Abstract
The type 1 fimbriae of Actinomyces naeslundii T14V mediate adhesion of this gram-positive species to the tooth surface. The present findings show that the locus for type 1 fimbria production in this strain includes three genes, fimQ for a minor fimbrial subunit that appears to be an adhesin, fimP for the major structural subunit, and srtC1 for a type 1 fimbria-specific sortase involved in the assembly of these structures.
Studies of the prominent oral microorganism Actinomyces naeslundii have provided important insights into both the properties of fimbriae or pili on gram-positive bacteria (16, 18) and the underlying mechanisms of dental plaque formation (4). The fimbriae of A. naeslundii, which were among the first observed for a gram-positive species (11), consist of two functionally distinct types (5). The type 1 fimbriae mediate adhesion of this species to the tooth surface through the binding of adsorbed salivary proline-rich proteins (PRPs) (7, 10, 13), whereas the type 2 fimbriae promote biofilm formation (14) through recognition of hostlike saccharide motifs in the surface polysaccharides of early colonizing streptococci (3). The major subunits of type 1 and type 2 fimbriae, encoded by the genes fimP (19) and fimA (20), respectively, are similar in size (56 kDa) and have sequences that are approximately 40% identical. The gene fimA of A. naeslundii T14V occurs between open reading frame 977 (ORF 977), which may encode the type 2 fimbria-associated adhesin (12), and ORF 365 for a sortase that is required for the covalent polymerization of FimA monomers (20). In contrast, fimP of this strain appears to be flanked by three essential upstream ORFs (i.e., ORF3-ORF2-ORF1) of unknown function and two essential downstream ORFs (i.e., ORF4-ORF5) (22), which include the partial coding sequence of a putative sortase (9). Moreover, the genes that reportedly flank fimP in strain T14V differ dramatically from those that flank this gene in type 1-fimbriated Actinomyces viscosus 19246 (13).
To verify the genes for type 1 fimbria production in A. naeslundii T14V, we amplified and sequenced a series of overlapping PCR products from genomic DNA of this strain by using primers designed with the previously published sequence of this region (22). Annotation of the resulting sequence revealed fimP as previously described (19). However, this gene in our amended sequence is flanked by one upstream gene (i.e., fimQ) rather than three (i.e., ORF3-ORF2-ORF1) and by two downstream genes (i.e., srtC1 and orfC) that differ from those (i.e., ORF4-ORF5-ORF6) previously described (22). For the most part, these differences appeared to reflect the existence of sequencing errors, introduced previously by manual sequencing of GC-rich regions. Importantly, the presently described genes in strain T14V (i.e., fimQ, fimP, srtC1, and orfC) are comparable to those previously identified (13) in A. viscosus 19246 (i.e., orfA, fimP, orfB and orfC), as well as those in the whole genome sequence of A. naeslundii MG1, which is available at The Institute for Genomic Research website (http://cmr.tigr.org).
The gene fimQ of A. naeslundii T14V (Fig. 1) and orfA in A. viscosus 19246 (13) encode 149-kDa proteins with sequences that are 83% identical. Both proteins have typical 45-amino-acid gram-positive leader sequences, predicted by the SignalP 3.0 server (http://www.cbs.dtu.dk/services/SignalP/), identical E box sequences (17), similar to the E box sequence present in FimP, and cell wall sorting motifs (LPLSG). These features raised the possibility that FimQ represented a previously unidentified type 1 fimbria-associated protein. Support for this possibility was gained from proteomic analysis of immunoaffinity-purified type 1 fimbriae, isolated by elution from a coupled FimP-specific monoclonal antibody (MAb) (2). Eluted fimbriae were digested either with pepsin or by dilute-acid hydrolysis, and the resulting peptides were analyzed by liquid chromatography-tandem mass spectrometry. Not surprisingly, most of the identified peptides (i.e., greater than 95%) had sequences identical to regions of FimP. However, several peptides with FimQ-like sequences were also identified, including the five described in Table 1 and Fig. S1 in the supplemental material. Representative tandem mass spectrometry spectra of two such peptides, VVRNSDGTF derived by pepsin digestion and PSTPGAKPLTD from dilute-acid hydrolysis of type 1 fimbriae, respectively, are shown in Fig. S2 and S3 in the supplemental material. These findings support the notion that FimQ is a minor type 1 fimbria-associated protein.
