Abstract
The tumor suppressor p53 is inactivated by multiple mechanisms that include mutations of the p53 gene itself and increased levels of the p53 inhibitors MDM2 and MDM4. Mice lacking Mdm2 or Mdm4 exhibit embryo-lethal phenotypes that are completely rescued by concomitant deletion of p53. Here we show that Mdm2 and Mdm4 haploinsufficiency leads to increased p53 activity, exhibited as increased sensitivity to DNA damage and decreased transformation potential. Moreover, in in vivo tumor development, Eμ-myc Mdm4+/− mice show a delayed onset of B-cell lymphomas compared to Eμ-myc mice. Additionally, Mdm2+/− Mdm4+/− double-heterozygous mice are not viable and exhibit defects in hematopoiesis and cerebellar development. The defects in Mdm2+/− Mdm4+/− mice are corrected by deletion of a single p53 allele. These findings highlight the exquisite sensitivity of p53 to Mdm2 and Mdm4 levels and suggest that some cell types may be more sensitive to therapeutic drugs that inhibit the Mdm-p53 interaction.
The tumor suppressor p53 is inactivated in most cancers by mutations or deletions of the p53 gene (10). Alternatively, increased levels of two p53 inhibitors, MDM2 and MDM4, common events in many cancers, also dampen p53 activity (5, 23). Two new p53 inhibitors have recently been identified, but their role in tumorigenesis remains unknown (6, 14). Other mechanisms of inactivating the p53 pathway include deletions of Arf, which encodes an Mdm2-interacting protein (22). Arf loss results in increased availability of Mdm2 to inhibit p53. Thus, a likely scenario is that inactivation of the p53 pathway by any number of means is an essential step in tumorigenesis.
While data showing high levels of MDM2 and MDM4 in human tumors are simply correlative, the essential role of Mdm2 and Mdm4 in the regulation of p53 activity in vivo is clear from mouse models. Mice lacking Mdm2 or Mdm4 exhibit embryo-lethal phenotypes that are completely rescued by concomitant deletion of p53 (13, 20, 21). Moreover, mice lacking Mdm2 and p53 have the same tumor phenotype as p53-null mice, suggesting that the only pivotal role of MDM2 in vivo is the negative regulation of p53 (16). Additionally, mice carrying a hypomorphic allele of Mdm2 that expressed ∼30% of Mdm2 levels had increased p53 activity (17). As a result, these mice were small, lymphopenic, and radiosensitive. Importantly, these phenotypes were rescued by the deletion of p53 (17). Mice with decreased levels of Mdm2 also showed a delay in tumor onset in a p53-dependent manner, emphasizing again the importance of p53 and Mdm2 gene dosage in normal cellular survival and tumor onset (18).
More recently, in humans, a single nucleotide polymorphism (SNP) in the MDM2 promoter that leads to increased Mdm2 levels and attenuation of p53 activity was discovered (3). The MDM2 SNP is associated with accelerated tumorigenesis in soft tissue sarcomas and in patients with Li-Fraumeni syndrome (3). These SNP data suggest that even small differences in Mdm2 levels may modify cancer risk (4).
To determine directly whether differences in Mdm2 or Mdm4 levels affect p53 response, we took advantage of Mdm2+/− and Mdm4+/− mice. These mice live normal life spans and lack any visible phenotypes. In this study, we asked whether haploinsufficiency at these loci contributed to a phenotype if the animal was stressed. Indeed, haploinsufficiency at either locus resulted in an increased p53-dependent response to ionizing radiation (IR) and decreased transformation potential in culture. Additionally, haploinsufficiency of Mdm4 significantly reduced lymphomagenesis in Eμ-myc transgenic mice, a model of non-Hodgkin's lymphoma that overexpresses c-myc in B cells (1). Thus, these data provide direct evidence that minor changes in Mdm2 or Mdm4 levels are mirrored by increased p53 levels and p53-dependent effects.
MATERIALS AND METHODS
Mice.
