Abstract
The regulatory network for the uptake of Escherichia coli autoinducer 2 (AI-2) is comprised of a transporter complex, LsrABCD; its repressor, LsrR; and a cognate signal kinase, LsrK. This network is an integral part of the AI-2 quorum-sensing (QS) system. Because LsrR and LsrK directly regulate AI-2 uptake, we hypothesized that they might play a wider role in regulating other QS-related cellular functions. In this study, we characterized physiological changes due to the genomic deletion of lsrR and lsrK. We discovered that many genes were coregulated by lsrK and lsrR but in a distinctly different manner than that for the lsr operon (where LsrR serves as a repressor that is derepressed by the binding of phospho-AI-2 to the LsrR protein). An extended model for AI-2 signaling that is consistent with all current data on AI-2, LuxS, and the LuxS regulon is proposed. Additionally, we found that both the quantity and architecture of biofilms were regulated by this distinct mechanism, as lsrK and lsrR knockouts behaved identically. Similar biofilm architectures probably resulted from the concerted response of a set of genes including flu and wza, the expression of which is influenced by lsrRK. We also found for the first time that the generation of several small RNAs (including DsrA, which was previously linked to QS systems in Vibrio harveyi) was affected by LsrR. Our results suggest that AI-2 is indeed a QS signal in E. coli, especially when it acts through the transcriptional regulator LsrR.
Bacteria communicate with each other through small “hormone-like” organic molecules referred to as autoinducers. Autoinducer-based bacterial cell-to-cell communication, enabling population-based multicellularity, has been termed quorum sensing (QS) (27). Cellular functions controlled by QS are varied and reflect the needs of a particular bacterial species for inhabiting a given niche (10, 38, 65).
QS among Escherichia coli and Salmonella strains has been a topic of great interest, and different intercellular signaling systems have been identified: that mediated by the LuxR homolog SdiA; the LuxS/autoinducer 2 (AI-2) system, an AI-3 system, and a signaling system mediated by indole (2, 19, 36, 57, 61, 68). Among these systems, the LuxS/AI-2 system possesses the unique feature of endowing cell population-dependent behavior while interacting with central metabolism through the intracellular activated methyl cycle (20, 21, 45, 65, 73). Therefore, it has the potential to influence both gene regulation and bacterial fitness.
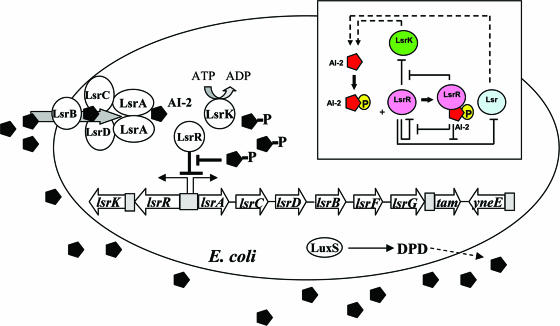
AI-2's function has been studied using luxS mutants and by adding either conditioned medium or in vitro-synthesized AI-2 to bacterial cultures. It is noteworthy that the luxS transcription profile is not synchronous with the accumulation profile of extracellular AI-2 in bacterial supernatants (5, 31, 75). In E. coli, extracellular AI-2 activity peaks during the mid- to late-exponential phase and rapidly decreases during entry into the stationary phase. A corresponding decrease in LuxS protein levels is not observed (31, 75). The disappearance of extracellular AI-2 activity in E. coli and Salmonella enterica serovar Typhimurium is due to its uptake, carried out by its import through an ATP-binding cassette (ABC) transporter named the luxS-regulated (Lsr) transporter (62, 69, 75). The transporter proteins are part of the lsr operon, which is regulated by cyclic AMP/cyclic AMP receptor protein and proteins transcribed from two genes, lsrK and lsrR, located immediately upstream of lsr and divergently transcribed in its own lsrRK operon (70). The cytoplasmic kinase LsrK phosphorylates AI-2 into an activated molecule that is suggested to bind and derepress the lsr repressor LsrR. A conceptual model of the LsrR/phospho-AI-2 circuit is provided in Fig. 1.
FIG. 1.
Regulatory mechanisms of the LsrR/phospho-AI-2 circuit in E. coli AI-2 uptake (modified from reference 70). The AI-2 uptake repressor LsrR represses many genes including the lsr operon (comprised of lsrACDBFG) and the lsrRK operon. AI-2 can be imported back inside the cell via LsrACDB. Imported AI-2 is processed as phospho-AI-2 via the kinase LsrK. Phospho-AI-2 has been reported to bind LsrR and relieve its repression of the lsr transporter genes, triggering their expression. This in turn stimulates additional AI-2 uptake. DPD, 4,5-dihydroxy-2,3-pentanedione.
LsrR and LsrK were among the first positively identified QS regulators in E. coli (19, 70, 75). Since QS regulators are responsible for mediating many cellular phenotypes and morphologies (18, 30, 34), the functions of LsrR and LsrK are of great interest. Furthermore, it is well known that AI-2 uptake is an integral part of the E. coli QS network; thus, it remains intriguing that these bacteria actively transport its QS autoinducer. In many other systems, the signal molecule is freely diffused or binds a cognate receptor, triggering a sensor-kinase couple. It is possible that AI-2-signaling bacteria import and internalize AI-2 to terminate extracellular AI-2-dependent cellular responses and alternatively trigger cytoplasmic AI-2-dependent gene expression, akin to a genetic switching mechanism.
The physiological functions associated with either extracellular or cytoplasmic AI-2 can be partially revealed using lsrK and lsrR mutants. In lsrK mutants, Lsr transporter expression is repressed, and AI-2 remains in the supernatant (extracellular AI-2) (69). In lsrR mutants, the Lsr transporter is expressed, and extracellular AI-2 is continuously imported into the cell (cytoplasmic AI-2), irrespective of its accumulation (62, 69). We carried out genome-wide transcriptome analyses of lsrR and lsrK mutants relative to the isogenic parent strain W3110. We further evaluated physiological changes (biofilm formation, motility, etc.) resulting from the mutations. We found that lsrR and lsrK serve as global regulators of gene expression and affect biofilm architecture through the coordinate regulation of biofilm-related genes such as wza (responsible for colanic acid) and the autoaggregation gene flu. While the expression of many important genes was found to be altered by lsrR and lsrK deletions (and are putatively regulated by LsrRK), those associated with host invasion, stress responses, and foreign DNA were most prevalent. For the first time, small riboregulators were shown to respond to the QS regulators lsrR and lsrK. Finally, and perhaps most importantly, our results suggest that lsrR and lsrK (or, more specifically, LsrR and AI-2) operate in tandem and in the inverse of their role in regulating AI-2 uptake. Positive identification of LsrR/AI-2 signaling sheds new light on the widely discussed differences between AI-2, the metabolic by-product, and AI-2, the QS signaling molecule (70, 73).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli K-12 strain W3110 (F− λ− in rrnD-rrnE) was obtained from the Genetic Stock Center (New Haven, CT). Details of its kanamycin-resistant isogenic mutants used in this study, W3110 ΔlsrR and W3110 ΔlsrK, were described elsewhere previously (69). E. coli strain ZK2686 [W3110 Δ(argF-lac)U169] and its isogenic agn43 mutant ZK2692 (ZK2686 agn43::cam) were kindly provided by R. Kolter (16). Luria-Bertani broth (LB) contains 5 g liter−1 yeast extract (Difco), 10 g liter−1 Bacto tryptone (Difco), and 10 g liter−1 NaCl. Cultures of E. coli (wild type and ΔlsrR and ΔlsrK mutants) grown overnight in LB were diluted to an optical density at 600 nm (OD600) of ∼0.03 in LB and subsequently incubated at 30°C and 250 rpm in two 50-ml shake flasks. When the cultures reached the appropriate OD600 (2.4), the cells were harvested for RNA extraction.
