Abstract
The process of implantation, necessary for all viviparous birth, consists of tightly regulated events, including apposition of the blastocyst, attachment to the uterine lumen, and differentiation of the uterine stroma. In rodents and primates the uterine stroma undergoes a process called decidualization. Decidualization, the process by which the uterine endometrial stroma proliferates and differentiates into large epithelioid decidual cells, is critical to the establishment of fetal-maternal communication and the progression of implantation. The role of bone morphogenetic protein 2 (Bmp2) in regulating the transformation of the uterine stroma during embryo implantation in the mouse was investigated by the conditional ablation of Bmp2 in the uterus using the (PR-cre) mouse. Bmp2 gene ablation was confirmed by real-time PCR analysis in the PR-cre; Bmp2fl/fl (termed Bmp2d/d) uterus. While littermate controls average 0.9 litter of 6.2 ± 0.7 pups per month, Bmp2d/d females are completely infertile. Analysis of the infertility indicates that whereas embryo attachment is normal in the Bmp2d/d as in control mice, the uterine stroma is incapable of undergoing the decidual reaction to support further embryonic development. Recombinant human BMP2 can partially rescue the decidual response, suggesting that the observed phenotypes are not due to a developmental consequence of Bmp2 ablation. Microarray analysis demonstrates that ablation of Bmp2 leads to specific gene changes, including disruption of the Wnt signaling pathway, Progesterone receptor (PR) signaling, and the induction of prostaglandin synthase 2 (Ptgs2). Taken together, these data demonstrate that Bmp2 is a critical regulator of gene expression and function in the murine uterus.
In rodents and primates the process of implantation consists of attachment and invasion of the uterine luminal epithelium. Successful embryo implantation in these species requires the rapid remodeling of the uterine stromal cells in a process termed decidualization (as reviewed previously [30]). Decidualization is a process characterized by morphological and functional changes in the uterine stromal cells that is characterized by endometrial stroma proliferation and differentiation into large epithelioid decidual cells. This process is critical for establishment of a fetal-maternal interface during implantation. Although the expression of many genes, including steroid hormone receptors, cytokines, growth factors, and several developmental factors, has been implicated in this process, direct in vivo evidence of gene function has been limited. This is largely due to the fact that the ablation of many of the genes implicated in this process result in early lethality or other developmental consequences that preclude further study. Here we investigate the role of a member of the bone morphogenetic protein family, Bmp2, in the process of embryo implantation.
Bone morphogenetic proteins (Bmps) are multifunctional growth factors that belong to the transforming growth factor β (TGF-β) superfamily. The roles of Bmps in embryonic development and cellular functions in postnatal and adult animals have been extensively studied in recent years. The activity of Bmp growth factors was first described in the induction of bone formation (56). Bmp2 was identified later as a soluble factor capable of inducing ectopic cartilage and bone formation in vivo when implanted into muscular tissues (58, 61). Numerous studies have characterized the role of Bmp2 as an essential osteoblast and osteoclast differentiation factor (reviewed in reference 5). Mice with a targeted deletion of Bmp2 are embryonic lethal due to a failure of proamniotic canal closure in the majority of mice or abnormal cardiac development in the surviving mice (67). In the uterus of pregnant mice, Bmp2 expression is absent in the preimplantation period and during implantation is initially detected in the stroma surrounding the site of blastocyst attachment. As implantation progresses, expression of Bmp2 is lost in the postmitotic primary decidual zone, and expression expands into the secondary decidual zone, which is still proliferating and undergoing hypertrophy. Although numerous Bmps are expressed in the uterus during pregnancy, only the expression of Bmp2 is tightly spatiotemporally correlated with implantation (45, 66). Due to its known effects in regulating proliferation and differentiation, as well as its correlated expression with implantation, we hypothesized that Bmp2 may be a critical regulator of decidualization in the murine uterus.
In order to further investigate the role of Bmp2 in the adult uterus, conditional ablation of Bmp2 was required to circumvent the embryo lethal phenotype. A line of mice in which Cre recombinase is under control of the progesterone receptor promoter (PR-cre) (52) was crossed with a mouse line containing the floxed Bmp2 allele (37) to provide a tissue-specific knockout of Bmp2 in PR-expressing tissues. The PR-cre mouse model has provided an effective method to elucidate gene function in the uterus (29, 42).
We have characterized here the effect of Bmp2 ablation on the murine uterus. We show that Bmp2 ablation leads to complete infertility, since the Bmp2d/d mice are unable to form implantation sites and demonstrate a complete lack of a decidual response. Microarray analysis demonstrates that ablation of Bmp2 leads to specific gene changes including disruption of the Wnt signaling pathway, PR activation, and the induction of prostaglandin synthase 2 (Ptgs2). These data demonstrate that Bmp2 is a critical regulator of gene expression and function in the murine uterus and that it cannot be compensated for by the other Bmp family members.
MATERIALS AND METHODS
Animals and hormone treatments.
Mice were maintained in the designated animal care facility at Baylor College of Medicine according to the institutional guidelines for the care and use of laboratory animals. The artificial decidual response has been previously described (15). Briefly, ovariectomized mice were treated with 3 daily injections of 100 ng of estrodial (E2) per mouse. After 2 days of rest, mice were then treated with daily injections of 1 mg of progesterone (P4) and 6.7 ng of E2 per mouse for 3 days, subcutaneously (s.c.). One uterine horn was traumatized by an intraluminal injection of 50 μl of sesame oil 6 h after the last injection. The contralateral horn was not traumatized and served as a control. Mice were given daily s.c. injections of 1 mg of P4 and 6.7 ng of E2 per mouse each day after the trauma. Mice were sacrificed 1, 2, or 5 days after the trauma by cervical dislocation while under anesthetic, Avertin (2,2-tribromoethyl alcohol; Sigma-Aldrich, St. Louis, MO). At the time of dissection, uterine tissues were placed in the appropriate fixative or flash frozen and stored at −80°C. In order to collect uteri from pregnant Bmp2f/f and Bmp2d/d mice, females were mated with wild-type males. The morning of the vaginal plug was designated as day 0.5.
Superovulation was induced in 3-week-old female mice by administering 5 IU of pregnant mares' serum gonadotropin intraperitoneally (EMD Biosciences, Inc., San Diego, CA), followed by 5 IU of human chorionic gonadotropin given intraperitoneally (Pregnyl; Organon International, Roseland, NJ) 48 h later. The mice were euthanized 24 h later, and oocytes were flushed from the oviducts and counted.
Embryo attachment was visualized in 6-week-old female mice mated with wild-type males at 4.5 days postcoitus (dpc). Mice were perfused with 4% paraformaldehyde (PFA), and uterine tissue was excised and fixed in ice-cold 4% PFA overnight. Tissues were then dehydrated through graded ethanol washes, processed for paraffin embedding, sectioned longitudinally, and stained with hematoxylin and eosin.
Bmp2 rescue experiments of decidualization were accomplished with the recombinant human BMP2 (rhBMP2) (R&D Systems, Inc., Minneapolis, MN). Ovariectomized mice were treated with 3 daily injections of 100 ng of E2 per mouse. After 2 days of rest, mice were each treated with daily s.c. injections of 1 mg of P4 and 6.7 ng of E2 per mouse. Both horns were traumatized by a needle scratch on the antimesometrial lumen 6 h after the third injection. At the time of the injection, 10 μl of vehicle (10% bovine serum albumin [BSA]) was injected intraluminally in one horn of the uterus, while 10 μl of rhBMP2 (167 ng/μl in 10% BSA) was injected into the other horn. Mice continued to receive daily s.c. injections of 1 mg of P4 and 6.7 ng of E2 per mouse each day after the trauma. Three days after the trauma, the mice were sacrificed, and the uteri were collected.
RNA isolation and microarray hybridization.