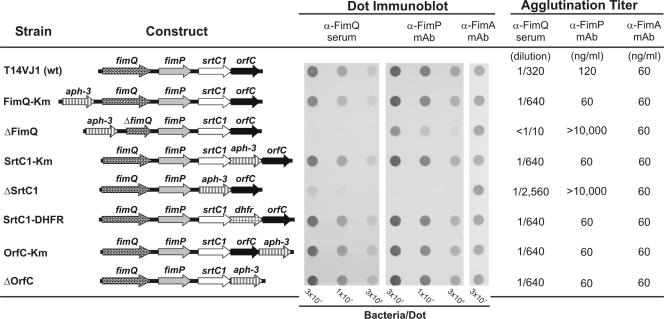
FIG. 1.
ORF diagrams of the type 1 gene clusters in A. naeslundii T14V and related mutant strains based on the amended sequence of this region obtained in the present study. Each strain was examined for surface expression of fimbria-associated proteins by dot immunoblotting and bacterial agglutination assays performed with rabbit anti-FimQ serum raised by immunization with a DNA vaccine, mouse MAb 8A (2) against FimP, the structural subunit of type 1 fimbriae, and MAb 5A against FimA (1), the structural subunit of type 2 fimbriae. For dot immunoblotting, nitrocellulose membranes were spotted with decreasing numbers of bacteria, incubated with anti-FimQ serum (1/400) or mouse MAb (250 ng/ml), followed by peroxidase-conjugated goat anti-rabbit or anti-mouse immunoglobulin G, and developed using a metal enhanced diaminobenzidine substrate kit (Pierce). Agglutination titers are expressed as the minimum concentration of antibody to achieve macroscopic agglutination of 5 × 108 bacteria/ml based on assays performed with serial twofold dilutions of antiserum (α-FimQ) or purified MAb (α-FimP or α-FimA).
TABLE 1.
FimQ peptides identified from type 1 fimbriae after pepsin and diluted-acid hydrolysis
| m/z | Charge | Mol wt
|
Sequence (positions) | |
|---|---|---|---|---|
| Exptl | Calculated | |||
| 497.80 | 2 | 993.58 | 993.49 | VVRNSDGTF (938-946)a |
| 419.93 | 3 | 1,256.77 | 1,256.62 | AAAPRAGADGGSRT (1023-1036)a |
| 575.83 | 2 | 1,149.64 | 1,149.54 | TVRENTPGYN (1044-1053)a |
| 399.28 | 2 | 796.54 | 796.37 | GSYRLSD (1167-1173)b |
| 542.37 | 2 | 1,082.73 | 1,082.56 | PSTPGAKPLTD (1277-1287)b |
Peptide obtained from pepsin digestion of purified A. naeslundii T14V type 1 fimbriae.
Peptide obtained from diluted-acid hydrolysis of the isolated type 1 fimbriae.
To further examine the possibility described above, we prepared antiserum against the central region of FimQ (amino acid residues 529 to 951) by immunization of rabbits (Aldevron, LLC, Fargo, ND) with the corresponding coding sequence cloned into the mammalian cell expression vector pJW4303, which was kindly provided by James I. Mullans (Department of Microbiology, University of Washington, Seattle). The anti-FimQ serum obtained labeled A. naeslundii T14V in dot immunoblot analysis and caused agglutination of this strain (Fig. 1). It did not, however, identify any bands on Western blots of mutanolysin digests prepared from this strain or any other strain described in this study. In other experiments (results not shown), the anti-FimQ antibody reacted with A. naeslundii 5519 cells, which has type 1 but not type 2 fimbriae, but did not react with A. naeslundii 5951 cells, which has type 2 but not type 1 fimbriae (5). Results obtained from immunogold labeling of type 1-fimbriated A. naeslundii 5519 (5) with rabbit anti-FimQ and mouse anti-FimP antibodies showed surface labeling of this strain with both antibodies (results not shown) but did not provide clear evidence for the localization of each antibody along individual fimbrial structures.
