Abstract
Nerve growth factor (NGF) acts through its receptor, TrkA, to elicit the neuronal differentiation of PC12 cells through the action of extracellular signal-regulated kinase 1 (ERK1) and ERK2. Upon NGF binding, TrkA translocates and concentrates in cholesterol-rich membrane microdomains or lipid rafts, facilitating formation of receptor-associated signaling complexes, activation of downstream signaling pathways, and internalization into endosomes. We have investigated the mechanisms responsible for the localization of TrkA within lipid rafts and its ability to activate ERK1 and ERK2. We report that NGF treatment results in the translocation of activated forms of TrkA to lipid rafts, and this localization is important for efficient activation of the ERKs. TrkA is recruited and retained within lipid rafts through its association with flotillin, an intrinsic constituent of these membrane microdomains, via the adapter protein, c-Cbl associated protein (CAP). Mutant forms of CAP that lack protein interaction domains block TrkA localization to lipid rafts and attenuate ERK activation. Importantly, suppression of endogenous CAP expression inhibited NGF-stimulated neurite outgrowth from primary dorsal root ganglion neurons. These data provide a mechanism for the lipid raft localization of TrkA and establish the importance of the CAP adaptor protein for NGF activation of the ERKs and neuronal differentiation.
Nerve Growth factor (NGF) is responsible for the development and survival of sympathetic and sensory neurons (17, 56) and plays an important role in neuronal plasticity in the central nervous system (11, 61). PC12 cells have been extensively employed to examine the mechanisms subserving the NGF-stimulated neuronal differentiation of these cells. A number of studies have demonstrated that the activation of the extracellular signal-regulated kinase (ERK) subfamily of mitogen-activated protein kinases (MAPKs) are responsible for directing the morphological and biochemical differentiation of these cells into a neuronal phenotype (12, 22, 33, 34, 42). NGF initiates its actions upon binding to its specific receptor, TrkA, which dimerizes and becomes autophosphorylated through its intrinsic tyrosine kinase activity. TrkA activation results in the association of a number of adapter proteins with the receptor, including those necessary for activation of the ERK MAPK cascade (28, 55, 69). NGF stimulates ERK activation via the recruitment and activation of the guanine nucleotide exchange factors SOS and C3G, which catalyze the exchange of GDP for GTP, activating the small G proteins Ras and Rap1, respectively. Ras and Rap1 then activate the MEK kinases, c-Raf and B-Raf, which in turn activate MEK-1 and -2. MEK-1 and -2 stimulate the activation of ERK1 and ERK2 (ERK1/2). NGF activation of the ERKs occurs predominately through a pathway involving the B-Raf isoform, with c-Raf playing a minor role (21). B-Raf is activated almost exclusively through Rap1 (70), leading to the prolonged stimulation of the ERKs due to the formation of a stable signaling complex involving the adaptor protein FRS2 (21). Persistent ERK activation results in its nuclear translocation, the activation of transcription factors, and induction of neural-specific gene expression leading to the neuronal differentiation of PC12 cells (33).
One of the central questions in understanding the initial steps in TrkA signal transduction is how signaling complexes are assembled following ligand binding. It has been appreciated that many molecules involved in the NGF signaling cascade, including TrkA, become localized to membrane subdomains, often referred to as lipid rafts. These membrane microdomains are proposed to facilitate signaling events by concentrating signal transducing elements and targeting them for internalization and incorporation into endocytic vesicles (41, 47). While the exact structure of lipid rafts is currently under debate (9, 10, 38), they have been characterized as cholesterol-enriched regions of the plasma membrane (16) that possess a high concentration of glycosphingolipids. Lipid rafts exhibit low buoyant density, resistance to solubilization by nonionic detergents (2, 44, 59, 62, 66), and in neurons the presence of the marker protein flotillin.
Flotillin-containing lipid rafts are enriched in many proteins essential to neuronal signaling cascades (16, 52, 67), and their constituents are targeted for endocytosis through both clathrin-dependent and -independent mechanisms (5, 26, 27, 31). In neurons these endosomes and their associated signaling complexes are then retrogradely trafficked from the nerve ending to the cell body (14, 15). These membrane microdomains appear to be the locus for the initiation of NGF signaling pathways. In order to effectively participate in intracellular signaling, these rafts must be dynamic in composition, allowing for the stimulation-dependent movement of molecules into and out of membrane domains (41, 63, 64), facilitating their functional interactions (31). Indeed, Howe and colleagues have shown that phosphorylated forms of TrkA are found in lipid rafts and recruited into clathrin-containing vesicles. Phosphorylated TrkA is localized to lipid raft membranes, and the binding of TrkA to its downstream effectors, Shc and phospholipase C, occurs within these domains (16). Suzuki et al. have recently reported that TrkB is recruited into lipid rafts in primary cortical neurons upon brain-derived neurotrophic factor (BDNF) binding (63). These data suggest that TrkA can be translocated to and retained within membrane microdomains that are targeted for internalization into endosomes.
We sought to determine the molecular mechanisms by which NGF receptors and signaling elements are localized and retained within lipid rafts. The c-Cbl-associated protein (CAP) is an adaptor protein that has been described as a lipid raft targeting molecule (4, 23, 50, 51). CAP contains a sorbin homology (SoHo) domain which specifically binds to flotillin in lipid rafts (23). Adaptor proteins containing this domain have been shown to bind flotillin and act to anchor signaling molecules within cholesterol-rich microdomains (4). CAP also has three C-terminal SH3 domains which can bind to proline-rich sequences in signaling effectors. Significantly, CAP has been found to associate with the TrkA-interacting adapter protein, APS (1, 30). APS is an adaptor protein that physically associates with activated Trk receptors by binding phosphotyrosine residues within their catalytic domain following NGF stimulation (49). APS exists in the cell as a pentamer and has been suggested to enhance TrkA phosphorylation and signaling by facilitating TrkA clustering and dimerization (48).
We report that following NGF stimulation, TrkA is concentrated within the lipid raft fraction of the plasma membrane owing to the adapter function of CAP which links TrkA-containing complexes to flotillin. Significantly, when the SoHo domain of CAP is deleted, TrkA and SOS do not undergo NGF-stimulated association with lipid rafts. This mislocalization of essential signaling molecules in the MAPK signaling pathway reduces TrkA phosphorylation, inhibits the activation of Rap1, and attenuates ERK activation. These data provide a mechanistic explanation of how, upon NGF binding, TrkA is incorporated into lipid rafts, thus focally concentrating the receptor in membrane microdomains targeted for internalization and facilitating its interaction with downstream effectors.
MATERIALS AND METHODS
Reagents.
Mouse beta-NGF was purchased from Austral Biologicals (San Ramon, CA). Anti-FLAG antibodies were purchased from Sigma (St. Louis, MO). Anti-phospho-ERK and anti-Myc-tag antibodies were purchased from Cell Signaling Technology (Beverly, MA). Antibodies to CAP, ERK2, Trk, Cbl, SOS, Rap1, FRS2, and flotillin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-pan-Ras antibody was purchased from Calbiochem (La Jolla, CA). The monoclonal antiflotillin and anti-phospho-TrkA antibodies were purchased from BD Biosciences (Palo Alto, CA). The AlexaFluor secondary antibodies were purchased from Invitrogen (Carlsbad, CA).
Cell culture.
PC12 cells were grown in Dulbecco's modified Eagle's Medium (DMEM) with 10% horse serum and 5% fetal bovine serum (FBS) in 10% CO2. MG139-2 cells, which are 3T3 cells that constitutively express TrkA (32), were a gift of Philip Barker (McGill University).
Cell transfection.
FLAG-CAP, FLAG-CAPΔSoHo (where CAPΔSoHo is a CAP mutant lacking the SoHo domain), and FLAG-CAPΔSH3 constructs were a gift of Alan Saltiel (University of Michigan). Myc-APS constructs were a gift of David Ginty (Johns Hopkins University). PC12 cells were plated at 70% confluence in 100-mm dishes and transfected with the CAP constructs using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Protein transduction.
The CAPΔSoHo construct was subcloned into the pET 3B vector (Stratagene, La Jolla, CA). Escherichia coli BL21(DE3) cells were transformed with this construct. Protein expression was induced by isopropyl-β-d-thiogalactopyranoside, and protein was purified by Anti-Flag M2 Affinity Gel (Sigma, St. Louis, MO) according to the manufacturer's instructions. Purified protein was transduced into PC12 cells in six-well plates at 50% confluence by using the Chariot protein delivery system (Active Motif, Carlsbad, CA) according to the manufacturer's instructions.
NGF stimulation and lysate preparation.
Cells were starved overnight in DMEM with 0.5% FBS and then collected and resuspended in solution with stimulation medium (DMEM with 5 mM HEPES and 0.1% bovine serum albumin [BSA]) in Eppendorf tubes. Cells were incubated in the absence or presence of 100 ng/ml NGF for the times indicated in each figure. Cells were rinsed with cold phosphate-buffered saline (PBS) and lysed in lysis buffer (20 mM Tris-HCl, pH 7.5, 137 mM NaCl, 1% Nonidet P-40, 10% glycerol, 1 mM MgCl2, 1 mM EGTA, 1 mM Na3VO4, 20 mM β-glycerol phosphate, 20 mM NaF, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 1 mg/ml aprotinin and leupeptin). Lysates were incubated on ice for 10 min and then centrifuged at 13,000 × g for 10 min at 4°C. Protein concentrations were determined by the Bradford method (6).