Total RNA was extracted from the uterine tissues by using the QIAGEN RNeasy total RNA isolation kit (QIAGEN, Valencia, CA). The RNA was pooled from the uteri of three mice per genotype. All RNA samples were analyzed with a Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA) before microarray hybridization. The fragmented, labeled cRNA (15 μg) was hybridized to Affymetrix mouse genome 430 2.0 arrays (Affymetrix, Santa Clara, CA). All experiments were performed in triplicate, with independent pools of RNA.
Data analysis.
Microarray data analysis was performed as described previously (26). After scanning and low-level quantification using Microarray Suite (Affymetrix, Santa Clara, CA), a DNA-Chip analyzer dChIP version 1.3 was utilized to adjust arrays to a common baseline using invariant set normalization (50). To estimate expression, the PM-only model of Li et al. was utilized (31, 33). All Genechips (n = 6) were normalized to the same baseline and modeled together. The data quality was reviewed by using present call rates from MAS5 (average = 49.5%, range = 44.9 to 52.5%), ratios of 3′ to 5′ GAPDH probe sets from MAS5 (average = 1.25, range = 0.87 to 1.95), and array outlier rates (average = 0.027, range = 0.010 to 0.084) from dChIP. Based on the parameters described above, all chips were considered of good quality and were included in subsequent analyses. We selected differentially expressed genes in Bmp2f/f and Bmp2d/d mice using a two-sample comparison according to the following criteria: lower bound of 90% confidence interval of fold change greater than 1.2 and an absolute value of difference between group means greater than 80. The software uses resampling and standard errors of model parameters to partially account for measurement error and unstable estimates of variability (32). Finally, the median number of detected genes in 50 permuted samples was used as an overall estimate of the false discovery rate. Differentially expressed genes were classified by pathway using GenMAPP (10) and Ingenuity Pathway analysis.
Quantitative real-time PCR.
In order to investigate the impact of Bmp2 ablation on gene expression changes in the postimplantation uterus, quantitative real-time PCR analysis was conducted on RNA isolated from mice given a steroid hormone regimen to mimic the postimplantation period. Briefly, ovariectomized mice were treated with three daily injections of 100 ng of E2 per mouse. After 2 days of rest, daily s.c. injections of 1 mg of P4 and 6.7 ng of E2 per mouse were given for 3 days. The uterus was traumatized by a needle scratch on the antimesometrial lumen 6 h after the last injection. Mice were given daily s.c. injections of 1 mg of P4 and 6.7 ng of E2 per mouse each day after the trauma. Prior to and 24 and 48 h after the trauma, the mice were sacrificed, and uteri were collected.
Expression levels of Bmp2 were validated by real-time reverse transcription-PCR (RT-PCR) TaqMan analysis using the ABI Prism 7700 sequence detector system according to manufacturer's instructions (Applied Biosystems, Foster City, CA). RT-PCRs were performed using One-Step RT-PCR Universal Master Mix reagent (Applied Biosystems) according to the manufacturer's instructions. Real-time probes and primers were purchased from (Applied Biosystems). For a complete list, see Table S4 in the supplemental material. Standard curves were generated by serial dilution of a preparation of total RNA isolated from whole mouse uteri. All real-time PCR was done by using RNA samples from three separate mice, and mRNA quantities were normalized against 18S RNA using ABI rRNA control reagents.
Immunofluorescence and immunohistochemistry.
For an assessment of cell proliferation, mice were killed at the time of the uterine trauma and 24 and 48 h later. Uteri were fixed in Bouin's fixative and then embedded in paraffin. Tissue sections (5 μm) were deparaffinized, rehydrated, and labeled with the rabbit anti-phospho-histone H3 (1:1,500; Upstate Billerica, MA). Tissues were counterstained with nuclear fast red.
Assessment of cellular differentiation by alkaline phosphatase staining was performed. Tissue sections were fixed at 4°C in 4% PFA for 2 h, followed by a sucrose gradient in phosphate-buffered saline (PBS) (10, 20, and 30%, respectively). Tissues were then embedded in OCT for sectioning. Slides were postfixed in 0.2% glutaraldehyde, rinsed in phosphate-buffered saline, and incubated with a 100 mM Tris buffer (pH 9.5) containing chromogenic substrates for alkaline phosphatase (168.5 μl of 100-mg/ml nitroblue tetrazolium salt in dimethylformamide and 175 μl of 50-mg/ml BCIP [5-bromo-4-chloro-3-indolylphosphate]/toluidinium salt in dimethylformamide added to 50 ml of the Tris buffer). The development of a purple color is indicative of alkaline phosphatase activity. Tissues were counterstained with nuclear fast red.
Vascularity in the uterus was determined by CD31 immunofluorescence. Rat-anti-mouse CD31 (1:100; BD Biosciences, San Jose, CA) was used. The specific signal was detected by the Alexa-Fluor-488 Donkey anti-rat immunoglobulin G (IgG; 1:200; Molecular Probes/Invitrogen, Carlsbad, CA). Immunofluorescence for Frap1 was performed using a rabbit anti-mTOR (Cell Signaling Technology, Inc., Danvers, MA). The specific signal was detected by the Alexa-Fluor-594 donkey anti-rabbit IgG (1:200; Molecular Probes/Invitrogen). Sections were counterstained with DAPI (4′,6′-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA) and visualized under fluorescence microscopy.
Immunohistochemical detection of PR has been described previously (36).
Western blotting.
Uteri were sonicated in 30 μl of radioimmunoprecipitation assay buffer with the addition of protease inhibitor cocktail tablets (Sigma Chemical, St. Louis, MO) at 4°C. Sonication was performed three times in 10-s bursts (20% duty cycle with a power level 2) on a Branson 250 sonicator. Samples were kept on ice for 30 min, and cellular debris was removed by centrifugation at 14,000 rpm for 15 min at 4 C. Protein concentration was determined by Bradford's method using BSA as the standard. Samples containing 20 μg of protein were subjected to sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis. The separated proteins were then transferred onto a polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA). Membranes were blocked overnight with 5% skim milk (wt/vol) in TBST (Tris-buffered saline with 10 mM Tris-HCl plus 150 mM NaCl plus 0.1% Tween 20 [pH 7.5]) and probed with antibodies for total and phosphorylated PR. Total PR was measured by using rabbit anti-PR (Dako, Carpenteria, CA). Phosphorylation specific antibodies for PR have previously been described (9, 44). Immunoreactivity was visualized by incubation with horseradish peroxidase-linked second antibodies (Pierce, Rockford, IL) and treatment with enhanced chemiluminescence reagents.
RESULTS
Bmp2d/d females are completely infertile and demonstrate no ovarian defects.
Bmp2 gene ablation was validated in the Bmp2d/d mice by measuring Bmp2 expression in the Bmp2f/f and Bmp2d/d mice after artificial induction of decidualization. No Bmp2 expression could be detected in the Bmp2d/d mice by real-time PCR 24 or 48 h after decidualization. Furthermore, real-time PCR confirmed that loss of Bmp2 expression in the uterus of the Bmp2d/d mouse also results in the loss in expression of known Bmp2 targets, Id1 and Fst (27, 28) (see Fig. S1 in the supplemental material). Taken together, these data show that Bmp2 signaling is active in the decidualizing uterus and effectively ablated in the Bmp2d/d uterus.
To detect possible reproductive phenotypes due to the ablation of Bmp2 in the uterus, we performed a 6-month breeding study and counted the number of pups and litters born. The Bmp2f/f females delivered 6.82 ± 0.23 pups per litter with an average time between litters of 37.0 ± 0.7 days for a total fecundity of approximately 38.7 pups over 6 months. The Bmp2d/d females were completely infertile, with no pups being born (Table 1). Apart from this lack of fertility, Bmp2d/d females are phenotypically normal, with no observable changes in size or behavior. Bmp2d/d females ovulate normally in response to a superovulatory regimen of gonadotropins (Bmp2f/f, 23.3 ± 3.3 oocytes; Bmp2d/d, 24.5 ± 5.1 oocytes). In addition, histological analysis of the Bmp2d/d uterus at 4.5 dpc shows that embryos in the uterine lumen attach normally to the uterine epithelium (see Fig. 2 in the supplemental material). Together, these data suggest that the observed infertility in Bmp2d/d females is not due to a disruption of ovarian function.
