Abstract
We designed an amebiasis subunit vaccine that is constructed by using four peptide epitopes of the galactose-inhibitable lectin heavy subunit that were recognized by intestinal secretory immunoglobulin A (IgA) antibodies from immune human subjects. These epitopes are contained in the region encompassing amino acids 758 to 1134 of the lectin heavy subunit, designated LC3. Baboons (Papio anubis) are natural hosts for Entamoeba histolytica; naturally infected baboons raised in captivity possess serum IgA antibodies to the same four LC3 epitopes as humans. Uninfected, seronegative baboons received four intranasal immunizations at 7-day intervals with the synthetic peptide vaccine (400, 800, or 1,600 μg per nostril) with cholera toxin (20 μg) as the adjuvant. As determined by an enzyme-linked immunosorbent assay (ELISA), each dose of the peptide vaccine elicited antipeptide serum IgA and IgG and intestinal IgA antibody responses in all six immunized baboons by day 28, 7 days after the last immunization (P, <0.01 for each dose compared to the cholera toxin control). The peptide vaccine elicited serum IgG and intestinal IgA antibodies that recognized purified recombinant LC3 protein (P, <0.008 and 0.02, respectively) and native lectin protein (P < 0.01). In addition, an indirect immunofluorescence assay with whole trophozoites (P < 0.01) and Western blot analysis confirmed that serum IgG antibodies from vaccinated baboons recognized native lectin protein on the surfaces of axenic E. histolytica trophozoites or from solubilized amebae. All four synthetic peptides were immunogenic; the vaccine elicited dose- and time-dependent responses, as determined by ELISA optical density readings indicating the production of serum and intestinal antibodies (P, <0.02 for antipeptide and antilectin antibodies). As a positive control, intranasal immunization with purified recombinant LC3 protein with cholera toxin as the adjuvant elicited a serum anti-LC3 IgA and IgG antibody response (P, 0.05 and <0.0001, respectively); however, no intestinal anti-LC3 IgA antibody response was observed (P = 0.4). Of interest, serum IgA and IgG antibodies elicited by the recombinant LC3 vaccine did not recognize any of the four putatively protective LC3 peptide epitopes. Both serum and fecal antibodies elicited by the peptide vaccine exhibited neutralizing activity, as determined by their dose-dependent inhibition of the galactose-specific adherence of E. histolytica trophozoites to Chinese hamster ovary cells in vitro (P, <0.001 for each group of antibodies compared to the control). In summary, a lectin-based intranasal polylysine-linked synthetic peptide vaccine was effective in eliciting an adherence-inhibitory, intestinal antilectin IgA antibody response in baboons. Future studies with the baboon model will determine vaccine efficacy against asymptomatic E. histolytica intestinal infection.
Invasive amebiasis, due to the enteric protozoan Entamoeba histolytica, manifests most commonly as amebic colitis or a liver abscess. E. histolytica infection is highly endemic in the most populated areas of the world, such as India, China, Africa, the Middle East, South and Central America, and southeast Asia (35). Recent epidemiological studies in Hue, Vietnam (8), South Africa (1, 36), and Bangladesh (20) revealed that E. histolytica infection and invasive disease are more prevalent than previously appreciated. Up to 70% of adults in an area where infection is highly endemic, such as Durban, South Africa, are challenged by the parasite each year (1); the prevalence of amebic liver abscesses (ALA) is as high as 21 per 100,000 people per year in Vietnam (8). It is estimated that E. histolytica is the third leading parasitic cause of death worldwide (43).
Presently, no vaccine to prevent asymptomatic E. histolytica infection or invasive disease is available. E. histolytica trophozoites attach to colonic mucins, host inflammatory cells, and epithelial cells by a galactose-inhibitable surface lectin (10, 32, 37). The lectin consists of three subunits (16); the heavy subunit contains the galactose-binding activity (33) and is highly antigenic (38). Purified native lectin protein (33) and a cysteine-rich recombinant protein encompassing amino acids 758 to 1134 of the lectin heavy subunit (designated LC3) (40) are effective as a vaccine (in combination with Freund's adjuvant) in the gerbil model of ALA. Monoclonal antibodies to the lectin heavy subunit inhibit amebic binding to colonic mucins (10), suggesting that an intestinal antilectin immunoglobulin A (IgA) antibody response may be effective in preventing parasite attachment to and invasion of the gut. Studies of asymptomatic E. histolytica intestinal infection in children in Bangladesh (19-21) and in adults in Durban, South Africa (1, 36), demonstrated that intestinal antilectin IgA antibodies mediate the rapid clearance of infection or immunity to new infections. Studies in Durban, South Africa, by our research group (1, 36) revealed that subjects cured of ALA develop high-titer peaks of intestinal antilectin IgA antibodies upon parasite challenge and that these antibody levels are sufficient to prevent or clear asymptomatic E. dispar and E. histolytica infections. In contrast, control subjects without a history of treatment for invasive amebiasis have lower-titer intestinal antilectin IgA antibody peaks that are delayed, less long-lasting, and insufficient to clear amebic infection (1).
Our strategy for vaccine design was to use synthetic peptides to elicit a mucosal immune response that mimics that of the South African ALA subjects. By using segments of the LC3 protein, expressed as overlapping recombinant proteins, we identified two LC3 fragments that are recognized by the antilectin IgA antibodies present in the sera and feces of immune human hosts (2). Fine mapping using overlapping synthetic peptides led to the discovery of four discrete LC3 peptide epitopes, referred to as peptides 2, 9, 11, and 12. Further studies have demonstrated that LC3 epitope recognition by human IgA antibodies is highly conserved and was exhibited by serum IgA antibodies from subjects cured of colitis in Cairo, Egypt (our unpublished data). Based on the epitope-mapping studies, we constructed a synthetic peptide vaccine containing the four putatively protective LC3 epitopes (peptides 2, 9, 11, and 12).
The systemic immunization of BALB/c mice with purified recombinant LC3 protein elicits murine IgA antibodies with LC3 epitope specificity different from that of human IgA antibodies, with little overlap (2). In addition, most mouse strains exhibit high levels of innate immunity to E. histolytica infection (22). Baboons were chosen for this vaccine study as they are naturally infected with E. histolytica in the wild (25, 42) and in captivity (45); in addition, baboons have an immune system that best approximates that of humans (39). Cholera toxin (CT) was selected as the adjuvant because of its known effectiveness in inducing a mucosal immune response (30) and because of our prior experience using recombinant LC3 protein to induce the secretion of intestinal adherence-inhibitory, antilectin IgA antibodies in BALB/c mice following oral immunization (7). Intranasal vaccination requires a smaller immunogen dose than oral vaccination (13) and obviates the need for sterile needles, an advantage considering the future application of a lectin-based subunit vaccine for humans.
MATERIALS AND METHODS
Selection of experimental subjects (baboons).
Female baboons, aged 1.5 and 3 years, were selected to participate in this study. Before being selected for the study, baboons (Papio anubis) of both sexes and various ages were housed in large breeding groups at the University of Oklahoma Health Sciences Center Comparative Medicine facility (which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International). Baboons were not utilized in other experiments before this study. During the immunization experiment, baboons were housed singly in aluminum cages. All experimental procedures were approved by the University of Oklahoma Health Sciences Center and the University of Minnesota Institutional Animal Care and Use Committees. Baboons were fed monkey chow twice daily and given fruit, popcorn, peanuts, and other treats once a day. Study baboons did not show any physical or behavioral abnormalities before selection. Vaccinated and control animals lived under identical conditions and were managed by the same personnel throughout the study.
