals (Irvine, CA). All buffers were prepared with high-performance liquid chromatography-grade chemicals in our laboratory as described below, employing our in-house distilled water that was additionally processed in four stages: charcoal, ion exchange, pyrogard filters, and, thereafter, redistillation (21, 28). All other chemicals were purchased from Sigma-Aldrich Co. (St. Louis, MO) or from Fisher Scientific (Pittsburgh, PA).
Probe preparation.
Indo-1-AM and BCECF-AM were dissolved in dry dimethyl sulfoxide (DMSO) and then aliquoted into glass Vacutainers at 1 mg/ml; they were kept frozen at −20°C until ready to use. One milligram of lyophilized H2HFF-OxyBURST-Green-BSA was dissolved in 1 ml phosphate-buffered saline (PBS) solution without glucose (125 mM NaCl, 2 mM NaH2PO4, 8 mM Na2HPO4, 5 mM KCl) and stored at 4°C until use. To avoid oxygen preexposure of the probe, preparations took place under a nitrogen atmosphere. Additionally, the buffers and solvents in use were deaerated via vacuum.
Participating individuals.
This study was approved by the Institutional Review Board of the Boston University School of Medicine. The participants were recruited through the Boston University School of Medicine. All donors were healthy, as determined by a detailed medical history review, were taking no medications known to affect neutrophil functions, and provided informed, written consent to participate.
Neutrophil preparation.
Peripheral venous blood (30 ml) was collected by venipuncture and instantly mixed with 3 ml of 4.3% sodium citrate. PMN were purified by dextran sedimentation followed by Ficoll-Hypaque centrifugation and brief hypotonic lysis of the remaining red blood cells, as previously described (28). As noted therein and above, special precautions were taken to ensure that PMN remained naïve and unprimed; this includes the use of distilled, three-cartridge-purified, and then redistilled H2O in the preparation of sterile buffers, rinsed plastic ware, and hypotonic lysis. PMN were kept rocking at 4°C in PBS with glucose (125 mM NaCl, 2 mM NaH2PO4, 8 mM Na2HPO4, 5 mM KCl, 5 mM glucose [pH 7.4]) until use. All experiments were performed within 6 h of blood drawing. PMN were loaded with the acetoxymethyl ester forms of the intracellular probes Indo-1 for cytoplasmic calcium and BCECF for intracellular pH, respectively, as previously described (5, 21, 37, 44). As we have also shown previously, full activatability without priming is achieved by incubating these PMN in Krebs-Ringer-phosphate (KRP) (PBS solution with glucose supplemented with 0.9 mM Ca2+ and 1.5 mM Mg2+ [pH 7.4]) for 2 min at 37°C before a stimulus was added.
Stimulus preparation.
fMLP was dissolved in dry DMSO at a concentration of 1 mM, aliquoted into glass Vacutainers, and frozen at −20°C until use. The final concentration of vehicle did not exceed 0.1% DMSO in fMLP-activated PMN suspensions. Possible effects of the vehicle DMSO on pHi or [Ca2+]i were monitored at their experimental concentrations and found to be absent. High-valency IC were prepared as the insoluble portion of a fourfold molar excess of rabbit anti-BSA compared to BSA as previously described (5, 35). As indicated above, all experiments shown here were performed with fPR1-saturating 10−7 M fMLP and/or FcγRII-saturating 120 μg/ml IC.
Simultaneous [Ca2+]i and pHi measurements.
Fluorescence (λex = 350 nm, λem+Ca2+ = 405, and λem2−Ca2+ = 485 nm [Indo-1]; λexpH dependent = 500 nm, λex2 pH independent = 450 nm, and λem = 530 nm [BCECF]) was measured continuously in a Hitachi F-4500 fluorimeter equipped with stirring and thermostating as previously described (21). The known Kd of Indo-1 and the Grynkiewicz equation allowed the calculation of [Ca2+]i from the Indo-1 ratio (F405/F485) (20). The fluorescence ratio of the pH probe BCECF (F500/F450) was converted into pH values using a calibration curve (37) and quadratic regression analysis (R2 = 0.997) by employing the statistical software SPSS. For stimulus response quantification, 2 × 106 PMN per ml were suspended in KRP at 37°C with stirring for 2 min; Δ[Ca2+]i and ΔpHi were monitored as previously described (21), and dose responses were determined (27, 44). Sequential stimulation of PMN was performed with the injection of fMLP or IC, as desired, at 120 and 420 s with or without the addition of 5 mM EGTA 15 s before the first or the second injection of the stimulus in order to chelate the extracellular Ca2+ pool without depleting the outer membrane of bound Ca2+ (37).
Elastase release by PMN.
A 5 mM solution of elastase substrate V was prepared in distilled H2O and aliquoted for storage at −20°C until use. Ten microliters of this 5 mM solution of MeO-Succ-Ala-Ala-Pro-Val-AMC was added to a cuvette containing KRP (pH 7.4) at 37°C, followed by 2 × 106 PMN (final volume, 1 ml), with stirring for 2 min (16, 22, 28, 43). After this equilibration phase, the desired volume of fMLP or IC was injected.
Fc-oxidative burst/superoxide production of PMN.
For the detection of oxidative product release, 10 μl of H2HFF-OxyBURST-Green-BSA stock solution was injected into KRP (pH 7.4) at 37°C, followed by 2 × 106 PMN (final volume, 1 ml), with stirring for 2 min. Special precautions to eliminate exposure to air were taken (see above).
RESULTS
Chemotactic receptor-mediated PMN responses.