TABLE 1.
Impact of Bmp2 ablation on female fertility
| Females tested
|
No. of:
|
Mean ± SE
|
|||
|---|---|---|---|---|---|
| Genotype | No. | Pups | Litters | Avg no. of pups per litter | No. of days between litters |
| Bmp2f/f | 3 | 116 | 17 | 6.82 ± 0.23 | 37.0 ± 2.7 |
| Bmp2d/d | 6 | 0 | 0 | 0 | NAa |
NA, not applicable.
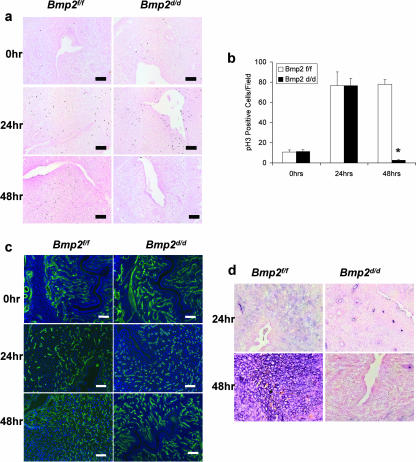
FIG. 2.
Bmp2 ablation affects differentiation and proliferation but not vascularization. (a) Proliferation as measured by immunohistochemical staining of phosphorylated histone H3 in Bmp2f/f and Bmp2d/d uteri prior to and 24 and 48 h after the decidual trauma. Slides are counterstained with nuclear fast red. (b) Quantification of phosphorylated histone H3-positive cells. The data are shown as means ± the standard error of the mean (SEM). Sections from three uteri of each genotype were counted at all time points. *, P = 8.36 e-5 from Bmp2f/f at 48 h. (c) Vascularization as measured by CD31 immunofluorescence in Bmp2f/f and Bmp2d/d uteri prior to and 24 and 48 h after the decidual trauma. Slides are counterstained with DAPI. (d) Differentiation as measured by alkaline phosphatase staining in Bmp2f/f and Bmp2d/d uteri 24 and 48 h after the decidual trauma. Slides are counterstained with nuclear fast red. Original magnification, ×20.
Bmp2d/d females are unable to form implantation sites due to an inability to undergo the decidual reaction.
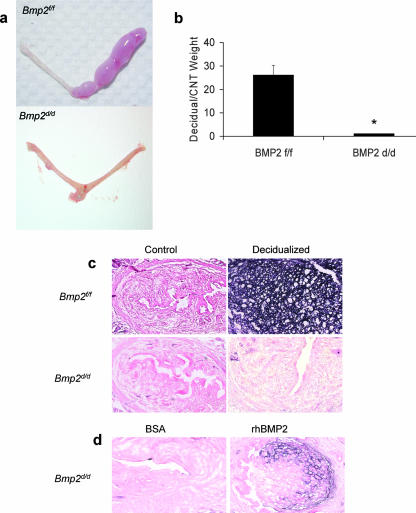
At 5.5 dpc, Bmp2d/d uteri failed to display implantation sites, which are visually scored by localized retention of Chicago blue dye (data not shown). In order to test whether Bmp2d/d mice are able to undergo a decidual reaction, a well-defined regimen of exogenous hormones was applied. Ovariectomized Bmp2f/f and Bmp2d/d mice were treated with estrogen (E2) and progesterone (P4), and then a decidualization reaction was initiated in the left uterine horn by an intraluminal injection of oil. The right horn was left unstimulated as a control. We then examined the gross anatomy of the stimulated and unstimulated uterine horns of Bmp2f/f and Bmp2d/d mice. As expected, the uterine horn of Bmp2f/f mice exhibited a robust decidual response 5 days after receiving the artificial stimulation (Fig. 1a). In contrast, the Bmp2d/d uteri under identical conditions failed to show any significant decidualization. A quantification of the decidual response by measurement of uterine wet weight shows the Bmp2d/d decidualized horn failed to increase in size in comparison to littermate controls (Fig. 1b). In addition, staining for alkaline phosphatase activity, a well-known marker for decidual cells, 48 h after the decidual trauma (14) shows no differentiation of stromal cells into decidual cells in the Bmp2d/d traumatized horn. Alkaline phosphatase activity, as indicated by the dark purple, can only be seen in the decidualized region of the traumatized Bmp2f/f uterine horn (Fig. 1c).
FIG. 1.
Decidualization in Bmp2f/f and Bmp2d/d mice. (a) Gross morphology of the decidual response in Bmp2f/f and Bmp2d/d uteri 5 days after the decidual stimulus. (b) Ratio of the decidual horn to the control horn weight of Bmp2f/f and Bmp2d/d uteri 5 days after the decidual stimulus. *, P = 3.8 e-4 from Bmp2f/f mice. (c) Differentiation as measured by alkaline phosphatase staining in the Bmp2f/f andBmp2d/d uterus 48 h after the decidual trauma. (d) Differentiation as measured by alkaline phosphatase staining in the Bmp2d/d uterus treated with 10% BSA or 167 ng of human recombinant Bmp2/μl in 10% BSA 48 h after the decidual trauma. Original magnification, ×10.
Many of the gene ablation mouse models that exhibit uterine implantation failure, including the homeobox genes Hoxa10 and Hoxa11, as well as Wnt7a (21, 40, 49), exhibit developmental defects that confound further analysis of these genes in vivo. To demonstrate that the effect of Bmp2 ablation in the Bmp2d/d uterus is due to the absence of expression in the peri-implantation period and not a developmental defect, we attempted to rescue the decidual response, absent in the Bmp2d/d, by administration of recombinant protein. At the time of the decidual trauma, 10 μl of vehicle (10% BSA) was injected intraluminally in one horn of the uterus, whereas 10 μl of human recombinant BMP2 (hrBMP2) (167 ng/μl in 10% BSA) was injected into the other horn. The uterine trauma utilized during the rescue experiment was a scratch with a burred needle rather than an intraluminal injection of oil. This method of traumatizing the uterus allowed us to deliver hrBMP2 to the uterine stroma without diluting the recombinant protein with oil. The mice were subsequently sacrificed 48 h later. This concentration of BMP2 has been shown to induce in vivo differentiation of osteoblasts (reviewed in reference 57). The Bmp2f/f uterus showed decidualization that was unaffected by hrBMP2 administration. As expected, the Bmp2d/d stroma showed no differentiation when injected with vehicle. However, administration of the hrBMP2 into the Bmp2d/d uteri partially restores stromal cell differentiation, as indicated by alkaline phosphatase-positive cells (Fig. 1d).
Taken together, these data indicate a severe impairment of the decidualization process in the Bmp2d/d mice and suggest a critical role for Bmp2 signaling in stromal cell differentiation. The phenotypic effects of Bmp2 ablation are likely due to a lack of Bmp2 induction during implantation and not due to developmental abnormalities.
Bmp2 ablation affects differentiation and proliferation but not vascularization.
Decidualization involves multiple changes in stromal cells adjacent to the implantation site. Initially, there is an increased localized proliferation of stromal cells, which then undergo postmitotic differentiation, forming the primary decidual zone. Subsequently, the stromal cells next to the primary decidual zone proliferate and form the secondary decidual zone. These cells in turn differentiate and undergo endoreduplication (12). Increased uterine vascular permeability and angiogenesis have long been observed hallmarks of uterine implantation (16). To further investigate the decidualization defect in Bmp2d/d mice, we monitored the proliferation, vascularization, and differentiation of steroid-hormone-primed stromal cells in response to a mechanical decidual stimulus, by using known markers of these events.
