Abstract
Physiological hypoxia extends the replicative life span of human cells in culture. Here, we report that hypoxic extension of replicative life span is associated with an increase in mitochondrial reactive oxygen species (ROS) in primary human lung fibroblasts. The generation of mitochondrial ROS is necessary for hypoxic activation of the transcription factor hypoxia-inducible factor (HIF). The hypoxic extension of replicative life span is ablated by a dominant negative HIF. HIF is sufficient to induce telomerase reverse transcriptase mRNA and telomerase activity and to extend replicative life span. Furthermore, the down-regulation of the von Hippel-Lindau tumor suppressor protein by RNA interference increases HIF activity and extends replicative life span under normoxia. These findings provide genetic evidence that hypoxia utilizes mitochondrial ROS as signaling molecules to activate HIF-dependent extension of replicative life span.
Human cells have a finite capacity to replicate, and after a critical number of cell divisions, they reach a state in which further division cannot occur, termed replicative senescence (25). Multiple mechanisms are postulated to explain the process of replicative senescence, including telomere attrition and accumulation of oxidative damage to DNA, lipids, and proteins from free radicals (2, 34). The latter hypothesis is known as the free radical theory. A seminal observation to support the free radical theory was made 30 years ago by Packer and Fuehr, who demonstrated that low oxygen concentration (hypoxia) extends replicative life span of cultured primary human fibroblasts (39). The canonical interpretation of this finding was that hypoxia decreases the generation of reactive oxygen species (ROS) due to limiting oxygen levels. This results in diminished oxidative damage to DNA, lipids, and proteins, thus prolonging replicative senescence and extending the life span of cells. However, we have previously reported that the levels of intracellular ROS paradoxically increase under hypoxia (7). Therefore, the increase in replicative life span observed during hypoxia is not consistent with the free radical theory.
The source of the increased ROS generated under hypoxia is the mitochondria (5, 8, 7, 24, 31). Hypoxia increases ROS via the transfer of electrons from ubisemiquinone to molecular oxygen at the Qo site of complex III of the mitochondrial electron transport chain (3). It has previously been demonstrated that these ROS are both necessary and sufficient to activate the transcription factor hypoxia-inducible factor (HIF) (7, 8). HIFs are transcription factors that regulate physiological responses to hypoxia, including placental development, and pathophysiological processes such as cancer (44). HIFs are basic helix-loop-helix transcription factors comprised of the constitutively stable HIF-β/arylhydrocarbon receptor nuclear translocator subunit and the oxygen-regulated HIF-α subunit. Under normal oxygen conditions, HIF-α is hydroxylated at two proline residues within the oxygen-dependent degradation domain (ODDD) by a family of prolyl hydroxylase enzymes. The hydroxylated prolines are recognized by the Von Hippel-Lindau tumor suppressor protein (pVHL), the recognition component of the VHL elongin B and elongin C E3 ligase, which targets the alpha subunit for rapid ubiquitin-mediated proteasomal degradation (42). Under hypoxic conditions, the generation of mitochondrial ROS prevents the hydroxylation of HIF-α, thereby stabilizing HIF-α and allowing it to translocate to the nucleus and dimerize with HIF-1β to initiate transcription of target genes (3, 5, 24, 31).
Recent studies in cancer cells demonstrate that telomerase activity and the mRNA of the rate-limiting catalytic subunit of telomerase, human telomerase reverse transcriptase (hTERT), increase during hypoxia, and that HIF is necessary for this increase (37, 38, 53). Telomerase is an enzyme that maintains telomere integrity, and it is a major regulator of replicative life span in human cells. Multiple studies have demonstrated that the overexpression of hTERT is sufficient to increase telomerase activity and replicative life span of human fibroblasts (4, 10, 11, 49). Conversely, the disruption of telomerase activity in normal human cells restricts replicative life span (33). Here, we demonstrate that stabilization of HIF under normal oxygen conditions is sufficient to increase hTERT mRNA and telomerase activity in primary cells. More importantly, HIF is necessary to increase replicative life span under hypoxic conditions, and it is sufficient to increase replicative life span under normal oxygen conditions, thereby providing a mechanism for the increase in replicative life span observed under hypoxic conditions.
MATERIALS AND METHODS
Cell culture.
Normal primary human diploid lung fibroblasts (PHLFs) were cultured in FGM-2 medium (Cambrex) at 37°C in 5% CO2 humidified incubators. To monitor population doublings (PDs), cells were split at approximately 70% confluence, counted, and seeded at a density of 4 × 104 cells. PD was calculated using the following formula: PD = log(number of cells counted/number of cells seeded)/log2 + previous PD. Representative curves are displayed from three independent experiments. Hypoxic conditions (1.5% O2 or 3% O2) were achieved using the variable humidified InVivo2 workstation (Biotrace).
Senescence-associated β-galactosidase staining.
PHLFs at various PDs were plated in 60-mm dishes. Three days later senescence-associated β-galactosidase activity was determined with a senescence-associated β-galactosidase staining kit (Cell Signaling) according to the manufacturer's protocol. Images were obtained with a Nikon Eclipse TE200 inverted microscope at a magnification of ×10.
Stable cell lines.
