Abstract
Enhancer elements modulate promoter activity over vast chromosomal distances, and mechanisms that ensure restrictive interactions between promoters and enhancers are critical for proper control of gene expression. The human β-globin locus control region (LCR) activates expression of five genes in erythroid cells, including the proximal embryonic ɛ- and the distal adult β-globin genes. To test for possible distance sensitivity of the genes to the LCR, we extended the distance between the LCR and genes by 2.3 kbp within the context of a yeast artificial chromosome, followed by the generation of transgenic mice (TgM). In these TgM lines, ɛ-globin gene expression decreased by 90%, while the more distantly located γ- or β-globin genes were not affected. Remarkably, introduction of a consensus EKLF binding site into the ɛ-globin promoter rendered its expression distance insensitive; when tested in an EKLF-null genetic background, expression of the mutant ɛ-globin gene was severely compromised. Thus, the ɛ-globin gene differs in its distance sensitivity to the LCR from the other β-like globin genes, which is, at least in part, determined by the transcription factor EKLF.
Proper temporal and spatial expression of genetic information is tightly regulated by DNA cis elements such as promoters, enhancers, and insulators. Specific combinations of enhancers and promoters often act synergistically to finely tune gene expression. In contrast, inappropriate communication between an enhancer and promoter can disturb their in vivo expression patterns. Enhancers were originally defined by their ability to activate transcription of cis-linked genes over considerable distances in an orientation-independent manner. Increasing evidence suggests that enhancers can physically and functionally interact with promoters over long distances, exceeding several hundreds of kilobase pairs in cis, or even with promoters located on different chromosomes in trans (reviewed in references 5 and 13). This suggests the presence of molecular mechanisms that allow specific enhancer-promoter interactions to take place while protecting promoters from being inappropriately activated by enhancer elements that might be located in neighboring gene loci or even on different chromosomes.
The human β-globin genes are organized within a 70-kbp span of chromosome 11, with the embryonic ɛ-globin gene located most 5′, followed by the two fetal γ-globin genes (Gγ and Aγ), while the adult δ- and β-globin genes are at the 3′ end of the locus (Fig. 1A). Expression of all the β-like globin genes, in primitive (embryonic) as well as definitive (fetal and adult) erythroid cells, requires the activity of the locus control region (LCR)/enhancer, which consists of five DNase I hypersensitive sites (HSs) and is located 6 or 48 kbp 5′ to the transcription initiation sites of the ɛ- and β-globin genes, respectively (19, 27).
FIG. 1.
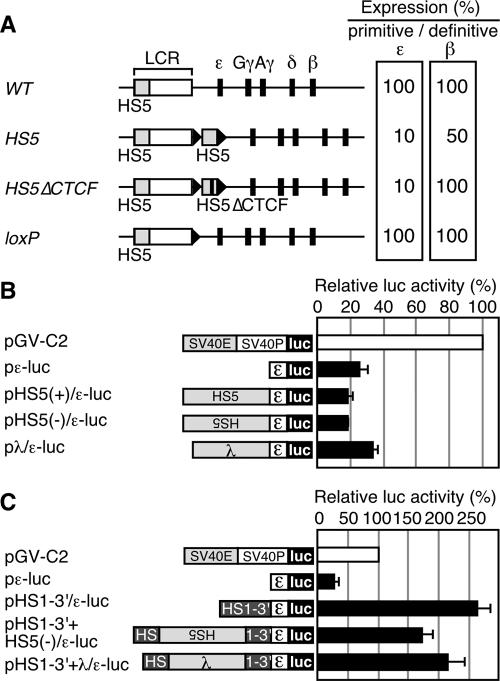
(A) Relative expression of the primitive ɛ-globin or the definitive β-globin genes in YAC TgM. The values in the WT TgM are set at 100. Results from the WT (28), HS5 (containing a 2.6 kbp HS5 fragment inserted 3′ to HS1) (30), HS5▵CTCF (containing the HS5 fragment lacking CTCF binding sites), and loxP (same as the WT, except that a ∼40-bp loxP footprint (arrowhead) is retained) TgM are shown. (B) Analysis of potential silencer activity associated with HS5 in K562 cells. Structures of the reporter constructs used in the transfection assays are shown on the left. The human ɛ-globin promoter region (174 to +38) was subcloned into the