Abstract
Bacillus anthracis, the causative agent of inhalational anthrax, enters a host through the pulmonary system before dissemination. We have previously shown that human alveolar macrophages participate in the initial innate immune response to B. anthracis spores through cell signal-mediated cytokine release. We proposed that the lung epithelia also participate in the innate immune response to this pathogen, and we have developed a human lung slice model to study this process. Exposure of our model to B. anthracis (Sterne) spores rapidly activated the mitogen-activated protein kinase signaling pathways ERK, p38, and JNK. In addition, an RNase protection assay showed induction of mRNA of several cytokines and chemokines. This finding was reflected at the translational level by protein peak increases of 3-, 25-, 9-, 34-, and 5-fold for interleukin-6 (IL-6), tumor necrosis factor alpha, IL-8, macrophage inflammatory protein 1α/β, and monocyte chemoattractant protein 1, respectively, as determined by an enzyme-linked immunosorbent assay. Inhibition of individual pathways by UO126, SP600125, and SB0203580 decreased induction of chemokines and cytokines by spores, but this depended on the pathways inhibited and the cytokines and chemokines induced. Combining all three inhibitors reduced induction of all cytokines and chemokines tested to background levels. An immunohistochemistry analysis of IL-6 and IL-8 revealed that alveolar epithelial cells and macrophages and a few interstitial cells are the source of the cytokines and chemokines. Taken together, these data showed the activation of the pulmonary epithelium in response to B. anthracis spore exposure. Thus, the lung epithelia actively participate in the innate immune response to B. anthracis infection through cell signal-mediated elaboration of cytokines and chemokines.
Infection with Bacillus anthracis causes three distinct clinical syndromes depending on the route of entry into the host. Inhalational anthrax results from entry through the respiratory system, cutaneous anthrax is caused by penetration through the skin, and ingestion of the bacterial spore results in gastrointestinal anthrax (9, 10). Inhalational anthrax is considered the most life-threatening form of the disease (12, 13) and was the main type that occurred during the 2001 bioterrorism attacks in the United States (35).
Inhalational anthrax is characterized by the unique finding that the spores do not germinate immediately in the lung but rather are consumed by phagocytes and are transported in these cells to the mediastinal lymph nodes. Although the exact mechanism is not well understood, during or at the end of this transit the spores germinate into metabolically active bacteria and can escape the host innate immune system and cause disseminated disease (9, 16, 17). B. anthracis bacteria penetrate into the blood and release three major virulence factors, lethal factor, edema factor, and protective antigen (34), which are thought to contribute to the massive tissue damage that occurs. There is a significant delay between spore exposure and the clinical disease known as anthrax (10), which suggests that the innate immune response plays a role in early containment of the pathogen.
Therefore, macrophages have been thought to be an integral component of the innate immune system that responds to exposure to B. anthracis spores (15). Our previous work has demonstrated that human alveolar macrophages rapidly internalize spores, triggering induction of cytokine and chemokine mRNA and protein through activation of the signaling pathways p38, ERK, and SAP/JNK (5). Lung epithelial cells may also play a role in activating the innate immune response; however, little is known regarding the interaction of lung epithelia with spores due to the fact that a practical model of the respiratory epithelium has been lacking. Most human lung epithelium studies have used single cell lines, such as A549 cells (25, 26). Studies using cell lines may not accurately reflect the course of an infection in human disease as cell lines differ from primary cells, and studies using single lines do not provide for interactions with other cell types.
We have developed a human lung slice tissue model in order to study the local lung response to human pathogens (2). Precision-cut lung slices have frequently been used in toxicology studies and have advantages over the use of isolated, cultured epithelial cells for infectious disease studies. The structural integrity of lung tissue is maintained (29), and this allows cell-cell interaction in a more complex and native three-dimensional system. Detailed mechanistic studies of intracellular processes such as signal pathway activation can be examined in human tissue without risk to the host.
In the current study, we sought to determine the lung innate immune response to exposure to B. anthracis spores using our lung slice model. We demonstrated that the human lung responds to B. anthracis spore exposure by induction of the mitogen-activated protein kinase (MAPK) pathways ERK, SAP/JNK, and p38. This is followed by an increase in the production of several cytokines and chemokines at both the transcriptional and translational levels. The importance of signal pathway activation in cytokine induction was determined by measurement of cytokine induction by spores in the presence or absence of chemical inhibitors of the pathways. The roles of specific chemokines in the lung innate immune response to spores were examined using neutrophil and monocyte chemotactic assays of supernatants of lung tissue exposed to B. anthracis spores. The source of these cytokines is both alveolar macrophages and epithelia, with a possible smaller contribution by interstitial cells. Taken together, the data indicated that the lung epithelia play a role in initiating the human innate immune response to B. anthracis spores. This is the first description of the initial human lung innate immune response to B. anthracis endospores.
MATERIALS AND METHODS
Preparation of B. anthracis spores.
B. anthracis Sterne strain 7702(pX01+ pX02−) was kindly provided by Jimmy Ballard (University of Oklahoma Health Sciences Center, Oklahoma City, OK). Bacteria were grown overnight at 37°C with continuous shaking in LB medium and were then streaked onto AK agar sporulating slants. Bacteria were incubated for 3 weeks at 30°C. The slants were washed with 10 ml of chilled, sterile, deionized water, spun at 10,000 × g for 10 min, and resuspended in 10 ml chilled water. The spore suspension was heated at 65°C for 30 min to kill vegetative bacteria. After heat treatment, the spores were centrifuged for 10 min at 10,000 × g. The pellet was washed five times to remove contaminating bacterial cell debris. The supernatant and the top layer of the pellet were aspirated and discarded following each wash. The spore preparation was resuspended in chilled, sterile, deionized water and centrifuged for 10 min. The titer of the spore preparation was determined by plate counting. The spores were diluted to a concentration of 1 × 109 spores/ml in distilled sterile deionized water and stored at 4°C. Titers were reconfirmed by plate counting before each use. There was no detectable endotoxin in the final spore preparations used in the experiments as determined by the Limulus amebocyte lysate assay (Cambrex, Walkersville, MD). The lower limit of detection of this assay is 0.1 endotoxin units/ml or approximately 20 pg/ml lipopolysaccharide (LPS).
Lung explant culture.
Human lung tissue was obtained from patients undergoing lung resection for cancer in accordance with protocols approved by the institutional review boards of the University of Oklahoma, Veterans Administration Hospital, Baptist-Integris Hospital, St. Anthony's Hospital, and Mercy Health Center, all of Oklahoma City, OK. Only tissue that did not contain tumors was used for experiments. The tumor-free lung tissue was transported on ice in sterile phosphate-buffered saline (PBS) (pH 7.2) containing 200 μg of gentamicin/ml, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2.5 μg of amphotericin B/ml (PBS+antibiotics), and the tissue was subsequently stored at 4°C in PBS+antibiotics for no longer than 4 h. The subsegmental bronchi of lung tissue with intact outer pleura were isolated and cannulated with a flexible Teflon catheter, and the lung segments were gently inflated with 37°C lung slice medium (LSM) containing 1.5% low-melting-point, low-gelling-point agarose (BioWhittaker, Rockland, ME). LSM consisted of minimal essential medium (Sigma Chemical Co., St. Louis, MO) supplemented with 1.0 μg of bovine insulin/ml, 0.1 μg of hydrocortisone/ml, 0.1 μg of retinyl acetate/ml, 200 μg of gentamicin/ml, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 1.25 μg of amphotericin B/ml (2). After the agarose-inflated lung was solidified, 1-cm-diameter tissue cores of nonemphysematous tissue were prepared and sliced into 500-μm-thick sections by using a Krumdieck tissue slicer (Alabama Research and Development, Munford, AL). During slicing, the cores were submerged in chilled PBS+antibiotics. Each slice was placed in 0.5 ml of LSM in a single well of a 24-well plate and then placed in a humidified incubator at 37°C in 5% CO2. The LSM was replaced before the slices were subjected to the experimental treatments.