Stable lines were generated in early-passage PHLFs (∼PD15 to PD25) using retroviral methods with the PT67 packaging cell line (Clontech). PT67 cells were transfected with 10 to 15 μg of plasmid using Transit-LT1 (Mirus) according to the manufacturer's protocol. Virus-containing medium was supplemented with 8 μg/ml of polybrene (Sigma) for infection. The pLXIN vector (Clontech) was used to overexpress a dominant negative HIF (HIF-DN; ATCC) (21) and the ODDDs (HIF-1α amino acids 531 to 575 fused to green fluorescent protein [GFP]). The pSiren vector (Clontech) was used to express short hairpin RNA (shRNA) sequences for pVHL (5′-GGAGCGCATTGCACATCAACG-3′) and Drosophila melanogaster HIF (dHIF) (5′-GCCTACATCCCGATCGATGATG-3′).
ROS measurement.
Intracellular ROS was measured using two independent assays. To measure acute ROS production, we used Amplex Red (Molecular Probes) according to the manufacturer's protocol. Briefly, 5 × 106 cells were lysed in Amplex Red solution (100 μM) supplemented with 200 mU/ml of superoxide dismutase (OXIS International) and incubated in the dark for 30 min. Fluorescence was measured in a Spectra Max Gemini (Molecular Probes) plate reader with excitation of 540 nm and emission of 590 nm. For longer time points we used the redox-sensitive GFP (roGFP) previously described (15). PHLFs were infected with 20 PFU of adenovirus encoding roGFP targeted to the cytosol. Cells were harvested for analysis with a CyanADP flow cytometry analyzer (Dako) 24 or 48 h after being placed under test conditions. The mean fluorescent channel for the ratio of violet excitable to blue excitable was determined with Summit software, version 4.2 (Dako). The percent oxidized probe is determined as the ratio of the sample mean to the mean from the probe oxidized by 1 mM H2O2. In the MitoQ experiment, cells were preincubated with MitoQ (gift from Michael Murphy) or methyltriphenyl-phosphonium bromide (TPMP; Sigma) for 30 min and then exposed to 21% O2 and 1.5% O2 for 4 h in the presence of either 2 μM MitoQ or TPMP.
Gene reporter assay.
HIF-mediated transcriptional activity was measured using three copies of a hormone response element (3×HRE) from the pgk-1 gene cloned into the pGL2-Basic plasmid (Promega). Cells were transfected with this plasmid using TransIT-LT1 (Mirus) according to the manufacturer's protocol. Cells were subjected to conditions for 16 h before lysates were collected. Luciferase values were determined using a dual-luciferase reporter assay kit (Promega) according to the manufacturer's protocol. Values for firefly luciferase were normalized to Renilla luciferase under the control of the thymidine kinase promoter in the pRLTK vector. In the MitoQ experiment, cells were preincubated with MitoQ or TPMP for 30 min and then exposed to 21% O2 and 1.5% O2 for 16 h in the presence of either 2 μM MitoQ or TPMP.
Real-time PCR.
PHLFs were cultured for 24 h at 21% O2 alone, after infection with 50 PFU of ODDD wild type (ODDDwt), ODDD with the mutation P564A [ODDD(P564A)], or roGFP adenoviruses, or at 1.5% O2. Adenoviral infections were performed in fibroblast basal medium (Cambrex) for 6 h. Total RNA was then isolated using an Aurum Mini Kit (Bio-Rad). cDNA was generated using the RNaqueous-4PCR system (Ambion) according to the manufacturer's protocol. Prepared cDNA was analyzed for hTERT mRNA using Platinum SYBR Green qPCR SuperMix UDG (Invitrogen). Cycle threshold (CT) values for hTERT were normalized to CT values for ribosomal protein L19, and data were analyzed using the Pfaffl method (41). Primers used were as follows: hTERT, 5′-CGTTTGGTGGATGATTTCTTGTT-3′ and 5′-TCGTCTTCTACAGGGAAGTTCA-3′; RPL19, 5′-AGTATGCTCAGGCTTCAGAAGA-3′ and 5′-CATTGGTCTCATTGGGGTCTAAC-3′.
Immunoblotting.
Nuclear extracts were prepared as previously described (7). Samples (30 μg) were resolved on a sodium dodecyl sulfate-7.5% polyacrylamide gel. HIF-1α protein was detected using a 1:250 dilution of HIF-1α antibody (clone 54; BD Biosciences), and RNA polymerase II (Pol II) protein was detected using a 1:200 dilution of RNA Pol II antibody (Santa Cruz). In the MitoQ experiment, cells were preincubated with MitoQ or TPMP for 30 min and then exposed to 21% O2 and 1.5% O2 for 4 h in the presence of either 5 μM MitoQ or TPMP.
DNA double-stranded break response.
PHLFs were incubated for 0, 4, or 8 h at 1.5 or 3% O2 in the variable hypoxic workstation InVivo2 (Biotrace Inc). Incubation with staurosporine (0.5 μM) for 6 h was used as a positive control. Cells were lysed in lysis buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 1.5 mM phenylmethylsulfonyl fluoride), and proteins were acid extracted. Cell lysates were incubated with 0.2 M HCl for 30 min on ice and centrifuged at 11,000 × g for 10 min at 4°C. Supernatant was dialyzed against 0.1 M acetic acid for 1 h twice and then against water for 1 h, 3 h, and overnight. Acid-extracted proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the presence of phosphorylated H2AX was determined by immunoblotting for γH2AX (clone JBW301; Upstate). Loading was controlled by immunoblotting for total H2AX (catalog no. 07-627; Upstate) and α-tubulin (Sigma).
Telomerase activity.
