Abstract
A novel and functional conjugative transfer system identified in O119:H2 enteropathogenic Escherichia coli (EPEC) strain MB80 by subtractive hybridization is encoded on a large multidrug resistance plasmid, distinct from the well-described EPEC adherence factor (EAF) plasmid. Variants of the MB80 conjugative resistance plasmid were identified in other EPEC strains, including the prototypical O111:NM strain B171, from which the EAF plasmid has been sequenced. This separate large plasmid and the selective advantage that it confers in the antibiotic era have been overlooked because it comigrates with the virulence plasmid on conventional gels.
Enteropathogenic Escherichia coli (EPEC) continues to be a major cause of diarrhea and death, predominantly in developing countries (8, 22). During infection, EPEC strains attach intimately to the intestinal epithelium and efface the absorptive microvilli, initiating a complex signaling cascade that ultimately leads to diarrhea by mechanisms that are only partially understood (8). These pathogenic effects manifest as an “attaching and effacing” histopathological hallmark, which is conferred by a large chromosomal island called the locus of enterocyte effacement (LEE). The LEE encodes a virulence gene regulator and a type III secretion system and effectors, as well as the intimin adhesin and its translocated receptor. The LEE is present in all EPEC strains, as well as in enterohemorrhaghic E. coli and some animal pathogens (reviewed in references 8 and 22).
EPEC strains are classified as being either typical or atypical based on their ability to form densely packed three-dimensional clusters, or microcolonies, a phenotype known as localized adherence (LA). LA is conferred by a large EPEC adherence factor (EAF) plasmid, associated with virulence in epidemiological and volunteer challenge studies, which is present in all typical EPEC strains and absent or altered in atypical EPEC strains (3, 16, 25, 35). The specific virulence factor responsible for LA is an EAF plasmid-encoded bundle-forming pilus (Bfp), which also contributes to antigenicity, autoaggregation, and biofilm formation (3, 11, 15, 20). On a separate region of the EAF plasmid are the perABC genes, encoding the plasmid-encoded regulator (a transcriptional activator for the bfp operon and the LEE, as well as its own promoter) and other virulence loci (9, 19). The EAF plasmid and the LEE are believed to have been acquired by different EPEC lineages in separate horizontal events (30).
EAF plasmids from two well-studied EPEC strains have been sequenced. The major difference between the EAF plasmids from isolates E2368/69 (of the EPEC1 lineage [30]) and B171 (representing the EPEC2 lineage [30]), is the presence of conjugative transfer (tra) genes on pMAR7, the E2348/69 EAF plasmid (7). The B171 EAF plasmid, pB171, does not contain tra genes (36), even though at least one earlier paper reported conjugative transfer of pB171 (31). Other than with respect to tra genes, the two sequenced EAF plasmids are highly conserved, and low-resolution mapping suggests that other EAF plasmids are similarly conserved (23). Many atypical EPEC isolates lack the entire EAF plasmid. Other strains harbor a plasmid that shares a conserved backbone, and is in many ways similar to pB171 and pMAR7, but with inactivating mutations in the bfp and per operons that are required for LA (5, 27). Such strains are phenotypically “atypical,” even though they carry probe-detectable EAF plasmids. We therefore hypothesized that the large plasmids of these strains would carry compensatory virulence and/or fitness genes.
An atypical O119:H2 EPEC strain, MB80, with a deletion in the bfp operon and inactivating point mutations in the plasmid-encoded regulator genes, was selected for study (5, 27). Plasmids used, identified, or constructed in the course of the study are listed in Table 1, and standard molecular biology procedures were used throughout (32). Alkaline lysis-extracted (4) EPEC plasmid bands in the range of 60 to 150 kb were gel-purified away from smaller plasmids and contaminating chromosomal DNA by electroelution. A PCR-select subtractive hybridization kit (Clontech/BD Biosciences) was used to subtract the RsaI-digested EPEC strain MB80 large-plasmid DNA (tester) from a similarly digested preparation from prototypical EPEC strain E2348/69 (driver), according to the manufacturer's instructions, essentially as described by Akopyants et al. (2). E2348/69 carries a larger virulence plasmid (98 kb versus 69 kb in B171 [7, 36]) and was therefore selected as the driver over the more closely related B171. Subtracted sequences were cloned into pGEM-T (Promega), sequenced, and compared to the GenBank nucleotide database (www.ncbi.nlm.nih.gov) by a nucleotide-nucleotide BLAST search.
