Abstract
Chromatin condensation is the most recognizable nuclear hallmark of apoptosis. Cleavage and activation of MST1 by caspases induce chromatin condensation. It was previously reported that, during apoptosis, activated MST1 induced c-Jun N-terminal kinase (JNK) activation and also phosphorylated histone H2B. However, which of these mechanisms underlies MST1's induction of chromatin condensation has yet to be clarified. Here, we report that MST1-mediated activation of JNK is both essential and sufficient for chromatin condensation. MST1 activation did not result in chromatin condensation in mitogen-activate protein kinase kinase 4 (MKK4)/MKK7 double knockout (MKK4/7 DKO) embryonic stem (ES) cells, which genetically lack the ability to activate JNK. On the other hand, constitutively active JNK was able to induce chromatin condensation in MKK4/7 DKO ES cells. In contrast, histone H2B phosphorylation did not correlate with chromatin condensation in wild-type ES cells. Finally, inhibition of JNK as well as inhibitor of caspase-activated DNase blocked chromatin condensation during Fas-mediated apoptosis of Jurkat cells. Taken together, our results indicate that caspase-mediated cleavage of MST1, followed by MST1-mediated activation of the JNK pathway, is the mechanism responsible for inducing chromatin condensation during apoptosis.
Apoptosis, or programmed cell death, is fundamental to both the development and homeostasis of multicellular organisms (14, 35). Apoptosis is an active process triggered by a broad range of stimuli, including death ligands, DNA damage, and cellular stresses. Although apoptosis-inducing stimuli are structurally and functionally diverse, they induce a common cell death process characterized by cell shrinkage and detachment, membrane blebbing, chromatin condensation, DNA fragmentation, and apoptotic body formation. This apoptotic program is regulated by a proteolytic cascade mediated by the caspase family of cysteine proteases (5, 24, 30). Caspases are originally expressed as inactive precursors, but when a cell receives an apoptotic stimulus, the signal induces the autoproteolytic activation of initiator caspases such as caspase-8 and -9. Once activated, the initiator caspases cleave and activate downstream effector caspases, such as caspase-3 and -6, which in turn cleave various cellular substrates that may be activated or inactivated (20). One of the critical substrates cleaved by effector caspases is inhibitor of caspase-activated DNase (ICAD). Cleavage of ICAD by caspase-3 releases the enzyme caspase-activated DNase (CAD), which is responsible for nucleosomal DNA fragmentation during apoptosis (8, 17, 18). However, the molecular mechanisms that underlie other characteristic morphologies of apoptotic cells have yet to be elucidated, and the biological functions of most caspase substrates remain unknown.
A striking hallmark of apoptosis is chromatin condensation, in which the cell's genomic DNA condenses to form small particles in the nucleus. Chromatin condensation progresses in two distinct steps: stage I involves chromatin compaction while stage II involves DNA fragmentation (27). Because caspase inhibitors can block chromatin condensation induced by apoptotic stimuli, it is believed that caspase-mediated cleavage of cellular substrates is important for one or both stages of this process (27, 37, 39). Among caspase substrates, CAD is considered to be a key regulator of DNA fragmentation (23, 25, 38). Inhibition of CAD allows discrimination between the two stages of chromatin condensation because loss of CAD function blocks only DNA fragmentation and not chromatin compaction (25, 27). The nucleus of the cell in which CAD is inhibited assumes a morphological pattern called “peripheral chromatin condensation,” so named because the chromatin condenses around the periphery of the nucleus rather than adopting the apoptotic condensation morphology that results when both stage I and stage II are completed. These results suggest that the digestion of nucleosomal DNA by CAD is not connected to the induction of chromatin compaction. Thus, it is likely that there is another caspase substrate that drives stage I of chromatin condensation during apoptosis.
MST1 is a prominent kinase cleaved by caspases during apoptosis (11, 16). MST1 was originally discovered as a mammalian homologue of the budding yeast gene STE20, which is the mitogen-activated protein kinase kinase kinase kinase (MAPKKKK) enzyme of the Saccharomyces cerevisiae MAPK cascade (4, 29). Human MST1 has two caspase cleavage sites situated between its N-terminal kinase domain and its C-terminal regulatory domain (10, 11). Once cleaved by a caspase, MST1 becomes constitutively active (CA) and activates the stress-response MAPK pathways mediated by p38 and c-Jun N-terminal kinase (JNK; also called the stress-activated protein kinase) (11). We previously reported that MST1 overexpression induces caspase activation and apoptotic morphology (34) and that activated MST1 translocates from the cytoplasm to the nucleus and enhances the induction of peripheral chromatin condensation (33). These data suggested that MST1 might be the caspase substrate that induces chromatin compaction (stage I) during apoptosis. We also showed that MST1-induced apoptotic morphology could be suppressed by expression of dominant-negative (DN) JNK (34), indicating that the JNK pathway is the main downstream target of caspase-cleaved MST1 during apoptosis. However, MST1 is also known to phosphorylate histone H2B in vitro (16) and was recently shown by Cheung et al. to directly phosphorylate Ser14 of histone H2B in vivo (3). Cheung and colleagues therefore hypothesized that histone H2B phosphorylation is the trigger that induces chromatin condensation (3). To clarify which signaling pathway downstream of MST1 induces chromatin condensation in apoptotic cells, we analyzed MST1-induced peripheral chromatin condensation in MKK4/MKK7 double knockout (MKK4/7 DKO) embryonic stem (ES) cells that lack function of the JNK pathway. We provide clear genetic evidence that MST1-mediated activation of JNK is both essential and sufficient for the induction of peripheral chromatin condensation. Furthermore, inhibition of JNK blocked peripheral chromatin condensation during Fas-mediated apoptosis. Our data therefore indicate that caspase-mediated cleavage of MST1 and the resulting activation of the JNK pathway are key elements of the mechanism by which chromatin condensation is induced during apoptosis.
MATERIALS AND METHODS
Plasmids.
