Abstract
Mycoplasma gallisepticum is an etiologic agent of chronic respiratory disease in chickens and infectious sinusitis in turkeys. Other than proteins important for cytadherence, few M. gallisepticum factors or pathways contributing to host cell interactions have been identified. In this study, an oligonucleotide-based microarray was utilized to investigate transcriptional changes in M. gallisepticum strain Rlow upon exposure to eukaryotic cells. Fifty-eight genes were either up- or downregulated upon exposure to MRC-5 lung fibroblasts grown in vitro, including genes encoding transport-, metabolism-, and translation-associated proteins. Twenty of the 58 regulated genes have no assigned function. These results indicate that M. gallisepticum regulates gene expression upon exposure to eukaryotic cells, revealing genes and pathways likely to be important for host-bacterium interaction.
Mycoplasma gallisepticum represents a major threat to the poultry industry, acting as an important pathogen in chronic respiratory disease and resulting in reduced weight gain and egg production in infected birds. M. gallisepticum is also pathogenic in other species, causing infectious sinusitis in turkeys and conjunctivitis in house finches (4, 15). With the exception of attachment proteins GapA and CrmA (10), and to a lesser extent dihydrolipoamide dehydrogenase (Lpd) (3), little is known about factors responsible for survival or persistence in the host.
Mycoplasmas lack obvious homologues of conventional elements of transcriptional regulation, including sigma factors, signaling factors, and transcription factors. This absence has led to the supposition that differences in gene expression in mycoplasma species are due to population selection and heterogeneity rather than more traditional mechanisms. Although several basic investigations into transcriptional responses have shown differences due to heat shock (5, 17) and iron depletion (6), changes that are not attributable to population selection, no study has thus far examined the whole transcriptomic response of mycoplasmas upon exposure to eukaryotic cells.
The availability of the genome sequence of M. gallisepticum strain R (11) enables a method of screening for transcriptomic changes; namely, an oligonucleotide-based microarray has been developed representing all known open reading frames (ORFs) based on this sequence. Utilizing this microarray, we investigated transcriptional changes when M. gallisepticum was incubated with a cell culture monolayer of human lung fibroblasts. In the absence of an established chicken trachea epithelial cell line, MRC-5 human lung fibroblasts have been used in previous studies as an in vitro model for M. gallisepticum interaction with host cells (9, 10, 12). This approach identified 25 upregulated and 33 downregulated transcripts that were differentially expressed upon incubation with MRC-5 cells and thus provide evidence of their function, suggesting a potential role in the interaction of M. gallisepticum with host cells in vivo.
MATERIALS AND METHODS
Microarray design.
An oligonucleotide-based microarray specific for M. gallisepticum strain Rlow was developed by MWG Biotech (Raleigh, NC). Oligonucleotides, each 50 nucleotides in length, were selected to represent each of the 756 putative ORFs. Thirty-six blank and eight Arabidopsis control features were included as negative controls. All features were spotted twice on glass slides, representing the genome in duplicate on each slide. Based on BLAST analysis (1), 21 features predicted to cross-hybridize with more than one genetic locus were excluded from further analysis.
Culture conditions and experimental design.
M. gallisepticum strain Rlow (passage 14) was cultured at 37°C in Hayflick's complete medium (2) until mid-log phase, as determined by color change and optical density. MRC-5 human lung fibroblasts (ATCC, Manassas, VA) were cultured to 95% confluence in 150-cm2 flasks (Fisher Scientific, Pittsburgh, PA) in minimal essential medium with 10% fetal bovine serum, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids at 37°C with 5% CO2. MRC-5 cell monolayers were washed three times in phosphate-buffered saline prior to exposure to mycoplasmas. Mid-log-phase Rlow cultures were pelleted by centrifugation at 10,000 × g for 10 minutes, resuspended in 10 ml of Hayflick's complete medium, and incubated with washed MRC-5 cells for 1 hour at 37°C. Mid-log-phase Rlow cultures, incubated 1 hour at 37°C, were used as reference samples for microarray and reverse transcriptase PCR (RT-PCR) analysis. Prior to RNA extraction, mycoplasma-fibroblast cocultures were washed three times with phosphate-buffered saline.
RNA extraction.
