Abstract
K+ transport in living cells must be tightly controlled because it affects basic physiological parameters such as turgor, membrane potential, ionic strength, and pH. In yeast, the major high-affinity K+ transporter, Trk1, is inhibited by high intracellular K+ levels and positively regulated by two redundant “halotolerance” protein kinases, Sat4/Hal4 and Hal5. Here we show that these kinases are not required for Trk1 activity; rather, they stabilize the transporter at the plasma membrane under low K+ conditions, preventing its endocytosis and vacuolar degradation. High concentrations (0.2 M) of K+, but not Na+ or sorbitol, transported by undefined low-affinity systems, maintain Trk1 at the plasma membrane in the hal4 hal5 mutant. Other nutrient transporters, such as Can1 (arginine permease), Fur4 (uracil permease), and Hxt1 (low-affinity glucose permease), are also destabilized in the hal4 hal5 mutant under low K+ conditions and, in the case of Can1, are stabilized by high K+ concentrations. Other plasma membrane proteins such as Pma1 (H+-pumping ATPase) and Sur7 (an eisosomal protein) are not regulated by halotolerance kinases or by high K+ levels. This novel regulatory mechanism of nutrient transporters may participate in the quiescence/growth transition and could result from effects of intracellular K+ and halotolerance kinases on membrane trafficking and/or on the transporters themselves.
The regulation of K+ transport is a fundamental property of living cells (18, 39). K+ is the major intracellular cation, and its concentration affects basic physiological parameters such as turgor pressure, electrical membrane potential, ionic strength, and pH (34). An important, and general, mechanism for regulation is feedback inhibition of K+ transport by high intracellular K+ levels (5, 34), but the molecular details remain unknown.
In the yeast Saccharomyces cerevisiae the high-affinity potassium uptake system is encoded by the partially redundant genes TRK1 and TRK2 (12, 24). TRK1 encodes the most active transporter, corresponding to a large protein, 1,235 amino acids long, containing eight membrane-spanning domains and with a four-MPM (membrane-pore-membrane) structure similar to that of shaker K+ channels (9, 12). Like the H+-ATPase Pma1, Trk1 is an integral plasma membrane protein localized to the “raft” domains (1, 45), which are glycolipid-enriched microdomains of the plasma membrane that are postulated to form a platform for lipid and protein sorting and trafficking (22, 32). TRK-type transporters are present in all fungi and plants studied to date (34) and are also present in most bacteria and archaea (9).
Genetic and phenotypic studies of yeast have identified several proteins involved in the regulation of potassium transport. For instance, the protein phosphatase Ppz1 deactivates Trk1 and is in turn deactivated by an inhibitory subunit, Hal3 (8, 46). Another phosphatase, calcineurin, has been reported to be required for the activation of Trk1 and Trk2 by sodium stress (27). Other proteins involved in the regulation of potassium transport include the protein kinase Sky1 (11); the osmo-induced protein Hal1 (33); the G protein of the Ras superfamily, Arl1 (29); and the Hal4/Sat4 and Hal5 kinases (28).
The partially redundant protein kinases Sat4/Hal4 and Hal5 were identified by their ability to confer salt tolerance upon overexpression, and genetic studies have suggested that they positively regulate Trk1. Hal4 and Hal5 belong to a family of kinases, including Npr1 and Ptk2, which have been postulated to be dedicated to the regulation of transporters (21, 23). For instance, Ptk2 increases the affinity of Pma1 for ATP in response to glucose metabolism (13). In vitro, this kinase can phosphorylate Ser 899 in the COOH-terminal tail of the ATPase (10). Npr1 operates by a different mechanism. It regulates the turnover and intracellular trafficking of several amino acid permeases, (7, 14, 37, 41) and the Gln3 transcription factor (6), but direct phosphorylation of these proteins has not been demonstrated.
In this study we show that the Hal4 and Hal5 kinases are not required for Trk1 activity; rather, they stabilize the K+ transporter at the plasma membrane. Unexpectedly, high concentrations of K+ maintain Trk1 at the plasma membrane in the hal4 hal5 mutant. Other nutrient transporters undergo a similar Hal4/Hal5- and high-K+-dependent regulation, and therefore this novel pathway may be of general relevance.
MATERIALS AND METHODS
Yeast strains and culture conditions.
All strains of S. cerevisiae used in this work are listed in Table 1. YPD contained 2% glucose, 2% peptone, and 1% yeast extract. Minimal medium (SD) contained 2% glucose, 0.7% yeast nitrogen base (Difco) without amino acids, 50 mM succinic acid adjusted to pH 5.5 with Tris, and the amino acids and purine and pyrimidine bases required by the strains. The minimal media with alternate nitrogen sources contained 2% glucose, 0.2% yeast nitrogen base without amino acids and without ammonium sulfate, and either 0.1% proline or 30 mg/ml urea. Growth assays were performed in solid media, spotting serial dilutions of saturated cultures onto plates with the indicated composition.
TABLE 1.
Strains used in this study
| Strain | Relevant genotype | Referencea |
|---|---|---|
| W303-1A | mataade2-1 can1-100 his3-11,15 leu2,3,112 trp1-1 ura3-1 | 13 |
| trk1 trk2 | W303-1A trk1::LEU2 trk2::HIS3 | 28 |
| hal4 hal5 | W303-1A hal4::LEU2 hal5::HIS3 | 28 |
| hal4 hal5 npi1 | W303-1A hal4::LEU2 hal5::HIS3 npi1::URA3 | |
| W303[YCp-TRK1-GFP] | W303-1A[p414-TRK1-GFP] | |
| hal4 hal5[YCp-TRK1-GFP] | W303-1A hal4::LEU2 hal5::HIS3[p414-TRK1-GFP] | |
| hal4 hal5[YEp][YCp-TRK1-GFP] | W303-1A hal4::LEU2 hal5::HIS3[YEp352][p414-TRK1-GFP] | |
| hal4 hal5[YCp-HAL5][YCp-TRK1-GFP] | W303-1A hal4::LEU2 hal5::HIS3[pUN50-HAL5][p414-TRK1-GFP] | |
| hal4 hal5[YEp-HAL5][YCp-TRK1-GFP] | W303-1A hal4::LEU2 hal5::HIS3[YEp352-HAL5][p414-TRK1-GFP] | |
| W303[YEp-CAN1-GFP] | W303-1A[pVTU100-CAN1-GFP] | |
| W303[YEp-HXT1-GFP] | W303-1A[pVTU100-HXT1-GFP] | |
| W303[YCp-FUR4-GFP] | W303-1A[pYCplac33-FUR4-GFP] | |
| W303[YCp-GAP1-GFP] | W303-1A[pYCp-GAP1-GFP] | |
| hal4 hal5[YEp-CAN1-GFP] | W303-1A hal4::LEU2 hal5::HIS3[pVTU100-CAN1-GFP] | |
| hal4 hal5[YEp-HXT1-GFP] | W303-1A hal4::LEU2 hal5::HIS3[pVTU100-HXT1-GFP] | |
| hal4 hal5[YCp-FUR4-GFP] | W303-1A hal4::LEU2 hal5::HIS3[pYCplac33-FUR4-GFP] | |
| hal4 hal5[YCp-GAP1-GFP] | W303-1A hal4::LEU2 hal5::HIS3[pYCp-GAP1-GFP] | |
| trk1 trk2[YEp-CAN1-GFP] | W303-1A trk1::LEU2 trk2::HIS3[pVTU100-CAN1-GFP] | |
| trk1 trk2[YEp-HXT1-GFP] | W303-1A trk1::LEU2 trk2::HIS3[pVTU100-HXT1-GFP] | |
| trk1 trk2[YCp-FUR4-GFP] | W303-1A trk1::LEU2 trk2::HIS3[pYCplac33-FUR4-GFP] | |
| trk1 trk2[YCp-GAP1-GFP] | W303-1A trk1::LEU2 trk2::HIS3[pYCp-GAP1-GFP] | |
| hal4 hal5 SUR7-GFP | W303-1A hal4::LEU2 hal5::HIS3 SUR7-GFP::URA3 | |
| hal4 hal5[YCp-TRK1] | W303-1A hal4::LEU2 hal5::HIS3[p414-TRK1-HA] | |
| hal4 hal5[YCp-TRK1Δ35] | W303-1A hal4::LEU2 hal5::HIS3[p414-TRK1Δ35-HA] | |
| hal4 hal5[YCp-URA3] | W303-1A hal4::LEU2 hal5::HIS3[pUN50] | |
| hal4 hal5[YCp-TRP1] | W303-1A hal4::LEU2 hal5::HIS3[p414] | |
| hal4 hal5[YCp-URA3][YCpTRP1] | W303-1A hal4::LEU2 hal5::HIS3[pUN50][p414] | |
| hal4 hal5[YCp-TRK1][YEp-CAN1-GFP] | W303-1A hal4::LEU2 hal5::HIS3[p414-TRK1-HA][pVTU100-CAN1-GFP] | |
| hal4 hal5[YCp-TRK1][YEp-HXT1-GFP] | W303-1A hal4::LEU2 hal5::HIS3[p414-TRK1-HA][pVTU100-HXT1-GFP] | |
| hal4 hal5[YCp-TRK1][YCp-FUR4-GFP] | W303-1A hal4::LEU2 hal5::HIS3[p414-TRK1-HA][pYCplac33-FUR4-GFP] | |
| hal4 hal5[YCp-TRK1Δ35][YEp-CAN1-GFP] | W303-1A hal4::LEU2 hal5::HIS3[p414-TRK1Δ35-HA][pVTU100-CAN1-GFP] | |
| hal4 hal5[YCp-TRK1Δ35][YEp-HXT1-GFP] | W303-1A hal4::LEU2 hal5::HIS3[p414-TRK1Δ35-HA][pVTU100-HXT1-GFP] | |
| hal4 hal5[YCp-TRK1Δ35][YCp-FUR4-GFP] | W303-1A hal4::LEU2 hal5::HIS3[p414-TRK1Δ35-HA][pYCplac33-FUR4-GFP] |
Unless otherwise indicated, the strains used are from this study.