Mdm2 and Mdm4 single-heterozygous mice were crossed to generate all four genotypes: wild-type, Mdm2+/− (20), Mdm4+/− (21), and Mdm2+/− Mdm4+/−. The resulting mice were maintained on a mixed 129S6/SvEv × C57BL/6 background. The p53-null allele was generated by Jacks et al. (12). The Eμ-myc transgenic mouse strain was kindly provided by C. Eischen (7). Mdm4+/− mice were crossed to the Eμ-myc transgenic mice (in a C57BL/6 background) to generate Eμ-myc Mdm4+/− mice and Eμ-myc Mdm4+/+ mice. Genotypes of weaned mice were determined by PCR analysis of tail DNA using published primer sets for Mdm2 (20), p53 (12), Mdm4 (21), and Eμ-myc (1). Embryos (13.5 to 14.5 days postcoitum [dpc]) and tissues from pups were fixed in 10% (vol/vol) phosphate-buffered formalin. Bones were decalcified in 10% formic acid. Tissues were embedded in paraffin, cut into 4-μm-thick sections, stained with hematoxylin and eosin, and analyzed for pathology. Wild-type, Mdm2+/−, and Mdm4+/− mice from the same litter aged 5 to 6 weeks were exposed to a sublethal dose of 4 or 6 Gy of whole-body γ-IR from a 137Cs source (1.4 Gy/min). The mice were monitored daily. Moribund animals were euthanized according to the guidelines of the Institutional Animal Care And Use Committee.
Statistics.
Log rank tests and Kaplan-Meier analyses were performed to assay statistical differences. A factor was considered statistically significant if it had a two-sided P value of < 0.05.
Western blotting.
Tissue lysates (100 μg of total protein) were prepared from the thymuses of irradiated (6Gy, 4 h) and nonirradiated male and female Mdm2+/− mice. Cell lysates (50 μg of total protein) were prepared from cells infected with Ras and Myc. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred for 1 h to polyvinylidene difluoride membranes (Amersham-Pharmacia, Piscataway, NJ). After being blocked for 1 h at room temperature in 5% skim milk in Tris-buffered saline-Tween 20, the membranes were incubated with a rabbit polyclonal antibody against p53 (CM5; dilution, 1:1,000; Novocastra, Norwell, MA), a rabbit polyclonal antibody against Mdm2 (20) (1:500 dilution), a mouse monoclonal antibody against H-Ras (F235; dilution, 1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA), or a rabbit polyclonal antibody against c-Myc (A-14; dilution, 1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. An anti-actin antibody (dilution, 1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA) was used as a loading control. Membranes were washed with Tris-buffered saline-Tween 20, incubated with a horseradish peroxidase-conjugated anti-rabbit secondary antibody, and visualized by ECL (Amersham-Pharmacia, Piscataway NJ).
Cell proliferation, p53 IHC, and apoptosis assays.
For cell proliferation assays in embryos, pregnant female mice were injected intraperitoneally with 100 μg bromodeoxyuridine (BrdU)/g of body weight and were sacrificed 2 h later, Sections (thickness, 5 μm) of paraffin-embedded embryos were analyzed using a BrdU staining kit (Zymed Laboratories, Carlsbad, CA). For pups, we used staining with Ki-67 (1:1,000 dilution; Vector Laboratories, Burlingame, CA) to assess cellular proliferation. Immunohistochemical (IHC) analysis of p53 was performed as previously described (8) on adjacent sections using the anti-p53 antibody CM5 at a 1:200 dilution overnight at 4°C. To detect apoptosis, we performed IHC with rabbit anti-caspase-3 as recommended by the manufacturer (Cell Signaling, Danvers, MA).
Immunostaining of Purkinje cells.
Fourteen-day-old mice were perfused in 4% formalin, and whole brains were fixed overnight at 4°C. A sucrose gradient of 5% sucrose in phosphate-buffered saline (1 h), 10% sucrose in H2O (1 h), 15% sucrose in H2O (1 h), 20% sucrose in H2O (1 h), and 25% sucrose (overnight) was applied. Brains were frozen in O.C.T. (Sakura Finetek USA, Torrance, CA). Frozen sections (thickness, 45-μm) were obtained, and floating sections were stained for 2 days with the Purkinje cell-specific marker, rabbit anti-calbindin D-28K (dilution, 1:1,200; Chemicon, Temecula, CA), and an antibody against mouse glial fibrillary acidic protein (GFAP) (1:400 dilution; Sigma, St. Louis, MO). Goat anti-rabbit antibody-conjugated Alexa 488 and goat anti-mouse antibody-conjugated Alexa 594 (Molecular Probes, Carlsbad, CA) were used as fluorochromes. Topro3 (Molecular Probes, Carlsbad, CA) was used as a nuclear stain.
Cell culture and focus formation assay.
Mouse embryonic fibroblasts (MEFs) were prepared from 13.5-dpc embryos and cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and penicillin (100 IU/ml)-streptomycin (100 μg/ml). Early-passage MEFs were assayed for colony formation potential by infection with a retrovirus vector containing an activated Ha-RasV12 cDNA and a puromycin resistance gene and with a retrovirus vector containing a c-myc cDNA with the hygromycin resistance gene. Two days after infection, puromycin (2 μg/μl) and hygromycin (100 μg/μl) were added to the cells in order to select cells expressing ras and myc. Following double selection, 5 × 104 cells were mixed with 9.5 × 105 noninfected cells of the same genotype, plated on a 10-cm-diameter dish, and maintained for 2 weeks. The medium was changed every other day. Cells were then fixed, stained with 0.05% crystal violet in 25% methanol, and washed with water. Foci were counted and plotted as a histogram. This experiment was performed in triplicate.
