Abstract
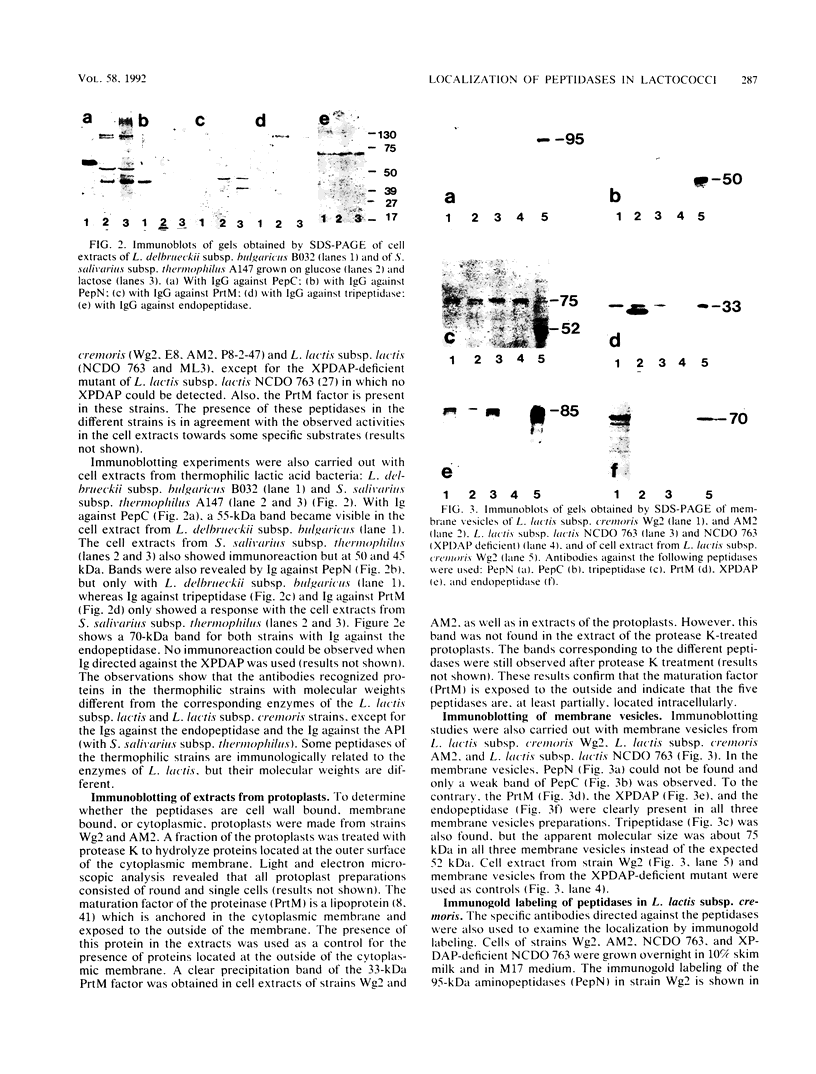
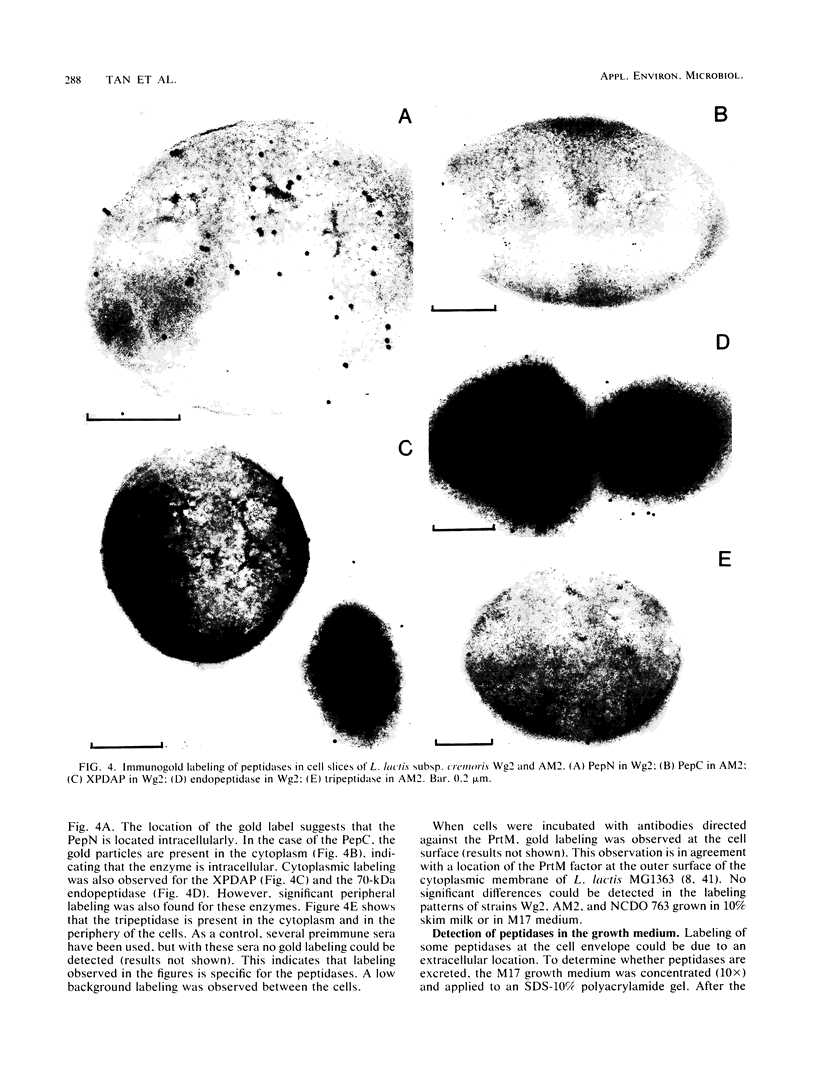
The localization of two aminopeptidases, an X-prolyl-dipeptidyl aminopeptidase, an endopeptidase, and a tripeptidase in Lactococcus lactis was studied. Polyclonal antibodies raised against each purified peptidase are specific and do not cross-react with other peptidases. Experiments were performed by immunoblotting after cell fractionation and by electron microscopy of immunogold-labeled peptidases. All peptidases were found to be intracellular. However, immunogold studies showed a peripheral labeling of the X-prolyl-dipeptidyl aminopeptidase, the tripeptidase, and the endopeptidase. This peripheral location was further supported by the detection of these three enzymes in cell membrane fractions in which none of the two aminopeptidases was present.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlan D., Laloi P., Portalier R. X-Prolyl-Dipeptidyl Aminopeptidase of Lactobacillus delbrueckii subsp. bulgaricus: Characterization of the Enzyme and Isolation of Deficient Mutants. Appl Environ Microbiol. 1990 Jul;56(7):2174–2179. doi: 10.1128/aem.56.7.2174-2179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman B. W., Tan P. S., Konings W. N. Purification and Characterization of a Tripeptidase from Lactococcus lactis subsp. cremoris Wg2. Appl Environ Microbiol. 1990 Jun;56(6):1839–1843. doi: 10.1128/aem.56.6.1839-1843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exterkate F. A. Location of Peptidases Outside and Inside the Membrane of Streptococcus cremoris. Appl Environ Microbiol. 1984 Jan;47(1):177–183. doi: 10.1128/aem.47.1.177-183.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exterkate F. A., de Veer G. J. Purification and Some Properties of a Membrane-Bound Aminopeptidase A from Streptococcus cremoris. Appl Environ Microbiol. 1987 Mar;53(3):577–583. doi: 10.1128/aem.53.3.577-583.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Lee E. Y., Welch R. A. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol. 1985 Jul;163(1):88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haandrikman A. J., Kok J., Laan H., Soemitro S., Ledeboer A. M., Konings W. N., Venema G. Identification of a gene required for maturation of an extracellular lactococcal serine proteinase. J Bacteriol. 1989 May;171(5):2789–2794. doi: 10.1128/jb.171.5.2789-2794.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz J., van Sinderen D., Kok J., Konings W. N. Cell Wall-Associated Proteases of Streptococcus cremoris Wg2. Appl Environ Microbiol. 1987 Apr;53(4):853–859. doi: 10.1128/aem.53.4.853-859.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid N. M., Marth E. H. Purification and Partial Characterization of a Prolyl-Dipeptidyl Aminopeptidase from Lactobacillus helveticus CNRZ 32. Appl Environ Microbiol. 1990 Feb;56(2):381–388. doi: 10.1128/aem.56.2.381-388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok J. Genetics of the proteolytic system of lactic acid bacteria. FEMS Microbiol Rev. 1990 Sep;7(1-2):15–42. doi: 10.1111/j.1574-6968.1990.tb04877.x. [DOI] [PubMed] [Google Scholar]
