Abstract
We identified a single open reading frame that is strongly similar to ArcR, a member of the Crp/Fnr family of bacterial transcriptional regulators, in all sequenced Staphylococcus aureus genomes. The arcR gene encoding ArcR forms an operon with the arginine deiminase (ADI) pathway genes arcABDC that enable the utilization of arginine as a source of energy for growth under anaerobic conditions. In this report, we show that under anaerobic conditions, S. aureus growth is subject to glucose catabolic repression and is enhanced by arginine. Likewise, glucose and arginine have reciprocal effects on the transcription of the arcABDCR genes. Furthermore, we show using a mutant deleted for arcR that the transcription of the arc operon under anaerobic conditions depends strictly on a functional ArcR. These findings are supported by proteome analyses, which showed that under anaerobic conditions the expression of the ADI catabolic proteins depends on ArcR. Bioinformatic analysis of S. aureus ArcR predicts an N-terminal nucleotide binding domain and a C-terminal helix-turn-helix DNA binding motif. ArcR binds to a conserved Crp-like sequence motif, TGTGA-N6-TCACA, present in the arc promoter region and thereby activates the expression of the ADI pathway genes. Crp-like sequence motifs were also found in the regulatory regions of some 30 other S. aureus genes mostly encoding anaerobic enzymatic systems, virulence factors, and regulatory systems. ArcR was tested and found to bind to the regulatory regions of four such genes, adh1, lctE, srrAB, and lukM. In one case, for lctE, encoding l-lactate dehydrogenase, ArcR was able to bind only in the presence of cyclic AMP. These observations suggest that ArcR is likely to play an important role in the expression of numerous genes required for anaerobic growth.
Staphylococcus aureus is an important human pathogen and one of the major causes of community- and hospital-acquired infections worldwide. It colonizes primarily the squamous epithelium of the anterior nares (11), causing a variety of diseases ranging from simple skin infections to life-threatening diseases such as endocarditis, toxic shock syndrome, and chronic infections that require the successful adaptation of the pathogen to the human host (33). In the absence of oxygen, S. aureus can grow by fermentation of glucose or by using an alternative terminal electron acceptor such as nitrate (39, 41). When neither glucose nor nitrate is available, arginine can serve as the sole energy source for growth. The main bacterial arginine catabolic pathway is the arginine deiminase (ADI) pathway, encoded by the arc operon (13). The pathway comprises three reactions catalyzed by ADI (EC 3.5.3.6), ornithine carbamoyl transferase (EC 2.1.3.3), and carbamate kinase (EC 2.7.2.2), resulting in the conversion of arginine into ornithine, ammonia, and CO2, with the concomitant production of 1 mol of ATP per mol of arginine consumed. The arc operon may also contain genes encoding ArgR and Crp/Fnr regulatory proteins as well as transport proteins (2). ADI operons have been described for a wide variety of bacteria, including mycoplasma, halobacteria, Pseudomonas spp., Bacillus spp., and lactic acid and dental bacteria. In Streptococcus rattus, the ADI pathway functions as a key defense mechanism against acidification (12). Ammonia generation by ADI catabolism of arginine is believed to be crucial for pH homeostasis and a major factor in promoting dental health (7). In those bacteria that lack a respiratory chain, the ADI system is subject to carbon catabolite repression, while arginine serves to induce its expression (16, 17, 24).
An ADI gene cluster is present in the genomes of all nine S. aureus strains that have been fully sequenced. We noted that they all possess an open reading frame (ORF) encoding a 234-amino-acid protein related to the Crp/Fnr family of regulatory proteins. The S. aureus ORF is significantly related to the Bacillus licheniformis and Lactobacillus sakei ArcR proteins, which control the expression of the ADI catabolic pathway (34, 35, 52). Accordingly, the S. aureus ORF was named ArcR. The S. aureus arcR gene, encoding ArcR, is, unlike other previously reported ADI gene clusters, located immediately downstream of the arcABDC genes. Inspection of the region upstream of arcABDC revealed a conserved palindromic sequence motif, TGTGA-N6-TCACA, that is the recognition site for Crp and some Fnr-related regulatory proteins (4). Bioinformatic analysis identified 15 more identical copies of this motif in the regulatory region of genes involved in anaerobic metabolism, including genes coding for lactate dehydrogenase, alcohol dehydrogenase, and pyruvate formate-lyase as well as the ADI pathway genes. These findings prompted us to analyze the role of ArcR in regulating the expression of the arc operon and of other genes that function in anaerobic growth. We show here that S. aureus ArcR positively controls the transcription of the arcABDC genes via binding to the upstream regulatory region and that a similar mechanism likely operates with some other anaerobic genes.
MATERIALS AND METHODS
Strains, media, and culture conditions.
Bacterial strains and plasmids used in this study are described in Table 1. S. aureus strains were grown at 37°C in tryptone soy broth (TSB) (Difco) and brain heart infusion broth (Difco) supplemented with erythromycin (12 μg ml−1) and kanamycin (200 μg ml−1) where appropriate. Phage transductions were carried out with ϕ11 as described previously (40). Escherichia coli was grown in Luria-Bertani medium with the addition of ampicillin (100 μg ml−1) or kanamycin (50 μg ml−1) as needed. S. aureus aerobic liquid cultures were grown at 37°C in an air orbital shaker at 250 rpm. For limiting oxygen conditions, the standard anaerobic growth conditions used for growth of cultures were agitation at 100 rpm in an orbital shaker at 37°C in TSB medium supplemented with cysteine (5.7 mM) to scavenge traces of oxygen and 0.001% resazurin as a redox indicator. Wheaton serum bottles (100-ml capacity) containing 60 ml of the above-described medium were purged with nitrogen gas for 4 min at a pressure of 0.75 atm prior to being autoclaved. Aerobic cultures were subcultured (0.5 ml) in 60 ml of the above-described medium and grown for 16 to 20 h to stationary phase (optical density at 600 nm [OD600] of ∼2), and 2 ml was used to inoculate 60 ml of the same medium. Anaerobic growth of S. aureus colonies on plates was carried out in a sealed anaerobic jar (Oxoid) equipped with an AnaeroGen (Oxoid) sachet and by employing an Anaerotest indicator strip (Merck) for verifying anaerobic conditions.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or relevant characteristic(s) | Source or reference |
|---|---|---|
| Bacterial strains | ||
| E. coli XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqlacZΔM15 Tn10(Tcr)] | Laboratory stock |
| S. aureus RN4220 (NCTC 8325) | Restriction-minus derivative of 8325-4, primary recipient for propagation of E. coli plasmids, contains an 11-bp deletion in the rsbU gene | 32 |
| S. aureus SH1000 | 8325-4 carrying an intact rsbU gene | S. Foster (28) |
| S. aureus SH1000 ΔarcR | SH1000 in which the arcR gene was deleted and replaced by the Ωkm-2 kanamycin cassette | This study |
| Plasmids | ||
| pUC18 | Cloning vector; lacZ Apr | MBI/Fermentas |
| PMUTIN-4 | pUC18-based suicidal vector for gram-positive bacteria; Pspac-lacZ PpenP-lacI Apr Emr | 51 |
| pAUL-A | Temperature-sensitive shuttle vector; lacZ Emr | 47 |
| pBR322::Ωkm-2 | pBR322 carrying the Ωkm-2 cassette; Apr Kmr | 43 |
| pGEM-T Easy vector | An EcoRV-linearized vector with 3′-added T bases at both ends | Promega |
| pET28a(+) | A T7-based protein expression vector carrying an N-terminal His tag/thrombin tag | Novagen |
DNA manipulations.