We then constructed the kan-containing control mutant, strain FimQ-Km, and the corresponding fimQ deletion mutant, strain ΔFimQ (Fig. 1), by allelic exchange. This involved electrotransformation (21) of A. naeslundii T14V with plasmids p3FimQ and p8FimQ, respectively (Table 2), and the growth of transformants on kanamycin-containing medium. Strains FimQ-Km and ΔFimQ contained the kan marker at the same position (i.e., 317 base pairs upstream of the putative ATG translation start codon of fimQ). However, strain ΔFimQ lacked the first 1,063 nucleotides of fimQ, which introduced a frame shift in the remaining fimQ sequence. The steps and primers used to prepare these and other plasmids are summarized in Table 2 in this paper and in Table S1 in the supplemental material. The integrity of all plasmid constructs listed in Table 2 was verified by restriction enzyme digestions and sequencing at the junctions of inserted DNA fragments. Likewise, the location and orientation of the allelic-exchange plasmid DNA in each mutant were confirmed by amplification of specific PCR products across the upstream and downstream boundaries of the introduced sequence by using appropriate primers.
TABLE 2.
Bacterial strains and plasmids
| Plasmid or strain | Descriptiona | Source or reference |
|---|---|---|
| Plasmids | ||
| pJRD215 | Template for PCR amplification (primers 1 and 2) of kan (the aph-3 gene for Kmr without its transcriptional terminator) | 8 |
| EZ-TN5<DHFR-1> | Template for PCR amplification of dhfr (primers 3 and 4) (the gene for Tmr without its transcriptional terminator) | Epicentre |
| pDONR/Zeo | Cloning vector; Zeor | Invitrogen |
| p1FimQ | pDONR/Zeo containing a PCR-amplified (primers 5 and 6) region extending from upstream of strain T14V fimQ to the middle of fimQ | This study |
| p2FimQ | p1FimQ with an XbaI site created 317 bp upstream of fimQ using a QuikChange site-directed mutagenesis kitb and primers 7 and 8 | This study |
| p3FimQ | p2FimQ with kan in the XbaI site | This study |
| p4FimQ | pDONR/Zeo containing a PCR-amplified (primers 5 and 9) region extending from upstream of strain T14V fimQ to the ATG starting codon of fimQ | This study |
| p5FimQ | p4FimQ with an XbaI site created at the same position as in p2FimQ by site-directed mutagenesis (primers 7 and 8) | This study |
| p6FimQ | p5FimQ with kan in the XbaI site | This study |
| p7FimQ | pDONR/Zeo containing a PCR-amplified (primers 10 and 11) region of strain T14V fimQ corresponding to nucleotides 1066 to 2348 of the complete gene | This study |
| p8FimQ | p7FimQ with the NdeI-HpaI fragment replaced with an NdeI-HpaI fragment from p6FimQ, resulting in a 1,063-bp deletion of fimQ (from ATG starting codon) | This study |
| p1Srt | pDONR/Zeo containing a PCR-amplified (primers 12 and 13) srtC1 region of strain T14V | This study |
| p2Srt | p1Srt with an XbaI site created 42 bp upstream of the 3′ end of srtC1 by site-directed mutagenesis (primers 14 and 15) | This study |