Total RNA was extracted from pelleted broth-grown Rlow, mycoplasma-MRC-5 cocultures, and MRC-5 monolayers using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RNA was treated with DNase (Sigma, St. Louis, MO) and purified using phenol-chloroform-isoamyl alcohol (Fisher Scientific), and concentration was determined based on absorbance at the 260-nm wavelength. Eukaryotic ribosomal and polyadenylated RNAs were removed from samples derived from infected monolayers using the MICROBEnrich kit according to the manufacturer's instructions (Ambion, Austin, TX). RNA extracted from broth-grown Rlow cultures was also treated once according to the protocol of the MICROBEnrich kit as a control. Each RNA sample was viewed in a 0.8% agarose gel to confirm RNA integrity.
Microarray hybridization.
Fifty micrograms of total RNA from each condition was reverse transcribed using the Amino Allyl cDNA labeling kit (Ambion) according to the manufacturer's instructions. Samples were labeled with either Cy3 or Cy5 (Amersham Biosciences, Buckinghamshire, United Kingdom), excess dye was removed using the Nuc-Away spin columns provided in the Amino Allyl cDNA labeling kit, and labeled cDNA was resuspended in hybridization buffer (MWG Biotech). Microarray slides were blocked in blocking buffer (1% bovine serum albumin and 2% sodium dodecyl sulfate in 1× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]) for 1 hour at 42°C and washed three times in 0.1× SSC buffer prior to hybridization. Labeled cDNA was resuspended in hybridization buffer, heated briefly at 95°C, cooled on ice, hybridized to the blocked slide at 42°C for 18 hours, and then washed three times with decreasing concentrations of SSC buffer (2× SSC with 0.1% sodium dodecyl sulfate, 1× SSC, and 0.1× SSC). Separate RNA extractions and hybridizations were repeated five times, switching sample dyes for two of the five experiments.
Data acquisition and analysis.
Microarray slides were scanned using a GenePix 4000B scanner (Axon Instruments, Sunnyvale, CA), and data were acquired with the GenePix 4.0 (Axon Instruments). Features were individually analyzed and normalized using the Bayesian Analysis of Gene Expression Level (BAGEL) model (8) (University of California, Berkeley, CA). Briefly, fluorescence levels for each feature in each data set are normalized, and then a relative expression level and a P value are determined across all data sets through the application of a specific algorithm by the BAGEL model. Up- or downregulated genes were selected by averaging values across duplicate features and filtering reproducible features that were significantly up- or downregulated more than 1.5-fold (P < 0.05). The relative expression level for each transcript was averaged across duplicate features.
Validation of microarray data.
Relative transcription levels of differentially regulated transcripts were investigated by RT-PCR. RNA was generated in three separate extractions from Rlow grown in broth culture and mycoplasma-MRC-5 cocultures as described above with the addition of RNALater (Ambion) according to the manufacturer's instructions. All primers (MWG Biotech) were used at a final concentration of 400 nM and checked for cross-hybridization with MRC-5 RNA. Reactions were performed using 100 ng and 10 ng of RNA and the SuperScript RT-PCR kit (Invitrogen). Cycling conditions for RT-PCRs were as follows: 50°C reverse transcription for 25 min, 94°C denaturation for 2 minutes, amplification for 35 cycles using 94°C denaturation for 30 seconds, 50°C annealing for 30 seconds, and 72°C extension for 45 seconds, followed by a final 72°C extension for 10 min. Contaminating DNA was detected by replacing the SuperScript Platinum Taq in the reaction mix with AmpliTaq (Applied Biosystems, Foster City, CA). Reactions were run in a 0.8% agarose gel with 0.01% ethidium bromide. Pictures of each gel were taken under UV light and scanned at 300 dpi. Bands of the same dilution were densitometrically compared using Adobe Photoshop 6.0 by measuring mean pixel intensity, subtracting background, and averaging the triplicate results for each condition.
Microarray data accession number.
Microarray data were submitted to the Gene Expression Omnibus database under the accession number GSE6717.
RESULTS
Transcriptional profiling of M. gallisepticum strain R interacting with host cells.
Transcriptomic comparisons of Rlow cultures grown in broth in relation to cultures incubated with MRC-5 cell monolayers were investigated by microarray analysis a total of five times. Duplicate features that reproducibly showed a significant (P < 0.05) increase or decrease in signal greater than 1.5-fold were included in the data set. A total of 25 upregulated (Table 1) and 33 downregulated (Table 2) transcripts met these criteria.