RNA isolation, cDNA generation, and microarray processing.
Total RNA was isolated using RNeasy Mini kits (QIAGEN Inc., Valencia, CA) according to the manufacturer's instructions. cDNA was synthesized and labeled according to the manufacturer's suggestions for E. coli Antisense genome arrays (Affymetrix Inc., Santa Clara, CA). Further preparation, hybridization, and scanning were carried out as previously described (70). Reverse transcription (RT)-PCR was also performed as previously reported (70), except that an Applied Biosystems 7300 real-time PCR system (Applied Biosystems) was used. 16S rRNA was used for normalizing all reactions; its transcript levels showed minimal variation between wild-type and mutant cells (data not shown).
Microarray data analysis.
Microarray data were analyzed with the Affymetrix Microarray Suite software 5.1 (Affymetrix Inc., Santa Clara, CA) and a four-comparison survival method (15). The fluorescence of each array was normalized by scaling total chip fluorescence intensities to a common value of 500. For each growth condition, two independent experimental cell cultures (wild type) were compared with two independent control groups (ΔlsrR or ΔlsrK mutant), and four comparisons were made. The change (n-fold) for each gene was calculated by dividing the signal intensity for these two mutants by the signal intensity for the wild type. The reported values for the change (n-fold) are the averages of the four comparisons. Genes with consistent increases or decreases in all comparisons were determined and used for the analysis. However, the induced genes with absent calls of the array signal in the experimental groups and the repressed genes with absent calls of the array signal in the control groups were eliminated. Determinations of functional categories were based on the E. coli K-12 MG1655 database from TIGR (http://www.tigr.org/tigr-scripts/CMR2/gene_table.spl?db=ntec01).
SEM.
Cells were collected and gently washed three times with Millonig's phosphate buffer (pH 7.3) (centrifugation at 2,000 × g for 10 min) and fixed with 2% glutaraldehyde (1 h at room temperature and 9 h at 4°C). Cells were collected with 0.2-μl filters, and residual glutaraldehyde was washed out using Millonig's phosphate buffer three times before cells were further fixed in 1% OsO4. The filters were then dehydrated with ethanol (70%, 95%, and 100%). The filters were fully dehydrated in a Denton vacuum freezer (Denton DCP-1 critical-point dryer) and coated with Ag-Pd (Denton DV 502/503 vacuum evaporator). Coated filters were examined by scanning electron microscopy (SEM) at the Biological Ultrastructure Laboratory of the University of Maryland (College Park, MD).
Autoaggregation assay.
Autoaggregation assays were performed as described previously (32), with slight modifications. Cultures grown overnight were adjusted to the same optical densities, and 10 ml of each culture was placed into a 15-ml Falcon tube and kept on ice. At each time point, 100-μl samples were taken from each tube, ∼1 cm from the top, and transferred into new tubes containing 1 ml 0.9% NaCl for measuring optical densities.
Flow cell biofilm experiments and image analysis.
E. coli K-12 W3110 cells constitutively expressing green fluorescent protein via pCM18 were streaked onto LB agar plates with erythromycin (300 μg/ml) and grown in the same medium overnight. E. coli K-12 W3110 ΔlsrK:Kanr/pCM18 and E. coli K12 W3110 ΔlsrR:Kanr/pCM18 were streaked onto LB agar plates with erythromycin (300 μg/ml) and kanamycin (50 μg/ml) and then grown overnight in the same medium. Cultures grown overnight were diluted into LB and erythromycin (300 μg/ml) to reach an OD600 of 0.05. The flow cell (22) was inoculated for 2 h at 30°C with 200 ml of these cells, and fresh medium was then added at a flow rate of 10 ml/h for 49 h. The number of cells in the culture after 2 h of inoculation was 1.4 × 105 to 3.2 × 105 cells/ml. For the wild-type strain, biofilm formation was not significant at 24 h, so only images at 49 h were taken for all three strains. Green fluorescent protein was visualized by excitation with an Ar laser at 488 nm (emission, 510 to 530 nm) using a TCS SP5 scanning confocal laser microscope with a 63× HCX PL FLUOTAR L dry objective with a correction collar and a numerical aperture of 0.7 (Leica Microsystems, Mannheim, Germany). Color confocal flow cell images were converted to gray scale using an Image Converter (Neomesh Microsystems, Wainuiomata, Wellington, New Zealand). Biomass, substratum coverage, surface roughness, and mean thickness were determined using COMSTAT image-processing software (22) written as a script in Matlab 5.1 (The MathWorks) and equipped with the Image Processing Toolbox. Thresholding was fixed for all image stacks. At each time point, nine different positions were chosen for microscope analysis, and 225 images were processed for each position. Values are means of data from the different positions at the same time point, and standard deviations were calculated based on these mean values for each position. Reconstructed three-dimensional images were obtained using IMARIS (BITplane, Zurich, Switzerland). Twenty-five pictures were processed for each three-dimensional image.
RESULTS
Deletion of lsrR and lsrK does not affect growth or motility.
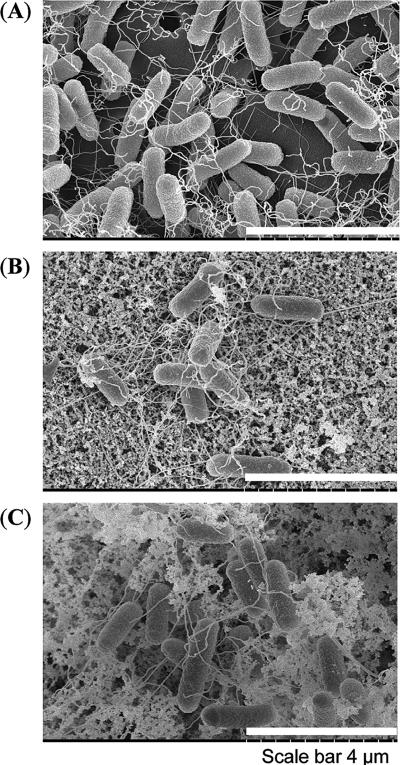
We observed no changes in growth rate and cell motility due to a deletion of either lsrR or lsrK when cells were grown in LB (data not shown). No significant differences in biofilm formation were found using a crystal violet assay for cells cultivated up to 24 h in various media (data not shown). We then used SEM to visualize biofilm morphology. W3110 ΔlsrR and ΔlsrK strains appeared with significantly different structures than that of the wild type: an extracellular matrix not present on the wild type was observed on the surface of both mutants (Fig. 2). To investigate associated gene regulation, we carried out a complete transcriptome analysis of the lsrR and lsrK mutants and later reexamined biofilm formation using a more comprehensive flow cell chamber and confocal microscopy (see below).
FIG. 2.