| p3Srt | p2Srt with kan in the XbaI site | This study |
| p4Srt | p2Srt with srtC1 deleted (all coding sequence except the 42 bp at the 3′ end) by inverse PCR (primers 16 and 17); the resultant plasmid contains an XbaI site at the same position as in p2Srt | This study |
| p5Srt | p4Srt with kan in the XbaI site | This study |
| p6Srt | p2Srt with dhfr in the XbaI site | This study |
| p1OrfC | pDONR/Zeo containing a PCR-amplified (primers 18 and 19) orfC region of A. naeslundii T14V | This study |
| p2OrfC | p1OrfC with an XbaI site created 6 bp downstream of the 3′ end of orfC by site-directed mutagenesis (primers 20 and 21) | This study |
| p3OrfC | p2OrfC with kan in the XbaI site | This study |
| p4OrfC | p2OrfC with orfC deleted (all coding sequence except the first 12 bp from the 5′ end) by inverse PCR (primers 22 and 23); the resultant plasmid contains an XbaI site at the same position as in p2OrfC | This study |
| p5OrfC | p4OrfC with kan in the XbaI site | This study |
| A. naeslundii strains | ||
| T14V | Wild-type strain; Kms Smr | 5 |
| FimQ-Km | Kmr transformant of T14V obtained with p3FimQ | This study |
| ΔFimQ | Kmr transformant of T14V obtained with p8FimQ | This study |
| SrtC1-Km | Kmr transformant of T14V obtained with p3Srt | This study |
| ΔSrtC1 | Kmr transformant of T14V obtained with p5Srt | This study |
| SrtC1-DHFR | Tmr transformant of ΔSrtC1 obtained with p6Srt | This study |
| OrfC-Km | Kmr transformant of T14V obtained with p3OrfC | This study |
| ΔOrfC | Kmr transformant of T14V obtained with p5OrfC | This study |
The sequences of all primers used in the present study are listed in Table S1 in the supplemental material. Antibiotic abbreviations: Km, kanamycin; Sm, streptomycin; Tm, trimethoprim; Zeo, zeocin.
From Stratagene, La Jolla, CA.
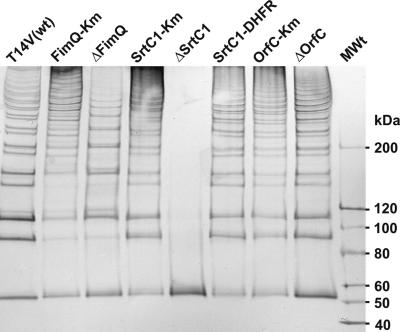
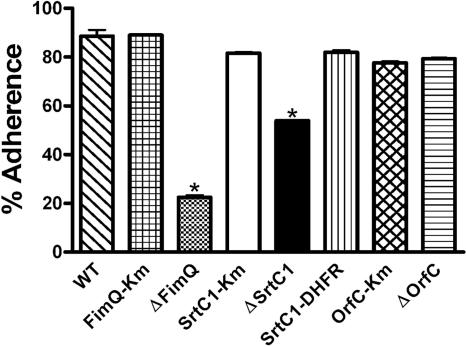
Unlike control strain FimQ-Km, strain ΔFimQ was unreactive with anti-FimQ antibody in both dot immunoblotting and bacterial agglutination assays (Fig. 1). The anti-FimP immunoreactivity of strain ΔFimQ in dot immunoblotting was also lower than that of strain FimQ-Km. Importantly, strain ΔFimQ agglutinated in the presence of anti-FimA MAb 5A against type 2 fimbriae but not in the presence of anti-FimP MAb 8A against type 1 fimbriae. The absence of typical type 1 fimbriae on strain ΔFimQ was further suggested by the different patterns of anti-FimP reactive bands seen in Western blots of mutanolysin digests prepared from strains FimQ-Km and ΔFimQ (Fig. 2). The percentages of adherence of bacteria to saliva-treated hydroxyapatite (SHA) were similar for wild-type strain T14V and control strain FimQ-Km. In contrast, the value of approximately 20% adherence for strain ΔFimQ was comparable to values obtained previously for strains that lacked type 1 fimbriae (5). Thus, the deletion in fimQ abolished type 1 fimbria-mediated adhesion.