TABLE 1.
Transcripts upregulated when Rlow is exposed to MRC-5 cells
| ORF product identity | ORF | Mean relative increase in expression | SD of relative increase in expression | P value | Validation by RT-PCRa |
|---|---|---|---|---|---|
| Unique hypothetical | MGA_122 | 1.81 | 0.19 | 0.023 | ND |
| Unique hypothetical | MGA_184 | 2.12 | 0.13 | 0.007 | Yes |
| SrmB ATP-dependent helicase | MGA_206 | 3.03 | 0.27 | 0.004 | ND |
| OppD oligopeptide transport ATP-binding protein | MGA_232 | 2.49 | 0.07 | 0.013 | ND |
| RpsB ribosomal protein S2 | MGA_242 | 2.08 | 0.02 | 0.048 | ND |
| 50S ribosomal protein I28 | MGA_253 | 3.62 | 0.16 | 0.003 | ND |
| RpsG ribosomal protein S7 | MGA_261 | 1.78 | 0.04 | 0.022 | ND |
| Unique hypothetical | MGA_267 | 6.63 | 1.59 | 0.000 | Yes |
| Unique hypothetical | MGA_324 | 1.61 | 0.02 | 0.045 | ND |
| Conserved hypothetical | MGA_340 | 1.87 | 0.04 | 0.046 | ND |
| Hypothetical macrophage-activating protein, p47 | MGA_398 | 2.95 | 0.54 | 0.013 | ND |
| RpsR ribosomal protein S18 | MGA_421 | 1.89 | 0.02 | 0.024 | ND |
| RpsP ribosomal protein S16 | MGA_440 | 1.77 | 0.07 | 0.006 | ND |
| RnpA RNase P protein component | MGA_630 | 2.01 | 0.38 | 0.030 | ND |
| RpsI ribosomal protein S10 | MGA_705 | 2.06 | 0.04 | 0.027 | ND |
| RpsT ribosomal protein S20 | MGA_844 | 2.88 | 0.14 | 0.014 | ND |
| RplJ ribosomal protein L10 | MGA_872 | 1.86 | 0.10 | 0.025 | Yes |
| Unique hypothetical | MGA_875 | 3.80 | 0.31 | 0.008 | ND |
| VlhA 4.01 pMGA family protein | MGA_966 | 2.41 | 0.39 | 0.024 | ND |
| Conserved hypothetical lipoprotein | MGA_993 | 3.56 | 0.22 | 0.002 | ND |
| Conserved hypothetical | MGA_1107 | 3.63 | 0.33 | 0.008 | Yes |
| ABC-type transport system, permease | MGA_1140 | 3.08 | 0.87 | 0.034 | ND |
| RplM ribosomal protein L13 | MGA_1154 | 1.98 | 0.06 | 0.012 | ND |
| RplU ribosomal protein L21 | MGA_1290 | 2.63 | 0.19 | 0.016 | ND |
| RpmA ribosomal protein L27 | MGA_1292 | 3.21 | 0.08 | 0.003 | ND |
ND, not done.
TABLE 2.