Construction of the Myc-tagged CA MST1 (CA-MST1) expression vector (pcMycMST1 containing residues 1 to 330 [pcMycMST1(1-330)]) and its kinase-dead (KD-MST1) mutant [pcMycMST1(1-330 KD)] were described previously (34). The histone H2B-enhanced green fluorescent protein (EGFP) fusion protein expression vector (pH2B-EGFP) was constructed by replacing DsRed1 of pH2B-DsRed1 with EGFP (13, 33). For expression in ES cells, the expression vector pCE-IRES2-EGFP was constructed by inserting the internal ribosome entry site 2 (IRES2) sequence between the EF-1 promoter and the EGFP sequence of pCE-EGFP, followed by insertion of the HindIII/EcoRI fragment of pcMycMST1(1-330) (containing Myc-tagged CA-MST1) before the IRES2 sequence to give pCE-MycMST1(1-330)-IRES2-EGFP. The KD mutant counterpart was constructed using the same method. The EGFP sequence of pCE-IRES2-EGFP was replaced by the histone H2B-EGFP fusion gene to construct pCE-IRES2-H2B-EGFP, and CA-MST1 was also inserted into this vector [pCE-MycMST1(1-330)-IRES2-H2B-EGFP]. The MKK7-Flag-JNK1 fragment and its KD mutant counterpart (bearing a mutation in the JNK1 kinase domain [32]) were subcloned from pEF-MKK7-Flag-JNK1 into pCE-IRES2-EGFP/H2B-EGFP. DN JNK [pcFlag-JNK1(APF)] (34) and DN c-Jun [pCS2-c-Jun(TAM)] (32) were described previously. The construction of the cleavage-negative ICAD (ICAD-CN) has also been described previously (34). The ICAD-CN fragment was subcloned into pIRES2-H2B-EGFP in which the EGFP sequence of pIRES2-EGFP (Clontech) was replaced by H2B-EGFP.
Cell culture and transfections.
293T and HeLa cells were cultured in Dulbecco's modified Eagle's medium (Nissui) with 10% fetal bovine serum (FBS) in a 5% CO2 humidified atmosphere at 37°C. Cells were plated 18 h before transfection at a density of 1 × 105 cells/35-mm dish. The culture medium was changed to 2 ml of OptiMEM-I (GIBCO/Invitrogen) just prior to transfection. Transfection was performed with FuGENE6 (Roche) for 293T cells and with Lipofectamine 2000 (Invitrogen) for HeLa cells. In some experiments, the caspase inhibitor Z-Asp-CH2-DCB (50 μM; Bachem) was added to the culture medium at 3 h posttransfection.
The generation of MKK4/7 DKO ES cells has been described previously (22). MKK4/7 DKO and wild-type (WT) E14K (parent strain) ES cells were maintained in Dulbecco's modified Eagle's medium (GIBCO/Invitrogen) containing 15% FBS and supplemented with leukemia inhibitory factor and β-mercaptoethanol as described previously (21). Cells were plated 18 h prior to transfection at a density of 5 × 105 cells/35-mm dish, and transfection was carried out as described for HeLa cells. In some experiments, Z-Asp-CH2-DCB (50 μM) was added to the ES culture medium at 3 h posttransfection.
HL60 cells and Jurkat cells were cultured in RPMI 1640 medium (Sigma) containing 10% FBS. HL60 cells were plated 18 h prior to apoptosis induction at a density of 5 × 105 cells/35-mm dish and treated with 20 μg/ml etoposide to induce apoptosis. Jurkat cells were plated 18 h prior to transfection at a density of 5 × 105 cells/35-mm dish. Then, transfection was performed with Lipofectamine 2000, and cells were cultured for 21 h prior to apoptosis induction. The JNK inhibitor SP600125 (5 μM; Sigma) was added at the same time as apoptosis was induced by the addition of 100 ng/ml anti-Fas antibody ([Ab] clone CH-11; MBL Japan).
Cell lysis and histone acid extraction.
For Western blotting, ES cells were transfected with expression vectors containing the IRES2-EGFP or IRES2-H2B-EGFP sequence (see above). For whole-cell lysis, harvested cells were lysed in a buffer containing 150 mM NaCl, 20 mM Tris-HCl (pH 7.5), 10 mM β-glycerophosphate, 5 mM EGTA, 1 mM NaPPi, 5 mM NaF, 0.5% Triton X-100, 1 mM NaVO4, 5 mM dithiothreitol, 0.5% (vol/vol) aprotinin, and 1 mM phenylmethylsulfonyl fluoride.
For the analysis of histone modifications, cells were suspended in an extraction buffer containing 10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 1.5 mM phenylmethylsulfonyl fluoride, and 0.1% (vol/vol) phosphatase inhibitor cocktail 1 and 2 (Sigma). The cells were acid extracted by the addition of a one-fifth volume of 1 N HCl and placed on ice for 30 min. The acid-extracted lysate was centrifuged, and the supernatant was neutralized by adding an appropriate volume of 3 N Tris base.
Both whole-cell lysates and acid-extracted lysates were boiled with Laemmli's sample buffer and subjected to Western blot analysis.
Western blot analysis.
Proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane that was then incubated in blocking solution (5% skim milk in Tris-buffered saline [TBS]) for 1 h. The blocked membrane was incubated with anti-c-Myc Ab (clone 9E10; Santa Cruz), anti-phospho-JNK, anti-JNK1 (which recognizes both splicing variants of JNK1: 46 kDa and 55 kDa), anti-phospho-c-Jun Ser63, anti-c-Jun (all from Cell Signaling Technology), anti-phospho-histone H2B Ser14, or anti-histone H2B (both from Upstate) in blocking solution for 1 h. The membrane was then washed in TBS-Tween 20 (0.05%), incubated with anti-mouse/rabbit horseradish peroxidase-conjugated Ab (Jackson ImmunoResearch Laboratory) for 30 min, and washed three times in TBS-Tween 20. Proteins were visualized using Immobilon HRP (Millipore) or the SuperSignal West Femto Kit (Pierce) and a ChemiDoc XRS system (Bio-Rad).
Nucleosomal DNA fragmentation assay.
Cells were incubated in 100 μl of lysis buffer (10 mM Tris-Cl, pH 7.5, 10 mM EDTA, 0.5% Triton X-100) for 10 min on ice followed by microcentrifugation for 15 min at 4°C. The supernatant was recovered and incubated first with 2 μl of RNase A (20 mg/ml) for 1 h at 37°C and then with 2 μl of proteinase K (20 mg/ml) for 1 h at 37°C. DNA in the supernatant was precipitated by adding 20 μl of 5 M NaCl plus 120 μl of isopropanol and incubating overnight at −20°C. Precipitated DNA was resuspended in Tris-EDTA buffer and subjected to 1.6% agarose gel electrophoresis in Tris-borate-EDTA buffer. Fractionated DNA was stained with ethidium bromide and observed under UV illumination using the ChemiDoc XRS system.