The hal4 hal5 npi1 strain was constructed by using a PCR-generated fragment containing the Kluyveromyces lactis URA3 gene, flanked by sequences designed to create a partial disruption of the RSP5 promoter (replacing the sequence corresponding to bp −800 to −500, relative to the start codon) upon integration, thus mimicking the npi1 mutant, as described previously (16). Correct integration was confirmed by genomic PCR.
Plasmid construction.
The TRK1-hemagglutinin (HA) and TRK1-green fluorescent protein (GFP) plasmids were previously described (45). The Trk1Δ35 allele was constructed using a similar approach, which generated a PstI/NdeI PCR fragment corresponding to the COOH-terminal half of the TRK1 gene, using the internal PstI site and the 3′ primer 5′-TAATATCTCGAGCATATGCATTGGGTCTTCTGTATTGGT-3′. The NdeI site used for cloning is indicated in boldface. For the construction of YCp-HAL5 and YEp-HAL5, a genomic fragment containing the HAL5 open reading frame (SphI/SacI), 500 bp of the promoter, and 250 bp of the terminator was inserted into the centromeric vector pUN50 or the multicopy vector YEp352 (17). The following plasmids were kindly provided by Widmar Tanner: pVTU100-CAN1-GFP, pYCplac33-FUR4-GFP, and pVTU100-HXT1-GFP, SUR7-GFP (15, 26, 26). The Gap1-GFP (centromeric, endogenous promoter) plasmid was kindly provided by Bruno André.
Confocal microscopy.
Fluorescence images were obtained as described previously (45).
Vacuolar staining.
Vacuolar membranes were stained with a lipophilic styryl dye, N-(3-triethylammoniumpropyl)-4-(p-diethylaminophenyl-hexatrienyl)pyridium dibromide (FM 4-64) Molecular Probes Inc., OR), using a protocol modified from that already described (42). Cells contained in 15 ml of a mid-log-phase culture (optical density at 660 nm of 0.4) were collected by centrifugation and washed twice with 5 ml of sterile water. The sample was resuspended to an optical density of 0.3 at 660 nm in minimal medium supplemented with potassium. A 500-μl aliquot of the sample was removed, and 7 μl of FM4-64 (2 mg/ml in dimethyl sulfoxide and PIPES buffer [piperazine-1,4-bis(2-ethanesulfonic acid), pH 6.8] was added to a final concentration of 20 mM. This mixture was then incubated in the dark at 28°C for 90 min. The yeast cells were collected by centrifugation and washed once in 1.5 ml of fresh medium to remove excess stain. The cells were then resuspended in 1 ml of fresh minimal medium without potassium and incubated for a further 1.5 to 2 h to allow internalization of the dye and staining of the vacuolar membranes. Fluorescence was observed by confocal microscopy at an excitation wavelength of 488 nm and a detection wavelength of between 620 and 660 nm, as described previously (45).
Cell fractionation.
The indicated yeast strains were grown in potassium-supplemented minimal medium to an optical density at 660 nm of 0.4, and cells were incubated for the indicated times in medium with or without supplementation, harvested by centrifugation, and frozen at −70°C. Cells were resuspended in homogenization buffer (50 mM Tris [pH 8.0], 0.1 M KCl, 5 mM EDTA, 5 mM dithioerythritol, 20% [wt/vol] sucrose, protease inhibitor cocktail [Roche Diagnostics GmbH, Mannheim, Germany]) and lysed by vortexing with glass beads. The lysate was collected after centrifugation for 5 min at 500 × g. The crude extract was separated into soluble and particulate fractions by centrifugation for 30 min at 16,000 × g at 4°C. The particulate fraction was either resuspended directly in Laemmli sample buffer or resuspended by homogenization in the buffer described above and was solubilized by incubating with 3 mM Zwittergent-14 (Calbiochem) for 15 min at 28°C. The remaining insoluble material was removed by centrifugation, and the protein present in the supernatant was precipitated by adding 15% trichloroacetic acid. The precipitated proteins were collected by centrifugation, washed, and resuspended in Laemmli sample buffer.
Western analysis.
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and immunoblotted with either a monoclonal anti-HA antibody (12CA5; Roche Diagnostics GmbH, Mannheim, Germany) for Trk1 detection, anti-Pma1 (1:20,000) (38), or anti-GFP (1:2,000) (Roche Diagnostics GmbH, Mannheim, Germany). The anti-Hal5 antiserum (1:5,000) was generated in-house using histidine-tagged Hal5 purified from Escherichia coli to immunize rabbits by standard procedures. The anti-Hal5-specific antibodies present in the crude antisera were affinity purified using antigen immobilized on nitrocellulose as described previously (25). Immunoreactive bands were visualized using the ECL-Plus chemiluminescence system and horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences UK Ltd., England). When necessary, membranes were stripped by incubation for 30 min at 50°C in a buffer containing 100 mM 2-mercaptoethanol, 2% sodium dodecyl sulfate, and 62.5 mM Tris-HCl (pH 6.7), followed by extensive washes, and reprobed as described above.
Rubidium uptake and internal potassium measurements.
Rubidium uptake and internal potassium were measured by atomic absorption spectrophotometry as described previously (35) or by high-pressure liquid chromatography as described previously (28).
RESULTS
Trk1 is mislocalized and destabilized in the hal4 hal5 mutant.
Genetic evidence suggests that the Hal4 and Hal5 kinases are involved in the positive regulation of the Trk1 potassium transporter (28). Northern analysis of TRK1 mRNA transcript levels shows that TRK1 expression does not change in the hal4 hal5 mutant, compared to the wild type (S. Merchan and L. Yenush, unpublished observations; see Fig. 3A). This result suggests that the regulation of Trk1 by Hal4 and Hal5 does not occur at the transcriptional level, which led to the hypothesis that the Hal4 and Hal5 kinases are involved in controlling the activity of the Trk1 potassium transporter by posttranslational modification or by affecting Trk1 sorting to, or stability at, the plasma membrane.
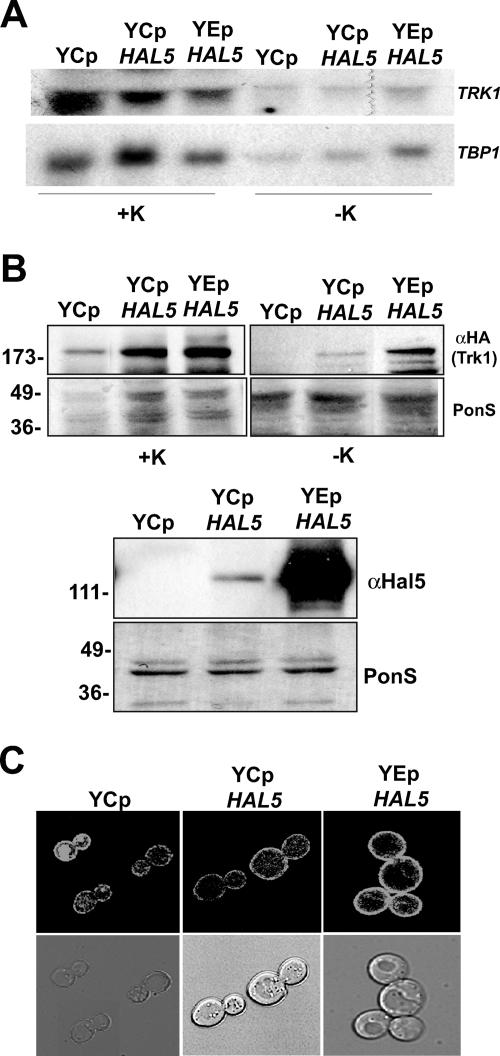
FIG. 3.
