Abstract
The granulomatous and intramural inflammation observed in cases of inflammatory bowel diseases (IBD) and veterinary Johne's disease suggests that Mycobacterium avium subsp. paratuberculosis is a causative agent. However, an incomplete understanding of the immunological steps responsible for the pathologies of IBD makes this conclusion uncertain. Sera from interleukin-10-deficient (IL-10−/−) mice with spontaneous colitis displayed significantly higher M. avium subsp. paratuberculosis-specific immunoglobulin G2a antibody responses than did sera from similar mice without disease. Pathogen-free IL-10−/− mice received control vehicle or the vehicle containing heat-killed or live M. avium subsp. paratuberculosis. Mucosal CD4+ T cells from the mice that developed colitis proliferated and secreted higher levels of gamma interferon and tumor necrosis factor alpha after ex vivo stimulation with a Vβ11+ T-cell receptor-restricted peptide from the MPT59 antigen (Ag85B) than those secreted from cells from mice before the onset of colitis. The data from this study provide important information regarding the mechanisms of colitis in IL-10−/− mice, which are driven in part by Ag85B-specific T cells. The data suggest a plausible mechanism of Ag-specific T-cell responses in colitis driven by potent Ags conserved in Mycobacterium species.
Crohn's disease (CD) and ulcerative colitis are chronic inflammatory bowel diseases (IBD) with severe morbidity and uncertain etiology. It is widely held that human IBD are multifactorial and are caused by immunologic, environmental, and genetic factors (25). It has been suggested that IBD may be the result of enhanced or aberrant responsiveness due to a microbial trigger (17) or an overall autoimmune dysregulation and imbalance of T cells (26). Among the microbial triggers postulated to have such a role, Mycobacterium avium subsp. paratuberculosis has received considerable attention because it is the cause of a chronic infectious colitis disease in livestock called Johne's disease (2). In particular, this postulate was stimulated in the 1980s by reports of M. avium subsp. paratuberculosis cells cultured from granulomatous lesions from patients with CD (6, 19). Subsequent studies reported the isolation of M. avium subsp. paratuberculosis, the detection of M. avium subsp. paratuberculosis antigens (Ags) in CD lesion material (1), blood (20), and other body fluids (22), and the presence of elevated M. avium subsp. paratuberculosis-specific serum antibody (Ab) levels in CD patients (21). Recently, in situ hybridization was used to detect M. avium subsp. paratuberculosis in the tissues of CD patients (12).
At ∼3 months of age, under conventional housing conditions, interleukin-10-deficient (IL-10−/−) mice develop spontaneous murine colitis with weight loss and a marked increase in serum acute-phase proteins (3). However, this disease does not readily occur when these mice are housed in a germfree environment, implicating a microbial trigger for colitis. The present study was undertaken to explore potential mechanisms by which M. avium subsp. paratuberculosis could accelerate the development of colitis in IL-10−/− mice. This study revealed that components of M. avium subsp. paratuberculosis might enhance the production of CXCL9, CXCL10, CXCL11, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) as well as promote the recruitment and/or expansion of Th1 cells during murine colitis.
MATERIALS AND METHODS
Immunogens.
M. avium subsp. paratuberculosis strain Ben (CIP 103966), a clinical isolate from a CD patient, was obtained from the American Type Culture Collection (ATCC 43544) (6). Bacteria were cultured in Middlebrook 7H9 broth supplemented with 10% albumin-dextrose-catalase (BD/Difco) and 2 μg/ml mycobactin J (Allied Monitor) to an optical density at 580 nm of 0.5 and then frozen in replicate stock aliquots. The viable titers of the stocks were determined by thawing replicates, serially diluting them in culture medium, and plating them on Middlebrook 7H10 agar supplemented with 2 μg/ml mycobactin J. An immunodominant epitope of Ag85B/MPT59 consisting of 15 amino acids, FQDAYNAAGGHNAVF, termed peptide 25 (33), was synthesized and purified by high-performance liquid chromatography (Biopeptide).
Animals and M. avium subsp. paratuberculosis challenge.
Female IL-10−/− or wild-type mice on a B6 background, aged 4 to 5 weeks, were purchased from the Jackson Laboratory. The animals were maintained in isolator cages under pathogen-free or conventional housing conditions at the Morehouse School of Medicine animal facility. The guidelines proposed by the Committee for the Care of Laboratory Animal Resources Commission of Life Sciences, National Research Council, were followed to minimize animal pain and distress. To determine the M. avium subsp. paratuberculosis reactivity of mice with spontaneous colitis, naïve IL-10−/− mice were removed from germfree housing and moved to conventional housing (i.e., without filter-top cages). After these mice lost 15% of their initial body weight, their blood was collected to evaluate the presence of M. avium subsp. paratuberculosis-reactive Abs.
In previous experiments (not shown), a live M. avium subsp. paratuberculosis dose-response experiment (with 10-fold increments, starting at 10 and ending with 1010 CFU) was performed to determine the lowest CFU required to induce colitis in IL-10−/− mice housed under germfree conditions. In this study, groups of 15 IL-10−/− mice (housed under germfree conditions) each received a single dose, by gavage, of 200 μl of cream, defined as milk containing >36% milk fat (heated at 65°C for 2 h), either by itself (control vehicle) or with either 104 CFU of live M. avium subsp. paratuberculosis or 104 CFU of heat-killed M. avium subsp. paratuberculosis (heated at 65°C for 2 h). The body weights and serum amyloid A (SAA) levels of the mice were subsequently monitored every week for 14 weeks after challenge. At the end of this period, mice were sacrificed by CO2 inhalation, and thereafter, systemic and mucosal Ag-specific T-cell responses were analyzed and leukocyte subpopulations were quantified by flow cytometry.
Cell isolation.