Standard procedures were employed for PCR amplification, restriction enzyme digestion, ligation, Southern blotting, and other DNA manipulations (46). Preparation of plasmids and electroporation of competent cells were done as described previously (46). S. aureus genomic DNA was prepared as described previously (40).
RNA extraction.
Total RNA was isolated from S. aureus exponential-phase cultures grown in TSB medium at 37°C, as described previously (27). Cells (50 mg [wet weight]) were lysed in 0.3 ml of TES buffer (100 mM Tris-HCl [pH 7.5], 8 mM EDTA, 150 mM NaCl) containing 100 μg ml−1 of lysostaphin (Sigma), and RNA was extracted using 1.5 ml of RNazol B (Tel-test). For reverse transcription reactions, residual DNA was removed by treatment with RQ1 RNase-free DNase (Promega). RNA concentrations were determined by A260 measurements, and RNA integrity was analyzed by agarose-formaldehyde gel electrophoresis (46).
Northern blot analysis.
S. aureus total RNA (5 μg) was electrophoresed in an agarose-formaldehyde gel and transferred onto NytranN nylon membranes (Schleicher & Schuell) as previously described (36). Internal DNA fragments of arcA, arcB, arcC, arcD, arcR, lctE, pflB, and trxB were amplified by PCR and labeled with the PCR DIG Probe Synthesis kit (Roche). Primers used for the preparation of probes are listed in Table 2. Chemiluminescent detection was carried out according to the manufacturer's instructions.
TABLE 2.
Oligonucleotide primers used for construction by PCR of DNA probes
| Primer | Primer sequencea | Description |
|---|---|---|
| arcR_FOR | 5′-CATATGACAGAAAACTTTATTTTGGG-3′ | Amplification of arcR gene |
| arcR_REV | 5′-GGATCCTTAAACACATACATCATTG-3′ | Amplification of arcR gene |
| arcA_for | 5′-GACCCAAAATACCTTTATTTATG-3′ | Amplification of 233-bp probe in arcA regulatory region |
| arcA_rev | 5′-CCAGGACGCTTAAGTAACAC-3′ | Amplification of 233-bp probe in arcA regulatory region |
| lctE_for | 5′-CACTGGCGAAGTACGAAGAC-3′ | Amplification of 210-bp probe in lctE regulatory region |
| lctE_rev | 5′-AAAGGTCATGTGTCATCCGC-3′ | Amplification of 210-bp probe in lctE regulatory region |
| adh1_for | 5′-TGTCTTAGATTGATTGGGAG-3′ | Amplification of 215-bp probe in adh1 regulatory region |
| adh1_rev | 5′-GACATAATCGATATGCTAACG-3′ | Amplification of 215-bp probe in adh1 regulatory region |
| lukM_for | 5′-ATTAATGACTTTGTACACAC-3′ | Amplification of 211-bp probe in lukM regulatory region |
| lukM_rev | 5′-GCACATGATAATGATGACGC-3′ | Amplification of 211-bp probe in lukM regulatory region |
| srrA_for | 5′-GTCATTTAGCAGAACATGGG-3′ | Amplification of 157-bp probe in srrAB regulatory region |
| srrA_for | 5′-ACAGGTCATACCTCCCACAC-3′ | Amplification of 157-bp probe in srrAB regulatory region |
| nrdD_for | 5′-ACATGTCGAAATGACGGACG-3′ | Amplification of 221-bp probe in nrdD regulatory region |
| nrdD_rev | 5′-AACCCGTTAATGCTTCTTCG-3′ | Amplification of 221-bp probe in nrdD regulatory region |
| arcA mut_for | 5′-TATGTGAATATAATGGGATGTAAGCGTTTGAAG-3′ | Mutagenesis |
| arcA mut_rev | 5′-AACGCTTACATCCCATTATATTCACATAAAG-3′ | Mutagenesis |
The mismatched bases for the mutations of primers used for mutagenesis are underlined.
Construction of the arcR deletion mutant.
Deletion of the S. aureus arcR gene was performed by homologous recombination in RN4220. A ∼1.1-kb DNA fragment from the arcR upstream region, containing the last 39 bp of arcD and arcC and 80 bp of the arc-arcR intergenic region, was amplified by PCR using forward primer 1250 (with a native PacI restriction site [underlined]) (5′-TCGGGTTAATTAAGTTATTGATGGG-3′) and reverse primer 1251 (with an added EcoRI restriction site [underlined]) (5′-TCTCGAATTCCTTGCAAAGTGTCAGCAGAC-3′). The fragment was cut with PacI and EcoRI, purified from an agarose gel, ligated with the ∼8.25-kb PacI-EcoRI fragment of pMUTIN-4 (51) to give pUP, and introduced into E. coli XL1-Blue by electroporation. A ∼1.1-kb DNA fragment from the arcR downstream region, containing 328 bp of the arcR-clfB intergenic region and 793 bp of clfB, was amplified by PCR using forward primer 1252 (with an added EcoRI restriction site [underlined]) (5′-TCTCGAATTCGTTGAACATGAGGTCTAACG-3′) and as reverse primer 1569 (with an added EheI restriction site [underlined]) (5′-TCTCGGCGCCAACTATCTGGTAACTTCGCTG-3′). The fragment was cut with EcoRI and EheI, purified, and inserted into the ∼4.9-kb EcoRI-EheI fragment of pUP to create pUP-DW. Digestion of pUP-DW with EcoRI and ligation with the 2.27-kb EcoRI-EcoRI Ωkm-2 cassette yielded pUP_Km_DW, with two possible orientations of the cassette. Restriction analysis identified an isolate in which the arcC and the kanamycin cassette possess the same orientation and was introduced into S. aureus RN4220 by electroporation. Transformants were selected on TSB plates containing erythromycin and kanamycin. The expected single integration event resulting from recombination between pUP_Km_DW and the host chromosomal region was confirmed by PCR and Southern analysis (data not shown). To select for spontaneous segregation of the wild-type arcR allele, one transformant was propagated for 100 generations in TSB liquid medium containing kanamycin but lacking erythromycin, and colonies were screened for a loss of the erythromycin marker on TSB plates containing kanamycin. One kanamycin-resistant, erythromycin-sensitive clone was chosen for further analysis by PCR and DNA sequencing to confirm the desired deletion-substitution in the arcR gene. The arcR substitution-deletion (ΔarcR) was introduced into S. aureus SH1000 (rsbU+) by ϕ11 phage transduction, and its presence was verified by PCR using forward primer 1250 and reverse primer 1252 (5′-AGAATAATCCACGTCTCC-3′) and by DNA sequencing.
Cloning, expression, and purification of ArcR.