Chromatin condensation assay.
For confocal laser scanning microscope (CLSM) studies, 293T cells were cotransfected with the nuclear marker expression vector pH2B-EGFP/pH2B-DsRed1, and ES cells and Jurkat cells were transfected with expression vectors containing the IRES2-H2B-EGFP sequence. After 24 h, cells were harvested by incubation in 5% EDTA-phosphate-buffered saline (PBS) for 10 min at 37°C and attached to poly-l-lysine-coated coverslips by centrifugation. Attached cells were fixed in 4% formaldehyde, and nuclear morphology was visualized by H2B-EGFP/DsRed1 fluorescence using a Zeiss LSM-510 CLSM. Cross-sectional images of each cell showing the nucleus were obtained, and the percentage of nuclei showing peripheral chromatin condensation was calculated.
Immunohistochemistry.
Etoposide-treated HL60 cells and MST1-transfected ES cells were suspended and attached to poly-l-lysine-coated coverslips by centrifugation. Attached cells were fixed in 4% formaldehyde and permeabilized using 0.5% Triton X-100 in PBS. Permeabilized cells were treated for 1 h with blocking solution (2% bovine serum albumin and 2% FBS in PBS) at room temperature and incubated with rabbit anti-phospho-histone H2B Ser14 monoclonal Ab (Upstate) in blocking solution overnight at 4°C. Cells were washed twice in PBS, incubated with Alexa 546-conjugated anti-rabbit secondary Ab (Invitrogen) for 30 min, and washed twice in PBS. The fluorescent signal indicating the presence of phospho-histone H2B Ser14 was visualized using a Zeiss LSM-510 CLSM.
RESULTS
MST1 induces peripheral chromatin condensation in a caspase-independent manner.
We previously reported that overexpression of MST1 in 293T cells induced peripheral chromatin condensation in the absence of an apoptotic stimulus (33, 34), leading us to hypothesize that MST1 might act downstream of caspases to induce chromatin compaction. To test this hypothesis, we introduced CA-MST1 [Myc-MST1(1-330)] into 293T cells and investigated the effect of the caspase inhibitor Z-Asp-CH2-DCB on MST1-induced peripheral chromatin condensation. Expression of CA-MST1 induced peripheral chromatin condensation, as expected (Fig. 1A), and this condensation was not affected by the addition of the caspase inhibitor (Fig. 1B). Thus, caspase activation downstream of MST1 is not necessary for the induction of peripheral chromatin condensation. This result strongly suggests that MST1 is a downstream effector of caspases.
FIG. 1.
Expression of CA-MST1 induces peripheral chromatin condensation in a caspase-independent manner. (A) Typical chromatin condensation morphology induced by MST1 expression in 293T cells. Control vector (Control) or CA-MST1 [Myc-MST1(1-330)] vector was transfected into 293T cells. pH2B-DsRed1 was cotransfected as a nuclear marker. After 18 h, nuclei were examined by CLSM. Nuclei of CA-MST1-transfected cells show the morphology typical of peripheral chromatin condensation. (B) Peripheral chromatin condensation induced by MST1 is caspase-independent. 293T cells were transfected with CA-MST1 in the presence of the caspase inhibitor Z-Asp-CH2-DCB (25 μM) or solvent alone (0.1% dimethyl sulfoxide). After 18 h, nuclei were examined by CLSM. Results shown are the mean percentage ± standard deviation of cells with nuclei showing peripheral chromatin condensation and are representative of three trials.
JNK is essential for MST1-induced peripheral chromatin condensation.
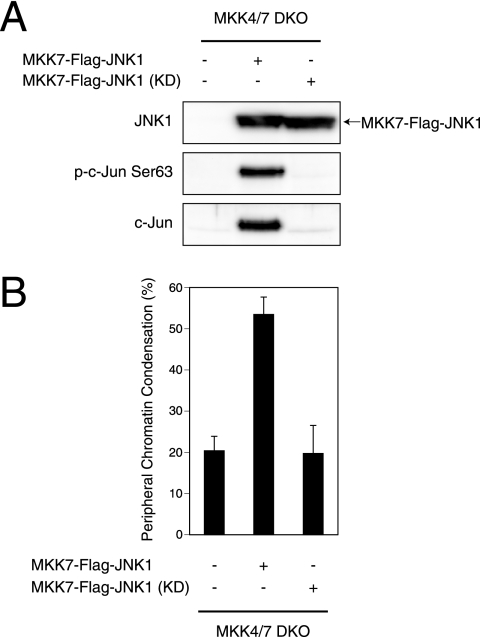
To clarify the involvement of JNK in MST1-induced peripheral chromatin condensation, we took advantage of our previously generated strain of MKK4/7 DKO ES cells (22). These cells lack all JNK-activating MAPKKs and cannot activate JNK in response to various cellular stimuli. It has previously been shown that apoptotic signaling pathways in WT ES cells are identical to those in somatic cells and that the usual hallmark morphologies are exhibited by apoptotic ES cells (37). To confirm that MST1 can induce peripheral chromatin condensation in ES cells as it does in somatic cells, we introduced CA-MST1 into WT ES cells in the presence of caspase inhibitor. We found that the pattern of peripheral chromatin condensation induced in ES cells (Fig. 2A) was strikingly similar to that in 293T cells (Fig. 1A). Next, we introduced CA-MST1 and KD-MST1 [Myc-MST1(1-330 KD)] into WT and MKK4/7 DKO ES cells and cultured these cells with caspase inhibitor. When we examined JNK activation in these cells, we found that CA-MST1 induced the phosphorylation of two splicing variants of JNK (approximately 46 kDa and 55 kDa) in WT ES cells in a manner that was dependent on MST1's kinase activity (Fig. 2B). In contrast, even the basal level of JNK phosphorylation was diminished in MKK4/7 DKO ES cells, and no MST1-induced elevation in JNK phosphorylation was observed in response to CA-MST1 expression (Fig. 2B). In addition, MST1-induced peripheral chromatin condensation was observed only when CA-MST1 was expressed in WT ES cells and not in MKK4/7 DKO ES cells (Fig. 2C). This chromatin condensation activity strongly correlated with the level of JNK phosphorylation. Thus, JNK is the essential target through which MST1 induces peripheral chromatin condensation.
FIG. 2.