Scanning electron micrographs of wild-type W3110 and isogenic lsrR and lsrK mutants. (A) Wild-type strain W3110. (B) Isogenic lsrR mutants. (C) Isogenic lsrK mutants. Length scale is indicated by bars.
lsrR and lsrK mutations reveal targets of AI-2 signaling.
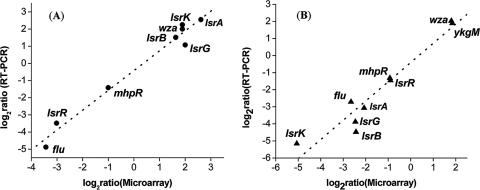
For transcriptome analysis, cells were grown in LB medium (without glucose) to an OD600 of 2.4 ± 0.1 (early stationary phase). At this point, extracellular AI-2 in cultures of wild-type cells being transported back into the cells is nearly completely depleted from ΔlsrR mutants and is retained at near-peak levels in ΔlsrK mutants (69). To report the number of genes differentially expressed, we took the commonly used twofold ratio as a cutoff limit (11, 37), together with a P value of <0.05 to ensure statistical significance. Furthermore, a set of selected genes whose expression was changed by the lsrR and/or lsrK gene was verified with real-time RT-PCR measurements. Figure 3 shows that there was a strong positive correlation (r2 = 0.98 for lsrR mutants and r2 = 0.96 for lsrK mutants) between the two techniques. Results indicate that there were 119 and 27 genes induced and repressed, respectively, at least twofold by lsrR, and there were 117 and 32 genes induced and repressed, respectively, by lsrK. Among these genes were 78 genes whose expression levels were changed by both lsrR and lsrK mutants (see Tables S1 to S3 in the supplemental material).
FIG. 3.
Correlation between microarray and real-time RT-PCR results. The differences in expression of eight lsrR-controlled genes and nine lsrK-controlled were log2 transformed and plotted (microarray versus real-time RT-PCR). (A) Symbols: •, W3110 ΔlsrR; dashed line, corresponding linear correlation between microarray data and real-time RT-PCR results. (B) Symbols: ▴, W3110 ΔlsrK; dotted line, corresponding linear correlation between microarray data and real-time RT-PCR results.
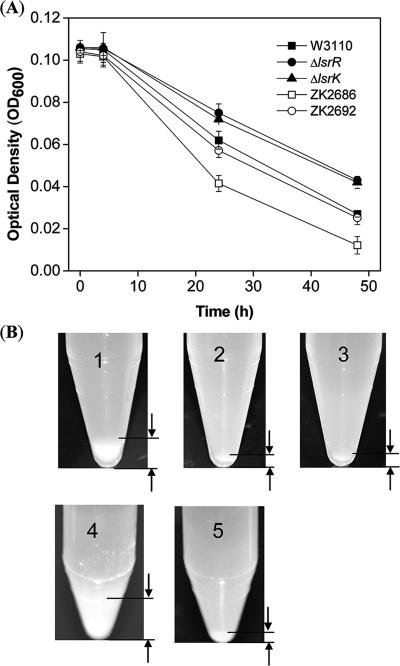
The coregulated genes comprise a number of genes that one might expect to be regulated by QS mechanisms. For example, flu, which encodes phase-variable protein antigen 43 (Ag43), was dramatically depressed, 10.8- and 6.3-fold in ΔlsrR and ΔlsrK mutants, respectively. Ag43 belongs to an autotransporter protein family, which regulates its own transport to the bacterial cell surface. Ag43 mediates cell-to-cell aggregation and thus enhances biofilm formation (16, 39, 53). As Ag43 plays an important role in the initial recognition and attachment to host tissue surfaces, it also plays a role in the pathogenesis of disease-causing E. coli (33). We carried out an autoaggregation assay to see if lsrRK in fact played a role in aggregation, presumably through Ag43. An additional test between ZK2686 and its isogenic (Δflu) mutant, ZK2692, was run to validate our assay as an indicator of Ag43 function. In Fig. 4, more wild-type cells settled to the bottom of the tubes, and faster, than both mutant strains (Fig. 4A), even though complete resolution of the assay took 2 days (Fig. 4B). We suspect that fimbrial blockage of autoaggregation may have contributed to this delay (32). The complete deficiency in autoaggregation of both lsrR and lsrK mutants is consistent with our microarray results and a regulatory model involving LsrR and LsrK.
FIG. 4.
Autoaggregation assay of W3110 and isogenic mutants ΔlsrR and ΔlsrK. (A) Time-resolved sedimentation results (autoaggregation assay) (see Materials and Methods). Symbols: ▪, W3110; •, W3110 ΔlsrR; ▴, W3110 ΔlsrK; □, ZK2686; ○, Δagn43 (isogenic mutant from parent strain ZK2686, called ZK2692). (B) Pictures of final pellets from the autoaggregation assay (A). 1, W3110; 2, W3110 ΔlsrR; 3, W3110 ΔlsrK; 4, ZK2686; 5, ZK2692.
There were 68 genes whose expression changed in an lsrR mutant alone (not changed in the lsrK mutant), and among these, 25 are hypothetical proteins with unknown function (see Table S2 in the supplemental material). We report a preponderance of genes associated with attachment, defense, and pathogenicity affected by the lsrR mutation. For example, a curli production assembly/transport component, csgE, from the second curli operon was repressed in the lsrR mutant. Curli is associated with biofilm formation, host cell adhesion and invasion, and immune system activation, where CsgA is the major fiber subunit and CsgE, CsgF, and CsgG are nonstructural proteins involved in curli biogenesis (4, 54). htrE, a homolog of papD involved in type II pilus assembly (51), was negatively regulated. Another putative fimbria-like protein, from b0942, was likely upregulated (twofold increase in expression in the lsrR mutant). A transmembrane domain, sapB of the SapABCD system (homologs of the S. enterica serovar Typhimurium SapABCD proteins) (49), which is required for virulence and resistance to the antimicrobial peptides melittin and protamine, was repressed in the lsrR mutant. Meanwhile, an increase in the transcription of a protamine-like protein, tpr, was observed. yheF (also called GspD), which belongs to a secretin protein family and is involved in virulence and filamentous phage extrusion (28), was upregulated by the lsrR deletion. YheF proteins are not normally expressed and are silenced by the nucleoid-structuring protein H-NS (26). The upregulation of yheF due to the lsrR deletion is likely an example of bacterial self-protection.
There were 71 genes whose expression changed in an lsrK mutant only, and among these genes, 38 are annotated as being hypothetical proteins with unclear functions (see Table S3 in the supplemental material). Like lsrR, a number of genes associated with attachment, defense, and pathogenicity were found to be regulated by lsrK. ppdD, which encodes a putative major type IV pilin, was repressed twofold in an lsrK mutant. PpdD was able to form type IV pili when expressed in Pseudomonas aeruginosa, as determined by immunogold labeling (55). PpdD also formed pili when pullulanase secretion proteins from Klebsiella oxytoca and E. coli K-12 ppdD were coexpressed in E. coli (56). Genes for two putative fimbrial proteins, yadK and yadN, were repressed 2.4- and 2-fold, respectively, in the lsrK mutant. mcrA, a type IV site-specific DNase defending cells against foreign DNA such as bacteriophages (3), was upregulated 2.4-fold. sieB, similar to a λ gene responsible for preventing phage superinfection (25), was also upregulated 2.5-fold.
lsrR and lsrK regulate biofilm architecture and formation.