The S. aureus arcR gene was amplified from genomic DNA by PCR using the primers listed in Table 2. The 714-bp fragment was purified using the QIAquick gel extraction kit (QIAGEN), ligated into the pGEM-T Easy vector (Promega), and introduced into E. coli XL1-Blue by electroporation, and transformants were selected on LB plates containing ampicillin, IPTG (isopropyl-β-d-thiogalactopyranoside) (0.5 mM), and X-gal (5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside) (80 μg/ml). After overnight incubation at 37°C, white colonies were picked and tested for the presence of the arcR gene by colony PCR. Positive clones were used for plasmid isolation and sequencing. pGEMT-Easy:arcR was cut with restriction endonucleases NdeI and BamH, ligated into the pET28a(+) expression vector, cut with the same enzymes, and electroporated into E. coli XL1-Blue. The resulting plasmid, pET28a(+):arcR, contains arcR fused in frame at its 3′ end to six histidine codons. To overexpress ArcR, pET28a(+):arcR was introduced into E. coli BL21(DE3) cells, and cultures were grown to an OD550 of 0.4 and treated with 0.5 mM IPTG for 3 h to induce synthesis. ArcR was purified by Ni2+-CAM affinity chromatography according to the manufacturer's instructions (Sigma). Purified proteins were dialyzed against buffer containing 50 mM Tris-HCl (pH 8), 300 mM NaCl, and 5 mM dithiothreitol using a PD-10 desalting column (Amersham Biosciences). Recovery of recombinant protein was monitored by the Bradford method (Sigma) using bovine serum albumin as a standard (6) and by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
DNA binding assays.
Electrophoretic gel mobility shift assays (EMSAs) were performed with DNA probes containing the arcA, lctE, adh1, lukM, srrA, and nrdD upstream regulatory regions. Probes were generated by PCR using primers listed in Table 2 and purified using the QIAquick gel extraction kit (QIAGEN). DNA fragments were labeled at the 3′ end with digoxigenin (DIG)-dUTP using the Terminal Transferase kit (Roche, Mannheim, Germany). Binding reactions were carried out in a final volume of 20 μl containing 3 fmol labeled DNA probe, 20 mM Tris-HCl (pH 8.5), 5% (vol/vol) glycerol, 1 mM MgCl2, 40 mM KCl, 1 mM dithiothreitol, purified wild-type or mutant ArcR (0.2 to 1.6 μg protein) or crude cell extracts (0.4 to 6 μg protein), 1 μg sonicated salmon sperm DNA, and 0.1 μg poly-l-lysine. The mixture was incubated for 30 min at 37°C and placed on ice, and 5 μl of loading buffer was added to each sample. Protein-DNA complexes were monitored on a preelectrophoresed 6% polyacrylamide gel in 0.5× TBE running buffer (89 mM Trizma base [Sigma], 89 mM boric acid, 2 mM EDTA) at room temperature for 2 h. Gels were contact blotted for 8 h onto a Hybond-N+ membrane (Amersham Biosciences). Cross-linking of oligonucleotides was carried out using a Stratalinker and baking at 120°C for 30 min. Chemiluminescent detection was performed by adding alkaline phosphatase conjugated with an anti-DIG antibody. The membrane was washed at room temperature in washing buffer and equilibrated in detection buffer. One milliliter of alkaline phosphatase chemiluminescent substrate (CSPD, ready to use; Roche Applied Science) was added to allow the visualization of the hybrids (5 min at room temperature). The membrane was exposed to X-ray film (FUJI) for 15 to 30 min at 37°C.
Preparative two-dimensional (2D) gels and protein identification by mass spectrometry.
Cytoplasmic proteins were prepared from 50-ml cultures of cells grown to an OD550 of 0.7 under anaerobic conditions. Cells were harvested by centrifugation for 10 min at 7,000 × g at 4°C, washed twice with ice-cold Tris-EDTA buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]), and resuspended in Tris-EDTA buffer containing 4 mM sodium azide. Cells were disrupted using a Ribolyser (Thermo Electron Corporation) for 30 s at 6.5 m/s. Cell debris and insoluble and aggregated proteins were removed by centrifugation for 25 min at 21,000 × g at 4°C, and the supernatant was centrifuged for 45 min at 4°C. The protein concentration was determined using Roti-Nanoquant (Roth, Karlsruhe, Germany). The protein solution was stored at −20°C.
Preparative 2D gel electrophoresis was performed using an immobilized pH gradient (IPG) technique as described previously (5, 20). Proteins were separated in the first dimension on IPG strips (GE Healthcare, Piscataway, NJ) with a pH range of 4 to 7, and proteins were separated in the second dimension on 12.5% polyacrylamide gels. Proteins were stained with colloidal Coomassie brilliant blue (9), and the gels were scanned with a Scanner X-Finity Ultra (Quato Graphic). For protein identification, Coomassie-stained proteins were cut from the gel using a spot cutter (Proteome Work) (30). Trypsin digestion of proteins, spotting of peptide samples onto matrix-assisted laser desorption ionization targets, and matrix-assisted laser desorption ionization-time of flight mass spectrometry analyses were preformed as described previously (20, 30).
RESULTS
S. aureus ArcR is a Crp/Fnr-like transcriptional regulator.
To identify whether S. aureus contains Crp/Fnr-like transcriptional regulators, the complete genomes of nine strains, NCTC 8325, N315, Mu50, MW2, MRSA252, MSSA476, COL, USA300, and RF122 (a bovine isolate), were screened with a BLAST algorithm (1) using the E. coli Fnr and Crp protein sequences and the Bacillus subtilis Fnr-like protein sequences as queries. A single ORF possessing limited but significant sequence conservation was detected in all strains. The S. aureus 234-amino-acid ORF SAOUHSC_02964 (NCTC 8325) has a calculated molecular mass of 27,428 Da. It is closely related to Staphylococcus epidermidis ORFs SE2214 (strain ATCC 12228) and SERP2246 (strain RP62A), with which it shares approximately 60% sequence identity. Among the Crp family of proteins, the S. aureus ORF is most similar to the B. licheniformis, Bacillus cereus ATCC 10987, and L. sakei ArcR proteins, which have been reported to regulate the expression of the ADI pathway genes (34, 56). Figure 1C shows a sequence alignment of the S. aureus ORF with B. licheniformis ArcR and E. coli Crp (also called CAP, for catabolite-activating protein). The S. aureus ORF shares overall 19% identity and 45% similarity with B. licheniformis ArcR but is much less similar to E. coli Crp. In this work, it is designated ArcR. Multiple alignments of the deduced amino acid sequences of 18 ArcR proteins revealed that they share remarkably low overall similarity, suggesting that they differ in their response to various environmental signals (see the supplemental material).
FIG. 1.
Organization of the S. aureus arc operon. (A) Schematic representation of the S. aureus RN4220 (NCTC 8325) arcABDCR gene cluster. (B) Nucleotide sequence of the arcA regulatory region. A putative ArcR 16-bp binding site is shown framed and in boldface type; a putative cre motif is indicated by a hyphenated box; two putative ARG boxes are highlighted in gray. The predicted arcA ribosomal binding site (RBS) and ATG initiation codon are shown in boldface type. Positions of primers used for PCR amplification of the arcA promoter probe in DNA binding studies are underlined. The putative −10 and −35 promoter elements are indicated by boldface type. The DNA sequence is reported under GenBank accession number AJ566750. (C) Alignment of the S. aureus ArcR (SAU), B. licheniformis ArcR (LIC), and E. coli Crp (ECO) deduced protein sequences. Predicted cyclic nucleotide binding and HTH domains are highlighted in gray and in a rectangular box, respectively. Identical amino acid residues are shown by asterisks, highly conserved residues are shown by colons, and weakly conserved residues are shown by dots. The cysteine that was mutated in this work is circled.
Organization of genes in the S. aureus ADI operon.
The S. aureus ADI metabolic pathway genes are organized in an operon, arcABDC (Fig. 1A). The arcR gene is located 98 bp downstream of arcC and is oriented in the same direction as the gene cluster. A second putative regulatory gene, argR, encoding an ArgR/AhrC repressor, is positioned upstream of arcA. S. epidermidis contains two arc gene clusters. Similar arc gene clusters occur in other gram-positive and gram-negative bacteria. We noted the presence of a second arc gene cluster but lacking arcA in S. aureus. A somewhat different ADI gene organization occurs in community-acquired methicillin-resistant S. aureus strain USA300, where it is termed the arginine catabolic mobile element and appears to have been acquired horizontally from S. epidermidis or other coagulase-negative staphylococci (15).