In order to examine the proliferation in the forming deciduae, we stained the uteri with a phosphorylated histone 3 antibody (pH3), which is a marker of endometrial mitotic activity (3). Unlike Ki67 and Brd-U, pH3 labels cells only in the M phase, thus giving an unbiased reflection of proliferation that does not include the growth and synthesis of DNA found in the endoreduplication by the forming decidual cells. As shown in Fig. 2a, uteri of Bmp2f/f mice exhibited basal levels of proliferation primarily in the stroma prior to application of the decidual stimulus. This measurement reflects the preimplantation proliferation mediated by P4 necessary for decidualization. At 24 h after receiving the decidual stimulus, the uteri of Bmp2f/f mice exhibited an intense staining for pH3 in the stroma surrounding the lumen. This is clearly indicative of extensive stromal cell proliferation, which is also seen during normal pregnancy in response to embryo attachment. This proliferation continued at 48 h after the scratch especially in the secondary decidual zone. Uterine sections from Bmp2d/d mice showed normal levels of preimplantation proliferation staining under identical conditions. In addition, 24 h after receiving the decidual stimulus, the Bmp2d/d uteri showed a similar surge in cellular proliferation as the controls in the primary decidual zone. These results indicate that the initial mitotic expansion at the time of implantation prior to differentiation is independent of Bmp2 effects. However, 48 h after the decidual stimulus, Bmp2d/d uteri showed little pH3 staining, demonstrating that elevated proliferation levels were not maintained. Quantification of the number of pH3 cells per field substantiates these observations (Fig. 2b).
Embryo implantation and development are critically dependent on the spatial and temporal regulation of angiogenesis and localized uterine vascular permeability. Other mouse models that have an impaired decidual response, such as prostaglandin synthase 2 (Ptgs2)−/− mice, have defects in uterine vascularization at the time of implantation and decidualization (39). When we investigated angiogenesis in uteri of Bmp2f/f and Bmp2d/d mice by CD31 immunofluorescence to label endothelial cells, no significant differences in the vascularity of the Bmp2d/d uterus compared to Bmp2f/f controls prior to or 24 or 48 h after the scratch were observed (Fig. 2c).
Upon closer examination of differentiation, as visualized by alkaline phosphatase staining, faint alkaline phosphatase staining is noted in the stroma at 24 h after the scratch (Fig. 2d). By 48 h, the traumatized horn of the Bmp2f/f uterus is strongly alkaline phosphatase positive, a finding indicative of a robust differentiation into decidual cells. In contrast, the Bmp2d/d uteri remain alkaline phosphatase negative, indicating a lack of differentiation (Fig. 2d). Since initial proliferation and vascularization of the uterus appear to be normal, we hypothesize that Bmp2 is a necessary factor for secondary proliferation and subsequent differentiation of endometrial stromal cells into decidual cells.
Microarray analysis of Bmp2f/f and Bmp2d/d uteri.
From total RNA extracts prepared from the decidualizing horns, Bmp2 target genes, Id1 and Fst, are both significantly downregulated by 24 h after the decidual trauma in the Bmp2d/d uteri compared to littermate controls (see Fig. S1 in the supplemental material). In order to further elucidate the role of Bmp2 in uterine physiology, high-density DNA microarray analysis was used to identify genes differentially expressed in the Bmp2d/d uterus compared to littermate controls at 24 h after the decidual trauma. Total RNA extracts were subjected to microarray analysis using the Affymetrix mouse genome 430 2.0 arrays, measuring the expression of approximately 39,000 transcripts. To generate reproducible gene expression data, three independent microarray experiments were performed. This analysis revealed 959 and 1,185 transcripts whose abundance is significantly increased or decreased, respectively, in the Bmp2d/d uterus, compared to Bmp2f/f controls. A complete list of the genes whose transcripts increase or decrease in abundance is presented in Tables S1 and S2 in the supplemental material. Grouping of differentially expressed genes into pathways was achieved by utilizing two different software platforms GenMAPP (10) and Ingenuity Pathway analysis (Ingenuity Systems). These programs showed known pathways whose members showed significantly altered expression patterns including Wnt signaling, galactose metabolism, arginine and proline metabolism, TGF-β signaling, DNA replication, cell cycle control, and focal adhesions. The expression of Bmp2, as well as its target genes Id1 and Fst1, was significantly downregulated in the Bmp2d/d uterus compared to controls. In addition, all subsequently evaluated targets of the microarray analysis were validated by real-time PCR. Together, these data allow us to conclude that Bmp2 ablation causes the deregulation of several genetic pathways.
Bmp2 ablation disrupts expression of Wnt pathway genes.
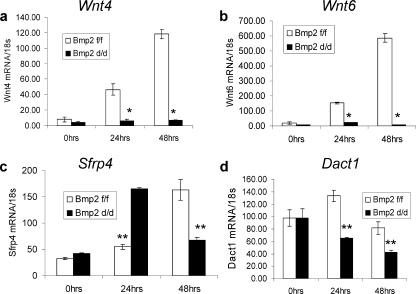
Wnt proteins form a family of highly conserved secreted glycoproteins that are critical for cell-cell communication, cell fate specification, growth, and differentiation during development of vertebrates and invertebrates (60, 63). During implantation, Wnt4, like Bmp2, is strongly induced in the primary decidual region, with expression expanding to the secondary decidual zone (11, 45). Wnt4-null animals exhibit perinatal lethal phenotype due to defects in kidney tubulogenesis (53). Wnt6 also has been implicated to play a role in kidney tubular development (25). Both Wnt4 and Wnt6 are highly induced in the forming decidua of the Bmp2f/f animals. However, in the Bmp2d/d uteri, no induction of the Wnt ligands is apparent (Fig. 3a and b). In addition, a clear deregulation of secreted frizzled related protein 4 (Sfrp4), a Wnt antagonist, is observed. This protein has been found to be differentially regulated in rodents, primates, and human myometrium (1, 11, 18, 47). Upon implantation, Sfrp4 expression is restricted to the undifferentiated stromal cells forming a dividing zone between the circular muscle layer and the forming decidua. As decidualization progresses, Sfrp4 expression becomes localized in the myometrium (11). In the Bmp2f/f uterus, the expression of Sfrp4 is slightly elevated 24 h and robustly expressed 48 h after the decidual stimulus. In contrast, in the Bmp2d/d uterus, expression levels were highest at 24 h after the scratch and sharply declined by 48 h (Fig. 3c). Dapper homolog 1 (Dact1) (also known as Frodo) is a recently discovered gene that either positively or negatively regulates Wnt signal transduction in a context-dependent manner (4, 20). In the Bmp2f/f uterus, Dact1 levels are slightly but significantly increased at 24 h after the decidual scratch. Ablation of Bmp2 leads to a reduction of Dact1 in the uterus (Fig. 3d). Together, these data demonstrate that Bmp2 ablation leads to a clear disruption of the expression of Wnt pathway genes in the decidualizing uterus.
FIG. 3.
Bmp2 ablation deregulates Wnt signaling. Real-time PCR in Wnt4 (a), Wnt6 (b), Sfrp4 (c), and Dact1 in Bmp2f/f and Bmp2d/d uteri. The data shown as means ± the SEM. *, P < 0.01 from Bmp2f/f at the corresponding time point; **, P < 0.05 from Bmp2f/f at the corresponding time point.
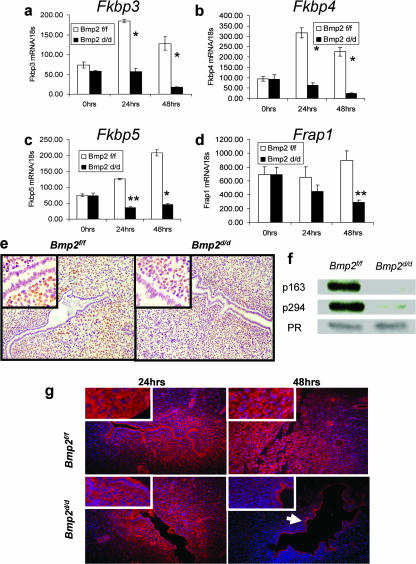
Bmp2 ablation disrupts expression of FK-506-binding proteins.