The mesenteric lymph nodes (MLN) from individual mice were mechanically dissociated, and red blood cells were lysed with ACK lysing buffer (Cambrex). Cell suspensions of the MLN were passed through a sterile wire screen (Sigma). Single-cell suspensions were washed twice with RPMI 1640 and stored on ice in complete medium, which consisted of RPMI 1640 supplemented with 10 ml/liter of nonessential amino acids (Mediatech), 1 mM sodium pyruvate (Sigma), 10 mM HEPES (Mediatech), 100 U/ml penicillin, 100 μg/ml streptomycin, 40 μg/ml gentamicin (Elkins-Sinn, Inc.), 50 μM mercaptoethanol (Sigma), and 10% fetal bovine serum (FBS; Atlanta Biologicals). After removal, Peyer's patches (PP) were kept on ice-cold RPMI containing 2% FBS. The intestines were cut into 1-cm segments and stirred in phosphate-buffered saline (PBS) containing 1 mM EDTA for 30 min. The cells from the intestinal lamina propria (LP) and PP were isolated as described previously (15). In brief, the LP lymphocytes were isolated by digesting intestinal tissue with 1 mg/ml of collagenase type IV (Sigma) in RPMI 1640 (collagenase solution) for 45 min, with stirring, at 37°C. After each 45-minute interval, the released cells were centrifuged and stored in complete medium, and the remaining mucosal pieces were replaced with fresh collagenase solution. LP lymphocytes were further purified using a discontinuous Percoll (Pharmacia) gradient, collecting small high-density cells at the 55%-75% interface. Subsequently, lymphocytes were maintained and cultured in a complete medium.
Cytokine analysis by ELISA.
Purified CD4+ T cells and γ-irradiated feeder cells were cultured at densities of 5 × 106 and 106 cells per ml, respectively, in complete medium containing 1 μg/ml of peptide 25 at 37°C in 5% CO2. After 3 days of peptide 25 stimulation, 106 cells/ml were transferred to polystyrene 96-well plates (Corning Glass Works). For the assessment of cytokine production, 1 ml culture supernatant from cells cultured in 12-well flat-bottomed plates (Corning Glass Works) was harvested after 3 days. The IL-2, IL-4, TNF-α, and IFN-γ levels in cell culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA) following the manufacturer's instructions (e-Biosciences, San Diego, CA). Briefly, 96-well microtiter plates were coated with 100 μl of 2.5-μg/ml rat anti-mouse IFN-γ, TNF-α, IL-2, or IL-4 in 0.1 M bicarbonate buffer (pH 8.2) overnight at 4°C and then blocked with 10% FBS at room temperature for 2 h. Next, 100 μl of serially diluted recombinant murine cytokines as standards or culture supernatant sample was added in duplicate and incubated for 4 h at room temperature. The plates were washed with PBS-T (PBS and 0.05% Tween 20) and incubated with 0.2 μg/ml of biotinylated murine cytokine secondary detection Abs in FBS-PBS-T for 3 h at room temperature. After being washed with PBS-T and PBS alone, the wells received 100 μl of 0.5-μg/ml peroxidase-conjugated anti-biotin Ab, and the plates were incubated for 2 h prior to development following the addition of 100 μl of 1.1 mM 2,2′-azino-bis(3)-ethylbenzthiazoline-6-sulfonic acid (Sigma) in 0.1 M citrate-phosphate buffer (pH 4.2) containing 0.01% H2O2 (ABTS solution). The cytokine ELISA assays were capable of detecting 8 pg/ml of IFN-γ and TNF-α, 2 pg/ml of IL-2, and 4 pg/ml of IL-4.
Cell proliferation.
Lymphocyte proliferation was measured by use of a 5-bromo-2′-deoxyuridine (BrdU) absorption detection kit per the manufacturer's instructions (Roche Diagnostics); subsequently, BrdU incorporation was detected using a scanning multiwell spectrophotometer (Spectra-Max 250 ELISA reader; Molecular Devices). In brief, purified CD4+ T cells were cultured at a density of 5 × 106 cells/ml, with 106 irradiated feeder cells/ml, in complete medium containing 1 μg/ml of peptide 25 at 37°C in 5% CO2. After 2 days of peptide 25 restimulation, 106 cells/ml were transferred to polystyrene 96-well plates (Corning Glass Works). Ten microliters of BrdU labeling solution (10 μM [final concentration]) was then added, and the plates were incubated for 18 h at 37°C with 5% CO2. The cells were fixed and incubated with 100 μl of nuclease in each well for 30 min at 37°C. Next, the cells were washed with complete medium and incubated with BrdU-peroxidase solution for 30 min at 37°C. To determine the incorporation of BrdU, the plates were developed by adding 100 μl of tetramethylbenzidine substrate. The substrate reaction was allowed to continue for 20 min, after which the optical density was read at 405 nm, with a reference wavelength of 490 nm.
Mycobacterium-specific Ab detection by ELISA.
Mycobacterium-specific immunoglobulin G (IgG) Ab responses in the sera of IL-10−/− mice with spontaneous colitis or those maintained under germfree housing conditions were measured by ELISA (16). Briefly, 96-well Falcon ELISA plates (Fisher Scientific, Pittsburgh, PA) were coated with 100 μl of 1-μg/ml heat-killed and paraformaldehyde-fixed M. avium subsp. paratuberculosis in coating buffer (sodium carbonate-bicarbonate buffer) overnight at 4°C and blocked with 200 μl of 10% FBS (Atlanta Biologicals) in PBS (FBS-PBS) for 2 h at room temperature. Individual samples were added and serially diluted in FBS-PBS. After 4 h of incubation at room temperature, the plates were washed (three times), and the concentrations of IgG subclass Abs were determined following the addition of 100 μl of biotin-conjugated rat anti-mouse γ1 (G1-7.3; 12.5 ng/ml), γ2a (R19-15; 125 ng/ml), γ2b (R12-3; 12.5 ng/ml), and γ3 (R40-82; 50 ng/ml) (BD Pharmingen) heavy-chain-specific monoclonal Abs (14). After incubation and washing steps, 100 μl of 0.5-μg/ml avidin-horseradish peroxidase (HRP) Ab (Vector Labs) in FBS-PBS was added to IgG subclass Ab detection wells, and the plates were incubated for 2 h at room temperature. Following incubation, the plates were washed six times, developed by adding 100 μl of ABTS solution, and read at 415 nm. The Mycobacterium-specific ELISAs were capable of detecting 10 pg/ml of mouse IgG subclass samples.