Transcripts downregulated when Rlow is exposed to MRC-5 cells
| ORF product identity | ORF | Mean relative decrease in expression | SD of relative decrease in expression | P value | Validation by RT-PCRa |
|---|---|---|---|---|---|
| PepB leucyl aminopeptidase | MGA_114 | 1.62 | 0.03 | 0.030 | ND |
| Conserved hypothetical | MGA_123 | 2.09 | 0.02 | 0.002 | ND |
| GroEL heat shock protein | MGA_152 | 1.67 | 0.08 | 0.040 | ND |
| Lpd dihydrolipoamide dehydrogenase | MGA_161 | 2.28 | 0.27 | 0.005 | Yes |
| AceF dihydrolipoamide acetyltransferase | MGA_162 | 2.22 | 0.10 | 0.024 | Yes |
| Hypothetical methyltransferase | MGA_249 | 1.93 | 0.08 | 0.011 | ND |
| Hlp1 cytoskeletal protein (HMW1-like protein) | MGA_306 | 2.21 | 0.18 | 0.005 | Yes |
| CpsG phosphomannomutase | MGA_358 | 1.64 | 0.07 | 0.031 | ND |
| DeoD purine-nucleoside phosphorylase | MGA_364 | 2.61 | 0.06 | 0.007 | ND |
| GatB glutamyl/aspartyl tRNA amidotransferase | MGA_412 | 1.52 | 0.02 | 0.045 | ND |
| TopA DNA topoisomerase I | MGA_454 | 1.74 | 0.07 | 0.037 | ND |
| Rpe pentose-5-phosphate-3-epimerase | MGA_455 | 2.41 | 0.43 | 0.020 | ND |
| Unique hypothetical | MGA_482 | 2.12 | 0.32 | 0.016 | ND |
| Conserved hypothetical | MGA_484 | 1.94 | 0.12 | 0.033 | ND |
| Unique hypothetical | MGA_487 | 2.14 | 0.13 | 0.024 | ND |
| HsdS restriction endonuclease S subunit | MGA_539 | 1.53 | 0.17 | 0.021 | ND |
| Unique hypothetical | MGA_573 | 2.56 | 0.18 | 0.000 | ND |
| SerS seryl-tRNA synthetase | MGA_608 | 3.33 | 0.31 | 0.001 | ND |
| Dihydroxyacetone kinase | MGA_661 | 3.44 | 0.26 | 0.002 | Yes |
| Hypothetical nuclease | MGA_676 | 1.92 | 0.32 | 0.027 | Yes |
| ABC transporter ATP-binding protein | MGA_677 | 2.36 | 0.22 | 0.010 | ND |
| Mdh lactate dehydrogenase | MGA_746 | 3.50 | 0.59 | 0.004 | Yes |
| Unique hypothetical | MGA_867 | 2.30 | 0.46 | 0.004 | ND |
| Hlp3 cytoskeletal protein (HMW3-like protein) | MGA_928 | 1.81 | 0.14 | 0.022 | No |
| Conserved hypothetical | MGA_1011 | 2.44 | 0.22 | 0.003 | ND |
| Exo 5′-3′ exonuclease | MGA_1052 | 1.84 | 0.24 | 0.026 | ND |
| AtpB ATP synthase a subunit | MGA_1164 | 3.38 | 1.14 | 0.015 | Yes |
| AtpF ATP synthase b subunit | MGA_1168 | 2.97 | 0.98 | 0.009 | Yes |
| AtpG ATP synthase g subunit | MGA_1174 | 3.93 | 2.32 | 0.012 | ND |
| AtpD ATP synthase b subunit | MGA_1177 | 3.03 | 0.72 | 0.011 | Yes |
| PlpA fibronectin-binding protein | MGA_1199 | 2.29 | 0.23 | 0.003 | Yes |
| Unique hypothetical | MGA_1224 | 2.90 | 1.20 | 0.037 | No |
| GrpE heat shock protein | MGA_1232 | 2.23 | 0.11 | 0.020 | ND |
ND, not done.
Validation of microarray data.
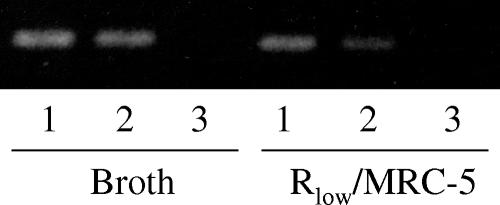
Sixteen transcripts identified as differentially regulated by the microarray were chosen randomly for validation by RT-PCR, representing 27% of the total data set. Specific primers were designed for each transcript (Table 3), and transcripts were compared between Rlow grown in broth culture and Rlow incubated with MRC-5 cells. Equal amounts of RNA were reverse transcribed, and band intensities of the resulting products were compared by measuring mean pixel intensity using Adobe Photoshop 6.0. This comparison was performed on three separate extractions of RNA for each condition. Fourteen of the 16 transcripts tested (88%) showed an upregulation or downregulation consistent with the microarray data. Figure 1 shows an example comparison of one replicate for both conditions at two different dilutions, displaying a clear downregulation of AtpB in Rlow associated with MRC-5 cells compared to the broth-grown culture. Densitometric analysis of the reaction shown indicated a 2.5-fold downregulation of this gene in Rlow incubated with MRC-5 cells compared with Rlow grown in broth culture. RT-PCR was performed on 23S rRNA and dnaK as housekeeping genes, as these genes would not be expected to be differentially regulated and were not predicted to be differentially regulated by the microarray results. These genes did not show any difference by RT-PCR (data not shown).