It is known that that flagella, fimbriae, type I pili, curli fibers, Ag43, exopolysaccharides (EPS), and other outer membrane adhesins are critical for biofilm development in E. coli (16, 17, 50, 53). However, several flagellum-related and motility-associated genes, such as the motility master regulon flhDC and type I adhesin, were unchanged in the lsrR and lsrK mutants compared to the parental strain. The complex nature by which EPS and capsular polysaccharides (CPS) exert influence on biofilm formation cannot be overstated, however. For example, in Pseudomonas aeruginosa and V. cholerae, QS is shown to control biofilm formation, in part, through the regulation of EPS synthesis (18, 30). Colanic acid synthesis is necessary for forming EPS and CPS during biofilm development (17, 48, 50). The product of the wza gene is associated with colanic acid synthesis for EPS and CPS surface expression and assembly (6, 23, 24, 47, 52, 72). Many reports have described the importance of wza in biofilm formation, and changes in wza expression affect biofilm formation (17, 48, 50). Remarkably, this gene was induced 3.7- and 3.5-fold in lsrR and lsrK mutants, respectively. Also, a putative regulator for colanic acid synthesis, wcaA, was induced 3.5-fold in lsrR mutants.
Another biofilm-related gene, flu, an autotransporter, was significantly repressed in both mutants (Table 1). The biofilm-related curli gene csgE and a putative fimbrial assembly gene, htrE, were both downregulated upon lsrR deletion. In lsrK mutants, two putative fimbria-related genes, yadK and yadN, were downregulated more than twofold. Our SEM analysis revealed significant differences in cell-based fimbriae and matrices of both mutants compared to the wild type (Fig. 2).
TABLE 1.
Biofilm-related genes from genomic profiling
| Locus tag | Gene | Gene product | Fold changea
|
|
|---|---|---|---|---|
| ΔlsrR/WT | ΔlsrK/WT | |||
| b2000 | flu | Outer membrane fluffing protein, similar to adhesin | −10.8 | −6.3 |
| b2062 | wza | Putative polysaccharide export protein | 3.7 | 3.5 |
| b0139 | htrE | Probable outer membrane porin protein involved in fimbrial assembly | −3.2 | |
| b1039 | csgE | Curli production assembly/transport component, second curli operon | −3.0 | |
| b0942 | Putative fimbria-like protein | 2.2 | ||
| b2059 | wcaA | Putative regulator | 3.5 | |
| b0141 | yadN | Putative fimbria-like protein | −2.0 | |
| b0136 | yadK | Putative fimbrial protein | −2.4 | |
WT, wild type.
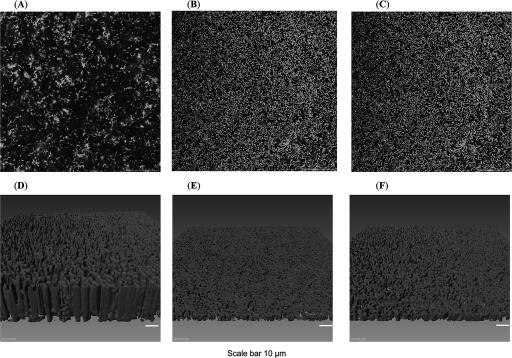
In order to further elucidate the variation in biofilm formation between the wild type and mutants, we utilized confocal scanning microscopy on flow-cell-derived biofilms (see Materials and Methods). Confocal images (Fig. 5) show for the first time that more biofilm was formed at the substrate surface in both mutant strains than in the wild type. Also, Imaris imaging demonstrated that the biofilm thicknesses of the lsrR and lsrK mutants were similar and much less than that of the wild type. Hence, the mean thickness and biomass of the wild type were higher, while the substratum coverage was lower than that of the mutants (Table 2) (because more of the mutant biofilms adhered to the bottom); that is, the bottom layer adjacent to the substrate appeared to be more substantive in the case of the mutants, and they tended to collapse and pack tightly onto the surface of the substrate.
FIG. 5.
Scanning confocal laser microscopic images of flow-cell-generated biofilms. (A and D) Wild-type W3110. (B and E) Isogenic lsrR mutant. (C and F) Isogenic lsrK mutant. We note that results for the lsrR mutant had a larger standard deviation than those for the lsrK mutant; the biofilm height was observed to fluctuate in the flow chamber (not shown here). A, B, and C are scanning confocal microscopic images; D, E, and F are reconstructed three-dimensional biofilm structures. The length scale is indicated by bars.
TABLE 2.
Confocal analysis report of biofilm flow cell assay
| Strain | Biomass (μm3/μm2) | Substratum coverage (%) | Mean thickness (μm) | Roughness coefficient |
|---|---|---|---|---|
| W3110 type/pCM18 | 32.02 ± 3.2 | 20.4 ± 6.4 | 25.45 ± 2.8 | 0.196 ± 0.08 |
| ΔlsrK:Kanr/pCM18 | 3.64 ± 1.05 | 50.8 ± 4.7 | 2.95 ± 1.24 | 0.235 ± 0.05 |
| ΔlsrR:Kanr/pCM18 | 13.43 ± 14.43 | 44.6 ± 21.4 | 10.86 ± 11.85 | 0.229 ± 0.11 |
In summary, two key functions known to affect biofilm formation and architecture, colanic acid synthesis and fimbria formation, were shown to be regulated by genes of the QS regulators LsrR and LsrK. Also, biofilm structures were altered significantly in the lsrR and lsrK mutants.
Deletion of lsrR and lsrK affected sRNA expression.
As shown recently by Lenz and colleagues, small RNA (sRNA) species are involved in QS (42). We searched the intergenic regions for known and putative sRNAs (Table 3) and found that the global sRNA regulator DsrA was induced 3.6- and 4.4-fold in ΔlsrR and ΔlsrK strains, respectively. DsrA is a riboregulator for RpoS and H-NS production, wherein DsrA enhances the translation of rpoS RNA by stabilizing rpoS mRNA. It also inhibits H-NS translation by sharply increasing hns mRNA turnover (40, 44) and thereby curtails H-NS-mediated transcriptional silencing. Correspondingly, DsrA RNA also affects CPS biosynthesis via increased production of the activator RcsA due its inhibitory effects on H-NS-mediated transcriptional silencing (58). DsrA also plays a regulatory role in acid resistance (41). The regulatory effects of DsrA are mediated by specific RNA-to-RNA pairing interactions, while its stability and activity require the recruitment of Hfq (8, 46, 59, 60).
TABLE 3.
sRNAs affected by lsrR and lsrK
| Protein | Position
|
Flanking gene | Fold changea
|
Condition | ||
|---|---|---|---|---|---|---|
| Start | End | ΔlsrR/WT | ΔlsrK/WT | |||
| DsrA | 2023233 | 2023532 | dsrB/yedP | 3.6 | 4.4 | Confirmed |
| DicF | 1647459 | 1647632 | rzpQ/dicB | 2.0 | 2.5 | Confirmed |
| RydB/tpe7 | 1762411 | 1762957 | sufA (ydiC)/ydiH | 4.9 | Confirmed | |
| IS102 | 2069234 | 2069404 | yeeP/flu | −2.3 | −1.5 | Confirmed |
| Tpke70 | 2494586 | 2496690 | ddg/yfdZ | 2.0 | Confirmed | |
| MicC (ISO63) | 1434918 | 1435283 | ompN/ydbK | 3.2 | Confirmed | |
| AyjiW | 4577468 | 4577637 | Opposite yjiW | 2.5 | 2.1 | Confirmed |
| SokX | 2885243 | 2885600 | 3.5 | Confirmed | ||
| Unknown | 2468480 | 2468778 | yfdI/tfaS | 2.3 | 3.2 | Predicted |
WT, wild type.