Cytokine quantitation by Luminex analysis.
The levels of T helper cell-derived cytokines IFN-γ and TNF-α in the sera were determined with a Beadlyte mouse multicytokine detection kit (Bio-Rad, Hercules, CA). Filter-bottomed ELISA plates were rinsed with 100 μl of Bio-Plex assay buffer, and liquid was removed using a Millipore multiscreen separation vacuum manifold system (Bedford, MA) set at 5 mm Hg. Analyte beads in assay buffer were added to the wells, followed by 50 μl of serum or standard solution. The plates were incubated for 30 min at room temperature with continuous shaking (at setting 3), using a Lab-Line Instrument titer plate shaker. The filter-bottomed plates were washed as described above and centrifuged at 300 × g for 30 s. Subsequently, 50 μl of anti-mouse IFN-γ or TNF-α Ab-biotin reporter solution was added to each well, after which the plates were incubated with continuous shaking for 30 min, followed by centrifugation and washing. Next, 50 μl streptavidin-phycoerythrin solution was added, and the plates were incubated with continuous shaking for 10 min at room temperature. One hundred twenty-five microliters of Bio-Plex assay buffer was added, and Beadlyte readings were measured using a Luminex system and calculated using Bio-Plex software (Bio-Rad). The cytokine Beadlyte assays were capable of detecting >5 pg/ml of each analyte.
SAA ELISA.
SAA levels were determined by ELISA (Biosource International). In brief, 50 μl of SAA-specific Ab solution was used to coat microtiter strips to capture SAA. Serum samples and standards were added to wells and incubated for 2 h at room temperature. After the plates were washed with the assay buffer, the HRP-conjugated anti-SAA Ab solution was added, and the plates were incubated for 1 h at 37°C. After washing of the plates, 100 μl tetramethylbenzidine substrate solution was added, and the reactions were stopped after incubation for 15 min at room temperature by the addition of stop solution. The plates were read for the optical density at 450 nm.
Chemokine ELISA.
Serum concentrations of CXCL9, CXCL10, and CXCL11 in mice were determined by ELISA (R&D Systems) according to the manufacturer's instructions. In brief, 96-well ELISA plates were coated with capture Abs, and the plates were incubated overnight at room temperature. After plate blocking, 100 μl of sample or standards was added to each well, and the plates were incubated for 2 h at room temperature. One hundred microliters of detection Ab solution was then added to each well, and the plates were further incubated for 2 h at room temperature. After washing of the plates, 100 μl of streptavidin-HRP solution was added, and the plates were incubated for 20 min in the dark. Next, 100 μl of substrate solution was added to the plates, which were incubated for an additional 20 min at room temperature in the dark. Finally, 50 μl of the stop solution was added, and the plates were read for the optical density at 450 nm after 30 min, using λ corrections of 540 and 570 nm. The ELISAs were capable of detecting >10 pg/ml of each chemokine.
Histology.
Intestinal tissues were preserved using Streck fixative (Streck Laboratories) and were embedded in paraffin. Fixed tissues were sectioned at 6 μm and stained with hematoxylin and eosin for microscopic examination. Intestinal lesions were multifocal and of variable severity. The grades given to intestinal sections took into account the number of lesions as well as their severity. A score (0 to 4) was given to each section, based on previously described criteria (30). The summation of these scores provided a total disease score per mouse. The disease score could range from 0 to a maximum of 12 (with grade 4 lesions in ascending, transverse, and descending intestinal segments).
Statistics.
The data were expressed as means ± standard errors of the means and were compared using a two-tailed paired Student t test or an unpaired Mann-Whitney U test. The results were analyzed using the Statview II statistical program (Abacus Concepts, Inc.) and Microsoft Excel (Microsoft) for Macintosh computers. Single-factor and two-factor analyses of variance were used to evaluate groups and subgroups, respectively. Hence, results were considered statistically significant if P values were <0.01.
RESULTS
M. avium subsp. paratuberculosis-specific IgG subclass Ab profile during murine colitis.
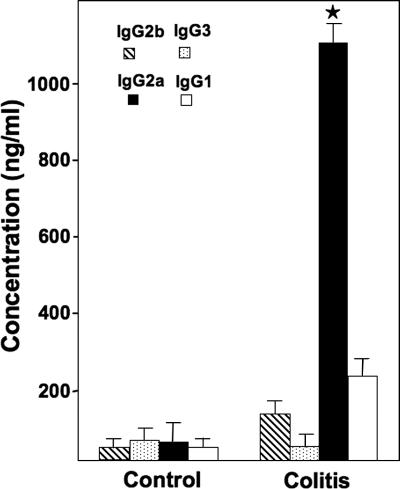
The previously described imbalance of cytokine levels (Th1 > Th2) during colitis suggests that there may be a Th1-biased humoral response associated with the progression of colitis. To test this hypothesis, we measured levels of M. avium subsp. paratuberculosis-specific IgG subclass Abs in the sera of IL-10−/− mice with spontaneous colitis. M. avium subsp. paratuberculosis-specific IgG2a Ab responses were significantly higher in mice with spontaneous colitis, kept under conventional housing, than in similar control mice without disease, which were housed under germfree conditions (Fig. 1).
FIG. 1.
M. avium subsp. paratuberculosis-specific serum Ab responses in IL-10−/− mice during spontaneous colitis. The data presented are mean concentrations (ng/ml) ± standard deviations (SD) of M. avium subsp. paratuberculosis-specific IgG subclasses from three separate experiments. Asterisks indicate statistically significant differences, i.e., P < 0.01, compared to controls. Mouse experimental groups consisted of 15 mice each. Assays were repeated three times.
Changes in colitis severity after M. avium subsp. paratuberculosis challenge.