TABLE 1.
Plasmids and constructs used in this study
| Plasmid(s) or construct | Description | Reference or source |
|---|---|---|
| pBR322 | Cloning vector | 32 |
| pBluescript | Cloning vector | Stratagene |
| pGEM-T | Ampr; TA cloning vector | Promega |
| pED208 | Derepressed form of IncFV plasmid F0lac | 10, 18 |
| pB171, pE2348 (pMAR7), and pMB80 | Wild-type EAF plasmids from EPEC strains B171 (O111:H2), E2348/69 (O127:H6), and MB80 (O119:H2), respectively | 5, 7, 23, 36 |
| pMB80-2 | Conjugative resistance plasmid from EPEC strain MB80 | This study |
| pB171-2 | Conjugative resistance plasmid from EPEC strain B171 | This study |
| pINKM1t | Miniplasmid generated from pMB80-2 and containing most of the resistance region | This study |
| pINkE15k, pINKE18t | Resistance region subclones of from pMB80-2 | This study |
We reproducibly subtracted six MB80-specific sequences and confirmed that all were absent from the driver genome by BLAST analysis of the in-process E2348/69 genome at http://www.sanger.ac.uk/cgi-bin/BLAST/submitblast/escherichia_shigella. Four of the six fragments mapped to a conjugative (tra) system with homology to the system from Salmonella enterica serovar Typhi plasmid pED208 (10, 18) (Table 2). We obtained single-strand end-sequence(s) from eight Pst1 and EcoRI fragments located outside the resistance region of plasmid pMB80, and seven of these mapped to the tra region (see Table S2 in the supplemental material). With the subtracted clones, this approach yielded clone coverage of about 12 kb of the predicted 32-kb region, at least one sequence hit for 15 of the 22 open reading frames between traB and orf4. From BLAST searches and restriction mapping, we are able to infer that this part of the MB80 transfer region shows similar gene organization and approximately 80% identity at the DNA level to that of Salmonella serovar Typhi plasmid pED208 (18) (Table 2; see Table S2 in the supplemental material).
TABLE 2.
Blast hits for MB80 plasmid-specific DNA fragments isolated by subtractive hybridization
| Subtracted clone | BLAST hit | GenBank accession no. (region) | BLAST identities (%), expect value |
|---|---|---|---|
| pAN1 | Insertion sequence IS26 from E. coli plasmid pAPEC-O2-R | AY214164.3 | 134/137 (97), 5e-63 |
| pAN2 | Insertion element IS2 from E. coli K-12 MG1655 | U00096 (region 380068..382096) | 187/193 (96), 4e-89 |
| pAN3 | traI gene from Salmonella serovar Typhi plasmid pED208 IncFV plasmid transfer region | AF411480.1 | 177/203 (87), 2e-47 |
| pAN4 | trbB-traH from Salmonella serovar Typhi plasmid pED208 IncFV plasmid transfer region | AF411480.1 | 56/61 (91), 1e-12 |
| pAN5 | traV-traC from Salmonella serovar Typhi plasmid pED208 IncFV plasmid transfer region | AF411480.1 | 273/312 (87), 3e-81 |
| pAN6 | traI from Salmonella serovar Typhi plasmid pED208 IncFV plasmid transfer region | AF411480.1 | 389/446 (87), 6e-118 |
We designed primers complementary to internal regions of the traI and the traC genes that are conserved between the MB80 tra system and pED208, as well as one primer pair whose product straddles traU and trbC in pED208 in a region not conserved in MB80 (27) (Table 3). None of the EPEC strains produced an amplicon with pED208-specific traU-trbC primers, but three other strains produced the predicted amplicon for the traC and traI loci (Table 3). A panel of 26 enteroaggregative E. coli strains, which belong to a pathotype for which plasmid-borne, transferable resistance is common (26, 28), were also screened, and all were negative for the traI and traC PCRs. Strain B171, a prototypic O111:NM outbreak isolate from the United States, was one of the EPEC strains that screened positive for traI and traC, even though there are no sequences homologous to pMB80 tra genes in its EAF plasmid (pB171) (36).