Prior to being selected for the study, baboons were screened for E. histolytica and E. dispar infection by PCR analysis of fecal samples (2) and an enzyme-linked immunosorbent assay (ELISA) of serum samples for anti-LC3 IgA and IgG antibodies. Baboons were sedated with an intramuscular injection of ketamine hydrochloride (approximately 10 mg/kg of body weight) for the collection of blood using a 20-gauge needle and a 10-ml syringe. Fecal and blood samples were collected from baboons prior to each vaccination and 7 days after the last immunization. Blood was divided between a serum separator tube and a heparin tube and then placed on ice until centrifuged. After clotting for about 2 h, the samples were centrifuged for 15 min at high speed. The sera were stored at −80°C and shipped in dry ice to laboratories at the University of Minnesota.
Detection of E. dispar or E. histolytica infection by fecal PCR assay.
Baboon stool samples were stored at −70°C, and extracted DNA was stored at −20°C in a fecal DNA bank. The QIAamp DNA stool mini kit was used to extract DNA from feces according to the protocol of the manufacturer (QIAGEN, Haldin, Germany). Four separate laboratory areas were used for PCR analysis to minimize the risk of contamination. DNA was extracted from stool in one area, and the PCR mix was prepared and samples were added in another area. The PCR was run in a third area, and the analysis of amplified PCR products and the storage of the PCR products and other materials (glassware and equipment) occurred in the remaining area.
PCR was performed using deoxynucleoside triphosphates (Amersham Pharmacia Biotech; catalog no. 27-2035-01) by mixing 100 μl of each nucleotide (G, C, T, and A) with 5 ml of PCR buffer and 4.6 ml of H2O. The mixture was divided into 1-ml aliquots and stored at −20°C. The Taq polymerase (Amersham Pharmacia Biotech; catalog no. 270799) was diluted at 1:20 immediately before use. The E. histolytica sense primer (5′-GTA CAA AAT GGC CAA TTC ATT CAA CG-3′), E. dispar sense primer (5′-GTA CAA AGT GGC CAA TTT ATG TAA GCA-3′), and E. histolytica/E. dispar antisense primer (5′-GAA TTG ATT TTA CTC AAC TCT AGA G-3′) (2) were prepared at concentrations of 10 Pmol/μl. A 500-μl volume of bovine serum albumin (BSA [Pierce; catalog no. 23210]) at 200 mg/dl was diluted with 500 μl of H2O and kept at 4°C. The DNA sample to be tested (5 μl) was added to 95 μl of the PCR mixture to yield a complete volume of 100 μl. Each DNA sample was tested twice, once using the E. histolytica sense primer and once using the E. dispar sense primer.
Conventional PCR machine thermocycling conditions were as described previously (2). The specific detection of amplified DNA was achieved by gel electrophoresis. Amplified DNA was separated on a 2% agarose gel containing ethidium bromide.
ELISA for detection of baboon antilectin, anti-LC3, and antipeptide antibodies.
ELISAs were performed as described previously (36). Recombinant 52-kDa LC3 protein was purified as described previously (40), and native lectin protein was purified by immunoaffinity chromatography from detergent-solubilized trophozoites (33, 40). ELISA 96-well microtiter flat-bottomed polystyrene plates were coated with LC3 protein, purified native lectin, or synthetic peptides, and nonreactive sites were blocked with 1% BSA. Serum samples at a 1:100 dilution in phosphate-buffered saline (PBS)-Tween (1% BSA) were analyzed for IgG or IgA by ELISAs and incubated for 2 h at room temperature or overnight at 4°C. Each sample was studied in triplicate and tested twice by ELISAs. Alkaline phosphatase-conjugated goat anti-human IgG (Sigma, St. Louis, MO) or IgA (ICN Biomedicals, Costa Mesa, CA) antibodies were diluted (1:5,000 for IgG and 1:2,500 for IgA) in PBS-Tween (1% BSA) to give a final volume of 100 μl/well, and the plates were incubated for 2 h at room temperature. Reading of the plates and correction for nonspecific background binding were performed as described previously (38).
ELISAs for the detection of fecal antilectin IgA antibodies were performed using purified native lectin protein as described previously (1, 3). We have previously reported that native lectin is more sensitive than recombinant LC3 protein for the detection of antilectin intestinal IgA antibodies in humans (3, 36). Flat-bottomed microtiter ELISA plates were coated with lectin protein (0.1 μg/well), and nonreactive sites were blocked with 1% BSA (3). Fecal samples were mixed with equal volumes of PBS containing 2.0 mM phenylmethylsulfonyl fluoride, and the mixtures were added at 100 μl/well and incubated for 2 h at room temperature or overnight at 4°C. Alkaline phosphatase-conjugated goat anti-human IgA antibodies (Sigma, St. Louis, MO) diluted (1:3,000) in PBS-Tween containing 1% BSA were added for incubation over 2 h at room temperature. Plate reading with correction for nonspecific background binding was performed as described previously (1, 3).
Preparation of the polylysine-linked synthetic peptides for use in the intranasal vaccine.
Based on previous findings (2), four peptides that included amino acids 891 to 903 (peptide 2), 918 to 936 (peptide 9), 1114 to 1138 (peptide 11), and 1128 to 1150 (peptide 12) of the LC3 protein were utilized. Peptides were synthesized using a PerkinElmer Pioneer peptide synthesizer by solid-phase 9-fluorenylmethoxy carbonyl chemistry. Peptides were cleaved from the resin, deprotected using reagent R, and then lyophilized. Lyophilized crude peptides were purified by preparative reverse-phase high-performance liquid chromatography using a Beckman 126 system with a Vydac C4 column. Solvent A consisted of 0.1% trifluoroacetic acid in water, and solvent B consisted of 0.1% trifluoroacetic acid in acetonitrile. The purification was carried out with a gradient of 0 to 60% solvent B in 30 min. Purity and quality control of the peptides was done by using an analytical high-performance liquid chromatography HP 1090 system with a C18 column and the same gradient and by using mass spectrometry as on an HP matrix-assisted laser desorption ionization-time of flight system (6). The levels of purity of the four peptides utilized were 97, 100, 90, and 75%, respectively, for peptides 2, 9, 11, and 12.
Peptide polylysine complexes were manufactured by Alpha Diagnostic International and shipped in a lyophilized form. Each polylysine molecule had a set of six peptides, a random mixture of all four peptides, linked to it. The polylysine-linked peptide vaccine preparation was suspended in saline, submitted to a quantitative assay (12), and adjusted with saline according to the desired vaccination protocol. Each aliquot tube contained one vaccine dose for one animal to avoid repeated thawing and freezing of peptides. The contents of each tube were mixed with the proper amount of adjuvant as defined by the vaccination protocol before use.
Experimental vaccine protocol.
Seronegative and PCR-negative baboons were selected by screening, isolated, and then immunized intranasally (in each nostril) four times at 7-day intervals. CT adjuvant (Sigma) at 20 μg per nostril served as a negative control, and purified recombinant LC3 protein (40) at 200 μg per nostril with CT adjuvant was the positive control. An analysis of dose-dependent responses to the polylysine-linked synthetic peptide vaccine at 400, 800, and 1,600 μg with 20 μg of CT per nostril per immunization was performed. Four separate experiments with 9, 9, 6, and 6 baboons, for a total of 30 baboons, were studied. Experiments 1 and 2 included three baboons immunized with adjuvant alone, three baboons immunized with LC3 protein, and three baboons immunized with 400 μg of the peptide vaccine. Experiments 3 and 4 included three baboons immunized with 800 μg and three immunized with 1,600 μg of the peptide vaccine. Therefore, a total of six baboons per experimental condition were studied. Blood and feces were collected from each baboon before vaccination and 7 days after each vaccination (days 7, 14, 21, and 28 of the study). All ELISA results were corrected based on prevaccination results for each individual baboon.
IFA.