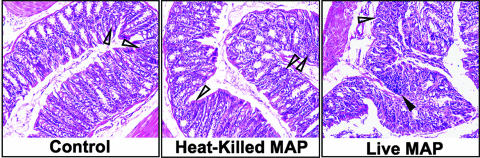
The histological severity of colitis in IL-10−/− mice challenged with live M. avium subsp. paratuberculosis yielded significantly higher colitis disease scores and SAA levels than those found for similar mice challenged either with heat-killed M. avium subsp. paratuberculosis or with the control (Table 1). While lesion scores and SAA levels for mice exposed to heat-killed M. avium subsp. paratuberculosis were elevated over those of controls, the differences were not significant. The intestinal tissues of mice challenged with live M. avium subsp. paratuberculosis showed increased levels of cellular infiltrates, which consisted of lymphocytes and, occasionally, polymorphonuclear cells (Fig. 2). The colitis progression was more aggressive in mice that received live M. avium subsp. paratuberculosis, as noted by multifocal lesions, or aggregates of cellular infiltrates, in all regions of their large intestines. In addition to the dramatic differences in the mean colonic disease scores between the mice challenged with live M. avium subsp. paratuberculosis and those challenged with heat-killed M. avium subsp. paratuberculosis or cream alone, epithelial cells in mice challenged with live M. avium subsp. paratuberculosis were hypertrophied, the intestinal crypt length was decreased, and elongated glandular cells were also present in both the mucosa and the submucosa. Tissues from mice receiving live M. avium subsp. paratuberculosis were also stained using the Ziehl-Neelsen method as well as cultured for the presence of M. avium subsp. paratuberculosis. However, neither resulted in positive identification of live M. avium subsp. paratuberculosis cells. While this does not rule out temporary intestinal colonization of M. avium subsp. paratuberculosis (i.e., <14 weeks), it suggests that live M. avium subsp. paratuberculosis is not present 14 weeks after challenge with 104 CFU.
TABLE 1.
Histological evaluation of Mycobacterium-induced colitis progression in IL-10−/− micea
| Treatment | No. of mice | Colitis disease score (0-12) | SAA concn (μg/ml) |
|---|---|---|---|
| Live M. avium subsp. paratuberculosis | 15 | 7.67 ± 1.23* | 287.6 ± 21* |
| Heat-killed M. avium subsp. paratuberculosis | 15 | 3.01 ± 1.01 | 151.3 ± 16 |
| Cream only | 15 | 1.87 ± 0.89 | 89.5 ± 4.6 |
| No treatment (wild-type B6 mice) | 5 | 0 | 13.4 ± 0.6 |
The persistence of colitis was monitored by evaluating SAA levels and histopathological changes in the colons of IL-10−/− mice with a B6 background. Mice received a single dose of 200 μl of control (cream), 104 CFU of live M. avium subsp. paratuberculosis in cream, or 104 CFU of heat-killed M. avium subsp. paratuberculosis in cream by gavage or were untreated (wild-type B6 mice) and housed under otherwise germfree conditions. Following euthanasia, colons were fixed, sectioned at 6 μm, and stained with hematoxylin and eosin. The sections were examined microscopically at a magnification of ×40 and scored according to the severity of colitis. The data presented for concentrations of SAA are the means ± standard errors of the means, as determined by ELISA in three separate experiments. Asterisks indicate statistically significant differences, i.e., P values of <0.01 between experimental groups. Experimental groups consisted of 15 mice each.
FIG. 2.
Histological characteristics of IL-10−/− mice challenged with M. avium subsp. paratuberculosis. At 14 weeks postchallenge, histopathological sections of colons from IL-10−/− mice that received a single dose of 200 μl of control vehicle (cream only), 104 CFU of live M. avium subsp. paratuberculosis (live MAP) in cream, or 104 CFU of heat-killed M. avium subsp. paratuberculosis (heat-killed MAP) in cream by gavage and were maintained under otherwise germfree conditions were fixed, sectioned at 6 μm, and stained with hematoxylin and eosin. Mild (open triangles) and heavy (solid triangles) cellular infiltrates were noted in the groups (i.e., live M. avium subsp. paratuberculosis ≫ heat-killed M. avium subsp. paratuberculosis > controls). For live M. avium subsp. paratuberculosis-challenged mice, aggregates of cellular infiltrates were typically associated with focal lesions, or hypertrophied epithelial cells with reduced crypt lengths. Sections were examined by light microscopy (magnification, ×40). Experimental groups consisted of 15 mice each. Representative samples are shown.
Characteristics of colitis progression in IL-10−/− mice.
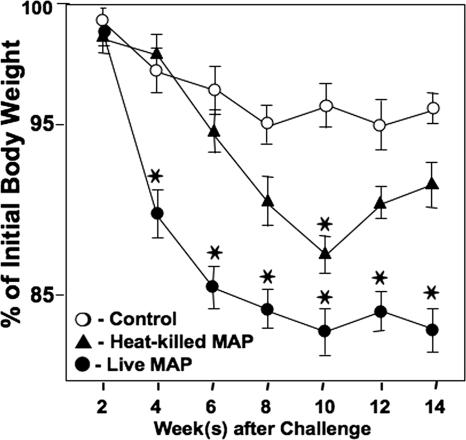
Severe colitis progression in IL-10−/− mice closely corresponded with increased SAA levels (>280 μg/ml) and with a 15% reduction in body weight compared with the initial body weight. IL-10−/− mice do not develop severe colitis under germfree conditions but develop spontaneous colitis, which is associated with weight loss, diarrhea, and ruffled fur, under conventional housing conditions (30). The results of the present study show that mice that were challenged with M. avium subsp. paratuberculosis and housed under otherwise germfree conditions lost more body weight and experienced higher SAA levels than did similar mice challenged with heat-killed M. avium subsp. paratuberculosis or given the control vehicle (Fig. 3 and Table 1). Mice exposed to heat-killed M. avium subsp. paratuberculosis had less weight loss than those exposed to live M. avium subsp. paratuberculosis but had only a marginal increase in the SAA level. The results indicate that mice challenged with live M. avium subsp. paratuberculosis show rapid colitis progression associated with elevated SAA levels and reductions in body weight compared with the control group.