luciferase reporter plasmid to generate pɛ-luc. The HS5 DNA fragment (2.6 kbp; same as the one used in the HS5 TgM) was inserted upstream of the ɛ-globin promoter in sense or antisense orientations to generate pHS5±/ɛ-luc. A 2.3-kbp fragment from λ DNA was inserted upstream of the ɛ-globin gene promoter to generate pλ/ɛ-luc. The reference reporter construct pGV-C2 is driven by the simian virus 40 (SV40) enhancer (E) and promoter (P). K562 cells were transfected with the luciferase reporter plasmids as well as the control pCMV-β-Gal. Luciferase activities were normalized to β-Gal activities to control for transfection efficiencies. The relative luciferase activity is expressed relative to that in the pGV-C2 (set at 100). Each value of luciferase activity represents the mean ± SD for at least three independent experiments. (C) HS5 DNA insertion does not attenuate the enhancer activity localized within the 3′ region of HS1 (HS1-3′). The HS1-3′ DNA fragment (∼1 kbp) was inserted upstream of the ɛ-globin promoter, within the context of pɛ-luc, to generate pHS1-3′/ɛ-luc. The HS5 (2.6 kbp) or the λ (2.3 kbp) DNA fragment was inserted in the HS1-3′ region (at the same position as the HS5 fragment was inserted in the HS5 TgM) of pHS1-3′/ɛ-luc to generate pHS1-3′+HS5(−)/ɛ-luc and pHS1-3′+λ/ɛ-luc, respectively. These reporter plasmids were transfected into K562 cells and assayed as described above.
How distal enhancer elements and LCRs activate gene expression has long been a subject of intense debate, and numerous models have been proposed. Among them, two models, “looping” and “tracking,” have been most often considered. The looping model predicts that proteins bound to the LCR (enhancer) and to the promoters physically associate to form a complex with the intervening DNA looped out (4). In the tracking model, an activating signal, such as RNA polymerase II (Pol II) or histone modifiers, launches from the enhancer and scans along the DNA toward a promoter (32). A compromise between these two models is the “facilitated tracking” model, in which enhancer-bound complexes track along chromatin until a promoter is encountered, at which stage a stable DNA loop structure is formed (5).
Recently, it has been shown by chromatin conformation capture and other assays that the LCR physically interacts with the β-globin gene in definitive erythroid cells of the fetal liver, an observation that is consistent with the looping or facilitated tracking models (3, 23, 31). Subsequently, detailed chromatin conformation capture analyses have shown that the whole β-globin locus forms a higher-order chromatin structure, dubbed a “chromatin hub,” which changes configurations as erythroid cell maturation proceeds (22). Based on these and other observations, it is now widely accepted that the LCR/enhancer activates β-globin gene expression at least in definitive erythroid cells by a looping mechanism. In this case, specific enhancer-promoter interactions may require association of enhancer-bound and promoter-bound protein complexes, possibly involving GATA-1, FOG-1 or EKLF (8, 33).
Another mechanism that ensures tightly regulated enhancer-promoter interactions involves enhancer-blocking insulators, which prevent an enhancer from activating a promoter when an insulator is located between them. The most extensively characterized vertebrate insulator was originally identified in the chicken β-globin LCR, which consists of four DNase I HSs. The 5′-most of these, HS4, bears a CTCF regulatory factor-dependent insulator activity. The human ortholog of chicken HS4 is LCR element HS5 (1, 11).
