Abstract
The typically recovered quantity of nontypeable Haemophilus influenzae (NTHi) bacteria in an ex vivo middle ear (ME) aspirate from the chinchilla model of experimental otitis media is insufficient for direct analysis of gene expression by microarray or of lipopolysaccharide glycoforms by mass spectrometry. This prompted us to investigate a strategy of multiple consecutive lavage samplings to increase ex vivo bacterial recovery. As multiple consecutive lavage samples significantly increased the total number of bacterial CFU collected during nasopharyngeal colonization or ME infection, this led us to evaluate whether bacteria sequentially acquired from consecutive lavages were similar. Comparative observation of complete ex vivo sample series by microscopy initially revealed ME inflammatory fluid consisting solely of planktonic-phase NTHi. In contrast, subsequent lavage samplings of the same infected ear revealed the existence of bacteria in two additional growth states, filamentous and biofilm encased. Gene expression analysis of such ex vivo samples was in accord with different bacterial growth phases in sequential lavage specimens. The existence of morphologically distinct NTHi subpopulations with varying levels of gene expression indicates that the pooling of specimens requires caution until methods for their separation are developed. This study based on multiple consecutive lavages is consistent with prior reports that NTHi forms a biofilm in vivo, describes the means to directly acquire ex vivo biofilm samples without sacrificing the animal, and has broad applicability for a study of mucosal infections. Moreover, this approach revealed that the actual burden of bacteria in experimental otitis media is significantly greater than was previously reported. Such findings may have direct implications for antibiotic treatment and vaccine development against NTHi.
As a major childhood illness, acute otitis media (AOM) is a primary reason for physician visits (about 24 million per year in the United States) and accounts for a substantial proportion of antibiotic use in general practice (45). Children experience, on average, two or more episodes of AOM by age 2 (73), with sequelae including impaired hearing (45) and delayed language development (64, 65).
The pathogenesis of AOM initially involves viral respiratory tract infection resulting in Eustachian tube dysfunction, permitting the ascension of otopathogens from the colonized nasopharynx into the middle ear (ME) (55). Streptococcus pneumoniae (∼50%), nontypeable Haemophilus influenzae (NTHi) (∼30%), and Moraxella catarrhalis (∼20%) are the three respiratory pathogens typically recovered from the MEs of children with AOM (46). With the introduction of the pneumococcal conjugate vaccine in 2000 (21), the number of AOM episodes involving vaccine-type S. pneumoniae decreased, while disease due to pneumococcal nonvaccine serotypes as well as to NTHi increased. NTHi has since emerged as the most common otopathogen in children aged 7 to 24 months failing initial antimicrobial therapy (7, 12).
Immunoprophylaxis remains the major strategy for reducing the burden of AOM in children and preventing subsequent morbidities. The decline in vaccine serotype-related episodes of AOM following the introduction of a heptavalent pneumococcal conjugate vaccine confirms the potential for disease prevention but also demonstrates the significance of replacement disease due to nonvaccine serotypes as well as NTHi (25). More recently, a pneumococcal otitis efficacy trial study demonstrated a reduction of 30% in AOM due to NTHi by using a vaccine formulation consisting of a decavalent pneumococcal vaccine conjugated to NTHi outer membrane protein D (63). This study (POET) suggests that targeting both S. pneumoniae and H. influenzae has the potential to increase the impact on AOM observed with a single-pathogen vaccine.
The development of vaccines for the prevention of AOM has proven more challenging than the development of vaccines against invasive disease. First, invasive disease is caused by few capsular serotypes of S. pneumoniae and H. influenzae relative to the large number of pneumococcal serotypes and NTHi strains causing AOM (32-35). In addition, there is the challenge of identifying noncapsular surface-exposed, immunogenic targets expressed during nasopharyngeal (NP) colonization and ME infection as conserved across species-wide diversity.
Genetic approaches, such as microarray analysis, offer the capacity to characterize gene expression in vivo. Successful comprehensive analysis at this level would provide critical insight into particular surface antigens and, thus, candidate vaccine targets associated with infection. For OM, this requires evaluation of bacterial gene expression during both NP colonization and ME infection. To date, efforts have been limited by the quantity of bacteria collected in vivo from these sites, providing insufficient yields of bacterial mRNA for direct analysis by microarray. To overcome this limitation, we investigated a strategy of multiple consecutive lavage samplings for increased recovery of bacteria from both the nasopharynx and the ME. The characterization of resultant ME lavage sample contents provided a new perspective on the burden of bacteria and their growth states found during NTHi infection, with implications for the pooling of specimens as well as for treatment and vaccine development.
MATERIALS AND METHODS
Bacterial strains.
Two nontypeable, i.e., capsule-negative, H. influenzae strains were used for animal inoculation, the genomically sequenced strain Rd KW20, an unencapsulated isolate derived from a type d parent strain (1, 2, 28, 79), and clinical strain 375, which was collected from a pediatric patient with documented OM (36). These strains were chosen to be evolutionarily divergent from our extensive, phylogenetically organized collection of about 600 independent H. influenzae isolates (Fig. 1). Our unpublished studies show that strain Rd is virulent at a low inoculum, reaches a density in the ME of 107 to 108 CFU/ml, and displays the same duration of ME infection as the NTHi 375 clinical isolate. Unlike disease due to encapsulated haemophilus, all animals survive Rd infection without evidence of sepsis.
FIG. 1.
Neighbor-joining phylogenetic dendrogram of ∼600 typeable and nontypeable strains of H. influenzae (10). Details of the dendrogram and its validation have been published previously (8, 13, 27). NTHi strains used for animal inoculation in this report are indicated with black arrows. (Reprinted from reference 10 with permission of the publisher.)
Animal model.
Procedures were carried out in accordance with the guidelines of the Boston University Medical Center Institutional Animal Care and Use Committee. Chinchillas (Chinchilla laniger) with no prior evidence of ME infection as determined by either otoscopy or tympanometry were used for this study.
NP colonization studies were carried out with NTHi strain 375 and data gathered during four independent studies involving identical care and housing conditions and with a total of 20 animals. Studies were composed of (i) four groups of four animals, (ii) one group of two animals, and (iii) two studies involving a single animal. In addition, a study involving four animals was carried out to evaluate the natural history of experimental otitis media (EOM) due to strain Rd.
ME infection studies were carried out with both NTHi strains 375 and Rd and involved a total of 22 animals. First, a comparative study between NTHi strains 375 and Rd, involving four and five animals in each group, respectively, was carried out. In addition, three studies involving (i) two groups of four animals, (ii) two groups of two animals, and (iii) a single animal were carried out with strain 375.
Animals were inoculated with bacteria either nasopharyngeally or directly into the ME cavity. For NP inoculation, bacteria were grown on chocolate agar plates (Remel) in a CO2-enriched atmosphere at 37°C. Bacteria were harvested from plates and resuspended in Hanks’ balanced salt solution (HBSS; GIBCO) to an optical density at 600 nm of 1.6 arbitrary units. Each nare was inoculated with 100 μl of this suspension, corresponding to a total bacterial inoculum of ∼108 CFU per animal. For ME inoculation, bacteria were grown to mid-log phase (optical density at 600 nm of 0.1 arbitrary units) in supplemented brain heart infusion broth (DIFCO) (10). Following serial dilutions in HBSS, less than 100 CFU in a final volume of 100 μl was injected through the superior bulla of each ear.
Direct fluid and lavage sample collection.
Prior to sampling, animals were assessed for signs of tympanic membrane inflammation by otoscopy and tympanometry. Both NP and ME samples were collected under anesthesia. Animals were sampled up to days 16 and 20 postinoculation in the nasopharynx or ME, respectively, until the samples were culture negative.
Ex vivo NP samples were collected by lavage with 1 ml HBSS flushed through one nare, with collection of effluent from the opposite nare. ME sample collection involved a small hole in the bullar bone, providing access to the ME. ME inflammatory fluid, when present, was collected by direct aspiration of the cavity. Lavages were carried out by flushing the ME cavity with 0.5 ml HBSS, followed by aspiration. Volumes acquired from the NP lavages ranged from 300 μl to 900 μl, while direct ME fluid aspirates ranged from 50 μl to 300 μl and ME lavage samples ranged from 200 μl to 500 μl. Multiple consecutive lavage samplings were collected from both the NP and the ME cavities, as described above, at intervals of less than 1 min between lavages. A maximum of 20 and 10 consecutive lavage samplings were collected from the nasopharynx and ME, respectively. Following collection, lavage samplings were briefly vortexed and a 15-μl aliquot was diluted in series in HBSS while the remainder of the sample was immediately processed for RNA extraction on site (within less than 1 min of sample collection). Bacterial titers were determined by colony counts from serial dilutions plated onto chocolate agar.
RNA isolation.