An indirect immunofluorescence assay (IFA) was used to evaluate the recognition of amebic surface lectin by serum IgG antibodies from peptide-vaccinated baboons. The procedure was performed according to the method of Strachan et al. (41). Axenic cultures of E. histolytica strain HMI:IMSS were grown in TYI-S-33 culture medium as described by Diamond et al. (14). E. histolytica trophozoites were suspended at 50,000/ml in PBS, and 5 μl was pipetted into each well of a Teflon-coated IFA slide. Within 15 min after drying at room temperature, the slide was fixed in methanol. Serial dilutions of baboon sera (1/4, 1/8, 1/16, 1/32, 1/64, and 1/128) from peptide-vaccinated and control baboons were prepared in 2% Marvel (dried skim milk) dissolved in PBS (PBSM) and added at 25 μl/well. Slides were incubated for 2 h in a moist chamber at room temperature. Excess antibodies were rinsed off with PBSM, and the slides were washed. After drying, fluorescein isothiocyanate-labeled goat anti-human IgG antibodies (Sigma) diluted 1:40 in PBSM (containing 33 μl of 1% Evan's blue/ml) were added, and the slides were incubated for 1 h. After washing, a drop of mounting medium (50% Citifluor and 50% PBS) was added to each well. Fluorescence density (FD) readings were obtained with an immunofluorescence microscope (E clips E 600; Nikon) under blue light; a Photometrics COOL SNAP ES camera was used to read the slides. Each trophozoite was traced separately, and an FD histogram was developed and corrected for background fluorescence. The cutoff point for immunofluorescence positivity was calculated as the mean + 2 standard deviations of the FD reading for control trophozoites submitted to the same procedure.
Effect of antipeptide serum and intestinal antibodies from vaccinated baboons on amebic galactose-inhibitable adherence in vitro.
E. histolytica trophozoites, strain HMI:IMSS, were maintained in culture as described above. Chinese hamster ovary (CHO) cells obtained from the American Type Culture Collection were grown in F-12 medium (GIBCO) supplemented with 10% fetal bovine serum (GIBCO), penicillin (100 μg/ml), and streptomycin (100 μg/ml) as described previously (37).
Amebic galactose-inhibitable adherence studies were performed by using a rosetting assay as described previously (37). Axenic E. histolytica trophozoites (105/ml) were incubated at 4°C in serial dilutions (1.25, 2.5, 5.0, and 10%) of baboon sera or fecal supernates. Sera and feces were obtained from baboons 7 days after the final vaccination with the 1,600-μg dose of the peptide vaccine. Amebae incubated with galactose (0.05, 0.1, 0.2, and 0.4% dilutions) in RPMI-10% fetal bovine serum were used as a positive control for the adherence-inhibitory effects of galactose. Amebae in RPMI-10% fetal bovine serum only or with sera or fecal supernates from naïve, uninfected seronegative baboons served as negative controls. Trophozoites were washed, and 104 were added to CHO cells (2 × 105) in 1 ml of M199S. The mixture was centrifuged at 250 × g and incubated for 2 h at 4°C. After incubation, 0.8 ml of supernate was removed and the cellular pellet was suspended. The total number of amebae that formed or did not form rosettes with CHO cells (three or more adherent CHO cells per ameba) was determined in a hemocytometer chamber. The results were expressed as the percentage of all the amebae counted that formed ameba-CHO cell rosettes.
Statistical methods.
We used repeated-measure models to analyze the mean change in antibody response compared to the baseline, as determined by ELISA, induced by vaccination with one of three different doses of synthetic peptides or with purified LC3 recombinant protein. The effects of each vaccination regimen both over the entire follow-up period, as assessed with a type 3 F− test, and at the last follow-up, as assessed with a two-sample t test, were compared to those of a saline control. Degrees of freedom for the statistical tests were determined using the between-within method. An additional repeated-measure analysis using data for all three doses of synthetic peptides was used to perform a type 3 F− test of dose-dependent responses. The analyses described above were repeated for the nine different combinations of antigen (synthetic peptides, recombinant LC3, and native lectin) and antibodies (IgA and IgG in sera and IgA in feces) we investigated.
Our repeated-measure models accounted for correlated errors that may have arisen due to the longitudinal measurements made for each baboon at the baseline time point and at 7, 14, 21, and 28 days after the baseline time point. We used a first-order autoregressive model for the variation in the immune response of each baboon. Since immune responses to experimental vaccines were expected to be more variable than those to the saline control, our analysis estimates separate residual variance parameters for synthetic peptide, LC3, and saline vaccination groups. The models were fit to the data by restricted maximum likelihood using PROC MIXED in SAS version 9.1.
The proportions of trophozoites adhering to CHO cells under different experimental conditions were compared using chi-square tests. For all statistical analyses, P of <0.05 was considered statistically significant.
RESULTS
We screened a total of 131 baboons from the colony and found that 16% were positive for serum antilectin IgG antibodies, 6.5% were positive for serum antilectin IgA antibodies, and 9.9% were positive for intestinal antilectin IgA antibodies. None of the baboons screened by fecal PCR were infected with E. histolytica; however, 22% were infected with E. dispar. We found that antilectin intestinal IgA antibodies from baboons naturally infected in captivity recognized all of the LC3 peptide epitopes included in the vaccine (optical density [OD] readings were 2.76, 2.08, 0.393, and 0.494 for LC3 peptides 2, 9, 11, and 12, respectively). Baboons selected for the study were seronegative and were not infected with E. histolytica or E. dispar.
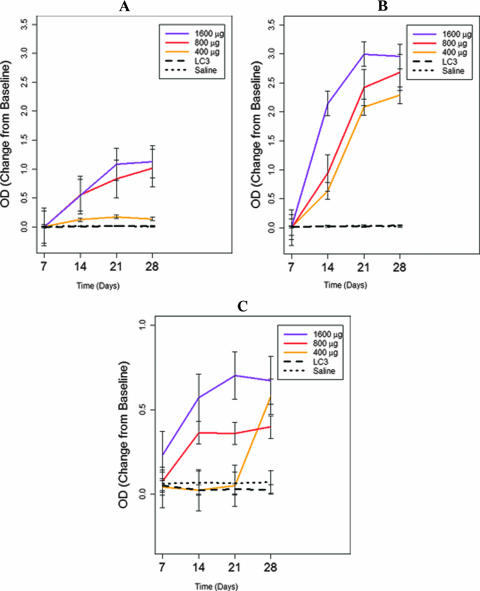
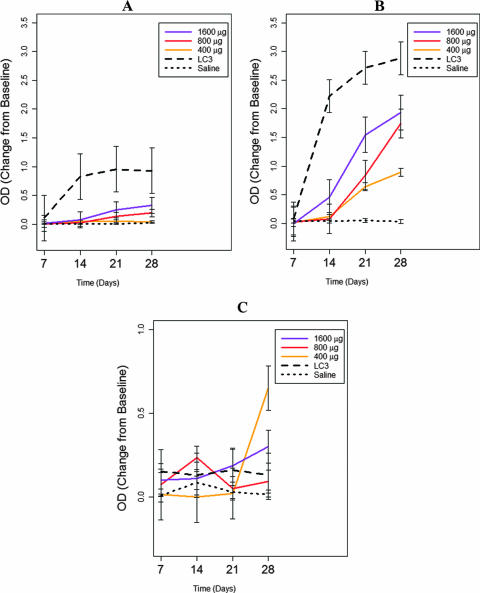
Antibody responses to the synthetic peptide and recombinant LC3 vaccines were examined by three separate ELISAs using either all four synthetic peptides, purified recombinant LC3 protein, or immunoaffinity chromatography-purified native lectin (obtained from solubilized trophozoites) as the capture antigen. We found that baboons immunized intranasally with the synthetic peptide vaccine demonstrated brisk serum antipeptide IgA, serum antipeptide IgG, and intestinal antipeptide IgA antibody responses in a dose-dependent fashion (P, <0.03 for each type of response compared to those in control baboons) (Fig. 1A, B, and C, respectively). Higher doses of the peptide vaccine (800 and 1,600 μg) elicited a more rapid (P < 0.02) and incrementally greater antibody response than the 400-μg dose (P, <0.003 for each antibody studied) (Fig. 1). Of interest, by day 28, 100% of the six baboons studied for each peptide vaccine dose (a total of 18 baboons) developed antipeptide serum IgA and serum IgG antibody responses (P, <0.01 for each group compared to controls); at the 800- and 1,600-μg doses, 100% of the baboons had intestinal antipeptide IgA antibody responses by 14 and 21 days after immunization (P, <0.01 compared to controls). The results of vaccination with purified recombinant LC3 protein are also illustrated in Fig. 1. Unexpectedly, the serum IgA and IgG antibodies elicited by the LC3 vaccine did not recognize the synthetic peptide antigen (Fig. 1). Using recombinant LC3 protein as the capture antigen in the ELISA, we found that the peptide vaccine elicited serum IgG antibodies to LC3 protein (P < 0.008) (Fig. 2B); antibody responses were maximal at 28 days and generally dose dependent (P, <0.001 compared to the baseline) (Fig. 2 B). Serum IgA antibodies elicited by the peptide vaccine had low levels of reactivity to LC3 protein (Fig. 2A). Intestinal IgA antibodies to LC3 protein were elicited after the fourth immunization with the 400- and 1,600-μg peptide doses (P < 0.02) (Fig. 2C). The recombinant LC3 vaccine induced high ODs indicating high levels of serum IgA and IgG antibodies to itself (P, <0.001 compared to controls) (Fig. 2A and B). However, no measurable intestinal IgA antibody response was elicited by the LC3 vaccine, even at 28 days (Fig. 2C) (P, <0.001 compared to the peptide vaccine and >0.5 compared to saline controls).