Domain structure of ArcR.
S. aureus ArcR contains the two structural domains that are characteristic of members of the Crp family of regulatory proteins. Sequence alignment of S. aureus ArcR with the B. licheniformis ArcR and E. coli Crp shows that that the N-terminal portion possesses a cyclic nucleotide binding domain (PF00027; IPR000595) (Fig. 1C). In E. coli, this domain is composed of about 120 amino acids that form a network of α-helices and an antiparallel β-barrel structure (37, 48). Figure 1C shows that the S. aureus ArcR cyclic nucleotide binding domain contains several key structural residues that are conserved in Crp-related proteins. The ArcR C-terminal portion contains a well-conserved 22-amino-acid helix-turn-helix (HTH) motif. It possesses several residues, including Arg201 and Glu202, which correspond to residues that are known from crystal structure and mutagenesis studies of E. coli Crp to be involved in protein-DNA interactions (42). Sequence comparison of the cyclic nucleotide binding and HTH domains in 18 ArcR proteins is presented in the supplemental material.
ArcR is necessary for utilization of arginine as an energy source for growth under anaerobic conditions.
To assess the role of S. aureus ArcR in generating energy for growth via the anaerobic ADI pathway, we constructed a deletion mutant of the arcR gene. The wild type (SH1000) and a kanamycin substitution-replacement mutant (ΔarcR) were plated onto TSB medium lacking glucose and containing 50 mM arginine as the main energy source. Plates containing glucose (25 mM) or glucose plus arginine served as controls. Plates were incubated for 72 h under aerobic and anaerobic conditions. Under aerobic conditions, no significant difference was found in the growth of the wild-type and mutant strains under any of the conditions (data not shown). Under anaerobic conditions, significant differences in growth occurred depending on the energy sources (Fig. 2). In the absence of supplements, wild-type and mutant strains exhibited limited growth. When glucose was added, the growth of both strains was improved. If the main energy source was arginine, growth of the arcR deletion mutant was considerably impaired compared with that of the wild type. When glucose and arginine were both present, the growth of the parent strain was further enhanced, whereas the growth of the arcR mutant was similar to that in medium containing glucose alone. These observations establish that under anaerobic conditions, ArcR is necessary for the utilization of arginine as an energy source for growth.
FIG. 2.
Growth of S. aureus SH1000 and the arcR deletion mutant under anaerobic conditions. Cultures were plated onto TSB solid medium supplemented with 25 mM glucose or 50 mM arginine or both as the main energy source and incubated for 72 h at 37°C. wt, wild type.
ArcR is a positive regulator of transcription of arcABDCR genes under anaerobic conditions.
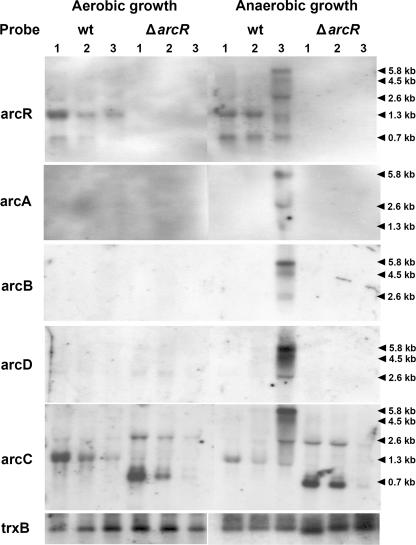
Northern blot analysis was performed to assess the role of ArcR in regulating the transcription of the arcABDC genes. Initially, we showed in SH1000 cells that arcABDC and arcR are cotranscribed. Total RNA was extracted from aerobically and anaerobically grown cultures of SH1000 grown in TSB medium, supplemented with glucose, and hybridized with arcA, arcB, arcC, arcD, and arcR PCR-labeled probes. Under anaerobic conditions, a ∼5.8-kb transcript was detected with each of the probes but only at the stationary growth phase (Fig. 3). The transcript corresponds in size to that expected for the cotranscription of the arcABDC and arcR genes, showing that they form an operon. We suppose that the inability to detect the ∼5.8-kb transcript during exponential growth is most likely due to glucose catabolic repression, which is relieved when, at the stationary phase, the medium becomes depleted of glucose. In contrast, under aerobic conditions, we were unable to detect the ∼5.8-kb transcript with any of the probes at any stage of growth.
FIG. 3.
Northern blot analysis of arcABDC and arcR genes under aerobic and anaerobic conditions. Total RNA was isolated from cultures of wild-type (wt) S. aureus SH1000 and the arcR deletion strain grown under aerobic and anaerobic conditions and hybridized with arcR, arcA, arcB, arcD, and arcC probes and a control trxB probe. Lanes 1, 2, and 3 indicate sampling times. Lanes for aerobic growth are as follows: 1, exponential phase with an OD600 of ∼0.4; 2, late exponential phase with an OD600 of ∼2.0; 3, stationary phase with an OD600 of ∼4.0. Lanes for anaerobic growth are as follows: 1, exponential phase with an OD600 of ∼0.3; 2, late exponential phase with an OD600 of ∼0.8; 3, stationary phase with an OD600 of ∼1.1.
While the ∼5.8-kb transcript is the major arc transcript made under anaerobic conditions, several smaller transcripts were also detected, suggesting a partial termination of transcription or processing of the full-length transcript (Fig. 3). Alternatively, some of the minor transcripts may arise from an additional promoter located within the arc operon. Thus, a ∼1.3-kb transcript was detected in total RNA isolated from anaerobic cultures using arcC and arcR probes but was not detected with arcA, arcB, and arcD probes, and a ∼0.7-kb transcript was detected with the arcR probe in each case, independent of the growth phase. Sequence analysis identified a putative promoter in the 3′ end of the arcC gene, which, if functional, would generate the ∼1.3-kb transcript terminating at the arcR terminator signal. The ∼0.7-kb transcript is likely the result of processing of the ∼1.3-kb transcript.
To assess the role of ArcR in the transcription of arcABDCR, parallel experiments were carried out with the arcR deletion mutant. In contrast to the wild-type strain, we were unable to detect full-length ∼5.8-kb transcripts in total RNA isolated from anaerobic cultures employing arcA, arcB, arcC, arcD, and arcR probes at any growth phase (Fig. 3). Hence, under anaerobic conditions, ArcR positively controls the transcription of the arcABDCR operon. Confirmation that arcABDCR is transcribed solely under anaerobic conditions and depends on a functional ArcR was obtained by reverse transcription-PCR analysis (data not shown). None of the smaller transcripts mentioned above that were detected in the wild type by the arcR probe were detected in the arcR deletion mutant. However, the arcC probe detected two transcripts of ∼0.7 kb and ∼2.4 kb (Fig. 3). These transcripts were also detected in RNA isolated from the arcR deletion mutant under aerobic conditions (Fig. 3). The putative promoter in arcC most likely accounts for the two transcripts. It is predicted to generate the ∼0.7-kb transcript through termination at a stem-loop structure in the upstream region of the kanamycin cassette, while the ∼2.3-kb transcript is predicted to arise from termination at the kanamycin terminator signal. Since both transcripts were detected under aerobic and anaerobic conditions in the wild type and the arcR deletion mutant (see below), the putative arcC promoter is independent of the oxygen level and independent of the ArcR regulator.