Telomerase activity was determined using a quantitative real-time telomerase repeat amplification protocol method previously described (50). Briefly, PHLFs were cultured for 24 h at 21% O2 alone, after infection with 100 PFU of ODDDwt or ODDD(P564A) adenovirus (ViraQuest Inc.), or at 1.5% O2. Cells were then harvested and suspended in CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate) buffer (10 mM Tris-HCl pH 7.5, 1 mM MgCl2, 1 mM EDTA, 5 μM β-mercaptoethanol, 10% glycerol, 0.1 mM phenylmethylsulfonyl fluoride, 0.5% CHAPS) at 105 cells/μl. The suspension was incubated on ice for 30 min and then centrifuged at 16,000 × g for 30 min. Supernatant was harvested, and 1 μl was mixed on ice with 0.1 μg of telomerase primer TS (5′-AATCCGTCGAGCAGAGTT-3′), 0.05 μg of anchored return primer ACX (5′-GCGCGG[CTTACC]3CTAACC-3′), 25 μl of SYBR Green PCR Mastermix (Bio-Rad), and water to 50 μl. Samples were incubated at 25°C for 20 min and then amplified by PCR using an iQ Icycler (Bio-Rad) and a two-step PCR for 35 cycles (30 s at 95°C and then 90 s at 60°C). Sample CT values were compared to a standard curve generated by serial dilution of extract obtained from telomerase-positive 293T cells.
Statistical analysis.
Data are presented as means ± standard error of mean. Data were analyzed using one-way analysis of variance. When analysis of variance indicated that a significant difference was present, we explored the individual difference with a Student's t test. Statistical significance was determined at a value of 0.05. Experimental samples were compared with the appropriate controls.
RESULTS
Hypoxia increases replicative life span and ROS.
Multiple reports have demonstrated that human fibroblasts extend their replicative life span during hypoxia (20, 39, 43). Consistent with previous reports, hypoxia (1.5% O2) increased the replicative life span of PHLFs by 10 PDs compared to culture conditions mimicking normal physiological oxygen conditions of lung cells (ambient air) (Fig. 1a). Cells cultured under hypoxia also displayed an increase in the rate of proliferation. Senescence of cells under normoxia and hypoxia was confirmed by staining for β-galactosidase activity, a hallmark of the senescence phenotype (13). As expected, proliferating cells under either normoxic or hypoxic conditions (PD29 and PD27, respectively) did not stain blue when fixed and treated with X-Gal (5-bromo-4-chloro-3-indolyl-β′′-d-galactopyranoside), indicating a lack of β-galactosidase activity. Normoxic cells that had stopped proliferating (PD56) clearly displayed β-galactosidase activity. In contrast, cells under hypoxic conditions at the same PD (PD55) failed to display β-galactosidase activity but eventually demonstrated β-galactosidase activity at PD66 (Fig. 1b).
FIG. 1.
Hypoxia increases replicative life span of human lung fibroblasts and mitochondrion-generated ROS. (a) PHLFs were cultured in either 21% O2 or 1.5% O2 and their PDs were monitored. (b) Senescence was detected by β-galactosidase activity in normoxia (PD29 and PD55) or hypoxia (PD27, PD55, and PD66). Scale bar, 132 μm. (c) Relative intracellular H2O2 levels of PHLFs, as determined by Amplex Red in 21% O2, 1.5% O2, or antimycin A (1 μg/ml) for 4 h in the presence of the mitochondrion-targeted antioxidant MitoQ (1 μM) or control compound TPMP (1 μM). *, P < 0.05 for TPMP at 21% O2 compared to TPMP at 1.5% O2 or antimycin A (n = 3). (d) Cytosolic ROS levels were detected by roGFP in PHLFs exposed to 21% O2 and 1.5% O2 for 24 and 48 h. Intracellular oxidant levels are displayed as a percentage of roGFP maximally oxidized by treating cells with 1 mM H2O2 for 5 min (n = 3).
In accordance with the free radical theory, the delay in replicative senescence under hypoxia is thought to be directly due to a decrease in ROS. Although hypoxia extends the replicative life span of PHLFs, the levels of intracellular ROS did not diminish under hypoxia. The levels of intracellular ROS, as determined by Amplex Red, increased in cells exposed to hypoxia compared to normoxic counterparts (Fig. 1c). As expected, PHLFs also increase intracellular ROS when incubated with the electron transport inhibitor antimycin A. Incubating these PHLFs with the mitochondria-targeted antioxidant MitoQ abrogated the increase in ROS observed in PHLFs treated with antimycin A, as well as in hypoxia, compared to cells incubated with the control compound TPMP (Fig. 1c). These results demonstrate that an increase in mitochondrial ROS production during hypoxia is associated with an extension of replicative life span.
Amplex Red is a nonphysiological target of ROS, while the target of ROS within the cell during hypoxia is likely a physiologically and biologically relevant protein. Therefore, we measured the level of ROS production in PHLFs using a biological target of ROS in living cells, roGFP. This roGFP protein contains GFP mutations with two surface-exposed cysteine residues placed at positions 147 and 204 (S147C and Q204C). In the presence of an oxidant, a disulfide bond forms between the two surface-exposed cysteines that increases the excitation at 400 nm at the expense of the peak near 490 nm. The ratio of fluorescence between 400 and 490 nm is proportional to the oxidant-induced disulfide bond formation. We infected PHLFs with an adenovirus expressing roGFP. This probe serves as a surrogate biological target of ROS in living cells (15). PHLFs displayed increased levels of ROS under hypoxic conditions at both 24 and 48 h (Fig. 1d). These data indicate that the ROS generated under hypoxic conditions are sufficient to react with protein targets in living cells.
Hypoxic increase in ROS does not result in DNA damage.