Since RpoS and H-NS play an important role in globally regulating genes in response to changing environments, it is not surprising that AI-2-related QS networks utilize their uptake regulators (e.g., LsrR and LuxP), together with DsrA, in a hierarchical modality for mediating prompt responses to environmental stimuli and extracellular stresses. Induction of components (yheE and yheF) in the type II secretion complex GspC to GspO is a good example of DsrA regulation: type II secretion was silenced by H-NS in wild-type E. coli (26), while DsrA antagonizes the H-NS-mediated silencing of numerous promoters (58). Therefore, the QS-mediated induction of DsrA resulting from the lsrR and lsrK deletions leads to amplified expression of yheE and yheF (seemingly in the lsrR mutant only).
The sRNA cell division inhibitor DicF was induced by at least twofold in both lsrR and lsrK mutants. A twofold increase in expression of the cell division gene dicB was also observed, which is not surprising since both genes belong to the same cell division operon (see the supplemental material) (Table 1) (7). dicF inhibits cell division in E. coli by decreasing the abundance and activity of FtsZ; therefore, DicF affects the septum formation and separation of the replicated chromosomes into daughter nucleoids (63, 64). However, this inhibition effect can be suppressed by an rpoB mutation, and the inhibition effect is partially counteracted by an rpoS mutation (9). We note, however, that we did not see elongated cells in lsrR and lsrK mutants in our SEM studies. This is probably because cell division is a complex process controlled by many modes of regulation (1, 12, 29, 67).
Another sRNA immediately upstream of the flu gene was repressed in both mutants. This might account for the dramatic decrease in flu expression, 10.8- and 6.3-fold, respectively, in the lsrR and lsrK mutants described above, although a monocistronic RNA has not been identified. Two other sRNAs were found to be coregulated by lsrR and lsrK: one is ayjiW, and the other, which is unnamed, is located between yfdI and tfaS. The function of these riboregulators remains unclear and awaits further research.
The remaining sRNAs revealed in our study (Table 3) include three from the lsrR deletion (RydB, MicC, and Tpke70) and one from the lsrK deletion (SokX). High-copy expression of RydB decreases rpoS expression during the stationary phase in LB medium (71). It is intriguing to speculate that LsrR associates with RydB to assist in fine-tuning QS circuitry through the regulation of the global regulator RpoS. MicC works similarly as an antisense mechanism and is induced when cells are grown at low temperatures or in minimal medium (13). MicC negatively regulates the translation of an outer membrane protein, OmpC (14). Consistent with this posttranscriptional regulation, we did not see transcriptional changes in ompC expression or changes in the expression of its regulator, ompF. Finally, Tpke70 is an antisense RNA with an unknown function. In lsrK mutants, SokX, of unknown function, was induced 3.5-fold.
DISCUSSION
In contrast to our previous microarray study of W3110 and a luxS mutant strain, LW7, where fewer than 50 genes were significantly affected by luxS mutation (70), our current study found many genes that were significantly affected by the lsrR and lsrK deletion (146 and 149 genes, respectively). Of these genes, only nine were regulated in exactly the same manner as that described previously for LuxS-regulated genes (Fig. 1) (70, 75). Deletion of lsrR results in the induction of the lsr operon (including the lsrACDBFG and tam genes), while deletion of lsrK results in the depression of those genes; that is, upon entry into the cell via the Lsr transporter, AI-2 is phosphorylated by LsrK. Phospho-AI-2 can bind the cognate transcriptional regulator LsrR and derepress gene expression. The immediate targets of this derepression are the very same AI-2 uptake genes (Fig. 1). Observations that AI-2 regulates its own uptake and transcriptome results indicating that few genes are impacted by the luxS mutation (70) have fueled speculation that AI-2 is limited in its role as a signal molecule in E. coli. Indeed, in our previous report, we suggested that the signaling role of AI-2 might require additional cellular factors (70).
A significant difference between the present lsrR and lsrK mutants and the luxS mutant is in the roles of the expressed proteins: signal perception versus signal generation. The present study enables a linkage between lsrR and lsrK to AI-2 as a signaling molecule. A key to this understanding was revealed previously but not reported for its importance (69, 70): the lsr-lacZ and lsrR-lacZ reporters in lsrR and lsr operon mutants were both upregulated manifold in both strains. Of note, the lsr transcription rate in an lsr operon mutant was upregulated to an almost equivalent extent to that in an lsrR mutant strain, suggesting that the cells still possessed phospho-AI-2 even though they did not possess the uptake complex. These cells did import AI-2 but at a much slower rate than the wild type and lsrR mutants. The same transcriptional reporter plasmid was nearly completely inactive in the lsrK mutant, as expected (consistent with the absence of derepression afforded by phospho-AI-2). Interestingly, the extracellular AI-2 level in LsrK mutants never dropped, suggesting that AI-2 was not taken up by the cells in the absence of LsrK. These results suggest that AI-2, taken in by an alternative transporter (alluded to in reference 70) or otherwise unsecreted AI-2, may still be phosphorylated by LsrK. These findings also suggest that (i) the Lsr transporter does not function without LsrK and (ii) LsrK can work with another transporter. These findings also give rise to the possibility that LsrK (and LsrR) may work on genes other than the lsr operon.
Indeed, the present analysis reveals a host of genes regulated by LsrR and LsrK, most of which did not appear in luxS mutants. Perhaps the most striking results of our current study are that (i) the majority of genes affected by the lsrR mutation are also affected by the lsrK mutation, (ii) the expression of the vast majority of these genes are identically affected (up or down) by both mutations, and (iii) these “coregulated” genes are not those of the lsr regulon (tam, metE, yneE, and lsr operons) (70). These findings suggest that AI-2, in addition to phospho-AI-2, is an LsrR regulator; that is, for the apparently coregulated genes, we suspect that a totally different regulatory mechanism than that shown in Fig. 1 exists (in which LsrR is a repressor [and at times an activator] and its repression is released by phospho-AI-2). We propose an extended hypothesis that LsrR is a QS regulator that acts in tandem with unphosphorylated AI-2 or its anomer (Fig. 6); namely, AI-2 binds to LsrR and derepresses the transcription of a variety of genes, those identified in Table S1 in the supplemental material, which one might expect should be under QS regulation (as opposed to metabolic genes associated with the activated methyl cycle) (70).
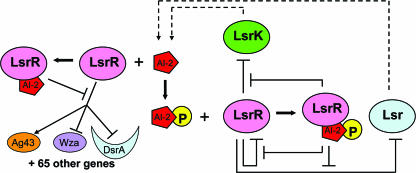
FIG. 6.