FIG. 1.
Effects of immunization of baboons with the polylysine-linked synthetic peptide vaccine (400, 800, or 1,600 μg), the recombinant LC3 protein vaccine (200 μg), or a control vaccine consisting of CT adjuvant (20 μg) alone as assessed by an ELISA using the synthetic peptides from the vaccine as the capture antigen. Compared to controls, immunized baboons exhibited serum antipeptide IgA (A) and IgG (B) responses over 4 weeks (P < 0.03) and at day 28 (P < 0.01). The serum antipeptide IgA and IgG antibody responses increased with the vaccine dose (P < 0.0001). (C) The synthetic peptide vaccine antigen at the 800- and 1,600-μg doses (P, <0.02 compared to the control), but not at the 400-μg dose (P = 0.26), induced an intestinal IgA antibody response to the peptide as measured over 4 weeks. However, at day 28, positive responses to all doses were observed (P, <0.02 compared to the control), and dose-dependent responses were observed over the 4 weeks (P = 0.004). The LC3 vaccine did not elicit any serum IgA (A) or IgG (B) antibodies to the peptide antigen (P = 0.80) compared to the control. The LC3 vaccine did not induce any intestinal antipeptide IgA antibody response (P = 0.46) (C).
FIG. 2.
Effects of immunization of baboons with the polylysine-linked synthetic peptide vaccine (400, 800, or 1,600 μg), the recombinant LC3 protein vaccine (200 μg), or the CT adjuvant (20 μg) alone as assessed by an ELISA using purified recombinant LC3 protein as the capture antigen. The peptide vaccine did not elicit a significant serum IgA antibody response (A) to LC3 antigen at any dose, over time or at 4 weeks (P > 0.233), but did elicit a substantial serum anti-LC3 IgG antibody response (B) at all doses (P < 0.001) and in a dose-dependent manner (P < 0.0001). The peptide vaccine at the 400- and 1,600-μg doses elicited an intestinal anti-LC3 IgA antibody response (C) after 4 weeks (P, 0.02 and 0.06 compared to the control, respectively). The LC3 vaccine elicited significant levels of serum anti-LC3 IgA (A) and IgG (B) antibodies over time and at 4 weeks (P, <0.001 compared to the control). The LC3 vaccine did not elicit any intestinal IgA antibody response (C) over 4 weeks or by the end of the study (P = 0.4).
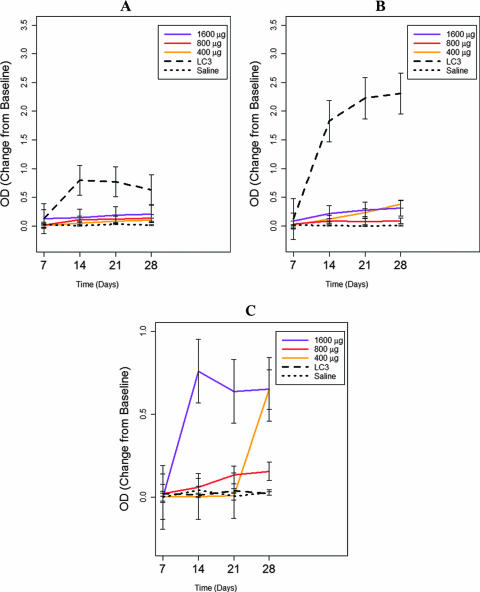
Lastly, we studied antibody responses using immunoaffinity chromatography-purified native lectin as the capture antigen in the ELISA. Serum IgA and IgG antibodies elicited by the peptide vaccine reacted with immunoaffinity chromatography-purified lectin, but at low levels as indicated by ODs (Fig. 3). However, the synthetic peptide at two doses (400 and 1,600 μg) elicited intestinal IgA antibodies in a dose-dependent manner (P = 0.03), and after four vaccinations, these antibodies demonstrated high levels of recognition of purified native lectin protein (P, <0.01 compared to those from control baboons at 28 days) (Fig. 3C). Lastly, vaccination with the recombinant LC3 protein elicited serum IgA and IgG antibodies to immunoaffinity chromatography-purified lectin protein at high levels as indicated by OD readings (P, <0.001 compared to the control) (Fig. 3A and B). However, no significant intestinal antilectin IgA antibody response was observed at 28 days after four intranasal vaccinations with LC3 protein (Fig. 3C) (P = 0.80).
FIG. 3.
Effects of intranasal immunization of baboons with the polylysine-linked synthetic peptide vaccine (400, 800, and 1,600 μg), the recombinant LC3 protein vaccine (200 μg), or CT adjuvant (20 μg) alone as assessed by an ELISA using immunoaffinity chromatography-purified galactose-inhibitable lectin as the capture antigen. The synthetic peptide vaccine did not elicit significant serum antilectin IgA antibodies (A) as determined by the ELISA (P > 0.11). There was a statistically significant but meager serum antilectin IgG antibody response (B) at the 1,600-μg dose (P, 0.003 over 4 weeks and 0.0004 at 4 weeks compared to the control). Of greatest interest, the synthetic peptide vaccine at the 400- and 1,600-μg doses elicited an intestinal antilectin IgA response (C) compared to the control after 4 weeks (P = 0.004). There was a positive dose-dependent response (P = 0.0002) and an increase in the ELISA OD reading over time (P = 0.002). The LC3 vaccine elicited substantial serum antilectin IgA (P = 0.02) (A) and IgG (P < 0.0002) (B) antibody responses over time and at 4 weeks. In contrast, the LC3 vaccine did not induce any intestinal antilectin IgA response (C) by 28 days (P = 0.80).