We have previously shown that 10−7 M fMLP saturates the high-affinity fPR1, as measured by a maximal Δ[Ca2+]i and ΔpHi response from all the cells (27), while others (7, 25) previously reached the same conclusion via direct binding studies, which also showed essentially no formation of a complex with the low-affinity fPR2 at concentrations below 10−6 M. We also demonstrated that lower doses of fMLP yielded smaller, dose-proportional responses from each cell (27). The early responses to 10−7 M fMLP, detectable within 4 to 10 s, consist of a rapid transient rise of 1,000 to 1,200 nM [Ca2+]i simultaneously with a decrease in cytoplasmic pH by 0.1 to 0.15 units from pH0. These are followed by a gradual decrease in free [Ca2+]i, as redistribution within the cell occurs, to an eventual level up to 100 nM above that of the naïve PMN, accompanied by a gradual rise in pHi to 0.2 to 0.3 units higher than its pH0 of 7.05 to 7.08, as the Na+/H+ antiport opens (2, 26, 27). We also confirm here, as has been reported previously (3, 6, 7, 13, 15, 25, 39, 48-50), that in unprimed naïve PMN in suspension, prepared as described in Materials and Methods, no detectable oxidative products or lytic enzymes are released when the cells are exposed to concentrations less than or equal to 10−7 M.
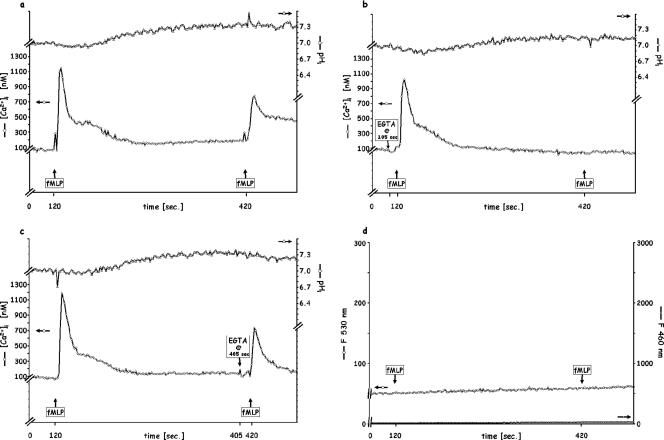
Since PMN moving up a chemoattractant gradient must recognize that chemoattractant repeatedly as its concentration rises, we mimicked the effect by injecting a second dose of fMLP (10−7 M final concentration) 3, 5, or 10 min after the first exposure to this dose. As there was no difference between the 5- and 10-min intervals (data not shown), we performed the remainder of our studies with a 5-min time lapse between the first and second additions of stimuli. As shown in Fig. 1a for a representative experiment (n = 6), upon injection of a second fMLP dose in the presence of extracellular Ca2+, a Δ[Ca2+]i somewhat smaller than the first (800 versus 1,200 nM) occurred, but virtually no further change in pHi could be detected. It is not clear from these experiments whether this consistent absence of reacidification upon restimulation is due to (i) the antiport still being open or (ii) a lack of further phospholipase D activation and consequent generation of phosphatidic acid (14, 15). Thus, since the 5-min presence of extracellular Ca2+ (Ca2+out) permitted the replenishment of that cation's intracellular stores, a situation likely to occur physiologically (37), the PMN response and its ability to reinitiate intracellular Ca2+ signaling remained intact, as did its H+ homeostasis. Furthermore, these results show that the low-affinity fPR2 are not engaged at the low fMLP doses and that no oxidative burst or elastase release could be detected whether saturating (10−7 M) fMLP was added once or twice (Fig. 1d).
FIG. 1.
Sequential stimulation of PMN by fPR1 (high-affinity)-saturating doses (10−7 M) of fMLP 5 min apart. (a) Control; (b) 5 mM EGTA added 15 s before the first fMLP injection; (c) 5 mM EGTA added before the second fMLP injection; (d) release of elastase (shown as F460) and of oxidative products (shown as F530). Each figure is representative of six independent experiments. In this and each of the following figures, the [Ca2+]i and pHi are indicated on the ordinates; F460 is proportional to the elastase release, and F530 is proportional to the release of oxidative products.
When Ca2+out was chelated with 5 mM EGTA 15 s prior to the first addition of 10−7 M fMLP (Fig. 1b), the ensuing Δ[Ca2+]i remained high (only 50 to 100 nM lower), while the response to a second addition of fMLP was abolished, implying that the availability of extracellular Ca2+ is required for the replenishment of the intracellular source of the transient Ca2+i “spike”; similar results have been reported when such replenishment was prevented by the blockage of Ca2+ reentry channels (32, 34). Furthermore, the final [Ca2+]i and pHi were both slightly lower, by 100 nM and 0.15 units, respectively, possibly due to the Ca2+/H+ channel, which would allow Ca2+ to exit the cell in the presence of EGTA and H+ to enter it (2). In contrast, when Ca2+out was chelated later, 15 s prior to the second addition of 10−7 M fMLP, the second Δ[Ca2+]i profile (Fig. 1c) was unaltered in comparison to that observed in the absence of EGTA (Fig. 1a), except for the lower final [Ca2+]i and pHi attributable to the Ca2+/H+ channel.