Overexpression of HAL5 leads to Trk1 accumulation at the plasma membrane. (A) Total RNA was extracted from the indicated strains grown in potassium-supplemented medium (+K) or incubated for 2.5 h in minimal medium not supplemented with KCl (−K). Northern analysis was performed to determine the levels of TRK1 mRNA using a specific probe corresponding to nucleotides 385 to 2319 (top panel). The membrane was reprobed with TBP1 as a loading control (bottom panel). (B) Western analysis of the insoluble protein fraction isolated from the same strains treated as described for panel A. The amounts of HA-tagged Trk1 (top panels) and Hal5 (bottom panels) are shown. The Ponceau S (PonS)-stained filter is shown as a control for protein loading in each case. (C) Confocal microscopy images of Trk1-GFP localization in the hal4 hal5 mutant strain transformed with either an empty plasmid or a centromeric (YCp) or multicopy (YEp) plasmid containing the HAL5 coding sequence under control of its own promoter.
In order to observe the subcellular localization of Trk1, we constructed a Trk1-GFP fusion protein in a centromeric plasmid. The function of this construct was confirmed by complementation of the LiCl sensitivity of the trk1 trk2 mutant (data not shown). Strains were grown to mid-log phase in minimal medium supplemented with 0.2 M KCl. This amount of KCl, although far above the physiologically relevant concentrations, is required for optimum growth of the hal4 hal5 mutant in minimal medium. As we were interested in studying the defect that occurs in the hal4 hal5 mutant under standard growth conditions, we analyzed the phenotypes of this mutant after incubation in unsupplemented medium. We observed the pattern of GFP fluorescence in live cells incubated in unsupplemented medium by confocal microscopy. As shown in Fig. 1A, Trk1-GFP is present at the plasma membrane in wild-type cells, as expected. However, in the hal4 hal5 mutant, less Trk1-GFP was observed at the plasma membrane, and a strong GFP signal was observed in the lumen of the vacuole in most cells. Vacuolar membranes were visualized using the styryl dye FM4-64 as previously described (42). This decrease in the amount of Trk1 at the plasma membrane explains the previously reported potassium dependence of the hal4 hal5 mutant, and the appearance of GFP signal in the lumen of the vacuole suggests that the transporter is targeted to the vacuole for degradation, as has been observed for other nutrient transporters (3, 7). We confirmed these results by Western analysis (Fig. 1B). We observed that the amount of full-length Trk1 protein present in the insoluble, membrane-associated fraction is markedly decreased in hal4 hal5 mutants after transfer to unsupplemented medium, compared to the wild type, confirming the rapid degradation of the Trk1 protein under these conditions. Interestingly, a considerable amount of Trk1 protein is present in cells grown in medium supplemented with potassium. Therefore, it appears that the Hal4 and Hal5 kinases play a more important role in Trk1 stability in medium with physiologically relevant potassium concentrations.
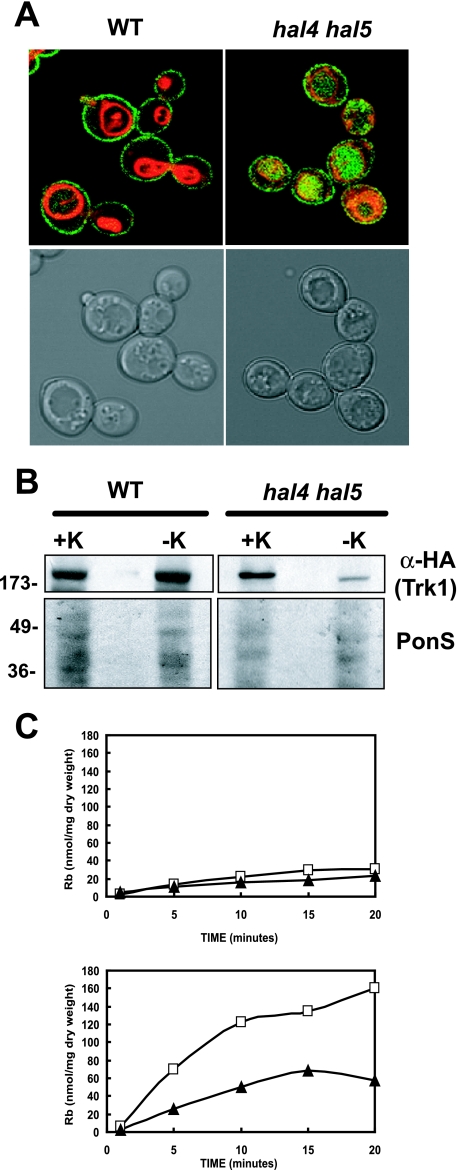
FIG. 1.
Trk1 is less stable in the hal4 hal5 mutant upon potassium starvation. (A) The wild-type (WT) strain and the hal4 hal5 mutant expressing a centromeric plasmid containing a TRK1-GFP fusion were grown to mid-log phase in potassium-supplemented medium and stained with the vacuolar dye FM4-64 as described in Materials and Methods. Gray scale and fluorescence overlays of representative confocal microscopy images depicting the localization of Trk1 (green) and the vacuolar membrane (red) are shown. (B) Western analysis of Trk1 levels in the membrane fractions of the wild-type and hal4 hal5 mutant strains maintained in potassium chloride-containing minimal medium (+K) or incubated in unsupplemented medium for 2 h (−K). Images of the Ponceau S-stained filters are shown in the bottom panels, as a control for protein loading. (C) The wild-type (□) and hal4 hal5 (▴) strains were grown to mid-log phase in minimal medium supplemented with potassium chloride and were analyzed for high affinity rubidium uptake (0.5 mM) immediately after washing (top panel) and after 2 hours of incubation in low-potassium medium (bottom panel). Similar results were observed using 50 mM RbCl.
We next analyzed the rubidium uptake activity under these experimental conditions. Cells were grown to mid-log phase in potassium-supplemented minimal medium, washed, and assayed for rubidium uptake. Under these conditions, virtually no rubidium uptake was observed initially, suggesting that the Trk1 transporter is not active. However, as expected, after 2 h of incubation in unsupplemented medium, a higher initial rate of rubidium uptake activity is observed in the wild-type strain than in the hal4 hal5 mutant (Fig. 1C). This result is in agreement with previously published data and the confocal and Western analyses presented here (28). This decrease in rubidium uptake is also reflected in the internal concentrations of potassium; after a 2-h incubation in medium without added potassium, we observed a modest decrease in the internal K+ concentration in the wild-type control strain (161 ± 11 mM versus 136 ± 7 mM), whereas in the hal4 hal5 mutant, the concentration of internal potassium decreased from wild-type levels in the presence of potassium supplementation (162 ± 18 mM) to 92 ± 8 mM after the 2-h incubation in unsupplemented medium, reflecting the defect in Trk1 activity due to the lack of transporter in the plasma membrane. The Trk1-independent potassium uptake is likely due to lower-affinity uptake systems, such as the NSC1 cation uptake activity, which appear to remain functional in the hal4 hal5 mutant (4).
The observed differences in Trk1 stability in the hal4 hal5 mutant are not due to changes in osmotic pressure caused by the removal of KCl.
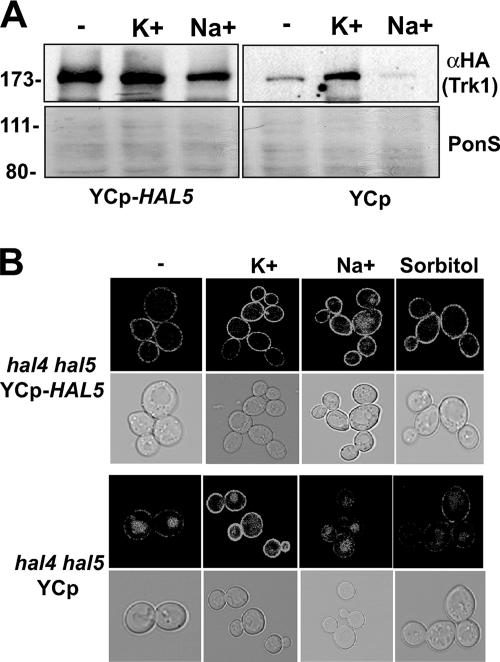
We next tested whether the observed instability of Trk1 in the hal4 hal5 mutant upon potassium starvation was due to the change in osmotic pressure, caused by removal of potassium from the medium, or an effect specific to potassium itself. We observed Trk1-GFP protein levels by Western analysis in strains grown in the presence of 0.2 M KCl and then transferred to unsupplemented minimal medium or to medium containing 0.2 M KCl or 0.2 M NaCl (Fig. 2A). In the hal4 hal5 mutant strain expressing the HAL5 gene from a centromeric plasmid, no changes in the Trk1-GFP protein levels are observed when the cells are incubated in the presence of potassium or sodium. As observed previously by Western analysis, considerable amounts of Trk1 are observed in the hal4 hal5 mutant when it is grown in the presence of excess potassium chloride (0.2 M). These protein levels are not maintained when potassium is replaced by sodium. These observations are further supported by confocal analysis of Trk1-GFP in the hal4 hal5 mutant strain compared to the complemented strain transformed with YCp-HAL5 after incubation in minimal medium or in medium supplemented with 0.2 M KCl, 0.2 M NaCl, or 0.3 M sorbitol (Fig. 2B). No changes in Trk1 distribution occur in the YCp-HAL5-containing complemented strain. In the hal4 hal5 mutant, Trk1-GFP accumulates in the vacuole in medium supplemented with 0.2 M NaCl or 0.3 M sorbitol, but Trk1-GFP is stable at the plasma membrane if maintained in 0.2 M KCl. These results suggest that the greater stability of Trk1 in the presence of 0.2 M KCl in the hal4 hal5 mutant is not due to changes in osmotic pressure caused by potassium removal, because when the osmotic pressure is maintained in the form of sodium or sorbitol, we observe the same destabilization of Trk1 as in unsupplemented medium. It is interesting to note that very little Trk1 activity (as measured by Rb+ uptake) was observed when cells were grown in the presence of a high external potassium concentration (Fig. 1C), suggesting that the transporter is not active under these conditions.