RESULTS
Radiosensitivity of Mdm4+/− mice.
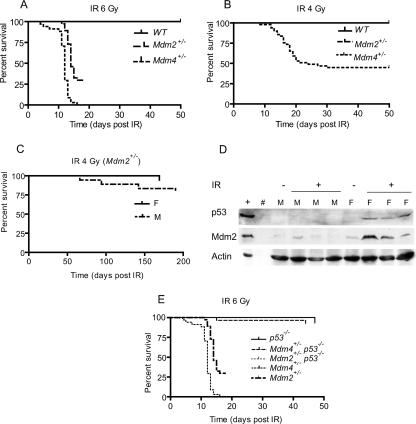
Mdm2+/− and Mdm4+/− mice are normal and live an average life span (20, 21). To determine if these mice contained an intact p53 pathway in response to DNA damage, wild-type, Mdm2+/−, and Mdm4+/− mice were subjected to a sublethal dose (6 Gy) of whole-body IR at the age of 5 to 6 weeks (Fig. 1). Mdm2+/− mice were more sensitive than wild-type mice to IR, as previously published (17). Surprisingly, Mdm4+/− mice were even more sensitive (Fig. 1A). Approximately 30% of Mdm2+/− mice were alive 20 days after IR, whereas all Mdm4+/− died within 14 days after IR. In both cases, the cause of death was a depletion of the hematopoietic system (17; also data not shown). None of the wild-type mice died after exposure to this sublethal dose of IR. Overall and pairwise comparisons of survival rates using log rank tests determined that the differences between Mdm2+/−, Mdm4+/−, and wild-type mice were statistically significant (P < 0.0001). Sex was not a significant factor when the mice were grouped by genotype (P values, 0.85 and 0.9087 for Mdm2+/− and Mdm4+/− mice, respectively, by the log rank test).
FIG. 1.
Radiosensitivity in Mdm2+/− and Mdm4+/− 5-week-old mice after a sublethal dose of whole-body IR. (A) Kaplan-Meier survival curves of age-matched wild-type (WT) (n = 65), Mdm2+/− (n = 37), and Mdm4+/− (n = 34) mice after 6 Gy of IR. (B) Kaplan-Meier survival curves of age-matched wild-type (n = 50), Mdm4+/− (n = 31), and Mdm2+/− (n = 39) mice after 4 Gy of IR. (C) Kaplan-Meier survival curves for Mdm2+/− mice by sex (18 females and 21 males). (D) Protein lysates from the thymuses of irradiated (+) and nonirradiated (−) male (M) and female (F) mice blotted with p53 and Mdm2 antibodies. Actin was used as a loading control. (E) Kaplan-Meier survival curves of Mdm2+/− (n = 37), Mdm4+/− (n = 34), Mdm2+/− p53−/− (n = 27), Mdm4+/− p53−/− (n = 28), and p53−/− (n = 9) mice.
The finding that all Mdm4+/− mice died within 14 days of whole-body IR at 6 Gy did not allow a clear resolution of the differences in response between these mice and the Mdm2+/− mice at this dose. We therefore examined the effects of exposure to a lower IR dose. At 4 Gy, 30% of Mdm4+/− mice died, but none of the Mdm2+/− mice died when examined within 50 days after IR (Fig. 1B). Thus, the difference between these two genotypes was even more prominent at 4 Gy of IR. Upon longer observation, some Mdm2+/− mice died, mainly of lymphomas, and gender differences became apparent. The Mdm2+/− female mice lived longer than males (P = 0.0332) (Fig. 1C). No influence of sex on survival was detected in the Mdm4+/− mice (P = 0.3577 [data not shown]). Analysis of a potential influence due to differences in animal weight showed no effect on the observed survival rate (data not shown). Thus, sex affects the tumor phenotype of Mdm2+/− mice in response to a low dose of IR.
To determine if the basis of this sexual dimorphism is due to differences in p53 levels or activity, we examined the status of p53 and Mdm2 in irradiated male and female Mdm2+/− mice after 4 h of 6-Gy IR (Fig. 1D). Western blot analysis of thymus tissue showed no detectable p53 or Mdm2 in nonirradiated male and female mice. An obvious increase in p53 levels was observed for females after irradiation. Increased p53 levels correlated well with increases in the level of the p53 target, Mdm2, in these animals (Fig. 1D). These data indicate that the p53 response is greater in females than in males among Mdm2+/− mice (Fig. 1C).