Infection of human lung slices with B. anthracis spores and determination of cytokine and chemokine induction.
After overnight incubation of the lung slices, the culture medium was replaced with fresh LSM containing 50 μg/ml gentamicin. Maintaining this dose of gentamicin effectively prevents any detectable bacterial survival or replication (5, 19, 36), and this was confirmed by microscopy. For each data point, multiple lung slices were exposed to 1 × 106 Sterne endospores and incubated at 37°C in the presence of 5% CO2 for the periods indicated below. Spore diluent served as a negative control, and LPS (1 μg/ml) was used as a positive control. Following stimulation for various times, medium supernatants and tissue extracts were harvested and stored at −20°C prior to an enzyme-linked immunosorbent assay (ELISA).
RNA preparation and RPA.
Lung slices were harvested by homogenization in TRIzol reagent (Invitrogen, Carlsbad CA), and the total RNA was isolated according to the manufacturer's protocol using glycogen (20 mg/ml) as the carrier. Triplicate slices yielded 8 to 10 μg total RNA, 6 μg of which was used for a single RNase protection assay (RPA) reaction.
Relative gene expression was determined with the RiboQuant multiprobe RPA system (BD Biosciences/Pharmingen, Franklin Lakes NJ). Two template sets were used, the hCK5 set containing probes for lymphotactin, RANTES, IP-10, macrophage inflammatory protein 1β (MIP-1β), MIP-1α, monocyte chemoattractant protein 1 (MCP-1), interleukin-8 (IL-8), and I-309/SCYA1 and a custom cytokine set containing probes for tumor necrosis factor alpha (TNF-α), IL-12/p35, IL-10, gamma interferon (IFN-γ), IL-1β, granulocyte-macrophage colony-stimulating factor (GM-CSF), transforming growth factor β, and IL-6. Both template sets contained probes for ribosomal protein (L32) and glyceraldehyde-3-phosphate dehydrogenase for normalization of RNA loading. Labeled riboprobe was made with an In Vitro transcription kit (BD Biosciences/Pharmingen) and [α-32P]UTP. The RPA kit (BD Biosciences/Pharmingen) was used for hybridization of the probe with the target RNA in the samples and for digestion of unpaired transcripts. Additional controls included a sample containing yeast total RNA, a sample with the hCK5 or custom template control RNA, and a sample with unprotected probe. The resulting mRNA duplexes were separated on a standard 50-cm-long, 0.4-mm-thick polyacrylamide gel. The gel was dried and imaged using a Phosphorimager (Molecular Dynamics, Sunnyvale, CA). The image was analyzed with the ImageQuant 5.0 software (Molecular Dynamics) using the volume quantitation method with histogram peak background subtraction. The identity of each protected band in a sample lane was determined from the positions of the bands in the unprotected probe lane. The fold increase for each RNA species compared with control samples prepared at the same time points was determined after correction for loading using the L32 and glyceraldehyde-3-phosphate dehydrogenase standards.
Signal pathway inhibition and cytokine and chemokine protein determination by ELISA.
After overnight incubation of the lung slices, the media were removed and fresh LSM containing 50 μg/ml gentamicin was added to kill vegetative bacteria (27). Lung slices were exposed to 1 × 106 Sterne spores in triplicate wells of a 24-well plate and incubated at 37°C for 8, 24, and 48 h. Gentamicin remained in the media during the entire incubation time. Spore diluent was used as a negative control, and LPS (1 μg/ml) was used as a positive control.
To determine the effect of inhibition of the ERK, p38, and JNK signaling pathways on cytokine induction, the specific inhibitors U0126, SB203580, and SP600125 (Calbiochem, San Diego CA), respectively, were used (6, 8, 18). Lung slices were preincubated with the inhibitors at 50 μM in LSM containing gentamicin for 4 h at 37°C. The medium was replaced with LSM containing 50 μg/ml gentamicin, and the lung slices were exposed to 1 × 106 B. anthracis Sterne spores for 24 h at 37°C. The final concentration of the inhibitors was maintained throughout the experiment. LPS (1 μg/ml) was used as a positive control, and mock-infected slices were exposed to the same concentration of the inhibitor solvent, dimethyl sulfoxide (DMSO), that was present when all three inhibitors were used. After incubation, the supernatants were collected, centrifuged at 10,000 × g for 2 min, transferred to new tubes, and stored at −20°C.
Cytokine ELISAs were performed as previously described (2), using anticytokine monoclonal primary antibodies and biotinylated anticytokine polyclonal secondary antibodies (R&D Systems, Minneapolis MN). As the MIP-1α monoclonal antibody cross-reacts with MIP-1β, the results were expressed as MIP-1α/β levels. Plates were developed using the 3,3,5′,5′-tetramethylbenzidine reagent (BD Biosciences).
Monocyte and neutrophil preparation for chemotaxis assays.
Heparinized whole blood was obtained by venipuncture from healthy donors, and the peripheral blood monocytes and neutrophils were separated by density gradient centrifugation over Lymphoprep (Axis-Shield PoC AS, Oslo, Norway). To isolate monocytes, the mononuclear cell layers were pooled, washed, and depleted of CD2-, CD7-, CD16a-, CD16b-, CD56-, and CD19-positive cells by incubation with specific antibody-magnetic particle beads, and the monocytes were isolated from a series of washes using a magnetic particle concentrator according to the manufacturer's recommendations (Invitrogen/Dynal). The monocyte viability was 98%, as determined by trypan blue exclusion, and the purity was greater than 90%, based on morphology as determined using preparations stained with Diff-Quick stain reagents (Baxter Healthcare, McGraw Park, IL). To purify neutrophils, the red blood cell-polymorphonuclear cell layer from the density gradient centrifugation was further separated by sedimentation through dextran, followed by hypotonic lysis to remove residual red blood cells. The neutrophil viability and purity were both more than 95%, based on trypan blue exclusion and morphology.
To label the cells, monocytes and neutrophils were resuspended at a concentration of 2 × 107 cells/ml in PBS containing 0.1% bovine serum albumin (fraction V; Sigma, St. Louis, MO) and incubated in 5 μM carboxyfluorescein diacetate succinimidyl ester (Invitrogen/Molecular Probes) for 10 min at room temperature. The cells were then washed twice in PBS containing 2% bovine serum albumin to remove unbound label and resuspended at a concentration of 2 × 106 cells/ml in Gey's salt solution. After labeling, the cells were 98% viable as determined by trypan blue exclusion. Purified, labeled monocytes and neutrophils were kept on ice until use.
Monocyte and neutrophil chemotaxis assays.