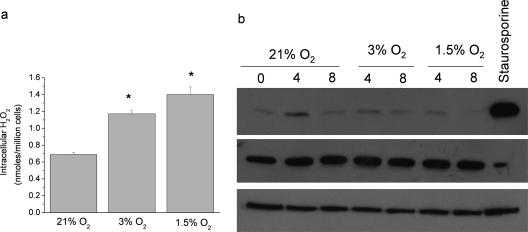
Previous studies have demonstrated that hypoxia increases ROS (3, 5, 14, 16, 24, 28, 31, 52). However, other studies have indicated that ROS are decreased under hypoxic conditions (18, 36, 48). Consistent with previously published data (7), intracellular ROS is increased in an oxygen dose-dependent manner (Fig. 2a). ROS have been shown to induce DNA double-stranded breaks. To explore whether the ROS generated under hypoxic conditions damaged DNA, we examined the phosphorylation of H2AX, a marker of DNA double-stranded breaks (40). Culturing PHLFs under hypoxic conditions that increase intracellular ROS, 3% O2 or 1.5% O2, does not induce the phosphorylation of H2AX (Fig. 2b.) However, treatment with the DNA damaging agent staurosporine does increase phosphorylation of H2AX. These data indicate that the ROS generated under hypoxic conditions do not act to damage DNA and likely rather serve as signaling molecules to initiate signal transduction cascades.
FIG. 2.
Hypoxic increase in cytosolic ROS does not induce DNA double-stranded break response. (a) Relative intracellular H2O2 levels of PHLFs, as determined by Amplex Red in 21% O2, 3% O2, or 1.5% O2. *, P < 0.05 for 3% O2 or 1.5% O2 compared to 21% O2 (n = 3). (b) Phosphorylation status of H2AX in acid-extracted proteins from PHLF exposed to 21% O2, 3% O2, 1.5% O2, or staurosporine.
Mitochondrion-generated ROS stabilize HIF-1α during hypoxia in PHLFs.
The mitochondrion-generated ROS produced under hypoxia initiate signaling cascades that result in cellular adaptation to environments with decreased oxygen levels (17). One such signaling cascade results in the activation of HIF to induce a transcriptional program. HIF is not activated in the absence of mitochondrion-generated ROS, indicating that the hypoxic activation of HIF during hypoxia requires mitochondrial ROS (3, 5, 7, 24, 31). Hypoxic activation of HIF-mediated transcription occurs in an oxygen dose-dependent manner (Fig. 3a). As expected, hypoxic stabilization of HIF-1α protein levels and HIF activity was abolished in the presence of the mitochondrion-targeted antioxidant MitoQ (Fig. 3b and c). These data indicate that PHLFs require hypoxic induction of mitochondrial ROS to activate HIF.
FIG. 3.
Hypoxia-induced generation of mitochondrial ROS triggers HIF activation in PHLFs. (a) PHLFs transiently transfected with the firefly luciferase reporter construct driven by 3×HRE and the Renilla luciferase construct driven by the thymidine kinase promoter and exposed to 21% O2, 3% O2, or 1.5% O2. *, P < 0.05 for 3% O2 or 1.5% O2 compared to 21% O2 (n = 3). (b) HIF-1α and RNA Pol II protein levels in nuclear extracts from PHLFs exposed to 21% O2 and 1.5% O2 for 4 h in the presence of either 5 μM MitoQ or TPMP. (c) PHLFs transiently transfected with the firefly luciferase reporter construct driven by 3×HRE and the Renilla luciferase construct driven by the thymidine kinase promoter. Cells were exposed to 2 μM MitoQ or TPMP under 21% O2 and 1.5% O2 for 16 h. Relative luciferase values were determined by normalizing firefly to Renilla luciferase. *, P < 0.05 for TPMP at 21% O2 compared to TPMP at 1.5% O2 (n = 3).
HIF is sufficient to increase hTERT and telomerase activity in PHLFs.
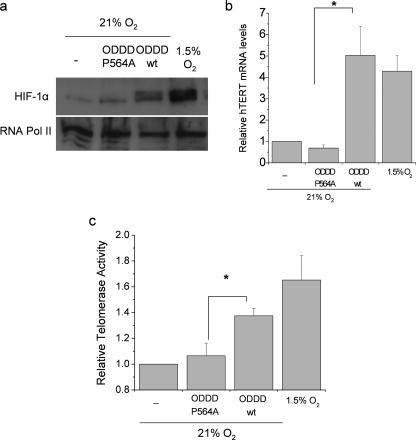
Hypoxia increases telomerase activity and hTERT mRNA, and HIF is necessary for this increase (53). However, it is not established if HIF alone is sufficient to mediate these increases. To explore this possibility, we utilized a technique that specifically stabilizes HIF protein under normal oxygen conditions. Overexpression of a peptide that corresponds to HIF ODDD effectively competes with endogenous HIF for its E3 ligase pVHL, allowing HIF to accumulate, translocate to the nucleus, and induce transcription. Stabilization of endogenous HIF under normoxia, achieved by overexpression of the HIF ODDD (ODDDwt) but not a mutant lacking proline 564 [ODDD(P564A)] was sufficient to increase HIF-1 α protein levels (Fig. 4a). Stabilization of HIF-1α protein by overexpressing the ODDDwt increased hTERT mRNA and telomerase activity compared to cells that overexpress ODDD(P564A) (Fig. 4b and c). These data demonstrate that HIF activation is sufficient to induce hTERT expression and telomerase activity in primary cells.
FIG. 4.