- Kok J., Leenhouts K. J., Haandrikman A. J., Ledeboer A. M., Venema G. Nucleotide sequence of the cell wall proteinase gene of Streptococcus cremoris Wg2. Appl Environ Microbiol. 1988 Jan;54(1):231–238. doi: 10.1128/aem.54.1.231-238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Poolman B., Driessen A. J. Bioenergetics and solute transport in lactococci. Crit Rev Microbiol. 1989;16(6):419–476. doi: 10.3109/10408418909104474. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laan H., Konings W. N. Mechanism of Proteinase Release from Lactococcus lactis subsp. cremoris Wg2. Appl Environ Microbiol. 1989 Dec;55(12):3101–3106. doi: 10.1128/aem.55.12.3101-3106.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Mayo B., Kok J., Venema K., Bockelmann W., Teuber M., Reinke H., Venema G. Molecular cloning and sequence analysis of the X-prolyl dipeptidyl aminopeptidase gene from Lactococcus lactis subsp. cremoris. Appl Environ Microbiol. 1991 Jan;57(1):38–44. doi: 10.1128/aem.57.1.38-44.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet V., Le Bars D., Gripon J. C. Purification and characterization of a cell wall proteinase from Streptococcus lactis NCDO 763. J Dairy Res. 1987 May;54(2):247–255. doi: 10.1017/s0022029900025383. [DOI] [PubMed] [Google Scholar]
- Nardi M., Chopin M. C., Chopin A., Cals M. M., Gripon J. C. Cloning and DNA sequence analysis of an X-prolyl dipeptidyl aminopeptidase gene from Lactococcus lactis subsp. lactis NCDO 763. Appl Environ Microbiol. 1991 Jan;57(1):45–50. doi: 10.1128/aem.57.1.45-50.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neviani E., Boquien C. Y., Monnet V., Thanh L. P., Gripon J. C. Purification and Characterization of an Aminopeptidase from Lactococcus lactis subsp. cremoris AM2. Appl Environ Microbiol. 1989 Sep;55(9):2308–2314. doi: 10.1128/aem.55.9.2308-2314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto R., Lageveen R. G., Veldkamp H., Konings W. N. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1982 Feb;149(2):733–738. doi: 10.1128/jb.149.2.733-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid E. J., Konings W. N. Relationship between utilization of proline and proline-containing peptides and growth of Lactococcus lactis. J Bacteriol. 1990 Sep;172(9):5286–5292. doi: 10.1128/jb.172.9.5286-5292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid E. J., Plapp R., Konings W. N. Peptide uptake is essential for growth of Lactococcus lactis on the milk protein casein. J Bacteriol. 