Total RNA from ex vivo samples was isolated using TRIzol LS (Invitrogen) and precipitated in the presence of 20 μg glycogen (Invitrogen), with the manufacturer's protocol modified to a 20-min incubation with glycogen and isopropyl alcohol and a 20-min subsequent centrifugation to increase RNA yield. RNA preparations were purified and DNase treated on QIAGEN RNeasy columns. Purified total RNA samples were resuspended in diethyl pyrocarbonate-treated, nuclease-free water (Invitrogen), aliquoted, and stored at −80°C. No RNA-stabilizing reagent was used as this proved more adverse than helpful. The presence and integrity of bacterial RNA extracted with this procedure were examined by Northern blot analysis (data not shown).
Quantitative RT-PCR.
The relative gene expression of ex vivo NTHi 375 ME samples obtained by multiple consecutive lavages of a single animal versus the expression of the in vitro-grown inoculum was assessed by quantitative reverse transcription-PCR (qRT-PCR) using the SuperScript III Platinum SYBR green one-step qRT-PCR kit (Invitrogen). Gene-specific mRNA was reverse transcribed and amplified using a SmartCycler II (Cepheid) with cycles of 59 to 60°C for 30 min and 95°C for 2 min, followed by 50 cycles at 95°C for 15 s, primer-specific annealing at the temperatures indicated in Table 1 for 30 s, and 72°C for 30 s, and the process was ended by a melting curve analysis from 60°C to 95°C with a ramping of 0.2°C/s. Primer (Table 1) specificity to target genes was evaluated by BLASTn analysis (4). All samples were analyzed in triplicate with parallel controls to verify the absence of DNA in the RNA preparation as well as the absence of primer dimers in control samples lacking template RNA. RT-PCR products were also analyzed using 2% agarose gel electrophoresis, and in all cases, a single product was observed at the expected base pair size, as estimated relative to a ready-load, 100-bp DNA ladder (Invitrogen). Amounts of bacterial RNA in different samples were normalized relative to the expression of gyrase (gyrA), as gyrA has been shown to be constitutively expressed by NTHi under various in vitro growth conditions (52). The calculation of relative expression ratios was carried out with the relative expression software tool-multiple condition solver (REST-MCS; http://rest.gene-quantification.info) using the pairwise fixed reallocation randomization test (58, 59).
TABLE 1.
qRT-PCR gene-specific primers
| Gene | Rd gene no. | Full name | Primer sequences | Taa (°C) | Product length (bp) |
|---|---|---|---|---|---|
| gyrA | HI1264 | DNA gyrase subunit A | 5′-ACTGCTGGCGGTATTGTG-3′, 5′-CGCGACCTTGTGATGAGAAC-3′ | 63 | 149 |
| hemR | HI0113 | Hemin receptor | 5′-GTGACGCTGAGGATGTTAGAGTG-3′, 5′-GCCACCATTACCATATTGAGGAG-3′ | 63 | 149 |
| frdB | HI0834 | Fumarate dehydrogenase subunit B | 5′-GTAACGAAGCACCAGCATTAG-3′, 5′-ACCACAGTTGATACACATTGAG-3′ | 60 | 125 |
| recA | HI0600 | Recombinase A | 5′-GGATTGGTGGTTTGCCTATG-3′, 5′-AATGACGGAAAGAGTTAATGTTG-3′ | 59 | 86 |
Ta, optimum annealing temperature, determined experimentally via temperature gradient.
Microscopy.
Ex vivo samples of NTHi strains Rd and 375 were observed by microscopy immediately following collection. Samples were examined either as recovered or following centrifugation at 10,000 × g for 10 min at 4°C and resuspension in 1/10 their original volume in HBSS. Antibody staining was carried out on NTHi Rd samples divided into two aliquots, collected by centrifugation at 10,000 × g for 10 min at 4°C, and resuspended in 100 μl phosphate-buffered saline, pH 7.4, 1% bovine serum albumin. Each aliquot was then incubated for 30 min at 4°C with a 1:100 dilution either of ascitic fluid containing 3.4 mg/ml of monoclonal antibody (MAb) D2 against NTHi lipopolysaccharide (LPS) structure Galα1-4Galβ1-4Glcβ1-2HepIII or of negative-control Shigella flexneri LPS MAb SYAJ (at 1.3 mg/ml) against the Rha1-3GlcNAc1-2Rha LPS epitope (Maine Biotechnology). Following incubation with the primary MAb, samples were washed with phosphate-buffered saline, pH 7.4, 1% bovine serum albumin and incubated for 20 min at 4°C with a 1:100 dilution of anti-mouse immunoglobulin G conjugated to fluorescein isothiocyanate (Sigma). NTHi Rd samples were also stained for double-stranded DNA (dsDNA) with Hoechst 33342 (Molecular Probes) at a final concentration of 0.35 μg/μl for 20 min. All samples were examined with an Axioskop 2 Plus Zeiss microscope using an α Plan-Fluar 100×/1.45 oil lens with Nomarski optics and under UV light with appropriate filters for fluorescein isothiocyanate and Hoechst 33342 fluorescence. Images were acquired using the AxioCam HRm and the AxioVs40, version 4.5.0.0, software (Carl Zeiss Imaging Solutions). All primary micrographs include a 10-μm size bar for comparative purposes. While samples of both NTHi strains Rd and 375 were observed under Nomarski optics microscopy, antibody and dsDNA staining assays were carried out on only NTHi Rd samples because of limited access to fluorescence microscopy (see Acknowledgments).
Statistical analysis.
In order to examine the effect of multiple consecutive lavages on CFU titer recovery (bacterial yield), a dependent variable was constructed to measure the difference in the log10 numbers of CFU for each of lavages 2 through 20 (with the majority measured through lavage 10 only) compared to lavage 1 for samples from the nasopharynx and lavages 2 through 10 for samples from the ME on each day of study per animal. These data being correlated serially within each animal, mixed linear models (23) were used to test the effect of multiple lavages and assess possible additional effects of other factors. These models have the advantage over standard analytic procedures for repeated measures, such as repeated-measures analysis of variance, in that they allow for the inclusion of unequal numbers of repeated observations per subject and can incorporate time-dependent information on predictive factors (independent variables) as well as complex time-related correlation structures in the data (for example, here an autoregressive [AR1] structure on the repeated log10 CFU values over time was assumed). Lavage number was treated as a grouping factor in the mixed linear models in order to allow for nonlinear relationships between the change from lavage 1 and subsequent lavages to be identified, e.g., lavage 1 to lavage 2, lavage 1 to lavage 3, etc. With respect to additional factors examined, the day postinoculation was tested in the models for both the nasopharynx and the ME data. These were modeled in a piecewise form, with separate slopes for intervals of days 1 to 4 and days 5 to 16 in the nasopharynx data and for intervals of days 1 to 5 and days 6 to 20 in the ME data. In each model, the interaction of lavage number and each of the other factors was formally tested for significance in order to rule out more complex relationships of lavage number with the dependent variable, change in log10 CFU from lavage 1. Differences in the model-based estimates of the log10 CFU change from lavage 1 for lavages subsequent to lavage 2 were compared and statistically tested to those for lavage 2 in analyses planned a priori. These statistical tests were interpreted as statistically significant or not after applying a Bonferroni correction (9, 42) to account for multiple-hypothesis testing. In the nasopharynx data, 18 such post hoc tests (i.e., lavage 3 versus 2, lavage 4 versus 2, and so on through lavage 20 versus 2) were performed, resulting in an adjusted significance criterion of a P value of 0.003 (0.05/18). In the model for the ME data, additional factors tested included the disease phase (early, peak, and late phases of the disease course), body side (left or right ear), and strain (375 or Rd) and eight post hoc tests (i.e., lavage 3 versus 2, lavage 4 versus 2, and so on to lavage 10 versus 2) were performed, resulting in an adjusted significance criterion of a P value of 0.006 (0.05/8).
RESULTS
Course of NP colonization and ME infection in the chinchilla model of EOM.
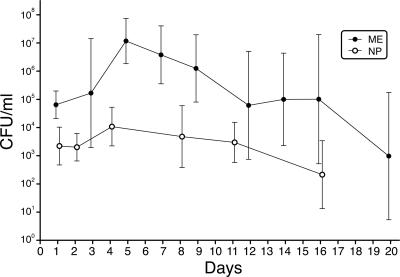
We determined bacterial sample titers (mean ± standard deviation CFU per milliliter) in the nasopharynx and ME in the chinchilla model of EOM over the course of disease, as shown in Fig. 2. Bacterial samples collected from the nasopharynges of 20 animals over 16 days showed a variation in mean titer from 103 CFU/ml 24 and 48 h postinoculation (2.2 × 103 ± 4.7 × 103 CFU/ml and 2.0 × 103 ± 3.1 × 103 CFU/ml, respectively) to 104 CFU/ml on day 4 (1.1 × 104 ± 4.9 × 104 CFU/ml), with titers then progressively decreasing until no bacteria were detectable in lavage samples (beginning day 16; lower detection limit of 102 CFU/ml). ME bacterial samples collected from 22 animals over the course of 20 days ranged in mean titer from about 105 CFU/ml during days 1 to 3, defined as the initial phase of the infection (day 1, 6.4 × 104 ± 3.1 × 104 CFU/ml; day 3, 1.7 × 105 ± 8.6 × 101 CFU/ml), to 107 CFU/ml (day 5, 1.2 × 107 ± 6.4 × 107 CFU/ml) when inflammatory fluid initially appeared between days 4 and 6 (defined as peak of infection). Bacterial titers steadily decreased thereafter until below the threshold of detection (beginning around day 20). Neither colonization nor infection resolved synchronously in all animals, resulting in greater variability in colony counts among animals later in the course of the disease, when some animals remained culture positive while others had resolved infection.