Culture medium supernatants from three independent experiments using lung slices exposed for 24 h to 1 × 106 B. anthracis endospores or the mock-treated media from the same experiments were used to assess monocyte and neutrophil chemotaxis. A portion of the medium samples was preincubated for 2 h at 37°C with chemokine-specific, neutralizing polyclonal antibodies (R&D Systems). For monocyte chemotaxis, spore-stimulated medium was treated with either 200 μg/ml goat anti-human MCP-1 polyclonal antibody, 100 μg/ml goat anti-human MIP-1α/β polyclonal antibody, or a mixture of the two antibodies at the same concentrations. For neutrophil chemotaxis, both mock- and spore-stimulated media were treated with 100 μg/ml goat anti-human IL-8 polyclonal antibody. As controls for chemokine-specific chemotactic activity and for the ability of specific neutralizing antibodies to prevent chemotaxis, fresh LSM was amended with recombinant human chemokines (R&D Systems). For neutrophil chemotaxis 50 ng/ml IL-8 was used, and for monocyte chemotaxis either 50 ng/ml MCP-1, 5 ng/ml MIP-1α, or a mixture of MCP-1 and MIP-1α at the same concentrations was used. Other control LSM samples contained mixtures of an antibody and corresponding chemokine at the concentrations mentioned above, and these control mixtures were preincubated for 2 h at 37°C. Chemotaxis was assayed using 96-well HTS FluoroBlok plates with 3-μm pores (BD Biosciences) by adding 5 × 105 purified, labeled cells (50 μl) to the upper insert chambers and 150 μl of the control or experimental media to the lower chambers. Each sample was assayed in triplicate. The plates were incubated for 2 h at 37°C in the presence of 5% CO2, after which the fluorescence of cells which had migrated through the membrane was measured with a model FLx800 fluorescent plate reader (Bio-Tek Instruments, Winooski, VT) using filter settings with a peak excitation wavelength of 460 nm and an emission wavelength of 528 nm. Chemotactic activity was expressed as a percentage of the IL-8 or MCP-1 plus MIP-1α chemokine-spiked LSM control minus the background activity of the unamended LSM control.
Signaling pathway kinase assay.
Human lung slices were maintained overnight at 37°C in the presence of 5% CO2 in 0.5 ml LSM containing antibiotics, and the medium was replaced prior to stimulation. Four to eight slices per condition were used for MAPK family assays. Slices were stimulated with either 1 × 106 Sterne spores, phorbol 12-myristate 13-acetate (PMA) (100 ng/ml), or LPS (1 μg/ml). Mock-infected, negative control slices were exposed to an equivalent volume of spore-free diluent. After incubation at 37°C in the presence of 5% CO2 for the times indicated below, the slices were harvested and homogenized in 100 μl cold lysis buffer as previously described (2). Clarified lung slice homogenates containing 20 to 30 μg protein were heat denatured in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. The samples were separated on a 10% SDS-PAGE gel and then electrophoretically transferred to polyvinylidene fluoride membranes. To detect activated, phosphorylated ERK1/2, p38, or SAPK/JNK, the membranes were blocked overnight in 5% powdered milk in Tris-buffered saline and then immunoblotted with specific affinity-purified rabbit polyclonal antibodies (Cell Signaling Technology, Beverly, MA). Identically prepared membranes were probed with either rabbit polyclonal anti-ERKl/2, anti-p38, or anti-SAPK/JNK antibodies that recognized both phosphorylated and nonphosphorylated forms of the signaling proteins (Cell Signaling Technology). The membranes were developed with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Cell Signaling Technology) and chemiluminescent reagents (Pierce Biotechnology, Rockford, IL). The developed membranes were exposed to X-ray film, and digital scans of the film were quantified using ImageQuant software (BD/Molecular Dynamics, Bedford, MA).
Cytokine immunohistochemistry of lung tissue explants.
To examine which cell types in the lung tissue produced IL-6 and IL-8 after spore infection, lung slices were exposed to 1 × 106 spores or spore diluent in fresh LSM containing 50 μg/ml of gentamicin and incubated at 37°C for 24 h. Brefeldin A (L C Laboratories, Wofford, MA) was added at a concentration of 5 μg/ml to block protein export in order to enhance cytokine detection. Following incubation, the lung slices were fixed with 4% paraformaldehyde in PBS at room temperature for 30 min and were then imbedded in paraffin. Sections (3 to 5 μm) were mounted on glass slides and immunoprobed overnight at 4°C with a goat anti-human polyclonal antibody for IL-6 or IL-8 (R&D Systems). After washing, the sections were probed with a donkey anti-goat secondary antibody conjugated to Alexa Fluor 546 (Molecular Probes), and the cell nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI). Transmitted light and fluorescent microscopy images were obtained using an Olympus BX51 microscope equipped with a Spot 4.2 digital camera.
Statistical analysis.
Where applicable, the data are expressed as the means ± standard errors of the means (SEM). Statistical significance was determined by one-way analysis of variance (ANOVA) with the Student-Newman-Keuls post hoc correction for multiple comparisons. A P value of <0.05 was considered significant (39).
RESULTS
RNA expression of cytokines and chemokines by human lung slices exposed to B. anthracis spores.
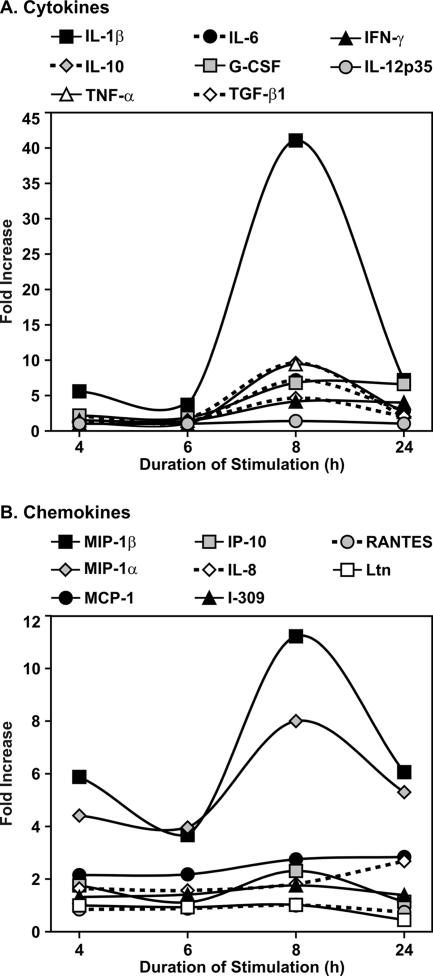
We first examined the innate immune cytokine and chemokine response of human lung tissue to B. anthracis spores using RPA. Lung slices were exposed to 1 × 106 spores or spore diluent (negative control) for 4, 6, 8, and 24 h. RPA for cytokines demonstrated that there was 4- to 43-fold induction of IL-6, IL-1β, IL-10, TNF-α, transforming growth factor β, GM-CSF, and IFN-γ. The peak fold increase occurred at 8 h postinfection for most of the cytokines, but GM-CSF and IFN-γ peak induction occurred at 24 h (Fig. 1A).
FIG. 1.
B. anthracis Sterne spores induce mRNA of cytokines and chemokines in human lung tissue. Human lung tissue slices (three slices per data point) were exposed to 1 × 106 spores in the presence of gentamicin for the times indicated. Equal volumes of spore diluent (sterile water) were used as a negative control. mRNA expression levels were determined using a custom cytokine template (A) and the hCK5 chemokine template (B) (Pharmingen) (see Materials and Methods). The fold increases in mRNA levels compared with controls were determined by normalization for levels of housekeeping genes present in each sample (see Materials and Methods). G-CSF, granulocyte-macrophage colony-stimulating factor; Ltn, lymphotactin.