TABLE 3.
Distribution of EPEC conjugative multiresistance plasmid loci among different EPEC strains
| Strain | Serotype | Year | Country | Result for gene with indicated primer target
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| traI (pMB80-2/ pED208) | traC (pMB80-2/ pED208) | traU-trbC (pED208) | Class 1 integron (5′- and 3′CS) | TEM bla (pHCM1) | sulII (dps) (pHCM1) | tetA (pHCM1) | cat (pHCM1) | merA (pHCM1) | csi (pB171-2) | ||||
| C54-58 (DEC 1a) | O55:H6 | 1958 | Dutch Guyana | − | − | − | − | − | − | − | − | − | − |
| Beta | O55:H6 | 1947 | Scotland | − | − | − | − | − | − | − | − | − | − |
| 5513-56 (DEC2b) | O55:NM | 1956 | United States | − | − | − | − | − | − | − | − | − | − |
| E990 | O86:NM | 1950 | England | − | − | − | − | − | − | − | − | − | − |
| 2966-56 (DEC 12b) | O111:H2 | 1956 | United States | − | − | − | aadA1 | − | − | − | − | + | − |
| DIF 043256 | O111:H2 | 1986 | Mexico | + | + | − | aadA1 | + | + | + | − | − | − |
| 2309-77 | O111ab:H2 | 1977 | United States | − | − | − | aadA1 | − | − | + | − | − | − |
| B171 | O111:NM | United States | + | + | − | aadA1 | − | − | − | − | + | + | |
| Stoke W | O111:NM | 1947 | Scotland | + | + | − | − | − | − | − | − | − | − |
| 009-271082 | O111 | 1982 | Peru | − | − | − | aadA1 | + | − | + | − | + | − |
| MB80 | O119:H2 | 1993 | Brazil | + | + | − | aadA1 | + | + | − | − | + | − |
| O119#6 | O119:H6 | Brazil | − | − | − | − | − | − | − | − | − | − | |
| C35 | O119:H6 | 1995 | Nigeria | − | − | − | − | − | + | − | − | − | − |
| 0659-79 | O119:H6 | 1979 | United States | − | − | − | − | + | + | − | − | − | − |
| E. coli 10 | O119:H6 | 1986 | Chile | − | − | − | aadA1 | − | − | − | − | − | − |
| 023-220982 | O126 | Peru | − | − | − | − | − | + | − | − | − | − | |
| E2348/69 | O127:H6 | 1969 | England | − | − | − | − | − | − | − | − | − | − |
| 1092-80 | O127:NM | − | − | − | − | + | + | − | − | − | − | ||
| MB21 | O128:H2 | Brazil | − | − | − | − | − | + | + | − | − | − | |
| E56/54 | O128ab:H2 | 1953 | England | − | − | − | − | − | − | − | − | − | − |
| 010-311082 | O128 | 1982 | Peru | − | − | − | − | − | + | − | + | − | − |
| #19 | O142:H6 | Brazil | − | − | − | − | + | + | − | − | + | − | |
| #15 | O142:H6 | Brazil | − | − | − | − | − | + | + | + | − | − | |
| G51 | O142:H6 | 1995 | Nigeria | − | − | − | − | + | + | + | + | + | − |
| C771 | O142:H6 | pre-1960 | Indonesia | − | − | − | − | − | − | + | − | + | − |
| E851/71 | O142:H6 | 1971 | Scotland | − | − | − | − | + | − | + | − | − | − |
| 012-050982 | O142:H6 | 1982 | Peru | − | − | − | − | − | + | + | + | − | − |
| Canine 4225 | 1989 | Canada | − | − | − | aadA1 | + | + | − | − | + | − | |
| pED208 in K12 | + | + | + | − | + | − | − | − | + | − | |||
| EAEC 042 | O44:H18 | − | − | − | aadA1 | − | − | − | + | + | − | ||
| S. enterica CT18 | Typhi | − | − | − | + | + | + | + | + | − | |||
Antimicrobial susceptibility testing was performed by the disk diffusion method (24), using disks containing ampicillin (10 μg), tetracycline (30 μg), trimethoprim (5 μg), nalidixic acid (30 μg), chloramphenicol (30 μg), sulfonamide (300 μg), streptomycin (10 μg), and ciprofloxacin (5 μg) (Remel, KS). The inhibition zone diameters were interpreted according to Clinical and Laboratory Standards Institute (formerly NCCLS) requirements (24), and E. coli NCTC 10418 was used as the control. All wild-type EPEC strains were nalidixic acid sensitive, but most were resistant to one or more other agents. Five of seven O111/O119:H2 EPEC strains were resistant to three or more antimicrobials, and three of these were positive for the pMB80-2 traI and traC genes. We were able to identify at least two of the resistance loci in each of these strains by PCR (Table 3). Only one strain, 1947 Scottish isolate Stoke W (O111:NM), from early in the antibiotic era, possessed the tra genes and was sensitive to all tested antimicrobials.