FIG. 2.
Western blot analyses of cell surface proteins extracted from A. naeslundii wild-type and mutant strains. Cell surface proteins were extracted by mutanolysin digestion of bacteria that were osmotically stabilized in the presence of 26% melizitose, separated on a NuPAGE Tris-acetate gradient gel (3 to 8%), transferred to a nitrocellulose membrane, probed with MAb 8A against an epitope of FimP, and developed with a chromogenic Western blot immunodetection kit (Invitrogen). Lane MWt, 10 μl of MagicMark XP Western protein standard (Invitrogen).
The genes designated srtC1 in A. naeslundii T14V (Fig. 1) and orfB in A. viscosus 19246 (13) encode putative class C sortases (9) that are 76% identical. To assess the involvement of srtC1 in type 1 fimbria production, we constructed the kan-containing control strain SrtC1-Km and the corresponding SrtC1 deletion mutant, strain ΔSrtC1 (Fig. 1), by an allelic-exchange strategy similar to that described above and outlined in Table 2. This involved the creation of a unique XbaI site 14 codons upstream from the 3′ end of srtC1 in p2Srt. This engineered site was used for the insertion of antibiotic markers alone or linked to appropriate srtC1 constructs. These insertions created a premature stop codon in srtC1 and fused the last 14 codons of this gene with the 3′ end of kan, thus preserving the putative ribosome binding site of downstream orfC.
In contrast to control strain SrtC1-Km, which was similar to the wild type, strain ΔSrtC1 specifically lacked type 1 fimbriae. This difference was evident from results of dot immunoblotting and agglutination assays performed with anti-FimP and anti-FimA reactive MAbs (Fig. 1). Although the presence of FimP on strain ΔSrtC1 was not detected by surface labeling, monomeric FimP was detected in the mutanolysin digest of this strain. In Western blots, this protein migrated as a sharp band between the 50- and 60-kDa markers (Fig. 2). Interestingly, the deletion of srtC1 reduced but did not abolish the binding of anti-FimQ antibody in dot immunoblots. Equivalent binding of this antibody to strains SrtC1-Km and ΔSrtC1 required approximately 10-fold more cells of the latter strain (Fig. 1). In addition, the agglutination titer of this antibody was higher in comparable assays performed with strain ΔSrtC1 than in those performed with strain SrtC1-Km. Moreover, the adhesion of strain ΔSrtC1 to SHA, although lower than that of control strain SrtC1-Km, was significantly greater than that observed with strain ΔFimQ (Fig. 3). In genetic complementation studies, the cell surface phenotype of strain ΔSrtC1 was restored to that of wild-type strain T14V by the expression of srtC1 linked to a selectable dhfr marker in strain SrtC1-DHFR (Fig. 1 to 3). The construction of strain SrtC1-DHFR is outlined in Table 2.
FIG. 3.
Adherence of A. naeslundii strains to beads (5 mg) of SHA determined by using the adsorption assay described by Clark et al. (6). A t test was used for comparison of adhesion levels between strains ΔFimQ and ΔSrtC1. The single asterisks indicate a statistically significant difference in adherence between strains ΔFimQ and ΔSrtC1 (P < 0.0001).
The protein encoded by orfC in the amended sequence of A. naeslundii T14V (Fig. 1) is 90% identical to the putative prepilin peptidase-like protein encoded by this gene in A. viscosus 19246 (13). To test the essential role of orfC in type 1 fimbria production, we constructed control strain OrfC-Km containing kan immediately downstream of orfC and strain ΔOrfC containing kan in place of this gene (Fig. 1). The strategy used to construct these strains (Table 2) was similar to that described above. The surface phenotypes of strains OrfC-Km and ΔOrfC were the same and indistinguishable from that of wild-type A. naeslundii T14V (Fig. 1 to 3). Thus, the production of functional type 1 fimbriae does not require orfC.