FIG. 2.
Dynamics of NP colonization and ME infection in CFU per milliliter following NP and ME inoculation with NTHi strain 375, over the course of disease (in days) in the chinchilla model of EOM. Mean titers and standard deviations were determined from 20 animals sampled over 16 days in the nasopharynx and from 22 animals sampled over 20 days in the ME. Error bars indicate standard deviations.
Multiple consecutive lavage samplings in the chinchilla model of EOM.
As demonstrated in Fig. 2, even the best CFU yields from a single sample at a single time point (day 4, with 104 CFU/ml in the nasopharynx, and day 5, with 107 CFU/ml in the ME) were not sufficient to provide the minimum quantity of mRNA currently required for direct microarray gene expression analysis or of LPS required for consistent mass spectrometry glycoform analysis (∼108 bacteria). To investigate whether more bacteria were present in vivo than in a single inflammatory fluid sample, as typically employed with the chinchilla model of EOM (5, 10, 52, 60, 66, 74, 81), we initially carried out two consecutive lavages of the nasopharynx as well as the ME and compared the titers recovered. Statistical analysis of samples from the two consecutive lavages from either site did not reveal significant differences (P was 0.8717 for the nasopharynx and 0.0817 for the ME). This observation prompted us to increase the number of consecutive lavages carried out on a single animal at each time point (up to 20 and 10 lavages in the nasopharynx and ME, respectively). An example of one such multiple consecutive lavage series collected on day 5 postinoculation in the nasopharynx and in the ME is displayed in Fig. 3A.
FIG. 3.
Titers of NTHi strain 375 (CFU/ml) collected by multiple consecutive samplings of the nasopharynx and of the ME. (A) Consecutive lavage samplings collected on day 5 postinoculation following NP or ME inoculation, respectively. Lavage samplings were numbered in the order of collection from 1 to 20 in the nasopharynx and 1 to 10 in the ME, with sample 0 being the initial, direct fluid sample collected from the ME cavity. (B) Lavage titer mean variation within consecutive sample series collected throughout the course of colonization. Consecutive lavage samplings were numbered in the order of collection from 1 to n, and their log10-transformed titers were compared to that of the first lavage sampling of the corresponding series. Titers are expressed as the mean difference to the baseline sample titer (lavage 1). The mean titer difference (y axis) between each lavage sampling and its baseline titer was averaged from series collected in different animals throughout the course of the disease, and the standard deviation (SD) (error bars) was calculated. The main data curves represent lavage titer variations within a series, while SD bars represent across-series titer variation. Reference lines at one log10 (y = −1.0), half a log10 (y = −0.5), and null (y = 0) mean variations are displayed parallel to the x axis.
Consecutive lavages, referred to as lavage series when collected in a single animal and on the same day, were numbered 1 through n in the order of collection. The nasopharynges or MEs of animals were sampled by multiple consecutive lavages on multiple days throughout the course of the studies. Log10-transformed titers of lavages 2 through n were compared to that of the first lavage of their respective series, and titers were expressed as the difference to the baseline log10-transformed titer of lavage 1 (Fig. 3B).
We evaluated the multiple consecutive lavage sampling method as a means to recover more ex vivo bacterial cells from both the nasopharynx and the ME of the chinchilla EOM model by analyzing consecutive lavage titer variation within NP and ME lavage series. Overall, consecutive lavage titers were within, on average, a log10 of the baseline titer both in the nasopharynx and in the ME. NP consecutive lavage titers were less variable within a series than those of ME lavages (Fig. 3B), but NP series were more variable across animals and days than ME series were (Fig. 3B), primarily due to the difference in the numbers of lavages in each data set (n = 167 for the nasopharynx; n = 361 for the ME).
NP lavage data.
The degree of differences in the log10 CFU changes from lavage 1 in the NP data can be seen in the nonmodel-based means plotted in Fig. 3B. To statistically evaluate changes over days 1 through 16, we analyzed 167 repeated observations on 20 animals in mixed linear models. The factors examined in these models included lavage number (2 through 20) and day following inoculation (intervals of days 1 to 4 and 5 to 16). A preliminary model examining the interactions among these factors found none to be statistically significant (P > 0.05). Thus, the final model included the above-mentioned factors only as main effects. This model was statistically significant (P < 0.0001), with lavage number (P = 0.02) and day for the interval of day 1 to day 4 (P = 0.02) as significant factors. In post hoc contrasts unadjusted for multiple testing but controlling for the study day, the log10 CFU change from lavage 1 for each of lavages 8 through 20 was significantly different from the change from lavage 1 to lavage 2, ranging from a P value of 0.046 for lavages 9 versus 2 to a P value of <0.0001 for lavage 19. After adjusting for multiple testing via the Bonferroni correction (9, 42), the contrasts for lavages 12 versus 2 (i.e., lavage 12-lavage 1 versus lavage 2-lavage 1; P = 0.002), 14 versus 2 (P = 0.0003), 15 versus 2 (P = 0.0004), 17 versus 2 (P = 0.0009), 19 versus 2 (P < 0.0001), and 20 versus 2 (P = 0.0025) were statistically significant. Based on this model, we found that the changes from lavage 1 for lavages 3 through 20 were each significantly different from zero, while the change from lavage 1 for lavage 2 was not.
ME lavage data.
The change in log10-transformed CFU from lavage 1 in the ME data can also be seen in the nonmodel-based means plotted in Fig. 3B. This depicts the number of CFU per milliliter determined by serial dilutions on chocolate agar plates and does not consider bacteria enmeshed in biofilm fragments subsequently revealed during the Nomarski optics microscopy studies described below. To statistically evaluate changes in the data obtained from the ME over days 1 through 20, we analyzed 361 repeated observations on 22 animals in mixed linear models. The interactions between the factors examined in these models, including lavage number (2 through 10), sampling day (interval, days 1 to 5 or 6 to 20), disease phase (early, peak or late), bacterial strain (Rd or 375), and sampling site (left or right ear), were not statistically significant (P > 0.05). Thus, the final model included the above-mentioned factors only as main effects. This model was statistically significant (P < 0.0001) and had lavage number as its sole significant factor (P < 0.0001). In post hoc contrasts unadjusted for multiple testing but controlling for the other factors in the model (day intervals, disease phase, bacterial strain, and body side), the change in log10 CFU from lavage 1 for each of lavages 3 through 10 was significantly different from the change from lavage 1 to lavage 2. These comparisons were statistically significant after adjusting for multiple testing via the Bonferroni correction (9, 42). Based on this model, we found that the changes from lavage 1 for lavages 8 through 10 were each significantly different from zero, but the changes from lavage 1 for lavages 2 through 7 were not.
The number of actual lavages and how they might affect the course of disease were considered in the development of the multiple consecutive lavage sampling method. We limited the number of lavages to 20 in the nasopharynx and 10 in the ME because of increased local trauma to the mucosal surfaces. A larger number of animals developed ruptures of the tympanic membrane during the multiple-lavage procedure in the ME than during fluid collection only. Within the limits of the number of lavages we carried out in this study, we did not observe a difference in the average durations of culture-positive disease or bacterial titers recovered from the ME between control animals sampled only later in the course of the study without prior handling and animals undergoing periodic multiple lavages (data not shown).
Gene expression profiles of NTHi acquired through a multiple consecutive lavage series of the ME.