Immunoprecipitation.
Cell lysates (1 to 2 mg of protein) were incubated with 40 μl of protein A-agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) and 2 μg of specific antibody. The samples were incubated for 2 h at 4°C with rocking. The agarose beads were collected by centrifugation and washed three times with lysis buffer, resuspended in Laemmli sample buffer, and boiled for 5 min at 90°C.
Immunoblotting.
Samples were electrophoresed on sodium dodecyl sulfate (SDS) polyacrylamide gels and transferred to a polyvinylidene difluoride membrane. Membranes were blocked in Tris-buffered saline, pH 7.2, with 0.1% Tween (TBST) containing 3% BSA for 1 h. Primary antibodies were added to blocking buffer, and the membranes were incubated overnight at 4°C. The membranes were washed three times for 5 min with TBST and incubated with horseradish peroxidase-conjugated secondary antibodies in TBST with 5% nonfat dry milk for 1 h at room temperature. Membranes were rinsed in TBST three times for 5 min, and proteins were visualized by chemiluminescence.
MβCD treatment.
PC12 cells in solution were pretreated with 10 mM methyl-β-cyclodextrin (MβCD) in DMEM with 5 mM HEPES and 0.1% BSA for 15 min. Cells were then stimulated with 100 ng/ml NGF in the presence of MβCD for the times indicated in each figure and lysed in lysis buffer (described above). The samples were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotted for detection of phospho-ERK, and then the membranes were stripped and reprobed using anti-ERK2 antibodies. Cellular viability was monitored in parallel using trypan blue exclusion.
Ras and Rap1 pull-down assays.
PC12 cells transiently expressing FLAG-CAP, FLAG-CAPΔSoHo, or green fluorescent protein (GFP) were starved overnight in DMEM with 0.5% FBS. Cells were stimulated with NGF for the times indicated in the figure legend and lysed in Raf binding domain (RBD) buffer (50 mM Tris, pH 7.5, 10 mM MgCl2, 0.3 M NaCl, 1% Triton X-100). Lysates were clarified by centrifugation at 8,000 × g for 5 min, and protein concentration was quantified by the Bradford assay. Lysates (2 mg of protein) were added to 33 μg of agarose-immobilized Raf-RBD protein (Cytoskeleton, Denver, CO) and incubated at 4°C for 1 h with rocking. Beads were washed with RBD buffer and resuspended in Laemmli sample buffer and resolved by SDS-PAGE. Samples were immunoblotted to detect Ras and Rap1.
Lipid raft isolation.
Lipid rafts were isolated by a sucrose gradient flotation assay modified from Rubin and Ismail-Beigi (53) and Sargiacomo et al. (54). PC12 cells were starved overnight in DMEM with 0.5% FBS. Cells were suspended in solution with DMEM with 5 mM HEPES and 0.1% BSA and then stimulated with 100 ng/ml NGF for 5 min or left untreated. Cells were rinsed with PBS (pH 7.5) and incubated on ice in 750 μl of lysis buffer (50 mM HEPES, 150 mM NaCl, 10 mM EDTA, 0.5% Triton X-100, 2 mM Na3VO4, 40 mM NaF, 1 mM PMSF, and 1 mg/ml aprotinin and leupeptin) for 15 min. Lysates were assayed for protein concentration using the Bradford assay (6). Lysates containing 9 mg of protein were mixed with 1.2 ml of a 65% sucrose solution in buffer A (20 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF) to create a final concentration of 40% sucrose. A sucrose step gradient was constructed in a 5-ml centrifuge tube by overlaying the lysate with 2.0 ml of a 35% sucrose solution in buffer A, followed by 800 μl of 5% sucrose solution in buffer A. The gradients were centrifuged in a 50.1 Ti rotor at 39,000 rpm for 18 h at 4°C. Gradients were separated into seven fractions. Gradient fractions were assayed for protein concentration using the Bradford assay. Equal amounts of protein from corresponding gradient fractions were resolved by SDS-PAGE and immunoblotted for the specified proteins.
Immunocytochemistry.
PC12 cells were plated on poly-l-lysine-coated coverslips and stimulated with NGF for 0, 5, or 15 min in DMEM with 5 mM HEPES and 0.1% BSA. The cells were rinsed with PBS and fixed with 4% paraformaldehyde for 30 min at room temperature (TrkA staining) or in 2% paraformaldehyde for 10 min at room temperature (flotillin/CAP staining). The cells were then permeabilized with 0.3% Triton X-100 in PBS for 20 min at 4°C. Cells were blocked in 5% nonfat powdered milk and 1% horse serum for at least 2 h at room temperature. The primary antibodies were added to PBS with 5% nonfat dry milk for 1 h at room temperature, followed by an overnight incubation at 4°C with the following dilutions: TrkA, 1:100; flotillin, 1:50; CAP, 1:20. The cells were rinsed twice in PBS for 10 min, and then incubated with AlexaFluor secondary antibodies diluted 1:200 in PBS containing 5% nonfat dry milk for 1 h at room temperature. The coverslips were washed four times for 10 min in PBS and then mounted on slides and imaged on a Zeiss 510 META confocal microscope. FLAG staining was done following TrkA/flotillin staining according to the manufacturer's instructions.
Biotinylation.
MG139-2 cells were transfected with constructs expressing FLAG-CAP, FLAG-CAPΔSoHo, or GFP using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After 24 h, cells were stimulated with 100 ng/ml NGF for 15 min in DMEM with 5 mM HEPES and 0.1% BSA. Cells were rinsed with cold PBS and incubated with 1.5 mg/ml EZ-Link Sulfo-NHS-LC-Biotin [sulfosuccinimidyl-6-(biotinamido)hexanoate] (Pierce, Rockford, IL) in biotinylation buffer (10 mM boric acid, 154 mM NaCl, 7.2 mM KCl, 1.8 mM CaCl2, pH 8.4) for 15 min. Cells were then rinsed two times with quenching buffer (192 mM glycine, 25 mM Tris-HCl, 1.8 mM CaCl2, 154 mM NaCl, pH 8.3) and lysed in lysis buffer (20 mM Tris-HCl, pH 7.5, 137 mM NaCl, 1% Nonidet P-40, 10% glycerol, 1 mM MgCl2, 1 mM EGTA, 1 mM Na3VO4, 20 mM β-glycerol phosphate, 20 mM NaF, 1 mM PMSF, and 1 mg/ml aprotinin and leupeptin). Lysates were incubated on ice for 10 min and then centrifuged at 13,000 × g for 10 min at 4°C. Protein concentrations were determined by the Bradford method (6). Lysates containing 1 mg of protein each were added to 100 μl of UltraLink Immobilized NeutrAvidin beads (Pierce, Rockford, IL). Samples were incubated with rocking at 4°C for 2 h. NeutrAvidin beads were rinsed three times with lysis buffer, and 50 μl of Laemmli buffer was added to each sample. Samples were resolved by SDS-PAGE and immunoblotted for Trk and FLAG.
Transfection of primary dorsal root ganglion neurons with fluorescent siRNA constructs.
Control and CAP small interfering RNA (siRNA) constructs, a pool of three target-specific 20- to 25-nucleotide siRNA constructs (Santa Cruz Biotechnologies, Santa Cruz, CA) were labeled with carboxyfluorescein (FAM) using a Silencer siRNA labeling kit (Ambion, Austin, TX). Dorsal root ganglia (DRG) were isolated from E14 mouse embryos, trypsinized, and transfected with the FAM siRNA-labeled constructs using the Amaxa nucleofector device and the neuron nucleofector kit (Amaxa, Gaithersburg, MD). DRG neurons were then plated on laminin-coated dishes in Ultra culture medium (Cambrex Inc) with 10% FBS, 2 mM l-glutamine, and 50 ng/ml NGF. After a 20-h incubation, cells were fixed with 4% paraformaldehyde and stained for Tuj1. Cells were examined for uptake of the siRNAs by evaluation of FAM fluorescence, and fluorescent cells were quantitated for neurite length. Individual neurites were examined using the Zeiss Axiovision Rel, version 4.5, program. The cells were classified by their longest individual neurite with short neurites defined as <250 μm and long neurites defined as >250 μm. More than 200 cells were counted, and statistical analysis was done to determine the percentage of control siRNA- or CAP siRNA-transfected neurons with short neurites versus long neurites. In sister cultures, the cells were collected, the cellular lysates were resolved by SDS-PAGE, and CAP expression levels were evaluated by Western analysis.
RESULTS
Depletion of membrane cholesterol decreases the magnitude and duration of NGF-stimulated ERK activation.