The Fkbps represent a diverse family of proteins that were initially characterized by the ability to bind FK506, an immunosuppressive drug, and have been known to participate in many cellular processes, such as cell signaling, protein transport, and transcription. Fkbp3 physically associates with the histone deacetylases and may modulate transcriptional activation (64). Fkbp4 and Fkbp5 have been shown to be a part of the molecular chaperone machinery that maintains hormone receptors in a functional state competent for binding hormone and transcriptional activation (2). Recently, Fkbp4 has been shown to be critical for embryo implantation. Fkbp4 is necessary for appropriate PR transcriptional activity and induction of critical P4-regulated genes (55, 65). Fkbp5, on the other hand, is a P4-responsive target gene that attenuates PR transcriptional activation (23). In the Bmp2d/d uterus, several members of the Fkbps show significant deregulation. Fkbp3, Fkbp4, and Fkbp5 are all induced in the Bmp2f/f controls after the decidual scratch. However, in the Bmp2d/d uterus, the expression of these genes is not induced (Fig. 4a to c). In addition, the expression of many known P4-regulated genes was compromised in the Bmp2d/d uterus (see Table S3 in the supplemental material). However, the expression pattern of PR in the Bmp2d/d mice 24 h after the decidual stimulus is comparable to that of the littermate controls (Fig. 4e). In addition, levels of total PR appear to be equal when quantified by Western blotting. Phosphorylation of PR is known to be necessary for their ligand-dependent activation. Phosphorylation at Ser162 and Ser294 are indicative of the ligand-dependent activity of human PR-B (9, 68). Phosphor-specific antibodies that specifically recognize PR phosphorylated at these sites have been shown to recognize the conserved serine phosphorylation sites, at Ser163 and Ser294, on the mouse PR protein (N. Weigel and D. Edwards, unpublished data). Western blot analysis demonstrates a drastic reduction of PR phosphorylated at Ser163 and Ser294 in the Bmp2d/d uteri (Fig. 4f). Together, these data show that, although PR levels are normal, the activity of the receptors is greatly reduced and suggest that Bmp2 is necessary for PR activation, possibly through modulation of the Fkbps.
FIG. 4.
Bmp2 ablation deregulates the expression and action of FK-506-binding proteins. Real-time PCR in Fkbp3 (a), Fkbp4 (b), Fkbp5 (c), and Frap1 (d) in Bmp2f/f and Bmp2d/d uteri. The data shown as means ± the SEM. *, P < 0.01 from Bmp2f/f at the corresponding time point; **, P < 0.05 from Bmp2f/f at the corresponding time point. (e) PR immunohistochemistry in Bmp2f/f and Bmp2d/d uteri 24 h after the decidual trauma. (f) Western blot for phosphorylated and total PR 24 h after the decidual trauma. (g) Expression of Frap1 by immunofluorescence. The arrow indicates retained epithelial expression. Original magnification, ×20. High-power insets are at ×40 magnification.
FK506-binding protein 12-rapamycin-associated protein 1 (Frap1) (also known as mTOR), a conserved kinase protein, is critical for the regulation of cell growth and proliferation (reviewed in reference 62). Ablation of Bmp2 leads to a gradual loss of Frap1 in the uterus (Fig. 4d). The expression of Frap1 in the uterus, when visualized by immunofluorescence, shows that although expression is retained in the epithelium at all time points, Bmp2 ablation leads to a loss of Frap1 in the stroma at 48 h (Fig. 4g). The established necessity of Frap1 for cellular proliferation and its loss in the stroma at 48 h suggests a potential explanation of loss of cellular proliferation observed in the Bmp2d/d uterus at 48 h (Fig. 2a and b).
Bmp2 Acts upstream of Ptgs2.
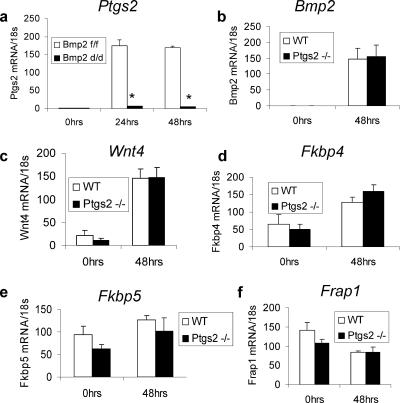
The cyclooxygenases (Ptgs1 and Ptgs2) are rate-limiting enzymes regulating the synthesis of prostaglandins from arachidonic acid. Ptgs2 is expressed in the luminal epithelium and subepithelial stromal cells at the antimesometrial pole exclusively surrounding the blastocyst at the time of attachment (6); targeted ablation of Ptgs2 leads to blunted decidual response that appears to be background dependent (7, 35, 59). Numerous gene products have been associated with specifically patterning the uterus during postnatal growth, such as Hoxa10 (34), and preparing the uterus for implantation. Genes with known importance during the preimplantation period include leukemia inhibitory factor (Lif) (51, 54), Cebpb (38), and Indian hedgehog (Ihh) (29). Ablation of these genes leads to a disruption of Ptgs2 expression at the time of decidualization. However, the molecular conduit through which these various factors affect Ptgs2 expression has yet to be elucidated. Importantly, the expression of genes known to be important in the preimplantation uterus including Lif, members of the Ihh signaling axis, and Hoxa10 are unchanged in the Bmp2d/d uteri prior to the decidual scratch (see Fig. S3 in the supplemental material). However, the induction of Ptgs2 in the Bmp2d/d uterus is severely blunted (Fig. 5a). On the other hand, the expression of Bmp2 in the Ptgs2−/− uterus is not affected (Fig. 5b). These results demonstrate that Bmp2 is acting upstream of Ptgs2. In addition, other factors deregulated in the Bmp2d/d uterus, including Wnt4, Fkbp4, Fkbp5, and Frap1, are unchanged in the Ptgs2−/− uterus compared to littermate controls (Fig. 5c-f). These data suggest that the phenotypic consequences of Bmp2 ablation may be in part due to disrupted Ptgs2 induction in the deciduae. In addition, the effect of Bmp2 ablation on the Wnt and Fkbp signaling pathways is independent of Ptgs2.
FIG. 5.
Bmp2 acts upstream of Ptgs2. (a) Real-time PCR in Ptgs2 in Bmp2f/f and Bmp2d/d uteri. Real-time PCR of Bmp2 (b), Wnt4 (c), Fkbp4 (d), Fkbp5 (e), and Frap1 (f), in Ptgs2−/− and littermate control uteri. The data shown as means ± the SEM. *, P < 0.01 from Bmp2f/f at the corresponding time point; *, P < 0.05 from Bmp2f/f at the corresponding time point.
DISCUSSION
Conditional ablation of Bmp2 in the mouse uterus results in female infertility due to the inability of the uterus to support postimplantation embryo development. Although embryo attachment, initial proliferation, and vascularization of the endometrium during this period appear to be normal, Bmp2d/d mice exhibit compromised endometrial stromal cell differentiation, which is required for completion of the endometrial decidual response. Microarray analysis demonstrates that ablation of Bmp2 leads to alteration of specific regulatory pathways including Wnt signaling pathway, PR signaling, and induction of Ptgs2. Taken together, these data demonstrate that Bmp2 is a critical regulator of gene expression and function in the murine uterus.
In addition to its expression in the stroma surrounding the site of blastocyst attachment (45, 66), Bmp2 is also expressed and regulated during the estrus cycle (13). Therefore, the phenotype observed in the Bmp2d/d uterus may be due to altered developmental programming that incapacitates the decidualization potential of the endometrial stroma or due to the acute action of the Bmp2 signaling axis necessary for induction of this process. A partial rescue of the decidual defect by administration of recombinant human BMP2 suggests that acute Bmp2 action is sufficient for uterine function.