Proposed AI-2 signaling with QS regulators LsrR and LsrK. During early and mid-exponential phases, AI-2 levels are low, insufficient for triggering rapid uptake. LsrR binds and represses many genes including lsr genes. As AI-2 accumulates extracellularly, it begins to be transported into the cells via a non-Lsr pathway or otherwise accumulates within the cells (or other pre-AI-2 anomers), which then bind to LsrR and derepress many QS genes including lsrR, flu, wza, and dsrA (crescents represent sRNA). Lsr-mediated AI-2 uptake remains repressed, as its derepression is due to phospho-AI-2. This temporal model is consistent with the activation of QS phenotypes that are switched on in late exponential phase. Finally, when the AI-2 concentration reaches the uptake “threshold” and cells sense nutrient depletion, lsr rapidly imports AI-2. Thereafter, cells phosphorylate the imported AI-2 signal, which results in the cessation of the LsrR/AI-2 regulation and amplification of LsrR/phopho-AI-2 regulation. Hence, a rapid QS switch is manifested by a change in the phosphorylation state of AI-2 and its binding to LsrR.
Our extended model is either supported by or consistent with all studies reported to date concerning AI-2, luxS, and lsr in E. coli and Salmonella. LsrR is a known transcriptional regulator that responds to the binding of QS signal AI-2 (62, 75). We suggest that the binding of phospho-AI-2 competes favorably with the binding of unphosphorylated AI-2 and that the interchange between AI-2 and phospho-AI-2 represents a switching mechanism for the affected genes. LsrR is transcribed in its own monocistronic operon with the kinase LsrK in a manner divergent from that of the lsr operon (70). It is therefore distinct from the uptake genes and can operate independently. AI-2 is internalized and phosphorylated by the Lsr/LsrK complex. We suggest this is the predominant mode of AI-2 entry into the cells and, by phosphorylation, prevents AI-2 efflux. However, AI-2 is taken up by Lsr mutants. Also, intracellular AI-2 by deletion of ydgG has been noted (35), and AI-2 is synthesized by non-LuxS/Pfs pathways (43). All are consistent with the existence of AI-2 (or its anomers) inside cells. An lsrR mutation leads to deficient LsrR expression and identification of LsrR-regulated genes. An lsrK mutation results in a lack of imported phospho-AI-2 (70), which is the principal signal for derepression of the lsr operon. We suggest that unphosphorylated AI-2 participates as a specific regulator of LsrR activity and that this mode of activity is the dominant feature of LsrR-mediated QS. We also suggest that the phosphorylated AI-2 acts as a “trigger” to terminate AI-2-mediated cellular processes and initiate the recycling of AI-2 through the lsr operon, which thus serves as the interconnect between the signaling process and a metabolic function. The metabolic functions of LuxS were described previously (70).
We prefer this signaling modality for AI-2-mediated QS for two reasons: first, QS signals have been shown to be global regulators, and hence, cells must have a purpose for importing and processing AI-2; second, lsrR and lsrK belong to an AI-2-mediated regulon (70). Thus, it is not surprising that E. coli utilizes the product of this operon to globally control the cellular phenotype. Our transcriptome results agree with this model, since most of the LsrRK-regulated genes are related to the cell's secretion systems (e.g., flu and yheE), biofilm formation (e.g., wza), transcriptional regulators (e.g., mhpR, tdcA, and envR), sRNAs or riboregulators (e.g., DsrA), and regulatory proteins for stress responses and nutrient depletion (e.g., adiY, glnK, and cspF). Indeed, AI-2 is perhaps a global regulator of E. coli (74) only when it is coupled to another regulator, as LuxP in Vibrio harveyi.
Our observation of biofilm phenotypes in lsrR and lsrK mutants cooperates with this model: AI-2 probably binds with LsrR to mediate biofilm architecture and formation by coordinately regulating interactions of biofilm-related genes, including the colanic acid synthesis regulator wza and the flu gene. Equally importantly, both of the genes affecting the phenotypes and the phenotypic outcomes were altered identically for the lsrR and lsrK strains. These findings suggest an intimate coordination between lsrR and lsrK, as shown in Fig. 6. The influence of these genes on biofilm structure has already been elucidated: their QS dependence is shown here for the first time.
Finally, our study provides the first evidence that sRNAs interact with QS regulators in E. coli K-12. A riboregulator, RsmZ, was found to control biofilm formation and type III secretion in Pseudomonas aeruginosa (66). Previous reports conclusively demonstrated that four sRNAs are intimately involved in the QS networks of V. harveyi and V. cholerae and act through the RNA chaperone Hfq (42). This finding is the first to suggest the convergence of an E. coli QS signaling system onto the Hfq/LuxO transduction process of V. harveyi and suggests yet one more modality for which bacterial autoinducer signal transduction occurs.
Supplementary Material
Acknowledgments
We thank R. Kolter for generously providing strains ZK2686 and ZK2692 for our studies. We also thank A. Godínez for assistance in the DNA microarray experiments and T. Maugel for scanning electron microscopy experiments. We are grateful for proofreading by R. Fernandes.
This work was supported by the National Science Foundation (grants BES-0124401 and BES-0222687) and the U.S. Army.
Footnotes
Published ahead of print on 8 June 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aarsman, M. E., A. Piette, C. Fraipont, T. M. Vinkenvleugel, M. Nguyen-Disteche, and T. den Blaauwen. 2005. Maturation of the Escherichia coli divisome occurs in two steps. Mol. Microbiol. 55:1631-1645. [DOI] [PubMed] [Google Scholar]
- 2.Ahmer, B. M. 2004. Cell-to-cell signalling in Escherichia coli and Salmonella enterica. Mol. Microbiol. 52:933-945. [DOI] [PubMed] [Google Scholar]
- 3.Anton, B. P., and E. A. Raleigh. 2004. Transposon-mediated linker insertion scanning mutagenesis of the Escherichia coli McrA endonuclease. J. Bacteriol. 186:5699-5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnhart, M. M., and M. R. Chapman. 2006. Curli biogenesis and function. Annu. Rev. Microbiol. 60:131-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beeston, A. L., and M. G. Surette. 2002. pfs-dependent regulation of autoinducer 2 production in Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:3450-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beis, K., R. F. Collins, R. C. Ford, A. B. Kamis, C. Whitfield, and J. H. Naismith. 2004. Three-dimensional structure of Wza, the protein required for translocation of group 1 capsular polysaccharide across the outer membrane of Escherichia coli. J. Biol. Chem. 279:28227-28232. [DOI] [PubMed] [Google Scholar]
- 7.Bouche, F., and J. P. Bouche. 1989. Genetic evidence that DicF, a second division inhibitor encoded by the Escherichia coli dicB operon, is probably RNA. Mol. Microbiol. 3:991-994. [DOI] [PubMed] [Google Scholar]