It has been postulated that recruitment and retention of signaling molecules within cholesterol-rich membrane microdomains are critical for receptor-mediated signal transduction (57). Specifically, signaling from TrkA to its downstream effectors has been argued to be reliant upon cholesterol-rich lipid rafts as disruption of these domains with filipin or MβCD abolished NGF-stimulated ERK activation in PC12 cells (43) or inhibited TrkA phosphorylation in TrkA-expressing 3T3 cells (16) when evaluated after 5 min of NGF stimulation. We examined this phenomenon in greater detail to establish if perturbation of lipid rafts affected the kinetics and magnitude of ERK activation in response to NGF in PC12 cells. To determine the role of these cholesterol-rich domains in NGF stimulation of the ERKs, PC12 cells were pretreated with the cholesterol binding drug MβCD. This drug extracts cholesterol from the plasma membrane and disrupts the structure and function of lipid rafts (60). We found that cholesterol-depleted PC12 cells exhibited a marked decrease in ERK phosphorylation after NGF stimulation (Fig. 1A and B). The magnitude of the inhibition of ERK activation, measured at 5 min, was less dramatic than reported by Peiro et al. (43), who reported a near complete suppression of ERK activation at this time. However, we found that the effect of drug treatment was most pronounced at later times, reflecting a reduction of the sustained phase of ERK signaling. To determine if this reduction of ERK activation was due to a reduction in the activation of the NGF receptor TrkA, we monitored the effect of cholesterol depletion on the levels of the phosphorylated and active TrkA following NGF stimulation (Fig. 1C). TrkA phosphorylation was approximately 25% less than that of the controls at 5-min stimulation and 50% lower at later points. These data indicate that the magnitude of both ERK and TrkA activation by NGF is attenuated by MβCD treatment. This latter finding verifies the findings of Huang et al. (16) but differs from that reported by Peiro et al. (43). Cell viability was monitored in parallel and was unaffected by MβCD throughout this time course, consistent with previous reports (3). These experiments establish that perturbation of membrane cholesterol content alters the magnitude of TrkA signaling to the ERKs.
FIG. 1.
MβCD treatment decreases ERK and TrkA activation. PC12 cells were treated with 10 mM MβCD, which depletes cholesterol from cell membranes, for 15 min prior to NGF stimulation (100 ng/ml) for the indicated times. (A) Western blots were probed for phospho-ERK1/2 (P-ERK). The blots were stripped and subsequently reprobed for ERK2 to control for protein loading. (B) The data are represented as mean pixel intensity normalized to the percentage of maximal phospho-ERK response. The data shown are an average (± standard error) of three independent experiments (*, P < 0.05). (C) Western blots were probed for phospho-TrkA (P-TrkA) and then stripped and reprobed for TrkA to control for protein loading.
NGF-stimulated translocation of TrkA, SOS, and CAP to lipid rafts.
MβCD treatment is a common method of examining lipid raft function, and although its effects are often interpreted as reflective of disruption of lipid rafts, it remains possible that cholesterol depletion has a broader range of effects (10). Previous studies report that TrkA is localized to lipid rafts (16). One difficulty in interpreting these results is that there are dramatic differences in the fraction of TrkA reported to be associated with lipid rafts in untreated PC12 cells when the membranes are isolated in the absence of detergent under conditions that are reported to preserve raft structure (18, 39). Moreover, TrkA is dynamically trafficked between the cell surface and diverse intracellular membrane compartments in PC12 cells (20), and only a subset of the receptor is localized at the cell surface (65). Thus, it seems unlikely that the membrane isolation procedures employed previously accurately reflect the degree of raft-associated TrkA. We have reinvestigated this question using a different, but widely accepted, method of fractionating cellular membranes and isolation of raft-containing, low-buoyant-density membrane fractions employing nonionic detergents. Indeed, recent investigations of receptor dynamics using nonionic detergent extraction of cellular membranes have allowed the detection of BDNF-stimulated TrkB translocation into lipid rafts (63) as well as a similar glial cell-derived neurotrophic factor-stimulated receptor translocation into these membrane microdomains (64). We have employed a modification of this procedure to fractionate membranes from untreated and NGF-treated PC12 cells. This method utilizes the low buoyant density of lipid raft-containing membranes to separate them from the bulk plasma membrane that differ in their detergent-resistant lipid composition, facilitating their resolution on sucrose gradients. We incubated PC12 cells with or without NGF for 5 min and then lysed the cells. We loaded equal amounts of protein onto sucrose gradients and centrifuged these gradients for 18 h. Following centrifugation, each gradient was divided into seven fractions, and these were examined by Western analysis. We probed the fractions with flotillin (5) to identify the light, lipid raft-containing membrane fraction (fraction 2) and transferrin receptor 1 (TfR1) (7, 58) to mark the heavy, bulk plasma membrane fraction (fractions 5 to 7) (Fig. 2). In PC12 cells TrkA was found predominately localized within high-density membranes, reflective of its principal localization within intracellular membrane compartments and nonraft plasma membrane (20, 65, 68), with only a small fraction present within the low-buoyant-density fraction in untreated cells. Importantly, upon 5 min of stimulation with NGF, a significant translocation of TrkA into the lipid raft membrane fraction was observed (Fig. 2). This finding is consistent with a recent report of the neurotrophin-stimulated recruitment of TrkB into lipid rafts (63). The binding of NGF to TrkA at the cell surface results in activation of its intrinsic tyrosine kinase activity and extensive autophosphorylation. We found that the majority of the active phospho-TrkA was in the low-density fraction, suggesting that this is the fraction from which TrkA signaling is initiated. This latter finding is consistent with work from Huang and coworkers, who have argued that phosphorylated TrkA signaling is initiated within the lipid rafts (16). However, since the translocation of phosphorylated TrkA was incomplete, it remains possible that signaling might also be initiated from non-raft-associated receptors.
FIG. 2.
TrkA, SOS, and CAP concentrate in the flotillin-containing membrane fraction following NGF treatment. PC12 cell lysates were fractionated using discontinuous sucrose gradients. Fraction 1 represents the top of the gradient. PC12 cells were left untreated or stimulated with NGF for 5 min and lysed, and the lysates were resolved on sucrose gradients. Gradients were fractionated, and equal amounts of protein from corresponding fractions were separated by SDS-PAGE. Western blots were probed for flotillin, TrkA, phospho-TrkA (P-TrkA), CAP, SOS, FRS2, and TfR1. Flotillin, which is a marker for lipid raft membranes, was used as a marker for the lipid raft fraction, while TfR1 was used to mark the heavy membrane fractions. These results are representative of three independent experiments.
In resting cells, the adaptor protein CAP, which contains a SoHo lipid raft-targeting domain, is present at very low levels in lipid rafts, but upon NGF stimulation CAP was rapidly translocated to these membrane domains (Fig. 2). NGF also stimulated the movement of the Ras-specific guanine nucleotide exchange factor SOS to the lipid raft membrane fraction in PC12 cells (Fig. 2). SOS has previously been shown to associate with lipid raft domains following epidermal growth factor (EGF) stimulation of Rat-1 cells (37). It should be noted that SOS and CAP, like other adapter proteins, are multivalent signaling molecules and have multiple functions in the cell. We expect that only a subset of these proteins are involved in TrkA signaling events, and the magnitude of their translocation to the membrane is reflective of the plasma membrane levels of activated TrkA. We also found that the adaptor protein FRS2, which binds TrkA following NGF stimulation (36), is constitutively localized to lipid rafts, and its distribution is unchanged following NGF treatment. This is consistent with previous data (52). The TfR is a marker of high-density membrane fractions.
NGF stimulates the formation of signaling complexes with CAP.
Since CAP has been identified as a lipid raft targeting protein and is also able to bind signaling elements (24, 51), it is possible that CAP is able to facilitate the concentration of SOS and TrkA in lipid rafts. To test this hypothesis, we identified CAP binding partners following NGF stimulation. First, we confirmed that CAP associates with flotillin in lipid raft domains. We used a polyclonal flotillin antibody to immunoprecipitate flotillin in PC12 cell lysates and then probed these immunoprecipitates with an antibody to CAP. We found only low levels of interaction between flotillin and CAP in unstimulated cells; however, this association was significantly enhanced upon 5 min of NGF treatment (Fig. 3A). The NGF-stimulated association of CAP and flotillin was transient, as longer treatment with NGF resulted in diminished levels of these complexes (Fig. 3A).
FIG. 3.
Following NGF stimulation, CAP becomes associated with proteins important in lipid rafts and in MAPK activation. PC12 cells were stimulated with NGF for the indicated times, and cell lysates were prepared. (A) Flotillin was immunoprecipitated (IP) from cell lysates and the resulting Western blot (IB) was probed for CAP and flotillin. These data are representative of three independent experiments. (B) TrkA-CAP interactions were investigated by immunoprecipitation of TrkA followed by Western blotting with antibodies to CAP. The blots were stripped and reprobed for TrkA. (C) PC12 cells expressing Myc-tagged APS or GFP were stimulated with NGF. The tagged proteins were then immunoprecipitated from cell lysates with anti-Myc antibodies and then analyzed by Western blotting. The blots were probed with anti-CAP antibodies and then stripped and reprobed with anti-Myc antibodies to control for protein expression levels and immunoprecipitation efficiency. (D) SOS was immunoprecipitated from cell lysates. Immunoprecipitates were resolved by SDS-PAGE. Western blots were probed for CAP. The blots were then stripped and reprobed with anti-SOS antibodies to control for immunoprecipitation efficiency. (E) PC12 cells were stimulated with NGF for the indicated times and then lysed. Cbl was immunoprecipitated from cell lysates. Immunoprecipitates were analyzed using Western blotting and probed for CAP and then reprobed with anti-Cbl antibodies.
We hypothesize that TrkA is recruited to lipid rafts through protein complexes incorporating the CAP adapter protein. We found that NGF stimulation of PC12 cells resulted in the association of CAP with TrkA, as evidenced by the coimmunoprecipitation of TrkA with CAP. This interaction was a transient effect of NGF action, with maximal levels of complex formation observed after 5 min of NGF treatment (Fig. 3B).