The RPA for chemokines also showed that there was RNA induction at 4 h postinfection, with the levels either peaking at 8 h and then decreasing slightly by 24 h postexposure or, in the case of IL-8 and MCP-1, gradually increasing during the course of the experiment. Both monocyte and neutrophil chemotaxins were induced, and the induction ranged from 2- to 12-fold for MIP-1α, MIP-1β, IL-8, MCP-1, and IP-10. In contrast, there was minimal induction of the lymphocyte chemotaxins RANTES and lymphotactin (Fig. 1B).
Thus, the RPA results suggest that there was a broad cytokine immune response and there was also a monocyte and neutrophil chemokine response after exposure of human lung tissue to B. anthracis spores.
Induction of cytokines in human lung tissue infected with B. anthracis spores.
RPA data indicated that there were increases in the mRNA levels of several cytokines and chemokines. To confirm that these increases in mRNA were reflected at the level of translation, we tested for the relevant proteins in supernatants of lung slices exposed to B. anthracis Sterne spores for 8, 24, and 48 h using ELISA. Human lung slices were mock treated with spore diluent, treated with 1 × 106 strain Sterne spores, or treated with LPS (1 μg/ml) as a positive control. Consistent with the RPA results, we saw increases in the cytokines IL-6 and TNF-α and the chemokines IL-8, MCP-1, and MIP-1α/β with spore exposure. Specifically, there were peak fold increases compared with the background in the cytokine and chemokine levels of IL-6, TNF-α, IL-8, MCP-1, and MIP-1α/β of 3-, 25-, 9-, 5-, and 34-fold, respectively. Maximum IL-6 and MIP-1α/β levels were seen at 24 h, while the maximum increases in IL-8 and MCP-1 were seen at 48 h. TNF-α levels were elevated at all time points tested, although there was a slight decline in the absolute levels from 8 to 48 h. At all the times tested MCP-1 was stimulated to a greater extent than the positive control, 1 μg/ml LPS (Fig. 2D). Although we saw significant induction of IL-1β mRNA in the RPA, the IL-1β protein levels were low (less than 100 pg/ml) in lung slice supernatants and undetectable in lung tissue extracts. There was no induction of cytokines or chemokines when human lung slices were exposed to undiluted supernatants of the final spore preparation, confirming that the active component of the spore preparation for cytokine induction was B. anthracis spores (data not shown).
FIG. 2.
B. anthracis spores stimulate chemokines and cytokines in the human lung slice tissue model. Lung slices were exposed to 1 × 106 spores in the presence of gentamicin for the times indicated. Spore diluent was used as a negative control, and LPS (1 μg/ml) was used as a positive control. Chemokine and cytokine protein levels were determined by ELISA using lung slice supernatants. The data are expressed as the means ± SEM from four separate lung slice donor experiments, and three tissue slices were used per experiment. Statistical significance was determined by ANOVA. Means were compared to data for the negative control group. One asterisk, P < 0.05; two asterisks, P < 0.01.
These results show that the induction of cytokine and chemokine genes, as determined by RPA, is consistently reflected at the protein level.
Induction of discrete neutrophil and monocyte chemotactic activity in lung tissue exposed to B. anthracis spores.
Exposure of human lung tissue to B. anthracis spores resulted in induction of multiple cytokines and chemokines. In order to determine whether these chemokines were biologically active, neutrophil and monocyte chemotactic activity in B. anthracis spore-exposed lung tissue supernatants was measured using a fluorescent assay as described above (see Materials and Methods) Briefly, cells were labeled with carboxyfluorescein diacetate succinimidyl ester and allowed to migrate through opaque 3-μm-pore-size membranes toward the target media, after which chemotaxis was assessed with a fluorescent plate reader. To determine whether the chemokines detected by RPA and ELISA represented the dominant neutrophil and monocyte chemokines induced, chemotactic activity was measured in the presence and absence of neutralizing antibodies to the chemokines detected. As a positive control, similar amounts of recombinant chemokine were added to unexposed media. To confirm the neutralizing capacity of the chemokine antibodies, recombinant chemokine was preincubated with antibody prior to the assay.
In terms of neutrophil chemotaxis, the predominant induced chemotaxin identified by RPA and ELISA was IL-8; thus, recombinant IL-8 protein and IL-8 neutralizing antibody were used for this experiment (Fig. 3A). The addition of recombinant IL-8 to unexposed medium at concentrations similar to those detected in spore-exposed LSM resulted in a fivefold increase in cell-associated fluorescence. The spore-exposed LSM contained similar amounts of neutrophil chemotactic activity. Preincubation of this medium with neutralizing IL-8 antibody significantly decreased neutrophil activity to a level that was approximately that seen with unexposed medium. These results suggest that the dominant neutrophil chemotaxin induced by B. anthracis in lung tissue is IL-8.
FIG. 3.
Lung innate immune cytokine response to B. anthracis spores contains discrete neutrophil and monocyte chemotactic activity. Supernatants from lung slices exposed to B. anthracis spores for 24 h were tested for neutrophil and monocyte chemotactic activity. Antibodies to specific known chemotaxins were employed to confirm the dominant neutrophil and monocyte chemokines present in the lung slice supernatants tested. LSM amended with recombinant chemokines as indicated and media exposed to spore buffer (Mock) were used as positive and negative controls, respectively. Selection of the antibodies and recombinant chemokines used as positive controls was based on RPA and ELISA of B. anthracis-exposed supernatants (see Materials and Methods). (A) Neutrophil chemotactic activity in the presence of IL-8 neutralizing antibody. (B) Monocyte chemotactic activity in the presence or absence of MCP-1 and MIP-1α/β neutralizing antibody. The data are expressed as the percentage of monocyte chemotaxis due to the positive control using a fluorescence-based chemotaxis assay and are the means ± SEM of three separate assays. Statistical significance was determined by ANOVA. Means were compared to data from mock-exposed control supernatants (asterisks) and B. anthracis-exposed supernatants (number signs) without antibody; an asterisk or a number sign indicates that the P value is <0.05.
Supernatants were also tested for monocyte chemotaxins. RPA and ELISA detected significant induction of the monocyte chemotaxins MCP-1 and MIP-1α/β. Addition of either or both of these proteins to unexposed media resulted in significant monocyte chemotaxis, as demonstrated by 3.5- to 5-fold increases in cell-associated fluorescence (Fig. 3B). B. anthracis spore-exposed LSM contained amounts of monocyte chemotactic activity similar to those seen with unexposed media to which either MCP-1 or both chemotaxins were added. Preincubation of the MCP-1- and MIP-1α/β-amended media with either MCP-1 or MIP-1α/β antibody diminished this activity, and when media were preincubated with both antibodies, the effects appeared to be additive. Similarly, preincubation of the spore-exposed LSM with MCP-1 or MIP-1α/β antibody inhibited monocyte chemotactic activity, and when media were preincubated with both antibodies, the effects also appeared to be additive. These results suggest that the predominant monocyte chemokines induced by B. anthracis spores in the human lung are MCP-1 and MIP-1α/β.
Induction of MAPK signaling pathways in human lung slices exposed to B. anthracis spores.