Uncertainty as to the homogeneity of the bacterial populations consecutively acquired by lavages led us to examine the nature of the in vivo bacterial population. We initially assessed expression levels of four genes representing different functional categories. A sample series was collected from the ME of an NTHi 375-inoculated animal upon initial appearance of fluid on day 6 after bacterial inoculation and was composed of the initial inflammatory fluid sample and 10 consecutive lavage samples. Total mRNA levels of fumarate dehydrogenase B (frdB), recombinase A (recA), hemin receptor (hemR), and DNA gyrase A (gyrA) were analyzed in the in vitro inoculum, ex vivo initial ME fluid, and subsequent lavages 1, 3, 5, 7, and 9 and were normalized relative to gyrA. The selection of a constitutively expressed gene with continuous, equivalent transcriptional levels in conditions as different as rich in vitro culture medium and in vivo inflamed ME environment was challenging. We chose gyrA, for which expression was reported to be constitutive under a spectrum of in vitro growth conditions (52) (K. Mason, personal communication), including our in vitro inoculum growth conditions. The relative gene expression levels in ex vivo samples were compared to that of the in vitro inoculum. As depicted in Fig. 4, levels of frdB, recA, and hemR were all down-regulated relative to their levels in the mid-log phase-grown inoculum. A difference in the general gene expression patterns was observed between the inflammatory fluid and samples from (i) lavages 1 through 3 (early lavages) and (ii) lavages 5 through 9 (late lavages). In the fluid and early lavage samples, the expression levels of the three genes analyzed were decreased in general compared to levels under in vitro growth conditions. In late lavage samples, frdB and recA showed a further down-regulation, while hemR expression was undetectable. Specifically, frdB was down-regulated ∼14-fold in the inflammatory fluid, ∼25-fold in lavage 1, and between ∼80- to 150-fold in lavages 3 through 9. recA was down-regulated ∼3-fold in the initial fluid sample and lavage 1, ∼14-fold in lavage 3, and ∼100- to 200-fold in lavages 5 through 9. hemR was minimally down-regulated in the inflammatory fluid and lavage 1, was down-regulated about threefold in lavage 3, and was no longer detectable in subsequent lavages. Considering the general pattern of down-regulation of the three genes assessed, it is possible that gyrA was differentially expressed as well. If gyrA was down-regulated in any in vivo samples compared to the in vitro reference, the levels of repression of frdB, recA, and hemR would be underestimated using this methodology.
FIG. 4.
Relative gene expression ratios in NTHi 375 ex vivo initial inflammation fluid and subsequent multiple consecutive lavage samples. The amounts of frdB, recA, and hemR mRNA were normalized against those of the gyrA genes used here as a reference, and relative ratios of expression were determined compared to exponentially grown in vitro inoculum conditions. Error bars indicate standard deviations.
Direct analysis of ME consecutive sample series using Nomarski optics microscopy.
NTHi biofilm formation in the chinchilla ME has been reported for fixed specimens of dissected bullar mucosa from a similar EOM animal model (24, 39, 61). To determine whether such biofilm was directly recovered by multiple consecutive lavage samplings, we used microscopy to examine the initial fluid sample as well as each consecutive lavage sample from the MEs of infected animals. Nomarski optics microscopy (56) was employed to avoid artifacts caused by drying, fixing, embedding, cryo-freezing, and/or staining, as may occur with sample preparation. In contrast, Nomarski optics based on differential interference contrast (DIC) allows for direct visualization of structures in fluid and lavage samples as they are recovered.
Microscopy was carried out on both NTHi Rd and 375 mid-log phase and plate-grown inocula as in vitro controls ( Fig. 5A) and on ex vivo inflammatory fluid from the ME and each of 10 consecutive lavage samples collected on days 5, 8, and 10 postinoculation. Ex vivo NTHi Rd and 375 samples from each of these series revealed similar findings. Neutrophils and red blood cells as well as planktonic bacteria were observed in the initial inflammatory fluid samples (Fig. 5B). Consecutive ME lavage samples were likewise found to contain neutrophils and red blood cells, although significantly fewer than the initial fluid samples, with the numbers decreasing with subsequent lavages. Bacteria in the planktonic phase were also observed. What samples from lavages 1 through 5 uniquely contained, i.e., what was never present in initial fluid samples, were various sizes of mucoid-appearing structures, typically amorphous and much larger than red blood cells and neutrophils. These structures often span the entire expanse of the microscopic field (Fig. 6A, B, and C). Samples from later lavages (typically beyond lavage 6) also contained such amorphous structures that tended to be less mucoid and were often smaller than those seen in samples from earlier lavages. Many structures with minimally mucoid areas appeared to be composed of bacterial cells organized in a “rippling pattern” (Fig. 7A and B) analogous to that reported for Myxococcus xanthus biofilms (67). In addition, large fragments seemingly denuded of mucous overlay were found mainly in the latter-most lavage samples. On close examination, these appeared to be a matrix composed of densely packed arrays of bacterial cells (Fig. 7C). Naïve, uninfected control animals had no ME inflammatory fluid, and no structures as described above were seen in any of 10 consecutive ME lavage samples examined (data not shown).
FIG. 5.
Micrograph galleries of control H. influenzae Rd in vitro culture and typical ex vivo ME inflammatory fluid. (A) An HBSS-resuspended, plate-grown in vitro sample was stained with NTHi LPS MAb D2 and Hoechst DNA dye. Microscopy examination of the unfixed sample was carried out with Nomarski optics (i.e., DIC [top left panel]). D2 monoclonal antibody (top right panel) and dsDNA staining (bottom left panel) were visualized under UV light with appropriate filters. Specific NTHi LPS structure and dsDNA localization were revealed by overlay of fluorescence upon the DIC frame (bottom right panel), with DNA localized to every bacterial cell and D2 antibody localized mainly to aggregated NTHi cells. (B) Ex vivo ME inflammation fluid stained with Hoechst DNA dye and visualized with Nomarski optics (top panel) and under UV light (center panel). An overlay of DIC and fluorescence is presented in the bottom panel, with planktonic bacteria denoted by white arrows. Scale bars, 10 μm. While fluorescent assays were not carried out with NTHi 375 samples because of a time constraint with microscopy equipment, observations made with ex vivo samples were similar between the two strains.
FIG. 6.
Micrographs and through focus gallery of mucoid biofilm structures observed in early lavage samples (1 to 5). (A and B) NTHi Rd mucoid biofilm structures collected in lavage 4 of infected ME were visualized with Nomarski optics. (C) Through focus of biofilm structure in panel B, revealing channels and rippling patterns. Scale bars, 10 μm. Similar biofilm fragment structures were observed in NTHi 375 ex vivo early lavage samples.
FIG. 7.
Nomarski optics micrographs of partially mucoid and denuded biofilm structures observed in late lavage samples (6 to 10). (A and B) NTHi 375 lavage 7 samples. Minimal mucoid dsDNA overlay allows for visualization of “rippling pattern” of an organized bacterial community (67). (C) NTHi 375 lavage 9 sample. Denuded biofilm structure photographed using differential contrast. A complete absence of mucoid overlay allows for visualization of dense bacterial packing as well as typical biofilm channels. Scale bars, 10 μm. Similar biofilm fragment structures were observed in NTHi Rd ex vivo late lavage samples.
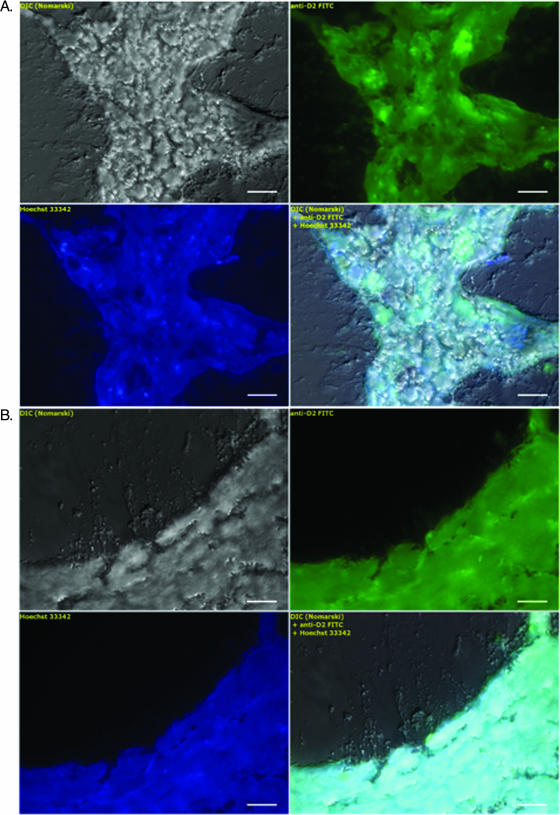
Any putative biofilm containing NTHi would be expected to incorporate the dsDNA-binding Hoechst stain. This minor groove-binding dye, however, would stain dsDNA derived from host tissue as well. In order to specifically identify NTHi in ME lavage samples, we used NTHi LPS MAb D2. Both stains colocalized to in vitro control NTHi Rd cells. While the Hoechst stain was incorporated by all bacteria, the D2 antibody predominantly bound to the surface of aggregated NTHi Rd cells (Fig. 5A). Ex vivo ME inflammatory fluid samples showed that neither D2 nor Hoechst stain interacted with red blood cells, while Hoechst stain appeared solely in the nuclei of neutrophils (Fig. 5B). To determine whether NTHi was present within the mucoid structures in ME lavage samples 1 through 5, we stained samples with Hoechst and MAb D2. Over 100 such putative fragments consistent with biofilm structures of various morphologies were examined. Micrograph galleries in Fig. 8 display typical results of the analysis, revealing the colocalization of both dsDNA (Hoechst blue positive) and NTHi LPS (MAb D2 green positive) (Fig. 8A and B). As a negative control for MAb D2, we used Shigella LPS Y MAb with secondary antibody identical to that used for D2 antibody, which did not show any binding to in vitro-grown NTHi bacterial cells or putative biofilm fragments (data not shown). Because of the time constraint for use of the loaner fluorescence microscope, Nomarski microscopy was carried out with both NTHi strains Rd and 375; however, DNA and antibody staining was carried out only on Rd samples because fluorescence microscopy was not available for subsequent assays on the 375 samples. Thus, the Rd samples were those selected to illustrate the fluorescence assays of recovered fragments consistent with biofilm structures. Such biofilm structures recovered by consecutive lavages were morphologically similar for both strains.