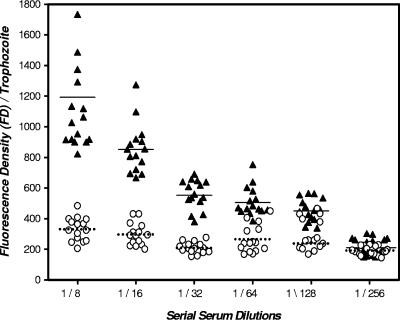
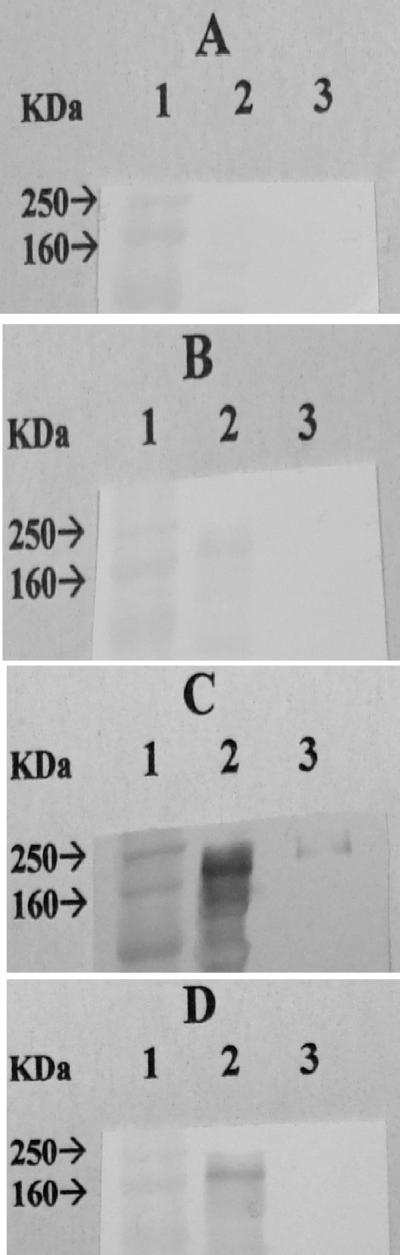
To determine whether the low level of recognition in the ELISA of immunoaffinity chromatography-purified lectin protein by peptide vaccine-elicited serum IgA and IgG antibodies compared to recombinant LC3 protein-elicited antibodies may have been due to the alteration of the protein epitopes during the detergent solubilization and protein purification process, we performed IFAs using intact axenic E. histolytica trophozoites. Serum IgG antibodies from peptide (1,600-μg-dose)-immunized baboons were reactive to trophozoite surface antigen at serial serum dilutions from 1/8 to 1/128 as evaluated by an IFA (P, >0.001 for each dilution compared to sera from control baboons) (Fig. 4). In addition, every baboon immunized with the peptide vaccine demonstrated a positive antilectin antibody response as determined by the IFA (P, >0.001 for the 1/8 dilution compared to the controls). To correlate with the ELISA and IFA results, we found by Western blot analysis that serum IgG antibodies from peptide-immunized baboons recognized the lectin 170-kDa heavy subunit to be present in whole axenic trophozoites better than in immunoaffinity chromatography-purified native lectin (Fig. 5C and D). As a positive control, immune human sera from ALA subjects demonstrated recognition of the 170-kDa lectin subunit in whole trophozoites and in the immunoaffinity chromatography-purified lectin protein (Fig. 5C and D). Control human and baboon sera were nonreactive (Fig. 5A and B).
FIG. 4.
Recognition of E. histolytica surface lectin by baboon serum IgG antibodies as assessed by an IFA following intranasal vaccination with the synthetic peptide vaccine (1,600-μg dose). Sera collected from vaccinated baboons (▴) at 28 days and prepared as serial dilutions from 1/8 to 1/128 demonstrated positive reactions to whole trophozoites as determined by an IFA (P, <0.001 for each dilution compared to control baboon sera [○]). (Means are represented by solid and dotted lines, respectively.) In addition, serial dilutions of immune baboon sera demonstrated a stepwise decrease in IFA-measured fluorescence intensity (P, <0.01 for 1/8 to 1/16 and 1/16 to 1/32). Importantly, 100% of study baboons receiving this vaccine dose had positive serum responses as determined by an IFA (P, <0.001 compared to control baboons). Control baboon sera did not show a positive IFA result at any dilution compared to results from IFA studies without serum present (data not shown).
FIG. 5.
Western blot analysis of responses of human and baboon control and immune sera to whole trophozoite protein and immunoaffinity chromatography-purified lectin. Lanes: 1, molecular mass standard; 2, whole amebic lysate; 3, immunoaffinity chromatography-purified protein lectin. Serum IgG antibodies from subjects cured of ALA recognized the lectin 170-kDa heavy subunit in both whole trophozoites (panel C, lane 2) and immunoaffinity chromatography-purified native lectin (panel C, lane 3) compared to control human sera (A). In contrast, serum IgG antibodies from peptide-immunized baboons recognized the 170-kDa lectin protein in whole trophozoites (panel D, lane 2) but under these experimental conditions were not reactive with immunoaffinity chromatography-purified native lectin (panel D, lane 3). Control baboon sera were entirely nonreactive (B).
To determine which of the peptides utilized in the vaccine were immunogenic, we preformed ELISA peptide dose analysis for intestinal IgA and serum IgA and IgG antibodies to each of the four peptides by using pooled sera and feces from baboons immunized at the 1,600-μg peptide dose (Table 1). We found that LC3 peptides 2, 9, and 12 elicited intestinal and serum IgA antibody responses; LC3 peptides 9, 11, and 12 elicited serum IgG antibody responses (Table 1).
TABLE 1.
Recognition of individual peptides by serum and intestinal antibodies from vaccinated baboonsa
| Antibody group | OD readingb for indicated LC3 peptide
|
|||
|---|---|---|---|---|
| 2 (aa 891-903) | 9 (aa 918-939) | 11 (aa 1114-1138) | 12 (aa 1128-1150) | |
| Fecal antipeptide IgA | 1.829 | 0.735 | 0.0 | 0.783 |
| Serum antipeptide IgA | 0.904 | 3.706 | 0.168 | 1.334 |
| Serum antipeptide IgG | 0.518 | 3.701 | 2.149 | 3.742 |
Serum and fecal samples from the six baboons immunized with the 1,600-μg dose of polylysine-linked synthetic peptides were pooled and studied in duplicate.
A positive result was defined as a reading ≥4-fold higher than the OD reading for control baboon sera. aa, amino acids.
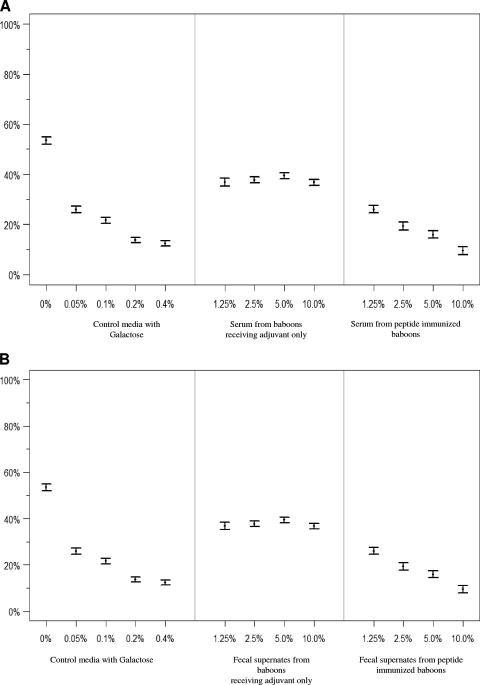
To determine whether serum and intestinal antilectin antibodies elicited by the peptide vaccine exhibited neutralizing activity against viable E. histolytica trophozoites, we studied galactose-specific adherence in vitro using the well-established ameba-CHO cell rosetting model (38). Trophozoites were incubated at 4°C with various dilutions of sera or fecal supernates, washed, and then pelleted with CHO cells to study adherence. A positive control with galactose and a negative control with test medium combined with sera or fecal supernates from control baboons (receiving adjuvant only) were studied. As expected, galactose exhibited a dose-dependent (0.05 to 0.4%) inhibition of trophozoite adherence to CHO cells compared to control media (P, <0.001 for each dilution) (Fig. 6). At each dilution, sera from peptide-immunized baboons inhibited trophozoite adherence to CHO cells compared to either control media (P, >0.0001) (Fig. 6) or control baboon sera (P, <0.001 for each dilution) (Fig. 6A). Increasing concentrations of immune sera, from 1.25 to 10%, demonstrated a stepwise enhancement of inhibition of amebic adherence to CHO cells (P, <0.03 for each dilution compared to the previous one) (Fig. 6A). Fecal supernates from baboons receiving adjuvant alone had some inhibitory effect on amebic adherence to CHO cells (P < 0.01) (Fig. 6B), but increasing concentrations of the supernates caused no change in the level of inhibition (P > 0.5) (Fig. 6B). Fecal supernates from peptide-immunized baboons exhibited greater inhibition of trophozoite adherence to CHO cells than supernate from control baboons (P, <0.001 at each dilution) (Fig. 6B). The inhibition of amebic adherence by fecal supernates from peptide-immunized baboons was also concentration dependent (P < 0.0004) (Fig. 6B). The results of these studies demonstrate that the synthetic peptide vaccine elicited serum and intestinal antibodies that recognized lectin molecules on the surfaces of viable E. histolytica trophozoites and that had substantial adherence-inhibitory activity in vitro, comparable to that of galactose monomers.