FIG. 2.
Vacuolar accumulation of Trk1 is not caused by changes in osmotic pressure during potassium starvation. (A) Western analysis of the insoluble proteins isolated from the indicated strains transformed with either an empty or a HAL5-containing centromeric plasmid incubated for 2 h in unsupplemented minimal medium (−) or medium supplemented with 0.2 M KCl or 0.2 M NaCl. The amount of Trk1 was analyzed using the anti-HA antibody (top panel), and the amount of protein present in each sample is shown in the Ponceau S (PonS)-stained filter (bottom panel). (B) Confocal microscopy images of Trk1-GFP localization in the hal4 hal5 mutant under the same experimental conditions, including an additional control using an osmotically equivalent amount of sorbitol (0.3 M).
Overexpression of HAL5 leads to Trk1 accumulation at the plasma membrane.
To examine the effect of HAL5 overexpression on TRK1 gene expression and Trk1 stability and localization, we employed the hal4 hal5 mutant containing either an empty plasmid, the centromeric plasmid containing the HAL5 open reading frame under the control of its own promoter described above, or an episomal plasmid carrying the same HAL5-containing genomic fragment. We compared the mRNA and protein expression of Trk1 in strains grown in potassium-supplemented medium or incubated for 2.5 h in unsupplemented medium. Northern analysis shows that no significant differences are observed in the amount of TRK1 mRNA in any of the experimental conditions tested (Fig. 3A). Therefore, we went on to examine the amount of Trk1 present in these same strains. We observed increased levels of Trk1 present in the insoluble fraction as the expression levels of Hal5 increased (Fig. 3B). This result was confirmed by observation of the fluorescence patterns of Trk1-GFP in these strains (Fig. 3C). These results suggest that as Hal5 levels increase, more Trk1 is present at the plasma membrane during incubation in standard minimal medium without potassium supplementation and that this regulation is likely due to posttranslational modification.
Other transporters accumulate in the vacuole in the hal4 hal5 mutant.
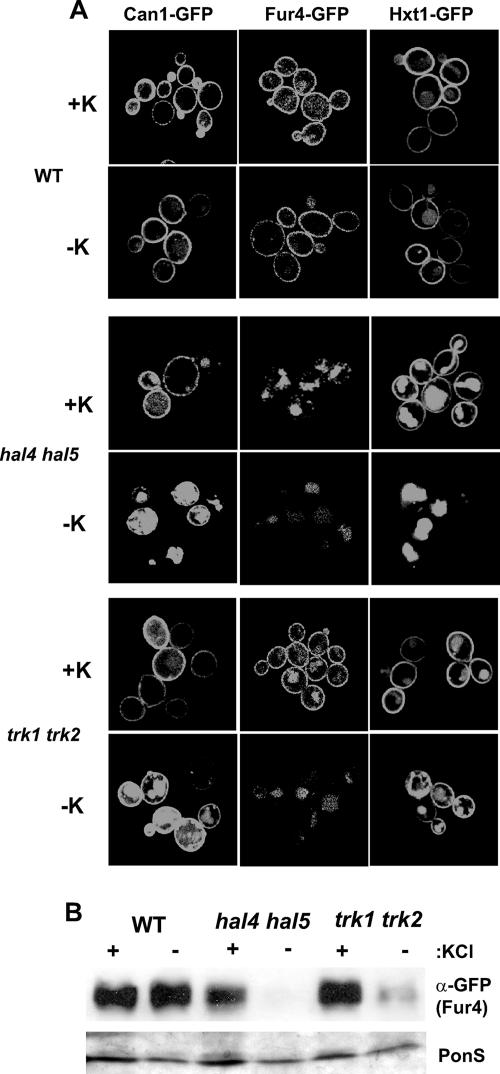
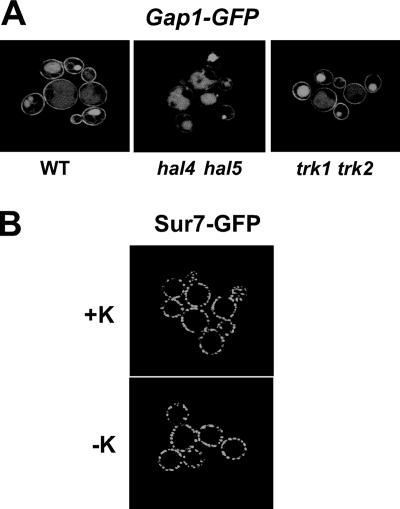
In order to test the specificity of the observed effects on Trk1 stability in hal4 hal5 strains incubated in medium without potassium supplementation, we examined the behavior of other nutrient transport proteins under the same conditions. As observed in Fig. 4A, the localization of three other nutrient transport proteins was also defective. The Can1 arginine permease, the Fur4 uracil permease, and the Hxt1 low-affinity glucose transporter were all present at the plasma membrane in wild-type strains regardless of the amount of potassium present, as expected (Fig. 4A, top panels). However, in the hal4 hal5 mutant, we observed a marked vacuolar accumulation of these GFP-fused permeases upon incubation in medium not supplemented with potassium (Fig. 4A, middle panels). Similar results were observed for Tat2 (data not shown). Moreover, in the case of Fur4 and Hxt1, this mislocalization was observed even in cells maintained in potassium-supplemented medium, suggesting a more general role for Hal4 and Hal5 in transporter trafficking.
FIG. 4.
Subcellular localization of nutrient transporters/permeases in the wild-type (WT), hal4 hal5, and trk1 trk2 strains. (A) The indicated strains were transformed with plasmids expressing the three different GFP fusion proteins, grown to mid-log phase in potassium-supplemented minimal medium, incubated for 2 h in medium supplemented or not with 0.2 M KCl, and analyzed by confocal microscopy. Representative images are shown. (B). Western analysis of insoluble proteins isolated from the indicated strains expressing the Fur4-GFP plasmid, treated as described for panel A. The anti-GFP antibody recognizes the Fur4-GFP fusion protein, and a portion of the Ponceau S (PonS)-stained filter is shown as a loading control.
We then wanted to test if the effects observed for nutrient transporter mislocalization in the hal4 hal5 mutant could be attributed specifically to these kinases or to the internal potassium depletion due to lack of Trk1 activity. Therefore, we examined the GFP fluorescence pattern of the various transporters in the trk1 trk2 mutant in the presence and absence of potassium supplementation. We observed similar, but not identical, fluorescence patterns for these GFP transporter fusion proteins in trk1 trk2 mutants (Fig. 4A, bottom panels). Specifically, we observed that upon potassium starvation, Can1, Fur4, and Hxt1 (to a lesser extent) appear to be less stable, as the GFP fluorescence was observed to accumulate in the lumen of the vacuole in the trk1 trk2 mutant. However, in the presence of potassium, the mislocalization of both Fur4 and especially Hxt1 was less apparent compared to those in the hal4 hal5 mutant. In order to confirm these results in a more quantitative assay, the amount of Fur4-GFP was analyzed in the same strains under the same experimental conditions. In agreement with the confocal images, less Fur4 was observed in the hal4 hal5 mutant, even in the presence of potassium supplementation, than in the wild-type strain, and these levels decreased drastically upon potassium withdrawal (Fig. 4B). Fur4 protein levels are comparable to those in the wild type in the trk1 trk2 mutant when grown in the presence of potassium supplementation. Upon potassium withdrawal, a decrease in Fur4 protein levels is observed in the trk1 trk2 strain, but the effect does not appear to be as dramatic as in the hal4 hal5 mutant. These results suggest that in the case of Fur4, both the Hal4/Hal5 kinases and internal potassium concentrations are important for its stability at the plasma membrane.