FIG. 8.
Micrograph galleries of mucoid biofilm structures observed in early lavage samples. Specimen fields are identical to those shown in Fig. 6A and B observed with Nomarski optics (top left panels) and are here stained with NTHi LPS MAb D2 (top right panels) and Hoechst DNA dye (bottom left panels). An overlay of DIC and fluorescence is presented in the bottom right panels, with both specific NTHi LPS structure and DNA colocalizing over the entire mucoid structure at variable intensities. Scale bars, 10 μm.
Not present in initial fluid samples, though likewise exclusively found in ME lavage samples, was a third NTHi growth state, segmented, filamentous bacterial cells (Fig. 9A) equivalent in number to the more typical, rod-shaped planktonic NTHi cells (Fig. 5B). These filamentous forms are 5- to 15-fold longer (5 to 15 μm) than expected for exponentially growing NTHi cells. dsDNA-binding Hoechst stain was found to be localized with the filamentous growth forms (Fig. 9B). No such filamentous bacteria were found among lavage samples from ME cavities of uninfected control animals, nor were they recovered from the NP lavage samples of inoculated animals. As expected for a segmented filamentous phenotype incapable of cell division (47), attempts to culture ME lavage samples containing this filamentous phenotype yielded only NTHi of the usual coccobacillary morphology when plated on agar medium.
FIG. 9.
Micrograph and gallery of filamentous NTHi Rd collected by ME lavage, visualized with Nomarski optics. (A) Bundle of filamentous bacterial cells collected by lavage 9 of an infected ME. (B) Top panel, DIC; middle panel, Hoechst DNA dye; bottom panel, DIC overlay with Hoechst fluorescence colocalizing with each filamentous bacterial cell. Scale bars, 10 μm. A filamentous phenotype was also observed in NTHi 375 ex vivo lavage samples.
As the natural course of OM begins with NP colonization, followed by ascension to the ME, we performed an analogous examination of consecutive NP lavage samples to determine whether similar biofilm structures and filamentous forms were resolved in NP-inoculated animals. While both NTHi bacteria in the planktonic phase and chinchilla epithelial cells could be readily seen in these NP lavage samples, no biofilm-like structure or segmented filamentous NTHi cells analogous to those resolved in ME lavage samples were observed (data not shown).
DISCUSSION
Nontypeable H. influenzae is frequently recovered from children with recurrent OM. Numerous studies support the causative role of NTHi in the recurrence of AOM (41, 43, 49). Barenkamp et al. (6) provided further insight by characterizing isolates from ME fluid aspirates of 30 children with recurrent AOM due to NTHi and found that early recurrent disease was usually a result of relapse with the initial infecting NTHi strain, while late recurrent disease was usually the result of infection with a different strain.
As hypothesized by Post and Ehrlich (62), a potential explanation for this propensity of NTHi to cause relapsing disease could be its organization into the biofilm-like structures. Results presented here involving direct isolation of NTHi ME biofilm support the proposed role while providing additional factors likely contributing to early recurrent disease, e.g., a previously unrecognized burden level of planktonic NTHi in the ME as well as the coexistence of NTHi in a static filamentous state.
Multiple consecutive lavage samplings reveal a larger in vivo bacterial population while providing a more representative sample.
From the chinchilla model of EOM, the typical titer of available ME bacterial sample found in inflammatory fluid at the peak of infection (usually around day 5) reached about 107 CFU/ml. Likewise, in the nasopharynx, a single lavage yielded titers of about 104 CFU/ml 5 days postinoculation (Fig. 2). These values translated to total viable bacterial yields of ∼106 and ∼103 CFU for the ME and nasopharynx, respectively. The amount of bacterial mRNA or membrane LPS that can be obtained from such a single sample currently precludes microarray or consistent mass spectrometry analyses on direct samples. An alternate strategy to increase sample quantity by combining samples from multiple animals has significant limitations as animals may not reach the same stage of disease concurrently, leaving it unclear as to whether samples collected on the same day represent the same stage of infection. This limitation of bacterial sample size from the chinchilla model of EOM has constrained genetic analysis of ex vivo NTHi samples to assays such as RT-PCR (52), thus precluding critical genome-wide analyses of expression patterns over the course of colonization and infection.
As described in Results, the ability to acquire NTHi consistently throughout 20 NP and 10 ME consecutive lavages (Fig. 3) demonstrated that a single sample underestimates the burden of infection, as considerably more bacteria are in fact present both in the nasopharynx and in the ME cavity, involved in colonization and infection, respectively. For the ME, this nonetheless represents a minimum burden, as bacteria enmeshed in the biofilm fragments revealed by DIC microscopy are not quantifiable by routine culture and therefore the true bacterial density is yet greater than that identified by multiple consecutive lavages. Our results also suggest that a limited number of consecutive lavages can be performed without significant alteration of the duration of culture-positive status of the animal and infectious load. As such, pooling consecutive lavage samples might be considered an effective means to increase sample size from a single animal at a single time point for specific hypothesis testing, but consideration of the heterogeneity of the bacterial population recovered in each lavage sample would dictate whether such a strategy is appropriate. Whether pooled or not, consecutive lavages provide a more representative sample of the in vivo bacterial population as a whole. This sampling method showed no significant statistical difference in the consistencies of consecutive lavage titers recovered within ME series between the two NTHi strains used in our studies (P = 0.91) and yielded results comparable to those reported for strains Rd and 375 with a third phylogenically divergent clinical isolate (NTHi 486) (Fig. 1) collected from a pediatric patient with documented OM (36), suggesting that the method is applicable to a wide array of NTHi isolates.
Analysis of ex vivo bacterial populations recovered by consecutive lavages.
Our studies reveal the existence of significantly more NTHi bacteria in the nasopharynx and ME than previously observed with the chinchilla model of EOM. Similarity or differences as to both gene expression profile and growth phase among the bacteria collected throughout a lavage series would determine whether the pooling of consecutive lavage samples would be appropriate to test a given hypothesis.
To address this question at the level of gene expression, relative levels were determined by qRT-PCR for NTHi 375 in the exponentially grown in vitro inoculum and for a consecutive series of ex vivo samples acquired on day 6 postinoculation, including initial inflammatory fluid plus samples from ME lavages 1, 3, 5, 7 and 9. Four genes representing different functional categories were selected for this analysis: (i) frdB, encoding one of the two catalytic units of the four-subunit fumarate reductase, a terminal electron acceptor under anaerobic growth conditions (15, 37); (ii) recA, encoding a catalyzer of homologous recombination, a regulatory protein and a required component of the SOS response to DNA damage (20, 48); (iii) hemR, encoding one of the TonB-dependent hemin receptors/transporters localized on the outer membrane, involved in scavenging of heme (29, 72), a required growth factor for H. influenzae (26); and (iv) gyrA, encoding one of the two subunits of the four-subunit DNA gyrase involved in DNA supercoiling and replication (70), used here as a reference for relative quantification as carried out previously for ex vivo NTHi acquired from the chinchilla model of EOM (52).
Relative expression levels for these ex vivo samples revealed that frdB, recA, and hemR were down-regulated compared to levels for the exponentially in vitro-grown NTHi inoculum. We observed differences in the levels of down-regulation of each gene among the fluid and lavage samples, with the greatest decrease in expression occurring in lavages 5 to 9 (Fig. 4), highlighting the considerable heterogeneity among bacteria in the ME. This result may be related to the recovery of bacteria in biofilms and/or the filamentous growth phase seen in later lavage samples by DIC microscopy, as discussed below. Such down-regulation of gene expression suggests that consecutive ME samples acquired at a single time point might be composed of NTHi bacteria in different metabolic states, indicative of different growth phases, limiting the potential for pooling consecutively acquired samples to specific hypotheses examining the entire population of bacteria within the ME.
We investigated whether differential gene expression correlated with differential growth phases by direct microscopy of consecutive samples. Many species of pathogenic bacteria form biofilms during the course of infection (17-19), and biofilm produced by NTHi has been observed by microscopy, both when grown in vitro (31, 39, 53, 71) and when grown in situ in the ME of the chinchilla model of EOM (24, 39, 61). The results from our microscopy studies of NTHi biofilm recovered by ME consecutive lavages are in accord with and expand on the latter in situ characterization of dissected fixed bulla mucosa.