To evaluate possible insulator activity of human HS5, we introduced a 2.5-kbp HS5 DNA fragment 3′ to LCR HS1 within the context of a yeast artificial chromosome (YAC) bearing the human β-globin locus and generated transgenic mice (TgM) (Fig. 1A, HS5) (30). In definitive erythroid cells of a TgM line, β-globin gene expression level was reduced by 50% of wild-type (WT) controls. Because this phenotype disappeared when the CTCF binding site was deleted from the HS5 fragment (HS5ΔCTCF), we concluded that HS5 carries a CTCF-dependent insulator activity in definitive erythroid cells. In primitive erythroid cells of the HS5 TgM, ɛ- but not γ-globin gene expression was also severely affected, resulting in a ∼90% reduction in ɛ-globin mRNA accumulation. Surprisingly, however, ɛ-globin gene expression was not rescued by the deletion of the HS5 CTCF site. The level of ɛ-globin gene expression recovered only after removing the whole HS5 fragment by in vivo Cre-loxP recombination (loxP). Thus, reduced ɛ-globin gene expression in primitive erythroid cells of the HS5 TgM was not solely attributable to HS5 insulator activity.
At least three hypotheses might explain the observed phenotype: (i) HS5 bears a previously unidentified, ɛ-globin gene-specific repressor activity; (ii) inserting the HS5 DNA fragment 3′ to HS1 impaired a previously unrecognized ɛ-globin gene-specific enhancer activity located at or near the insertion site; or (iii) extending the distance between the LCR and the ɛ-globin promoter somehow interfered with appropriate enhancer-promoter communication.
In the current work, we employed both cell-based transfection assays and YAC-TgM methodology to test the above-stated hypotheses. We show the following: (i) that no significant repressor activity resides in HS5; (ii) that although enhancer activity was found within the HS1 region in K562 cells, that activity was not impaired by insertion of the HS5 DNA fragment; and (iii) that inserting a non-insulator-containing DNA fragment (a piece of bacteriophage λ DNA) at two independent sites located between the LCR and the ɛ-globin gene attenuated ɛ-globin gene expression by more than 10-fold in YAC TgM. These results are consistent with the hypothesis that ɛ-globin gene transcription is sensitive to its distance from the LCR.
We also introduced the binding motif for the transcription factor EKLF into the ɛ-globin promoter in order to mimic the promoter architecture of the adult β-globin gene (a mutant previously termed Bepsi) (29) and then tested the distance sensitivity of this mutant promoter, since EKLF activity is known to be essential for β-globin transcription in definitive erythroid cells (24, 34) and is additionally required for the formation of an active chromatin hub, which positions the β-globin gene in close proximity to the LCR (8). Analysis of these YAC TgM lines revealed that the distance sensitivity of the Bepsi promoter became less pronounced in primitive erythroid cells. Importantly, when the same mice were tested in an EKLF-null genetic background, Bepsi mutant promoter sensitivity to its distance from the LCR was restored. Thus, although they evolved from a single ancestral gene (27), the ɛ- and β-globin promoters fundamentally differ in that the former is much more sensitive to its distance from the LCR. We speculate that evolution of the present-day β-globin gene promoter involved the creation of a binding site for EKLF, which mediates loop formation and renders the β-globin gene insensitive to distance from the LCR.
MATERIALS AND METHODS
Reporter plasmid construction and transfection assay.