We reasoned the CAP may provide a critical linkage between the lipid raft and TrkA; however, this linkage was unlikely to be direct but, rather, required other adapter molecules. It seemed possible that the adapter protein APS might mediate this linkage, given that APS has been reported to directly bind to phosphorylated, activated forms of TrkA (49). Moreover, it has been reported that CAP directly binds to the APS in 3T3-L1 adipocytes (1, 30). To determine if CAP and APS interact following NGF stimulation, we overexpressed a Myc-tagged APS construct in PC12 cells. We then incubated these cells with or without NGF for 5 min. Using the Myc tag, we immunoprecipitated APS from the lysates and probed for CAP association. We found a constitutive association of CAP with APS (Fig. 3C) which was not modified by NGF treatment. As APS binds phospho-TrkA following NGF stimulation, we postulate that CAP is able to associate with TrkA through the APS adaptor protein (49).
CAP has been shown to bind the SOS guanine nucleotide exchange factor in 3T3-L1 adipocytes (24, 51). To determine whether this interaction occurs in NGF-stimulated PC12 cells and could account for the activation of Ras, we immunoprecipitated SOS from PC12 cell lysates. We determined that NGF drives an association between SOS and CAP within 5 min of NGF stimulation (Fig. 3D); this association was coincident with the appearance of SOS in the lipid raft fraction (Fig. 2).
Finally, we examined the ability of CAP to bind to the adaptor protein Cbl in NGF-treated PC12 cells. Cbl is a well-characterized CAP binding protein, and the interaction of these molecules is essential to signaling cascades in other cell types (4, 23). Cbl was immunoprecipitated from the lysates of PC12 cells which had been stimulated with NGF, and these were analyzed for CAP association by Western blotting. CAP interacts with Cbl in untreated cells; however, NGF treatment resulted in a dramatic increase in this association (Fig. 3E). This experiment serves to verify that in PC12 cells Cbl exhibits interactions with CAP and documents that this adapter protein associates with one of its most prominent interacting proteins, CAP, in an NGF-dependent manner. Cbl has been postulated to play a number of roles in NGF signaling owing to its multifunctional roles as an adapter protein and ubiquitin ligase (13, 25, 35, 40), and it is unknown exactly how Cbl contributes to formation of complexes associated with TrkA.
Together, these data (Fig. 3A to E) indicate that, following NGF stimulation, CAP is the central molecule in a large protein complex that includes both members of the MAPK cascade and flotillin, a constituent of lipid rafts.
Both TrkA and CAP colocalize with flotillin on the cell surface following NGF stimulation.
To further examine the interaction of these molecules following NGF stimulation, immunocytochemistry and confocal microscopy were performed on NGF-stimulated PC12 cells. In unstimulated cells, CAP is distributed diffusely throughout the cytoplasm; however, following NGF stimulation for 5 and 10 min, CAP moves to the cell surface, where it colocalizes with flotillin (Fig. 4A). Flotillin is distributed throughout the cytoplasm and cell membrane, consistent with its role as a cholesterol binding protein. CAP and flotillin demonstrate little colocalization before stimulation, but following NGF stimulation, they colocalize in distinct regions of the plasma membrane; and this colocalization persists following 5 and 10 min of stimulation, consistent with the immunoprecipitation data shown in Fig. 3A.
FIG. 4.
Both TrkA and CAP colocalize with flotillin on the cell surface following NGF stimulation. (A) PC12 cells were treated with NGF for 0, 5, and 10 min. Cells were fixed and stained for CAP (red) and flotillin (green). Cells were then examined by confocal microscopy. (B) PC12 cells were treated with NGF for 0, 5, and 10 min. Cells were fixed and stained for TrkA (red) and flotillin (green). Cells were examined by confocal microscopy. Arrows indicate areas of colocalization. Scale bar, 5 μm.
TrkA also colocalizes with flotillin at the cell surface following 5 min of NGF stimulation (Fig. 4B); however, unlike CAP, this colocalization is transient and is diminished by 10 min of NGF stimulation. These experiments serve to verify, using direct imaging of the molecules, the NGF-stimulated interactions of TrkA, flotillin, and the adapter protein CAP.
CAPΔSoHo inhibits the movement of TrkA and SOS to the lipid raft fraction while overexpression of wild-type CAP has no effect.
We sought to determine if the sorbin homology domain of CAP, which specifically interacts with flotillin in lipid rafts, was responsible for the concentration of TrkA and SOS in the lipid raft domain following NGF stimulation. We first examined the effect of full-length CAP on protein localization in lipid rafts. We expressed a FLAG-CAP construct in PC12 cells which were then incubated in the presence or absence of NGF for 5 min and fractionated on sucrose gradients. The expression of FLAG-CAP had no effect on the NGF-stimulated translocation of TrkA, SOS, or CAP into lipid rafts (Fig. 5A). We detected a small increase in the basal level of CAP in raft fractions, as well as an overall increase in CAP protein levels, as a result of the overexpression of the full-length CAP construct. FLAG-CAP expression had no effect on the constitutive localization of FRS2 in the lipid raft fraction.
FIG. 5.
CAPΔSoHo but not full-length CAP inhibits the concentration of TrkA, SOS, and CAP in the lipid raft membrane fraction. PC12 cells expressing FLAG-CAP (A) or FLAG-CAPΔSoHo (B) were left untreated or stimulated with NGF for 5 min. The lipid raft membrane fractions were then isolated by sucrose gradient fractionation. Fraction 1 represents the top of the gradient. Lipid raft fractions were analyzed using Western blotting with antiflotillin, anti-Trk, anti-CAP, anti-SOS, anti-FLAG, anti-FRS2, and anti-TfR1 antibodies. The CAP antibody recognizes both the endogenous and the transfected CAP proteins. In panel A, FLAG antibodies are used to detect the expressed full-length CAP protein which is the same size as the endogenous protein. In panel B, the upper CAP band represents endogenous CAP levels, while the lower band shows the CAPΔSoHo-transfected protein. These results are representative of three independent experiments.
However, when we expressed CAPΔSoHo, a CAP mutant that lacks the SoHo domain, the NGF-stimulated translocation of TrkA and SOS into lipid rafts was blocked (Fig. 5B). These data indicate that the SoHo domain of CAP is responsible for the targeting of TrkA and SOS to lipid rafts in response to NGF treatment and support a mechanism in which TrkA concentrates in membrane microdomains, where it is autophosphorylated and interacts with its downstream effectors. Expression of CAPΔSoHo had no effect on the constitutive localization of FRS2 within lipid rafts. These data argue that CAP is responsible for the stimulation-dependent localization of TrkA-related signaling molecules in lipid rafts.
Using confocal microscopy, we verified that expression of full-length CAP had no effect on the ability of TrkA to form complexes with flotillin in response to NGF; however, CAPΔSoHo inhibited this interaction (Fig. 6). Following 5 min of NGF stimulation, full-length CAP localized to the cell membrane where it exhibited a punctate distribution. However, in unstimulated cells, CAP distribution was diffuse and mostly concentrated in the cytoplasm. The CAPΔSoHo mutant distributed a diffuse localization regardless of NGF stimulation (Fig. 6).
FIG. 6.
CAPΔSoHo inhibits colocalization of TrkA and flotillin following NGF stimulation. PC12 cells were transfected with FLAG-CAP or FLAG-CAPΔSoHo; 48 h later they were either left untreated or stimulated with NGF for 5 min. Cells were fixed and stained for TrkA (red), flotillin (green), or FLAG (blue) and examined by confocal microscopy. Arrows indicate points of colocalization. Open arrowheads denote transfected cells. Scale bar, 5 μm.
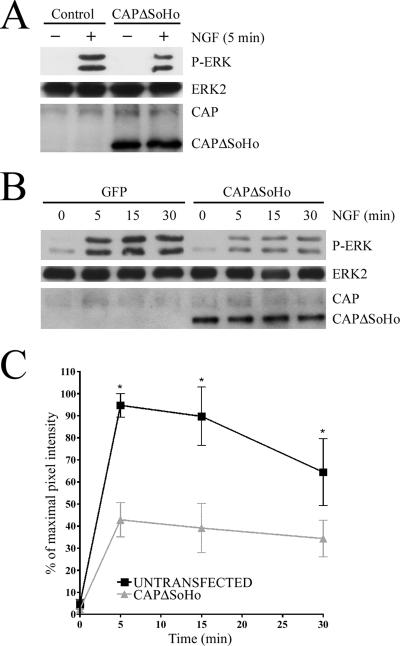
CAPΔSoHo decreases NGF activation of ERK1/2.
We tested whether inhibition of CAP localization to flotillin-containing lipid rafts perturbed TrkA-dependent activation of ERK1/2. We found that expression of the CAPΔSoHo mutant attenuated ERK activation in response to NGF (Fig. 7A to C). We determined this by two different methods. First, we employed a protein transduction system where recombinant CAPΔSoHo protein was introduced into PC12 cells by the Chariot protein delivery reagent. We observed an approximately 20% reduction in ERK phosphorylation by this method (Fig. 7A). Next, we transiently transfected a CAPΔSoHo expression construct into PC12 cells (Fig. 7B and 8A) and found diminished ERK activation in response to NGF. We saw no change in ERK activation when full-length CAP was transfected into the cells (Fig. 8A). These data demonstrate that the signaling pathways upstream of ERK activation are intact and not influenced by full-length CAP expression. In CAPΔSoHo-transfected cells, we saw about a 60% decrease in ERK phosphorylation after 5 min of NGF stimulation (Fig. 8A). To determine if the reduction in ERK phosphorylation observed after CAPΔSoHo transfection was due to a change in the activation kinetics of ERK, we examined a time course of ERK activation in CAPΔSoHo-transfected cells. We found that CAPΔSoHo does not alter the activation kinetics of ERK in response to NGF but significantly reduced the magnitude of ERK activation at all time points (Fig. 7B and C). These data support a mechanism of ERK activation that depends on CAP-facilitated TrkA movement into the lipid rafts.