Similar experiments were carried out to determine whether ArcR controls the expression of genes encoding lactate dehydrogenase (lctE) and pyruvate formate-lyase (pflBA), genes that are expressed under conditions of anaerobic growth. As described below, both sets of genes contain a consensus Crp binding site in their upstream regulatory regions and, for this reason, were considered to be likely to be regulated by ArcR. Northern blot analysis performed with lctE and pflB probes revealed the presence of ∼1.2-kb and ∼3-kb transcripts, respectively, corresponding to the expected full-size lctE and pflBA transcripts. However, unlike arcABDCR, no significant difference was found in their transcription under aerobic and anaerobic growth conditions, nor was there any appreciable difference in lctE and pflBA transcription between the wild type and the arcR deletion mutant. Similar results were obtained using S. aureus RN4220 and its arcR mutant (data not shown).
Glucose and arginine exert reciprocal effects on arcABDCR transcription.
To assess the effect of glucose and arginine on the transcription of the arc operon, total RNA was isolated from aerobically and anaerobically grown exponential cultures of SH1000 and SH1000ΔarcR grown to an OD550 of 0.4 in media containing or lacking glucose and/or arginine. Northern blot analysis performed with arcA and arcR probes showed that full-length ∼5.8-kb transcripts were readily detected under anaerobic conditions in medium containing arginine and lacking glucose (Fig. 4). Transcription was perceptibly weaker when arginine was omitted from the medium. In the presence of glucose (with or without arginine), arcABDCR transcription was strongly reduced, showing that the arc operon is subject to catabolic repression. In the arcR deletion mutant, we were unable to detect full-length arcABDCR transcripts using arcA or arcR probes under any of the growth conditions tested (Fig. 4). As described above, no full-length arc transcripts were detected under aerobic conditions in both parent and mutant strains with either probe; two minor ∼0.7-kb and ∼1.3-kb transcripts were detected in the wild type by the arcR probe under aerobic and anaerobic conditions but were not detected in the arcR deletion mutant.
FIG. 4.
Northern blot analysis of arcABDC and arcR transcripts in wild-type (wt) SH1000 and the arcR deletion strain in different growth media under aerobic and anaerobic conditions. Total RNA isolated from cultures of wild-type SH1000 and the arcR deletion mutant grown in TSB medium to an OD550 of 0.4 was hybridized with arcR and arcA probes and a control trxB probe. The lanes indicate different growth media. Lanes: 1, TSB medium (without glucose); 2, TSB medium plus 25 mM glucose and 50 mM arginine; 3, TSB medium plus 25 mM glucose; 4, TSB medium plus 50 mM arginine.
Synthesis of ArcA, ArcB, and ArcC under anaerobic conditions requires a functional ArcR.
Further evidence that ArcR positively regulates the expression of the arc operon under anaerobic conditions was obtained from proteome analyses. S. aureus SH1000 and the arcR deletion mutant were grown anaerobically in TSB medium to late exponential phase (OD540 of 0.8), and cytoplasmic proteins were prepared as described in Materials and Methods. Figure 5 shows that the amounts of ArcA, ArcB, and ArcC catabolic proteins were markedly reduced in the arcR mutant. Surprisingly, ArcA, ArcB, and ArcC were the only proteins whose amounts were significantly affected by the elimination of ArcR, and the amounts of other proteins encoded by genes known to be expressed specifically in anaerobic conditions, such as lactate dehydrogenase and alcohol dehydrogenase, were not appreciably different in the wild-type and arcR mutant strains.
FIG. 5.
Effect of ArcR on the synthesis of cytoplasmic proteins under anaerobic conditions. Shown is a false-colored dual-channel image of 2D gels of cytoplasmic proteins extracted from the wild type (SH1000) (red) and the arcR deletion mutant grown to an OD540 of 0.8 in TSB medium under anaerobic conditions. Three hundred micrograms of protein of crude cell extracts was separated on 2D gels using IPG strips in the pH range of 4 to 7. Proteins were stained with colloidal Coomassie brilliant blue. Proteins whose amounts were positively affected by ArcR appear in red, and proteins not affected by ArcR appear in yellow. Spots of interest are labeled with their protein names, as annotated for S. aureus N315.
ArcR binds to the regulatory region of the arc ABDCR operon.
The HTH domain in Crp regulatory proteins recognizes the symmetric sequence TGTGA-N6-TCACA (8). B. licheniformis ArcR recognizes a similar target (34). In a screen of the Mu50 genome, we identified 15 copies of Crp-like binding sites, one in the upstream regulatory region of arcA and the others in similar locations of genes involved in anaerobic metabolism. To determine if S. aureus ArcR controls the expression of the arc operon by binding to the arc promoter region, EMSAs were performed by employing crude cell extracts and purified recombinant His-ArcR proteins. DNA binding reactions were carried out with a DIG-labeled 232-bp PCR-amplified arcA fragment containing the Crp consensus binding motif TGTGA-N6-TCACA. Incubation of wild-type crude cell extracts with labeled DNA probe resulted in a shift in the mobility of the fragment, with it migrating more slowly than the free fragment, presumably due to the formation of a DNA-protein complex (Fig. 6A). Increasing the amount of cell extract led to the formation of higher-order complexes and a further reduction in mobility of the fragment. When binding was carried out with cell extracts from the arcR deletion mutant, a slight change in mobility of the fragment occurred but did not change with increasing amounts of extract.
FIG. 6.
EMSAs of ArcR binding to the arc operon regulatory region. (A) Crude cell extracts prepared from S. aureus SH1000 and the arcR deletion mutant (0.37 μg to 6 μg protein) incubated with a 232-bp DIG-labeled arcA fragment (3 fmol). (B) Purified recombinant His-ArcR (0.1 to 1.0 μg protein) incubated with the 232-bp arcA fragment (3 fmol). (C) Competition assay. His-ArcR (0.8 μg protein) and a DIG-labeled arcA fragment (3 fmol) were incubated in the presence of increasing amounts of unlabeled arcA fragment. Lane 1, 3 fmol unlabeled fragment; lane 2, 30 fmol unlabeled fragment; lane 3, 300 fmol unlabeled fragment. (D). Control assays. Lane 1, purified His-NrdR (5 μg) incubated with a DIG-labeled arcA fragment (3 fmol); lane 2, purified His-ArcR (1 μg) incubated with a DIG-labeled nrdD fragment (3 fmol).
When purified His-tagged ArcR was used in DNA binding reactions, a single DNA-protein complex was formed, the amount of which increased with increasing amounts of ArcR in the reaction mixture. The specificity of the reaction was shown in competition experiments in which the addition of a large excess of cold probe in the binding reaction mixture resulted in the disappearance of the signal (Fig. 6C). Control reactions employing an unrelated His-tagged NrdR protein had no effect on probe mobility (Fig. 6D). Also, ArcR did not bind to an unrelated 221-bp nrdD control promoter probe that lacks Crp binding site DNA. These results demonstrate that ArcR binds to the upstream regulatory region of the arcA promoter.
Multiple Crp consensus binding sites are present in the S. aureus genome and occur predominantly in the regulatory regions of genes required for anaerobiosis.