Numerous factors have been shown to be important in the preimplantation period to render the uterus capable of undergoing decidualization, including Ihh, Lif, and Hoxa10. Each of these steroid hormone regulated factors is essential for the preparation of the uterus to decidualize (29, 34, 54). Importantly, Bmp2 expression has been found to be dependent on both Ihh and Lif (17, 29), and the reciprocal experiment (see Fig. S3 in the supplemental material) demonstrates that the chicken ovalbumin transcription factor II (COUP-TFII; a known downstream marker of active Hh signaling), Lif, and Hoxa10 are not altered in the preimplantation period in the Bmp2d/d uterus. Therefore, Bmp2 appears to be an important molecular target that coordinates preimplantation signaling for appropriate uterine function.
Targeted ablation of Ptgs2 leads to a reduced decidual response that appears to be background dependent (7, 35, 59). In cultured osteoblastic precursor cells, previous work has demonstrated that Bmp2 directly regulates Ptgs2 (8). We present here in vivo evidence that Bmp2 is acting upstream of Ptgs2 in the uterus. In addition, other factors deregulated in the Bmp2d/d uterus, including Wnt4, Fkbp4, Fkbp5, and Frap1, are unchanged between Ptgs2−/− and littermate controls. These data suggest that Bmp2 coordinates a decidual program independently from Ptgs2.
Bmp and Wnt signaling pathways have been extensively studied in the roles of regulation of embryonic development and control of cellular differentiation and proliferation in adult tissues (reviewed in references 5 and 24). Genetic interaction between these pathways has been observed across species in multiple settings, including osteoblast differentiation and kidney development. In particular, previous studies have shown that Bmp2 enhances Wnt-dependent transcriptional activation in both the bone and the kidney (22, 43). In the present study, we demonstrate that ablation of Bmp2 leads to deregulation of the Wnt signaling pathway. The induction of Wnt4 and Wnt6 are both abrogated in the Bmp2d/d uterus. Wnt4 and Wnt6 act cooperatively in the process of kidney tubular development (25), and the coordinate induction during decidualization suggests a possible conservation of a cooperative function in the uterus as well. Both Wnt4 and Wnt6 are so-called “noncanonical” Wnts whose activity is independent of β-catenin (reviewed in reference 5). These findings correlate well with previous work that demonstrates that β-catenin is not activated in the stroma during implantation (41) and indicate that noncanonical Wnt may play an important role in uterine function.
In addition, a clear deregulation of secreted frizzled related protein 4 (Sfrp4), a Wnt antagonist, is observed. This protein has been found to be differentially regulated in rodents, primates, and human myometrium (1, 11, 18, 47). Sfrp4 is dynamically expressed factor in the undifferentiated stroma cells and myometrium. Deregulated expression of Sfrp4 has also been found in the uteri of Hoxa10−/− mice, which also show defects in decidualization (11). These results reinforce the idea that Bmp2, acting downstream of Hoxa10, is a coordinator of preimplantation events. In addition, the tightly controlled regulation and evolutionary conserved expression of both positive and negative regulators of Wnt pathway molecules in the endometrium suggest essential roles for these proteins that have yet to be elucidated.
Four Fkbp family members showed altered expression between the Bmp2d/d uterus and littermate mate controls. Fkbp3, Fkbp4, and Fkbp5 are all induced in the Bmp2f/f controls after the decidual scratch but showed no such induction in the Bmp2d/d uterus. The known functions of these genes, including interaction with histone deacetylases to alter gene transcription (64) and maintenance of functional hormone receptors (2, 23, 55, 65), suggests that these factors may be critical to appropriate, localized hormone action. The deregulation of known P4 target genes, as well as the lack of ligand-dependent phosphorylation of PR, suggests that Bmp2 induction of Fkbps is critical for appropriate hormone responsiveness of the uterus. Bmp2 modulation of Fkbps may also be relevant to other Bmp2 target tissues. Fkbps have also been shown to affect glucocorticoid receptor (GR) signaling, as well as PR signaling (46). Although the synergistic action between the GR ligand, dexamethasone, and Bmp2 in osteoblastic differentiation from bone marrow stromal cells has been well established (48), the molecular mechanisms of this interaction have yet to be fully elucidated. An increase in Fkbp levels may also play a role in the process.
Bmp2 ablation leads to a gradual loss of Frap1 in the uterus (Fig. 4d). The expression of Frap1 in the uterus, visualized by immunofluorescence, is lost in stroma by 48 h after the decidual scratch in the Bmp2d/d uterus (Fig. 4g). The loss of Frap1 expression in the stroma suggests a potential explanation of loss of cellular proliferation observed in the Bmp2d/d uterus at 48 h (Fig. 2a and b). Previous work has demonstrated that Bmp2 activity is dependent on phosphatidylinositol 3-kinase (PI3K) and Akt serine/threonine kinase (19). Frap1 is a known integrator of PI3K and Akt kinase cascades and is a critical factor for proliferation that exerts its effects by regulating cell growth morphology (reviewed in reference 62). The necessity of PI3K and Akt signaling for Bmp2 activity and the known effects of these kinase cascades on Frap1 activation suggests that Bmp2-dependent proliferation may be dependent upon Frap1 in the uterus as well as other tissues.
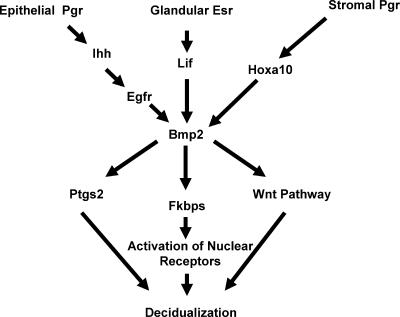
In summary, the present study has demonstrated that the Bmp2 signaling axis is critical for regulating stromal cell differentiation. We have also provided evidence that Bmp2 ablation deregulates genes of known importance in uterine biology, including Ptgs2 and the Fkbp family. Our model depicting the role of Bmp2 in uterine implantation is shown schematically in Fig. 6. We hypothesize that Bmp2 serves as a molecular integrator of preimplantation events to coordinately regulate a molecular program necessary for stromal cell differentiation. Our studies promise to provide a broader conceptual framework for understanding not only uterine implantation but also the molecular mechanisms by which Bmp signaling accomplishes disparate objectives in other tissues.
FIG. 6.
Model depicting the relationships of genes during implantation.
Supplementary Material
Acknowledgments
We thank Jinghua Li, Bryan Ngo, Jie Li, and Janet L. DeMayo for technical assistance. We also thank Ming-Jer Tsai for comments, Dean Edwards for the phosphorylation specific PR antibodies, and Heather Franco for manuscript preparation.
This study was supported by NICHD/NIH as part of the Cooperative Program on Trophoblast-Maternal Tissue Interactions (U01HD042311) (to F.J.D.), a Reproductive Biology Training Grant (5 T32 HD07165) (to K.Y.L.), grant R01 DE012324-11 to (J.M.), grant R01-DK55636 to (S.Y.T.), and grant RO1-CA77530 and Susan G. Komen Award BCTR0503763 (to J.P.L.).