In a first step towards localizing tra genes in the MB80 and B171 genomes, mating reactions were set up with traI- and traC-positive EPEC strains as donors and a nalidixic acid-resistant plasmidless K-12 E. coli strain, C600, as the recipient. Transconjugants, selected on plates containing tetracycline and nalidixic acid, harbored an 80- to 120-kb plasmid. In all conjugation reactions, using all traI- and traC-positive EPEC strains as donors, we were able to cotransfer multiple antimicrobial resistance markers along with the tra loci. Transfer frequencies from MB80 and B171 were 2 × 10−5 and 7 × 10−5, respectively, by liquid mating and 6 × 10−5 and 7 × 10−6 by solid mating (18). In most but notably not all transconjugants, the EAF plasmid-encoded perA gene had not been cotransferred. The plasmids could be transferred from C600 transconjugants to a rifampin-resistant derivative of E. coli ORN172, verifying that the plasmids were sufficient to mediate their own transfer. Plasmid extraction by alkaline lysis or boiling protocols followed by horizontal gel electrophoresis resulted in a single band or close doublet for large plasmids from wild-type strains MB80 and B171, consistent with profiles presented by earlier investigators (31).
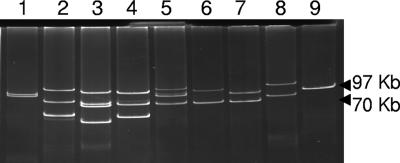
Our own results and other published results for horizontally electrophoresed plasmid preparations suggest that although additional plasmids smaller than 12 kb can be visualized in some EPEC strains, only one large plasmid band is discernible by conventional electrophoresis methods (31). To verify that the second large plasmids recovered from strains MB80 and B171 were present in the wild-type strain (and not, for example, excised from the chromosome during conjugation), we performed plasmid profiling by an alternate method. Vertical electrophoresis of Kado and Liu preparations on E-buffer gels (14) demonstrated in every case that the strains contained a smaller EAF plasmid of 69 to 90 kb and a larger conjugative resistance plasmid of 100 to 120 kb for MB80 and B171 (Fig. 1). EAF-negative transconjugants carried only the larger plasmid, which we designate pB171-2. Other O111 strains showed 2 to 4 large-plasmid bands.
FIG. 1.
Resolution of Kado and Liu (14) plasmid preparations by vertical electrophoresis demonstrates multiple plasmids in EPEC2 strains. Loaded (left to right) are plasmid preparations from control strain BA337 with two plasmids (lane 1); O111 strains 009-271082 (lane 2), Stoke W (lane 3), 2309-77 (lane 4), DIF043256 (lane 5), 2966-5 (lane 6), and B171 (lane 7); O119:H2 strain MB80 (lane 8); and control O127:H6 strain E2348/69 with one plasmid (lane 9). Molecular mass markers are on the right.
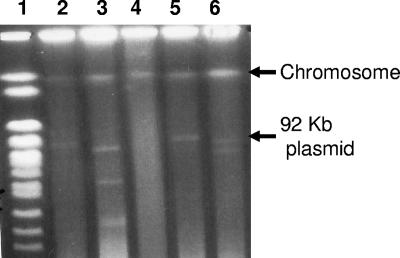
Pulsed-field gel electrophoresis has been successfully used to resolve large circular replicons (37). We performed pulsed-field gel electrophoresis of DNA extracted from agarose-embedded B171 and compared the profile obtained with that of genome-sequenced E. coli strains. Again the data demonstrate that B171 has two large plasmids, the previously sequenced EAF plasmid, pB171, and a second replicon, pB171-2 (Fig. 2). This finding was supported by MluI restriction analysis of large-plasmid DNA. Thus, the EPEC conjugative system identified in this study, unlike that of strain E2348/69 (7), is present on a plasmid separate from the virulence plasmid, and this second plasmid also bears multiple antimicrobial resistance genes.