Having observed a general effect on all the nutrient transport proteins tested, we next examined the localization of two additional plasma membrane proteins. First, we examined the localization of Gap1-GFP. The trafficking of this general amino acid permease is known to be regulated by the quality of the nitrogen source and by the Npr1 protein kinase (7, 30). It has been previously reported that Gap1 is localized to the plasma membrane only when strains are grown in poor nitrogen sources, such as proline or urea. Therefore, we analyzed the localization of the Gap1-GFP protein in the wild-type, hal4 hal5, and trk1 trk2 strains grown to mid-log phase in a proline-based medium supplemented with potassium. As shown in Fig. 5A, under these conditions, some GFP is present in the vacuolar lumen; however, a considerable amount of Gap1-GFP can be observed in the plasma membrane in both the wild-type and trk1 trk2 mutant strains. In contrast, we observed less accumulation of Gap1-GFP in the plasma membrane in the hal4 hal5 mutant strain. Similar results were observed in medium containing urea as the sole nitrogen source (data not shown).
FIG. 5.
Subcellular localization of the Gap1 permease and the integral membrane protein, Sur7. (A) The subcellular localization of Gap1-GFP was analyzed in the indicated strains grown to mid-log phase in potassium-supplemented (50 mM) minimal medium with proline as the sole nitrogen source. Representative micrographs are shown. WT, wild type. (B) The subcellular localization of the eisosomal-associated integral membrane protein Sur7 in the hal4 hal5 mutant was analyzed by confocal microscopy after 2 h of incubation in minimal medium (without KCl). Identical Sur7-GFP patterns were observed in the wild-type strain and in the hal4 hal5 strain grown in the presence of KCl (data not shown).
Having observed a general defect in the plasma membrane stability of several transporters and permeases in the hal4 hal5 mutant, we analyzed a different class of plasma membrane proteins to rule out a general effect on plasma membrane structure. For this experiment, we examined the fluorescence pattern of the Sur7-GFP fusion protein in the hal4 hal5 mutant in the presence or absence of potassium supplementation. We chose this protein because it is an integral plasma membrane protein but not a transporter. Sur7 is localized to plasma membrane cortical actin patches associated with sites of endocytosis, called eisosomes (43). As shown in Fig. 5B, Sur7 is stable at the plasma membrane in the hal4 hal5 mutant 2 h after the removal of potassium supplementation. Identical localization patterns were observed in the wild-type strain (data not shown).
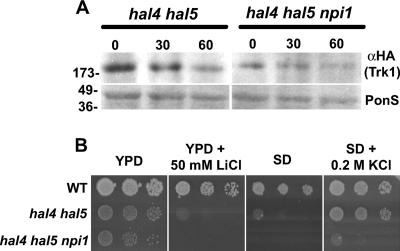
Based on previous results demonstrating a role for the Rsp5 ubiquitin ligase in the sorting and/or stability of many nutrient transporters in yeast, including Fur4, Can1, Tat2, and Gap1 (36), we tested whether the stability of Trk1 is subject to a similar regulatory mechanism. We analyzed the stability of Trk1 in hal4 hal5 mutants with decreased expression of Rsp5 by constructing a strain with a partial disruption of the RSP5 promoter (mimicking the npi1 mutant) and monitoring the Trk1 protein levels 30 and 60 min after potassium withdrawal (Fig. 6A). As observed, upon potassium withdrawal, the Trk1 protein levels markedly decrease in the hal4 hal5 mutant. In hal4 hal5 mutants with decreased expression of RSP5, we observed less accumulation of Trk1 at the plasma membrane in the hal4 hal5 npi1 strain grown in the presence of potassium, and the expression levels decreased slightly during the potassium starvation. Growth assays in solid medium were performed in order to determine whether the increased plasma membrane stability of various nutrient transporters due to the reduced expression of Rsp5 affected the growth phenotypes of the hal4 hal5 mutant. As shown in Fig. 6B, we observed a reduced growth rate for the hal4 hal5 npi1 mutant compared to the parental hal4 hal5 strain in all conditions tested, suggesting that reduction of Rsp5 expression levels is not able to improve the growth defects of the hal4 hal5 mutant. This reduced growth rate was most evident in minimal medium not supplemented with KCl, in agreement with the reduced levels of Trk1 protein observed in this mutant. However, the npi1 mutant has pleiotropic phenotypes, thus complicating further interpretation of these results.
FIG. 6.
Reduction of Rsp5 expression leads to a decrease in the amount of Trk1 in a hal4 hal5 strain and does not improve its growth defects. (A) Western analysis of Trk1 protein levels in the insoluble fraction isolated from the indicated strains. The amount of Trk1 protein was detected using anti-HA antibodies, and the Ponceau S (PonS)-stained filter is shown as a loading control. The time after potassium withdrawal (0, 30, and 60 min) is indicated. (B) The indicated strains were grown to saturation in selective minimal medium supplemented with potassium, serially diluted, and spotted onto the indicated solid media. Growth was recorded after 48 to 72 h of incubation at 28°C. WT, wild type.
Deletion of the last 35 amino acids of Trk1 leads to increased protein stability and toxic cation tolerance.
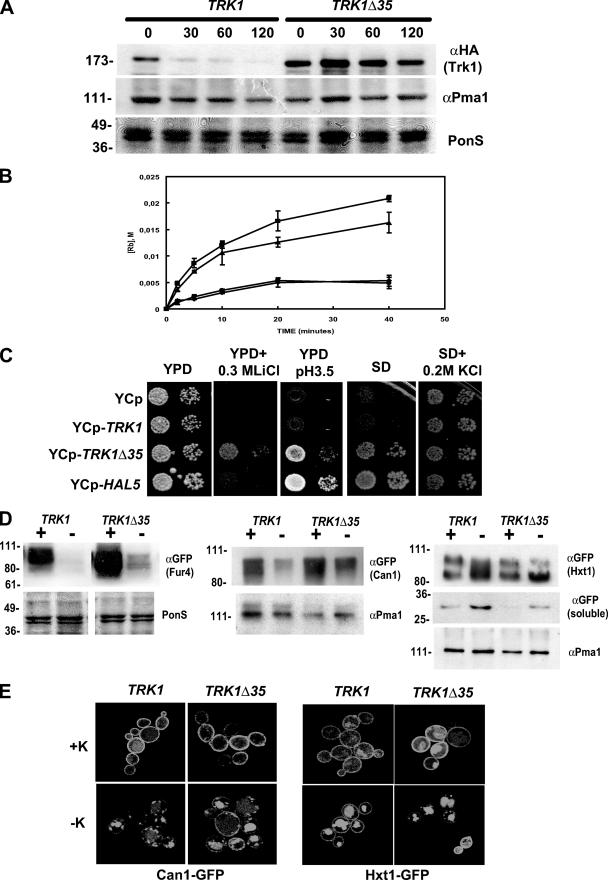
In order to begin to define the molecular mechanism of Hal4/Hal5-mediated Trk1 stability, we constructed a TRK1 deletion mutant lacking the nucleotides encoding the last 35 amino acids. We chose this region based on previous experiments done with Schizosaccharomyces pombe showing that only a COOH-terminal deletion mutant, and not the full-length Trk1 homologue, was able to restore the salt sensitivity of a strain lacking the HAL4 homologue (44). We postulated that this rescue phenotype could be due to an increased stability of the mutant form of the Trk1 homologue. We constructed a similar deletion of the Saccharomyces cerevisiae TRK1 gene and analyzed its stability in the hal4 hal5 mutant during potassium starvation. As observed in the Western analysis presented in Fig. 7A, the truncated version is much more stable than the full-length Trk1 in the hal4 hal5 mutant. We also determined the stability of the H+-ATPase Pma1 under these conditions, and we observed no significant changes in the levels of this protein (compare Pma1 amounts with the total amount of protein in the Ponceau S-stained filter). Thus, the subcellular localization and trafficking of Pma1 and Sur7 do not appear to be influenced by either potassium or the Hal4/Hal5 kinases.
FIG. 7.