Numerous putative Crp-like binding sites were identified in the S. aureus genome and are listed in Table S1 of the supplemental material. Fifteen binding sites contain the identical sequence motif of the consensus Crp binding site, another 14 contain a single change, and another 5 contain two changes in either one of the two half-sites of the consensus motif. Many of these sites occur in front of genes that are thought to be required for anaerobic growth. For example, potential Crp binding sites were found in the upstream regions of genes encoding the two-component systems SrrAB (50, 53) and VicRK (18), for the cytochrome oxidase homolog CtaB, for the aerolysin/leukocidin toxin LukM, and for various other functions. Most of these sequences were found within intergenic regions and positioned in front of genes coding for putative anaerobic regulatory and enzymatic systems, suggesting that they may be transcriptionally regulated by ArcR.
ArcR binds to the regulatory regions of adh1, lctE, srrAB, and lukM.
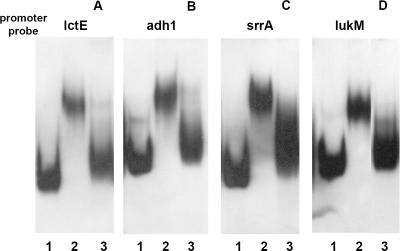
To assess whether S. aureus ArcR potentially controls the expression of the genes encoding alcohol dehydrogenase (adh1), lactate dehydrogenase (lctE), and aerolysin/leukocidin toxin (lukM), each of which contains a Crp consensus binding site in the upstream regulatory region, and the two-component signal transduction system srrAB, which contains a single change in the Crp motif, we examined the ability of ArcR to bind to DNA fragments containing the regulatory regions. EMSAs showed that S. aureus wild-type cell extracts caused similar shifts in mobility of the adh1, srrA, and lukM probes, whereas cell extracts of the arcR deletion mutant had no discernible effect on mobility (Fig. 7). The ability of ArcR to bind to the adh1, srrA, and lukM regulatory regions was confirmed by using purified His-ArcR, which formed, in each case, a single DNA-protein complex (data not shown). Unexpectedly, while cell extracts caused a mobility shift with the lctE DNA fragment, this was not observed with purified ArcR, suggesting that additional cellular factors might be necessary for binding.
FIG. 7.
EMSAs of ArcR binding to the lctE, adh1, and lukM regulatory regions. Crude cell extracts prepared from S. aureus SH1000 and the arcR deletion mutant were incubated with DIG-labeled DNA fragments (3 fmol). (A) lctE fragment (210 bp). (B) adh1 fragment (215 bp). (C) srrA fragment (157 bp). (D) lukM fragment (211 bp). Lane 1, negative control (DNA probe only); lane 2, SH1000 crude extract (6 μg); lane 3, S. aureus crude extract of arcR deletion mutant (6 μg).
ArcR binding to the lctE regulatory region is cAMP dependent.
Crp proteins typically form a complex with cyclic AMP (cAMP) that facilitates binding to its target promoter region. To determine whether cAMP influences the binding of ArcR to the lctE regulatory region, 0.5 mM cAMP was included in DNA binding reactions. EMSAs showed that cAMP significantly increased the formation of an ArcR-DNA complex (Fig. 8A). The specificity of ArcR binding to the lctE DNA fragment was shown by competitive inhibition of binding by the addition of an excess of unlabeled fragment (Fig. 8B). In contrast, cAMP had little or no effect on stimulating ArcR binding to the arcA, adh1, and srrAB DNA fragments (data not shown).
FIG. 8.
EMSAs of His-ArcR binding to the lctE regulatory region in the absence and presence of cAMP (0.5 mM). (A) Incubation of His-ArcR with the lctE fragment (3 fmol). His-ArcR protein concentrations were as follows: lane 1, 0 μg; lane 2, 0.1 μg; lane 3, 0.2 μg; lane 4, 4 μg. (B) Incubation of His-ArcR and the lctE fragment (3 fmol) in the presence of cAMP (0.5 mM). His-ArcR protein concentrations were as follows: lane 1, 0 μg; lane 2, 0.1 μg; lane 3, 0.2 μg; lane 4, 0.4 μg; lane 5, 0.5 μg. Lanes 6 to 8 show a competition assay. His-ArcR (0.4 μg) and the DIG-labeled lctE fragment (3 fmol) were incubated in the presence of increasing amounts of unlabeled lctE fragment. Lane 6, 3 fmol; lane 7, 30 fmol; lane 8, 300 fmol.
DISCUSSION
A systematic search of the S. aureus genome databases identified a single ORF homologous to ArcR, a member of the Crp-Fnr family of transcriptional regulators. ArcR was shown in several bacteria to govern expression of the ADI pathway in response to arginine, providing a source of ATP for energy under anaerobic conditions (21, 44, 56) and generating ammonia to protect against the deleterious effects of acidic environments (10). In this study, we show that S. aureus ArcR is not essential for growth under aerobic conditions but is necessary to support growth under anaerobic conditions when arginine is the sole energy source. The significance of the S. aureus ADI system for anaerobic growth is also indicated by studies that showed that hemB mutants that are deficient in citric acid cycle and electron transport pathways can compensate to produce ATP by upregulating the ADI pathway (29). Similarly, transcription of the ADI pathway genes was reported to be stimulated in an agr- and cell density-dependent manner (19), thereby enabling anaerobic growth in the presence of arginine. Recent studies suggest that S. aureus ArcR may play an important role in anaerobic biofilm formation, since the ADI and urease pathway genes are significantly induced in biofilm cells compared to planktonic conditions and possibly are the basis for survival under anoxic conditions (3, 45).
The S. aureus arcR and arcABDC genes are coexpressed and form an operon. Full-length transcripts were detected only under anaerobic conditions. In medium containing glucose, arcABDC transcription was observed only in the stationary state, indicating that the operon is subject to catabolite control and that expression is induced when glucose is depleted. In contrast, in the absence of glucose, arginine strongly enhanced the transcription of arc genes. Glucose and arginine therefore have reciprocal effects on regulating the expression of the arc genes: arginine acts as a positive regulator, and glucose acts as a negative regulator. Similar observations were reported previously for other bacteria (13, 14). To determine whether ArcR is essential for the expression of arc genes under conditions of anaerobic growth, we deleted arcR and showed that the expression of the arcABDC gene cluster was abolished. Thus, under conditions when arginine is the main source of carbon for energy, ArcR is essential for anaerobic growth. Proteomic analysis confirmed that Arc proteins are synthesized under the control of ArcR under anaerobic conditions. ArcA, ArcB, and ArcC were all detected under anaerobic conditions in the wild type but were absent in the arcR deletion mutant. ArcD, which encodes the arginine-ornithine antiporter, was not detected, presumably because it is a membrane protein. Unexpectedly, the expression of several other proteins encoded by genes implicated in anaerobic metabolism, pflB, hmp, lctE, ddh, and srrA, was not significantly different in the wild-type and mutant strains even though all were recently shown to be transcriptionally upregulated in the transition from aerobic to anaerobic growth (22). Thus, while the regulatory regions of these genes have a consensus Crp motif and bind ArcR (see below), their activation during anaerobiosis is likely to be mediated by a Crp-like activator other than ArcR. Alternatively, ArcR may exert a much less pronounced effect on inducing the expression of these genes compared to the arc genes under the physiological conditions employed in these experiments.
Crp regulatory proteins act by binding to specific sequences in the promoter regions of the genes that they control (31, 38). The consensus Crp DNA binding sequence is TGTGA-N6-TCACA. We assumed that since S. aureus ArcR is a Crp-like protein, it would bind to a similar motif in the arc promoter region. The arc promoter region contains a consensus Crp binding site to which ArcR binds. Possibly, S. aureus ArcR functions in conjunction with ArgR to activate the expression of the arc operon. A putative argR gene coding for an ArgR repressor and two putative ARG boxes are located immediately upstream of the arcA gene.