TABLE 3.
Primers used in RT-PCR
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| 23S rRNA | AGTCAAACCGTGAGGATTGG | CAAATCCGATAATGGGGATG |
| DnaK | GACCTTGGTGGTACGTT | CCACGTAATACCCCACCTTG |
| MGA_184 | TGAACTCATCAAGGGTTGGTT | CCAGCGCGACAATTCATAA |
| MGA_267 | CGGTTCTGCACAAGCTAACA | CTTTCATGCACCAGAAAGCA |
| RplJ | AGCTGGCATTGATGGATTTG | ACTGGAGCTTGAAGCACTGA |
| MGA_1107 | CTATTGCGCAGCAAAATGAA | CGTTGGTAGTTATGCCTTCG |
| Lpd | GGGTGAATTGCTTGTTCT | GTGGTTTCTAAGATGCCA |
| AceF | CCAGTTGCGACTCCTTTAGC | GCTGGAATTTCTTCGTGAGC |
| Hlp1 | TCGAAGTAACCAGTTCAAACTCA | AGGCACGGAATTTATTGTGG |
| MGA_661 | GGGGATTTTGTTGATTCA | CTGCCATTAACTCTTGAG |
| MGA_676 | CCAGCTGATAGCAAACCGTT | GCTGCAATCCCAATTGGTCTA |
| Mdh | TGTGACGTAATGGCTGGTGT | TAACTTCCATCCGTGCTTGC |
| Hlp3 | TGATTACTACCCACCAGCTTATGA | CTTCGCACTCTTGGTTGTTG |
| AtpB | TTCCGACTGCTCATGTCTTG | TTTTCTCGCCCAATAGATCG |
| AtpF | CAAGCACGCGAGATTATCAA | ACATCAACGATCCGACCTTC |
| AtpD | CAACCGTTCTTTGTTGCTGA | CTTCATCGATCGAACCAACA |
| PlpA | AAAGAAGAGATCGACAGCTTGC | TAACGGTTATTGTAAGGGTC |
| MGA_1224 | GGGAATAGTCGATGGATA | CGACTTGGGGTTCTTCTAGG |
FIG. 1.
RT-PCR amplification of AtpB transcript from M. gallisepticum Rlow grown in broth culture and attached to MRC-5 cells. RT-PCR was performed on serially diluted RNA extracted from Rlow grown in broth and from Rlow exposed to MRC-5 cells. A representative one of three replicates is shown here including the following serial dilutions: lane 1, 100 ng; lane 2, 10 ng; lane 3, control for DNA contamination. Downregulation of AtpB, 1 of the 16 transcripts tested, in the presence of MRC-5 cells is shown here.
DISCUSSION
This study presents, for the first time, evidence of transcriptional regulation in M. gallisepticum in response to environmental conditions and substantiates existing reports of transcriptional regulation in mycoplasmas (5, 6, 17). The 1-hour incubation time in these experiments was specifically chosen to be shorter than a generation time (approximately 2 hours) to eliminate the influence of population selection or outgrowth. Criteria, specifically a P of <0.05 and at least a 1.5-fold change, were selected in order to include genes that were significantly and clearly differentially regulated upon exposure to lung fibroblasts in culture. These genes included 25 upregulated and 33 downregulated transcripts, representing approximately 8% of the predicted M. gallisepticum transcriptome. When 16 of these transcripts were examined by RT-PCR, 14 (88%) demonstrated relative transcriptional differences that were consistent with the microarray results.
These experiments verified the upregulated expression of 10 ORFs annotated as being hypothetical, supporting their role as functional genes. Six of these ORFs are unique to M. gallisepticum, and three are conserved among other mycoplasmas. Additionally, one hypothetical protein (p47) was identified by Markham et al. as having sequence homology to a macrophage-activating lipoprotein but was determined to have no role in pathogenicity or colonization based on the behavior of an isogenic mutant in a tracheal explant model (7). Similarly, 10 hypothetical transcripts were downregulated, 5 of which are conserved primarily among other mycoplasmas and other related bacterial species. Two conserved hypothetical transcripts were further described to contain predicted functional domains based on BLAST analysis: MGA_676 contains a predicted nuclease domain, while MGA_249 contains a predicted methylase domain. This annotation does not assign specific functions to these transcripts, however.