FIG. 7.
CAPΔSoHo inhibits ERK activation. (A) Purified FLAG-CAPΔSoHo protein was introduced into PC12 cells using the Chariot protein transduction reagent. Cells were incubated with or without NGF for 5 min and then lysed. Cell lysates were probed for phospho-ERK (P-ERK) to examine differences in ERK activation and for ERK2 to control for protein loading. Anti-CAP was used to probe for the incorporation of CAPΔSoHo into the cells. This antibody recognizes both endogenous (upper band) and mutant CAP proteins (lower band) which differ in their molecular weights. (B) PC12 cells were transfected with FLAG-CAPΔSoHo or GFP constructs using Lipofectamine 2000. Cells were stimulated with NGF for indicated times. Western blots were probed for phospho-ERK, ERK 2, and CAP. (C) Graphical analysis of data from three independent experiments. Data are represented as the average (± standard error) mean pixel intensity normalized to the percentage of maximal activation (*, P < 0.05).
FIG. 8.
CAP-mediated protein interactions are necessary for NGF but not EGF stimulation of the ERKs. (A) PC12 cells were transfected with constructs expressing full-length FLAG-CAP, FLAG-CAPΔSoHo, or GFP. Cells were incubated with or without NGF for 5 min. Cells were lysed, and the lysates were examined by Western blotting analysis. Western blots were probed for phospho-ERK (P-ERK), ERK2, and FLAG. (B) PC12 cells were transfected with constructs expressing FLAG-CAPΔSH3 or GFP. Cells were either treated with NGF for 5 min or left untreated. Cells were lysed and analyzed for protein concentration. Equal amounts of protein from each sample were separated by SDS-PAGE and analyzed by Western blotting. Blots were probed for phospho-ERK, ERK2, and FLAG. (C) PC12 cells were transfected with constructs expressing FLAG-CAP, FLAG-CAPΔSoHo, or GFP and then treated with EGF for 5 min or left untreated. Cells were lysed, and lysates were analyzed for protein concentration. Equal amounts of protein from each sample were separated by SDS-PAGE and analyzed by Western blotting. Blots were probed for phospho-ERK, ERK 2, and FLAG.
CAP-mediated protein interactions are necessary for NGF, but not EGF, stimulation of ERK.
CAP promotes the localization of its interacting proteins to lipid raft domains (4, 23); thus, both its flotillin binding domain and SH3 protein interaction domains should be essential for functional signaling complex formation. To determine if the three SH3 protein-protein interaction domains of CAP are essential to ERK activation, we transfected a CAP protein lacking all three SH3 domains (CAPΔSH3) into PC12 cells (Fig. 8B). Following transfection the cells were incubated with or without NGF for 5 min. The cells were lysed and analyzed by Western blotting for ERK activation. Transfection of FLAG-CAPΔSH3 reduces ERK activation by NGF (Fig. 8B). However, there was no change in ERK activation with the expression of full-length CAP compared to vector controls (Fig. 8A). These data indicate that both protein interaction domains of CAP, SoHo and SH3, are required for NGF stimulation of the ERKs and that CAP acts as an essential adaptor protein in this signaling pathway.
To determine if the CAP adaptor protein is specific for NGF activation of the ERKs, we transfected PC12 cells with GFP, FLAG-CAP, or FLAG-CAPΔSoHo and incubated the cells with or without EGF for 5 min. In contrast to the effect of NGF, there was no noticeable difference in EGF-stimulated activation of the ERKs between control, full-length CAP-, and CAPΔSoHo-transfected cells (Fig. 8C). This finding demonstrates that CAP acts specifically to facilitate TrkA action and does not participate in EGF-stimulated ERK activation.
CAPΔSoHo inhibits TrkA phosphorylation and internalization.
To determine if translocation of TrkA into lipid rafts is important for receptor activation, GFP, full-length CAP, and CAPΔSoHo expression constructs were transfected into PC12 cells. Cells were treated with NGF, and TrkA phosphorylation was examined using a phospho-specific antibody for Y490 of TrkA. Phosphorylation of the Y490 residue is correlated with the activation status of the receptor. TrkA phosphorylation was reduced, but not completely inhibited, in cells expressing CAPΔSoHo compared to controls, while the expression of full-length CAP had no effect (Fig. 9A). Phospho-Trk levels were quantified from three independent experiments and normalized for protein loading. In all experiments at least a 50% decrease in Trk phosphorylation was observed at 5 min in CAPΔSoHo-transfected cells. TrkA activation was examined to determine if its activation was reduced or simply delayed (Fig. 9B). Reduction in TrkA activation was observed at all time points. These data indicate that TrkA concentration in lipid rafts is required for the activation of the receptor and suggests that clustering of TrkA in lipid rafts facilitates receptor dimerization and transphosphorylation in response to NGF.
FIG. 9.
CAPΔSoHo inhibits TrkA phosphorylation and internalization. (A) PC12 cells were transfected with GFP, FLAG-CAP, or FLAG-CAPΔSoHo. Cells were left untreated or stimulated with NGF for 5 min. Cells were lysed, and lysates were probed with antibodies to phospho-Trk (P-Trk), TrkA, and FLAG. (B) PC12 cells were transfected with FLAG-CAPΔSoHo- or GFP-expressing constructs. Cells were left untreated or treated with NGF for 5, 15, or 30 min. Cells were then lysed, and lysates were analyzed for protein concentration using the Bradford protein assay. Equal amounts of protein from each sample were resolved using SDS-PAGE. Western blots were probed for phospho-TrkA, TrkA, and CAP. These results are representative of three independent experiments. Cells were transfected with FLAG-CAP (C) or FLAG-CAPΔSoHo (D) and were treated with NGF for 15 min or left untreated. Cells were incubated with biotin to label cell surface proteins. Cell lysates were then incubated with neutravidin beads to specifically isolate the biotin-labeled proteins. Western blotting was performed, and blots were probed for TrkA. Lysates were probed with anti-FLAG antibodies to detect expression of the transfected proteins.
As Trk receptor activation is necessary for its internalization, we sought to determine whether CAPΔSoHo had any effect on the internalization of the TrkA receptor. Using a 3T3 cell line that constitutively expresses TrkA (32) and responds to NGF stimulation, we used biotinylation of cell surface proteins to determine the amount of TrkA on the cell surface before and after 15 min of NGF stimulation in control, CAP-, and CAPΔSoHo-expressing cells. Following transfection with constructs for CAP, CAPΔSoHo, and GFP, cells were stimulated with NGF for 15 min and incubated with biotin, which labels all proteins on the cell surface. NeutrAvidin beads were then used to isolate the biotin-linked proteins. Proteins bound to the NeutrAvidin beads were examined by Western blots probed for TrkA. Following NGF stimulation of GFP-transfected cells, the amount of TrkA located on the cell surface was significantly reduced (Fig. 9C), signifying the ligand-dependent internalization of the receptor. Transfection with CAP-expressing constructs did not have any effect on NGF-stimulated internalization of TrkA (Fig. 9C). However, following transfection of CAPΔSoHo-expressing constructs, the amount of TrkA located on the cell surface was not significantly reduced following NGF stimulation (Fig. 9D), indicating that CAPΔSoHo is able to inhibit the internalization of the TrkA receptor. These data support the dependence of TrkA activation and downstream signaling on the CAP adaptor protein.
CAPΔSoHo influences the activation of the small G protein Rap1.
NGF stimulation results in the activation of the small G proteins, Ras and Rap1 (70). In PC12 cells, Rap1 is the predominant activator of ERK1/2, while Ras plays a relatively minor role in NGF-stimulated ERK activation (21, 33, 70). To determine whether CAPΔSoHo affects the activation of Ras or Rap1, we isolated active Ras and Rap1 from NGF-stimulated cells. Active Ras and Rap1 were isolated using agarose beads preconjugated to the Raf-RBD, which specifically associates with GTP-bound Ras and Rap1.
Transfection of CAPΔSoHo into PC12 cells caused a significant inhibition of Rap1 (Fig. 10B and C) following NGF stimulation compared to GFP-transfected and full-length CAP-transfected controls. This loss of Rap1 activation is likely responsible for the inhibition of ERK in CAPΔSoHo-expressing cells. In contrast, the expression of CAPΔSoHo did not decrease the activation of Ras following NGF treatment compared to GFP-transfected and full-length CAP-transfected controls (Fig. 10A). These findings support the observation that Rap1 is the principal regulator of NGF-stimulated ERK activation, with Ras playing a qualitatively minor role (19, 70), and that Rap1 activation of its downstream targets is reliant upon the localization of TrkA within lipid rafts.
FIG. 10.
CAPΔSoHo affects the activation of the small G proteins Ras and Rap1. PC12 cells were transfected with constructs expressing FLAG-CAP, FLAG-CAPΔSoHo, or GFP. Cells were left untreated or stimulated with NGF for 5 min. Lysates were incubated with agarose-immobilized Raf-RBD protein to specifically isolate activated forms of Ras and Rap1. The Raf-RBD associated proteins were analyzed using Western blots probed for Ras (A) and Rap1 (B). Lysates were probed for total Ras and Rap1 protein and for expression of the FLAG-tagged proteins. (C) Quantitative analysis of Rap1 activation following 5 min of NGF stimulation from three independent experiments. Data are represented as the average (± standard error) mean pixel intensity derived from Western blots of Rap1 bands normalized to the vector control.