To further examine the role of p53 in this radiosensitivity, we generated Mdm2+/−, and Mdm4+/− mice lacking p53. All Mdm2+/− p53−/− mice and all but one of the Mdm4+/− p53−/− mice survived for at least 50 days after 6 Gy of IR (Fig. 1E). Thus, these data indicate that the sensitivities of Mdm2+/−, and Mdm4+/− mice to death induced by IR are p53 dependent.
Hematopoietic failure and cerebellar hypoplasia in Mdm2+/− Mdm4+/− mice.
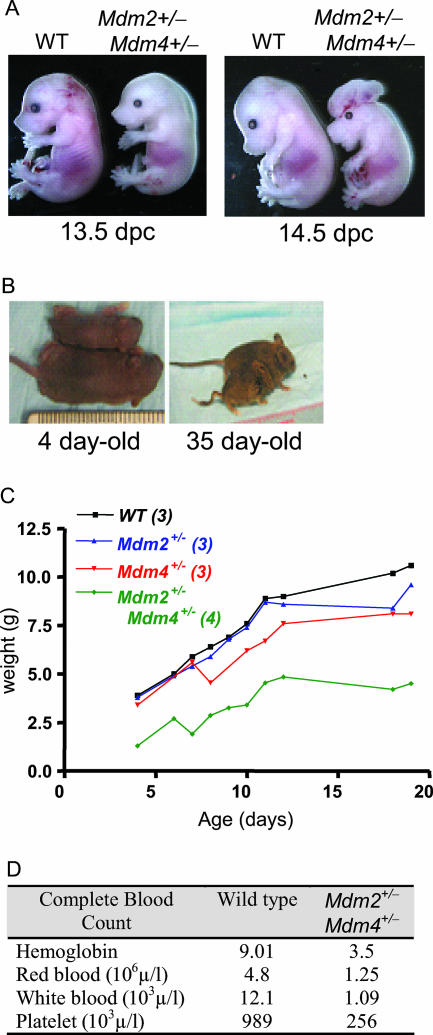
We also wanted to examine the sensitivities of Mdm2+/− Mdm4+/− double-heterozygous mice to IR. However, of 100 mice born from Mdm2+/− × Mdm4+/− crosses, none were double heterozygous at postnatal day 21 (P21) (the expected frequency is 25%). Additionally, only 70% of the expected number of these embryos were born. Embryos examined at midgestation appeared pale, suggestive of anemia, and were smaller than wild-type embryos (Fig. 2A). Additionally, 10 to 20% of them had exencephaly associated with cleft palate, or other types of neural tube closure defects, such as kinky tails (Fig. 2A and B). Immediately after birth, double-heterozygous pups were smaller than their wild-type littermates (Fig. 2B and C) and anemic (Fig. 2D and data not shown).
FIG. 2.
Embryonic and postnatal phenotypes of Mdm2+/− Mdm4+/− mice. (A) Twenty percent of Mdm2+/− Mdm4+/− embryos show exencephaly associated with cleft palate or other neural tube defects. WT, wild type. (B) Delay in postnatal development of Mdm2+/− Mdm4+/− mice compared to wild-type mice. (C) Weights of mice with various genotypes during early postnatal development. (D) Complete blood counts for 13-day-old wild-type and Mdm2+/− Mdm4+/− pups.
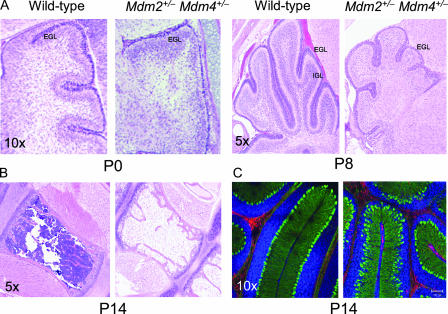
A full necropsy was performed on mice that survived as long as 20 days. Histologic examination of tissue sections from these mice showed marked hypoplasia in the bone marrow and cerebellum (Fig. 3A and B). Additional defects included hypoplasia and atrophy of the spleen and thymus, decreased extramedullary hematopoiesis in the liver, and fewer and smaller glomeruli in the kidneys (data not shown). The blood compartment was also examined by using blood smears, and complete blood counts were performed whenever possible. All lineages of blood cells were observed with normal morphological characteristics and at appropriate stages of maturity. A complete blood count suggested widespread depletion of all blood cell types (Fig. 2D). Bone marrow pathology revealed systemic and severe depletion of all blood cells in the double-heterozygous animals (Fig. 3B). These abnormalities were never detected in Mdm2 or Mdm4 single-heterozygous animals.