Stabilization of HIF under normal oxygen conditions is sufficient to increase hTERT transcription and telomerase activity. HIF-1α protein levels (a), relative hTERT mRNA levels normalized to the ribosomal protein L19 (b), and telomerase activity (c) in PHLF exposed to 21% O2, PHLF infected with adenovirus encoding the ODDDwt or ODDD(P564A) of HIF-1α (amino acids 531 to 575) in 21% O2, or PHLF in 1.5% O2 *, P < 0.05 for ODDDwt compared to ODDD(P564A) (n = 3).
HIF transcriptional activity is necessary for hypoxic increase in replicative life span.
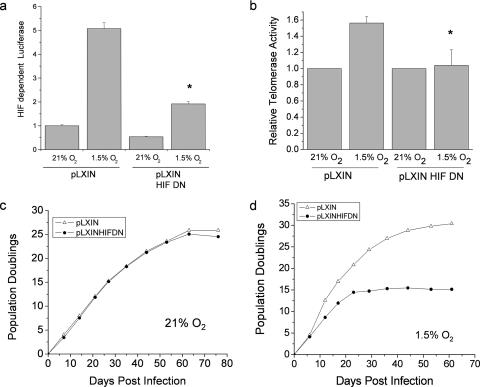
Since HIF is sufficient to increase telomerase activity in primary cells and telomerase activity increases replicative life span, we examined whether HIF transcriptional activity is necessary for the hypoxic increase in replicative life span. PHLFs stably expressing HIF-DN (21) demonstrated impaired HIF transcriptional activity under hypoxia (Fig. 5a). Furthermore, these cells were not able to increase telomerase activity under hypoxic conditions (Fig. 5b). No difference in PDs was observed between cells containing the HIF-DN and the vector control cells (pLXIN) cultured under normoxic conditions (Fig. 5c). By contrast, cells expressing the HIF-DN cultured under hypoxia had a significantly lower PD than control cells (Fig. 5d), indicating that HIF is necessary for the hypoxic increase in replicative life span.
FIG. 5.
HIF is necessary for the hypoxic increase in replicative life span. (a) Transcriptional activity of PHLFs stably expressing HIF-DN or the vector control pLXIN was assayed with a firefly luciferase reporter construct driven by 3×HRE and with a control Renilla luciferase construct driven by the thymidine kinase promoter. Relative luciferase values were determined by normalizing values of firefly to Renilla luciferase (n = 3). (b) Telomerase activity of PHLFs stably expressing HIF-DN or pLXIN exposed to 21% O2 or 1.5% O2 (n = 3). PHLFs stably expressing HIF-DN or pLXIN were grown in 21% O2 (c) or 1.5% O2 (d), and their PDs were monitored postinfection.
Activation of HIF or loss of pVHL is sufficient to extend replicative life span.
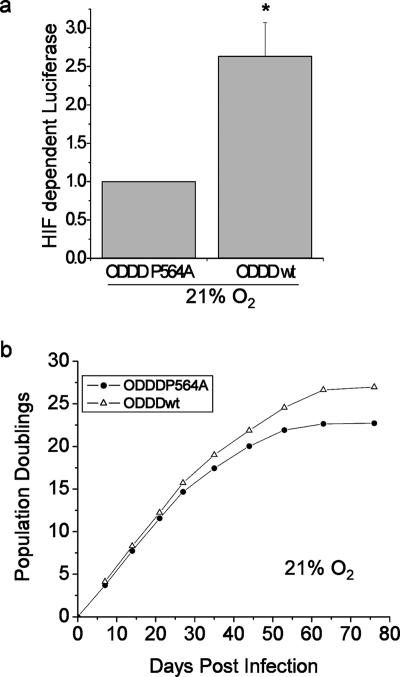
To determine whether stabilization of endogenous HIF under normoxia is sufficient to extend replicative life span, PHLFs stably expressing ODDDwt or ODDD(P564A) were cultured until they senesced. Cells expressing ODDDwt display an increase in HIF transcriptional activity and undergo five more PDs than cells that express the ODDD(P564A) (Fig. 6a and b). These data clearly demonstrate that HIF is sufficient to increase replicative life span under normal oxygen conditions, providing a novel role for HIF in the regulation of replicative life span.
FIG. 6.
HIF is sufficient for the increase in replicative life span. PHLF cells with activated endogenous HIF were generated by stably expressing the ODDDwt construct, with ODDD(P564A) as a control. Transcriptional activity of HIF (a) and PDs (b) were monitored under 21% O2 at the indicated days postinfection. * P < 0.05 for ODDDwt compared to ODDD(P564A) (n = 3).
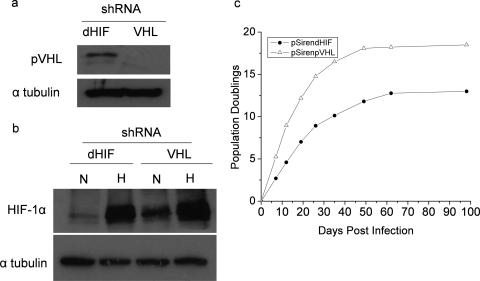
The loss of tumor suppressor VHL increases HIF activity under normoxia. To test whether the loss of pVHL would regulate senescence, PHLFs were created to stably express an shRNA against pVHL (Fig. 7a). Cells expressing the shRNA against pVHL displayed an increase in HIF-1α protein under normal oxygen conditions (Fig. 7b). These cells also displayed an increase in PDs compared to cells that expressed the control shRNA against dHIF under normal oxygen conditions (Fig. 7c). Thus, loss of pVHL is sufficient to increase replicative life span under normoxic conditions.