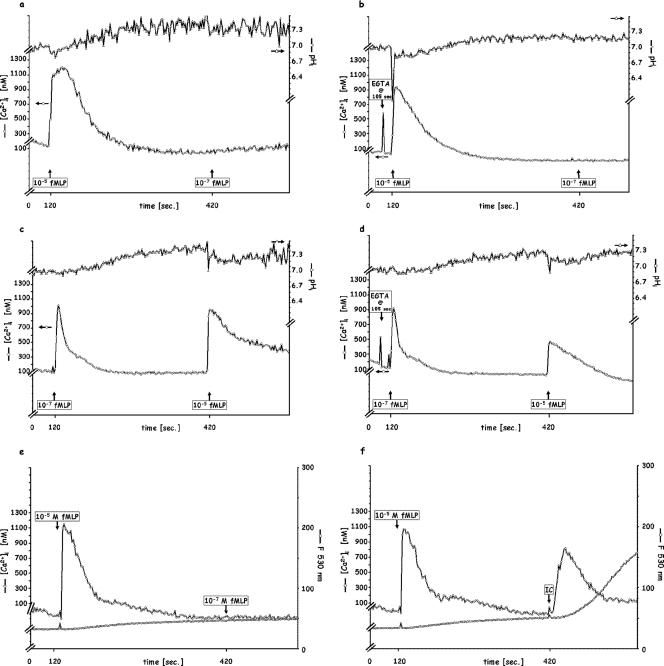
In contrast to the findings when fPR1 was the only fMLP receptor engaged, i.e., in conditions corresponding to the usual physiological environment encountered by PMN, a supersaturating amount of fMLP, such as 10−5 M, exhibited a broader but not higher Δ[Ca2+]i if added to naïve PMN (Fig. 2a), which became narrower and sharper in the presence of EGTA (Fig. 2b). PMN exposed to 10−5 M fMLP were then unable to respond to a subsequent exposure to 10−7 M fMLP with either the Δ[Ca2+]i or the pH signal, whether extracellular calcium was available or not (Fig. 2a, b, and e). If the fMLP additions were reversed, i.e., 10−7 M followed by 10−5 M after 5 min (Fig. 2c), the second Δ[Ca2+]i transient was higher, and acidification was present, both contrasting with findings when only enough fMLP to saturate fPR1 was added (compare Fig. 2c with 1a). Furthermore, while a second response to a second addition of 10−7 M fMLP in the presence of EGTA yielded no second Ca2+ transient (Fig. 1b), a significant second response was elicited with 10−5 M fMLP (Fig. 2d), as was a greater acidification. These high doses of fMLP, in contrast to those involving only fPR1, also activated the PMN bactericidal functions. As shown in Fig. 2e, 10−5 M fMLP initiates a slow oxidation, unchanged by a second addition of 10−7 M peptide. In contrast, if the initial 10−5 M fMLP stimulus is followed by 120 μg/ml IC, a full oxidation response is achieved (Fig. 2f). The Ca2+ transient does not appear upon the addition of the second fMLP following the initial 10−5 M peptide stimulation (Fig. 2e) but does appear upon the addition of IC (Fig. 2f). These observations would be consistent with an fPR2-mediated mechanism involving the Ca2+/H+ channel (since a Ca2+ outflow toward the chelator would involve an electrically neutral H+ inflow) and/or a different intracellular Ca2+ store as well as signals which activate the fusion of granules with the phagosome and the initiation of lytic enzyme release and of the oxidative burst.
FIG. 2.
Sequential stimulation of PMN by high doses (10−5 M) of fMLP, followed 5 min later by 10−7 M fMLP. (a) Control; (b) 5 mM EGTA added 15 s before the first fMLP injection; (e) control and oxidative product release (see Materials and Methods); (f) 10−5 M fMLP followed by 120 μg/ml IC. (c and d) Reversal of the order of sequential stimulation, 10−7 M fMLP, followed by 10−5 M fMLP 5 min later. (c) Control; (d) 5 mM EGTA added 15 s before the first fMLP injection. Each figure is representative of four independent experiments.
While these findings are interesting and could help to elucidate the functions of the low-affinity receptor, they also deviate from normal physiological conditions and imply the desensitization or destruction of fMLP receptors previously suggested by Sklar and colleagues (40-43). For these reasons, the remainder of our studies were carried out with fPR1-saturating 10−7 M fMLP.
Chemotactic-mediated PMN responses followed by FcγR-mediated PMN responses.
In order to mimic the chemoattraction followed by stimulation of PMN effector (bactericidal) functions, we performed experiments using 10−7 M fMLP for the first addition and 120 μg/ml IC (FcγRIII saturating) as the second dose.
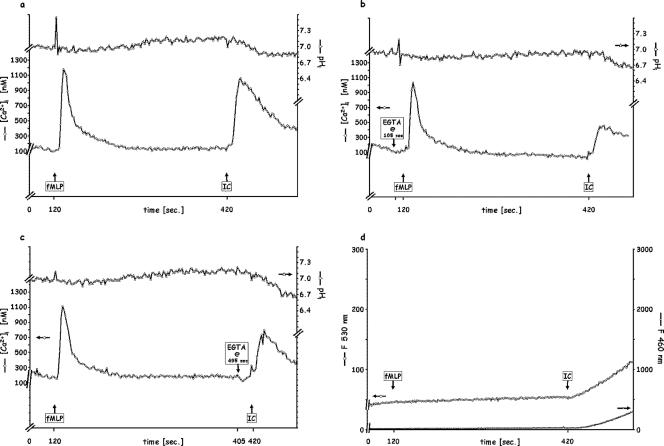
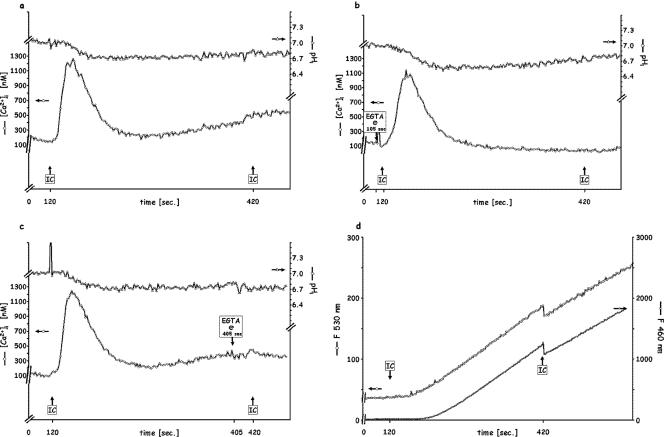
Since fMLP and IC stimulate PMN via different receptors, stimulation with fMLP, followed by IC after 5 min (Fig. 3a), elicited transient Δ[Ca2+]i and ΔpHi signals for both (Fig. 3a), comparable to those observed for each stimulus when added to naïve PMN (Fig. 1a and 4a, respectively) at the same concentration.
FIG. 3.
Sequential stimulation of PMN by saturating doses (10−7 M) of fMLP followed by 120 μg/ml IC 5 min later. (a) Control; (b) 5 mM EGTA added 15 s before fMLP injection; (c) 5 mM EGTA added before IC injection; (d) release of elastase (shown as F460) and of oxidative products (shown as F530). Each figure is representative of five independent experiments.