Knock-down of endogenous CAP expression results in decreased neurite length in cultured DRG neurons.
To determine the biological significance of CAP in TrkA-expressing neurons, we used CAP siRNA constructs to knock down the expression of endogenous CAP in primary DRG neurons isolated from E14 mouse embryos (Fig. 11E). We first labeled the CAP siRNA or control siRNAs with FAM in order to detect the siRNA constructs in the cells. We used the Amaxa nucleofection system to incorporate the siRNA into the freshly isolated DRG neurons. The neurons were then plated on laminin-coated coverslips and incubated with NGF for 20 h. The neurons were stained for Tuj1, and neurite length was examined (Fig. 11A and B). Neurite length was scored as short (under 250 μm) or long (above 250 μm). DRG that incorporated CAP siRNA typically had short axons, while cells that incorporated control siRNA predominantly displayed long axons (Fig. 11C and D). These data indicate that a reduction in CAP expression affects neurite outgrowth in TrkA-expressing primary sensory neurons.
FIG. 11.
CAP siRNA reduces neurite outgrowth in cultured primary DRG neurons. DRG neurons were cultured from E14 mouse embryos and transfected using Amaxa nucleofection with FAM-labeled control siRNA (A) or FAM-labeled CAP siRNA (B) and incubated in NGF-containing medium. (C and D) Cells transfected with FAM-labeled siRNA were counted and scored for longest individual neurite length (short neurites, <250 μm; long neurites, >250 μm). Statistical analysis was done to determine the percentage of total transfected neurons extending short neurites or long neurites. In >200 cells the ranges were as follows: control siRNA, 21.5% ± 5.5% short and 78% ± 6% long; CAP siRNA, 66.5% ± 1.5% short and 33.5% ± 1.5% long. (E) Lysates from DRG neurons transfected with control and CAP siRNAs were probed for CAP protein expression by Western blotting.
DISCUSSION
One of the principal unresolved issues in neurotrophin signaling is establishing how Trk receptors are translocated into membrane compartments that facilitate the assembly of signaling complexes, their subsequent internalization, and finally the propagation of signals to intracellular effectors. PC12 cells have been a particularly valuable model for establishing how TrkA elicits neuronal differentiation through regulation of the activity of the ERKs (12, 22, 34, 42). The capacity of NGF and other neural differentiating stimuli to stimulate ERK activity over extended intervals leads to the physical translocation of the kinases into the nucleus, where they phosphorylate transcription factors responsible for the expression of neuron-specific genes. Moreover, the necessity to propagate signals from the membrane to the nucleus in neurons that have highly asymmetric morphology has led to the evolution of mechanisms that allow the sustained activation of intracellular signaling molecules such as the ERKs. Howe and colleagues have argued that receptor-containing internalized vesicles, or “signaling endosomes,” are responsible for much of neurotrophin-mediated signaling in neurons through the retrograde transport of receptor-bearing endosomes from the membrane of neuronal processes to the nucleus (14, 15).
We have focused our attention on the initial step in the cellular compartmentalization of neurotrophin signaling, the focal concentration of Trk receptors within cell surface membrane microdomains. The mechanisms subserving the clustering of receptors and their associated signaling elements within membrane domains that are subsequently targeted for internalization are not well understood. The present study addressed several unresolved issues regarding the dynamics of TrkA redistribution within membrane compartments following NGF binding. Earlier studies had reported that TrkA was constitutively associated with low-buoyant-density membrane fractions using fractionation procedures employing carbonate buffers at basic pH, and the degree to which TrkA was localized into the light membrane fractions was reported to be between approximately 50% to 90% (16, 39, 43) in untreated PC12 cells. This distribution was reported not to be dramatically affected by NGF treatment (16). These findings are difficult to reconcile with the known cellular distribution of TrkA (65), which is found predominately associated with intracellular membranous compartments, with only a fraction present at the cell surface. We have employed a different method of lipid raft isolation that has been demonstrated by others to allow detection of ligand-regulated redistribution of membrane receptors into lipid rafts (63, 64) and has the additional advantage of preserving receptor-associated protein complexes which were postulated to be disrupted at the high pH used in previous studies (16). We report that NGF stimulates the rapid translocation of TrkA into cholesterol-rich lipid rafts. This observation is similar to the recent finding that the TrkB is translocated into lipid rafts in cortical neurons upon BDNF treatment (63). While the fraction of TrkA that is associated with the lipid rafts is a small fraction of total cellular TrkA, it represents the biologically active, phosphorylated species that are responsible for initiating downstream signaling cascades. Indeed, Suzuki et al. have provided evidence that Trk tyrosine kinase activity is required for translocation into these membrane domains (63), consistent with the phosphorylation-dependent assembly of receptor-associated signaling complexes.
The localization of TrkA into lipid rafts has been recognized as important for downstream signaling as perturbation of these structures by cholesterol removal (16), cholesterol binding drugs (43), or ganglioside overexpression (39) resulted in inhibition of TrkA phosphorylation and ERK activation, and our findings are consistent with these observations. These findings raise the question of how, exactly, TrkA is targeted to and retained within lipid raft domains. We have demonstrated that disruption of the SoHo domain of CAP, preventing the linkage of TrkA to the lipid rafts via flotillin, blocks the ability of the receptor to localize to these membrane microdomains. We postulate that the adaptor protein CAP acts through its interaction with flotillin to anchor TrkA to cholesterol-rich rafts. Flotillin avidly binds to cholesterol, is concentrated in cholesterol-rich membrane microdomains, and is an integral membrane protein of lipid rafts (5). The adaptor protein CAP binds to flotillin through its SoHo domain, and this interaction is enhanced upon NGF treatment of the cells. CAP is linked to TrkA, likely through its interaction with the adapter protein APS, which binds to TrkA through a phosphotyrosine residue within the catalytic domain of the receptor (49). APS has an N-terminal multimerization domain that is postulated to play a role in the clustering of activated TrkA, which seems to be critical for the propagation of downstream signaling and specifically for the sustained activation of the ERKs (48).
Despite this documented role for APS in ERK activation, APS may not represent the only link between CAP and Trk as there is evidence that other molecules such as Cbl bind both of these proteins. Furthermore, CAP's effects on TrkA signaling may not be limited to its association with the receptor. As a multipotent adaptor protein, CAP has the capacity to associate with many signaling molecules. The deletion of the protein interaction domains of CAP may interfere with the formation of multiple protein complexes and suppress ERK activation through more than one mechanism.
TrkA activation stimulates two parallel signaling pathways that can lead to the activation of the ERKs. NGF binding to TrkA results in the rapid activation of Ras and c-Raf through a mechanism which is thought to occur principally at the plasma membrane. In PC12 cells this pathway is a quantitatively minor contributor to overall ERK activation (21, 70). TrkA activates the ERKs principally through a parallel signaling pathway mediated by Rap1 and B-Raf (19, 21, 70). Rap1 is localized almost exclusively in endosomes (68), and this pathway is responsible for >90% of signaling to the ERKs in NGF-stimulated PC12 cells (21). This latter pathway is reliant upon the formation of a stable, endosomally associated (68) signaling complex centered on the scaffolding protein FRS2 (21).
We found that the principal effect of perturbation of the localization of TrkA and its signaling complexes to lipid rafts is the dramatic inhibition of Rap1 activation, leading to the attenuation of ERK activation. Rap1 activation of B-Raf is the central signaling pathway for ERK activation in PC12 cells (19). Significantly, FRS2, an essential adaptor protein for TrkA activation of Rap1, is constitutively associated with lipid raft domains (52). It is possible that CAPΔSoHo interferes with the ability of TrkA to associate with FRS2 in lipid rafts.
Although TrkA and SOS do not concentrate in lipid rafts following NGF stimulation of CAPΔSoHo-expressing cells, Ras activation in these cells is not decreased. This effect may be explained by the fact that Ras localization to lipid rafts is reported to be isoform specific. While H-Ras preferentially localizes to membrane microdomains, K-Ras is excluded from them (45, 46). The loss of TrkA and SOS from lipid rafts may allow for the preferential activation of K-Ras. As most TrkA binding partners, i.e., SHC, phospholipase C-γ, and FRS-2, are sequestered in lipid rafts, TrkA may highly couple to SOS and activate Ras outside of lipid rafts, resulting in CAPΔSoHo expression having little effect on Ras activation.
The CAP adaptor protein has a well-described role in insulin signaling pathways (1, 4, 8, 23, 29, 30, 51). Following insulin stimulation, CAP facilitates the translocation of Cbl away from the insulin receptor and into lipid raft complexes where Cbl is able to recruit downstream signaling elements (4, 23). This translocation is absolutely required to maintain the integrity of insulin signaling to its downstream effectors (4, 23). In this study we have presented data indicating that CAP has a broader role in cellular signaling. CAP is a versatile adaptor, whose lipid raft targeting ability is essential to multiple signaling pathways. Recently, CAP has been shown to regulate adhesion-dependent ERK activation in fibroblasts and has been implicated as a protein involved in cellular differentiation (71).