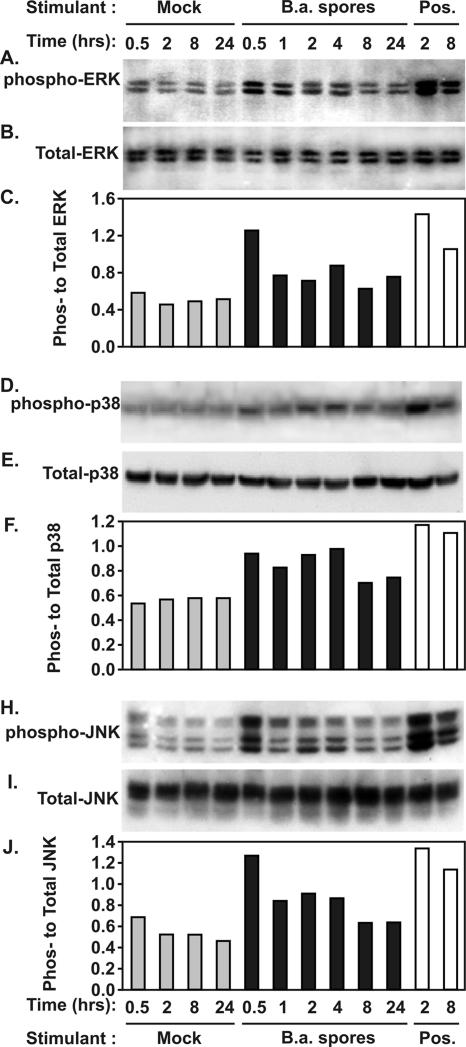
To determine the role of signal pathway activation in cytokine and chemokine induction by B. anthracis Sterne strain spores in human lung slices, we next studied the kinetics of spore-induced activation of the MAPK signaling cascades. We assessed ERK1/2, SAPK/JNK, and p38 activation as exhibited by phosphorylation in human lungs infected with Sterne spores (1 × 106 spores). Stimulation with a mixture of PMA (100 ng/ml) and LPS (1 μg/ml) for 2 and 8 h served as positive controls for phosphorylation, and mock-infected lysates were prepared at 0.5, 2, 8, and 24 h. Gentamicin (50 μg/ml) was present in the growth medium throughout the course of the experiment to prevent the growth of vegetative bacteria and the resultant production of anthrax toxins, which are known to cleave and inactivate MAPKs. At various times after infection, lung lysates were prepared, and total and phosphorylated ERK1/2, SAPK/JNK, and p38 levels were assessed by Western blot analysis. Phosphorylation was assessed by determining the ratio of phosphorylated kinase (Fig. 4A, D, and H) to total kinase (Fig. 4B, E, and I), and the resultant activities were graphed (Fig. 4C, F, and J). This ratio corrects for variations in the amount of sample protein loaded onto SDS-PAGE gels. The results revealed that ERK1/2, SAPK/JNK, and p38 were activated by Sterne spores.
FIG. 4.
Kinetics of ERK1/2, SAPK/JNK, and p38 phosphorylation by B. anthracis Sterne strain spores (B.a. spores) in human lung slices. Human lung tissue slices were exposed to 1 × 106 Sterne spores for the times indicated in the presence of gentamicin. Lung lysates were prepared, and the levels of total and phosphorylated ERK1/2 (A, B, and C), p38 (D, E, and F), and SAPK/JNK (H, I, and J) were assessed by Western blot analysis. PMA (100 ng/ml) and LPS (1 μg/ml) were used as positive controls, and spore diluent was used as a negative control. Western blots were probed with an antibody specific for phosphorylated ERK1/2 (A), p38 (D), or SAPK/JNK (H). Identically prepared blots were also probed with pan-antikinase antibody against ERK1/2 (B), p38 (E), or SAPK/JNK (I). Activation as determined by the ratio of phosphorylated kinase to total kinase for ERK1/2 (C), p38 (F), and SAPK/JNK (J) is also shown.
Phosphorylation of ERK and JNK peaked within 30 min of spore contact with the lungs, with ERK1/2 and SAPK/JNK exhibiting 2.1- and 1.8-fold increases, respectively, compared with mock-infected controls. Spore-induced p38 phosphorylation exhibited a very slight increase compared with the negative control at all times tested. By 8 h postinfection, the levels of spore-induced activation of all three kinases had reached their nadirs, declining to less than 1.5-fold greater than those of the negative controls. Spore-induced kinase activation remained slightly elevated at 24 h postinfection, with ERK1/2, SAPK/JNK, and p38 phosphorylation reaching 1.6-, 1.44-, and 1.1-fold increases, respectively, compared with mock-infected negative controls at 24 h. These results indicate that exposure to B. anthracis Sterne spores elicits a modest increase in ERK1/2, SAPK/JNK, and p38 activation which precedes cytokine mRNA and protein production in human lung slices.
Importance of multiple signaling pathways in cytokine induction by B. anthracis spores.
The findings described above demonstrated that the MAPK signaling pathways are activated when human lung slices are exposed to B. anthracis spores. We next sought to determine whether the activation of these signaling pathways is essential for the induction of cytokines. Lung slices were preincubated for 4 h in medium with 50 μmol of the ERK pathway inhibitor U0126, the p38 pathway inhibitor SB203580, or the SAPK/JNK pathway inhibitor SP600125. For the human lung, these doses were sufficient to inhibit induction of the corresponding signaling pathways by spores (not shown). A combination of the three inhibitors was also used. The inhibitors remained in the medium throughout the duration of the experiment, and slices that were not treated with inhibitors were exposed to an inhibitor solvent (DMSO) as an additional control. The lung slices were incubated for 24 h either with spores (1 × 106 spores) or with LPS (1 μg/ml) prior to collection of the supernatants for cytokine and chemokine determination.
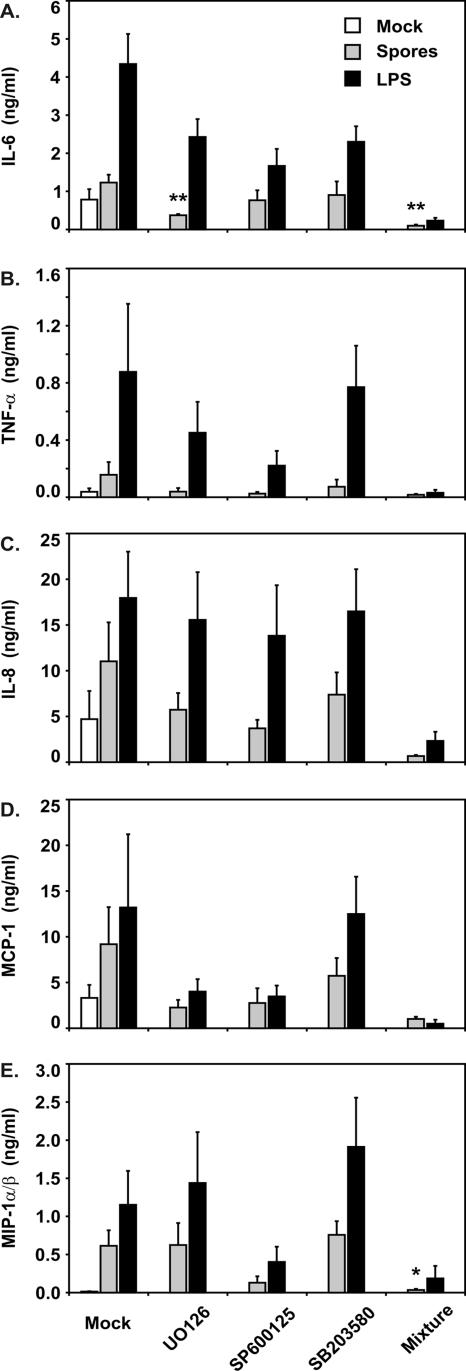
The data revealed differences in the requirements for signaling pathway activation for the induction of specific cytokines by B. anthracis spores (Fig. 5). With the exception of spore induction of MIP-1α/β, addition of any of the three inhibitors diminished cytokine and chemokine induction by spores. In the case of MIP-1α/β, inhibition of the ERK and p38 pathways individually did not alter induction of this chemokine by spores. For all cytokines and chemokines tested, inhibition of all three pathways at the same time appeared to enhance blockade of spore cytokine and chemokine induction. Due to the low levels seen, this additional effect frequently did not reach statistical significance.