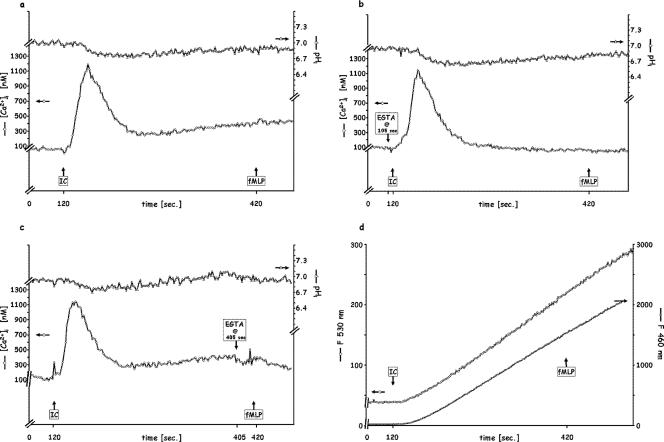
FIG. 4.
Sequential stimulation of PMN by saturating doses (120 μg/ml) of IC followed 5 min later by 10−7 M fMLP. (a) Control; (b) 5 mM EGTA added 15 s before IC injection; (c) 5 mM EGTA added before fMLP injection; (d) release of elastase (shown as F460) and of oxidative products (shown as F530). Each figure is representative of five independent experiments.
In the absence of Ca2+out (EGTA 15 s before the first addition) (Fig. 3b) and unlike the effect of the addition of fMLP 5 min after an initial fMLP stimulation (Fig. 1b), the addition of IC after fMLP elicited a smaller (500 nM) but reproducible Ca2+ transient. This implies that either a different intracellular pool was involved or a greater mobilization from the same pool occurred with FcγRIII-saturating IC than with fPR1-saturating fMLP. Interestingly, the pHi signaling elicited by IC, including the greater acidification (0.35 to 0.40 units) and the absence (or delay of more than 2 min) of alkalinization, was independent of the availability of extracellular Ca2+ (Fig. 3b and c), in contrast to the fMLP-fMLP additions (Fig. 1b and c), where the acidification was virtually abolished in the absence of Ca2+out. Whether or not Ca2+out was available, there was an acidification in response to IC, added 5 min after fMLP, accompanied by an oxidative burst and elastase release (Fig. 3d). Besides reconfirming the difference in the mechanisms mediated via different classes of receptors, these observations indicate that FcγRIII accesses an intracellular Ca2+ pool that is inaccessible to the fPR1-mediated pathway and controls pHi differently. These results are in agreement with our previous findings (14) showing that, in contrast to fPR1, FcγRIII does not initiate the activation of phospholipase D and the consequent formation of phosphatidic acid, which then ionizes in the cytoplasm, leading to a lower pHi. Additionally, in contrast to the fPR1-saturating 10−7 M fMLP, the FcγRIII-saturating 120 μg/ml IC (4, 5, 35, 44, 45) does stimulate PMN phagocytosis and degranulation, initiating the release of elastase and of oxidative entities (Fig. 3d). This is the sequence of responses occurring physiologically as a phagocyte is attracted to a foreign entity, which it then phagocytizes and destroys, although as noted in the introduction, other receptors in addition to FcR may be involved (for example, if that entity has been opsonized).
Fcγ-mediated PMN responses followed by chemotactic receptor-mediated PMN responses.
Can PMN be restimulated after degranulation has been activated? Although we showed above that a subpopulation of PMN responds to subsaturating doses of IC, leaving the remainder of cells to respond to further exposure, the fluorimetric technique used here does not allow us to study subpopulation responses. As shown in Fig. 4, PMN exposed to 120 μg/ml IC exhibit the previously reported Δ[Ca2+]i and ΔpHi (Fig. 4a), as well as degranulation (Fig. 4d), in the presence of Ca2+out. However, in contrast to the inverse order (fMLP and then IC), fMLP added 5 min after IC elicited neither signal, whether or not Ca2+out was available (Fig. 4a, b, and c), and no additional release of oxidative products or elastase was detected (Fig. 4d).
Fcγ-mediated PMN responses followed by FcγR-mediated PMN responses.
We next investigated whether PMN exposed to 120 μg/ml of IC, so that all of its FcγRIII is liganded, can respond again to a second dose of IC 5 min after the first dose. Again, subpopulation responses were avoided by using saturating doses, and therefore, the ability of PMN to phagocytize individual particles (29) one at a time does not apply. Whether Ca2+out was present (Fig. 5a), chelated before the first stimulation (Fig. 5b) or the second stimulation (Fig. 5c), neither cytoplasmic signal was elicited by the second addition, nor was there any increase in oxidative product or elastase release (Fig. 5d). It should be noted that the sharp decrease in fluorescence at a single wavelength upon the addition of IC (Fig. 5d) is attributable to increased light scatter, since the IC stimulus is particulate; no scatter occurs with soluble fMLP. The light-scattering effect is more pronounced when observations are made at a single wavelength (e.g., oxidative burst and elastase release), as ratios ([Ca2+]i or pHi) partially correct for the light scatter by canceling out the concentration of particulate matter. This effect becomes more pronounced at increased fluorescence readings; the small change that occurs with the addition of the first IC stimulus, at baseline fluorescence (compare Fig. 4d and 5d at 120 s), is below the detection threshold of the instrument.
FIG. 5.
Sequential stimulation of PMN by saturating doses (120 μg/ml) of IC 5 min apart. (a) Control, (b) 5 mM EGTA added 15 s before the first IC injection; (c) 5 mM EGTA added before the second IC injection; (d) release of elastase (shown as F460) and of oxidative products (shown as F530). In panel d, the sharp decrease upon injection is due to light scatter (see Results). Each figure is representative of six independent experiments.