We demonstrate a novel role for CAP in TrkA activation and its downstream signaling. As an essential adaptor protein, the disruption of either of the protein interaction domains of CAP reduces TrkA activation of the ERKs. CAP appears to act specifically to regulate NGF signaling through TrkA, as activation of the ERKs by EGF is unaffected by the loss of CAP protein-protein interaction domains. Thus, unlike NGF, EGF signaling is not dependent on CAP-mediated signaling complexes. These data may provide an explanation for previous reports that EGF-stimulated activation of the ERKs was insensitive to cholesterol depletion (43) and that EGF receptors were reduced in raft fractions following EGF stimulation (16, 37). However, EGF only transiently activates the ERKs and is unable to support sustained ERK signaling, reinforcing our view that the CAP adaptor protein contributes to prolonged signaling events. Importantly, we demonstrate that CAP is an essential element in signaling through the ERKs to regulate neurite outgrowth in neurons, as inhibition of endogenous CAP expression inhibits NGF-stimulated neurite outgrowth from primary DRG neurons. Our data demonstrate that CAP-mediated localization of signaling elements to lipid rafts is an important mechanism for signal propagation.
We have described a novel mechanism of TrkA localization and activation following NGF stimulation. This mechanism elucidates the biological significance of lipid rafts in NGF signaling and establishes a previously unknown role for the adaptor protein CAP in TrkA-dependent ERK activation. Although the biological activities of TrkA receptors in PC12 cells are likely to differ from those in primary neurons, these data underscore the importance of the localization of signaling to distinct compartments in the cell and support a mechanism of signal transduction where signaling molecules are required to move into specific membrane domains for internalization and intracellular signal propagation. This mechanism supports the concept of “signaling organelles” which possess complexes of active signaling molecules that are trafficked to other intracellular locations to interact with their effectors (14).
Acknowledgments
We thank Alan Saltiel for providing us with the Flag-CAP, Flag-CAPΔSoHo, and Flag-CAPΔSH3 constructs; David Ginty for providing us with the Myc-APS construct; and Philip Barker for providing the MG139-2 cells.
This work was supported by a grant from the National Science Foundation (IBN-97-28317). A.L. was supported by a training grant from the NIH (TD-HD07104).
Footnotes
Published ahead of print on 4 June 2007.
REFERENCES
- 1.Ahn, M. Y., K. D. Katsanakis, F. Bheda, and T. S. Pillay. 2004. Primary and essential role of the adaptor protein APS for recruitment of both c-Cbl and its associated protein CAP in insulin signaling. J. Biol. Chem. 279:21526-21532. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, R. G. 1998. The caveolae membrane system. Annu. Rev. Biochem. 67:199-225. [DOI] [PubMed] [Google Scholar]
- 3.Arispe, N., and M. Doh. 2002. Plasma membrane cholesterol controls the cytotoxicity of Alzheimer's disease AbetaP (1-40) and (1-42) peptides. FASEB J. 16:1526-1536. [DOI] [PubMed] [Google Scholar]
- 4.Baumann, C. A., V. Ribon, M. Kanzaki, D. C. Thurmond, S. Mora, S. Shigematsu, P. E. Bickel, J. E. Pessin, and A. R. Saltiel. 2000. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 407:202-207. [DOI] [PubMed] [Google Scholar]
- 5.Bickel, P. E., P. E. Scherer, J. E. Schnitzer, P. Oh, M. P. Lisanti, and H. F. Lodish. 1997. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J. Biol. Chem. 272:13793-13802. [DOI] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain, L. H., and G. W. Gould. 2002. The vesicle- and target-SNARE proteins that mediate Glut4 vesicle fusion are localized in detergent-insoluble lipid rafts present on distinct intracellular membranes. J. Biol. Chem. 277:49750-49754. [DOI] [PubMed] [Google Scholar]
- 8.Chiang, S. H., C. A. Baumann, M. Kanzaki, D. C. Thurmond, R. T. Watson, C. L. Neudauer, I. G. Macara, J. E. Pessin, and A. R. Saltiel. 2001. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 410:944-948. [DOI] [PubMed] [Google Scholar]
- 9.Douglass, A. D., and R. D. Vale. 2005. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 121:937-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edidin, M. 2003. The state of lipid rafts: from model membranes to cells. Annu. Rev. Biophys. Biomol. Struct. 32:257-283. [DOI] [PubMed] [Google Scholar]
- 11.Frade, J. M., and Y. A. Barde. 1998. Nerve growth factor: two receptors, multiple functions. Bioessays 20:137-145. [DOI] [PubMed] [Google Scholar]
- 12.Friedman, W. J., and L. A. Greene. 1999. Neurotrophin signaling via Trks and p75. Exp. Cell Res. 253:131-142. [DOI] [PubMed] [Google Scholar]
- 13.Galisteo, M. L., I. Dikic, A. G. Batzer, W. Y. Langdon, and J. Schlessinger. 1995. Tyrosine phosphorylation of the c-cbl proto-oncogene protein product and association with epidermal growth factor (EGF) receptor upon EGF stimulation. J. Biol. Chem. 270:20242-20245. [DOI] [PubMed] [Google Scholar]
- 14.Howe, C. L., and W. C. Mobley. 2004. Signaling endosome hypothesis: a cellular mechanism for long distance communication. J. Neurobiol. 58:207-216. [DOI] [PubMed] [Google Scholar]
- 15.Howe, C. L., J. S. Valletta, A. S. Rusnak, and W. C. Mobley. 2001. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron 32:801-814. [DOI] [PubMed] [Google Scholar]
- 16.Huang, C. S., J. Zhou, A. K. Feng, C. C. Lynch, J. Klumperman, S. J. DeArmond, and W. C. Mobley. 1999. Nerve growth factor signaling in caveolae-like domains at the plasma membrane. J. Biol. Chem. 274:36707-36714. [DOI] [PubMed] [Google Scholar]
- 17.Huang, E. J., and L. F. Reichardt. 2003. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72:609-642. [DOI] [PubMed] [Google Scholar]
- 18.Huang, E. J., G. A. Wilkinson, I. Farinas, C. Backus, K. Zang, S. L. Wong, and L. F. Reichardt. 1999. Expression of Trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of TrkA and TrkB in addition to TrkC. Development 126:2191-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaiswal, R. K., S. A. Moodie, A. Wolfman, and G. E. Landreth. 1994. The mitogen-activated protein kinase cascade is activated by B-Raf in response to nerve growth factor through interaction with p21ras. Mol. Cell. Biol. 14:6944-6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jullien, J., V. Guili, E. A. Derrington, J. L. Darlix, L. F. Reichardt, and B. B. Rudkin. 2003. Trafficking of TrkA-green fluorescent protein chimerae during nerve growth factor-induced differentiation. J. Biol. Chem. 278:8706-8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kao, S., R. K. Jaiswal, W. Kolch, and G. E. Landreth. 2001. Identification of the mechanisms regulating the differential activation of the mapk cascade by epidermal growth factor and nerve growth factor in PC12 cells. J. Biol. Chem. 276:18169-18177. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan, D. R., and F. D. Miller. 1997. Signal transduction by the neurotrophin receptors. Curr. Opin. Cell Biol. 9:213-221. [DOI] [PubMed] [Google Scholar]
- 23.Kimura, A., C. A. Baumann, S. H. Chiang, and A. R. Saltiel. 2001. The sorbin homology domain: a motif for the targeting of proteins to lipid rafts. Proc. Natl. Acad. Sci. USA 98:9098-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurakin, A., N. G. Hoffman, and B. K. Kay. 1998. Molecular recognition properties of the C-terminal Sh3 domain of the Cbl associated protein, Cap. J. Pept. Res. 52:331-337. [DOI] [PubMed] [Google Scholar]
- 25.Lachyankar, M. B., P. J. Condon, M. C. Daou, A. K. De, J. B. Levine, A. Obermeier, and A. H. Ross. 2003. Novel functional interactions between Trk kinase and p75 neurotrophin receptor in neuroblastoma cells. J. Neurosci. Res. 71:157-172. [DOI] [PubMed] [Google Scholar]
- 26.Lang, D. M., S. Lommel, M. Jung, R. Ankerhold, B. Petrausch, U. Laessing, M. F. Wiechers, H. Plattner, and C. A. Stuermer. 1998. Identification of reggie-1 and reggie-2 as plasma membrane-associated proteins which cocluster with activated GPI-anchored cell adhesion molecules in non-caveolar micropatches in neurons. J. Neurobiol. 37:502-523. [DOI] [PubMed] [Google Scholar]
- 27.Le Roy, C., and J. L. Wrana. 2005. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell. Biol. 6:112-126. [DOI] [PubMed] [Google Scholar]
- 28.Liu, H. Y., and S. O. Meakin. 2002. ShcB and ShcC activation by the Trk family of receptor tyrosine kinases. J. Biol. Chem. 277:26046-26056. [DOI] [PubMed] [Google Scholar]