FIG. 2.
Pulsed-field gel electrophoresis of undigested DNA from EPEC strain B171 and controls. Lane 1: yeast chromosome markers. Lanes 2 to 6: undigested DNA from E. coli strains (with plasmid size in kb, if confirmed by genome sequence) EDL933 (lane 2; single large plasmid, 92 kb); H30 (lane 3); MG1655 (lane 4; no plasmid); E2348/69 (lane 5; single large plasmid, 98 kb); and B171 (lane 6).
We searched the GenBank database for genetic loci that are present in EPEC but are not present on sequenced EAF plasmids or the LEE. We identified one locus, predicted to be a csi (calcium sequestration inhibitor) gene for the well-studied strain B171 (GenBank accession no. Y08258). PCR and sequencing demonstrated that the csi gene was unique to strain B171 and encoded on the conjugative resistance plasmid. We did not find epithelial cell adherence, autoagglutination, or biofilm formation associated with the pMB80-2 or pB171-2 plasmids, although it is possible that virulence functions conferred by the plasmid could be identified in the future. The genome of EPEC strain B171 is currently being sequenced (http://msc.tigr.org/e_coli_and_shigella/index.shtml), and any other genes of interest carried by pB171-2 should soon be in the public domain.
Through restriction analysis, end sequencing of MluI miniplasmid pINKM1t subcloned from pMB80-2, and PCR using primers for resistance genes on Salmonella serovar Typhi plasmid pHCM1 (Table 3; see Table S2 in the supplemental material), we were able to determine the resistance gene content and order within the pMB80-2 resistance region. The pMB80-2 plasmid bears a β-lactamase gene flanked by IS26 elements, a sulII gene encoding dihydropterate synthase, which mediates sulfonamide resistance, strAB genes encoding streptomycin resistance, a trimethoprim resistance gene, and a mercury resistance (mer) operon. These loci are 99% identical to the resistance genes on plasmid pHCM1 (29), and their organization is similar. Downstream of this 30-kb cluster lies a tetracycline resistance operon, bordered by pem genes and identical to the Tn1721 tetracycline resistance determinant found on the E. coli multiresistant plasmid pC15-1a (6).
Initial optimism that resistance would spread slowly among EPEC strains (34) has been overshadowed by reports of multidrug-resistant EPEC strains, particularly O111 strains, from diverse parts of the world (1, 12, 13, 17, 21, 33, 38, 39). We have identified a clandestine plasmid that accounts for this resistance in a subset of O111:H2/NM and O119:H2 EPEC strains, but which is absent from most other EPEC strains. The conjugative multidrug resistance plasmid identified in this study has been previously undetected or ignored due to its similarity in size with the EAF virulence plasmid in B171 and other strains. Its presence explains a number of properties that have been experimentally or anecdotally assigned to O111 and related EPEC strains but which were not found on the B171 virulence plasmid (36), including antimicrobial resistance, conjugative transfer, and the putative virulence gene csi (13, 31). The revelation that this plasmid is present but has been previously unreported from a well-studied strain caused us to examine the literature on strain B171 and other EPEC strains closely.
Nataro et al. (23) found that resistance was a common feature of EPEC strains but were unable to select plasmid cotransformants that harbored resistance genes and hybridized to a genetic marker on the EAF virulence plasmid in multiple strains, not including strain B171. In contrast, Riley et al. (31) were able to demonstrate conjugative transfer of resistance and LA in strain B171, but they identified only one 54-MDa plasmid in their transconjugants and therefore concluded that these genes were on the same plasmid. However, when the large plasmid from strain B171 was later sequenced, no tra genes were found. The inability of the whole-plasmid sequencing project to detect the second plasmid, pB171-2, is explained by the fact that the sequence was assembled from subclones, rather than by more-commonly employed shotgun protocols (36). A retrospective assessment of the data presented in these papers suggests that resistance and EAF plasmids in strains studied by both groups were, as in this study, separate entities and that the element was not excised from the chromosome during more recent storage or transport. It is likely that the conjugative multiresistance plasmid has been overlooked or ignored because it does not carry key EPEC virulence genes and because, in routine plasmid profiles, it overlaps or runs closely with the EAF plasmid.