All of the results described above were obtained in multiple separate experiments (n ≥ 4), with observations made by fluorimetry after confirmation by flow cytometry that the entire population of PMN was responding to each stimulus when a saturating concentration of that stimulus was injected (data not shown).
DISCUSSION
Polymorphonuclear neutrophils constitute one of the body's primary defenses against invading bacteria or foreign entities. Drawn towards the latter by increasing gradients of the chemoattractants that the organisms generate, the neutrophils’ task is, once they reach them, to phagocytize and destroy the organisms. Their bactericidal functions lie in the contents of their granules and the oxidative products, which the combination of granules, plasma membrane, and cytoplasmic contents allows them to generate in, or inject into, the phagosome enclosing the organism.
The roles of chemoattractants and phagocytizable entities are very different, and their effect on the neutrophils proceeds through different receptors and receptor-mediated processes. While such differences have long been recognized and some of the intermediates involved have been identified, the specific roles of each separate receptor-mediated early signaling in the cytoplasm, which lead to the so-called PMN effector functions, have not yet been delineated. We have addressed the question here, taking advantage of the indicators that we have developed in collaboration with Molecular Probes, which permit the simultaneous observation of intracellular signals and released bactericidal products. The time scale of these signals is rapid, with maximal fMLP- or IC-elicited Δ[Ca2+]i and minimal pHi occurring within <10 s and <30 s, respectively (2, 10, 14, 19, 21, 24, 38, 50). The effector functions can be detected within 30 to 45 s in IC-stimulated PMN (9, 35, 37) but are not initiated by 10−7 M fMLP in naïve unprimed PMN in suspension. Although there have been reports of capacitative changes in [Ca2+]i (18), these are smaller and occur much later, with maxima occurring at 5 to 8 min, and are not addressed in our current studies.
As shown previously by our group as well as by others (18, 23, 26, 27, 32, 34, 37, 50), chemoattractants such as fMLP elicit rapid dose-dependent signaling in the cytoplasm as the cells move, i.e., as the adhesion proteins are expressed and cytoskeletal motility (including actin and myosin) (31, 46) functions affect the motion of the PMN towards their targets. In this process, the same cell must respond multiple times to the occupancy of its chemoattractant receptors by increasing concentrations of the attractant until the origin of that attractant is reached. Results reported previously (16, 22) and shown here by our techniques, as well as binding and oxidative burst elicitation studies reported previously by others (25, 49), have shown that the high-affinity fMLP receptor fPR1 of naïve, unprimed PMN is saturated and elicits maximal cytoplasmic signals at 10−7 M fMLP and that higher concentrations bind to the low-affinity fPR2 (7, 25, 49) and initiate the oxidative burst and elastase release.
The fPR1-mediated rapid and transient rise in [Ca2+]i originates mostly from intracellular stores (32, 34, 37), although a small fraction comes via influx from the extracellular milieu (2, 32), as confirmed in the current study (Fig. 1b versus a and c). When calcium is available from the exterior, these intracellular stores are replenished, and our studies here confirm that the same cells can respond to further chemoattractant liganding by resignaling with a smaller Δ[Ca2+]i. Furthermore, we show, for the first time, that the pHi signals (14, 15, 19, 37, 48), which are significant in the first response of PMN to 10−7 M fMLP, are virtually absent if the same cells are reexposed to the same amount of fMLP but do occur if 100-fold more fMLP is added to them. Although our studies reported here focus on the high-affinity fPR1-initiated functions, our preliminary investigations with 10−5 M fMLP-initiated fPR2 functions show a broader but similar Δ[Ca2+]i, implicating either a second Ca2+ storage source or a more extensive depletion of the same store. Since for this higher dose, there is a more rapid and deeper acidification (to pHi 6.85 rather than 6.95) and a lower eventual pHi (7.15 versus 7.3), a Ca2+/H+ channel such as the one that we described recently (2) could be responsible. While the Ca2+ changes are probably necessary to affect cytoskeletal rearrangement and cell motility, the role of pHi signals in the chemoattractant response is not yet clear, although it has been documented that these pHi changes are also a vital component of the receptor-mediated signaling pathway in phagocytic cells (2, 14, 15, 19, 21, 24, 37, 38, 39). It is clear from the present study as well as previous studies that these pHi signals are important for the activation of the PMN bactericidal functions via either FcR or fPR2. It should be noted that our observations of [Ca2+]i changes concur qualitatively with those reported previously by Nowak et al. (32) and Rosales and Brown (34) (no pHi data were given), although the magnitudes of these changes, in our hands (14, 15, 21, 35, 37) as well as others’ (11, 12), are much higher, 1,000 to 1,200 nM versus 100 to 300 nM.
If extracellular calcium is chelated immediately before the initial chemoattractant addition, so that the cells have no time to lose their homeostatic equilibrium (37), the initial response to 10−7 M fMLP is almost unimpaired, but in the absence of intracellular calcium restoration, no cytoplasmic signaling response (Δ[Ca2+]i or ΔpHi) to further exposure to 10−7 M fMLP can be detected. In contrast, if the chelation occurs only after replenishment, i.e., immediately before the reexposure to chemoattractant, these responses to fMLP are restored, albeit reduced. As noted in our previous studies (2, 15, 37), we have found that prolonged (>1 min) depletion of extracellular Ca2+ profoundly perturbs the PMN functions, presumably by removing the cation not only from extracellular but also from intracellular binding sites via the Ca2+ channels; we therefore restricted depletion to 15 s before the stimulus addition, a time that we found to be too short for such a perturbation to occur.