- 29.Liu, J., S. M. DeYoung, J. B. Hwang, E. E. O'Leary, and A. R. Saltiel. 2003. The roles of Cbl-b and c-Cbl in insulin-stimulated glucose transport. J. Biol. Chem. 278:36754-36762. [DOI] [PubMed] [Google Scholar]
- 30.Liu, J., A. Kimura, C. A. Baumann, and A. R. Saltiel. 2002. APS facilitates c-Cbl tyrosine phosphorylation and GLUT4 translocation in response to insulin in 3T3-L1 adipocytes. Mol. Cell. Biol. 22:3599-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucero, H. A., and P. W. Robbins. 2004. Lipid rafts-protein association and the regulation of protein activity. Arch. Biochem. Biophys. 426:208-224. [DOI] [PubMed] [Google Scholar]
- 32.MacPhee, I., and P. A. Barker. 1999. Extended ceramide exposure activates the trkA receptor by increasing receptor homodimer formation. J. Neurochem. 72:1423-1430. [DOI] [PubMed] [Google Scholar]
- 33.Marshall, C. J. 1998. Signal transduction. Taking the Rap. Nature 392:553-554. [DOI] [PubMed] [Google Scholar]
- 34.Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 35.McCarty, J. H., and S. C. Feinstein. 1999. The TrkB receptor tyrosine kinase regulates cellular proliferation via signal transduction pathways involving SHC, PLCγ, and CBL. J. Recept. Signal Transduct. Res. 19:953-974. [DOI] [PubMed] [Google Scholar]
- 36.Meakin, S. O., J. I. MacDonald, E. A. Gryz, C. J. Kubu, and J. M. Verdi. 1999. The signaling adapter FRS-2 competes with Shc for binding to the nerve growth factor receptor TrkA. A model for discriminating proliferation and differentiation. J. Biol. Chem. 274:9861-9870. [DOI] [PubMed] [Google Scholar]
- 37.Mineo, C., G. L. James, E. J. Smart, and R. G. Anderson. 1996. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J. Biol. Chem. 271:11930-11935. [DOI] [PubMed] [Google Scholar]
- 38.Munro, S. 2003. Lipid rafts: elusive or illusive? Cell 115:377-388. [DOI] [PubMed] [Google Scholar]
- 39.Nishio, M., S. Fukumoto, K. Furukawa, A. Ichimura, H. Miyazaki, S. Kusunoki, and T. Urano. 2004. Overexpressed GM1 suppresses nerve growth factor (NGF) signals by modulating the intracellular localization of NGF receptors and membrane fluidity in PC12 cells. J. Biol. Chem. 279:33368-33378. [DOI] [PubMed] [Google Scholar]
- 40.Ohrt, T., A. Mancini, T. Tamura, and R. Niedenthal. 2004. c-Cbl binds to tyrosine-phosphorylated neurotrophin receptor p75 and induces its ubiquitination. Cell. Signal. 16:1291-1298. [DOI] [PubMed] [Google Scholar]
- 41.Paratcha, G., and C. F. Ibanez. 2002. Lipid rafts and the control of neurotrophic factor signaling in the nervous system: variations on a theme. Curr. Opin. Neurobiol. 12:542-549. [DOI] [PubMed] [Google Scholar]
- 42.Patapoutian, A., and L. F. Reichardt. 2001. Trk receptors: mediators of neurotrophin action. Curr. Opin. Neurobiol. 11:272-280. [DOI] [PubMed] [Google Scholar]
- 43.Peiro, S., J. X. Comella, C. Enrich, D. Martin-Zanca, and N. Rocamora. 2000. PC12 cells have caveolae that contain TrkA. Caveolae-disrupting drugs inhibit nerve growth factor-induced, but not epidermal growth factor-induced, MAPK phosphorylation. J. Biol. Chem. 275:37846-37852. [DOI] [PubMed] [Google Scholar]
- 44.Pike, L. J. 2003. Lipid rafts: bringing order to chaos. J. Lipid Res. 44:655-667. [DOI] [PubMed] [Google Scholar]
- 45.Prior, I. A., and J. F. Hancock. 2001. Compartmentalization of Ras proteins. J. Cell Sci. 114:1603-1608. [DOI] [PubMed] [Google Scholar]
- 46.Prior, I. A., A. Harding, J. Yan, J. Sluimer, R. G. Parton, and J. F. Hancock. 2001. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat. Cell Biol. 3:368-375. [DOI] [PubMed] [Google Scholar]
- 47.Puri, C., D. Tosoni, R. Comai, A. Rabellino, D. Segat, F. Caneva, P. Luzzi, P. P. Di Fiore, and C. Tacchetti. 2005. Relationships between EGFR signaling-competent and endocytosis-competent membrane microdomains. Mol. Biol. Cell 16:2704-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian, X., and D. D. Ginty. 2001. SH2-B and APS are multimeric adapters that augment TrkA signaling. Mol. Cell. Biol. 21:1613-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qian, X., A. Riccio, Y. Zhang, and D. D. Ginty. 1998. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron 21:1017-1029. [DOI] [PubMed] [Google Scholar]
- 50.Ribon, V., R. Herrera, B. K. Kay, and A. R. Saltiel. 1998. A role for CAP, a novel, multifunctional Src homology 3 domain-containing protein in formation of actin stress fibers and focal adhesions. J. Biol. Chem. 273:4073-4080. [DOI] [PubMed] [Google Scholar]
- 51.Ribon, V., J. A. Printen, N. G. Hoffman, B. K. Kay, and A. R. Saltiel. 1998. A novel, multifuntional c-Cbl binding protein in insulin receptor signaling in 3T3-L1 adipocytes. Mol. Cell. Biol. 18:872-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ridyard, M. S., and S. M. Robbins. 2003. Fibroblast growth factor-2-induced signaling through lipid raft-associated fibroblast growth factor receptor substrate 2 (FRS2). J. Biol. Chem. 278:13803-13809. [DOI] [PubMed] [Google Scholar]
- 53.Rubin, D., and F. Ismail-Beigi. 2003. Distribution of Glut1 in detergent-resistant membranes (DRMs) and non-DRM domains: effect of treatment with azide. Am. J. Physiol. Cell Physiol. 285:C377-C383. [DOI] [PubMed] [Google Scholar]
- 54.Sargiacomo, M., M. Sudol, Z. Tang, and M. P. Lisanti. 1993. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J. Cell Biol. 122:789-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlessinger, J., and M. A. Lemmon. 2003. SH2 and PTB domains in tyrosine kinase signaling. Sci. STKE 2003:RE12. [DOI] [PubMed]
- 56.Segal, R. A., and M. E. Greenberg. 1996. Intracellular signaling pathways activated by neurotrophic factors. Annu. Rev. Neurosci. 19:463-489. [DOI] [PubMed] [Google Scholar]
- 57.Shaul, P. W., and R. G. Anderson. 1998. Role of plasmalemmal caveolae in signal transduction. Am. J. Physiol. 275:L843-L851. [DOI] [PubMed] [Google Scholar]
- 58.Shogomori, H., and D. A. Brown. 2003. Use of detergents to study membrane rafts: the good, the bad, and the ugly. Biol. Chem. 384:1259-1263. [DOI] [PubMed] [Google Scholar]
- 59.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 60.Smart, E. J., and R. G. Anderson. 2002. Alterations in membrane cholesterol that affect structure and function of caveolae. Methods Enzymol. 353:131-139. [DOI] [PubMed] [Google Scholar]
- 61.Sofroniew, M. V., C. L. Howe, and W. C. Mobley. 2001. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 24:1217-1281. [DOI] [PubMed] [Google Scholar]
- 62.Sternberg, P. W., and S. L. Schmid. 1999. Caveolin, cholesterol and Ras signalling. Nat. Cell Biol. 1:E35-E37. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki, S., T. Numakawa, K. Shimazu, H. Koshimizu, T. Hara, H. Hatanaka, L. Mei, B. Lu, and M. Kojima. 2004. BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. J. Cell Biol. [DOI] [PMC free article] [PubMed]
- 64.Tansey, M. G., R. H. Baloh, J. Milbrandt, and E. M. Johnson, Jr. 2000. GFRalpha-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron 25:611-623. [DOI] [PubMed] [Google Scholar]
- 65.Urdiales, J. L., E. Becker, M. Andrieu, A. Thomas, J. Jullien, L. A. van Grunsven, S. Menut, G. I. Evan, D. Martin-Zanca, and B. B. Rudkin. 1998. Cell cycle phase-specific surface expression of nerve growth factor receptors TrkA and p75(NTR). J. Neurosci. 18:6767-6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waugh, M. G., S. Minogue, J. S. Anderson, M. dos Santos, and J. J. Hsuan. 2001. Signalling and non-caveolar rafts. Biochem. Soc. Trans. 29:509-511. [DOI] [PubMed] [Google Scholar]
- 67.Wu, C., S. Butz, Y. Ying, and R. G. Anderson. 1997. Tyrosine kinase receptors concentrated in caveolae-like domains from neuronal plasma membrane. J. Biol. Chem. 272:3554-3559. [DOI] [PubMed] [Google Scholar]
- 68.Wu, C., C. F. Lai, and W. C. Mobley. 2001. Nerve growth factor activates persistent Rap1 signaling in endosomes. J. Neurosci. 21:5406-5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan, K. S., M. Kuti, S. Yan, S. Mujtaba, A. Farooq, M. P. Goldfarb, and M. M. Zhou. 2002. FRS2 PTB domain conformation regulates interactions with divergent neurotrophic receptors. J. Biol. Chem. 277:17088-17094. [DOI] [PubMed] [Google Scholar]
- 70.York, R. D., H. Yao, T. Dillon, C. L. Ellig, S. P. Eckert, E. W. McCleskey, and P. J. Stork. 1998. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature 392:622-626. [DOI] [PubMed] [Google Scholar]
- 71.Zhang, M., J. Liu, A. Cheng, S. M. Deyoung, X. Chen, L. H. Dold, and A. R. Saltiel. 2006. CAP interacts with cytoskeletal proteins and regulates adhesion-mediated ERK activation and motility. EMBO J. 25:5284-5293. [DOI] [PMC free article] [PubMed] [Google Scholar]