The human ɛ-globin promoter (from nucleotides [nt] 19314 to 19525; GenBank, HUMHBB locus) was PCR-amplified with the primers EPSIR-5S (5′-CCTCAGATCTCCGCGGCACACATTATCACAAACTT-3′ with BglII and SacII sites) and EPSIR-3A (5′-GGCCTGAAGCTTGCTAGTGAT-3′ with an HindIII site). This fragment was then digested with BglII and HindIII enzymes (sites are underlined) and subcloned into the BglII/HindIII-digested luciferase (luc) reporter plasmid, pGV-B2 (TOYOINK), to generate pɛ-luc. The HS5 DNA was excised as a BamHI fragment from the plasmid pHS1/loxPw+/HS5 (30) and subcloned into BglII-digested pɛ-luc in sense or antisense orientations to generate pHS5+/ɛ-luc and pHS5−/ɛ-luc, respectively (pHS5±/ɛ-luc). The 2.3-kbp HindIII λ DNA fragment was excised as a BamHI fragment from the plasmid pHS1/loxPw+/λ (described below) and subcloned into BglII-digested pɛ-luc to generate pλ/ɛ-luc. The HS1-3′ fragment was excised as an XhoI-SacII fragment from the pHS1/loxP(+), which was generated by introducing the annealed HS1-LX1-5S and HS1-LX1-3A oligonucleotides (30) into HindIII-digested pHS1 (30) in the forward orientation. This fragment was subcloned into XhoI/SacII-digested pɛ-luc to generate pHS1-3′/ɛ-luc. The HS1-3′+HS5(−) DNA fragment was excised as an XhoI-SacII fragment from the plasmid pHS1/loxPw+/HS5(−) (30), subcloned into XhoI/SacII-digested pɛ-luc to generate pHS1-3′+HS5(−)/ɛ-luc. The HS1-3′+λ DNA fragment was excised as an XhoI-SacII fragment from the pHS1/loxPw+/λ (described below) and subcloned into XhoI/SacII-digested pɛ-luc to generate pHS1-3′+λ/ɛ-luc.
K562 cells expressing endogenous ɛ-globin gene were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum. Transfections were done by using Lipofectamine (Invitrogen). A β-galactosidase reporter plasmid was included to control for transfection efficiency. Luciferase and β-galactosidase assays were performed at least in triplicate, and average values with standard deviations (SD) were calculated.
YAC mutagenesis.
The 2.3-kbp HindIII-digested λ DNA fragment was subcloned into HindIII-digested pHS1/loxPw+ (30) to generate pHS1/loxPw+/λ. This plasmid DNA was linearized with SpeI and used for mutagenizing the human β-globin YAC (A201F4.3) to make A201F4.3/HS1/λ.
The human ɛ-globin 5′ upstream region (from nt 17174 to 17864; HUMHBB, GenBank) was PCR amplified with the primer set EP-5S (5′-TGCGGATCCTGAAGTGCTGGGATTATAGG-3′; BamHI site underlined) and EP-3A (5′-AGTAAGCTTCCTATGTTCAGGCCCTAGAGG-3′; HindIII site underlined), which was then digested with BamHI and HindIII and subcloned into BamHI/HindIII-digested pRS306 vector (pTAG/ɛ). Oligonucleotides (loxP sequences are italicized) EPLX-5S (5′-AATTGCATAACTTCGTATAGCATACATTATACGAAGTTATGAAGCTTC-3′; HindIII site underlined) and EPLX-3A (5′-AATTGAAGCTTCATAACTTCGTATAATGTATGCTATACGAAGTTATGC-3′; HindIII site underlined) were annealed, thus generating EcoRI-compatible ends, and ligated to EcoRI (at nt 17482; HUMHBB)-digested pTAG/ɛ, to generate pTAG/loxP(+)/ɛ (29). The ɛ-globin 5′ upstream fragment (from 17482 to 17864) containing the second loxP sequence was PCR amplified with the primer set EP-loxP-5S2 (5′-CCCAAGCTTGGATCCATAACTTCGTATAGCATACATTATACGAAGTTATCTGGTTTTGTCTGTGTTAGCC-3′; HindIII and BamHI sites underlined) and EP-3A, digested with HindIII, and ligated to HindIII-digested pTAG/loxP(+)/ɛ replacing the corresponding portion of it, to generate pTAG/loxPw+/ɛ. For convenience, one of the two BamHI sites (the one in the EP-5S oligonucleotide) in the pTAG/loxPw+/ɛ was removed by partial digestion with BamHI, generation of blunt ends, and religation. The 2.3-kbp λ DNA was excised as a BamHI fragment from the plasmid pHS1/loxPw+/λ and ligated to BamHI-digested pTAG/loxPw+/ɛ to generate pTAG/loxPw+/ɛ/λ. This plasmid DNA was linearized with BglII and used for the A201F4.3 mutagenesis, generating A201F4.3/ɛ/λ.