Multiple consecutive lavages generated ex vivo samples immediately available for microscopy analysis. This allowed us to compare ME inflammatory fluid samples with subsequent lavage samples containing fragments of biofilm-like structures from the same ME cavity. Our observations revealed that inflammatory fluid consisting of NTHi bacteria solely in planktonic phase represents only a small proportion of the in vivo bacterial population and is devoid of the biofilm-like fragments and the filamentous growth phase observed in subsequent lavage samples. As expected, NTHi bacteria in the planktonic phase appear to be metabolically more active (based on our qRT-PCR results) than bacteria obtained by later lavages. Whether the NTHi RNA isolated from these later lavages was derived from biofilm-encased and/or planktonic bacteria released from the biofilm remains to be determined.
Based upon insights from microscopy, multiple consecutive lavage CFU counts, and qRT-PCR, we can conclude that (i) the initial ME fluid sample that is typically characterized is not broadly representative of the total bacterial population involved in ME infection; (ii) multiple consecutive lavages provide a more accurate picture of the bacterial burden and populations in the ME, with potential implications for antibiotic treatment and vaccine development; and (iii) heterogeneity of bacteria (planktonic, filamentous, and biofilm encased) recovered from the different lavage samples restricts the use of pooling to investigation of the bacterial population as a whole until methods are developed to separate the distinct growth phases.
The developed multiple-lavage method also made possible the partial dissection of biofilm structures as consecutively recovered from the ME without sacrificing the animal. The use of Nomarski optics microscopy allowed for direct visualization of ex vivo samples as collected in lavage with no fixation, embedding, or cryopreservation, methods prone to artifacts. While the earliest lavage samples in an ME series contained large, amorphous expanses of NTHi biofilm with seemingly dense mucoid dsDNA and LPS overlay (Fig. 6 and 8), later lavages from the ME cavity yielded biofilm fragments with part or all of the mucoid overlay apparently washed off the surface (Fig. 7). Here, we identify several different patterns of bacterial cell-to-cell packing. As seen in Fig. 7A and B, where only minimal amounts of mucous cover remain, we observed dense aggregates similar to that seen with the gram-negative delta-proteobacterium Myxococcus xanthus, a pattern referred to as “rippling,” with ridge-like aggregates of bacterial cells surrounded by troughs of low density (67), a well-characterized phenomenon based on cell-surface-associated signaling for the case of M. xanthus (38, 44, 75). As seen in Fig. 7C, fragments of biofilm apparently devoid of dsDNA overlay were typically found in later lavage samples. In addition to what appeared to be densely packed arrays of bacteria, crevices similar to channels previously described for biofilm structures can be seen (68). The colocalization of Hoechst dsDNA-binding stain and NTHi LPS MAb D2 with biofilm recovered by ME lavages (Fig. 8) as well as a total absence of biofilm in multiple consecutive ME lavage samples of an uninfected animal control and NP lavage samples from infected animals strongly supports our findings as an integral feature of ME infection by NTHi. Whether the double-stranded DNA associated with the observed mucoid biofilm matrix (Fig. 8) might be released from NTHi, as has been described for biofilms of Pseudomonas aeruginosa (3), or from polymorphonuclear and epithelial host cells (Fig. 5) remains to be clarified. Whatever the source, observations depicted in Fig. 6 and 8 versus that in Fig. 7 indicate that unfixed, native NTHi ME biofilm extracted directly from the ME consists at least in part of a dense overlay of mucoid dsDNA and LPS, a common feature of gram-negative bacterial biofilms (22), beneath which there exists a complex matrix containing vast organized arrays of bacteria. Whether in this case the shroud of mucoid DNA shelters bacteria hidden underneath from detrimental aspects of the host defense system remains to be determined. As well, the discovery of both the rippling patterns of NTHi cells (Fig. 7A and B) and the organized arrays of NTHi bacteria (Fig. 7C) raises the possibility of sophisticated social motility and multicellular developmental processes analogous to that found for environmentally resistant M. xanthus (38, 44, 67, 75).
A number of other important questions remain unanswered in the interpretation of the results described above, particularly when considering both the extraordinary complexity of the native, ex vivo NTHi biofilm revealed by Nomarski optics microscopy (Fig. 6 and 7) and the known protective effect of such a biofilm matrix against antibodies (69), white blood cells (57, 77), antimicrobials (54), surfactants (30), and toxics in general (50). From this perspective we need to determine (i) the efficacy of the phenol-chloroform RNA extraction procedure on biofilm-encased NTHi for future studies involving qRT-PCR and microarray analyses of gene expression in vivo and (ii) whether CFU counts of the multiple-lavage samples (Fig. 3) accurately reflect the total bacterial load in the ME or solely represent those in the planktonic state. The unexpectedly high ME bacterial load revealed by multiple consecutive lavages would nonetheless represent the minimum number of bacteria present in vivo (16).
Unexpectedly, in the lavage samples from otherwise sterile ME cavities inoculated with NTHi, we also observed segmented, filamentous bacterial cells (Fig. 9) similar to those reported for H. influenzae following exposure to trimethoprim-sulfamethoxazole (80). Precedent exists for such an association of filamentous bacterial growth forms with established biofilm during the course of disease, as found in vivo for biofilm-forming uropathogenic Escherichia coli (40). Depletion of nutrients needed for cell division (e.g., thymine starvation [14]) as well as the down-regulation of genes involved with septum formation (e.g., homologs to the fitZ family) can prevent cell division, thus giving rise to filamentation (for a recent review, see reference 47). The regulatory basis for the appearance of filamentous forms of NTHi we observed and their possible role during ME infection are to be explored in future studies.
Clinical implications.
Our findings reveal a significantly higher bacterial burden in ME disease than previously estimated by a single sampling of ME inflammation fluid. This may have implications in the outcome of antibiotic treatment. For example, beta-lactam antibiotics may not be as effective in the presence of such higher bacterial density, a phenomenon referred to as the inoculum effect (78). Equally relevant, the presence of biofilms containing bacteria that are less metabolically active may also impact outcomes of treatment with beta-lactam drugs that are most potent against rapidly dividing organisms (11, 51, 76). As well, the morphological transition of certain gram-negative bacteria to the filamentous phase has been attributed to the down-regulation of expression of select penicillin-binding proteins that are important for septum formation and cell division and is accompanied by a concomitant resistance to cephalosporins (47). While it remains to be characterized, it is possible that the filamentous forms of NTHi that we have identified (Fig. 9) may confer a survival advantage to the bacteria, including resistance to such antibiotics.
Bacteria that dwell in biofilms are also likely to be refractory to clearance by direct bactericidal activity and opsonophagocytosis (57, 69, 77) and may be responsible for recalcitrant infections and disease relapses. When considered together, these findings suggest that (i) antimicrobial therapy capable of killing bacterial pathogens in all states of metabolic activity and growth should be evaluated to determine whether the outcome of AOM can be improved and (ii) vaccines for prevention of AOM would be most effective when directed against NTHi found in the nasopharynx or very early-stage ME infection, i.e., prior to development of biofilms.
Acknowledgments
Studies were supported in part by NIH NIDCD research grant awards DC04583, DC05564, and DC005855 to R.G. Chinchilla studies were funded in part by a research grant to S.I.P. from The Shereta R. Seelig Charitable Foundation Trust. S.R. was supported in part by NIH award AI054544.
We gratefully acknowledge Thomas M. Johnson and Bracie Watson, NIDCD Program Administrators, for encouragement, enthusiasm and support; Michael DuBow (Institut de Génétique et Microbiologie, Université Paris-Sud 11) for arrangements made allowing M.L. to carry out doctoral thesis research studies in the laboratory of the corresponding author; and Douglas Fishkind of Carl Zeiss MicroImaging International, Inc., for the loan of a Nomarski optics Axioskop 2 Plus microscope. Richard Moxon and Derek Hood (Molecular Infectious Diseases Group, Oxford University), James Richards (Institute for Biological Sciences, National Research Council of Canada), Rick Malley (Children's Hospital Medical Center, Boston, MA), Michael DuBow (Université Paris-Sud 11), and Janet Geisselsoder provided thoughtful discussions and helpful criticism during the course of these studies. Lauren Bakaletz (Columbus Children's Research Institute, Ohio State University) provided critical expertise with the chinchilla model of EOM, and Kevin Manson (Columbus Children's Research Institute, Ohio State University) provided unpublished data regarding growth conditions for constitutive expression of gyrA. Norman Gerry and Sarika Agarwal provided technical advice on RNA procedures, Jim Richards provided monoclonal antibodies, and Loc Truong and Bei Ma provided expert practical assistance with the animal model.