- 8.Brescia, C. C., P. J. Mikulecky, A. L. Feig, and D. D. Sledjeski. 2003. Identification of the Hfq-binding site on DsrA RNA: Hfq binds without altering DsrA secondary structure. RNA 9:33-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cam, K., A. Cuzange, and J. P. Bouche. 1995. Sigma S-dependent overexpression of ftsZ in an Escherichia coli K-12 rpoB mutant that is resistant to the division inhibitors DicB and DicF RNA. Mol. Gen. Genet. 248:190-194. [DOI] [PubMed] [Google Scholar]
- 10.Camilli, A., and B. L. Bassler. 2006. Bacterial small-molecule signaling pathways. Science 311:1113-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canales, R. D., Y. Luo, J. C. Willey, B. Austermiller, C. C. Barbacioru, C. Boysen, K. Hunkapiller, R. V. Jensen, C. R. Knight, K. Y. Lee, Y. Ma, B. Maqsodi, A. Papallo, E. H. Peters, K. Poulter, P. L. Ruppel, R. R. Samaha, L. Shi, W. Yang, L. Zhang, and F. M. Goodsaid. 2006. Evaluation of DNA microarray results with quantitative gene expression platforms. Nat. Biotechnol. 24:1115-1122. [DOI] [PubMed] [Google Scholar]
- 12.Chen, J. C., and J. Beckwith. 2001. FtsQ, FtsL and FtsI require FtsK, but not FtsN, for co-localization with FtsZ during Escherichia coli cell division. Mol. Microbiol. 42:395-413. [DOI] [PubMed] [Google Scholar]
- 13.Chen, S., E. A. Lesnik, T. A. Hall, R. Sampath, R. H. Griffey, D. J. Ecker, and L. B. Blyn. 2002. A bioinformatics based approach to discover small RNA genes in the Escherichia coli genome. Biosystems 65:157-177. [DOI] [PubMed] [Google Scholar]
- 14.Chen, S., A. Zhang, L. B. Blyn, and G. Storz. 2004. MicC, a second small-RNA regulator of Omp protein expression in Escherichia coli. J. Bacteriol. 186:6689-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, Y. W., P. Zhao, R. Borup, and E. P. Hoffman. 2000. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J. Cell Biol. 151:1321-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danese, P. N., L. A. Pratt, S. L. Dove, and R. Kolter. 2000. The outer membrane protein, antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol. Microbiol. 37:424-432. [DOI] [PubMed] [Google Scholar]
- 17.Danese, P. N., L. A. Pratt, and R. Kolter. 2000. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 182:3593-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 19.De Keersmaecker, S. C., K. Sonck, and J. Vanderleyden. 2006. Let LuxS speak up in AI-2 signaling. Trends Microbiol. 14:114-119. [DOI] [PubMed] [Google Scholar]
- 20.DeLisa, M. P., and W. E. Bentley. 2002. Bacterial autoinduction: looking outside the cell for new metabolic engineering targets. Microb. Cell Fact. 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLisa, M. P., J. J. Valdes, and W. E. Bentley. 2001. Quorum signaling via AI-2 communicates the “metabolic burden” associated with heterologous protein production in Escherichia coli. Biotechnol. Bioeng. 75:439-450. [DOI] [PubMed] [Google Scholar]
- 22.Domka, J., J. Lee, and T. K. Wood. 2006. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signaling. Appl. Environ. Microbiol. 72:2449-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummelsmith, J., and C. Whitfield. 1999. Gene products required for surface expression of the capsular form of the group 1 K antigen in Escherichia coli (O9a:K30). Mol. Microbiol. 31:1321-1332. [DOI] [PubMed] [Google Scholar]
- 24.Drummelsmith, J., and C. Whitfield. 2000. Translocation of group 1 capsular polysaccharide to the surface of Escherichia coli requires a multimeric complex in the outer membrane. EMBO J. 19:57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faubladier, M., and J. P. Bouche. 1994. Division inhibition gene dicF of Escherichia coli reveals a widespread group of prophage sequences in bacterial genomes. J. Bacteriol. 176:1150-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francetic, O., D. Belin, C. Badaut, and A. P. Pugsley. 2000. Expression of the endogenous type II secretion pathway in Escherichia coli leads to chitinase secretion. EMBO J. 19:6697-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuqua, W. C., S. C. Winans, and E. P. Greenberg. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genin, S., and C. A. Boucher. 1994. A superfamily of proteins involved in different secretion pathways in gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol. Gen. Genet. 243:112-118. [DOI] [PubMed] [Google Scholar]
- 29.Hale, C. A., and P. A. de Boer. 2002. ZipA is required for recruitment of FtsK, FtsQ, FtsL, and FtsN to the septal ring in Escherichia coli. J. Bacteriol. 184:2552-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammer, B. K., and B. L. Bassler. 2003. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 50:101-104. [DOI] [PubMed] [Google Scholar]
- 31.Hardie, K. R., C. Cooksley, A. D. Green, and K. Winzer. 2003. Autoinducer 2 activity in Escherichia coli culture supernatants can be actively reduced despite maintenance of an active synthase, LuxS. Microbiology 149:715-728. [DOI] [PubMed] [Google Scholar]
- 32.Hasman, H., T. Chakraborty, and P. Klemm. 1999. Antigen-43-mediated autoaggregation of Escherichia coli is blocked by fimbriation. J. Bacteriol. 181:4834-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henderson, I. R., F. Navarro-Garcia, M. Desvaux, R. C. Fernandez, and D. Ala'Aldeen. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68:692-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henke, J. M., and B. L. Bassler. 2004. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 186:3794-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herzberg, M., I. K. Kaye, W. Peti, and T. K. Wood. 2006. YdgG (TqsA) controls biofilm formation in Escherichia coli K-12 through autoinducer 2 transport. J. Bacteriol. 188:587-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houdt, R., A. Aertsen, P. Moons, K. Vanoirbeek, and C. W. Michiels. 2006. N-Acyl-l-homoserine lactone signal interception by Escherichia coli. FEMS Microbiol. Lett. 256:83-89. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa, J. K., A. Norris, M. G. Bangera, G. K. Geiss, A. B. van 't Wout, R. E. Bumgarner, and S. Lory. 2000. Interaction of Pseudomonas aeruginosa with epithelial cells: identification of differentially regulated genes by expression microarray analysis of human cDNAs. Proc. Natl. Acad. Sci. USA 97:9659-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keller, L., and M. G. Surette. 2006. Communication in bacteria: an ecological and evolutionary perspective. Nat. Rev. Microbiol. 4:562. [DOI] [PubMed] [Google Scholar]
- 39.Kjaergaard, K., M. A. Schembri, C. Ramos, S. Molin, and P. Klemm. 2000. Antigen 43 facilitates formation of multispecies biofilms. Environ. Microbiol. 2:695-702. [DOI] [PubMed] [Google Scholar]
- 40.Lease, R. A., and M. Belfort. 2000. A trans-acting RNA as a control switch in Escherichia coli: DsrA modulates function by forming alternative structures. Proc. Natl. Acad. Sci. USA 97:9919-9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lease, R. A., D. Smith, K. McDonough, and M. Belfort. 2004. The small noncoding DsrA RNA is an acid resistance regulator in Escherichia coli. J. Bacteriol. 186:6179-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenz, D. H., K. C. Mok, B. N. Lilley, R. V. Kulkarni, N. S. Wingreen, and B. L. Bassler. 2004. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118:69-82. [DOI] [PubMed] [Google Scholar]