FIG. 3.
Hypoplasia of bone marrow and cerebellum in Mdm2+/− Mdm4+/− mice. (A) Cerebellums from newborn (P0) and 8-day-old (P8) mice. Note the obvious hypoplasia of the EGL and the rudimentary internal granular layer (IGL) in P8 mice. (B) Bone marrow from the sternums of P14 Mdm2+/− Mdm4+/− mice. (C) Immunofluorescence staining showing the disorganization of Purkinje cells stained green for calbindin. Red, GFAP; blue, Topro3 nuclear stain.
Detailed histopathology also identified an underdeveloped cerebellum with marked hypoplasia of the granule layer, which included defects in both the external granule layer (EGL) and internal granule layer in postnatal double-heterozygous animals (Fig. 3A). In the early postnatal cerebellum (P0 to P4), the Purkinje neurons are required in order to induce proliferation of the granule neurons (15). To determine if the Purkinje cells were present and in the correct anatomical position, sections of the cerebellum were stained with the Purkinje cell-specific marker calbindin. The Purkinje cells were intact and present in the appropriate number, but disorganized, in the lobes of the cerebellums of Mdm2+/− Mdm4+/− mice (Fig. 3C). Thus, the appropriate cells are present and juxtaposed, but they cannot contribute to the development and expansion of the organ.
Since Mdm2 and Mdm4 are critical negative regulators of p53, we asked whether these phenotypes were p53 dependent. All Mdm2+/− Mdm4+/− phenotypes were completely abolished by the deletion of a single p53 allele. Mdm2+/− Mdm4+/− p53+/− mice were born at the expected Mendelian ratio, 6 of 12 when Mdm2−/− Mdm4−/− p53−/− mice were crossed to p53+/− mice. This clearly demonstrates that the underlying mechanism of lethality is entirely p53 dependent and that the stoichiometry between these loci is critically important to survival.
Increased p53 levels and apoptosis in Mdm2+/− Mdm4+/− mice.
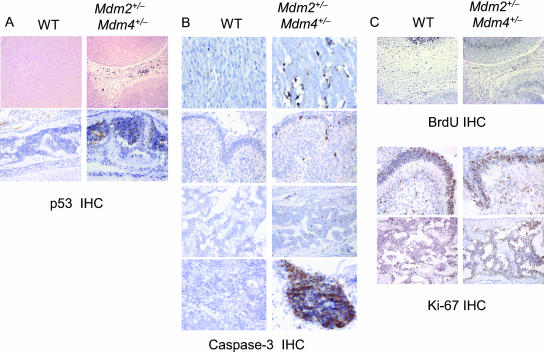
To further explore the mechanisms of cell loss, we performed p53 IHC analysis, cell proliferation, and apoptosis assays on 13.5-dpc mouse embryos and newborn mice. The p53 IHC of double-heterozygous mice showed increased p53 levels in the aberrantly developing brain of the embryo and in bone marrow of the P4 brain (Fig. 4A). An IHC assay for caspase-3 on paraffin-embedded sections of double heterozygote embryos showed a higher apoptotic index for these mice than for wild-type embryos (Fig. 4B). No notable difference in the proliferation index was seen after BrdU labeling of embryos and IHC with Ki-67 on pups (Fig. 4C).
FIG. 4.
p53 IHC, apoptosis, and proliferation assays. Paraffin-embedded sections from wild-type (WT) and Mdm2+/− Mdm4+/− mice were examined. (A) p53 IHC. (Upper row) Brains of 13.5-dpc embryos; (lower row) cranial bone marrow of 3-day-old pups. (B) For apoptosis, caspase-3 IHC of 13.5-dpc embryo brains (top row) and 1-day-old pup cerebellums (second row), cranial bone marrow (third row), and spleens (bottom row) is shown. (C) For proliferation, BrdU labeling of 13.5-dpc embryo brains (top row) and Ki-67 IHC of 3-day-old cerebellums (center row) and cranial bone marrow (bottom row) are shown. Note that the embryonic tissues do not compare due to developmental differences.
Mdm4 haploinsufficiency decreased transformation and lymphomagenesis.