1989 Nov;171(11):6135–6140. doi: 10.1128/jb.171.11.6135-6140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid E. J., Poolman B., Konings W. N. Casein utilization by lactococci. Appl Environ Microbiol. 1991 Sep;57(9):2447–2452. doi: 10.1128/aem.57.9.2447-2452.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P. S., Konings W. N. Purification and Characterization of an Aminopeptidase from Lactococcus lactis subsp. cremoris Wg2. Appl Environ Microbiol. 1990 Feb;56(2):526–532. doi: 10.1128/aem.56.2.526-532.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P. S., Pos K. M., Konings W. N. Purification and characterization of an endopeptidase from Lactococcus lactis subsp. cremoris Wg2. Appl Environ Microbiol. 1991 Dec;57(12):3593–3599. doi: 10.1128/aem.57.12.3593-3599.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhuis M., van Dijken J. P., Pilon S. A., Harder W. Development of crystalline peroxisomes in methanol-grown cells of the yeast Hansenula polymorpha and its relation to environmental conditions. Arch Microbiol. 1978 May 30;117(2):153–163. doi: 10.1007/BF00402303. [DOI] [PubMed] [Google Scholar]
- Vos P., van Asseldonk M., van Jeveren F., Siezen R., Simons G., de Vos W. M. A maturation protein is essential for production of active forms of Lactococcus lactis SK11 serine proteinase located in or secreted from the cell envelope. J Bacteriol. 1989 May;171(5):2795–2802. doi: 10.1128/jb.171.5.2795-2802.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Delepelaire P., Letoffe S., Schwartz M. Characterization of Erwinia chrysanthemi extracellular proteases: cloning and expression of the protease genes in Escherichia coli. J Bacteriol. 1987 Nov;169(11):5046–5053. doi: 10.1128/jb.169.11.5046-5053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T. R., Azuma N., Kaminogawa S., Yamauchi K. Purification and Characterization of a Substrate-Size-Recognizing Metalloendopeptidase from Streptococcus cremoris H61. Appl Environ Microbiol. 1987 Oct;53(10):2296–2302. doi: 10.1128/aem.53.10.2296-2302.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T. R., Azuma N., Kaminogawa S., Yamauchi K. Purification and characterization of a novel metalloendopeptidase from Streptococcus cremoris H61. A metalloendopeptidase that recognizes the size of its substrate. Eur J Biochem. 1987 Mar 2;163(2):259–265. doi: 10.1111/j.1432-1033.1987.tb10796.x. [DOI] [PubMed] [Google Scholar]
- van Boven A., Tan P. S. T., Konings W. N. Purification and Characterization of a Dipeptidase from Streptococcus cremoris Wg2. Appl Environ Microbiol. 1988 Jan;54(1):43–49. doi: 10.1128/aem.54.1.43-49.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]