Biochemical and phenotypic analysis of a truncated version of Trk1. (A) The hal4 hal5 strain was transformed with a centromeric plasmid containing either the full-length or the truncated version of TRK1 lacking the last 35 amino acids (Trk1Δ35). Strains were grown to mid-log phase in potassium-supplemented minimal medium and then transferred to unsupplemented minimal medium, samples were removed at the indicated times, and the amounts of Trk1 (top panel) and Pma1p (middle panel) present were analyzed by Western blotting. The Ponceau S (PonS)-stained filter is shown as a control for protein loading. (B) The indicated strains (hal4 hal5 mutant transformed with the empty vector [⧫], full-length TRK1 [•], TRK1Δ35 [▴], or HAL5 [▪]) were grown to mid-log phase in potassium-supplemented medium, washed, and incubated for 2 h in a low-potassium buffer. Rubidium uptake was measured by high-pressure liquid chromatography analysis, as described in Materials and Methods, using 5 mM RbCl. (C) The indicated strains were grown to saturation in potassium-supplemented minimal medium, serially diluted, and spotted onto the indicated plates. Growth was recorded after 48 to 72 h at 28°C. (D) The hal4 hal5 strains expressing the indicated fusion proteins were grown to mid-log phase in potassium-supplemented medium and then transferred to medium supplemented (+K) or not (−K) with 0.2 M KCl for 2 hours. Samples were processed for Western analysis as described in Materials and Methods and analyzed for the amount of Fur4-GFP (left panels), Can1-GFP (middle panels), or Hxt1-GFP (right panels). In the case of Hxt1, the appearance of free GFP in the soluble fraction was also analyzed [GFP (soluble)]. The Ponceau S (PonS)-stained filters or the amount of Pma1 is shown as a control for protein loading. (E) The hal4 hal5 strains expressing the indicated proteins were grown as described for panel D, and confocal microscopy images were generated. Representative micrographs are shown.
We also measured the rubidium uptake activity of the truncated version of Trk1 in the hal4 hal5 mutant. As shown in Fig. 7B, expression of either the empty vector or the wild-type version of TRK1 does not improve the defect in rubidium uptake observed for the hal4 hal5 mutant. However, the truncated version of Trk1 mediates rubidium uptake in a manner similar to that for the strain expressing a single copy of HAL5 under control of its own promoter. Therefore, under these experimental conditions, the truncated version of Trk1 restores the rubidium uptake of the hal4 hal5 mutant to nearly wild-type levels. Thus, the presence of HAL4 and HAL5 is not required for the activation of this truncated version of the Trk1 potassium transporter.
We next examined the growth phenotypes of strains expressing the mutated version of Trk1. The truncated version of Trk1, but not the full-length protein, is able not only to complement the salt and low-pH sensitivity of the hal4 hal5 mutant but also to confer tolerance to toxic LiCl concentrations in YPD medium (Fig. 7C). The truncated version of Trk1 also improves the growth of the hal4 hal5 mutant in minimal medium not supplemented with potassium. Similar results are observed in minimal medium containing urea as the sole nitrogen source, suggesting that both the defect in high-affinity potassium uptake observed in the hal4 hal5 mutant and the growth improvement conferred by the truncated Trk1 are independent of the quality of the nitrogen source (data not shown). However, the hal4 hal5 mutant expressing this more stable, active version of Trk1 does not grow as well as the wild-type strain in minimal medium, indicating that the rescue is only partial.
Since the truncated version of Trk1 appears active in the hal4 hal5 mutant, it provides an experimental system to begin to distinguish between the contributions of potassium and the Hal4 and Hal5 kinases in nutrient transporter stability. Accordingly, we began by analyzing the localization/stability of the Can1, Hxt1, and Fur4 transporters in hal4 hal5 mutants expressing either full-length TRK1 or the more stable, truncated version (Trk1Δ35) by either Western analysis (Fig. 7D) or confocal microscopy (Fig. 7E). We observed a similar defect in the localization/stability of these transporters upon removal of potassium supplementation in strains expressing Trk1Δ35, compared to the strains expressing full-length Trk1, suggesting that Hal4 and Hal5 may influence transporter localization and/or stability independently of Trk1. In the case of both Fur4 and Can1, a marked decrease in the amount of protein is observed after 2 h of incubation in medium not supplemented with KCl. In the case of Hxt1, the amount of full-length protein decreases, while the amount of an apparent product of protein degradation increases. In order to confirm this interpretation, we analyzed the amount of free GFP present in the soluble fraction. As expected, as the amount of full-length Hxt1-GFP decreases in the insoluble fractions, the amount of free GFP increases in the soluble fraction, likely corresponding to the signal observed in the lumen of the vacuole.
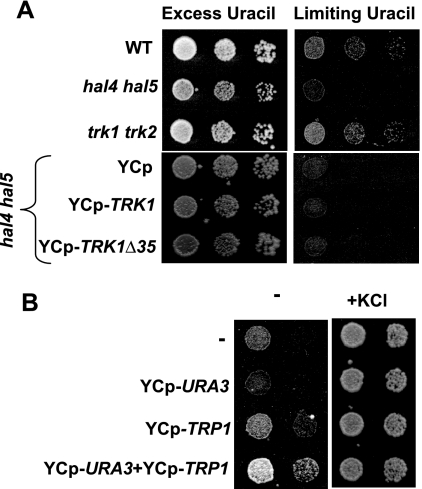
In order to investigate the possibility that the hal4 hal5 mutant shows reduced uptake of other nutrients, in addition to potassium, we assayed the growth of the hal4 hal5 strain in minimal medium supplemented with 0.1 M KCl and either excess uracil (300 μg/ml) or limiting amounts of uracil (1.2 μg/ml). As observed in Fig. 8A, the hal4 hal5 mutant, but not the trk1 trk2 mutant, is not able to grow in limiting amounts of uracil, suggesting that high-affinity uracil uptake is defective in this mutant. These results are consistent with the confocal microscopy, where we observed a defective localization of the Fur4 transporter in the hal4 hal5 mutant (but not the trk1 trk2 mutant) even in the presence of potassium supplementation. In addition, we observed that expression of the truncated version of Trk1 is not able to improve growth under these conditions, as expected from the results of the Western analysis of Fur4 stability (Fig. 7D).
FIG. 8.
Growth requirements of the hal4 hal5 mutant. (A) The indicated strains were grown to saturation in potassium-supplemented minimal medium, and serial dilutions were spotted onto plates containing minimal medium supplemented with 0.1 M KCl and either 300 μg/ml (excess) or 1.2 μg/ml (limiting) of uracil. Growth was recorded after 48 to 72 h. WT, wild type. (B) The hal4 hal5 strain was transformed with the indicated plasmids and grown to saturation in potassium-supplemented medium, and serial dilutions were spotted onto minimal medium plates containing uracil, tryptophan, and adenine (to complement auxotrophies of the nontransformed or singly transformed strains) without (−) or with (+KCl) 0.1 M KCl. Growth was recorded after 72 to 96 h.
We further corroborated these results suggesting that defects in nutrient uptake may contribute to the hal4 hal5 slow-growth phenotype by complementing the biosynthetic pathways for both uracil and tryptophan with centromeric plasmids. We postulated that if these nutrient transporters are less stable in the hal4 hal5 mutant, as observed by confocal microscopy, then complementation of the biosynthetic routes should improve the growth of this strain in medium not supplemented with potassium. As shown in Fig. 8B, we observed a slight improvement in the growth of the hal4 hal5 mutant transformed with both URA3- and TRP1-containing centromeric plasmids, compared to the control strains carrying only one plasmid or no plasmid at all, after 5 days of growth. It is important to note that these growth assays were performed in the presence of exogenous uracil and tryptophan and suggest that the hal4 hal5 mutant is defective in uracil and tryptophan uptake from the external medium.
DISCUSSION
Here, we describe a novel role for the Hal4 and Hal5 kinases in the regulation of the stability of a diverse subset of plasma membrane nutrient transporters. The Trk1 high-affinity potassium transporter is rapidly degraded in the hal4 hal5 mutant strain upon removal of potassium supplementation, whereas overexpression of the HAL5 gene results in increased accumulation of the Trk1 protein at the plasma membrane. These results explain both the potassium dependency of the hal4 hal5 strain and the mechanism of salt tolerance observed upon HAL5 overexpression. Furthermore, we show that although the hal4 hal5 mutant exhibits no defect in the overall structure of the plasma membrane during potassium withdrawal (as documented by the Sur7-GFP localization), the stability of other nutrient permeases undergoes a similar decrease. These data suggest that the Hal4 and Hal5 kinases perform a function analogous to that proposed for the Npr1 kinase (7). However, the physiological conditions under which these kinases are important appear to be different. The Hal4 and Hal5 kinases are required for growth in medium containing ammonia as the nitrogen source and physiologically relevant potassium concentrations, whereas the activity of the Npr1 kinase is important during nitrogen starvation. Many other plasma membrane proteins are known to be routed to the vacuole for degradation by an ubiquitylation-dependent mechanism. However, in these cases, nutrient starvation, rapamycin treatment, or substrate-mediated down-regulation are involved (reviewed in reference 20). Here, we present evidence that the Hal4 and Hal5 kinases are involved in modulating the trafficking of at least a subset of plasma membrane proteins under standard growth conditions.