FIG. 5.
Inhibition of B. anthracis spore induction of cytokines and chemokines by signal pathway inhibitors. Human lung tissue slices were preincubated with either 50 μM of one of the signaling pathway inhibitors (U0126, SB203580, or SP600125), all three inhibitors together (Mixture), or the inhibitor solvent (Mock), and the concentrations of these reagents were maintained throughout the experiment. Slices were then exposed to spore diluent, 1 × 106 spores, or LPS (1 μg/ml) for 24 h in the presence of gentamicin prior to measurement of cytokines and chemokines by ELISA. The data are expressed as the means ± SEM of three experiments, and three tissue slices were used per experiment. Statistical significance was determined by ANOVA. Means were compared to data from the spore-infected control group without inhibitors. One asterisk, P < 0.05; two asterisks, P < 0.01.
We observed lower fold induction by spores of all cytokines and chemokines tested except MIP-1α/β during our signaling pathway inhibition experiments. Additional experiments in which lung slices were exposed to spores or spore buffer in the presence or absence of the inhibitor solvent (DMSO) revealed that this was due to suppression of the maximal lung cytokine and chemokine response to spores by DMSO (data not shown). A similar cytokine-suppressing effect of DMSO has been observed in A549 pulmonary epithelial cells and human bronchial epithelial cells and has been attributed to the properties of DMSO as a scavenger of free radicals (21, 22).
Cellular source of cytokine and chemokine induction by B. anthracis spores.
To determine the lung cellular elements that participate in the lung innate immune cytokine response to B. anthracis spores, we performed an immunohistochemistry analysis of spore-exposed lung slices. Lung slices were exposed to 1 × 106 spores or spore diluent for 24 h in the presence of brefeldin A to enhance detection of cytokines. The slices were then processed for immunohistochemistry for detection of the cytokine IL-6 and the chemokine IL-8 using goat polyclonal antibodies as described in Materials and Methods. Tissues exposed to spore diluent were used to establish unstimulated cytokine and chemokine detection. An additional negative control was performed for IL-6 and IL-8 detection by using the same staining protocol but with omission of the IL-6 and IL-8 primary antibody. There was minimal background immunofluorescence in the absence of IL-6 and IL-8 primary antibody (Fig. 6N and O). IL-6 and IL-8 detection was significantly enhanced by spore exposure (Fig. 6E, F, K, and L). IL-6 was detected in both epithelial cells and intraalveolar cells which morphologically resembled alveolar macrophages (Fig. 6F). IL-8 was also detected both in epithelial cells and in alveolar macrophages (Fig. 6L). There were also scattered interstitial cells that stained positive for IL-6 and IL-8. The results indicate that both lung epithelia and alveolar macrophages contribute to the innate immune response through induction of cytokines and chemokines. Additional interstitial cells may also contribute to this response.
FIG. 6.
Cellular source of cytokine and chemokine induction by B. anthracis spores. Lung slices were exposed to 1 × 106 spores or spore diluent for 24 h in the presence of brefeldin A to enhance detection of cytokines. The slices were then processed for immunohistochemistry analysis for detection of the cytokine IL-6 (A to F) and the chemokine IL-8 (G to L) using goat polyclonal antibodies as described in Materials and Methods. Panels A, D, G, J, and M are bright-field images that demonstrate that lung architecture was preserved during the experiment. Panels B, E, H, and K are fluorescent images that show staining of nuclei by DAPI (blue) and IL-6 (B and E) or IL-8 (H and K) detection by Alexa Fluor 546 (red) secondary antibody. Panels C, F, I, L are overlays of the bright-field and fluorescent images and demonstrate that the primary cellular sources of the cytokines are alveolar epithelial cells (AE) and alveolar macrophages (AM). Some interstitial cells (IC) are also positive for IL-6 or IL-8. Panels M, N, and O are bright-field, fluorescent, and overlaid images of spore-exposed tissue stained with only secondary antibody (2′ Ab) and confirm that omission of the primary antibody results in a loss of signal.
DISCUSSION
The innate immune response to B. anthracis spores is important in early containment of infection as there is a significant delay (average length, 10 days) between exposure to spores and the onset of the clinical disease known as anthrax (9). On occasion, this delay may be as long as 43 days (23). Also noteworthy is the fact that germination does not initially occur in the lungs but rather occurs in the regional mediastinal lymph nodes or in transit to the lymph nodes (7, 15). Macrophages and dendritic cells have been the traditional focal points for study of the initial innate immune response to B. anthracis infection (3, 28). These cells are thought of as sentinel cells that protect the lungs during the initial infection with spores (31).
However, very little work has been performed on lung epithelial cells and their interaction with B. anthracis spores. The current study addresses this issue. The lung epithelium is the first site of exposure for spores in inhalational anthrax. It has a large (80-m2) surface area (38) for spore contact. Several bacterial infectious agents, including Legionella pneumophila and group B streptococci, as well as several viral infectious agents, including respiratory syncytial virus and adenovirus, stimulate lung epithelia to release cytokines and chemokines that activate and recruit inflammatory cells and antigen-presenting cells which are important in the transition to adaptive immunity (1, 20, 24, 32).
Our lung slice model, unlike differentiated cell lines, reproduces the normal lung architecture as it maintains the three-dimensional structure present in native tissue. Also, current cell culture models lack the diversity of cell types found in the normal lung. Although the number of alveolar macrophages is decreased in the final preparation, our model contains all of the parenchymal cell types present in the human lung (2). Thus, the complex interactions of the different cell types are not accurately modeled in cultured cell lines but are reproduced in our model.
The current study examined the interaction of B. anthracis spores with human lung tissue. We used B. anthracis Sterne strain spores for this study, and gentamicin was used throughout the experiments to ensure that only the spore response was examined. B. anthracis spores produce a strong proinflammatory cytokine and neutrophil and monocyte chemokine response in human lung tissue, as shown by our RPA and ELISA results (Fig. 1 and 2). Specifically, this response includes induction of the cytokines IL-6 and TNF-α, the monocyte chemokines MIP-1α/β and MCP-1, and the neutrophil chemokine IL-8. The kinetics of the response of the lung slice model to B. anthracis spores differs from the kinetics seen in human alveolar macrophages, as the increase in mRNA levels in macrophages peaks at 5 h (5), while the lung slice induction peaks at 8 h for MIP-1α and MIP-1β and at 24 h for other chemokines. Several cytokines are also induced in lung slices, including TNF-α, IL-10, IFN-γ, IL-1β, and GM-CSF, and this occurs at later times than it occurs in macrophages.
An interesting finding with lung slices was the large, 44-fold increase in IL-1β mRNA levels at 8 h, which is much greater than the increase seen in human macrophages. However, we saw only a modest increase in IL-1β protein levels in lung slice supernatant (less than 100 pg/ml) and no increase in IL-1β levels in lung tissue extracts. The most likely explanation for this apparent discrepancy may be the fact that the RPA reveals only relative fold increases in RNA concentrations and does not determine the absolute levels of RNA species. Thus, if IL-1β RNA levels are increased by spores, but only at low total levels, the total amount of IL-1β protein produced may be equally low, as we found by ELISA.