Close inspection of the S. aureus genome revealed the existence of numerous additional Crp-like binding sites, some which are identical to the consensus site and others which differ by just one or two bases (see Table S1 in the supplemental material). Interestingly, nearly all of the Crp-like sites are located in front of genes thought to have a role in anaerobiosis. Some of these sites show strong similarity to B. subtilis Fnr and Rex binding sites (26). Michel et al. (38) previously showed that the expression of many of these genes, and that of other genes presumed to be involved in anaerobic growth, is affected in a ClpP protease mutant. Several of the genes containing Crp-like binding sites were chosen as candidates to determine if ArcR is able to bind to their promoter regions. Promoter probes for four of these genes, lctE, adh1, srrAB, and lukM, all exhibited binding with cell extracts, and with the exception of lctE, all showed binding with purified ArcR. Plausibly, additional cellular factors might be necessary for ArcR binding to the lctE promoter region.
Crp is a DNA binding protein that assists RNA polymerase in binding to promoters to activate transcription. Crp binds cAMP and adopts a conformation that stimulates its interaction with RNA polymerase to establish active transcription complexes at Crp-dependent promoters (23). The ArcR N-terminal region contains a predicted cyclic nucleotide binding domain, and we therefore tested whether cAMP stimulates the binding of ArcR to the lctE promoter probe. cAMP significantly improved the formation of a complex between ArcR and the lctE probe. However, we failed to detect an effect of cAMP on Crp binding with any of the other probes. We analyzed the lctE promoter probe in more detail and found that it contains two sets of Crp-like binding sites, whereas each of the other promoter regions contains a single Crp-like binding site. cAMP stimulation of ArcR binding to the lctE promoter may involve a cooperative interaction between two Crp molecules. Lactate dehydrogenase is a key enzyme in anaerobic fermentation. Plausibly, the lctE gene is subject to glucose catabolic repression. Under anaerobic conditions, when glucose is limiting, the binding of cAMP to ArcR results in a conformational change that enhances its ability to bind to the lctE regulatory region and thus to activate transcription, allowing the anaerobic degradation of alternative carbon sources to obtain energy.
Catabolite repression of the arc operon has been reported previously for various species such as oral streptococci (16), L. sakei (55), and Streptococcus suis (25). In these bacteria, the expression of the ADI pathway genes is induced in the presence of arginine and repressed by glucose. A recent study shows that CcpA (catabolite control protein A) is involved in the catabolic repression of transcription of the S. gordonii arc operon (54). A similar mechanism may operate on the S. aureus arc operon, since we noted the presence of a putative cre sequence upstream of arcA, and a ccpA ortholog in S. aureus was recently described (49). In S. gordonii, ArcR, which is closely related to ArgR transcriptional regulators, appears to recognize high-affinity and low-affinity binding sites in the arc promoter region (54). In this respect, the S. aureus and S. gordonii ArcR proteins differ from E. coli Crp, which binds to two symmetrical sites. The presence of what may be high- and low-affinity ArcR binding sites in the S. aureus arcA promoter region may allow the fine-tuning of the regulation of the ADI pathway in response to different environmental signals.
Supplementary Material
Acknowledgments
We thank Batia Gorovitz-Harris, Rachel Schreiber, and Michaela Yanku for technical assistance.
Footnotes
Published ahead of print on 8 June 2007.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barcelona-Andres, B., A. Marina, and V. Rubio. 2002. Gene structure, organization, expression, and potential regulatory mechanisms of arginine catabolism in Enterococcus faecalis. J. Bacteriol. 184:6289-6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beenken, K. E., P. M. Dunman, F. McAleese, D. Macapagal, E. Murphy, S. J. Projan, J. S. Blevins, and M. S. Smeltzer. 2004. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 186:4665-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, O. G., and P. H. von Hippel. 1988. Selection of DNA binding sites by regulatory proteins. II. The binding specificity of cyclic AMP receptor protein to recognition sites. J. Mol. Biol. 200:709-723. [DOI] [PubMed] [Google Scholar]
- 5.Bernhardt, J., K. Buttner, C. Scharf, and M. Hecker. 1999. Dual channel imaging of two-dimensional electropherograms in Bacillus subtilis. Electrophoresis 20:2225-2240. [DOI] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Burne, R. A., and R. E. Marquis. 2000. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol. Lett. 193:1-6. [DOI] [PubMed] [Google Scholar]
- 8.Cameron, A. D., and R. J. Redfield. 2006. Non-canonical CRP sites control competence regulons in Escherichia coli and many other gamma-proteobacteria. Nucleic Acids Res. 34:6001-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Candiano, G., M. Bruschi, L. Musante, L. Santucci, G. M. Ghiggeri, B. Carnemolla, P. Orecchia, L. Zardi, and P. G. Righetti. 2004. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 25:1327-1333. [DOI] [PubMed] [Google Scholar]
- 10.Casiano-Colon, A., and R. E. Marquis. 1988. Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl. Environ. Microbiol. 54:1318-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clements, M. O., and S. J. Foster. 1999. Stress resistance in Staphylococcus aureus. Trends Microbiol. 7:458-462. [DOI] [PubMed] [Google Scholar]
- 12.Cotter, P. D., and C. Hill. 2003. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67:429-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunin, R., N. Glansdorff, A. Pierard, and V. Stalon. 1986. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50:314-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curran, T. M., Y. Ma, G. C. Rutherford, and R. E. Marquis. 1998. Turning on and turning off the arginine deiminase system in oral streptococci. Can. J. Microbiol. 44:1078-1085. [DOI] [PubMed] [Google Scholar]
- 15.Diep, B. A., S. R. Gill, R. F. Chang, T. H. Phan, J. H. Chen, M. G. Davidson, F. Lin, J. Lin, H. A. Carleton, E. F. Mongodin, G. F. Sensabaugh, and F. Perdreau-Remington. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet 367:731-739. [DOI] [PubMed] [Google Scholar]
- 16.Dong, Y., Y. Y. Chen, and R. A. Burne. 2004. Control of expression of the arginine deiminase operon of Streptococcus gordonii by CcpA and Flp. J. Bacteriol. 186:2511-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong, Y., Y. Y. Chen, J. A. Snyder, and R. A. Burne. 2002. Isolation and molecular analysis of the gene cluster for the arginine deiminase system from Streptococcus gordonii DL1. Appl. Environ. Microbiol. 68:5549-5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubrac, S., and T. Msadek. 2004. Identification of genes controlled by the essential YycG/YycF two-component system of Staphylococcus aureus. J. Bacteriol. 186:1175-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunman, P. M., E. Murphy, S. Haney, D. Palacios, G. Tucker-Kellogg, S. Wu, E. L. Brown, R. J. Zagursky, D. Shlaes, and S. J. Projan. 2001. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J. Bacteriol. 183:7341-7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eymann, C., A. Dreisbach, D. Albrecht, J. Bernhardt, D. Becher, S. Gentner, T. Tam le, K. Buttner, G. Buurman, C. Scharf, S. Venz, U. Volker, and M. Hecker. 2004. A comprehensive proteome map of growing Bacillus subtilis cells. Proteomics 4:2849-2876. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez, M., and M. Zuniga. 2006. Amino acid catabolic pathways of lactic acid bacteria. Crit. Rev. Microbiol. 32:155-183. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs, S., J. Pané-Farré, C. Kohler, M. Hecker, and S. Engelmann. 2007. Anaerobic gene expression in Staphylococcus aureus. J. Bacteriol. 189:4275-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green, J., C. Scott, and J. R. Guest. 2001. Functional versatility in the CRP-FNR superfamily of transcription factors: FNR and FLP. Adv. Microb. Physiol. 44:1-34. [DOI] [PubMed] [Google Scholar]