Construction of the targeting vector that introduces the Bepsi promoter mutation into the ɛ-globin promoter region was described elsewhere (29). This plasmid DNA was linearized with EcoNI and used for the A201F4.3/HS1/λ mutagenesis generating A201F4.3/HS1/λ/Bepsi.
All the vector sequences were verified by DNA sequencing, and successful homologous recombination in Saccharomyces cerevisiae was confirmed by Southern blot analyses with several combinations of restriction enzymes and probes.
TgM.
Generation and structural analysis of human β-globin YAC TgM has been described elsewhere (29). Removal of the ectopic λ DNA fragment and inversion of the human β-globin locus were conducted by mating β-globin YAC TgM with Cre recombinase TgM and was confirmed by Southern blot analysis. Genotyping of WT and mutant EKLF loci (21) was performed by PCR analysis of genomic DNA from embryos, as described elsewhere (29). The protocol was approved by the Animal Experiment Committee, University of Tsukuba, and experiments were performed according to the University of Tsukuba's Regulation of Animal Experiment.
Semiquantitative reverse transcription-PCR (RT-PCR).
Total RNA was extracted from yolk sacs (9.5 days postcoitum [dpc]), fetal livers (14.5 dpc), or phenylhydrazine-induced anemic adult spleens (1 to 2 months old) by using ISOGEN (Nippon Gene). First-strand cDNA was synthesized from 2.5 μg of RNA with ReverTra Ace (Toyobo). One-twentieth of the reaction mixture was used for PCR amplification using the following parameters: 94°C for 30 s, 58°C for 1 min, and 72°C for 1 min. Cycle numbers used for PCR analyses, unless otherwise noted, were as follows: 12 cycles for both the β and α cDNAs in spleen; 18 and 12 for the γ and β/α cDNAs, respectively, in the liver; and 18 and 12 for the ɛ and γ/α cDNAs, respectively, in the yolk sac. An aliquot of each PCR product was electrophoresed on 8% polyacrylamide gels, dried, and subjected to X-ray autoradiography and phosphorimaging for quantitative analysis. The sequences of the primers used have been described elsewhere (29).
RESULTS
Cell transfection assay.
To test the hypothesis that HS5 harbors intrinsic ɛ-globin gene silencing activity, we inserted a 2.5-kbp HS5 or a random 2.3-kbp λ DNA fragment upstream of an ɛ-globin promoter-luciferase reporter construct (pɛ-luc) to generate pHS5(±)/ɛ-luc and pλ/ɛ-luc, respectively (Fig. 1B). These constructs were assayed for their transcriptional activities in transiently transfected K562 cells. Luciferase activity showed that addition of either HS5 or λ DNA did not severely affect ɛ-globin promoter activity (Fig. 1B).
To test the hypothesis that an ɛ-globin enhancer is located 3′ to HS1 near the HS5 integration site, we assayed this region for any intrinsic enhancer activity in K562 cells. As shown in Fig. 1C, addition of a 1-kbp DNA fragment from this region to the pɛ-luc (pHS1-3′/ɛ-luc) construct increased its transcriptional activity 10-fold, and thus an enhancer activity is readily detected at this site. However, this enhancer activity was not severely affected by inserting the HS5 or λ DNA fragments into the site used to generate the HS5 TgM. Thus, reduction of ɛ-globin expression in the HS5 TgM is unlikely to be a consequence of disruption of an enhancer element.
Generation of YAC TgM.