In contrast to the physiological need for PMN to keep moving towards the source of chemoattractants, their ability to phagocytize and destroy via immune complex-mediated processes depends on the concentration of phagocytizable stimuli that they encounter. We showed a number of years ago (4, 5, 35, 44, 45) that IC bind equally to all PMN in a given suspension but that all respond with the above-mentioned intracellular [Ca2+]i and pHi signals only if all available FcR is bound. If subsaturating amounts of these IC are added to PMN, all cells appear to bind IC equally, but only a fraction, i.e., a subpopulation, of PMN exhibit signaling and eventual effector functions (oxidative burst and elastase release), while the remaining cells exhibit neither. The responding fraction is dose dependent, while its response is not; i.e., each responding cell exhibits maximal cytoplasmic signaling and degranulation, and each nonresponding cell exhibits neither. These observations implied that a second bimolecular step, after initial binding to FcR, was required for the initiation of PMN signaling. These observations fit well with, for example, the films of crawling PMN engulfing one opsonized particle after another since these are subsaturating concentrations of those stimuli. Unfortunately, when those observations were made in real time, labeling of those stimuli so that they could indicate oxidative burst (35) or elastase release (13, 14, 22) was not yet available. Our present studies, which rely on measurements of average cell responses across whole populations, do not provide insights into the mechanisms involved in single-cell responses. Studies of such single-cell responses would involve multiple non-mutually-interfering fluorescent indicators of the cell-associated parameters involved on each PMN (i.e., [Ca2+]i, pHi, receptor identity, receptor occupancy, oxidative burst, and elastase release) measured simultaneously, rapidly, and in real time, with at least one detector per parameter and three or more lasers for excitation. We are currently modifying an instrument for such investigations. It should be noted that in our previous, present, and planned studies, the emphasis is on initial signaling and, if present, effector function initiating time and rate but not on the extent of effector functions (e.g., total oxidative burst and elastase release), since these take much longer, up to 10 min, to reach completion.
The data in the current study confirm that signaling and degranulation initiated via FcR can follow and are independent of chemoattraction initiated via fPR. However, when PMN have been exposed to saturating doses of IC (i.e., all available FcR liganded), the data imply that a second saturating dose of IC, or exposure to fMLP, does not elicit either response in those previously fully IC-stimulated cells.
The oxidative component of PMN activation and its relation to Ca2+ signaling have previously been studied for adherent PMN exposed to opsonized zymosan particles by Dewitt et al. (11). We previously reported that there are major differences in signaling and effector responses, such as oxidative burst, between adherent and nonadherent monocytes (1), and similar differences exist for PMN, at least in part, since the expression of adhesion proteins involves partial activation such as that elicited by fMLP. Because these early events were not measured by Dewitt and colleagues, it is not possible to compare their results with ours.
Several studies have examined fMLP stimulation of human PMN and reported no or minimal reactive oxygen species release unless the PMN were preincubated with high concentrations of priming agents such as lipopolysaccharides, chemokines, cytokines, and tumor necrosis factor alpha platelet-activating factor (3, 6, 13). Our findings are in full agreement with those reports and extend them to the physiological situation-mimicking consequence, when the migrating cells then encounter the source of the chemoattractant and FcR are engaged.
Conclusions.
Taken together, our findings demonstrate the early signaling processes required for PMN attraction to and destruction of an invading entity, the importance of separate mechanisms mediated through the chemoattractant versus the FcR, and the reliance on intracellular calcium stores for the large, rapid cytoplasmic calcium transient and the concomitant pH change, both of which play an important role in PMN functions. As mentioned above, studies dealing with subsaturating doses of IC, which will provide further insights into early phagocytic signaling mechanisms, are currently under way.
Acknowledgments
We gratefully acknowledge the support of the Alexander von Humboldt-Foundation (Feodor Lynen Program) as well as NIH grants DK31056, HL76463, DE16191, and RR00533.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 25 May 2007.
REFERENCES
- 1.Bernardo, J., A. M. Billingslea, M. F. Ortiz, K. F. Seetoo, J. Macauley, and E. R. Simons. 1997. Adherence-dependent calcium signaling in monocytes: induction of a CD14-high phenotype, stimulus-responsive subpopulation. J. Immunol. Methods 209:165-175. [DOI] [PubMed] [Google Scholar]
- 2.Bernardo, J., H. Hartlaub, X. Yu, H. Long, and E. R. Simons. 2002. Immune complex stimulation of human neutrophils involves a novel Ca2+ /H+ exchanger that participates in the regulation of cytoplasmic pH: flow cytometric analysis of Ca2+/pH responses by subpopulations. J. Leukoc. Biol. 72:1172-1179. [PubMed] [Google Scholar]
- 3.Brown, G. E., M. Q. Stewart, S. A. Bissonnette, A. E. Elia, E. Wilker, and M. B. Yaffe. 2004. Distinct ligand-dependent roles for p38 MAPK in priming and activation of the neutrophil NADPH oxidase. J. Biol. Chem. 279:27059-27068. [DOI] [PubMed] [Google Scholar]