FIG. 3.
Changes in body weight of IL-10−/− mice after M. avium subsp. paratuberculosis challenge. The wasting disease associated with murine colitis was observed by monitoring the body weight during colitis progression. IL-10−/− mice with a B6 background received a single dose of 200 μl of control (cream; open circles), 104 CFU of live M. avium subsp. paratuberculosis in cream (solid circles), or 104 CFU of pasteurized M. avium subsp. paratuberculosis in cream (triangles) and were maintained under otherwise germfree conditions. Percentages of initial body weight of IL-10−/− mice were recorded biweekly. The data presented are the means ± SD for three separate experiments. Asterisks indicate statistically significant differences, i.e., P < 0.01, compared to controls. Experimental groups consisted of 15 mice each, and assays were repeated three times.
M. avium subsp. paratuberculosis challenge enhances systemic CXCR3 ligand and Th1 cytokine levels.
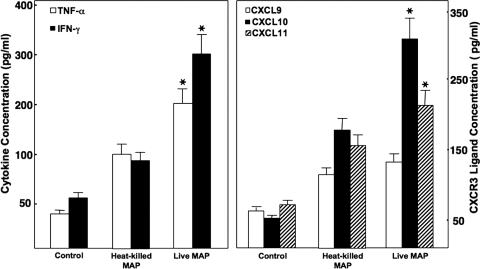
IFN-γ has been shown to be elevated during intestinal inflammation, and TNF-α is overproduced during IBD (29). We next examined the expression of these cytokines and CXCR3 ligands after M. avium subsp. paratuberculosis challenge. IFN-γ and TNF-α levels were significantly higher (∼6-fold) in sera of IL-10−/− mice challenged with live M. avium subsp. paratuberculosis than in control mice; mice exposed to heat-killed M. avium subsp. paratuberculosis had ∼2-fold greater TNF-α and IFN-γ responses than those of controls, but these differences were not significant (Fig. 4). Serum levels of CXCL10 and CXCL11, but not CXCL9, were significantly increased in mice challenged with live or heat-killed M. avium subsp. paratuberculosis compared with those for mice in the control group. These results indicate that exposure to M. avium subsp. paratuberculosis increased the production of systemic IFN-γ, TNF-α, CXCL10, and CXCL11.
FIG. 4.
Serum cytokine levels in IL-10−/− mice after M. avium subsp. paratuberculosis challenge. IL-10−/− mice with a B6 background received a single dose of 200 μl of the control vehicle (i.e., cream), 104 CFU of live M. avium subsp. paratuberculosis (live MAP) in cream, or 104 CFU of heat-killed M. avium subsp. paratuberculosis (heat-killed MAP) in cream by gavage and were maintained under otherwise germfree conditions. The levels of serum TNF-α, IFN-γ, CXCL9, CXCL10, and CXCL11 14 weeks after challenge were determined by ELISA, which was capable of detecting >10 pg/ml TNF-α, IFN-γ, or CXCR3 ligand. The data presented are the mean TNF-α, IFN-γ, and CXCR3 ligand concentrations ± SD (ng/ml). Asterisks indicate statistically significant differences, i.e., P < 0.01, compared to controls. Experimental groups consisted of 15 mice each. Assays were repeated three times.
Vβ11+ T-cell receptor (TCR)-restricted peptide from Ag85B/MPT59 enhances proliferation and T helper cytokine response.
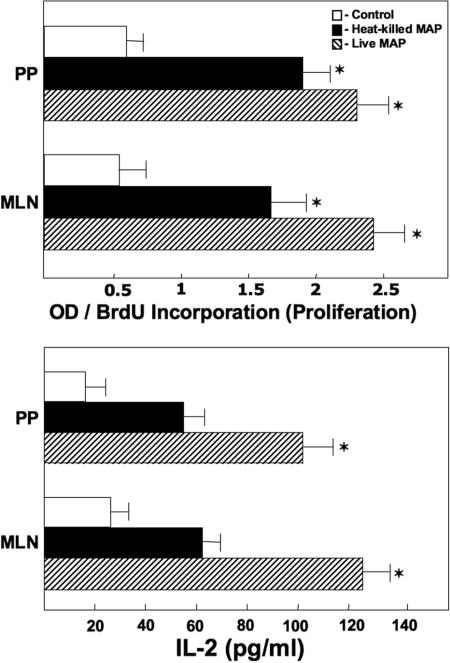
To determine if M. avium subsp. paratuberculosis challenge promoted Ag-specific T helper responses, we next examined the ability of peptide 25 to stimulate proliferative responses by CD4+ T cells isolated from the MLN and PP of mice previously challenged with live M. avium subsp. paratuberculosis, heat-killed M. avium subsp. paratuberculosis, and/or control vehicle. BrdU is incorporated into newly synthesized DNA strands of actively proliferating cells. Peptide 25-stimulated CD4+ T cells from the MLN and PP of mice previously challenged with either live or heat-killed M. avium subsp. paratuberculosis exhibited marked increases in BrdU incorporation compared with similar CD4+ T cells from mice challenged with cream alone (Fig. 5). These results suggest that Ag restimulation after exposure to M. avium subsp. paratuberculosis enhances CD4+ T-cell proliferation.
FIG. 5.
Anti-peptide 25 Ag (from MPT59)-induced proliferation of and IL-2 production by CD4+ T cells from IL-10−/− mice. IL-10−/− mice with a B6 background received a single dose of 200 μl of control vehicle (cream only; open bars), 104 CFU of live M. avium subsp. paratuberculosis in cream (hatched bars), or 104 CFU of heat-killed M. avium subsp. paratuberculosis in cream (solid bars) and were maintained under otherwise germfree conditions. CD4+ lymphocytes derived from the MLN and PP of the mice were purified and cultured at a density of 5 × 106 cells/ml with peptide 25 (1 μg/ml) for 3 days with γ-irradiated APCs (106 cells/ml). Cytokines present in culture supernatants were determined by ELISA. Proliferation was measured by BrdU incorporation. The data presented are the mean OD450 values for proliferative responses and the mean concentrations of IL-2 secretion (pg/ml) ± SD for quadruplicate cultures. Asterisks indicate statistically significant differences, i.e., P < 0.01, compared to controls. Experimental groups consisted of 15 mice each, and experiments were repeated three times.