The present and previous findings (19) identify three essential genes for type 1 fimbria production by A. naeslundii T14V: fimQ for a minor type 1 fimbria-associated protein, fimP for the major subunit, and srtC1 for a type 1 fimbria-specific sortase. In accordance with the model of pilus assembly in Corynebacterium diphtheriae (15, 18), we suspect that the SrtC1-dependent type 1 fimbria production in A. naeslundii T14V involves cleavage of FimP cell wall sorting motifs by SrtC1 and linkage of nascent C termini to ɛ-amino groups of lysine in the pilin motifs of adjacent FimP monomers. In previous studies (2), type 1 fimbria-mediated adhesion of strain T14V to SHA was not blocked by Fab fragments of various FimP-reactive monoclonal and polyclonal antibodies, thereby raising the possibility that the putative PRP-binding adhesin that mediates this interaction is a minor fimbrial component. Clearly, the present findings identify FimQ as an attractive candidate for the long-sought type 1 fimbrial adhesin. In this regard, it is interesting that cells of strain ΔSrtC1, which possess detectable cell surface FimQ but not FimP, were more adherent to SHA than cells of strain ΔFimQ, which possess detectable cell surface FimP but are devoid of FimQ (Fig. 1 and 3). While these findings are consistent with the notion that FimQ mediates adhesion, it is important to note that anti-FimP antibody did not agglutinate strain ΔFimQ, thereby indicating that the polymerized FimP detected from this strain (Fig. 2) does not exist in a form comparable to that of fully assembled fimbriae. Further studies are needed to assess the role of FimQ both in the assembly of type 1 fimbriae and in the adhesion of A. naeslundii to adsorbed salivary PRPs.
Nucleotide sequence accession number.
The amended sequence of the gene cluster for A. naeslundii T14V type 1 fimbria production and flanking regions has been deposited in GenBank under accession number DQ658412.
Supplementary Material
Acknowledgments
This work was supported by the U.S. Army Medical Research and Materiel Command and by the Intramural Research Program of the NIDCR, NIH.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, the Department of Defense, or the U.S. Government.
Editor: A. Camilli
Footnotes
Published ahead of print on 7 May 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Cisar, J. O., E. L. Barsumian, S. H. Curl, A. E. Vatter, A. L. Sandberg, and R. P. Siraganian. 1981. Detection and localization of a lectin on Actinomyces viscosus T14V by monoclonal antibodies. J. Immunol. 127:1318-1322. [PubMed] [Google Scholar]
- 2.Cisar, J. O., E. L. Barsumian, R. P. Siraganian, W. B. Clark, M. K. Yeung, S. D. Hsu, S. H. Curl, A. E. Vatter, and A. L. Sandberg. 1991. Immunochemical and functional studies of Actinomyces viscosus T14V type 1 fimbriae with monoclonal and polyclonal antibodies directed against the fimbrial subunit. J. Gen. Microbiol. 137:1971-1979. [DOI] [PubMed] [Google Scholar]
- 3.Cisar, J. O., A. L. Sandberg, C. Abeygunawardana, G. P. Reddy, and C. A. Bush. 1995. Lectin recognition of host-like saccharide motifs in streptococcal cell wall polysaccharides. Glycobiology 5:655-662. [DOI] [PubMed] [Google Scholar]
- 4.Cisar, J. O., Y. Takahashi, S. Ruhl, J. A. Donkersloot, and A. L. Sandberg. 1997. Specific inhibitors of bacterial adhesion: observations from the study of gram-positive bacteria that initiate biofilm formation on the tooth surface. Adv. Dent. Res. 11:168-175. [DOI] [PubMed] [Google Scholar]