Footnotes
Published ahead of print on 21 May 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ace, C. I., and W. C. Okulicz. 2004. Microarray profiling of progesterone-regulated endometrial genes during the rhesus monkey secretory phase. Reprod. Biol. Endocrinol. 2:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barent, R. L., S. C. Nair, D. C. Carr, Y. Ruan, R. A. Rimerman, J. Fulton, Y. Zhang, and D. F. Smith. 1998. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol. Endocrinol. 12:342-354. [DOI] [PubMed] [Google Scholar]
- 3.Brenner, R. M., O. D. Slayden, W. H. Rodgers, H. O. Critchley, R. Carroll, X. J. Nie, and K. Mah. 2003. Immunocytochemical assessment of mitotic activity with an antibody to phosphorylated histone H3 in the macaque and human endometrium. Hum. Reprod. 18:1185-1193. [DOI] [PubMed] [Google Scholar]
- 4.Brott, B. K., and S. Y. Sokol. 2005. Frodo proteins: modulators of Wnt signaling in vertebrate development. Differentiation 73:323-329. [DOI] [PubMed] [Google Scholar]
- 5.Canalis, E., A. N. Economides, and E. Gazzerro. 2003. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocrinol. Rev. 24:218-235. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty, I., S. K. Das, J. Wang, and S. K. Dey. 1996. Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J. Mol. Endocrinol. 16:107-122. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, J. G., and C. L. Stewart. 2003. Loss of cyclooxygenase-2 retards decidual growth but does not inhibit embryo implantation or development to term. Biol. Reprod. 68:401-404. [DOI] [PubMed] [Google Scholar]
- 8.Chikazu, D., X. Li, H. Kawaguchi, Y. Sakuma, O. S. Voznesensky, D. J. Adams, M. Xu, K. Hoshio, V. Katavic, H. R. Herschman, L. G. Raisz, and C. C. Pilbeam. 2002. Bone morphogenetic protein 2 induces cyclo-oxygenase 2 in osteoblasts via a Cbfal binding site: role in effects of bone morphogenetic protein 2 in vitro and in vivo. J. Bone Miner. Res. 17:1430-1440. [DOI] [PubMed] [Google Scholar]
- 9.Clemm, D. L., L. Sherman, V. Boonyaratanakornkit, W. T. Schrader, N. L. Weigel, and D. P. Edwards. 2000. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol. Endocrinol. 14:52-65. [DOI] [PubMed] [Google Scholar]
- 10.Dahlquist, K. D., N. Salomonis, K. Vranizan, S. C. Lawlor, and B. R. Conklin. 2002. GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nat. Genet. 31:19-20. [DOI] [PubMed] [Google Scholar]
- 11.Daikoku, T., H. Song, Y. Guo, A. Riesewijk, S. Mosselman, S. K. Das, and S. K. Dey. 2004. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol. Endocrinol. 18:1238-1250. [DOI] [PubMed] [Google Scholar]
- 12.Das, R. M., and L. Martin. 1978. Uterine DNA synthesis and cell proliferation during early decidualization induced by oil in mice. J. Reprod. Fertil. 53:125-128. [DOI] [PubMed] [Google Scholar]
- 13.Erickson, G. F., L. Fuqua, and S. Shimasaki. 2004. Analysis of spatial and temporal expression patterns of bone morphogenetic protein family members in the rat uterus over the estrous cycle. J. Endocrinol. 182:203-217. [DOI] [PubMed] [Google Scholar]
- 14.Finn, C. A., and J. R. Hinchliffe. 1964. Reaction of the mouse uterus during implantation and deciduoma formation as demonstrated by changes in the distribution of alkaline phosphatase. J. Reprod. Fertil. 8:331-338. [DOI] [PubMed] [Google Scholar]
- 15.Finn, C. A., and L. Martin. 1972. Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol. Reprod. 7:82-86. [DOI] [PubMed] [Google Scholar]
- 16.Finn, C. A., and L. Martin. 1967. Patterns of cell division in the mouse uterus during early pregnancy. J. Endocrinol. 39:593-597. [DOI] [PubMed] [Google Scholar]
- 17.Fouladi-Nashta, A. A., C. J. Jones, N. Nijjar, L. Mohamet, A. Smith, I. Chambers, and S. J. Kimber. 2005. Characterization of the uterine phenotype during the peri-implantation period for LIF-null, MF1 strain mice. Dev. Biol. 281:1-21. [DOI] [PubMed] [Google Scholar]
- 18.Fujita, M., S. Ogawa, H. Fukuoka, T. Tsukui, N. Nemoto, O. Tsutsumi, Y. Ouchi, and S. Inoue. 2002. Differential expression of secreted frizzled-related protein 4 in decidual cells during pregnancy. J. Mol. Endocrinol. 28:213-223. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh-Choudhury, N., S. L. Abboud, R. Nishimura, A. Celeste, L. Mahimainathan, and G. G. Choudhury. 2002. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J. Biol. Chem. 277:33361-33368. [DOI] [PubMed] [Google Scholar]
- 20.Gloy, J., H. Hikasa, and S. Y. Sokol. 2002. Frodo interacts with Dishevelled to transduce Wnt signals. Nat. Cell Biol. 4:351-357. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh-Li, H. M., D. P. Witte, M. Weinstein, W. Branford, H. Li, K. Small, and S. S. Potter. 1995. Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development 121:1373-1385. [DOI] [PubMed] [Google Scholar]
- 22.Hu, M. C., and N. D. Rosenblum. 2005. Smad1, beta-catenin and Tcf4 associate in a molecular complex with the Myc promoter in dysplastic renal tissue and cooperate to control Myc transcription. Development 132:215-225. [DOI] [PubMed] [Google Scholar]
- 23.Hubler, T. R., W. B. Denny, D. L. Valentine, J. Cheung-Flynn, D. F. Smith, and J. G. Scammell. 2003. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology 144:2380-2387. [DOI] [PubMed] [Google Scholar]
- 24.Huelsken, J., and W. Birchmeier. 2001. New aspects of Wnt signaling pathways in higher vertebrates. Curr. Opin. Genet. Dev. 11:547-553. [DOI] [PubMed] [Google Scholar]
- 25.Itaranta, P., Y. Lin, J. Perasaari, G. Roel, O. Destree, and S. Vainio. 2002. Wnt-6 is expressed in the ureter bud and induces kidney tubule development in vitro. Genesis 32:259-268. [DOI] [PubMed] [Google Scholar]
- 26.Jeong, J. W., K. Y. Lee, I. Kwak, L. D. White, S. G. Hilsenbeck, J. P. Lydon, and F. J. Demayo. 2005. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology 146:3490-3505. [DOI] [PubMed] [Google Scholar]
- 27.Katagiri, T., M. Imada, T. Yanai, T. Suda, N. Takahashi, and R. Kamijo. 2002. Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells 7:949-960. [DOI] [PubMed] [Google Scholar]
- 28.Kearns, A. E., and M. B. Demay. 2000. BMP-2 induces the expression of activin betaA and follistatin in vitro. J. Cell Biochem. 79:80-88. [PubMed] [Google Scholar]
- 29.Lee, K., J. Jeong, I. Kwak, C. T. Yu, B. Lanske, D. W. Soegiarto, R. Toftgard, M. J. Tsai, S. Tsai, J. P. Lydon, and F. J. Demayo. 2006. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat. Genet. 38:1204-1209. [DOI] [PubMed] [Google Scholar]
- 30.Lee, K. Y., and F. J. DeMayo. 2004. Animal models of implantation. Reproduction 128:679-695. [DOI] [PubMed] [Google Scholar]
- 31.Li, C., and W. Hung Wong. 2001. Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol. 2:RESEARCH0032. [DOI] [PMC free article] [PubMed]
- 32.Li, C., and W. H. Wong. 2003. DNA-Chip analyzer (dChip), p. 120-141. In G. Parmigiani, E. S. Garrett, R. Irizarry, and S. L. Zeger (ed.), The analysis of gene expression data: methods and software. Springer, New York, NY.