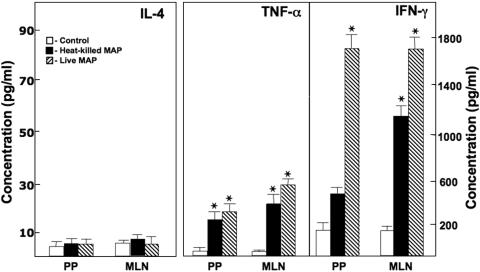
We next examined cytokine production following peptide 25 stimulation. IL-2 levels were significantly increased in CD4+ T cells from the MLN and PP of mice previously challenged with live or heat-killed M. avium subsp. paratuberculosis compared with those for control mice. However, IL-4 was not significantly expressed following ex vivo Ag stimulation (Fig. 6). IFN-γ and TNF-α secretion by peptide 25-stimulated CD4+ T cells from the PP and MLN of mice challenged with live or heat-killed M. avium subsp. paratuberculosis was significantly higher than that by similar cells from controls. These results indicate that M. avium subsp. paratuberculosis Ag85 presentation by Ag-presenting cells (APCs) induces CD4+ T cells from MLN and PP to produce IFN-γ, IL-2, and TNF-α.
FIG. 6.
MPT59/Ag85B peptide 25-specific T helper cytokine production by CD4+ T cells in IL-10−/− mice. IL-10−/− mice with a B6 background received a single dose of 200 μl of control vehicle (cream only; open bars), 104 CFU of live M. avium subsp. paratuberculosis in cream (hatched bars), or 104 CFU of heat-killed M. avium subsp. paratuberculosis in cream (solid bars) and were maintained under otherwise germfree conditions. CD4+ T cells from the MLN and PP of the mice were purified and cultured at a density of 5 × 106 cells/ml with peptide 25 (1 μg/ml) for 3 days along with 106 cells/ml of γ-irradiated APCs. Cytokine production by these ex vivo Ag-stimulated T helper cells was determined by ELISA. The data presented are the mean IL-4, TNF-α, and IFN-γ secretions (pg/ml) ± SD for quadruplicate cultures. Asterisks indicate statistically significant differences, i.e., P < 0.01, compared to controls. Groups consisted of 15 mice each, and experiments were repeated three times.
DISCUSSION
Although there is no recognized etiology for IBD, interest in M. avium subsp. paratuberculosis as a possible causative agent for IBD began due to similarities in the chronic idiopathic granulomatous and intramural inflammation observed in cases of both CD and veterinary Johne's disease, with M. avium subsp. paratuberculosis being the recognized cause of Johne's disease. In addition, identical strains of M. avium subsp. paratuberculosis were obtained from three CD patients, one of which was used in this study (5). In the present study, anti-M. avium subsp. paratuberculosis serum IgG2a Ab levels were higher in IL-10−/− mice with spontaneous colitis. These Th1-associated Ab responses correlated with relatively high levels of serum TNF-α and IFN-γ. A similar elevated Ab response of IBD patients to a purified protein from M. avium subsp. paratuberculosis has been reported (24). It has also been shown that Ab reactivity of sera to the P36 mycobacterial Ag was higher for IBD patients (10). These results illustrate a plausible link between Mycobacterium and IBD.
To study the nature of this association, we used a mouse model to explore the Th1-based responses to M. avium subsp. paratuberculosis and, more precisely, M. avium subsp. paratuberculosis Ag exposure. We hypothesized that exposure to M. avium subsp. paratuberculosis would enhance the development of colitis in IL-10−/− mice by modulating the production of Th1 and inflammatory cytokines. The study results demonstrate that IL-10−/− mice show M. avium subsp. paratuberculosis-specific Ab responses in serum and that exposure to M. avium subsp. paratuberculosis under otherwise germfree conditions in the murine model enhances the progression of colitis by decreasing body weight and increasing the SAA level as well as local and/or systemic CXCL10, CXCL11, IFN-γ, and TNF-α levels in IL-10−/− mice compared with those of control IL-10−/− mice exposed only to the control vehicle. Furthermore, we demonstrated that the changes in CXCR3 ligands and Th1 cytokines are driven in part by peptide 25-specific CD4+ T-cell responses.
It is well established that IL-12 drives Th1 differentiation and subsequent IFN-γ production (32). Indeed, IL-12, IL-23 (with the IL-12p40 subunit), and IFN-γ play a critical role in the induction and progression of colitis (25). We demonstrated that local and/or systemic expression of TNF-α, IFN-γ, and CXCR3 ligand(s) is increased in live M. avium subsp. paratuberculosis-challenged mice compared with that for the other groups. The presence of CXCL10 interacting with CXCR3 is considered an important signal for selective homing or activation of effector cells, which preferentially accumulate at some inflammatory sites (28). Perhaps the production of the CXCR3 ligands, IFN-γ, and TNF-α in the sera during colitis progression creates a situation in which Th1 cells are recruited, further differentiated, and expanded to initiate or maintain a state of colitis.
SAA is elevated in and alters many inflammatory conditions, such as IBD (8). TNF-α levels are also elevated in tissues and produced by mucosal cells of the LP during inflammation (4, 27). While TNF-α produced by CD4+ T cells is neither sufficient nor required for the induction of murine colitis, its production by APCs is essential for colitis (7). In the present study, the amount of TNF-α from MLN, PP, and LP lymphocytes was increased following M. avium subsp. paratuberculosis challenge compared with that for the control group. The interaction between specifically sensitized T cells and activated macrophage effector cells is considered a hallmark of protective immunity against pathogenic mycobacteria (2).