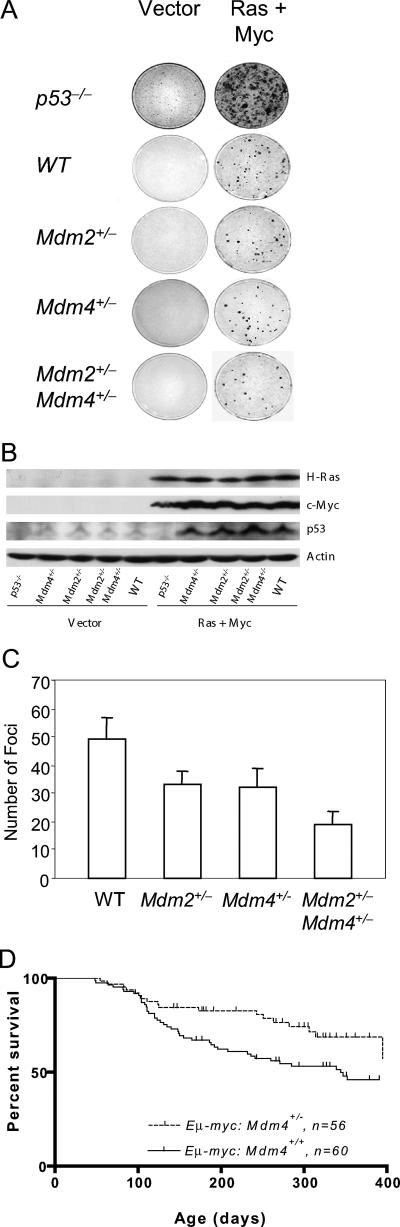
Although Mdm2+/−, and Mdm4+/− mice live normal life spans, they clearly have increased p53 activity in response to DNA damage compared to wild-type mice. These data suggest that Mdm2+/−, Mdm4+/−, and Mdm2+/− Mdm4+/− mice may have inherently higher than normal levels of the p53 tumor suppressor. We therefore examined the role of Mdm2 and Mdm4 haploinsufficiency in tumorigenesis in cell culture using a focus-forming assay. MEFs generated from wild-type, p53−/−, Mdm2+/−, Mdm4+/−, and Mdm2+/− Mdm4+/− mice were transformed with c-myc and activated Ras oncogenes (Fig. 5). Comparable levels of c-myc and Ras were present in all infected cells. In this assay, wild-type cells produce a quantifiable number of foci while p53-null MEFs produce many. A decrease in the number of foci in MEFs heterozygous for either Mdm2 or Mdm4 compared to wild-type cells was clear. Both Mdm2+/− and Mdm4+/− MEFs had 33% fewer foci than wild-type MEFs (Fig. 5A and C). The decrease in the number of foci is accentuated in double-heterozygote MEFs, suggesting that haploinsufficiency at both Mdm loci further inhibits focus formation (Fig. 5A and C). Lastly, Western blot analyses indicate that p53 levels are higher in Mdm2+/− Mdm4+/− MEFs than in all other MEFs after c-myc and Ras infection (Fig. 5B).
FIG. 5.
(A) Focus-forming assays with wild-type (WT), p53−/−, Mdm2+/−, Mdm4+/−, and Mdm2+/− Mdm4+/− MEFs. Early-passage MEFs were infected with two retrovirus vectors: one containing activated Ha-rasV12 and the second containing c-myc. p53−/− MEFs were used as a positive control. MEFs of different genotypes were also infected with vector alone as a negative control. (B) Western blotting of infected MEFs with c-myc and Ras antibodies. (C) Quantitative representation of the numbers of foci shown in panel A. (D) Delay of Myc-induced lymphomagenesis by Mdm4 haploinsufficiency. Kaplan-Meier survival curves of Eμ-myc Mdm4+/+ (n = 60) and Eμ-myc Mdm4+/− (n = 56) transgenic mice are shown.
The role of Mdm2 haploinsufficiency in tumorigenesis, more specifically in lymphoma development, has already been established (2). Eμ-myc Mdm2+/− mice show delayed onset of lymphomagenesis compared to Eμ-myc mice. The role of Mdm4 haploinsufficiency in tumor development has not been tested. To directly address this question, we crossed Mdm4+/− mice with the Eμ-myc transgenic mice. As shown in Fig. 5D, Mdm4 haploinsufficiency significantly delayed Eμ-myc-induced lymphomagenesis in Eμ-myc Mdm4+/− compared to Eμ-myc Mdm4+/+ transgenic mice (P = 0.04).
DISCUSSION
In this study, we asked whether haploinsufficiency of Mdm2 or Mdm4 loci generated differences in p53 activity in response to DNA damage or transformation. Our data clearly show that haploinsufficiency at Mdm2, Mdm4, or both loci yielded phenotypes consistent with increased p53 function. In response to DNA damage, Mdm2+/− and Mdm4+/− mice showed enhanced sensitivity that was p53 dependent. Increased sensitivity to Mdm2 loss using a hypomorphic allele has already been demonstrated (17). On the other hand, the lesser-known p53 negative regulator, Mdm4, was even more potent as a p53 inhibitor, suggesting that Mdm4 is a major regulator of p53 in response to DNA damage in vivo.