- 33.Li, C., and W. H. Wong. 2001. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc. Natl. Acad. Sci. USA 98:31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim, H., L. Ma, W. G. Ma, R. L. Maas, and S. K. Dey. 1999. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol. Endocrinol. 13:1005-1017. [DOI] [PubMed] [Google Scholar]
- 35.Lim, H., B. C. Paria, S. K. Das, J. E. Dinchuk, R. Langenbach, J. M. Trzaskos, and S. K. Dey. 1997. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91:197-208. [DOI] [PubMed] [Google Scholar]
- 36.Lydon, J. P., G. Ge, F. S. Kittrell, D. Medina, and B. W. O'Malley. 1999. Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res. 59:4276-4284. [PubMed] [Google Scholar]
- 37.Ma, L., and J. F. Martin. 2005. Generation of a Bmp2 conditional null allele. Genesis 42:203-206. [DOI] [PubMed] [Google Scholar]
- 38.Mantena, S. R., A. Kannan, Y. P. Cheon, Q. Li, P. F. Johnson, I. C. Bagchi, and M. K. Bagchi. 2006. C/EBPβ is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc. Natl. Acad. Sci. USA 103:1870-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto, H., W. G. Ma, T. Daikoku, X. Zhao, B. C. Paria, S. K. Das, J. M. Trzaskos, and S. K. Dey. 2002. Cyclooxygenase-2 differentially directs uterine angiogenesis during implantation in mice. J. Biol. Chem. 277:29260-29267. [DOI] [PubMed] [Google Scholar]
- 40.Miller, C., and D. A. Sassoon. 1998. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 125:3201-3211. [DOI] [PubMed] [Google Scholar]
- 41.Mohamed, O. A., M. Jonnaert, C. Labelle-Dumais, K. Kuroda, H. J. Clarke, and D. Dufort. 2005. Uterine Wnt/beta-catenin signaling is required for implantation. Proc. Natl. Acad. Sci. USA 102:8579-8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukherjee, A., S. M. Soyal, R. Fernandez-Valdivia, M. Gehin, P. Chambon, F. J. Demayo, J. P. Lydon, and B. W. O'Malley. 2006. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol. Cell. Biol. 26:6571-6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakashima, A., T. Katagiri, and M. Tamura. 2005. Cross-talk between Wnt and bone morphogenetic protein 2 (BMP-2) signaling in differentiation pathway of C2C12 myoblasts. J. Biol. Chem. 280:37660-37668. [DOI] [PubMed] [Google Scholar]
- 44.Narayanan, R., D. P. Edwards, and N. L. Weigel. 2005. Human progesterone receptor displays cell cycle-dependent changes in transcriptional activity. Mol. Cell. Biol. 25:2885-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paria, B. C., W. Ma, J. Tan, S. Raja, S. K. Das, S. K. Dey, and B. L. Hogan. 2001. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc. Natl. Acad. Sci. USA 98:1047-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ratajczak, T., B. K. Ward, and R. F. Minchin. 2003. Immunophilin chaperones in steroid receptor signalling. Curr. Top. Med. Chem. 3:1348-1357. [DOI] [PubMed] [Google Scholar]
- 47.Rehman, K. S., S. Yin, B. A. Mayhew, R. A. Word, and W. E. Rainey. 2003. Human myometrial adaptation to pregnancy: cDNA microarray gene expression profiling of myometrium from non-pregnant and pregnant women. Mol. Hum. Reprod. 9:681-700. [DOI] [PubMed] [Google Scholar]
- 48.Rickard, D. J., T. A. Sullivan, B. J. Shenker, P. S. Leboy, and I. Kazhdan. 1994. Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Dev. Biol. 161:218-228. [DOI] [PubMed] [Google Scholar]
- 49.Satokata, I., G. Benson, and R. Maas. 1995. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature 374:460-463. [DOI] [PubMed] [Google Scholar]
- 50.Schadt, E. E., C. Li, B. Ellis, and W. H. Wong. 2001. Feature extraction and normalization algorithms for high-density oligonucleotide gene expression array data. J. Cell Biochem. Suppl. 37:120-125. [DOI] [PubMed] [Google Scholar]
- 51.Song, H., H. Lim, S. K. Das, B. C. Paria, and S. K. Dey. 2000. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol. Endocrinol. 14:1147-1161. [DOI] [PubMed] [Google Scholar]
- 52.Soyal, S. M., A. Mukherjee, K. Y. Lee, J. Li, H. Li, F. J. DeMayo, and J. P. Lydon. 2005. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41:58-66. [DOI] [PubMed] [Google Scholar]
- 53.Stark, K., S. Vainio, G. Vassileva, and A. P. McMahon. 1994. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372:679-683. [DOI] [PubMed] [Google Scholar]
- 54.Stewart, C. L., P. Kaspar, L. J. Brunet, H. Bhatt, I. Gadi, F. Kontgen, and S. J. Abbondanzo. 1992. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359:76-79. [DOI] [PubMed] [Google Scholar]
- 55.Tranguch, S., J. Cheung-Flynn, T. Daikoku, V. Prapapanich, M. B. Cox, H. Xie, H. Wang, S. K. Das, D. F. Smith, and S. K. Dey. 2005. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc. Natl. Acad. Sci. USA 102:14326-14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Urist, M. R. 1965. Bone: formation by autoinduction. Science 150:893-899. [DOI] [PubMed] [Google Scholar]
- 57.Valentin-Opran, A., J. Wozney, C. Csimma, L. Lilly, and G. E. Riedel. 2002. Clinical evaluation of recombinant human bone morphogenetic protein-2. Clin. Orthop. Relat. Res. 395:110-120. [DOI] [PubMed] [Google Scholar]
- 58.Wang, E. A., V. Rosen, J. S. D'Alessandro, M. Bauduy, P. Cordes, T. Harada, D. I. Israel, R. M. Hewick, K. M. Kerns, P. LaPan, et al. 1990. Recombinant human bone morphogenetic protein induces bone formation. Proc. Natl. Acad. Sci. USA 87:2220-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, H., W. G. Ma, L. Tejada, H. Zhang, J. D. Morrow, S. K. Das, and S. K. Dey. 2004. Rescue of female infertility from the loss of cyclooxygenase-2 by compensatory up-regulation of cyclooxygenase-1 is a function of genetic makeup. J. Biol. Chem. 279:10649-10658. [DOI] [PubMed] [Google Scholar]
- 60.Wodarz, A., and R. Nusse. 1998. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14:59-88. [DOI] [PubMed] [Google Scholar]
- 61.Wozney, J. M., V. Rosen, A. J. Celeste, L. M. Mitsock, M. J. Whitters, R. W. Kriz, R. M. Hewick, and E. A. Wang. 1988. Novel regulators of bone formation: molecular clones and activities. Science 242:1528-1534. [DOI] [PubMed] [Google Scholar]
- 62.Wullschleger, S., R. Loewith, and M. N. Hall. 2006. TOR signaling in growth and metabolism. Cell 124:471-484. [DOI] [PubMed] [Google Scholar]
- 63.Yamaguchi, T. P. 2001. Heads or tails: Wnts and anterior-posterior patterning. Curr. Biol. 11:R713-R724. [DOI] [PubMed] [Google Scholar]
- 64.Yang, W. M., Y. L. Yao, and E. Seto. 2001. The FK506-binding protein 25 functionally associates with histone deacetylases and with transcription factor YY1. EMBO J. 20:4814-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang, Z., I. M. Wolf, H. Chen, S. Periyasamy, Z. Chen, W. Yong, S. Shi, W. Zhao, J. Xu, A. Srivastava, E. R. Sanchez, and W. Shou. 2006. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Mol. Endocrinol. 20:2682-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ying, Y., and G. Q. Zhao. 2000. Detection of multiple bone morphogenetic protein messenger ribonucleic acids and their signal transducer, Smad1, during mouse decidualization. Biol. Reprod. 63:1781-1786. [DOI] [PubMed] [Google Scholar]
- 67.Zhang, H., and A. Bradley. 1996. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122:2977-2986. [DOI] [PubMed] [Google Scholar]
- 68.Zhang, Y., C. A. Beck, A. Poletti, D. P. Edwards, and N. L. Weigel. 1994. Identification of phosphorylation sites unique to the B form of human progesterone receptor. In vitro phosphorylation by casein kinase II. J. Biol. Chem. 269:31034-31040. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.