FIG. 7.
Loss of the pVHL tumor suppressor protein that regulates HIF increases replicative life span in PHLF. (a) pVHL protein levels from whole-cell lysates of PHLF stably expressing an shRNA against pVHL. (b) HIF-1α protein levels in cells stably expressing shRNA for dHIF and pVHL that were exposed to 21% O2 or 1.5% O2. N, normoxia; H, hypoxia. (c) PHLFs expressing an shRNA against pVHL or dHIF (control) were grown in 21% O2, and their PDs were monitored postinfection.
DISCUSSION
Our results reveal that mitochondrial ROS behave as signaling molecules to activate an HIF-dependent increase in telomerase activity, thus contributing to the positive regulation of replicative life span in human cells. Paradoxically, ROS have been previously demonstrated to increase during hypoxia (3, 5, 7, 24, 31). The increase in ROS during hypoxia occurs at the Qo site of mitochondrial complex III (3). Consistent with previous observations, our current study indicates that hypoxia increases both ROS and HIF activity as a function of decreasing oxygen levels (7). Typically, ROS are thought to be exclusively damaging and are associated with the induction of senescence (2). However, we demonstrate in the current study that mitochondrial ROS generated under hypoxic conditions do not activate the DNA double-stranded break response. Instead, they activate HIF, resulting in the induction of hTERT mRNA and subsequent telomerase activity. Previous studies have demonstrated that hTERT is an HIF target gene. Therefore, our study highlights the finding that ROS can serve as signaling molecules to regulate HIF-dependent replicative senescence.
Multiple studies have demonstrated that low levels of ROS can serve as signaling molecules and are required for cell proliferation and gene expression by inactivating phosphatases and activating kinases (1, 19, 26, 27, 35, 47). Our results suggest that there is a graded response to senescence with respect to ROS. Accordingly, increased levels of oxidative stress during hypoxia would function as adaptive signaling molecules to extend replicative life span via activation of HIF, whereas higher levels of oxidative stress would trigger premature senescence. We speculate that the generation of mitochondrial ROS has not been completely eliminated in evolution because they are involved in activating genes that help in the adaptation to stress, such as changes in oxygen levels. In fact, ROS have been shown to activate transcription factors OxyR and Yap1 in Escherichia coli and Saccharomyces cerevisiae, respectively (for a review, see reference 22). Furthermore, there are multiple studies indicating redox-based activation of transcription factors in mammalian cells as well (for a review, see reference 30).
The finding that HIF is involved in the regulation of senescence is significant with respect to cancer. Reports indicate that overcoming senescence is critical for tumor suppression (6). Activation of oncogenes such as Ras or loss of the tumor suppressor PTEN can activate p53-dependent senescence to prevent the progression of cells to a transformed state (9, 46). Dysregulation of p53 occurs frequently in cancer, as does HIF overexpression (45). A causal role of HIF has been established in renal cell carcinoma, where a genetic defect results in loss of function of the tumor suppressor pVHL (29, 32). The loss of pVHL function results in an increase in HIF levels under normoxia, thereby contributing to the tumorigenicity of renal cell carcinoma via aberrant activation of HIF (29). The finding that HIF is sufficient under normal oxygen conditions to increase replicative life span demonstrates that HIF is capable of regulating replicative capacity to further promote the ability of precancerous cells to overcome the tumor-suppressing mechanism of senescence. The ability of HIF to increase replicative life span would provide a growth advantage in cancers that have aberrant upregulation of HIF due to loss of tumor suppressors or activation of oncogenes. A recent report also indicated that HIF regulates senescence in mouse cells (51). Collectively, these data identify a novel role of HIF in the regulation of cancer in addition to its established role in regulating tumor angiogenesis and metabolism.
In summary, our data demonstrating that HIF is necessary for hypoxic increase in replicative life span provide an alternative mechanism to the free radical theory for explaining the observed increase in replicative life span. Moreover, the finding that HIF is sufficient to increase replicative life span under normal oxygen conditions demonstrates that HIF is capable of regulating replicative capacity and would further implicate HIF as a positive regulator in diseases associated with increased proliferation, such as cancer and pulmonary hypertension. Finally, hypoxia and HIF have been implicated in maintaining stem cells in an undifferentiated state (12, 23). The ability of hypoxia and HIF to extend replicative life span would further provide an environmental advantage for stem cells to reside in hypoxic niches like the bone marrow.
Acknowledgments
This work was supported in part by National Institutes of Health grants (GM60472-07, P01HL071643-03004, and R21AG027093) to N. S. Chandel. E. Bell is supported by American Heart Association grant 0515563Z.
We thank Michael Murphy from University of Cambridge, United Kingdom, for providing us with MitoQ.
Footnotes
Published ahead of print on 11 June 2007.