Editor: D. L. Burns
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Alexander, H. E., and G. Leidy. 1951. Induction of heritable new type in type specific strains of H. influenzae. Proc. Soc. Exp. Biol. Med. 78:625-626. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, H. E., G. Leidy, and E. Hahn. 1954. Studies on the nature of hemophilus influenzae cells susceptible to heritable changes by desoxyribonucleic acids. J. Exp. Med. 99:505-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allesen-Holm, M., K. B. Barken, L. Yang, M. Klausen, J. S. Webb, S. Kjelleberg, S. Molin, M. Givskov, and T. Tolker-Nielsen. 2006. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 59:1114-1128. [DOI] [PubMed] [Google Scholar]
- 4.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 5.Babl, F. E., S. I. Pelton, and Z. Li. 2002. Experimental acute otitis media due to nontypeable Haemophilus influenzae: comparison of high and low azithromycin doses with placebo. Antimicrob. Agents Chemother. 46:2194-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barenkamp, S. J., P. A. Shurin, C. D. Marchant, R. B. Karasic, S. I. Pelton, V. M. Howie, and D. M. Granoff. 1984. Do children with recurrent Haemophilus influenzae otitis media become infected with a new organism or reacquire the original strain? J. Pediatr. 105:533-537. [DOI] [PubMed] [Google Scholar]
- 7.Block, S. L., J. Hedrick, C. J. Harrison, R. Tyler, A. Smith, R. Findlay, and E. Keegan. 2004. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr. Infect. Dis. J. 23:829-833. [DOI] [PubMed] [Google Scholar]
- 8.Bolduc, G. R., V. Bouchet, R. Z. Jiang, J. Geisselsoder, Q. C. Truong-Bolduc, P. A. Rice, S. I. Pelton, and R. Goldstein. 2000. Variability of outer membrane protein P1 and its evaluation as a vaccine candidate against experimental otitis media due to nontypeable Haemophilus influenzae: an unambiguous, multifaceted approach. Infect. Immun. 68:4505-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonferroni, C. E. 1936. Teoria statistica delle classi e calcolo delle probabilità. Pubbl. R Ist. Super. Sci. Econ. Commer. Fir. 8:3-62. [Google Scholar]
- 10.Bouchet, V., D. W. Hood, J. Li, J. R. Brisson, G. A. Randle, A. Martin, Z. Li, R. Goldstein, E. K. Schweda, S. I. Pelton, J. C. Richards, and E. R. Moxon. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc. Natl. Acad. Sci. USA 100:8898-8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, M. R., D. G. Allison, and P. Gilbert. 1988. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J. Antimicrob. Chemother. 22:777-780. [DOI] [PubMed] [Google Scholar]
- 12.Casey, J. R., and M. E. Pichichero. 2004. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr. Infect. Dis. J. 23:824-828. [DOI] [PubMed] [Google Scholar]
- 13.Cody, A. J., D. Field, E. J. Feil, S. Stringer, M. E. Deadman, A. G. Tsolaki, B. Gratz, V. Bouchet, R. Goldstein, D. W. Hood, and E. R. Moxon. 2003. High rates of recombination in otitis media isolates of non-typeable Haemophilus influenzae. Infect. Genet. Evol. 3:57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen, S. S., and H. D. Barner. 1954. Studies on unbalanced growth in Escherichia coli. Proc. Natl. Acad. Sci. USA 40:885-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole, S. T., C. Condon, B. D. Lemire, and J. H. Weiner. 1985. Molecular biology, biochemistry and bioenergetics of fumarate reductase, a complex membrane-bound iron-sulfur flavoenzyme of Escherichia coli. Biochim. Biophys. Acta 811:381-403. [DOI] [PubMed] [Google Scholar]
- 16.Costerton, J. W., K. J. Cheng, G. G. Geesey, T. I. Ladd, J. C. Nickel, M. Dasgupta, and T. J. Marrie. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 17.Costerton, J. W., Z. Lewandowski, D. DeBeer, D. Caldwell, D. Korber, and G. James. 1994. Biofilms, the customized microniche. J. Bacteriol. 176:2137-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 19.Costerton, W., R. Veeh, M. Shirtliff, M. Pasmore, C. Post, and G. Ehrlich. 2003. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Investig. 112:1466-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courcelle, J., and P. C. Hanawalt. 2003. RecA-dependent recovery of arrested DNA replication forks. Annu. Rev. Genet. 37:611-646. [DOI] [PubMed] [Google Scholar]
- 21.Darkes, M. J., and G. L. Plosker. 2002. Pneumococcal conjugate vaccine (Prevnar; PNCRM7): a review of its use in the prevention of Streptococcus pneumoniae infection. Paediatr. Drugs 4:609-630. [DOI] [PubMed] [Google Scholar]
- 22.Davey, M. E., and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diggle, P. J., K. Y. Liang, and S. L. Zeger. 1994. Analysis of longitudinal data. Clarendon Press, Oxford, United Kingdom.
- 24.Ehrlich, G. D., R. Veeh, X. Wang, J. W. Costerton, J. D. Hayes, F. Z. Hu, B. J. Daigle, M. D. Ehrlich, and J. C. Post. 2002. Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA 287:1710-1715. [DOI] [PubMed] [Google Scholar]
- 25.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Kayhty, P. Karma, R. Kohberger, G. Siber, and P. H. Makela. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 26.Evans, N. M., D. D. Smith, and A. J. Wicken. 1974. Haemin and nicotinamide adenine dinucleotide requirements of Haemophilus influenzae and Haemophilus parainfluenzae. J. Med. Microbiol. 7:359-365. [DOI] [PubMed] [Google Scholar]
- 27.Feil, E. J., E. C. Holmes, D. E. Bessen, M. S. Chan, N. P. Day, M. C. Enright, R. Goldstein, D. W. Hood, A. Kalia, C. E. Moore, J. Zhou, and B. G. Spratt. 2001. Recombination within natural populations of pathogenic bacteria: short-term empirical estimates and long-term phylogenetic consequences. Proc. Natl. Acad. Sci. USA 98:182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J. F. Tomb, B. A. Dougherty, J. M. Merrick, K. McKenney, G. Sutton, W. FitzHugh, C. Fields, J. D. Gicyne, J. Scott, R. Shirley, L. Liu, A. Glodek, J. M. Kelley, J. F. Weidman, C. A. Phillips, T. Spriggs, E. Hedblom, M. D. Cotton, T. R. Utterback, M. C. Hanna, D. T. Nguyen, D. M. Saudek, R. C. Brandon, L. D. Fine, J. L. Fritchman, J. L. Fuhrmann, N. S. M. Geoghagen, C. L. Gnehm, L. A. McDonald, K. V. Small, C. M. Fraser, H. O. Smith, and J. C. Venter. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 29.Fountoulakis, M., J. F. Juranville, D. Roder, S. Evers, P. Berndt, and H. Langen. 1998. Reference map of the low molecular mass proteins of Haemophilus influenzae. Electrophoresis 19:1819-1827. [DOI] [PubMed] [Google Scholar]
- 30.Govan, J. R. 1975. Mucoid strains of Pseudomonas aeruginosa: the influence of culture medium on the stability of mucus production. J. Med. Microbiol. 8:513-522. [DOI] [PubMed] [Google Scholar]
- 31.Greiner, L. L., H. Watanabe, N. J. Phillips, J. Shao, A. Morgan, A. Zaleski, B. W. Gibson, and M. A. Apicella. 2004. Nontypeable Haemophilus influenzae strain 2019 produces a biofilm containing N-acetylneuraminic acid that may mimic sialylated O-linked glycans. Infect. Immun. 72:4249-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanage, W. P., K. Auranen, R. Syrjanen, E. Herva, P. H. Makela, T. Kilpi, and B. G. Spratt. 2004. Ability of pneumococcal serotypes and clones to cause acute otitis media: implications for the prevention of otitis media by conjugate vaccines. Infect. Immun. 72:76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hausdorff, W. 2001. Haemophilus, meningococcus and pneumococcus: comparative epidemiologic patterns of disease. Int. J. Clin. Pract. Suppl. 118:2-4. [PubMed] [Google Scholar]
- 34.Hausdorff, W. P. 2002. Invasive pneumococcal disease in children: geographic and temporal variations in incidence and serotype distribution. Eur. J. Pediatr. 161(Suppl. 2):S135-S139. [DOI] [PubMed] [Google Scholar]
- 35.Hausdorff, W. P., G. Yothers, R. Dagan, T. Kilpi, S. I. Pelton, R. Cohen, M. R. Jacobs, S. L. Kaplan, C. Levy, E. L. Lopez, E. O. Mason, Jr., V. Syriopoulou, B. Wynne, and J. Bryant. 2002. Multinational study of pneumococcal serotypes causing acute otitis media in children. Pediatr. Infect. Dis. J. 21:1008-1016. [DOI] [PubMed] [Google Scholar]
- 36.Hood, D. W., K. Makepeace, M. E. Deadman, R. F. Rest, P. Thibault, A. Martin, J. C. Richards, and E. R. Moxon. 1999. Sialic acid in the lipopolysaccharide of Haemophilus influenzae: strain distribution, influence on serum resistance and structural characterization. Mol. Microbiol. 33:679-692. [DOI] [PubMed] [Google Scholar]
- 37.Iverson, T. M., C. Luna-Chavez, G. Cecchini, and D. C. Rees. 1999. Structure of the Escherichia coli fumarate reductase respiratory complex. Science 284:1961-1966. [DOI] [PubMed] [Google Scholar]
- 38.Jelsbak, L., and L. Sogaard-Andersen. 2002. Pattern formation by a cell surface-associated morphogen in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 99:2032-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jurcisek, J., L. Greiner, H. Watanabe, A. Zaleski, M. A. Apicella, and L. O. Bakaletz. 2005. Role of sialic acid and complex carbohydrate biosynthesis in biofilm formation by nontypeable Haemophilus influenzae in the chinchilla middle ear. Infect. Immun. 73:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Justice, S. S., C. Hung, J. A. Theriot, D. A. Fletcher, G. G. Anderson, M. J. Footer, and S. J. Hultgren. 2004. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA 101:1333-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karma, P., J. Luotonen, J. Pukander, M. Sipila, E. Herva, and P. Gronroos. 1983. Haemophilus influenzae in acute otitis media. Acta Otolaryngol. 95:105-110. [DOI] [PubMed] [Google Scholar]
- 42.Keppel, G. 1991. Design and analysis: a researcher's handbook, 3rd ed. Prentice-Hall, Englewood Cliffs, NJ.