FIG. 6.
Inhibition of amebic galactose-specific adherence to CHO cells by sera (A) or fecal supernates (B) from peptide (1,600-μg-dose)-immunized or control baboons. Axenic E. histolytica trophozoites were incubated at 4°C with or without increasing concentrations of galactose, sera, or fecal supernates. As expected, increasing concentrations of galactose demonstrated a dose-dependent inhibition of ameba-CHO cell rosette formation (P, <0.01 for each concentration compared to control media). Sera from peptide-immunized baboons (P, <0.01 at each dilution) and sera from baboons receiving adjuvant only (P, <0.001 at each comparable dilution) demonstrated significant inhibition of amebic galactose-specific adherence compared to control media. The adherence-inhibitory effects of immune baboon sera were no different from those of galactose. Fecal supernates from peptide-immunized baboons inhibited amebic adherence to CHO cells compared to fecal supernates from baboons receiving CT adjuvant only (P, <0.01 at each dilution). The adherence-inhibitory effects of fecal supernates from immunized baboons were also concentration dependent (P, <0.01 for each dilution compared to the proceeding one) and comparable to the adherence-inhibitory effects of galactose.
DISCUSSION
In summary, we demonstrated that an intranasal synthetic peptide vaccine which includes the four LC3 epitopes recognized by IgA antibodies from immune human subjects, with CT as the adjuvant, was highly immunogenic in baboons as determined by ELISAs for peptide-specific serum IgA, serum IgG, and intestinal IgA antibodies. The peptide vaccine elicited intestinal IgA antibodies that reacted strongly with immunoaffinity chromatography-purified native lectin protein as demonstrated by ELISA; peptide vaccine-elicited serum IgA or IgG antibodies exhibited low levels of reactivity to LC3 or immunoaffinity chromatography-purified lectin. To further address the recognition of lectin molecules by peptide vaccine-elicited antibodies, we performed IFAs and Western blot analysis and studied in vitro amebic adherence to CHO cells. IFAs demonstrated serum IgG antibody recognition of lectin molecules on the surfaces of axenic trophozoites, and immunoblotting demonstrated the recognition of the lectin heavy-subunit protein present in amebic lysates. Therefore, the lower level of reactivity of peptide vaccine-elicited serum antibodies than of recombinant LC3 protein-elicited antibodies to purified lectin protein as evaluated by ELISAs may have been due to the alteration of the peptide epitopes on the native lectin molecule by the detergent solubilization and immunoaffinity chromatography purification process. The recombinant LC3 protein, as a positive control, was effective in eliciting serum IgG and IgA antibodies to itself and to purified native lectin protein. However, the recombinant LC3 vaccine did not elicit a mucosal immune response. In addition, the serum anti-LC3 IgG and IgA antibodies elicited by the LC3 vaccine did not recognize the four peptide epitopes recognized by serum and intestinal IgA antibodies from immune human subjects (2). Therefore, recombinant LC3 protein would not be suitable for use as an intranasal subunit vaccine to elicit a protective mucosal immune response. Importantly, the synthetic peptide vaccine elicited neutralizing serum and fecal antilectin antibodies as evidenced by a dose-dependent inhibitory effect on the galactose-specific in vitro adherence of viable E. histolytica trophozoites to CHO cells.
To our knowledge, this is the first experimental study of an intranasal synthetic peptide amebiasis subunit vaccine. Investigators from our laboratory were also the first to report that a purified E. histolytica protein, the galactose-inhibitable lectin, is highly efficacious in a gerbil model of ALA (33). Gerbils immunized with native lectin protein exhibited a protective efficacy of 70% against intrahepatic challenge with axenic trophozoites; however, a subset of vaccinated animals developed larger abscesses than controls (33). Concern that vaccination with purified lectin may induce adherence-enhancing antibodies (34) or be immunosuppressive were raised. Therefore, the need for a more defined amebiasis subunit vaccine became evident. We subsequently reported that purified recombinant LC3 protein under the same experimental conditions was equally effective in the gerbil ALA model (40) without any paradoxical worsening of disease in those immunized (40). The gerbil ALA model has been utilized for the study of numerous amebiasis subunit vaccines, including native and recombinant forms of the galactose-inhibitable lectin (34, 40), the EhADH112 protein (29), and recombinant Yersinia enterocolitica expressing hybrid type III proteins containing the cysteine-rich section of the lectin heavy subunit (26). A DNA vaccine expressing the serine-rich protein SREHP (45) and a 24-mer derived from the lectin heavy subunit chemically coupled to keyhole limpet hemocyanin (27) are also protective in experimental gerbil or hamster liver abscess models. However, immunity to experimental ALA is dependent on cellular rather than mucosal immune mechanisms (24), bypasses the natural gastrointestinal route of infection, and thus is not analogous to the study of asymptomatic intestinal infection in humans.
In a susceptible-mouse (C3H) model, native galactose-inhibitable lectin and, to a lesser extent, a 64-kDa fragment of the lectin heavy subunit with Freund's adjuvant provide protection against experimental cecal inoculation (22). Studies with resistant mouse strains indicated that neutrophil and innate immune mechanisms are protective (5). Passive transfer with antibodies to the amebic proteophosphoglycan, an abundant surface molecule, prevented colitis in human intestinal xenografts placed in SCID mice (46). As mice are not natural hosts of E. histolytica and have vaccine immunity mediated by secretory IgA-independent mechanisms and epitope recognition differing from that observed following human infection (2, 40), we chose to use a primate model.
Baboons get infected with E. histolytica and E. dispar in the wild and in captivity (23, 25, 42) and are susceptible to amebic colitis (25, 42). They have the same immunoglobulin subclasses as humans (39). Upon the screening of a large, asymptomatic baboon colony, we found that antilectin IgA antibodies from naturally infected baboons recognized the same four LC3 peptide epitopes as antibodies from seropositive humans, further confirming the suitability of baboons as an experimental vaccine host. The nasal route of vaccination offers many advantages, including easy access, lack of the need for sterile syringes, and the successful inducement of mucosal and systemic immune responses, and requires lower doses of antigen than the oral route (13, 31). Antigen-specific B and T lymphocytes migrate from nose-associated lymphoid tissues to regional lymph nodes and distant effector sites (31, 45). Intranasal vaccination is more effective than the intragastric route in generating a rapid, retained, and strong mucosal immune response (44); mucosal immune responses in the gastrointestinal tract do occur after intranasal vaccination (11).