- 24.Griswold, A., Y. Y. Chen, J. A. Snyder, and R. A. Burne. 2004. Characterization of the arginine deiminase operon of Streptococcus rattus FA-1. Appl. Environ. Microbiol. 70:1321-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruening, P., M. Fulde, P. Valentin-Weigand, and R. Goethe. 2006. Structure, regulation, and putative function of the arginine deiminase system of Streptococcus suis. J. Bacteriol. 188:361-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gyan, S., Y. Shiohira, I. Sato, M. Takeuchi, and T. Sato. 2006. Regulatory loop between redox sensing of the NADH/NAD+ ratio by Rex (YdiH) and oxidation of NADH by NADH dehydrogenase Ndh in Bacillus subtilis. J. Bacteriol. 188:7062-7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart, M. E., M. S. Smeltzer, and J. J. Iandolo. 1993. The extracellular protein regulator (xpr) affects exoprotein and agr mRNA levels in Staphylococcus aureus. J. Bacteriol. 175:7875-7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457-7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohler, C., C. von Eiff, G. Peters, R. A. Proctor, M. Hecker, and S. Engelmann. 2003. Physiological characterization of a heme-deficient mutant of Staphylococcus aureus by a proteomic approach. J. Bacteriol. 185:6928-6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohler, C., S. Wolff, D. Albrecht, S. Fuchs, D. Becher, K. Buttner, S. Engelmann, and M. Hecker. 2005. Proteome analyses of Staphylococcus aureus in growing and non-growing cells: a physiological approach. Int. J. Med. Microbiol. 295:547-565. [DOI] [PubMed] [Google Scholar]
- 31.Korner, H., H. J. Sofia, and W. G. Zumft. 2003. Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol. Rev. 27:559-592. [DOI] [PubMed] [Google Scholar]
- 32.Kreiswirth, B. N., S. Lofdahl, M. J. Betley, M. O'Reilly, P. M. Schlievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709-712. [DOI] [PubMed] [Google Scholar]
- 33.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 34.Maghnouj, A., A. A. Abu-Bakr, S. Baumberg, V. Stalon, and C. Vander Wauven. 2000. Regulation of anaerobic arginine catabolism in Bacillus licheniformis by a protein of the Crp/Fnr family. FEMS Microbiol. Lett. 191:227-234. [DOI] [PubMed] [Google Scholar]
- 35.Maghnouj, A., T. F. de Sousa Cabral, V. Stalon, and C. Vander Wauven. 1998. The arcABDC gene cluster, encoding the arginine deiminase pathway of Bacillus licheniformis, and its activation by the arginine repressor argR. J. Bacteriol. 180:6468-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masalha, M., I. Borovok, R. Schreiber, Y. Aharonowitz, and G. Cohen. 2001. Analysis of transcription of the Staphylococcus aureus aerobic class Ib and anaerobic class III ribonucleotide reductase genes in response to oxygen. J. Bacteriol. 183:7260-7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKay, D. B., and T. A. Steitz. 1981. Structure of catabolite gene activator protein at 2.9 A resolution suggests binding to left-handed B-DNA. Nature 290:744-749. [DOI] [PubMed] [Google Scholar]
- 38.Michel, A., F. Agerer, C. R. Hauck, M. Herrmann, J. Ullrich, J. Hacker, and K. Ohlsen. 2006. Global regulatory impact of ClpP protease of Staphylococcus aureus on regulons involved in virulence, oxidative stress response, autolysis, and DNA repair. J. Bacteriol. 188:5783-5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neubauer, H., and F. Gotz. 1996. Physiology and interaction of nitrate and nitrite reduction in Staphylococcus carnosus. J. Bacteriol. 178:2005-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novick, R. P. 1991. Genetic systems in staphylococci. Methods Enzymol. 204:587-636. [DOI] [PubMed] [Google Scholar]
- 41.Pantel, I., P. E. Lindgren, H. Neubauer, and F. Gotz. 1998. Identification and characterization of the Staphylococcus carnosus nitrate reductase operon. Mol. Gen. Genet 259:105-114. [DOI] [PubMed] [Google Scholar]
- 42.Parkinson, G., C. Wilson, A. Gunasekera, Y. W. Ebright, R. E. Ebright, and H. M. Berman. 1996. Structure of the CAP-DNA complex at 2.5 angstroms resolution: a complete picture of the protein-DNA interface. J. Mol. Biol. 260:395-408. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Casal, J., M. G. Caparon, and J. R. Scott. 1991. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J. Bacteriol. 173:2617-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Proctor, R. A. 2000. Toward an understanding of biomaterial infections: a complex interplay between the host and bacteria. J. Lab. Clin. Med. 135:14-15. [DOI] [PubMed] [Google Scholar]
- 45.Resch, A., R. Rosenstein, C. Nerz, and F. Gotz. 2005. Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl. Environ. Microbiol. 71:2663-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 47.Schaferkordt, S., and T. Chakraborty. 1995. Vector plasmid for insertional mutagenesis and directional cloning in Listeria spp. BioTechniques 19:720-722, 724-725. [PubMed] [Google Scholar]
- 48.Schultz, S. C., G. C. Shields, and T. A. Steitz. 1991. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science 253:1001-1007. [DOI] [PubMed] [Google Scholar]
- 49.Seidl, K., M. Stucki, M. Ruegg, C. Goerke, C. Wolz, L. Harris, B. Berger-Bachi, and M. Bischoff. 2006. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 50:1183-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Throup, J. P., F. Zappacosta, R. D. Lunsford, R. S. Annan, S. A. Carr, J. T. Lonsdale, A. P. Bryant, D. McDevitt, M. Rosenberg, and M. K. Burnham. 2001. The srhSR gene pair from Staphylococcus aureus: genomic and proteomic approaches to the identification and characterization of gene function. Biochemistry 40:10392-10401. [DOI] [PubMed] [Google Scholar]
- 51.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097-3104. [DOI] [PubMed] [Google Scholar]
- 52.Wohlkonig, A., V. Stalon, and C. Vander Wauven. 2004. Purification of ArcR, an oxidation-sensitive regulatory protein from Bacillus licheniformis. Protein Expr. Purif. 37:32-38. [DOI] [PubMed] [Google Scholar]
- 53.Yarwood, J. M., D. J. Bartels, E. M. Volper, and E. P. Greenberg. 2004. Quorum sensing in Staphylococcus aureus biofilms. J. Bacteriol. 186:1838-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeng, L., Y. Dong, and R. A. Burne. 2006. Characterization of cis-acting sites controlling arginine deiminase gene expression in Streptococcus gordonii. J. Bacteriol. 188:941-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zúñiga, M., M. Champomier-Verges, M. Zagorec, and G. Pérez-Martínez. 1998. Structural and functional analysis of the gene cluster encoding the enzymes of the arginine deiminase pathway of Lactobacillus sake. J. Bacteriol. 180:4154-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zúñiga, M., C. de Carmen Miralles, and G. Pérez-Martínez. 2002. The product of arcR, the sixth gene of the arc operon of Lactobacillus sakei, is essential for expression of the arginine deiminase pathway. Appl. Environ. Microbiol. 68:6051-6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.