Multidrug-resistant O111 EPEC strains have been predominant causes of nursery outbreaks since the 1970s (1, 12, 13, 17, 21, 33, 38, 39). The efficiently transferred conjugative resistance plasmid in MB80, B171, and other O111 strains could have assisted in maintaining this epidemiologic importance, due to the selective advantage conferred by antimicrobial resistance. This opens the question of whether the plasmid, or at least one of its precursors, such as the 1947 Scottish Stoke W plasmid, was acquired vertically, before clonal expansion of this EPEC lineage, or horizontally in relatively recent times. If the latter is the case, the question of how the resistance plasmid spread rapidly and apparently selectively within the O111:H2/NM and O119:H2 EPEC lineages arises. Epidemic spread of resistance plasmids within a pathotype has been documented for successful incH plasmids among Salmonella serovar Typhi isolates in Asia (40). The ability of the resistance plasmid to occasionally mobilize the EAF plasmid means that it can, at least in theory, play some role in pathogen evolution. Finally, the identification of these EPEC conjugation plasmids represents the first example of an IncFV pED208 (or F0lac)-like conjugative system reported from nature since the 1960s. More expansive studies of the epidemiology of this plasmid family in EPEC strains, using markers identified in this study, could address the question of its origin and potential for persistence or further spread.
Supplementary Material
Acknowledgments
This work was supported by a Branco Weiss fellowship from the Society in Science, Zurich, to I.N.O. A.I.N. was a Howard Hughes Medical Institute multicultural scholar and T.M. a Loewy-Santer-Finger scholar at Haverford College. D.J.P. is a postdoctoral fellow supported by the Wellcome Trust of Great Britain.
We thank James Kaper and Carl Brinkley for helpful discussions and Gordon Dougan for mentorship and support for D.J.P. Strain MB80 was a gift from Vanessa Sperandio. We thank Laura Frost for the gift of conjugative plasmid pED208 and John Wain for genomic DNA from Salmonella serovar Typhi strain CT18. The validation of subtracted targets for this study was greatly assisted by access to in-process sequence data produced by the Escherichia coli and Shigella spp. comparative sequencing group at the Sanger Institute, which can be accessed at http://www.sanger.ac.uk/Projects/Escherichia_Shigella/.
Footnotes
Published ahead of print on 15 June 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ahmed, A., H. Osman, A. Mansour, H. Musa, A. Ahmed, Z. Karrar, and H. Hassan. 2000. Antimicrobial agent resistance in bacterial isolates from patients with diarrhea and urinary tract infection in the Sudan. Am. J. Trop. Med. Hyg. 63:259-263. [PubMed] [Google Scholar]
- 2.Akopyants, N. S., A. Fradkov, L. Diatchenko, J. E. Hill, P. D. Siebert, S. A. Lukyanov, E. D. Sverdlov, and D. E. Berg. 1998. PCR-based subtractive hybridization and differences in gene content among strains of Helicobacter pylori. Proc. Natl. Acad. Sci. USA 95:13108-13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bieber, D., S. Ramer, C.-Y. Wu, W. Murray, T. Tobe, R. Fernandez, and G. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280:2114-2118. [DOI] [PubMed] [Google Scholar]
- 4.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bortolini, M., L. Trabulsi, R. Keller, G. Frankel, and V. Sperandio. 1999. Lack of expression of bundle-forming pili in some clinical isolates of enteropathogenic Escherichia coli (EPEC) is due to a conserved large deletion in the bfp operon. FEMS Microbiol. Lett. 179:169-174. [DOI] [PubMed] [Google Scholar]