REFERENCES
- 1.Abe, J., M. Takahashi, M. Ishida, J. D. Lee, and B. C. Berk. 1997. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J. Biol. Chem. 272:20389-20394. [DOI] [PubMed] [Google Scholar]
- 2.Beckman, K. B., and B. N. Ames. 1998. The free radical theory of aging matures. Physiol. Rev. 78:547-581. [DOI] [PubMed] [Google Scholar]
- 3.Bell, E. L., T. A. Klimova, J. Eisenbart, C. T. Moraes, M. Murphy, G. R. S. Budinger, and N. S. Chandel. 11 June 2007, posting date. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J. Cell Biol. doi: 10.1083/jcb.200609074v1. [DOI] [PMC free article] [PubMed]
- 4.Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. [DOI] [PubMed] [Google Scholar]
- 5.Brunelle, J. K., E. L. Bell, N. M. Quesada, K. Vercauteren, V. Tiranti, M. Zeviani, R. C. Scarpulla, and N. S. Chandel. 2005. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 1:409-414. [DOI] [PubMed] [Google Scholar]
- 6.Campisi, J. 2005. Suppressing cancer: the importance of being senescent. Science 309:886-887. [DOI] [PubMed] [Google Scholar]
- 7.Chandel, N. S., E. Maltepe, E. Goldwasser, C. E. Mathieu, M. C. Simon, and P. T. Schumacker. 1998. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 95:11715-11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandel, N. S., D. S. McClintock, C. E. Feliciano, T. M. Wood, J. A. Melendez, A. M. Rodriguez, and P. T. Schumacker. 2000. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 275:25130-25138. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Z., L. C. Trotman, D. Shaffer, H. K. Lin, Z. A. Dotan, M. Niki, J. A. Koutcher, H. I. Scher, T. Ludwig, W. Gerald, C. Cordon-Cardo, and P. P. Pandolfi. 2005. Crucial role of p53-dependent cellular senescence in suppression of PTEN-deficient tumorigenesis. Nature 436:725-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Counter, C. M., W. C. Hahn, W. Wei, S. D. Caddle, R. L. Beijersbergen, P. M. Lansdorp, J. M. Sedivy, and R. A. Weinberg. 1998. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc. Natl. Acad. Sci. USA 95:14723-14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Counter, C. M., M. Meyerson, E. N. Eaton, L. W. Ellisen, S. D. Caddle, D. A. Haber, and R. A. Weinberg. 1998. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene 16:1217-1222. [DOI] [PubMed] [Google Scholar]
- 12.Cowden Dahl, K. D., B. H. Fryer, F. A. Mack, V. Compernolle, E. Maltepe, D. M. Adelman, P. Carmeliet, and M. C. Simon. 2005. Hypoxia-inducible factors 1α and 2α regulate trophoblast differentiation. Mol. Cell. Biol. 25:10479-10491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimri, G., X. Lee, G. Basile, M. Acosta, G. Scott, C. Roskelley, E. Medrano, M. Linskens, I. Rubelj, O. Pereira-Smith, M. Peacocke, and J. Campisi. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dirmeier, R., K. M. O'Brien, M. Engle, A. Dodd, E. Spears, and R. O. Poyton. 2002. Exposure of yeast cells to anoxia induces transient oxidative stress. Implications for the induction of hypoxic genes. J. Biol. Chem. 277:34773-34784. [DOI] [PubMed] [Google Scholar]
- 15.Dooley, C. T., T. M. Dore, G. T. Hanson, W. C. Jackson, S. J. Remington, and R. Y. Tsien. 2004. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 279:22284-22293. [DOI] [PubMed] [Google Scholar]
- 16.Duranteau, J., N. S. Chandel, A. Kulisz, Z. Shao, and P. T. Schumacker. 1998. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J. Biol. Chem. 273:11619-11624. [DOI] [PubMed] [Google Scholar]
- 17.Emerling, B. M., and N. S. Chandel. 2005. Oxygen sensing: getting pumped by sterols. Sci. STKE 2005:pe30. [DOI] [PubMed] [Google Scholar]
- 18.Fandrey, J., S. Frede, and W. Jelkmann. 1994. Role of hydrogen peroxide in hypoxia-induced erythropoietin production. Biochem. J. 303:507-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finkel, T. 2003. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15:247-254. [DOI] [PubMed] [Google Scholar]
- 20.Forsyth, N. R., A. P. Evans, J. W. Shay, and W. E. Wright. 2003. Developmental differences in the immortalization of lung fibroblasts by telomerase. Aging Cell 2:235-243. [DOI] [PubMed] [Google Scholar]
- 21.Forsythe, J. A., B. H. Jiang, N. V. Iyer, F. Agani, S. W. Leung, R. D. Koos, and G. L. Semenza. 1996. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16:4604-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgiou, G. 2002. How to flip the (redox) switch. Cell 111:607-610. [DOI] [PubMed] [Google Scholar]
- 23.Gustafsson, M. V., X. Zheng, T. Pereira, K. Gradin, S. Jin, J. Lundkvist, J. L. Ruas, L. Poellinger, U. Lendahl, and M. Bondesson. 2005. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell 9:617-628. [DOI] [PubMed] [Google Scholar]
- 24.Guzy, R. D., B. Hoyos, E. Robin, H. Chen, L. Liu, K. D. Mansfield, M. C. Simon, U. Hammerling, and P. T. Schumacker. 2005. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 1:401-408. [DOI] [PubMed] [Google Scholar]
- 25.Hayflick, L. 1965. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37:614-636. [DOI] [PubMed] [Google Scholar]
- 26.Irani, K., Y. Xia, J. L. Zweier, S. J. Sollott, C. J. Der, E. R. Fearon, M. Sundaresan, T. Finkel, and P. J. Goldschmidt-Clermont. 1997. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 275:1649-1652. [DOI] [PubMed] [Google Scholar]