Adjuvants known to be effective in animal models of intranasal vaccination include CT, the B subunit of CT (CTB), Escherichia coli heat-labile entertoxin, CTA1-DD (a protein produced by the fusion of the A1 subunit of CT [CTA1] with a staphylococcal protein A-derived protein that targets B cells), interleukin-10 [IL-10], and IL-12 (4, 11, 30). Microbially derived antigens that have been successful in experimental intranasal vaccines include E. coli outer membrane particles, the human papillomavirus, Mycobacterium bovis BCG, tetanus toxoid, the VP6 protein of rotavirus, influenza virus, schistosoma antigen, and human immunodeficiency virus, among others (11, 18, 30). CT is a very effective mucosal adjuvant for vaccines administered via the intranasal route (18, 31) due to the induction of CD4+ T helper cells secreting IL-4 and IL-5; these TH2 cells support the development of systemic IgG1 and IgG2b subclasses and mucosal secretory IgA responses (28). However, when delivered to mice, CT and CTB accumulate in the olfactory nerves and the olfactory bulb (17). This accumulation is due to the binding of CTB to gangliosides and the direct neural connections of the olfactory nerves to the olfactory bulb, raising the possibility of nervous tissue damage (9). CTA1-DD holds promise as a genetically engineered B-cell-specific adjuvant that should avoid such toxicity, as it does not traffic to the olfactory bulb (15). Therefore, intranasal vaccination appears promising as a safe method for the induction of protective mucosal immunity with the minimum use of vaccine antigen.
In summary, based on investigations of antilectin mucosal immunity in prospective field studies (1, 36), followed by IgA epitope mapping (2), we designed a polylysine-linked synthetic peptide amebiasis subunit vaccine. When delivered intranasally to baboons with CT as the adjuvant, this peptide vaccine was successful in eliciting high levels of neutralizing intestinal antilectin IgA antibodies. Studies to develop a reproducible baboon model of asymptomatic E. histolytica intestinal infection so as to determine vaccine efficacy are in progress. In addition, the use of other biomaterials and adjuvants to enhance the immunogenicity and safety of this subunit peptide vaccine will be necessary before conducting studies with humans.
Acknowledgments
This research was supported by NIAID grants U01-AI 35840 to Jonathan I. Ravdin and PO1-AI 36359 to Michael Lamm (principal investigator) for work on a project led by Jonathan I. Ravdin. The Baboon Resource Center is supported by NIH Baboon Research Resource Program grant P40 RR12317 to Gary L. White.
We thank Yvette Massey for expert secretarial assistance.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 25 May 2007.
REFERENCES
- 1.Abd-Alla, M. D., T. F. G. H. Jackson, T. Rogers, S. Reddy, and J. I. Ravdin. 2006. Mucosal immunity to asymptomatic Entamoeba histolytica and Entamoeba dispar infection is associated with peak intestinal anti-lectin immunoglobulin A antibody response. Infect. Immun. 74:3897-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abd-Alla, M. D., T. F. G. H. Jackson, G. C. Soong, M. Mazinac, and J. I. Ravdin. 2004. Identification of the Entamoeba histolytica galactose-inhibitable lectin epitopes recognized by human immunoglobulin A antibodies following cure of amebic liver abscess. Infect. Immun. 72:3974-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abou-el-Magd, I., C. J. Soong, A. M. El-Hawey, and J. I. Ravdin. 1996. Humoral and mucosal IgA antibody response to a recombinant 52-kDa cysteine-rich portion of the Entamoeba histolytica galactose-inhibitable lectin correlates with detection of native 170-kDa lectin antigen in serum of patients with amebic colitis. J. Infect. Dis. 174:157-162. [DOI] [PubMed] [Google Scholar]
- 4.Agren, L. C., L. Ekman, B. Lowenadler, J. G. Nedrud, and N. Y. Lycke. 1999. Adjuvanticity of the cholera toxin A1-based gene fusion protein, CTA1-DD, is critically dependent on the ADP-ribosyltransferase and Ig-binding activity. Immunology 162:2432-2440. [PubMed] [Google Scholar]
- 5.Asgharpour, A., C. Gilchrist, D. Baba, S Hamano, and E. Houpt. 2005. Resistance to intestinal Entamoeba histolytica infection is conferred by innate immunity and Gr-1+ cells. Infect. Immun. 73:4522-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atherton, E., H. Fox, C. Harkiss, R. C. Sheppard, and J. Williams. 1978. A mild procedure for solid phase peptide synthesis: use of fluorenylmethoxy carbonyl amino acids. J. Chem. Soc. Chem. Commun. 13:537-543. [Google Scholar]
- 7.Beving, D. E., C. J. Soong, and J. I. Ravdin. 1996. Oral immunization with a recombinant cysteine-rich section of the Entamoeba histolytica galactose-inhibitable lectin elicits an intestinal secretory immunoglobulin A response that has in vitro adherence inhibition activity. Infect. Immun. 64:1473-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blessmann, J., L. Van, P. Nu, H. Thi, B. Myhsok, H. Buss, and E. Tannich. 2002. Epidemiology of amebiasis in a region of high incidence of amebic liver abscess in central Vietnam. Am. J. Trop. Med. Hyg. 66:578-583. [DOI] [PubMed] [Google Scholar]
- 9.Bourguignon, P., M. Bisteau, J. Abarca, S. Veenstra, P. Hermand, V. Henderickx, et al. 1999. Reactogenicity in the nose and the brain of enterotoxins administered intranasally to mice, abstr. 23. In Proceedings of the Cold Spring Harbor Conference on Molecular Approaches to Vaccine Design. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 10.Chadee, K., W. A. Petri, Jr., D. J. Innes, and J. I. Ravdin. 1987. Rat and human colonic mucins bind to and inhibit the adherence lectin of Entamoeba histolytica. J. Clin. Investig. 80:1245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi, A. H., M. M. McNeal, J. A. Flint, M. Basu, N. Y. Lycke, J. D. Clements, J. A. Bean, H. L. Davis, M. J. McCluskie, J. L VanCott, and R. L. Ward. 2002. The level of protection against rotavirus shedding in mice following immunization with a chimeric VP6 protein is dependent on the route and the coadministered adjuvant. Vaccine 20:1733-1740. [DOI] [PubMed] [Google Scholar]
- 12.Cleland, W. W. 1964. Dithiothreitol, a new protective reagent for SH groups. Biochemistry 35:480-482. [DOI] [PubMed] [Google Scholar]
- 13.Davis, S. S. 2001. Nasal vaccines. Adv. Drug Delivery Rev. 51:21-42. [DOI] [PubMed] [Google Scholar]
- 14.Diamond, L. S., D. R. Harlow, and C. C. Cunnick. 1978. A new medium for the axenic cultivation of Entamoeba histolytica. Trans. R. Soc. Trop. Med. Hyg. 72:431-432. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson, A. M., K. M. Schon, and N. Y. Lycke. 2004. The cholera toxin-derived CTA1-DD vaccine adjuvant administered intranasally does not cause inflammation or accumulate in the nervous tissues. J. Immunol. 173:3310-3319. [DOI] [PubMed] [Google Scholar]
- 16.Frederick, J. R., and W. A. Petri, Jr. 2005. Roles for the galactose-/N-acetylgalactosamine-binding lectin of Entamoeba in parasite virulence and differentiation. Glycobiology 15(12):53R-59R. [DOI] [PubMed] [Google Scholar]