- 6.Boyd, D. A., S. Tyler, S. Christianson, A. McGeer, M. P. Muller, B. M. Willey, E. Bryce, M. Gardam, P. Nordmann, M. R. Mulvey, et al. 2004. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob. Agents Chemother. 48:3758-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkley, C., V. Burland, R. Keller, D. J. Rose, A. T. Boutin, S. A. Klink, F. R. Blattner, and J. B. Kaper. 2006. Nucleotide sequence analysis of the enteropathogenic Escherichia coli adherence factor plasmid pMAR7. Infect. Immun. 74:5408-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke, S. C., R. D. Haigh, P. P. E. Freestone, and P. H. Williams. 2003. Virulence of enteropathogenic Escherichia coli, a global pathogen. Clin. Microbiol. Rev. 16:365-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott, S., V. Sperandio, J. Giron, S. Shin, J. Mellies, L. Wainwright, S. Hutcheson, T. McDaniel, and J. Kaper. 2000. The locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE- and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 68:6115-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falkow, S., and L. S. Baron. 1962. Episomic element in a strain of Salmonella typhosa. J. Bacteriol. 84:581-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girón, J. A., A. S. Ho, and G. K. Schoolnik. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254:710-713. [DOI] [PubMed] [Google Scholar]
- 12.Gross, R. J., L. R. Ward, E. J. Threlfall, H. King, and B. Rowe. 1982. Drug resistance among infantile enteropathogenic Escherichia coli strains isolated in the United Kingdom. Br. Med. J. Clin. Res. 285:472-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerra, B., E. Junker, A. Schroeter, R. Helmuth, B. E. Guth, and L. Beutin. 2006. Phenotypic and genotypic characterization of antimicrobial resistance in Escherichia coli O111 isolates. J. Antimicrob. Chemother. 57:1210-1214. [DOI] [PubMed] [Google Scholar]
- 14.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knutton, S., R. K. Shaw, R. P. Anantha, M. S. Donnenberg, and A. A. Zorgani. 1999. The type IV bundle-forming pilus of enteropathogenic Escherichia coli undergoes dramatic alterations in structure associated with bacterial adherence, aggregation and dispersal. Mol. Microbiol. 33:499-509. [DOI] [PubMed] [Google Scholar]
- 16.Levine, M. M., J. P. Nataro, H. Karch, M. M. Baldini, J. B. Kaper, R. E. Black, M. L. Clements, and A. D. O'Brien. 1985. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J. Infect. Dis. 152:550-559. [DOI] [PubMed] [Google Scholar]
- 17.Lim, Y. S., C. C. Ngan, and L. Tay. 1992. Enteropathogenic Escherichia coli as a cause of diarrhoea among children in Singapore. J. Trop. Med. Hyg. 95:339-342. [PubMed] [Google Scholar]
- 18.Lu, J., J. Manchak, W. Klimke, C. Davidson, N. Firth, R. A. Skurray, and L. S. Frost. 2002. Analysis and characterization of the IncFV plasmid pED208 transfer region. Plasmid 48:24-37. [DOI] [PubMed] [Google Scholar]
- 19.Mellies, J., S. Elliott, V. Sperandio, M. Donnenberg, and J. Kaper. 1999. The Per regulon in enteropathogenic Escherichia coli: identification of a regulatory cascade and a novel transcriptional activator, the locus of enterocyte effacement (LEE)-encoded regulator (Ler). Mol. Microbiol. 33:296-306. [DOI] [PubMed] [Google Scholar]
- 20.Moreira, C. G., K. Palmer, M. Whiteley, M. P. Sircili, L. R. Trabulsi, A. F. P. Castro, and V. Sperandio. 2006. Bundle-forming pili and EspA are involved in biofilm formation by enteropathogenic Escherichia coli. J. Bacteriol. 188:3952-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moyenuddin, M., I. K. Wachsmuth, S. L. Moseley, C. A. Bopp, and P. A. Blake. 1989. Serotype, antimicrobial resistance, and adherence properties of Escherichia coli strains associated with outbreaks of diarrheal illness in children in the United States. J. Clin. Microbiol. 27:2234-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nataro, J. P., K. O. Maher, P. Mackie, and J. B. Kaper. 1987. Characterization of plasmids encoding the adherence factor of enteropathogenic Escherichia coli. Infect. Immun. 55:2370-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NCCLS. 1990. Performance standards for antimicrobial disk susceptibility tests, 4th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Villanova, PA.