JNK is essential for MST1-induced peripheral chromatin condensation in ES cells. (A) Typical chromatin condensation morphology induced by MST1 expression in ES cells. Control vector or CA-MST1 was transfected into WT ES cells in the presence of 50 μM Z-Asp-CH2-DCB. After 24 h, nuclei were examined by CLSM. (B) Activation of JNK by MST1 is completely abolished in MKK4/7 DKO ES cells. WT and MKK4/7 DKO ES cells were transfected with control vector, CA-MST1, or KD-MST1 [Myc-MST1(1-330 KD)] in the presence of 50 μM Z-Asp-CH2-DCB. After 24 h, expression levels of MST1 (Myc) and endogenous levels of phospho-JNK (p-JNK) and JNK1 were detected by Western blotting. (C) Peripheral chromatin condensation is not induced by MST1 in MKK4/7 DKO ES cells. Cells were transfected as described for panel B, and nuclei were examined 24 h later by CLSM. Results shown are the mean percentage ± standard deviation of cells with nuclei showing peripheral chromatin condensation and are representative of three trials.
Activation of JNK is sufficient to induce peripheral chromatin condensation.
We next examined whether JNK activation on its own was sufficient to induce peripheral chromatin condensation. It was recently reported that JNK1 fused to MKK7 functions as a CA form of JNK (32). We transfected MKK4/7 DKO ES cells with either the MKK7-JNK1 fusion protein or its KD counterpart bearing a mutation in the JNK domain. Because JNK can reportedly induce caspase activation through proapoptotic Bcl-2 family proteins (32), we performed these experiments in the presence of caspase inhibitor to exclude any effects of caspase activation downstream of the MKK7-JNK1 fusion protein. We first analyzed these cells for phosphorylation and expression levels of c-Jun, a transcription factor known to be phosphorylated and activated by JNK. Our results showed that expression of the MKK7-JNK1 fusion protein in MKK4/7 DKO ES cells both induced c-Jun phosphorylation and increased c-Jun expression (Fig. 3A). Notably, expression of the MKK7-JNK1 KD mutant did not induce c-Jun phosphorylation (Fig. 3A), confirming the necessity of intact JNK function for c-Jun activation and ruling out the possibility that the MKK7 domain of MKK7-JNK1 might have phosphorylated endogenous JNK. When we examined peripheral chromatin condensation in the MKK7-JNK1-transfected MKK4/7 DKO ES cells, we found that expression of the fusion protein induced peripheral chromatin condensation in a manner dependent on the kinase activity of JNK (Fig. 3B). These data clearly show that JNK activity is sufficient to induce peripheral chromatin condensation.
FIG. 3.
JNK is sufficient for the induction of peripheral chromatin condensation in MKK4/7 DKO ES cells. (A) Ectopic expression of MKK7-JNK1 fusion protein activates the JNK pathway. MKK4/7 DKO ES cells were transfected with control vector, the MKK7-JNK1 fusion gene (MKK7-Flag-JNK1), or its KD mutant in the presence of 50 μM Z-Asp-CH2-DCB. After 24 h, the expression levels of the MKK7-JNK1 fusion protein and endogenous levels of phospho-c-Jun (p-c-Jun Ser63) and total c-Jun were detected by Western blotting. (B) CA JNK induces peripheral chromatin condensation. MKK4/7 DKO ES cells were transfected as described for panel A, and nuclei were examined 24 h later by CLSM. Results shown are the mean percentage ± standard deviation of cells with nuclei showing peripheral chromatin condensation and are representative of three trials.
Histone H2B Ser14 phosphorylation does not correlate with MST1-induced peripheral chromatin condensation.
Histone H2B is commonly phosphorylated at Ser14 in apoptotic cells (1, 3). Moreover, it was recently shown that MST1 can directly phosphorylate histone H2B Ser14 in vivo (3). These observations prompted us to determine whether MST1-mediated phosphorylation of histone H2B Ser14 correlated with chromatin condensation. Because various apoptotic stimuli (including etoposide and UV) are known to trigger substantial histone H2B Ser14 phosphorylation in HL60 cells (3), we employed this cell line to examine the capability of the Ab against histone H2B phosphorylation. When we treated HL60 cells with the DNA-damaging agent etoposide, the cells underwent apoptosis within several hours and showed extensive nucleosomal DNA fragmentation (Fig. 4A). We then extracted the core histones from HL60 cells treated with etoposide for 3 h and noted that the phosphorylation of histone H2B Ser14 was strongly elevated (Fig. 4B), confirming the previous report (3). Immunofluorescent staining using a monoclonal Ab recognizing phospho-histone H2B Ser14 also showed a strong elevation of histone H2B phosphorylation in the nuclei of etoposide-treated HL60 cells (Fig. 4C). In contrast, when we examined histone H2B Ser14 phosphorylation in WT ES cells in which peripheral chromatin condensation was induced by expression of CA-MST1 in the presence of caspase inhibitor, no increase in histone H2B Ser14 phosphorylation could be detected (Fig. 4D).
FIG. 4.
Phosphorylation of histone H2B Ser14 is mediated by caspases acting downstream of MST1. (A) DNA fragmentation in HL60 cells. HL60 cells were treated for the indicated times with 20 μg/ml etoposide to induce apoptosis, and nucleosomal DNA fragmentation was examined by agarose gel electrophoresis. M, λ/EcoT14I markers. (B) Histone H2B Ser14 phosphorylation in HL60 cells. HL60 cells were treated with 20 μg/ml etoposide for 3 h and acid extracted. Endogenous levels of phospho-histone H2B Ser14 and total histone H2B (H2B) were analyzed by Western blotting. (C) Immunostaining of histone H2B Ser14 phosphorylation in HL60 cells. HL60 cells were treated with 20 μg/ml etoposide for 3 h and immunostained using anti-phospho-histone H2B Ser14 Ab. Levels of phospho-histone H2B were substantially increased in the nuclei of etoposide-treated cells. (D) Histone H2B Ser14 phosphorylation in MST1-expressing ES cells. WT ES cells were transfected with CA-MST1 as described in the legend of Fig. 2B in the presence of 50 μM Z-Asp-CH2-DCB. After 24 h, cells were acid extracted, and levels of MST1 (Myc), endogenous phospho-histone H2B Ser14, and total histone H2B were analyzed by Western blotting. (E) Histone H2B Ser14 phosphorylation in ES cells coexpressing MST1 and H2B-EGFP fusion protein. WT ES cells were transfected as described for panel D except that the expression vector also carried H2B-EGFP as a transfection marker. Western blotting was performed as described for panel D. (F) Immunostaining of histone H2B Ser14 phosphorylation in MST1-expressing ES cells. WT ES cells were transfected as described for panel E and immunostained using anti-phospho-histone H2B Ser14 Ab as described for panel C. No significant histone H2B Ser14 phosphorylation was observed in MST1-expressing ES cells showing peripheral chromatin condensation (white arrows). (G) Dependence of MST1-induced histone H2B Ser14 phosphorylation on caspases. HeLa cells were transfected with control vector or CA-MST1 in the presence of solvent alone (0.1% dimethyl sulfoxide) or 25 μM Z-Asp-CH2-DCB. After 24 h, cells were acid extracted, and levels of MST1 (Myc), endogenous phospho-histone H2B Ser14, and total histone H2B were analyzed as described for panel D. p-H2B Ser14, phospho-histone H2B Ser14; DIC, differential interference contrast.