- 4.Brunkhorst, B. A., G. Strohmeier, K. Lazzari, G. Weil, D. Melnick, H. B. Fleit, and E. R. Simons. 1992. Differential roles of Fc gamma RII and Fc gamma RIII in immune complex stimulation of human neutrophils. J. Biol. Chem. 267:20659-20666. [PubMed] [Google Scholar]
- 5.Brunkhorst, B. A., K. G. Lazzari, G. Strohmeier, G. Weil, and E. R. Simons. 1991. Calcium changes in immune complex-stimulated human neutrophils. Simultaneous measurement of receptor occupancy and activation reveals full population stimulus binding but subpopulation activation. J. Biol. Chem. 266:13035-13043. [PubMed] [Google Scholar]
- 6.Cadwallader, K. A., A. M. Condliffe, A. McGregor, T. R. Walker, J. F. White, L. R. Stephens, and E. R. Chilvers. 2002. Regulation of phosphatidylinositol 3-kinase activity and phosphatidylinositol 3,4,5-trisphosphate accumulation by neutrophil priming agents. J. Immunol. 169:3336-3344. [DOI] [PubMed] [Google Scholar]
- 7.Cavicchioni, G., A. Fraulini, M. Turchetti, K. Varani, S. Falzarano, B. Pavan, and S. Spisani. 2005. Biological activity of for-Met-Leu-Phe-OMe analogs: relevant substitutions specifically trigger killing mechanisms in human neutrophils. Eur. J. Pharm. 512:1-8. [DOI] [PubMed] [Google Scholar]
- 8.Coffer, P. J., and L. Koenderman. 1997. Granulocyte signal transduction and priming: cause without effect? Immunol. Lett. 57:27-31. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, H. J., P. E. Newburger, M. E. Chovaniec, J. C. Whitin, and E. R. Simons. 1981. Opsonized zymosan-stimulated granulocytes-activation and activity of the superoxide-generating system and membrane potential changes. Blood 58:975-982. [PubMed] [Google Scholar]
- 10.Demaurex, N., A. Monod, D. P. Lew, and K. H. Krause. 1994. Characterization of receptor-mediated and store-regulated Ca2+ influx in human neutrophils. Biochem. J. 297:595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewitt, S., I. Laffafian, and M. B. Hallett. 2003. Phagosomal oxidative activity during beta2 integrin (CR3)-mediated phagocytosis by neutrophils is triggered by a non-restricted Ca2+ signal: Ca2+ controls time not space. J. Cell Sci. 116:2857-2865. [DOI] [PubMed] [Google Scholar]
- 12.Downey, G. P., C. K. Chan, S. Trudel, and S. Grinstein. 1990. Actin assembly in electropermeabilized neutrophils: role of intracellular calcium. J. Cell Biol. 110:1975-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elbim, C., C. Guichard, P. M. Dang, M. Fay, E. Pedruzzi, H. Demur, C. Pouzet, J. El Benna, and M. A. Gougerot-Pocidalo. 2005. Interleukin-18 primes the oxidative burst of neutrophils in response to formyl-peptides: role of cytochrome b558 translocation and N-formyl peptide receptor endocytosis. Clin. Diagn. Lab. Immunol. 12:436-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gewirtz, A. T., and E. R. Simons. 1997. Phospholipase D mediates Fc gamma receptor activation of neutrophils and provides specificity between high-valency immune complexes and fMLP signaling pathways. J. Leukoc. Biol. 61:522-528. [DOI] [PubMed] [Google Scholar]
- 15.Gewirtz, A. T., K. F. Seetoo, and E. R. Simons. 1998. Neutrophil degranulation and phospholipase D activation are enhanced if the Na+/H+ antiport is blocked. J. Leukoc. Biol. 64:98-103. [DOI] [PubMed] [Google Scholar]
- 16.Gonzales, J. R., J. M. Herrmann, R. H. Boedeker, P. I. Francz, H. Biesalski, and J. Meyle. 2001. Concentration of interleukin-1beta and neutrophil elastase activity in gingival crevicular fluid during experimental gingivitis. J. Clin. Periodontol. 28:544-549. [DOI] [PubMed] [Google Scholar]
- 17.Goulding, N. J., S. M. Knight, J. L. Godolphin, and P. M. Guyre. 1992. Increase in neutrophil Fc gamma receptor I expression following interferon gamma treatment in rheumatoid arthritis. Ann. Rheum. Dis. 51:465-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granfeldt, D., M. Samuelsson, and A. Karlsson. 2002. Capacitative Ca2+ influx and activation of the neutrophil respiratory burst. Different regulation of plasma membrane- and granule-localized NADPH-oxidase. J. Leukoc. Biol. 71:611-617. [PubMed] [Google Scholar]
- 19.Grinstein, S., W. Furuya, and W. D. Biggar. 1986. Cytoplasmic pH regulation in normal and abnormal neutrophils. Role of superoxide generation and Na+/H+ exchange. J. Biol. Chem. 261:512-514. [PubMed] [Google Scholar]
- 20.Grynkiewicz, G., M. Poenie, and R. Y. Tsien. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440-3450. [PubMed] [Google Scholar]
- 21.Herrmann, J. M., A. Kantarci, H. Long, J. Bernardo, H. Hasturk, L. V. Wray, Jr., E. R. Simons, and T. E. Van Dyke. 2005. Simultaneous measurements of cytoplasmic Ca2+ responses and intracellular pH in neutrophils of localized aggressive periodontitis (LAP) patients. J. Leukoc. Biol. 78:612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann, J. M., J. R. Gonzales, J. Vonholdt, R. H. Boedeker, and J. Meyle. 2001. Microassay for the detection of elastase activity in the gingival crevice. J. Clin. Periodontol. 28:31-37. [DOI] [PubMed] [Google Scholar]
- 23.Ishibashi, K., S. Okazaki, and M. Hiramatsu. 2006. Simultaneous measurement of superoxide generation and intracellular Ca2+ concentration reveals the effect of extracellular Ca2+ on rapid and transient contents of superoxide generation in differentiated THP-1 cells. Biochem. Biophys. Res. Commun. 344:571-580. [DOI] [PubMed] [Google Scholar]
- 24.Jankowski, A., C. C. Scott, and S. Grinstein. 2002. Determinants of the phagosomal pH in neutrophils. J. Biol. Chem. 277:6059-6066. [DOI] [PubMed] [Google Scholar]