Genes encoding ribosomal or translation-associated proteins were upregulated in the mycoplasmas associated with MRC-5 cells. These proteins included 10 ribosomal proteins as well as an RNA helicase (SrmB), which is involved in ribosome assembly, and RNase P (RnpA), which completes the development of tRNAs. A similar pattern was observed in Campylobacter jejuni cultured in rabbit ileal loops: the majority of ribosomal proteins were upregulated when attached to the intestinal epithelium (16). A number of ribosomal transcripts were previously found to be upregulated in Mycoplasma pneumoniae following heat shock (17), indicating that this response may be due to environmental stress; however, a similar response was not observed in a heat shock study on another mycoplasma species, Mycoplasma hyopneumoniae (5). Whether in response to the host cell specifically or a general stress response, this pattern may simply reflect a global increase in translation.
Eleven metabolism-associated transcripts are downregulated upon exposure to MRC-5 cells, including four of the eight ATP synthase subunits and two of the four components of the pyruvate dehydrogenase complex. One of these components, Lpd, had been shown previously to be a virulence-related determinant by virtue of the attenuation of an isogenic mutant (3). The observed downregulation in these experiments is not necessarily contradictory to the previous report, however, as the Lpd mutant is introduced to the host with this pyruvate dehydrogenase deficiency, affecting its survival. Wild-type Rlow, conversely, will have a normal level of activity upon introduction to the host and may downregulate the pyruvate dehydrogenase complex subsequently, corresponding to the decrease observed here. Additional metabolism-related enzymes that are downregulated include a hypothetical protein possessing a dihydroxyacetone kinase domain (MGA_661), phosphomannomutase (CpsG), pentose-5-phosphate-3-epimerase (Rpe), purine-nucleoside phosphorylase (DeoD), and malate/lactate dehydrogenase (Mdh). With the exception of Rpe, all of these enzymes are in pathways involved in the metabolism of substrates other than glucose. Specifically, dihydroxyacetone kinase is involved in glycerol metabolism, DeoD is involved in nucleotide metabolism, and CpsG is involved in GDP-mannose metabolism (14). This pattern suggests that when M. gallisepticum is in association with the host cell, glucose may be available as a primary energy source, allowing alternative pathways to be downregulated.
As mentioned above, four of the eight ATP synthase subunits were downregulated when M. gallisepticum was in contact with host cells. In the majority of prokaryotes, the ATP synthase complex maintains a proton gradient through catabolism and hydrolysis of ATP; however, since mycoplasmas lack an electron transport chain necessary for further generation of ATP, the complex is believed to function primarily to maintain the electrochemical gradient. Published reports also suggest that the “reverse” ATP-generating function of this complex is active in mycoplasmas; namely, ATP may be generated by electron transfer to flavoproteins using oxygen as an intermediate rather than a terminator (flavin-terminated respiration) (13), and the b subunit of the complex, shown to be downregulated in this study, is likely surface exposed and thus potentially able to act in reverse (18). The observed transcriptional decrease of ATP synthase genes could, alternatively, reflect uptake of metabolic precursors from the host cell.
The data presented here are the first assessment of transcriptional responses of M. gallisepticum associated with a eukaryotic cell. The specific roles of the identified transcripts in vivo will have to be further investigated. In particular, the transcriptional responses presented here are specifically a result of an interaction with the host cell, but whether the attachment mechanism, the invasion of the host cells (19), or another component of the host cell is responsible remains to be determined. The downregulation of metabolism-associated genes when mycoplasmas are in contact with a host cell could be described as a shift to parasitism with the uptake of substrates from the host cell compared to the noncompetitive environment of a nutrient-rich growth medium. This change is particularly supported by the streamlining of metabolic pathways to possibly focus on a greater availability of glucose (or more directly, ATP) in association with the eukaryotic cell. The hypothetical ORFs upregulated in the presence of eukaryotic cells may be involved in colonization of the host. These data provide insight into the responses of M. gallisepticum to interaction with the host cell and provide candidates for further investigation into their roles in M. gallisepticum-host interaction.
Acknowledgments
We thank Theresa Smith, of the University of Connecticut Biotechnology/Bioservices Center, Animal Cell Culture Facility, for cell culture services and Edan Tulman for helpful discussions.
This work was supported by USDA grant 58-1940-0-007.