- 5.Cisar, J. O., A. E. Vatter, W. B. Clark, S. H. Curl, S. Hurst-Calderone, and A. L. Sandberg. 1988. Mutants of Actinomyces viscosus T14V lacking type 1, type 2, or both types of fimbriae. Infect. Immun. 56:2984-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark, W. B., L. L. Bammann, and R. J. Gibbons. 1978. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect. Immun. 19:846-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark, W. B., T. T. Wheeler, and J. O. Cisar. 1984. Specific inhibition of adsorption of Actinomyces viscosus T14V to saliva-treated hydroxyapatite by antibody against type 1 fimbriae. Infect. Immun. 43:497-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davison, J., M. Heusterspreute, N. Chevalier, V. Ha-Thi, and F. Brunel. 1987. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene 51:275-280. [DOI] [PubMed] [Google Scholar]
- 9.Dramsi, S., P. Trieu-Cuot, and H. Bierne. 2005. Sorting sortases: a nomenclature proposal for the various sortases of Gram-positive bacteria. Res. Microbiol. 156:289-297. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons, R. J., D. I. Hay, J. O. Cisar, and W. B. Clark. 1988. Adsorbed salivary proline-rich protein 1 and statherin: receptors for type 1 fimbriae of Actinomyces viscosus T14V-J1 on apatitic surfaces. Infect. Immun. 56:2990-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard, A. E., and B. H. Jacius. 1974. Ultrastructure of Actinomyces viscosus and Actinomyces naeslundii. Arch. Oral Biol. 19:71-79. [DOI] [PubMed] [Google Scholar]
- 12.Hoflack, L., and M. K. Yeung. 2001. Actinomyces naeslundii fimbrial protein Orf977 shows similarity to a streptococcal adhesin. Oral Microbiol. Immunol. 16:319-320. [DOI] [PubMed] [Google Scholar]
- 13.Li, T., M. K. Khah, S. Slavnic, I. Johansson, and N. Stromberg. 2001. Different type 1 fimbrial genes and tropisms of commensal and potentially pathogenic Actinomyces spp. with different salivary acidic proline-rich protein and statherin ligand specificities. Infect. Immun. 69:7224-7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer, R. J., Jr., S. M. Gordon, J. O. Cisar, and P. E. Kolenbrander. 2003. Coaggregation-mediated interactions of streptococci and actinomyces detected in initial human dental plaque. J. Bacteriol. 185:3400-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swierczynski, A., and H. Ton-That. 2006. Type III pilus of corynebacteria: pilus length is determined by the level of its major pilin subunit. J. Bacteriol. 188:6318-6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Telford, J. L., M. A. Barocchi, I. Margarit, R. Rappuoli, and G. Grandi. 2006. Pili in gram-positive pathogens. Nat. Rev. Microbiol. 4:509-519. [DOI] [PubMed] [Google Scholar]
- 17.Ton-That, H., L. A. Marraffini, and O. Schneewind. 2004. Sortases and pilin elements involved in pilus assembly of Corynebacterium diphtheriae. Mol. Microbiol. 53:251-261. [DOI] [PubMed] [Google Scholar]
- 18.Ton-That, H., and O. Schneewind. 2004. Assembly of pili in Gram-positive bacteria. Trends Microbiol. 12:228-234. [DOI] [PubMed] [Google Scholar]
- 19.Yeung, M. K., B. M. Chassy, and J. O. Cisar. 1987. Cloning and expression of a type 1 fimbrial subunit of Actinomyces viscosus T14V. J. Bacteriol. 169:1678-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeung, M. K., J. A. Donkersloot, J. O. Cisar, and P. A. Ragsdale. 1998. Identification of a gene involved in assembly of Actinomyces naeslundii T14V type 2 fimbriae. Infect. Immun. 66:1482-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeung, M. K., and C. S. Kozelsky. 1994. Transformation of Actinomyces spp. by a gram-negative broad-host-range plasmid. J. Bacteriol. 176:4173-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeung, M. K., and P. A. Ragsdale. 1997. Synthesis and function of Actinomyces naeslundii T14V type 1 fimbriae require the expression of additional fimbria-associated genes. Infect. Immun. 65:2629-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.