- 43.Li, J., L. Wang, Y. Hashimoto, C. Y. Tsao, J. J. Valdes, T. K. Wood, E. Zafiriou, and W. E. Bentley. 2006. A stochastic model of Escherichia. coli AI-2 quorum signal circuit reveals alternative synthesis pathways. Mol. Syst. Biol. 2:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majdalani, N., C. Cunning, D. Sledjeski, T. Elliott, and S. Gottesman. 1998. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc. Natl. Acad. Sci. USA 95:12462-12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.March, J. C., and W. E. Bentley. 2004. Quorum sensing and bacterial cross-talk in biotechnology. Curr. Opin. Biotechnol. 15:495-502. [DOI] [PubMed] [Google Scholar]
- 46.Moll, I., T. Afonyushkin, O. Vytvytska, V. R. Kaberdin, and U. Blasi. 2003. Coincident Hfq binding and RNase E cleavage sites on mRNA and small regulatory RNAs. RNA 9:1308-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nesper, J., C. M. Hill, A. Paiment, G. Harauz, K. Beis, J. H. Naismith, and C. Whitfield. 2003. Translocation of group 1 capsular polysaccharide in Escherichia coli serotype K30. Structural and functional analysis of the outer membrane lipoprotein Wza. J. Biol. Chem. 278:49763-49772. [DOI] [PubMed] [Google Scholar]
- 48.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 49.Parra-Lopez, C., M. T. Baer, and E. A. Groisman. 1993. Molecular genetic analysis of a locus required for resistance to antimicrobial peptides in Salmonella typhimurium. EMBO J. 12:4053-4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prigent-Combaret, C., G. Prensier, T. T. Le Thi, O. Vidal, P. Lejeune, and C. Dorel. 2000. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: role of flagella, curli and colanic acid. Environ. Microbiol. 2:450-464. [DOI] [PubMed] [Google Scholar]
- 51.Raina, S., D. Missiakas, L. Baird, S. Kumar, and C. Georgopoulos. 1993. Identification and transcriptional analysis of the Escherichia coli htrE operon which is homologous to pap and related pilin operons. J. Bacteriol. 175:5009-5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reid, A. N., and C. Whitfield. 2005. Functional analysis of conserved gene products involved in assembly of Escherichia coli capsules and exopolysaccharides: evidence for molecular recognition between Wza and Wzc for colanic acid biosynthesis. J. Bacteriol. 187:5470-5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reisner, A., J. A. Haagensen, M. A. Schembri, E. L. Zechner, and S. Molin. 2003. Development and maturation of Escherichia coli K-12 biofilms. Mol. Microbiol. 48:933-946. [DOI] [PubMed] [Google Scholar]
- 54.Robinson, L. S., E. M. Ashman, S. J. Hultgren, and M. R. Chapman. 2006. Secretion of curli fibre subunits is mediated by the outer membrane-localized CsgG protein. Mol. Microbiol. 59:870-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sauvonnet, N., P. Gounon, and A. P. Pugsley. 2000. PpdD type IV pilin of Escherichia coli K-12 can be assembled into pili in Pseudomonas aeruginosa. J. Bacteriol. 182:848-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sauvonnet, N., G. Vignon, A. P. Pugsley, and P. Gounon. 2000. Pilus formation and protein secretion by the same machinery in Escherichia coli. EMBO J. 19:2221-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sitnikov, D. M., J. B. Schineller, and T. O. Baldwin. 1996. Control of cell division in Escherichia coli: regulation of transcription of ftsQA involves both rpoS and sdiA-mediated autoinduction. Proc. Natl. Acad. Sci. USA 93:336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sledjeski, D., and S. Gottesman. 1995. A small RNA acts as an antisilencer of the H-NS-silenced rcsA gene of Escherichia coli. Proc. Natl. Acad. Sci. USA 92:2003-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sledjeski, D. D., C. Whitman, and A. Zhang. 2001. Hfq is necessary for regulation by the untranslated RNA DsrA. J. Bacteriol. 183:1997-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sonnleitner, E., J. Napetschnig, T. Afonyushkin, K. Ecker, B. Vecerek, I. Moll, V. R. Kaberdin, and U. Blasi. 2004. Functional effects of variants of the RNA chaperone Hfq. Biochem. Biophys. Res. Commun. 323:1017-1023. [DOI] [PubMed] [Google Scholar]
- 61.Sperandio, V., A. G. Torres, B. Jarvis, J. P. Nataro, and J. B. Kaper. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. USA 100:8951-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taga, M. E., S. T. Miller, and B. L. Bassler. 2003. Lsr-mediated transport and processing of AI-2 in Salmonella typhimurium. Mol. Microbiol. 50:1411-1427. [DOI] [PubMed] [Google Scholar]
- 63.Tetart, F., R. Albigot, A. Conter, E. Mulder, and J. P. Bouche. 1992. Involvement of FtsZ in coupling of nucleoid separation with septation. Mol. Microbiol. 6:621-627. [DOI] [PubMed] [Google Scholar]
- 64.Tetart, F., and J. P. Bouche. 1992. Regulation of the expression of the cell-cycle gene ftsZ by DicF antisense RNA. Division does not require a fixed number of FtsZ molecules. Mol. Microbiol. 6:615-620. [DOI] [PubMed] [Google Scholar]
- 65.Vendeville, A., K. Winzer, K. Heurlier, C. M. Tang, and K. R. Hardie. 2005. Making ‘sense’ of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat. Rev. Microbiol. 3:383-396. [DOI] [PubMed] [Google Scholar]
- 66.Ventre, I., A. L. Goodman, I. Vallet-Gely, P. Vasseur, C. Soscia, S. Molin, S. Bleves, A. Lazdunski, S. Lory, and A. Filloux. 2006. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc. Natl. Acad. Sci. USA 103:171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vicente, M., and A. I. Rico. 2006. The order of the ring: assembly of Escherichia coli cell division components. Mol. Microbiol. 61:5-8. [DOI] [PubMed] [Google Scholar]
- 68.Walters, M., and V. Sperandio. 2006. Quorum sensing in Escherichia coli and Salmonella. Int. J. Med. Microbiol. 296:125-131. [DOI] [PubMed] [Google Scholar]
- 69.Wang, L., Y. Hashimato, C. Y. Tsao, J. J. Valdes, and W. E. Bentley. 2005. Cyclic AMP (cAMP) and cAMP receptor protein influence both synthesis and uptake of extracellular autoinducer 2 in Escherichia coli. J. Bacteriol. 187:2066-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang, L., J. Li, J. C. March, J. J. Valdes, and W. E. Bentley. 2005. luxS-dependent gene regulation in Escherichia coli K-12 revealed by genomic expression profiling. J. Bacteriol. 187:8350-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wassarman, K. M., F. Repoila, C. Rosenow, G. Storz, and S. Gottesman. 2001. Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev. 15:1637-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whitfield, C., and A. Paiment. 2003. Biosynthesis and assembly of group 1 capsular polysaccharides in Escherichia coli and related extracellular polysaccharides in other bacteria. Carbohydr. Res. 338:2491-2502. [DOI] [PubMed] [Google Scholar]
- 73.Winzer, K., K. R. Hardie, and P. Williams. 2003. LuxS and autoinducer-2: their contribution to quorum sensing and metabolism in bacteria. Adv. Appl. Microbiol. 53:291-396. [DOI] [PubMed] [Google Scholar]
- 74.Xavier, K. B., and B. L. Bassler. 2003. LuxS quorum sensing: more than just a numbers game. Curr. Opin. Microbiol. 6:191-197. [DOI] [PubMed] [Google Scholar]
- 75.Xavier, K. B., and B. L. Bassler. 2005. Regulation of uptake and processing of the quorum-sensing autoinducer AI-2 in Escherichia coli. J. Bacteriol. 187:238-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.