Another possibility is a failure in IL-1β processing (11). The stimulation of IL-1β mRNA may come from spore contact with the lung cells and result in production of pro-IL-1β. However, the processing of pro-IL-1β to mature, biologically active IL-1β requires activation of caspase-1, which occurs during intracellular bacterial infection (4). It is not known whether caspase is induced by B. anthracis spores. In any case, the amounts of mature IL-1β that we detected may be significant biologically as small amounts of this cytokine are important in the induction of IL-8 by lung cells in response to viral exposure (33). We did find significant induction of the neutrophil chemotaxin IL-8 protein by lung slices, which was not seen in our previous studies with macrophages.
Our findings significantly differ from those of Radyuk and colleagues (30). In their study, these workers cultured primary small airway epithelial cells on collagen matrices and exposed this model to B. anthracis Sterne spores in the presence or absence of isolated peripheral blood monocytes. Although they did see enhancement of IL-6 and IL-8 production after the addition of monocytes, there was no effect of spore exposure on cytokine production per se. There was modest enhancement (twofold) of TNF-α induction by spores, but only when the model was prestimulated with Bacillus cell wall preparations. Our data not only show that IL-6 and IL-8 are induced in the lung by spores but also show that both alveolar macrophages and lung epithelia respond to exposure with elaboration of these cytokines. We recognize that the final macrophage number in our model is somewhat decreased, and this may have some effect on the total response. However, clearly our model more closely replicates native lung tissue structurally, and it is likely that the diverse cytokine and chemokine response to spores presented here more accurately describes the actual lung innate immune response to B. anthracis.
Induction of the neutrophil chemotaxin IL-8 is consistent with the neutrophil infiltration in mediastinal lymph nodes seen in humans and in animal models of inhalational anthrax (14, 37). The induction of monocyte chemotaxins is consistent with the monocyte infiltration seen in human lungs in patients suffering from inhalational anthrax (14). The recruitment of additional monocytes to the site of infection may be advantageous to B. anthracis as these monocytes/macrophages transport spores to the mediastinum, from which dissemination of the vegetative bacteria occurs. It is important to recognize that strict correlation of the autopsy findings with our present study is limited by the fact that autopsy studies represent findings of the terminal stages of anthrax, while the current study examined early B. anthracis-host interactions.
Activation of cell signaling pathways is critical for cytokine induction by pathogens. Dendritic cells infected with Sterne strain spores show strong activation of p38, weak activation of ERK1/2, and no activation of JNK (3). We have shown that in human alveolar macrophages there is rapid activation of all three MAKP signaling pathways after spore exposure (5). The fact that the lung slices exhibited activation of all three pathways at all the times tested (Fig. 4) suggests that signaling activation occurs throughout spore infection and could be responsible for the prolonged elevation of cytokine and chemokine protein secretion observed, especially for IL-8 and MCP-1. It also suggests that activation of these pathways extends beyond the initial contact with spores and may be due to secondary activation through intercellular communication, which is possible in our model. Inhibition experiments showed that individual pathways are important in induction of specific cytokines and chemokines. However, as addition of all three inhibitors together provided additional suppression of the cytokine and chemokine responses, it is likely that multiple pathways play a role in cytokine and chemokine induction by B. anthracis spores (Fig. 5).
Taken together, our results indicate that the human lung responds to infection by the B. anthracis spore by producing a robust cytokine and chemokine response which recruits monocytes and neutrophils to participate in the innate immune response. We also demonstrate here that the production of the proinflammatory cytokines and the monocyte and neutrophil chemokines is causally related to the activation of the signaling pathways ERK, JNK, and p38. The source of these cytokines is both alveolar macrophages and epithelia, with a possible smaller contribution by interstitial cells. This is the first description of the early innate immune cytokine response of the human lung to B. anthracis spore exposure.
Acknowledgments
The research described in this paper was partially supported by National Institute of Allergy and Infectious Diseases project 1U19 AI62629 (to J.P.M. and K.M.C.).
We acknowledge the kind assistance of the Departments of Pathology of the Veterans Administration Hospital, the University of Oklahoma Medical Center, Baptist-Integris Hospital, St. Anthony's Hospital, and Mercy Health Center, all of Oklahoma City, OK. We also acknowledge the assistance and expertise of Julie Maier of the Oklahoma Medical Research Foundation Imaging Analysis core facility.
Editor: F. C. Fang
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Arnold, R., B. Humbert, H. Werchau, H. Gallati, and W. Konig. 1994. Interleukin-8, interleukin-6, and soluble tumour necrosis factor receptor type I release from a human pulmonary epithelial cell line (A549) exposed to respiratory syncytial virus. Immunology 82:126-133. [PMC free article] [PubMed] [Google Scholar]
- 2.Booth, J. L., K. M. Coggeshall, B. E. Gordon, and J. P. Metcalf. 2004. Adenovirus type 7 induces interleukin-8 in a lung slice model and requires activation of Erk. J. Virol. 78:4156-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brittingham, K. C., G. Ruthel, R. G. Panchal, C. L. Fuller, W. J. Ribot, T. A. Hoover, H. A. Young, A. O. Anderson, and S. Bavari. 2005. Dendritic cells endocytose Bacillus anthracis spores: implications for anthrax pathogenesis. J. Immunol. 174:5545-5552. [DOI] [PubMed] [Google Scholar]
- 4.Burns, K., F. Martinon, and J. Tschopp. 2003. New insights into the mechanism of IL-1beta maturation. Curr. Opin. Immunol. 15:26-30. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarty, K., W. Wu, J. L. Booth, E. S. Duggan, K. M. Coggeshall, and J. P. Metcalf. 2006. Bacillus anthracis spores stimulate cytokine and chemokine innate immune responses in human alveolar macrophages through multiple mitogen-activated protein kinase pathways. Infect. Immun. 74:4430-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuenda, A., J. Rouse, Y. N. Doza, R. Meier, P. Cohen, T. F. Gallagher, P. R. Young, and J. C. Lee. 1995. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364:229-233. [DOI] [PubMed] [Google Scholar]
- 7.Dalldorf, F. G., A. F. Kaufmann, and P. S. Brachman. 1971. Woolsorters' disease. An experimental model. Arch. Pathol. Lab. Med. 92:418-426. [PubMed] [Google Scholar]