Footnotes
Published ahead of print on 8 June 2007.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Hayflick, L. 1965. Tissue cultures and mycoplasmas. Tex. Rep. Biol. Med. 23(Suppl. 1):285. [PubMed] [Google Scholar]
- 3.Hudson, P., T. S. Gorton, L. Papazisi, K. Cecchini, S. Frasca, Jr., and S. J. Geary. 2006. Identification of a virulence-associated determinant, dihydrolipoamide dehydrogenase (lpd), in Mycoplasma gallisepticum through in vivo screening of transposon mutants. Infect. Immun. 74:931-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ley, D. H., J. E. Berkhoff, and J. M. McLaren. 1996. Mycoplasma gallisepticum isolated from house finches (Carpodacus mexicanus) with conjunctivitis. Avian Dis. 40:480-483. [PubMed] [Google Scholar]
- 5.Madsen, M. L., D. Nettleton, E. L. Thacker, R. Edwards, and F. C. Minion. 2006. Transcriptional profiling of Mycoplasma hyopneumoniae during heat shock using microarrays. Infect. Immun. 74:160-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madsen, M. L., D. Nettleton, E. L. Thacker, and F. C. Minion. 2006. Transcriptional profiling of Mycoplasma hyopneumoniae during iron depletion using microarrays. Microbiology 152:937-944. [DOI] [PubMed] [Google Scholar]
- 7.Markham, P. F., A. Kanci, G. Czifra, B. Sundquist, P. Hains, and G. F. Browning. 2003. Homologue of macrophage-activating lipoprotein in Mycoplasma gallisepticum is not essential for growth and pathogenicity in tracheal organ cultures. J. Bacteriol. 185:2538-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meiklejohn, C. D., and J. P. Townsend. 2005. A Bayesian method for analysing spotted microarray data. Brief. Bioinform. 6:318-330. [DOI] [PubMed] [Google Scholar]
- 9.Mudahi-Orenstein, S., S. Levisohn, S. J. Geary, and D. Yogev. 2003. Cytadherence-deficient mutants of Mycoplasma gallisepticum generated by transposon mutagenesis. Infect. Immun. 71:3812-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papazisi, L., S. Frasca, Jr., M. Gladd, X. Liao, D. Yogev, and S. J. Geary. 2002. GapA and CrmA coexpression is essential for Mycoplasma gallisepticum cytadherence and virulence. Infect. Immun. 70:6839-6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papazisi, L., T. S. Gorton, G. Kutish, P. F. Markham, G. F. Browning, D. K. Nguyen, S. Swartzell, A. Madan, G. Mahairas, and S. J. Geary. 2003. The complete genome sequence of the avian pathogen Mycoplasma gallisepticum strain R(low). Microbiology 149:2307-2316. [DOI] [PubMed] [Google Scholar]
- 12.Papazisi, L., K. E. Troy, T. S. Gorton, X. Liao, and S. J. Geary. 2000. Analysis of cytadherence-deficient, GapA-negative Mycoplasma gallisepticum strain R. Infect. Immun. 68:6643-6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollack, J. D. 1992. Carbohydrate metabolism and energy conservation, p. 181-200. In J. Maniloff (ed.), Mycoplasmas: molecular biology and pathogenesis. American Society for Microbiology, Washington, DC.
- 14.Pollack, J. D. 1997. Mycoplasma genes: a case for reflective annotation. Trends Microbiol. 5:413-419. [DOI] [PubMed] [Google Scholar]
- 15.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stintzi, A., D. Marlow, K. Palyada, H. Naikare, R. Panciera, L. Whitworth, and C. Clarke. 2005. Use of genome-wide expression profiling and mutagenesis to study the intestinal lifestyle of Campylobacter jejuni. Infect. Immun. 73:1797-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiner, J., III, C. U. Zimmerman, H. W. Gohlmann, and R. Herrmann. 2003. Transcription profiles of the bacterium Mycoplasma pneumoniae grown at different temperatures. Nucleic Acids Res. 31:6306-6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weislander, A., and M. Rosen. 2002. The cell membrane and transport, p. 131-162. In S. Razin and R. Herrmann (ed.), Molecular biology and pathogenicity of mycoplasmas. Kluwer Academic/Plenum Publishers, New York, NY.
- 19.Winner, F., R. Rosengarten, and C. Citti. 2000. In vitro cell invasion of Mycoplasma gallisepticum. Infect. Immun. 68:4238-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]