- 27.Kamata, H., S. Honda, S. Maeda, L. Chang, H. Hirata, and M. Karin. 2005. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120:649-661. [DOI] [PubMed] [Google Scholar]
- 28.Killilea, D. W., R. Hester, R. Balczon, P. Babal, and M. N. Gillespie. 2000. Free radical production in hypoxic pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 279:L408-412. [DOI] [PubMed] [Google Scholar]
- 29.Kondo, K., J. Klco, E. Nakamura, M. Lechpammer, J. Kaelin, and G. William. 2002. Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell 1:237-246. [DOI] [PubMed] [Google Scholar]
- 30.Liu, H., R. Colavitti, I. I. Rovira, and T. Finkel. 2005. Redox-dependent transcriptional regulation. Circ. Res. 97:967-974. [DOI] [PubMed] [Google Scholar]
- 31.Mansfield, K. D., R. D. Guzy, Y. Pan, R. M. Young, T. P. Cash, P. T. Schumacker, and M. C. Simon. 2005. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-α activation. Cell Metab. 1:393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maranchie, J. K., J. R. Vasselli, J. Riss, J. S. Bonifacino, W. M. Linehan, and R. D. Klausner. 2002. The contribution of VHL substrate binding and HIF1-α to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell 1:247-255. [DOI] [PubMed] [Google Scholar]
- 33.Masutomi, K., E. Y. Yu, S. Khurts, I. Ben-Porath, J. L. Currier, G. B. Metz, M. W. Brooks, S. Kaneko, S. Murakami, J. A. DeCaprio, R. A. Weinberg, S. A. Stewart, and W. C. Hahn. 2003. Telomerase maintains telomere structure in normal human cells. Cell 114:241-253. [DOI] [PubMed] [Google Scholar]
- 34.McEachern, M. J., A. Krauskopf, and E. H. Blackburn. 2000. Telomeres and their control. Annu. Rev. Genet. 34:331-358. [DOI] [PubMed] [Google Scholar]
- 35.Meng, T. C., T. Fukada, and N. K. Tonks. 2002. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 9:387-399. [DOI] [PubMed] [Google Scholar]
- 36.Michelakis, E. D., I. Rebeyka, X. Wu, A. Nsair, B. Thebaud, K. Hashimoto, J. R. B. Dyck, A. Haromy, G. Harry, A. Barr, and S. L. Archer. 2002. O2 Sensing in the human ductus arteriosus: regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ. Res. 91:478-486. [DOI] [PubMed] [Google Scholar]
- 37.Minamino, T., S. A. Mitsialis, and S. Kourembanas. 2001. Hypoxia extends the life span of vascular smooth muscle cells through telomerase activation. Mol. Cell. Biol. 21:3336-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishi, H., T. Nakada, S. Kyo, M. Inoue, J. W. Shay, and K. Isaka. 2004. Hypoxia-inducible factor 1 mediates upregulation of telomerase (hTERT). Mol. Cell. Biol. 24:6076-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Packer, L., and K. Fuehr. 1977. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature 267:423-425. [DOI] [PubMed] [Google Scholar]
- 40.Paull, T. T., E. P. Rogakou, V. Yamazaki, C. U. Kirchgessner, M. Gellert, and W. M. Bonner. 2000. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10:886-895. [DOI] [PubMed] [Google Scholar]
- 41.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Safran, M., and W. G. Kaelin, Jr. 2003. HIF hydroxylation and the mammalian oxygen-sensing pathway. J. Clin. Investig. 111:779-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito, H., A. T. Hammond, and R. E. Moses. 1995. The effect of low oxygen tension on the in vitro-replicative life span of human diploid fibroblast cells and their transformed derivatives. Exp. Cell Res. 217:272-279. [DOI] [PubMed] [Google Scholar]
- 44.Semenza, G. L. 2000. HIF-1 and human disease: one highly involved factor. Genes Dev. 14:1983-1991. [PubMed] [Google Scholar]
- 45.Semenza, G. L. 2003. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3:721-732. [DOI] [PubMed] [Google Scholar]
- 46.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 47.Tobiume, K., A. Matsuzawa, T. Takahashi, H. Nishitoh, K. Morita, K. Takeda, O. Minowa, K. Miyazono, T. Noda, and H. Ichijo. 2001. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2:222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaux, E. C., E. Metzen, K. M. Yeates, and P. J. Ratcliffe. 2001. Regulation of hypoxia-inducible factor is preserved in the absence of a functioning mitochondrial respiratory chain. Blood 98:296-302. [DOI] [PubMed] [Google Scholar]
- 49.Vaziri, H., and S. Benchimol. 1998. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 8:279-282. [DOI] [PubMed] [Google Scholar]
- 50.Wege, H., M. S. Chui, H. T. Le, J. M. Tran, and M. A. Zern. 2003. SYBR Green real-time telomeric repeat amplification protocol for the rapid quantification of telomerase activity. Nucleic Acids Res. 31:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welford, S. M., B. Bedogni, K. Gradin, L. Poellinger, M. Broome Powell, and A. J. Giaccia. 2006. HIF1α delays premature senescence through the activation of MIF. Genes Dev. 20:3366-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wood, J. G., J. S. Johnson, L. F. Mattioli, and N. C. Gonzalez. 1999. Systemic hypoxia promotes leukocyte-endothelial adherence via reactive oxidant generation. J Appl. Physiol. 87:1734-1740. [DOI] [PubMed] [Google Scholar]
- 53.Yatabe, N., S. Kyo, Y. Maida, H. Nishi, M. Nakamura, T. Kanaya, M. Tanaka, K. Isaka, S. Ogawa, and M. Inoue. 2004. HIF-1-mediated activation of telomerase in cervical cancer cells. Oncogene 23:3708-3715. [DOI] [PubMed] [Google Scholar]