- 8.DeSilva, D. R., E. A. Jones, M. F. Favata, B. D. Jaffee, R. L. Magolda, J. M. Trzaskos, and P. A. Scherle. 1998. Inhibition of mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy. J. Immunol. 160:4175-4181. [PubMed] [Google Scholar]
- 9.Dixon, T. C., A. A. Fadl, T. M. Koehler, J. A. Swanson, and P. C. Hanna. 2000. Early Bacillus anthracis-macrophage interactions: intracellular survival and escape. Cell. Microbiol. 2:453-463. [DOI] [PubMed] [Google Scholar]
- 10.Dixon, T. C., M. Meselson, J. Guillemin, and P. C. Hanna. 1999. Anthrax. N. Engl. J. Med. 341:815-826. [DOI] [PubMed] [Google Scholar]
- 11.Franchi, L., C. McDonald, T. D. Kanneganti, A. Amer, and G. Nunez. 2006. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J. Immunol. 177:3507-3513. [DOI] [PubMed] [Google Scholar]
- 12.Friedlander, A. M., S. L. Welkos, M. L. Pitt, J. W. Ezzell, P. L. Worsham, K. J. Rose, B. E. Ivins, J. R. Lowe, G. B. Howe, P. Mikesell, et al. 1993. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 167:1239-1243. [DOI] [PubMed] [Google Scholar]
- 13.Fritz, D. L., N. K. Jaax, W. B. Lawrence, K. J. Davis, M. L. Pitt, J. W. Ezzell, and A. M. Friedlander. 1995. Pathology of experimental inhalation anthrax in the rhesus monkey. Lab. Investig. 73:691-702. [PubMed] [Google Scholar]
- 14.Guarner, J., J. A. Jernigan, W. J. Shieh, K. Tatti, L. M. Flannagan, D. S. Stephens, T. Popovic, D. A. Ashford, B. A. Perkins, S. R. Zaki, and the Inhalational Anthrax Pathology Working Group. 2003. Pathology and pathogenesis of bioterrorism-related inhalational anthrax. Am. J. Pathol. 163:701-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidi-Rontani, C. 2002. The alveolar macrophage: the Trojan horse of Bacillus anthracis. Trends Microbiol. 10:405-409. [DOI] [PubMed] [Google Scholar]
- 16.Guidi-Rontani, C., M. Levy, H. Ohayon, and M. Mock. 2001. Fate of germinated Bacillus anthracis spores in primary murine macrophages. Mol. Microbiol. 42:931-938. [DOI] [PubMed] [Google Scholar]
- 17.Guidi-Rontani, C., M. Weber-Levy, E. Labruyere, and M. Mock. 1999. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 31:9-17. [DOI] [PubMed] [Google Scholar]
- 18.Han, Z., D. L. Boyle, L. Chang, B. Bennett, M. Karin, L. Yang, A. M. Manning, and G. S. Firestein. 2001. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Investig. 108:73-81. (Erratum, 108:1883.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ireland, J. A., and P. C. Hanna. 2002. Macrophage-enhanced germination of Bacillus anthracis endospores requires gerS. Infect. Immun. 70:5870-5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leland Booth, J., and J. P. Metcalf. 1999. Type-specific induction of interleukin-8 by adenovirus. Am. J. Respir. Cell Mol. Biol. 21:521-527. [DOI] [PubMed] [Google Scholar]
- 21.Loitsch, S. M., C. von Mallinckrodt, S. Kippenberger, D. Steinhilber, T. O. Wagner, and J. Bargon. 2000. Reactive oxygen intermediates are involved in IL-8 production induced by hyperosmotic stress in human bronchial epithelial cells. Biochem. Biophys. Res. Commun. 276:571-578. [DOI] [PubMed] [Google Scholar]
- 22.Mastronarde, J. G., M. M. Monick, N. Mukaida, K. Matsushima, and G. W. Hunninghake. 1998. Activator protein-1 is the preferred transcription factor for cooperative interaction with nuclear factor-kappaB in respiratory syncytial virus-induced interleukin-8 gene expression in airway epithelium. J. Infect. Dis. 177:1275-1281. [DOI] [PubMed] [Google Scholar]
- 23.Meselson, M., J. Guillemin, M. Hugh-Jones, A. Langmuir, I. Popova, A. Shelokov, and O. Yampolskaya. 1994. The Sverdlovsk anthrax outbreak of 1979. Science 266:1202-1208. [DOI] [PubMed] [Google Scholar]
- 24.Mikamo, H., A. K. Johri, L. C. Paoletti, L. C. Madoff, and A. B. Onderdonk. 2004. Adherence to, invasion by, and cytokine production in response to serotype VIII group B streptococci. Infect. Immun. 72:4716-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosser, E. M., and R. F. Rest. 2006. The Bacillus anthracis cholesterol-dependent cytolysin, anthrolysin O, kills human neutrophils, monocytes and macrophages. BMC Microbiol. 6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey, J., and D. Warburton. 2004. Knock-on effect of anthrax lethal toxin on macrophages potentiates cytotoxicity to endothelial cells. Microbes Infect. 6:835-843. [DOI] [PubMed] [Google Scholar]
- 27.Pickering, A. K., and T. J. Merkel. 2004. Macrophages release tumor necrosis factor alpha and interleukin-12 in response to intracellular Bacillus anthracis spores. Infect. Immun. 72:3069-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickering, A. K., M. Osorio, G. M. Lee, V. K. Grippe, M. Bray, and T. J. Merkel. 2004. Cytokine response to infection with Bacillus anthracis spores. Infect. Immun. 72:6382-6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Placke, M. E., and G. L. Fisher. 1987. Adult peripheral lung organ culture—a model for respiratory tract toxicology. Toxicol. Appl. Pharmacol. 90:284-298. [DOI] [PubMed] [Google Scholar]
- 30.Radyuk, S. N., P. A. Mericko, T. G. Popova, E. Grene, and K. Alibek. 2003. In vitro-generated respiratory mucosa: a new tool to study inhalational anthrax. Biochem. Biophys. Res. Commun. 305:624-632. [DOI] [PubMed] [Google Scholar]
- 31.Ribot, W. J., R. G. Panchal, K. C. Brittingham, G. Ruthel, T. A. Kenny, D. Lane, B. Curry, T. A. Hoover, A. M. Friedlander, and S. Bavari. 2006. Anthrax lethal toxin impairs innate immune functions of alveolar macrophages and facilitates Bacillus anthracis survival. Infect. Immun. 74:5029-5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmeck, B., P. D. N′Guessan, M. Ollomang, J. Lorenz, J. Zahlten, B. Opitz, A. Flieger, N. Suttorp, and S. Hippenstiel. 2007. Legionella pneumophila-induced NF-kappaB- and MAPK-dependent cytokine release by lung epithelial cells. Eur. Respir. J. 29:25-33. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz, Y. A., R. S. Amin, J. M. Stark, B. C. Trapnell, and R. W. Wilmott. 1999. Interleukin-1 receptor antagonist inhibits interleukin-8 expression in A549 respiratory epithelial cells infected in vitro with a replication-deficient recombinant adenovirus vector. Am. J. Respir. Cell Mol. Biol. 21:388-394. [DOI] [PubMed] [Google Scholar]
- 34.Shafazand, S., R. Doyle, S. Ruoss, A. Weinacker, and T. A. Raffin. 1999. Inhalational anthrax: epidemiology, diagnosis, and management. Chest 116:1369-1376. [DOI] [PubMed] [Google Scholar]
- 35.Spencer, R. C. 2003. Bacillus anthracis. J. Clin. Pathol. 56:182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tochikubo, K., Y. Hayakawa, and K. Kojima. 1981. Mutual relationship between antibiotics and resting spores of Bacillus subtilis: binding of cyclic polypeptide and aminoglycoside antibiotics to spores and their inhibitory effect on outgrowth and vegetative growth. Microbiol. Immunol. 25:113-126. [DOI] [PubMed] [Google Scholar]
- 37.Vasconcelos, D., R. Barnewall, M. Babin, R. Hunt, J. Estep, C. Nielsen, R. Carnes, and J. Carney. 2003. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis). Lab. Investig. 83:1201-1209. [DOI] [PubMed] [Google Scholar]
- 38.Weibel, E. 1963. Morphometry of the human lung. Springer-Verlag, Berlin, Germany.
- 39.Zar, J. H. 1996. Biostatistical analysis. Prentice-Hall, Englewood Cliffs, NJ.