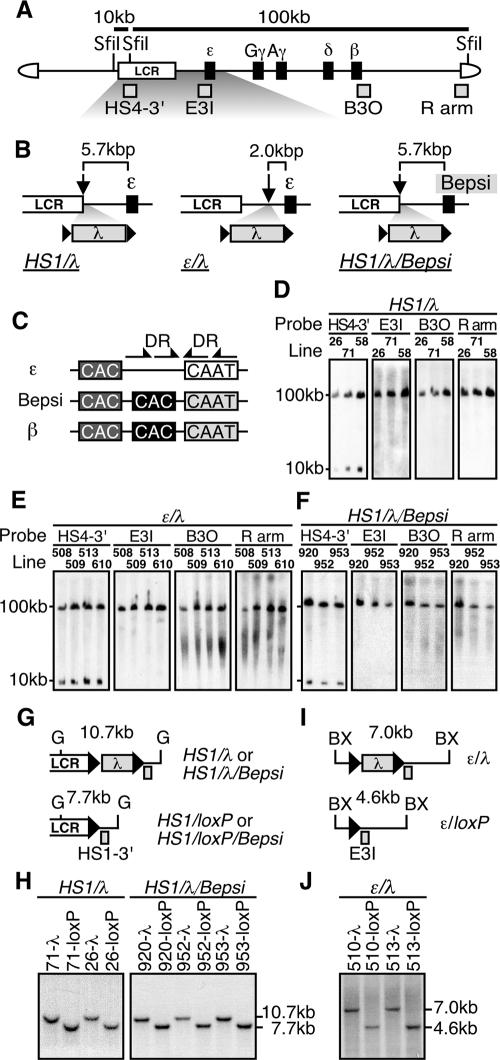
We next tested the hypothesis that HS5 diminishes ɛ-globin gene expression in TgM when it is located between HS1 and the ɛ-globin gene because it increases the distance between the LCR and the promoter. In order to increase the distance, as well as to confirm that the HS5 fragment does not harbor intrinsic silencer activity, we inserted a 2.3-kbp λ DNA fragment between HS1 and the ɛ-globin gene in the context of the human β-globin YAC. This fragment of λ DNA is similar in size to the HS5 DNA fragment and does not significantly modify transcriptional activity of the ɛ-globin reporter construct in K562 cells (Fig. 1B and C). In the first construct (Fig. 2A and B, HS1/λ), the λ DNA was floxed (flanked by directly repeated loxP sites) and inserted 3′ to HS1, which places it about 5.7 kbp upstream from the ɛ-globin gene transcriptional initiation site. In the second construct (Fig. 2B, ɛ/λ), we inserted the floxed λ DNA 2.0 kbp upstream from the start site of ɛ-globin gene transcription. To generate the HS1/λ/Bepsi construct, we introduced mutations into the ɛ-globin promoter to mimic adult β-globin gene promoter sequences (Bepsi) in the context of the HS1/λ construct (Fig. 2B and C).
FIG. 2.