Mdm2+/− Mdm4+/− mice did not survive long after birth and exhibited some of the same phenotypes previously seen in mice with a 70% decrease in Mdm2 expression (17). In our experiments, the hematopoietic defects were exacerbated, highlighting the crucial role of these p53 inhibitors in maintaining appropriate p53 levels in hematopoieisis. Additionally, a cerebellar defect was also visible in Mdm2+/− Mdm4+/− mice. At birth, the EGL of the cerebellum undergoes massive proliferation. Thus, two cellular compartments that contain highly proliferative cells were most affected by haploinsufficiency of Mdm2 and Mdm4, suggesting an increased sensitivity of cells that are actively proliferating. In both embryos and newborn mice, p53-dependent apoptosis appears to be the major mechanism of cell loss. These phenotypes were completely rescued by the deletion of one p53 allele, emphasizing the sensitivity of the p53 dose. This study clearly identifies cell types that are most sensitive to increased p53 levels in vivo and that may also be sensitive to inhibitors of the Mdm-p53 interaction.
At present, three Mdm4 loss-of-function alleles exist. The first Mdm4 allele used in this study makes a truncated carboxyl-terminal protein that cannot interact with p53 (21). The second allele was generated by viral integration within the Mdm4 promoter and shows as slightly weaker embryo-lethal phenotype (9, 19). The third Mdm4-null allele generated was a conditional inactivation of Mdm4 and deletes exon 2 (11). These alleles exhibit slight differences in p53-dependent phenotypes (11, 19, 21). Nevertheless, all three alleles share obvious similarities and manifest phenotypic variations that are relative to gradients of p53 levels (G. Lozano, unpublished observations).
Given the increased sensitivity to DNA damage, we also addressed a possible effect on transformation and clearly show that haploinsufficiency at Mdm2, Mdm4, or both loci results in decreased transformation potential. Lastly, in vivo, Mdm4+/− mice show delayed onset of lymphomagenesis in the Eμ-myc model. Mdm2+/− haploinsufficiency also delays tumor onset in Eμ-myc mice (2). Additionally, the Mdm2 hypomorphic mouse exhibits a decrease in tumorigenesis in the gut (18). These data suggest that potential polymorphisms at these loci, as have been observed in the human MDM2 promoter, may also be important effectors of tumorigenesis. Clearly, genetic changes that do not affect the survival of a mouse sensitize that mouse to DNA damage and modify a cancer phenotype.
Acknowledgments
This study was supported by a SPORE grant in Head and Neck Cancer, the Cancer Center Support Grant to MDACC, and a grant from the NCI to G.L. N.F.B. was supported by a Dermatology Foundation research career development award.
We thank the following individuals for guidance and helpful discussions: Gary Rosner, Simone Lunagomaz, Tomoo Iwakuma, Juan Barboza, and Arlette Audiffred. We thank Jason Grier, Sean Post, and Elizabeth L. Hess for critical reading of the manuscript and Angelito De Villa and Maurice J. Difulho IV for histology. We also thank Mong-Hong Lee for providing Ha-RasV12 and myc cDNAs and Christine Eischen for the Eμ-myc mouse.
Footnotes
Published ahead of print on 25 May 2007.