To take into account the background phosphorylation of histone H2B that naturally occurs by transfection, we performed parallel experiments using a vector expressing a histone H2B-EGFP fusion protein and examined the phosphorylation of this protein as a marker of transfection. When we examined the phosphorylation of histone H2B-EGFP in transfected, CA-MST1-expressing ES cells, no increase in histone H2B-EGFP phosphorylation was observed (Fig. 4E). Consistent with this result, histone H2B Ser14 phosphorylation could not be detected when CA-MST1-expressing cells that showed peripheral chromatin condensation were subjected to immunofluorescence staining (Fig. 4F). These results strongly indicate that MST1 can induce peripheral chromatin condensation in the absence of histone H2B phosphorylation and also suggest that MST1 does not directly phosphorylate histone H2B. We pursued this latter issue using HeLa cells, which are known to exhibit histone H2B phosphorylation by MST1 transfection (3). In line with this previous report, we found that expression of CA-MST1 in HeLa cells was able to induce high levels of histone H2B Ser14 phosphorylation (Fig. 4G). In both control and CA-MST1-expressing HeLa cells, this phosphorylation of histone H2B Ser14 was totally abrogated by the addition of caspase inhibitor (Fig. 4G). These results indicate that caspase activation is required downstream of MST1 to induce histone H2B Ser14 phosphorylation. Taken together, our data suggest that histone H2B Ser14 is not directly phosphorylated by MST1 and that histone H2B Ser14 phosphorylation does not correlate with MST1-induced peripheral chromatin condensation.
c-Jun transcriptional activity is not necessary for MST1-induced peripheral chromatin condensation.
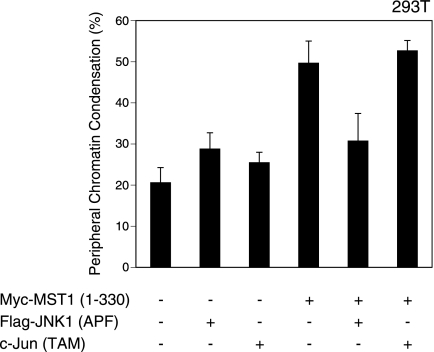
The best known target of JNK is c-Jun. JNK phosphorylates the N-terminal Ser63 and Ser73 of c-Jun, an event that activates the AP-1 transcription complex comprised of c-Jun and c-Fos (6). To clarify whether AP-1 activation and the expression of AP-1 target genes are required to induce peripheral chromatin condensation, we introduced CA-MST1 into 293T cells expressing either a DN form of JNK [JNK1(APF)] or a DN form of c-Jun [c-Jun(TAM)]. Consistent with our earlier results obtained for ES cells, MST1-induced peripheral chromatin condensation was suppressed in 293T cells expressing DN-JNK (Fig. 5). In contrast, expression of DN c-Jun had no effect on peripheral chromatin condensation (Fig. 5). Thus, AP-1-mediated transcription is not required for chromatin condensation. This result implies that a direct phosphorylation target of JNK exists that is important for the induction of peripheral chromatin condensation.
FIG. 5.
c-Jun-mediated transcription is not required for MST1-induced chromatin condensation. 293T cells were transfected with control vector or CA-MST1 plus either DN-JNK [Flag-JNK1(APF)] or DN c-Jun [c-Jun(TAM)]. After 24 h, nuclei were examined by CLSM. Results shown are the mean percentage ± standard deviation of cells with nuclei showing peripheral chromatin condensation and are representative of three trials.
JNK is involved in the induction of peripheral chromatin condensation during Fas-mediated apoptosis.
Our experiments showed that JNK activation is sufficient to induce peripheral chromatin condensation. We therefore examined the role of JNK in both stages of the chromatin condensation process: stage I, in which chromatin compaction leads to peripheral chromatin condensation, and stage II, in which nucleosomal DNA fragmentation leads to complete apoptotic chromatin condensation. We used anti-Fas Ab to trigger apoptosis as this stimulus directly activates the caspase cascade via Fas multimerization without involving any intracellular signaling pathways (15). We chose Jurkat cells for these experiments because these cells show a greater sensitivity to anti-Fas.
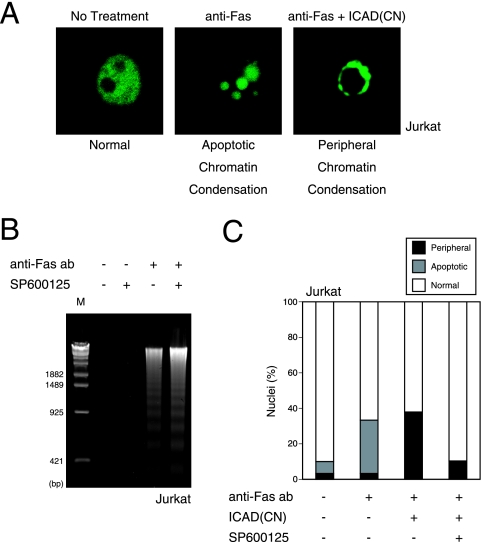
In our first experiments, we introduced ICAD-CN into Jurkat cells and treated the cells for 4 h with anti-Fas Ab to induce apoptosis. Anti-Fas-treated Jurkat cells expressing the control vector showed the apoptotic chromatin condensation pattern characteristic of the completion of both stages of chromatin condensation (Fig. 6A). In contrast, anti-Fas-treated Jurkat cells expressing ICAD-CN showed only peripheral chromatin condensation because the nucleosomal DNA fragmentation step was inhibited by ICAD-CN (Fig. 6A). This result demonstrated that we could discriminate between stage I and stage II of chromatin condensation in Jurkat cells. Next, we examined the effect of the JNK inhibitor SP600125 on the anti-Fas-induced chromatin condensation morphologies. Addition of JNK inhibitor did not affect nucleosomal DNA fragmentation in anti-Fas-treated control cells (Fig. 6B), indicating that caspase-3 activation and ICAD cleavage are not affected by JNK inhibition. In contrast, almost 38% of nuclei in anti-Fas-treated Jurkat cells expressing ICAD-CN showed only peripheral chromatin condensation, a level that dropped to about 10% of nuclei in the presence of JNK inhibitor (Fig. 6C). Thus, during Fas-mediated apoptosis, caspase activation leads to JNK activation that is required for the CAD-independent stage I of chromatin condensation. On the basis of these findings and our earlier results, we can conclude that MST1-induced chromatin compaction during apoptosis depends on JNK activation and not on histone H2B phosphorylation.