- 25.Nunes, E. B., H. O. Saridakis, K. Irino, and J. S. Pelayo. 2003. Genotypic and phenotypic characterization of attaching and effacing Escherichia coli (AEEC) isolated from children with and without diarrhoea in Londrina, Brazil. J. Med. Microbiol. 52:499-504. [DOI] [PubMed] [Google Scholar]
- 26.Okeke, I. N., A. Lamikanra, J. Czeczulin, F. Dubovsky, J. B. Kaper, and J. P. Nataro. 2000. Heterogeneous virulence of enteroaggregative Escherchia coli strains isolated from children in southwest Nigeria. J. Infect. Dis. 181:252-260. [DOI] [PubMed] [Google Scholar]
- 27.Okeke, I. N., J. A. Borneman, S. Shin, J. L. Mellies, L. E. Quinn, and J. B. Kaper. 2001. Comparative sequence analysis of the plasmid-encoded regulator of enteropathogenic Escherichia coli strains. Infect. Immun. 69:5553-5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okeke, I. N., I. C. Scaletsky, E. H. Soars, L. R. Macfarlane, and A. G. Torres. 2004. Molecular epidemiology of the iron utilization genes of enteroaggregative Escherichia coli. J. Clin. Microbiol. 42:36-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 30.Reid, S., C. Herbelin, A. Bumbaugh, R. Selander, and T. Whittam. 2000. Parallel evolution of virulence in pathogenic Escherichia coli. Nature 406:64-67. [DOI] [PubMed] [Google Scholar]
- 31.Riley, L. W., L. N. Junio, L. B. Libaek, and G. K. Schoolnik. 1987. Plasmid-encoded expression of lipopolysaccharide O-antigenic polysaccharide in enteropathogenic Escherichia coli. Infect. Immun. 55:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 33.Senerwa, D., L. N. Mutanda, J. M. Gathuma, and O. Olsvik. 1991. Antimicrobial resistance of enteropathogenic Escherichia coli strains from a nosocomial outbreak in Kenya. APMIS 99:728-734. [PubMed] [Google Scholar]
- 34.Slocombe, B., and R. Sutherland. 1973. Transferable antibiotic resistance in enteropathogenic Escherichia coli between 1948 and 1968. Antimicrob. Agents Chemother. 4:459-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sunabe, T., and Y. Honma. 1998. Relationship between O-serogroup and presence of pathogenic factor genes in Escherichia coli. Microbiol. Immunol. 42:845-849. [DOI] [PubMed] [Google Scholar]
- 36.Tobe, T., T. Hayashi, C. Han, G. Schoolnik, E. Ohtsubo, and C. Sasakawa. 1999. Complete DNA sequence and structural analysis of the enteropathogenic Escherichia coli adherence factor plasmid. Infect. Immun. 67:5455-5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trucksis, M., J. Michalski, Y. K. Deng, and J. B. Kaper. 1998. The Vibrio cholerae genome contains two unique circular chromosomes. Proc. Natl. Acad. Sci. USA 95:14464-14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vignoli, R., G. Varela, M. I. Mota, N. F. Cordeiro, P. Power, E. Ingold, P. Gadea, A. Sirok, F. Schelotto, J. A. Ayala, and G. Gutkind. 2005. Enteropathogenic Escherichia coli strains carrying genes encoding the PER-2 and TEM-116 extended-spectrum β-lactamases isolated from children with diarrhea in Uruguay. J. Clin. Microbiol. 43:2940-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vila, J., M. Vargas, C. Casals, H. Urassa, H. Mshinda, D. Schellemberg, and J. Gascon. 1999. Antimicrobial resistance of diarrheagenic Escherichia coli isolated from children under the age of 5 years from Ifakara, Tanzania. Antimicrob. Agents Chemother. 43:3022-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wain, J., L. T. Diem Nga, C. Kidgell, K. James, S. Fortune, T. Song Diep, T. Ali, P. O. Gaora, C. Parry, J. Parkhill, J. Farrar, N. J. White, and G. Dougan. 2003. Molecular analysis of incHI1 antimicrobial resistance plasmids from Salmonella serovar Typhi strains associated with typhoid fever. Antimicrob. Agents Chemother. 47:2732-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.