- 17.Fujihashi, K., T. Koga, F. W. van Ginkel, Y. Hagiwara, and J. R. McGhee. 2002. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine 20:2431-2438. [DOI] [PubMed] [Google Scholar]
- 18.Gluck, U., J. Gebbers, and R. Gluck. 1999. Phase 1 evaluation of intranasal virosomal influenza vaccine with and without Escherichia coli heat-labile toxin in adult volunteers. J. Virol. 73:7780-7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haque, R., A. S. G. Faruque, P. Hahn, D. M. Lyerly, and W. A. Petri, Jr. 1997. Entamoeba histolytica and Entamoeba dispar infection in children in Bangladesh. J. Infect. Dis. 175:734-736. [DOI] [PubMed] [Google Scholar]
- 20.Haque, R., I. M. Ali, R. B. Sack, B. M. Farr, G. Ramakrishnan, and W. A. Petri, Jr. 2001. Amebiasis and mucosal IgA antibody against Entamoeba histolytica adherence lectin in Bangladeshi children. J. Infect. Dis. 183:1787-1793. [DOI] [PubMed] [Google Scholar]
- 21.Haque, R., P. Duggal, I. M. Ali, M. B. Hossain, D. Mondal, R. B. Sack, B. M. Farr, T. H. Beaty, and W. A. Petri, Jr. 2002. Innate and acquired resistance to amebiasis in Bangladeshi children. J. Infect. Dis. 186:547-552. [DOI] [PubMed] [Google Scholar]
- 22.Houpt, E., L. Barroso, L. Lockhardt, R. Wright, C. Cramer, D. Lyerly, and W. A. Petri, Jr. 2004. Prevention of intestinal amebiasis by vaccination with the Entamoeba histolytica Gal/GalNac lectin. Vaccine 22:611-617. [DOI] [PubMed] [Google Scholar]
- 23.Jackson, T. F. G. H., P. G. Sargeaunt, P. S. Visser, V. Gathiram, S. Suparsad, and C. B. Anderson. 1990. Entamoeba histolytica: naturally occurring infections in baboons. Arch. Investig. Med. 21(Suppl. 1):153-156. [PubMed] [Google Scholar]
- 24.Ivory, C. P., K. Keller, and K. Chadee. 2006. CpG-oligodeoxynucleotide is a potent adjuvant with an Entamoeba histolytica Gal-inhibitable lectin vaccine against amebic liver abscess in gerbils. Infect. Immn. 74:528-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legesse, M., and B. Erko. 2004. Zoonotic intestinal parasites in Papio anubis (baboon) and Cercopithecus aethiops (vervet) from four localities in Ethiopia. Acta Trop. 90:231-236. [DOI] [PubMed] [Google Scholar]
- 26.Lotter, H., H. Russman, J. Heesemann, and E. Tannich. 2004. Oral vaccination with recombinant Yersinia enterocolitica expressing hybrid type III proteins protects gerbils from amebic liver abscess. Infect. Immun. 72:7318-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lotter, H., T. Zhang, K. B. Seydel, S. L. Stanley, Jr., and E. Tannich. 1997. Identification of an epitope on the Entamoeba histolytica 170-kD lectin conferring antibody-mediated protection against invasive amebiasis. J. Exp. Med. 185:1793-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marinaro, M., H. F. Staats, T. Hiroi, R. J. Jackson, M. Coste, P. N. Boyaka, et al. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 155:4621-4629. [PubMed] [Google Scholar]
- 29.Martinez-Lopez, C., E. Orozco, T. Sanchez, R. M. Garcia-Perez, F. Hernandez, and M. A. Rodriguez. 2004. The EhADH112 recombinant polypeptide inhibits cell destruction and liver abscess formation by Entamoeba histolytica trophozoites. Cell. Microbiol. 6:367-376. [DOI] [PubMed] [Google Scholar]
- 30.McGhee, J. R., and W. Strober. 2005. Mucosal immunology, 3rd ed., p. 371-373. Elsevier Academic Press, London, United Kingdom.
- 31.Mestecky, J., Z. Moldoveanu, S. M. Michalek, C. D. Morrow, R. W. Compans, D. P. Schafer, and M. W. Russell. 1997. Current options for vaccine delivery systems by mucosal routes. J. Control. Release 48:243-257. [Google Scholar]
- 32.Petri, W. A., Jr., R. Smith, P. Schlesinger, and J. I. Ravdin. 1987. Isolation of galactose-binding lectin which mediates the in vitro adherence of Entamoeba histolytica. J. Clin. Investig. 80:1238-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petri, W. A., Jr., and J. I. Ravdin. 1991. Protection of gerbils from amebic liver abscess by immunization with the galactose-specific adherence lectin of Entamoeba histolytica. Infect. Immun. 59:97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petri, W. A., Jr., T. L. Snodgrass, T. F. G. H. Jackson, et al. 1990. Monoclonal antibodies directed against the galactose-binding lectin of Entamoeba histolytica enhance adherence. J. Immunol. 144:4803-4809. [PubMed] [Google Scholar]
- 35.Ravdin, J. I., and W. Stauffer. 2005. Entamoeba histolytica (amebiasis), p. 3097-3111. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 6th ed. Churchill Livingstone, Inc., New York, NY.
- 36.Ravdin, J. I., M. D. Abd-Alla, S. L. Welles, S. Reddy, and T. F. G. H. Jackson. 2003. Intestinal antilectin immunoglobulin A antibody response and immunity to Entamoeba dispar infection following cure of amebic liver abscess. Infect. Immun. 71:6899-6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravdin, J. I., and R. L. Guerrant. 1981. Role of adherence in cytopathogenic mechanism of Entamoeba histolytica. J. Clin. Investig. 68:1305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravdin, J. I., T. F. G. H. Jackson, W. A. Petri, Jr., C. F. Murphy, B. L. Ungar, V. Gathiram, J. Skilogiannis, and A. E. Simjee. 1990. Association of serum antibodies to adherence lectin with invasive amebiasis and asymptomatic infection with pathogenic Entamoeba histolytica. J. Infect. Dis. 162:768-772. [DOI] [PubMed] [Google Scholar]
- 39.Shearer, M. H., R. D. Dark, J. Chodosh, and R. C. Kennedy. 1999. Comparison and characterization of immunoglobulin G subclasses among primate species. Clin. Diagn. Lab. Immunol. 6:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soong, G., K. Kain, M. Abd-Alla, T. F. G. H. Jackson, and J. I. Ravdin. 1995. A recombinant cysteine-rich section of Entamoeba histolytica galactose-inhibitable lectin is efficacious as a subunit vaccine in the gerbil model of amebic liver abscess. J. Infect. Dis. 171:645-651. [DOI] [PubMed] [Google Scholar]
- 41.Strachan, W. C., W. M. Spice, P. L. Chiodini, A. H. Moody, and J. P. Ackers. 1988. Immunological differentiation of pathogenic and non-pathogenic isolates of Entamoeba histolytica. Lancet i:561-563. [DOI] [PubMed] [Google Scholar]
- 42.Verweij, J. J., J. Vermeer, E. A. Brienen, C. Blotkamp, D. Laeijendecker, L. van Lieshout, and A. M. Polderman. 2003. Entamoeba histolytica infections in captive primates. Parasitol. Res. 90:100-103. [DOI] [PubMed] [Google Scholar]
- 43.Walsh, J. A. 1988. Prevalence of Entamoeba histolytica infection, p. 93-105. In J. I. Ravdin (ed.), Amebiasis: human infection by Entamoeba histolytica. Churchill Livingstone, New York, NY.
- 44.Wu, H. Y., and M. W. Russell. 1997. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol. Res. 16:187-201. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, T., E. Li, and S. L. Stanley, Jr. 1995. Protection of gerbils from amebic liver abscess by immunization with a recombinant Entamoeba histolytica antigen. Infect. Immun. 62:225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, Z., M. Duchêne, and S. L. Stanley, Jr. 2002. A monoclonal antibody to the amebic lipophosphoglycan-proteophosphoglycan antigens can prevent disease in human intestinal xenografts infected with Entamoeba histolytica. Infect. Immun. 70:5873-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]