Genetic evidence suggests a complex regulation of the Trk1 transporter. Work from several groups has identified protein kinases (such as Hal4, Hal5, and Sky1), protein phosphatases (such as Ppz1, Ppz2, and calcineurin), G proteins (such as Arl1), ion transport proteins (such as Nha1), and proteins of unknown function (such as Hal1) in Trk1-mediated potassium transport (2, 11, 27-29, 33, 45). However, in most cases, the mechanism of regulation is unknown. We previously reported that inactivation of two genes encoding type 1-like protein phosphatases, Ppz1 and Ppz1, increases the levels of phosphor ylation of Trk1 and leads to potassium accumulation. However, Trk1 protein stability and/or subcellular localization is not altered in the ppz1 ppz2 mutant strain (reference 45 and data not shown). Similarly, we observed no marked effect on Trk1-GFP protein levels or subcellular distribution in strains lacking SKY1, CNB1, ARL1, NHA1, HAL1, HOG1, BUL1, or BUL2 (data not shown). We tested HOG1 based on its reported role in the regulation of ion transporters, such as Nha1 and Tok1, during osmotic stress, (31). The BUL1 and BUL2 genes encode proteins which are ubiquitin-binding components of the Rsp5 E3-ubiquitin ligase complex and are proposed to act as E4 enzymes important for the multiubiquitylation of specific substrates (19). Rsp5-mediated ubiquitylation has been established as a key step in the regulation of intracellular trafficking of many plasma membrane proteins, such as the Gap1 amino acid permease (40). Indeed, we observed that Trk1 delivery to and/or stability at the plasma membrane also appears to be regulated by Rsp5; the amount of Trk1 protein present at the plasma membrane in hal4 hal5 strains with reduced expression of Rsp5 (hal4 hal5 npi1) is markedly decreased. Moreover, we observed that reduction of Rsp5 expression, which leads to the stabilization of some plasma membrane transport proteins, does not improve the growth defects observed for the hal4 hal5 strain.
In addition, we have provided evidence showing that the destabilization of Trk1 and Can1 at the plasma membrane of the hal4 hal5 mutant is not observed in medium supplemented with a high K+ concentration, whereas especially Fur4 and Gap1 and to a lesser extent Hxt1 do not accumulate at the plasma membrane in the hal4 hal5 mutant even in medium supplemented with potassium. Therefore, these data are consistent with a novel mechanism of regulation of the turnover of a subset of nutrient transporters influenced by both protein kinases and intracellular K+ levels. However, until the molecular details of this regulation are elucidated, we cannot rule out an indirect, stress-related effect in the case of Fur4, for example.
Analysis of the stability and function of the truncated version of Trk1 may provide clues for this novel mechanism. We observed that despite its increased stability and potassium uptake activity in the absence of Hal4 and Hal5, expression of this truncated version of Trk1 is not able to ameliorate many of the defects observed in the hal4 hal5 mutant, including destabilization of the Fur4, Can1, and Hxt1 nutrient transporters. These results suggest that, in addition to the regulation of the Trk1 potassium transporter, the Hal4 and Hal5 kinases may play a more general role in the regulation of the stability and/or trafficking of other nutrient transporters.
The last 35 amino acids of Trk1 contain 10 potential phosphorylation sites (S or T) and five lysine residues that could be ubiquitylated to regulate its delivery to the plasma membrane or its degradation via the vacuole. Detailed mutagenesis of this complex region will identify the residues implicated in this regulation. Our working model to explain the observed results contends that the last 35 amino acids contain regulatory sequences involved in the normal cycling and/or turnover of the transporter and that when they are deleted, the equilibrium is shifted towards accumulation at the plasma membrane. This model suggests that the regulation of transporter activity plays a less prominent role under our experimental conditions and that Hal4 and Hal5, or high K+ levels, are not necessary for this activation per se but are required to maintain Trk1 levels, possibly by impeding posttranslational modifications, such as ubiquitylation, that lead to vacuolar sorting. Both the stability and activity of the truncated version of Trk1 in the hal4 hal5 mutant and the increased stability of the Trk1 transporter upon overexpression of HAL5 are consistent with this model.
We previously reported that the in vivo phosphorylation of Trk1 increases upon HAL5 overexpression (45). However, in this case, the increase is likely to be due to the increase in protein levels. We have not observed Trk1 phosphorylation by Hal5 in vitro in the case of the full-length protein or a GST fusion containing the last 68 amino acids. Therefore, at this time, we cannot conclude whether the Hal4/Hal5-mediated regulation of Trk1 is direct or indirect. A recent report demonstrates that a Hal4/Hal5-related kinase, Ptk2, present in purified plasma membrane-containing fractions can phosphorylate Ser 899 in the COOH terminus of Pma1 expressed as a GST fusion protein in vitro (10). In the case of Npr1-mediated regulation of the plasma membrane amino acid permease Gap1 or the transcription factor Gln3, direct phosphorylation of these substrates has not been demonstrated. Indeed, in both cases, Npr1 is not strictly necessary for substrate phosphorylation (6, 7). In this respect, it is interesting to note that the Hal4 and Hal5 kinases also appear to play a role in Gap1 stability. Npr1 has been proposed to antagonize the activity of the Rsp5-Bul1/Bul2 E3/E4 ubiquitin ligase complex in the case of Gln3 nuclear translocation, but the molecular mechanism is still unknown (6). The fact that Gap1 is known to be regulated by the same ubiquitin ligase complex suggests that Npr1 may influence the activity of the Rsp5-Bul1/Bul2 complex. Two other nutrient transporters shown here to become destabilized in the hal4 hal5 mutant, Fur4 and Can1, are know to be ubiquitylated in an Rsp5-dependent manner, although a role for Bul1 and Bul2 has not been reported in these cases. Here, we observed a decrease in the amount of Trk1 present at the plasma membrane in hal4 hal5 strains with reduced RSP5 expression and that the amount of transporter decreases slightly during potassium starvation. Therefore, it appears that Rsp5-dependent ubiquitylation may influence the plasma membrane delivery of this high-affinity potassium transporter.
In this report, we have identified the mechanism by which the Hal4 and Hal5 kinases regulate Trk1. However, future work is needed to identify the specific residues implicated in the regulation of this complex region of the Trk1 potassium transporter. In addition, we show that Hal4 and Hal5 activity influences the stability of other nutrient transporters, thus providing a more general view of the function of this family of kinases and demonstrating that their activity may be required under distinct physiological conditions. Future experiments will determine whether the Hal4 and Hal5 kinases act by directly phosphorylating plasma membrane transport proteins, as suggested for Ptk2-dependent regulation of Pma1, or whether they antagonize an ubiquitin ligase complex, as proposed for Npr1. In the case of Trk1, the fact that the protein kinases can be replaced by a high K+ level suggests that, under normal growth conditions (high internal K+ level), phosphor ylation of Trk1 may not be required for stability.
Acknowledgments
This work was supported by grant BMC2002-04011-C05-02 from the Spanish Ministry of Education and Science (Madrid). L. Yenush was supported by the Ramón y Cajal program (Spanish Ministry of Science and Technology). S. Merchan and J. Pérez-Valle are supported by predoctoral fellowships from the Spanish Ministry of Science and Technology. S. Sharma was supported by a Biotech Overseas Fellowship from the Department of Biotechnology, Panjab University, Government of India, New Delhi.
We thank J. M. Mulet for providing crude anti-Hal5 antisera and Widmar Tanner and Bruno André for providing plasmids.
Footnotes
Published ahead of print on 4 June 2007.