FIG. 6.
JNK activity is involved in peripheral chromatin condensation during Fas-mediated apoptosis. (A) Discrimination between stages I and II of chromatin condensation. Jurkat cells were transfected with either control vector or vector expressing ICAD-CN. After 21 h, cells were left untreated or treated with 100 ng/ml anti-Fas Ab for 4 h to induce apoptosis, and nuclei were examined by CLSM. In the absence of ICAD cleavage, chromatin condensation remained at stage I (peripheral chromatin condensation) instead of completing stage II (apoptotic chromatin condensation). (B) JNK inhibition does not affect anti-Fas-induced DNA fragmentation. Jurkat cells were treated for 4 h with 100 ng/ml anti-Fas Ab in the presence of solvent alone (0.1% dimethyl sulfoxide) or 5 μM JNK inhibitor SP600125. Nucleosomal DNA fragmentation was then examined as described in the legend of Fig. 4A. (C) JNK inhibition blocks anti-Fas-induced peripheral chromatin condensation in Jurkat cells expressing ICAD. Jurkat cells were transfected with control vector or ICAD-CN. After 21 h, transfected cells were treated for 4 h with anti-Fas Ab and SP600125 as described for panel B. Nuclei were examined by CLSM. The data are expressed as the percentages of cells with nuclei showing normal morphology, apoptotic chromatin condensation, or peripheral chromatin condensation, as exemplified in panel A.
DISCUSSION
Chromatin condensation is a morphological hallmark of apoptosis and occurs in two stages: chromatin compaction leading to peripheral chromatin condensation and nucleosomal DNA fragmentation leading to complete apoptotic chromatin condensation. However, the underlying mechanisms driving these stages are poorly understood. We previously reported that MST1, a kinase activated upon caspase-mediated cleavage, can induce peripheral chromatin condensation (33, 34). Because peripheral chromatin condensation is a nuclear morphology commonly observed in CAD-inhibited apoptotic cells (23, 25, 38), we considered it likely that MST1 played an important role in the CAD-independent stage I of chromatin condensation. Here, we report that MST1-induced activation of the JNK pathway is essential and sufficient for the induction of peripheral chromatin condensation. In MKK4/7 DKO cells, both MST1-induced JNK activation and peripheral chromatin condensation were blocked. Furthermore, constitutive activation of JNK alone was sufficient to induce peripheral chromatin condensation. During Fas-mediated apoptosis, inhibition of CAD prevented the progression of chromatin condensation from stage I to stage II, and inhibition of JNK blocked stage I. Our results suggest the following sequence of events (Fig. 7): (i) stimulation of apoptosis leads to the activation of caspases that cleave MST1; (ii) activated MST1 phosphorylates and activates JNK; (iii) activated JNK drives chromatin compaction that results in a peripheral chromatin condensation morphology (stage I); (iv) activated caspases cleave ICAD, resulting in activation of CAD; (v) activated CAD induces nucleosomal DNA fragmentation to generate the complete apoptotic chromatin condensation morphology (stage II).
FIG. 7.
Schematic model of the roles of MST1 and JNK in chromatin condensation during apoptosis. Chromatin condensation during apoptosis occurs in two distinct steps: stage I, in which chromatin compaction results in peripheral chromatin condensation, and stage II, in which DNA fragmentation results in complete apoptotic chromatin condensation. In a cell undergoing Fas-mediated apoptosis, Fas ligand binding to Fas induces caspase-8 activation, which in turn activates caspase-3. Caspase-3 cleaves and activates MST1, which induces the JNK activation required for chromatin compaction. Caspase-3 also cleaves ICAD to release CAD, which promotes DNA fragmentation and the progression to complete apoptotic chromatin condensation.
Recent studies have revealed that specific histone modifications regulate chromatin dynamics and thus various physiological events (26). These histone modifications recruit specific proteins to the chromatin, resulting in transcription, DNA methylation, and chromatin condensation. During apoptosis, a commonly observed specific histone modification is the phosphorylation of histone H2B. It was therefore proposed that histone H2B phosphorylation might play an important role in regulating chromatin dynamics, particularly those of chromatin condensation during apoptosis (1, 7). Because MST1 induces peripheral chromatin condensation and can directly phosphorylate histone H2B Ser14 (3), it was hypothesized that MST1 might regulate chromatin condensation by phosphorylating histone H2B Ser14. However, our data indicate that MST1 can induce peripheral chromatin condensation in the absence of histone H2B phosphorylation and that caspase activity is necessary to induce histone H2B phosphorylation downstream of MST1. In other words, MST1 does not directly phosphorylate histone H2B in vivo. We previously reported that overexpression of MST1 induces caspase activation through the JNK pathway (34). In the absence of caspase inhibitors, MST1 may induce caspase activation through JNK, which induces histone H2B phosphorylation. However, peripheral chromatin condensation induced by MST1 is caspase independent, in line with our observation that histone H2B phosphorylation does not correlate with chromatin condensation in the presence of a caspase inhibitor.
Although not required for the induction of peripheral chromatin condensation, histone H2B phosphorylation remains a good biochemical marker of apoptosis. Furthermore, it remains possible that the phosphorylation of histone H2B plays a role in stage II and contributes to the regulation of chromatin dynamics by facilitating the recruitment of necessary factors. Another histone whose modification may be relevant to nuclear morphologies of apoptosis is H2AX. When phosphorylated by JNK, H2AX enhances DNA fragmentation and may recruit caspase-activated CAD to the nucleosome (19). The roles of various phosphorylated histones in apoptosis await clarification in future studies.