Unlike nominal Ags, which stimulate a small fraction of T cells, super-Ags stimulate a majority of T cells bearing a TCR carrying a specific V region polypeptide chain (9). Peptide 25 is not a super-Ag per se because it requires Ag processing in order to stimulate Vβ11+ Th1 cells. It is tempting to speculate that since Ag85B aids bacillus survival by inhibiting delayed-type hypersensitivity responses, perhaps hosts susceptible to Mycobacterium infection have evolved mechanisms targeting the Ag85 complex to inhibit this function. The present study shows that Ag85B/MPT59 peptide 25 induces proliferation and cytokine production by CD4+ T cells from M. avium subsp. paratuberculosis-challenged mice.
The transfer of CD4+ CD45RBhigh T cells into immunodeficient mice leads to colitis, with expansion of these T cells via Ag-specific activation. Donor CD4+ CD45RBhigh T cells from I-Ab-restricted mice are Vβ3, Vβ4, Vβ15, Vβ18, and Vβ11 positive and polyclonal, which allows them to respond to multiple Ags (18). Once transferred, the polyclonal Vβ11+ T cells home to the large intestine; next, specific Vβ11+ clonal or oligoclonal populations expand and colitis develops. While these observations and our findings do not support the notion that there is a single predominant Ag that mediates colitis, they do not exclude this possibility or the possibility that multiple Ags act to expand Vβ11+ clonal or oligoclonal populations. It is possible that peptide 25 is a mycobacterial TCR-specific Ag that may contribute to colitis pathogenesis by inducing a local release of cytokines from Ag85/MPT59-reactive T cells bearing the Vβ11-encoded mouse TCR. This has far-reaching implications considering that the peptide 25 epitope is conserved among several Mycobacterium species (31).
Activation of Toll-like receptors (TLRs) leads to the induction of antimicrobial pathways central to innate defense as well as to upregulation of Ag presentation and secretion of cytokines. It was recently shown that TLR-activated dendritic cells generally favor Th1 responses, due largely to TLR ligation inducing the production of IL-12 (13). M. avium subsp. paratuberculosis may provide TLR ligands to stimulate IL-12 production, which is necessary to facilitate the induction and progression of colitis in IL-10−/− mice (11, 23). Mycobacterium-induced colitis in IL-10−/− mice might be a highly relevant model for future study. While additional studies will be required to ascertain the role of M. avium subsp. paratuberculosis during the induction and progression of colitis, we report that live or heat-killed M. avium subsp. paratuberculosis challenge leads to colitis in IL-10−/− mice housed under germfree conditions. The results further suggest that M. avium subsp. paratuberculosis functions in an immunologic rather than infectious capacity and that it may not be essential for M. avium subsp. paratuberculosis organisms to be alive to exert their immunopathogenic potential on susceptible hosts.
Acknowledgments
The content of this paper benefited from many fruitful conversations with colleagues at the Morehouse School of Medicine, the UGA College of Veterinary Medicine, the Food and Drug Administration, and the University of Louisville as well as from editing by Andrew Marsh.
This work was supported in part by the Crohn's & Colitis Foundation of America; by National Institutes of Health grants RR03034, GM08248, MD000525, and AI57808; by the Southeast Center for Emerging Biologic Threats; and by the University of Louisville.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 14 May 2007.
REFERENCES
- 1.Autschbach, F., S. Eisold, U. Hinz, S. Zinser, M. Linnebacher, T. Giese, T. Loffler, M. W. Buchler, and J. Schmidt. 2005. High prevalence of Mycobacterium avium subspecies paratuberculosis IS900 DNA in gut tissues from individuals with Crohn's disease. Gut 54:944-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes, P. F., S. Lu, J. S. Abrams, E. Wang, M. Yamamura, and R. L. Modlin. 1993. Cytokine production at the site of disease in human tuberculosis. Infect. Immun. 61:3482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg, D. J., N. Davidson, R. Kuhn, W. Muller, S. Menon, G. Holland, L. Thompson-Snipes, M. W. Leach, and D. Rennick. 1996. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J. Clin. Investig. 98:1010-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breese, E. J., C. A. Michie, S. W. Nicholls, S. H. Murch, C. B. Williams, P. Domizio, J. A. Walker-Smith, and T. T. MacDonald. 1994. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology 106:1455-1466. [DOI] [PubMed] [Google Scholar]
- 5.Chiodini, R. J. 1989. Crohn's disease and the mycobacterioses: a review and comparison of two disease entities. Clin. Microbiol. Rev. 2:90-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiodini, R. J., H. J. Van Kruiningen, W. R. Thayer, R. S. Merkal, and J. A. Coutu. 1984. Possible role of mycobacteria in inflammatory bowel disease. I. An unclassified Mycobacterium species isolated from patients with Crohn's disease. Dig. Dis. Sci. 29:1073-1079. [DOI] [PubMed] [Google Scholar]
- 7.Corazza, N., S. Eichenberger, H. P. Eugster, and C. Mueller. 1999. Nonlymphocyte-derived tumor necrosis factor is required for induction of colitis in recombination activating gene (RAG)2(−/−) mice upon transfer of CD4(+)CD45RB(hi) T cells. J. Exp. Med. 190:1479-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Beer, F. C., R. K. Mallya, E. A. Fagan, J. G. Lanham, G. R. Hughes, and M. B. Pepys. 1982. Serum amyloid-A protein concentration in inflammatory diseases and its relationship to the incidence of reactive systemic amyloidosis. Lancet ii:231-234. [DOI] [PubMed] [Google Scholar]