REFERENCES
- 1.Bagnat, M., S. Keranen, A. Shevchenko, A. Shevchenko, and K. Simons. 2000. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 97:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banuelos, M. A., M. C. Ruiz, A. Jimenez, J. L. Souciet, S. Potier, and J. Ramos. 2002. Role of the Nha1 antiporter in regulating K(+) influx in Saccharomyces cerevisiae. Yeast 19:9-15. [DOI] [PubMed] [Google Scholar]
- 3.Beck, T., A. Schmidt, and M. N. Hall. 1999. Starvation induces vacuolar targeting and degradation of the tryptophan permease in yeast. J. Cell Biol. 146:1227-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bihler, H., C. L. Slayman, and A. Bertl. 2002. Low-affinity potassium uptake by Saccharomyces cerevisiae is mediated by NSC1, a calcium-blocked non-specific cation channel. Biochim. Biophys. Acta 1558:109-118. [DOI] [PubMed] [Google Scholar]
- 5.Borst-Pauwels, G. W. 1981. Ion transport in yeast. Biochim. Biophys. Acta 650:88-127. [DOI] [PubMed] [Google Scholar]
- 6.Crespo, J. L., S. B. Helliwell, C. Wiederkehr, P. Demougin, B. Fowler, M. Primig, and M. N. Hall. 2004. NPR1 kinase and RSP5-BUL1/2 ubiquitin ligase control GLN3-dependent transcription in Saccharomyces cerevisiae. J. Biol. Chem. 279:37512-37517. [DOI] [PubMed] [Google Scholar]
- 7.De Craene, J. O., O. Soetens, and B. Andre. 2001. The Npr1 kinase controls biosynthetic and endocytic sorting of the yeast Gap1 permease. J. Biol. Chem. 276:43939-43948. [DOI] [PubMed] [Google Scholar]
- 8.de Nadal, E., J. Clotet, F. Posas, R. Serrano, N. Gomez, and J. Arino. 1998. The yeast halotolerance determinant Hal3p is an inhibitory subunit of the Ppz1p Ser/Thr protein phosphatase. Proc. Natl. Acad. Sci. USA 95:7357-7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durell, S. R., and H. R. Guy. 1999. Structural models of the KtrB, TrkH, and Trk1,2 symporters based on the structure of the KcsA K(+) channel. Biophys. J. 77:789-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eraso, P., M. J. Mazon, and F. Portillo. 2006. Yeast protein kinase Ptk2 localizes at the plasma membrane and phosphorylates in vitro the C-terminal peptide of the H+-ATPase. Biochim. Biophys. Acta 1758:164-170. [DOI] [PubMed] [Google Scholar]
- 11.Forment, J., J. M. Mulet, O. Vicente, and R. Serrano. 2002. The yeast SR protein kinase Sky1p modulates salt tolerance, membrane potential and the Trk1,2 potassium transporter. Biochim. Biophys. Acta 1565:36-40. [DOI] [PubMed] [Google Scholar]
- 12.Gaber, R. F., C. A. Styles, and G. R. Fink. 1988. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol. Cell. Biol. 8:2848-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goossens, A., N. de La Fuente, J. Forment, R. Serrano, and F. Portillo. 2000. Regulation of yeast H+-ATPase by protein kinases belonging to a family dedicated to activation of plasma membrane transporters. Mol. Cell. Biol. 20:7654-7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grenson, M. 1983. Study of the positive control of the general amino-acid permease and other ammonia-sensitive uptake systems by the product of the NPR1 gene in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 133:141-144. [DOI] [PubMed] [Google Scholar]
- 15.Grossmann, G., M. Opekarova, J. Malinsky, I. Weig-Meckl, and W. Tanner. 2007. Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast. EMBO J. 26:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hein, C., J. Y. Springael, C. Volland, R. Haguenauer-Tsapis, and B. Andre. 1995. NPl1, an essential yeast gene involved in induced degradation of Gap1 and Fur4 permeases, encodes the Rsp5 ubiquitin-protein ligase. Mol. Microbiol. 18:77-87. [DOI] [PubMed] [Google Scholar]
- 17.Hill, J. E., A. M. Myers, T. J. Koerner, and A. Tzagoloff. 1986. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast 2:163-167. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman, J. F. 1964. The cellular functions of membrane transport. Prentice-Hall Inc., Englewood Cliffs, NJ.
- 19.Hoppe, T. 2005. Multiubiquitylation by E4 enzymes: ‘one size’ doesn't fit all. Trends Biochem. Sci. 30:183-187. [DOI] [PubMed] [Google Scholar]
- 20.Horak, J. 2003. The role of ubiquitin in down-regulation and intracellular sorting of membrane proteins: insights from yeast. Biochim. Biophys. Acta 1614:139-155. [DOI] [PubMed] [Google Scholar]
- 21.Hunter, T., and G. D. Plowman. 1997. The protein kinases of budding yeast: six score and more. Trends Biochem. Sci. 22:18-22. [DOI] [PubMed] [Google Scholar]
- 22.Ikonen, E. 2001. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 13:470-477. [DOI] [PubMed] [Google Scholar]
- 23.Kaouass, M., M. Audette, D. Ramotar, S. Verma, D. De Montigny, I. Gamache, K. Torossian, and R. Poulin. 1997. The STK2 gene, which encodes a putative Ser/Thr protein kinase, is required for high-affinity spermidine transport in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:2994-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko, C. H., and R. F. Gaber. 1991. TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:4266-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lillie, S. H., and S. S. Brown. 1987. Artifactual immunofluorescent labelling in yeast, demonstrated by affinity purification of antibody. Yeast 3:63-70. [DOI] [PubMed] [Google Scholar]
- 26.Malinska, K., J. Malinsky, M. Opekarova, and W. Tanner. 2003. Visualization of protein compartmentation within the plasma membrane of living yeast cells. Mol. Biol. Cell 14:4427-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendoza, I., F. Rubio, A. Rodriguez-Navarro, and J. M. Pardo. 1994. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 269:8792-8796. [PubMed] [Google Scholar]
- 28.Mulet, J. M., M. P. Leube, S. J. Kron, G. Rios, G. R. Fink, and R. Serrano. 1999. A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter. Mol. Cell. Biol. 19:3328-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munson, A. M., D. H. Haydon, S. L. Love, G. L. Fell, V. R. Palanivel, and A. G. Rosenwald. 2004. Yeast ARL1 encodes a regulator of K+ influx. J. Cell Sci. 117:2309-2320. [DOI] [PubMed] [Google Scholar]
- 30.Nikko, E., A. M. Marini, and B. Andre. 2003. Permease recycling and ubiquitination status reveal a particular role for Bro1 in the multivesicular body pathway. J. Biol. Chem. 278:50732-50743. [DOI] [PubMed] [Google Scholar]
- 31.Proft, M., and K. Struhl. 2004. MAP kinase-mediated stress relief that precedes and regulates the timing of transcriptional induction. Cell 118:351-361. [DOI] [PubMed] [Google Scholar]
- 32.Rajendran, L., and K. Simons. 2005. Lipid rafts and membrane dynamics. J. Cell Sci. 118:1099-1102. [DOI] [PubMed] [Google Scholar]
- 33.Rios, G., A. Ferrando, and R. Serrano. 1997. Mechanisms of salt tolerance conferred by overexpression of the HAL1 gene in Saccharomyces cerevisiae. Yeast 13:515-528. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Navarro, A. 2000. Potassium transport in fungi and plants. Biochim. Biophys. Acta 1469:1-30. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Navarro, A., and J. Ramos. 1984. Dual system for potassium transport in Saccharomyces cerevisiae. J. Bacteriol. 159:940-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotin, D., O. Staub, and R. Haguenauer-Tsapis. 2000. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J. Membr. Biol. 176:1-17. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt, A., T. Beck, A. Koller, J. Kunz, and M. N. Hall. 1998. The TOR nutrient signalling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J. 17:6924-6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serrano, R., B. C. Monk, J. M. Villalba, C. Montesinos, and E. W. Weiler. 1993. Epitope mapping and accessibility of immunodominant regions of yeast plasma membrane H(+)-ATPase. Eur. J. Biochem. 212:737-744. [DOI] [PubMed] [Google Scholar]
- 39.Serrano, R., J. M. Mulet, G. Rios, J. A. Marquez, I. F. de Larrinoa, M. P. Leube, I. Mendizabal, A. Pascual-Ahuir, M. Proft, R. Ros, and Montesinos. 1999. A glimpse of the mechanisms of ion homeostasis during salt stress in plants. J. Exp. Bot. 50:1023-1036. [Google Scholar]
- 40.Soetens, O., J. O. De Craene, and B. Andre. 2001. Ubiquitin is required for sorting to the vacuole of the yeast general amino acid permease, Gap1. J. Biol. Chem. 276:43949-43957. [DOI] [PubMed] [Google Scholar]
- 41.Vandenbol, M., J. C. Jauniaux, and M. Grenson. 1990. The Saccharomyces cerevisiae NPR1 gene required for the activity of ammonia-sensitive amino acid permeases encodes a protein kinase homologue. Mol. Gen. Genet. 222:393-399. [DOI] [PubMed] [Google Scholar]
- 42.Vida, T. A., and S. D. Emr. 1995. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128:779-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walther, T. C., J. H. Brickner, P. S. Aguilar, S. Bernales, C. Pantoja, and P. Walter. 2006. Eisosomes mark static sites of endocytosis. Nature 439:998-1003. [DOI] [PubMed] [Google Scholar]
- 44.Wang, L. Y., K. Shimada, M. Morishita, and K. Shiozaki. 2005. Response of fission yeast to toxic cations involves cooperative action of the stress-activated protein kinase Spc1/Sty1 and the Hal4 protein kinase. Mol. Cell. Biol. 25:3945-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yenush, L., S. Merchan, J. Holmes, and R. Serrano. 2005. pH-responsive, posttranslational regulation of the Trk1 potassium transporter by the type 1-related Ppz1 phosphatase. Mol. Cell. Biol. 25:8683-8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yenush, L., J. M. Mulet, J. Arino, and R. Serrano. 2002. The Ppz protein phosphatases are key regulators of K+ and pH homeostasis: implications for salt tolerance, cell wall integrity and cell cycle progression. EMBO J. 21:920-929. [DOI] [PMC free article] [PubMed] [Google Scholar]