Our results show that new gene expression triggered by AP-1 is not required for MST1-induced peripheral chromatin condensation, suggesting that JNK directly phosphorylates a target other than c-Jun to facilitate chromatin compaction. A candidate suspected to be involved in chromatin condensation is apoptosis-inducing factor, a mitochondrial protein that is released from the mitochondria in response to various apoptotic stimuli (28). When recombinant apoptosis-inducing factor protein is added to isolated nuclei, peripheral chromatin condensation is induced (28). However, we previously reported that MST1 translocates to the nucleus upon caspase-mediated cleavage and that nuclear localization of MST1 enhances peripheral chromatin condensation (33). These results suggest that activation of JNK in the nucleus, and not around the mitochondria, is important for chromatin condensation.
How chromatin condenses during apoptosis remains a mystery. Isolated nuclei treated with active CAD undergo chromatin condensation even when ATP is depleted, but this process is inhibited by excess CaCl2 (36). These effects are quite the opposite of those seen during mitotic condensation, where ATP is required and calcium binding is needed to stabilize the packed chromatin structure. These observations suggest that the mechanisms underlying apoptotic chromatin condensation and mitotic chromatin condensation differ in important ways. However, peripheral chromatin condensation can occur without CAD-mediated nucleosomal DNA fragmentation, and little is known about the molecules involved in chromatin compaction during the earliest phase of apoptosis. Further analysis of the compaction process and the identification of the relevant JNK target whose existence has been revealed in this study are required to fully understand the mechanisms of chromatin condensation during apoptosis.
JNK is a member of the stress-response MAPK superfamily, proteins that are potently activated by a variety of physiological stimuli. Many studies have reported that JNK can activate caspases in response to cellular stress (12), implying that JNK is a regulator of the initiation of apoptosis. On the other hand, JNK is also frequently activated in the course of the apoptotic cascade (2, 9, 31). Our results suggest that this latter type of JNK activation may constitute part of the death-executing machinery and promote chromatin condensation. Thus, we believe JNK has a bilateral character and contributes not only to the cell death decision but also to the execution of that death. Further research is needed to explore this new aspect of JNK's role as an executioner during apoptosis.
Acknowledgments
We thank Tetsuo Moriguchi, Tadao Usui, and Mary Saunders for useful advice and numerous members of the Nishina and Katada laboratories for critical reading and helpful discussions.
This work was partly supported by a Grant-in-Aid for Scientific Research on a Priority Area from the Ministry of Education, Culture, Sport, Science and Technology of Japan and the Ministry of Health, Labor and Welfare of Japan.
Footnotes
Published ahead of print on 4 June 2007.
REFERENCES
- 1.Ajiro, K. 2000. Histone H2B phosphorylation in mammalian apoptotic cells. An association with DNA fragmentation. J. Biol. Chem. 275:439-443. [DOI] [PubMed] [Google Scholar]
- 2.Cahill, M. A., M. E. Peter, F. C. Kischkel, A. M. Chinnaiyan, V. M. Dixit, P. H. Krammer, and A. Nordheim. 1996. CD95 (APO-1/Fas) induces activation of SAP kinases downstream of ICE-like proteases. Oncogene 13:2087-2096. [PubMed] [Google Scholar]
- 3.Cheung, W. L., K. Ajiro, K. Samejima, M. Kloc, P. Cheung, C. A. Mizzen, A. Beeser, L. D. Etkin, J. Chernoff, W. C. Earnshaw, and C. D. Allis. 2003. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell 113:507-517. [DOI] [PubMed] [Google Scholar]
- 4.Creasy, C. L., and J. Chernoff. 1995. Cloning and characterization of a member of the MST subfamily of Ste20-like kinases. Gene 167:303-306. [DOI] [PubMed] [Google Scholar]
- 5.Cryns, V., and J. Yuan. 1998. Proteases to die for. Genes Dev. 12:1551-1570. [DOI] [PubMed] [Google Scholar]
- 6.Curran, T., and B. R. Franza, Jr. 1988. Fos and Jun: the AP-1 connection. Cell 55:395-397. [DOI] [PubMed] [Google Scholar]
- 7.de la Barre, A. E., D. Angelov, A. Molla, and S. Dimitrov. 2001. The N terminus of histone H2B, but not that of histone H3 or its phosphorylation, is essential for chromosome condensation. EMBO J. 20:6383-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enari, M., H. Sakahira, H. Yokoyama, K. Okawa, A. Iwamatsu, and S. Nagata. 1998. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43-50. [DOI] [PubMed] [Google Scholar]
- 9.Frisch, S. M., K. Vuori, D. Kelaita, and S. Sicks. 1996. A role for Jun-N-terminal kinase in anoikis; suppression by Bcl-2 and CrmA. J. Cell Biol. 135:1377-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graves, J. D., K. E. Draves, Y. Gotoh, E. G. Krebs, and E. A. Clark. 2001. Both phosphorylation and caspase-mediated cleavage contribute to regulation of the Ste20-like protein kinase Mst1 during CD95/Fas-induced apoptosis. J. Biol. Chem. 276:14909-14915. [DOI] [PubMed] [Google Scholar]
- 11.Graves, J. D., Y. Gotoh, K. E. Draves, D. Ambrose, D. K. Han, M. Wright, J. Chernoff, E. A. Clark, and E. G. Krebs. 1998. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J. 17:2224-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ip, Y. T., and R. J. Davis. 1998. Signal transduction by the c-Jun N-terminal kinase (JNK)-from inflammation to development. Curr. Opin. Cell Biol. 10:205-219. [DOI] [PubMed] [Google Scholar]
- 13.Kanda, T., K. F. Sullivan, and G. M. Wahl. 1998. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 8:377-385. [DOI] [PubMed] [Google Scholar]