- 9.Dellabona, P., J. Peccoud, J. Kappler, P. Marrack, C. Benoist, and D. Mathis. 1990. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell 62:1115-1121. [DOI] [PubMed] [Google Scholar]
- 10.El-Zaatari, F. A., S. A. Naser, K. Hulten, P. Burch, and D. Y. Graham. 1999. Characterization of Mycobacterium paratuberculosis p36 antigen and its seroreactivities in Crohn's disease. Curr. Microbiol. 39:115-119. [DOI] [PubMed] [Google Scholar]
- 11.Fuss, I. J., T. Marth, M. F. Neurath, G. R. Pearlstein, A. Jain, and W. Strober. 1999. Anti-interleukin 12 treatment regulates apoptosis of Th1 T cells in experimental colitis in mice. Gastroenterology 117:1078-1088. [DOI] [PubMed] [Google Scholar]
- 12.Hulten, K., H. M. El-Zimaity, T. J. Karttunen, A. Almashhrawi, M. R. Schwartz, D. Y. Graham, and F. A. El-Zaatari. 2001. Detection of Mycobacterium avium subspecies paratuberculosis in Crohn's diseased tissues by in situ hybridization. Am. J. Gastroenterol. 96:1529-1535. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987-995. [DOI] [PubMed] [Google Scholar]
- 14.Lillard, J. W., Jr., P. N. Boyaka, J. A. Hedrick, A. Zlotnik, and J. R. McGhee. 1999. Lymphotactin acts as an innate mucosal adjuvant. J. Immunol. 162:1959-1965. [PubMed] [Google Scholar]
- 15.Lillard, J. W., Jr., P. N. Boyaka, D. D. Taub, and J. R. McGhee. 2001. RANTES potentiates antigen-specific mucosal immune responses. J. Immunol. 166:162-169. [DOI] [PubMed] [Google Scholar]
- 16.Lillard, J. W. J., P. N. Boyaka, O. Chertov, J. J. Oppenheim, and J. R. McGhee. 1999. Mechanism for induction of acquired host immunity by neutrophil peptide defensins. Proc. Natl. Acad. Sci. USA 96:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald, T. T., and S. Pettersson. 2000. Bacterial regulation of intestinal immune responses. Inflamm. Bowel Dis. 6:116-122. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda, J. L., L. Gapin, B. C. Sydora, F. Byrne, S. Binder, M. Kronenberg, and R. Aranda. 2000. Systemic activation and antigen-driven oligoclonal expansion of T cells in a mouse model of colitis. J. Immunol. 164:2797-2806. [DOI] [PubMed] [Google Scholar]
- 19.McFadden, J. J., P. D. Butcher, R. J. Chiodini, and J. Hermon-Taylor. 1987. Determination of genome size and DNA homology between an unclassified Mycobacterium species isolated from patients with Crohn's disease and other mycobacteria. J. Gen. Microbiol. 133:211-214. [DOI] [PubMed] [Google Scholar]
- 20.Naser, S. A., G. Ghobrial, C. Romero, and J. F. Valentine. 2004. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet 364:1039-1044. [DOI] [PubMed] [Google Scholar]
- 21.Naser, S. A., K. Hulten, I. Shafran, D. Y. Graham, and F. A. El-Zaatari. 2000. Specific seroreactivity of Crohn's disease patients against p35 and p36 antigens of M. avium subsp. paratuberculosis. Vet. Microbiol. 77:497-504. [DOI] [PubMed] [Google Scholar]
- 22.Naser, S. A., D. Schwartz, and I. Shafran. 2000. Isolation of Mycobacterium avium subsp. paratuberculosis from breast milk of Crohn's disease patients. Am. J. Gastroenterol. 95:1094-1095. [DOI] [PubMed] [Google Scholar]
- 23.Neurath, M. F., I. Fuss, B. L. Kelsall, E. Stuber, and W. Strober. 1995. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 182:1281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsen, I., H. G. Wiker, E. Johnson, H. Langeggen, and L. J. Reitan. 2001. Elevated antibody responses in patients with Crohn's disease against a 14-kDa secreted protein purified from Mycobacterium avium subsp. paratuberculosis. Scand. J. Immunol. 53:198-203. [DOI] [PubMed] [Google Scholar]
- 25.Parronchi, P., P. Romagnani, F. Annunziato, S. Sampognaro, A. Becchio, L. Giannarini, E. Maggi, C. Pupilli, F. Tonelli, and S. Romagnani. 1997. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am. J. Pathol. 150:823-832. [PMC free article] [PubMed] [Google Scholar]
- 26.Powrie, F., and M. W. Leach. 1995. Genetic and spontaneous models of inflammatory bowel disease in rodents: evidence for abnormalities in mucosal immune regulation. Ther. Immunol. 2:115-123. [PubMed] [Google Scholar]
- 27.Reimund, J. M., C. Wittersheim, S. Dumont, C. D. Muller, J. S. Kenney, R. Baumann, P. Poindron, and B. Duclos. 1996. Increased production of tumour necrosis factor-alpha interleukin-1 beta, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn's disease. Gut 39:684-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallusto, F., A. Lanzavecchia, and C. R. Mackay. 1998. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol. Today 19:568-574. [DOI] [PubMed] [Google Scholar]
- 29.Simpson, S. J., G. A. Hollander, E. Mizoguchi, D. Allen, A. K. Bhan, B. Wang, and C. Terhorst. 1997. Expression of pro-inflammatory cytokines by TCR alpha beta+ and TCR gamma delta+ T cells in an experimental model of colitis. Eur. J. Immunol. 27:17-25. [DOI] [PubMed] [Google Scholar]
- 30.Singh, U. P., S. Singh, D. D. Taub, and J. W. Lillard, Jr. 2003. Inhibition of IFN-gamma-inducible protein-10 abrogates colitis in IL-10(−/−) mice. J. Immunol. 171:1401-1406. [DOI] [PubMed] [Google Scholar]
- 31.Tasaka, H., and Y. Matsuo. 1984. Specificity and distribution of alpha antigens of Mycobacterium kansasii and Mycobacterium marinum. Am. Rev. Respir. Dis. 130:647-649. [DOI] [PubMed] [Google Scholar]
- 32.Trinchieri, G. 1994. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 84:4008-4027. [PubMed] [Google Scholar]
- 33.Yanagisawa, S., M. Koike, A. Kariyone, S. Nagai, and K. Takatsu. 1997. Mapping of V beta 11+ helper T cell epitopes on mycobacterial antigen in mouse primed with Mycobacterium tuberculosis. Int. Immunol. 9:227-237. [DOI] [PubMed] [Google Scholar]