REFERENCES
- 1.Adams, J. M., A. W. Harris, C. A. Pinkert, L. M. Corcoran, W. S. Alexander, S. Cory, R. D. Palmiter, and R. L. Brinster. 1985. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318:533-538. [DOI] [PubMed] [Google Scholar]
- 2.Alt, J. R., T. C. Greiner, J. L. Cleveland, and C. M. Eischen. 2003. Mdm2 haplo-insufficiency profoundly inhibits Myc-induced lymphomagenesis. EMBO J. 22:1442-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bond, G. L., W. Hu, E. E. Bond, H. Robins, S. G. Lutzker, N. C. Arva, J. Bargonetti, F. Barte, H. Taubert, P. Wuerl, K. Onel, L. Yip, S. J. Hwang, L. C. Strong, G. Lozano, and A. J. Levine. 2004. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 119:591-602. [DOI] [PubMed] [Google Scholar]
- 4.Bond, G. L., W. Hu, and A. Levine. 2005. A single nucleotide polymorphism in the MDM2 gene: from a molecular and cellular explanation to clinical effect. Cancer Res. 65:5481-5484. [DOI] [PubMed] [Google Scholar]
- 5.Danovi, D., E. Meulmeester, D. Pasini, D. Migliorini, M. Capra, R. Frenk, P. de Graaf, S. Francoz, P. Gasparini, A. Gobbi, K. Helin, P. G. Pelicci, A. G. Jochemsen, and J.-C. Marine. 2004. Amplification of Mdmx (or Mdm4) directly contributes to tumor formation by inhibiting p53 tumor suppressor activity. Mol. Cell. Biol. 24:5835-5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dornan, D., S. Bheddah, K. Newton, W. Ince, G. D. Frantz, P. Dowd, H. Koeppen, V. M. Dixit, and D. M. French. 2004. COP1, the negative regulator of p53, is overexpressed in breast and ovarian adenocarcinomas. Cancer Res. 64:7226-7230. [DOI] [PubMed] [Google Scholar]
- 7.Eischen, C. M., J. D. Weber, M. F. Roussel, C. J. Sherr, and J. L. Cleveland. 1999. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 13:2658-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans, S. C., M. Viswanathan, J. D. Grier, M. Narayana, A. K. El-Naggar, and G. Lozano. 2001. An alternatively spliced HDM2 product increases p53 activity by inhibiting HDM2. Oncogene 20:4041-4049. [DOI] [PubMed] [Google Scholar]
- 9.Finch, R. A., D. B. Donoviel, D. Potter, M. Shi, A. Fan, D. D. Freed, C. Y. Wang, B. P. Zambrowicz, R. Ramirez-Solis, A. T. Sands, and N. Zhang. 2002. mdmx is a negative regulator of p53 activity in vivo. Cancer Res. 62:3221-3225. [PubMed] [Google Scholar]
- 10.Greenblatt, M. S., W. P. Bennett, M. Hollstein, and C. C. Harris. 1994. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 54:4855-4878. [PubMed] [Google Scholar]
- 11.Grier, J. D., S. Xiong, A. C. Elizondo-Fraire, J. M. Parant, and G. Lozano. 2006. Tissue-specific differences of p53 inhibition by Mdm2 and Mdm4. Mol. Cell. Biol. 26:192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacks, T., L. Remington, B. O. Williams, E. M. Schmitt, S. Halachmi, R. T. Bronson, and R. A. Weinberg. 1994. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4:1-7. [DOI] [PubMed] [Google Scholar]
- 13.Jones, S. N., A. E. Roe, L. A. Donehower, and A. Bradley. 1995. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 378:206-208. [DOI] [PubMed] [Google Scholar]
- 14.Leng, R. P., Y. Lin, W. Ma, H. Wu, B. Lemmers, S. Chung, J. M. Parant, G. Lozano, R. Hakem, and S. Benchimol. 2003. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell 112:779-791. [DOI] [PubMed] [Google Scholar]
- 15.Lewis, P. M., A. Gritli-Linde, R. Smeyne, A. Kottmann, and A. P. McMahon. 2004. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev. Biol. 270:393-410. [DOI] [PubMed] [Google Scholar]
- 16.McDonnell, T. J., R. Montes de Oca Luna, S. Cho, L. L. Amelse, A. Chavez-Reyes, and G. Lozano. 1999. Loss of one but not two mdm2 null alleles alters the tumour spectrum in p53 null mice. J. Pathol. 188:322-328. [DOI] [PubMed] [Google Scholar]
- 17.Mendrysa, S. M., M. K. McElwee, J. Michalowski, K. A. O'Leary, K. M. Young, and M. E. Perry. 2003. Mdm2 is critical for inhibition of p53 during lymphopoiesis and the response to ionizing irradiation. Mol. Cell. Biol. 23:462-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendrysa, S. M., K. A. O'Leary, M. K. McElwee, J. Michalowski, R. N. Eisenman, D. A. Powell, and M. E. Perry. 2006. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev. 20:16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Migliorini, D., E. Lazzerini Denchi, D. Danovi, A. Jochemsen, M. Capillo, A. Gobbi, K. Helin, P. G. Pelicci, and J. C. Marine. 2002. Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Mol. Cell. Biol. 22:5527-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montes de Oca Luna, R., D. S. Wagner, and G. Lozano. 1995. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378:203-206. [DOI] [PubMed] [Google Scholar]
- 21.Parant, J., A. Chavez-Reyes, N. A. Little, W. Yan, V. Reinke, A. G. Jochemsen, and G. Lozano. 2001. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat. Genet. 29:92-95. [DOI] [PubMed] [Google Scholar]
- 22.Sherr, C. J. 2001. Cell cycle control and cancer. Harvey Lect. 96:73-92. [PubMed] [Google Scholar]
- 23.Vogelstein, B., and K. W. Kinzler. 2001. Achilles' heel of cancer? Nature 412:865-866. [DOI] [PubMed] [Google Scholar]