- 14.Kerr, J. F., A. H. Wyllie, and A. R. Currie. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 4:239-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruidering, M., and G. I. Evan. 2000. Caspase-8 in apoptosis: the beginning of “the end”? IUBMB Life 50:85-90. [DOI] [PubMed] [Google Scholar]
- 16.Lee, K. K., M. Murakawa, E. Nishida, S. Tsubuki, S. Kawashima, K. Sakamaki, and S. Yonehara. 1998. Proteolytic activation of MST/Krs, STE20-related protein kinase, by caspase during apoptosis. Oncogene 16:3029-3037. [DOI] [PubMed] [Google Scholar]
- 17.Liu, X., P. Li, P. Widlak, H. Zou, X. Luo, W. T. Garrard, and X. Wang. 1998. The 40-kDa subunit of DNA fragmentation factor induces DNA fragmentation and chromatin condensation during apoptosis. Proc. Natl. Acad. Sci. USA 95:8461-8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, X., H. Zou, C. Slaughter, and X. Wang. 1997. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89:175-184. [DOI] [PubMed] [Google Scholar]
- 19.Lu, C., F. Zhu, Y. Y. Cho, F. Tang, T. Zykova, W. Y. Ma, A. M. Bode, and Z. Dong. 2006. Cell apoptosis: requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol. Cell 23:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholson, D. W. 1999. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 6:1028-1042. [DOI] [PubMed] [Google Scholar]
- 21.Nishina, H., K. D. Fischer, L. Radvanyi, A. Shahinian, R. Hakem, E. A. Rubie, A. Bernstein, T. W. Mak, J. R. Woodgett, and J. M. Penninger. 1997. Stress-signalling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature 385:350-353. [DOI] [PubMed] [Google Scholar]
- 22.Nishitai, G., N. Shimizu, T. Negishi, H. Kishimoto, K. Nakagawa, D. Kitagawa, T. Watanabe, H. Momose, S. Ohata, S. Tanemura, S. Asaka, J. Kubota, R. Saito, H. Yoshida, T. W. Mak, T. Wada, J. M. Penninger, N. Azuma, H. Nishina, and T. Katada. 2004. Stress induces mitochondria-mediated apoptosis independent of SAPK/JNK activation in embryonic stem cells. J. Biol. Chem. 279:1621-1626. [DOI] [PubMed] [Google Scholar]
- 23.Sakahira, H., M. Enari, Y. Ohsawa, Y. Uchiyama, and S. Nagata. 1999. Apoptotic nuclear morphological change without DNA fragmentation. Curr. Biol. 9:543-546. [DOI] [PubMed] [Google Scholar]
- 24.Salvesen, G. S., and V. M. Dixit. 1997. Caspases: intracellular signaling by proteolysis. Cell 91:443-446. [DOI] [PubMed] [Google Scholar]
- 25.Samejima, K., S. Tone, and W. C. Earnshaw. 2001. CAD/DFF40 nuclease is dispensable for high molecular weight DNA cleavage and stage I chromatin condensation in apoptosis. J. Biol. Chem. 276:45427-45432. [DOI] [PubMed] [Google Scholar]
- 26.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 403:41-45. [DOI] [PubMed] [Google Scholar]
- 27.Susin, S. A., E. Daugas, L. Ravagnan, K. Samejima, N. Zamzami, M. Loeffler, P. Costantini, K. F. Ferri, T. Irinopoulou, M. C. Prevost, G. Brothers, T. W. Mak, J. Penninger, W. C. Earnshaw, and G. Kroemer. 2000. Two distinct pathways leading to nuclear apoptosis. J Exp. Med. 192:571-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Susin, S. A., H. K. Lorenzo, N. Zamzami, I. Marzo, B. E. Snow, G. M. Brothers, J. Mangion, E. Jacotot, P. Costantini, M. Loeffler, N. Larochette, D. R. Goodlett, R. Aebersold, D. P. Siderovski, J. M. Penninger, and G. Kroemer. 1999. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441-446. [DOI] [PubMed] [Google Scholar]
- 29.Taylor, L. K., H. C. Wang, and R. L. Erikson. 1996. Newly identified stress-responsive protein kinases, Krs-1 and Krs-2. Proc. Natl. Acad. Sci. USA 93:10099-10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 31.Toyoshima, F., T. Moriguchi, and E. Nishida. 1997. Fas induces cytoplasmic apoptotic responses and activation of the MKK7-JNK/SAPK and MKK6-p38 pathways independent of CPP32-like proteases. J. Cell Biol. 139:1005-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuruta, F., J. Sunayama, Y. Mori, S. Hattori, S. Shimizu, Y. Tsujimoto, K. Yoshioka, N. Masuyama, and Y. Gotoh. 2004. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 23:1889-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ura, S., N. Masuyama, J. D. Graves, and Y. Gotoh. 2001. Caspase cleavage of MST1 promotes nuclear translocation and chromatin condensation. Proc. Natl. Acad. Sci. USA 98:10148-10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ura, S., N. Masuyama, J. D. Graves, and Y. Gotoh. 2001. MST1-JNK promotes apoptosis via caspase-dependent and independent pathways. Genes Cells 6:519-530. [DOI] [PubMed] [Google Scholar]
- 35.Vaux, D. L., and S. J. Korsmeyer. 1999. Cell death in development. Cell 96:245-254. [DOI] [PubMed] [Google Scholar]
- 36.Widlak, P., O. Palyvoda, S. Kumala, and W. T. Garrard. 2002. Modeling apoptotic chromatin condensation in normal cell nuclei. Requirement for intranuclear mobility and actin involvement. J. Biol. Chem. 277:21683-21690. [DOI] [PubMed] [Google Scholar]
- 37.Woo, M., R. Hakem, M. S. Soengas, G. S. Duncan, A. Shahinian, D. Kagi, A. Hakem, M. McCurrach, W. Khoo, S. A. Kaufman, G. Senaldi, T. Howard, S. W. Lowe, and T. W. Mak. 1998. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 12:806-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuste, V. J., I. Sanchez-Lopez, C. Sole, R. S. Moubarak, J. R. Bayascas, X. Dolcet, M. Encinas, S. A. Susin, and J. X. Comella. 2005. The contribution of apoptosis-inducing factor, caspase-activated DNase, and inhibitor of caspase-activated DNase to the nuclear phenotype and DNA degradation during apoptosis. J. Biol. Chem. 280:35670-35683. [DOI] [PubMed] [Google Scholar]
- 39.Zheng, T. S., S. F. Schlosser, T. Dao, R. Hingorani, I. N. Crispe, J. L. Boyer, and R. A. Flavell. 1998. Caspase-3 controls both cytoplasmic and nuclear events associated with Fas-mediated apoptosis in vivo. Proc. Natl. Acad. Sci. USA 95:13618-13623. [DOI] [PMC free article] [PubMed] [Google Scholar]