- 43.Kilpi, T., E. Herva, T. Kaijalainen, R. Syrjanen, and A. K. Takala. 2001. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr. Infect. Dis. J. 20:654-662. [DOI] [PubMed] [Google Scholar]
- 44.Kim, S. K., and D. Kaiser. 1991. C-factor has distinct aggregation and sporulation thresholds during Myxococcus development. J. Bacteriol. 173:1722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein, J. O. 2000. The burden of otitis media. Vaccine 19(Suppl. 1):S2-S8. [DOI] [PubMed] [Google Scholar]
- 46.Klein, J. O. 1994. Otitis media. Clin. Infect. Dis. 19:823-833. [DOI] [PubMed] [Google Scholar]
- 47.Koch, A. L. 2005. Shapes that Escherichia coli cells can achieve, as a paradigm for other bacteria. Crit. Rev. Microbiol. 31:183-190. [DOI] [PubMed] [Google Scholar]
- 48.Kuzminov, A. 1999. Recombinational repair of DNA damage in Escherichia coli and bacteriophage lambda. Microbiol. Mol. Biol. Rev. 63:751-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leibovitz, E., D. Greenberg, L. Piglansky, S. Raiz, N. Porat, J. Press, A. Leiberman, and R. Dagan. 2003. Recurrent acute otitis media occurring within one month from completion of antibiotic therapy: relationship to the original pathogen. Pediatr. Infect. Dis. J. 22:209-216. [DOI] [PubMed] [Google Scholar]
- 50.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mah, T. F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 52.Mason, K. M., R. S. Munson, Jr., and L. O. Bakaletz. 2003. Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect. Immun. 71:3454-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy, T. F., and C. Kirkham. 2002. Biofilm formation by nontypeable Haemophilus influenzae: strain variability, outer membrane antigen expression and role of pili. BMC Microbiol. 2:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nickel, J. C., I. Ruseska, J. B. Wright, and J. W. Costerton. 1985. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 27:619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nokso-Koivisto, J., T. Hovi, and A. Pitkaranta. 2006. Viral upper respiratory tract infections in young children with emphasis on acute otitis media. Int. J. Pediatr. Otorhinolaryngol. 70:1333-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nomarski, G. 1957. From phase contrast to contrast by interference. Rev. Hematol. 12:439-442. [PubMed] [Google Scholar]
- 57.Peterson, P. K., B. J. Wilkinson, Y. Kim, D. Schmeling, and P. G. Quie. 1978. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect. Immun. 19:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:2002-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfaffl, M. W., G. W. Horgan, and L. Dempfle. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plontke, S. K., A. W. Wood, and A. N. Salt. 2002. Analysis of gentamicin kinetics in fluids of the inner ear with round window administration. Otol. Neurotol. 23:967-974. [DOI] [PubMed] [Google Scholar]
- 61.Post, J. C. 2001. Direct evidence of bacterial biofilms in otitis media. Laryngoscope 111:2083-2094. [DOI] [PubMed] [Google Scholar]
- 62.Post, J. C., and G. D. Ehrlich. 2000. The impact of the polymerase chain reaction in clinical medicine. JAMA 283:1544-1546. [DOI] [PubMed] [Google Scholar]
- 63.Prymula, R., P. Peeters, V. Chrobok, P. Kriz, E. Novakova, E. Kaliskova, I. Kohl, P. Lommel, J. Poolman, J. P. Prieels, and L. Schuerman. 2006. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 367:740-748. [DOI] [PubMed] [Google Scholar]
- 64.Roberts, J., L. Hunter, J. Gravel, R. Rosenfeld, S. Berman, M. Haggard, J. Hall, C. Lannon, D. Moore, L. Vernon-Feagans, and I. Wallace. 2004. Otitis media, hearing loss, and language learning: controversies and current research. J. Dev. Behav. Pediatr. 25:110-122. [DOI] [PubMed] [Google Scholar]
- 65.Roberts, J. E., R. M. Rosenfeld, and S. A. Zeisel. 2004. Otitis media and speech and language: a meta-analysis of prospective studies. Pediatrics 113:e238-e248. [DOI] [PubMed] [Google Scholar]
- 66.Sawchuk, R. J., B. W. Cheung, P. Ji, and L. L. Cartier. 2005. Microdialysis studies of the distribution of antibiotics into chinchilla middle ear fluid. Pharmacotherapy 25:S140-S145. [DOI] [PubMed] [Google Scholar]
- 67.Stevens, A., and L. Sogaard-Andersen. 2005. Making waves: pattern formation by a cell-surface-associated signal. Trends Microbiol. 13:249-252. [DOI] [PubMed] [Google Scholar]
- 68.Stoodley, P., D. Debeer, and Z. Lewandowski. 1994. Liquid flow in biofilm systems. Appl. Environ. Microbiol. 60:2711-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 70.Sugino, A., N. P. Higgins, and N. R. Cozzarelli. 1980. DNA gyrase subunit stoichiometry and the covalent attachment of subunit A to DNA during DNA cleavage. Nucleic Acids Res. 8:3865-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swords, W. E., M. L. Moore, L. Godzicki, G. Bukofzer, M. J. Mitten, and J. VonCannon. 2004. Sialylation of lipooligosaccharides promotes biofilm formation by nontypeable Haemophilus influenzae. Infect. Immun. 72:106-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tatusov, R. L., A. R. Mushegian, P. Bork, N. P. Brown, W. S. Hayes, M. Borodovsky, K. E. Rudd, and E. V. Koonin. 1996. Metabolism and evolution of Haemophilus influenzae deduced from a whole-genome comparison with Escherichia coli. Curr. Biol. 6:279-291. [DOI] [PubMed] [Google Scholar]
- 73.Teele, D. W., J. O. Klein, B. Rosner, L. Bratton, G. R. Fisch, O. R. Mathieu, P. J. Porter, S. G. Starobin, L. D. Tarlin, and R. P. Younes. 1983. Middle ear disease and the practice of pediatrics. Burden during the first five years of life. JAMA 249:1026-1029. [PubMed] [Google Scholar]
- 74.Tong, H. H., I. Grants, X. Liu, and T. F. DeMaria. 2002. Comparison of alteration of cell surface carbohydrates of the chinchilla tubotympanum and colonial opacity phenotype of Streptococcus pneumoniae during experimental pneumococcal otitis media with or without an antecedent influenza A virus infection. Infect. Immun. 70:4292-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Welch, R., and D. Kaiser. 2001. Cell behavior in traveling wave patterns of myxobacteria. Proc. Natl. Acad. Sci. USA 98:14907-14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wentland, E. J., P. S. Stewart, C. T. Huang, and G. A. McFeters. 1996. Spatial variations in growth rate within Klebsiella pneumoniae colonies and biofilm. Biotechnol. Prog. 12:316-321. [DOI] [PubMed] [Google Scholar]
- 77.Whitnack, E., A. L. Bisno, and E. H. Beachey. 1981. Hyaluronate capsule prevents attachment of group A streptococci to mouse peritoneal macrophages. Infect. Immun. 31:985-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiegand, I., and S. Burak. 2004. Effect of inoculum density on susceptibility of Plesiomonas shigelloides to cephalosporins. J. Antimicrob. Chemother. 54:418-423. [DOI] [PubMed] [Google Scholar]
- 79.Wilcox, K. W., and H. O. Smith. 1975. Isolation and characterization of mutants of Haemophilus influenzae deficient in an adenosine 5′-triphosphate-dependent deoxyribonuclease activity. J. Bacteriol. 122:443-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yogev, R., and E. R. Moxon. 1982. Elaboration of type b capsule by Haemophilus influenzae as a determinant of pathogenicity and impaired killing by trimethoprim-sulfamethoxazole. J. Clin. Investig. 69:658-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu, T., B. W. Cheung, L. L. Cartier, G. S. Giebink, and R. J. Sawchuk. 2003. Simultaneous intravenous and intramiddle-ear dosing to determine cefditoren influx and efflux clearances in middle ear fluid in freely moving chinchillas. J. Pharm. Sci. 92:1947-1956. [DOI] [PubMed] [Google Scholar]