- 25.Kinzer-Ursem, T., K. L. Sutton, A. Waller, G. M. Omann, and J. J. Linderman. 2006. Multiple receptor states are required to describe both kinetic binding and activation of neutrophils via N-formyl peptide receptor ligands. Cell. Signal. 18:1732-1747. [DOI] [PubMed] [Google Scholar]
- 26.Lazzari, K. G., P. J. Proto, and E. R. Simons. 1986. Simultaneous measurement of stimulus-induced changes in cytoplasmic Ca2+ and in membrane potential of human neutrophils. J. Biol. Chem. 261:9710-9713. [PubMed] [Google Scholar]
- 27.Lazzari, K. G., P. Proto, and E. R. Simons. 1990. Neutrophil hyperpolarization in response to a chemotactic peptide. J. Biol. Chem. 265:10959-10967. [PubMed] [Google Scholar]
- 28.Luscinskas, F. W., D. E. Mark, B. Brunkhorst, F. J. Lionetti, E. J. Cragoe, Jr., and E. R. Simons. 1988. The role of transmembrane cationic gradients in immune complex stimulation of human polymorphonuclear leukocytes. J. Cell. Physiol. 134:211-219. [DOI] [PubMed] [Google Scholar]
- 29.Melly, M. A., J. B. Thominson, and D. E. Rogers. 1960. Fate of staphylococci within human leukocytes. J. Exp. Med. 112:1121-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathan, C. 2006. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6:173-182. [DOI] [PubMed] [Google Scholar]
- 31.Niggli, V. 2003. Signaling to migration in neutrophils: importance of localized pathways. Int. J. Biochem. Cell Biol. 35:1619-1638. [DOI] [PubMed] [Google Scholar]
- 32.Nowak, D., P. Bialasiewicz, A. Antczak, M. Krol, and G. Piasecka. 1995. Changes of intracellular free calcium concentration in human polymorphonuclear leukocytes after repeated stimulations with N-formyl-methionyl-leucyl-phenylalanine. Immunobiology 192:343-352. [DOI] [PubMed] [Google Scholar]
- 33.Omann, G. M., and L. A. Sklar. 1988. Response of neutrophils to stimulus infusion: differential sensitivity of cytoskeletal activation and oxidant production. J. Cell Biol. 107:951-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosales, C., and E. J. Brown. 1992. Calcium channel blockers nifedipine and diltiazem inhibit Ca2+ release from intracellular stores in neutrophils. J. Biol. Chem. 267:1443-1448. [PubMed] [Google Scholar]
- 35.Ryan, T. C., G. J. Weil, P. E. Newburger, R. Haugland, and E. R. Simons. 1990. Measurement of superoxide release in the phagovacuoles of immune complex-stimulated human neutrophils. J. Immunol. Methods 130:223-233. [DOI] [PubMed] [Google Scholar]
- 36.Reference deleted.
- 37.Seetoo, K. F., J. E. Schonhorn, A. T. Gewirtz, M. J. Zhou, M. E. McMenamin, L. Delva, and E. R. Simons. 1997. A cytosolic calcium transient is not necessary for degranulation or oxidative burst in immune complex-stimulated neutrophils. J. Leukoc. Biol. 62:329-340. [DOI] [PubMed] [Google Scholar]
- 38.Simchowitz, L., and A. Roos. 1985. Regulation of intracellular pH in human neutrophils. J. Gen. Physiol. 85:443-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simchowitz, L. 1985. Chemotactic factor-induced activation of Na+/H+ exchange in human neutrophils. II. Intracellular pH changes. J. Biol. Chem. 260:13248-13255. [PubMed] [Google Scholar]
- 40.Sklar, L. A., P. A. Hyslop, Z. G. Oades, G. M. Omann, A. J. Jesaitis, R. G. Painter, and C. G. Cochrane. 1985. Signal transduction and ligand-receptor dynamics in the human neutrophil. Transient responses and occupancy-response relations at the formyl peptide receptor. J. Biol. Chem. 260:11461-11467. [PubMed] [Google Scholar]
- 41.Sklar, L. A., G. M. Omann, and R. J. Painter. 1985. Relationship of actin polymerization and depolymerization to light scattering in human neutrophils: dependence on receptor occupancy and intracellular Ca++. J. Cell Biol. 101:1161-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sklar, L. A. 1986. Ligand-receptor dynamics and signal amplification in the neutrophil. Adv. Immunol. 39:95-143. [DOI] [PubMed] [Google Scholar]
- 43.Sklar, L. A., H. Mueller, G. M. Omann, and Z. G. Oades. 1989. Three states for the formyl peptide receptor on intact cells. J. Biol. Chem. 264:8483-8486. [PubMed] [Google Scholar]
- 44.Strohmeier, G. R., B. A. Brunkhorst, K. F. Seetoo, J. Bernardo, G. J. Weil, and E. R. Simons. 1995. Neutrophil functional responses depend on immune complex valency. J. Leukoc. Biol. 58:403-414. [DOI] [PubMed] [Google Scholar]
- 45.Strohmeier, G. R., B. A. Brunkhorst, K. F. Seetoo, T. Meshulam, J. Bernardo, and E. R. Simons. 1995. Role of the Fc gamma R subclasses Fc gamma RII and Fc gamma RIII in the activation of human neutrophils by low and high valency immune complexes. J. Leukoc. Biol. 58:415-422. [DOI] [PubMed] [Google Scholar]
- 46.Swanson, J. A., and A. D. Hoppe. 2004. The coordination of signaling during Fc receptor-mediated phagocytosis. J. Leukoc. Biol. 76:1093-1103. [DOI] [PubMed] [Google Scholar]
- 47.Van de Winkel, J. G., and C. L. Anderson. 1991. Biology of human immunoglobulin G Fc receptors. J. Leukoc. Biol. 49:511-524. [DOI] [PubMed] [Google Scholar]
- 48.Verploegen, S., C. M. van Leeuwen, H. W. van Deutekom, J. W. Lammers, L. Koenderman, and P. J. Coffer. 2002. Role of Ca2+/calmodulin regulated signaling pathways in chemoattractant induced neutrophil effector functions. Comparison with the role of phosphotidylinositol-3 kinase. Eur. J. Biochem. 269:4625-4634. [DOI] [PubMed] [Google Scholar]
- 49.Waller, A., K. L. Sutton, T. L. Kinzer-Ursem, A. Absood, J. R. Traynor, J. J. Linderman, and G. M. Omann. 2004. Receptor binding kinetics and cellular responses of six N-formyl peptide agonists in human neutrophils. Biochemistry 43:8204-8216. [DOI] [PubMed] [Google Scholar]
- 50.Zurier, R. B., S. Hoffstein, and G. Weissmann. 1973. Cytochalasin B: effect on lysosomal enzyme release from human leukocytes. Proc. Natl. Acad. Sci. USA 70:844-848. [DOI] [PMC free article] [PubMed] [Google Scholar]