(A) Structure of the 150-kbp human β-globin YAC. The positions of the β-like globin genes (solid rectangles) are shown relative to the LCR. SfiI restriction enzyme sites are located 5′ to HS5, between HS4 and HS3, and in the right arm of the YAC. Probes (gray rectangles) used for long-range fragment analyses shown in panels D, E, and F, and expected restriction enzyme fragments with their sizes are shown. (B) Schematic representation of the mutant β-globin loci. The λ DNA fragment (2.3 kbp) was floxed (solid triangles) and inserted 3′ to HS1 (HS1/λ and HS1/λ/Bepsi) or introduced between HS1 and the ɛ-globin gene (ɛ/λ). In the HS1/λ/Bepsi construct, the promoter sequence of the ɛ-globin gene was mutated to mimic that of the β-globin gene, in addition to the λ insertion. (C) The positions of the distal (dark gray) or proximal (solid) CAC boxes are shown relative to the CAAT box in the human ɛ-globin, β-globin, and Bepsi gene promoters. Arrows indicate direct repeat (DR) elements, present only in the ɛ-globin promoter (29). (D, E, and F) Long-range structural analyses of the human β-globin YAC in TgM. The whole β-globin locus is contained within two SfiI fragments (10 and 100 kbp) as described for panel A above. DNAs from thymus cells were digested with SfiI in agarose plugs, separated by pulsed-field gel electrophoresis, and hybridized separately to probes (shown in panel A). A schematic representation of the transgene loci around the inserted λ DNA (G and I) and Southern blot analyses for confirming Cre-loxP-mediated in vivo recombination (H and J) are shown. Cre-loxP recombination removes the 2.3-kbp λ DNA insert from each mutated locus, which creates a 7.7-kbp BglII (G, HS1/loxP, and HS1/loxP/Bepsi) or a 4.6-kbp BstXI (BX, ɛ/loxP) fragment in each locus. Tail DNAs from each mutant and loxP footprint TgM lines were digested with BglII (HS1/λ and HS1/λ/Bepsi series) or BstXI (ɛ/λ series), separated on agarose gels, and hybridized to probes HS1-3′ or E3I. Arrowheads, loxP sequences.
We established several independent TgM lines for each YAC construct, all of which bear intact, single-copy YACs, except for TgM line 58 that carries two copies (Fig. 2D to F). By in vivo Cre-loxP recombination, we generated pseudo-WT lines from some of the mutant TgM lines to serve as controls, the structures of which were confirmed by Southern blot analysis (Fig. 2G to J).
Expression of the human β-like globin genes in HS1/λ TgM.
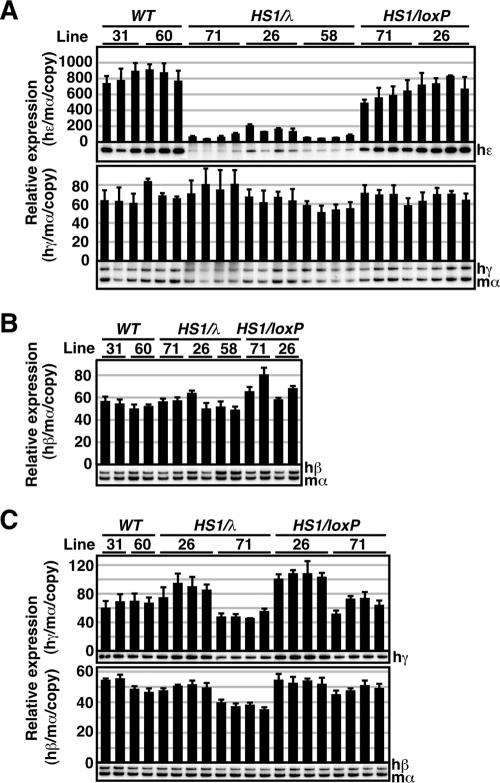
We first analyzed human ɛ- and γ-globin gene expression in primitive erythroid cells of the HS1/λ TgM. Total RNA was recovered from the yolk sacs (9.5 dpc) of at least two litters for each TgM line. Expression of globin mRNAs was measured by semiquantitative RT-PCR using endogenous mouse α-globin gene expression as an internal control (Fig. 3A). In a comparison of the expression levels between mutant (HS1/λ) and WT (28), as well as the pseudo-WT (HS1/loxP) TgM, ɛ-globin gene expression fell by more than 90% (Fig. 3A, top) in the mutant lines. This phenotype was gene specific since γ-globin gene expression was not affected by the insertion of λ in the same lines (Fig. 3A, bottom).
FIG. 3.
Expression of the human β-like globin genes in HS1/λ TgM. (A) Total RNA was prepared from the yolk sacs of more than two embryos (9.5 dpc) derived from the intercross of male transgenic and female WT animals. Samples were collected from two independent litters of each mutant line. Expression of human ɛ (hɛ)- and human γ (hγ)-globin compared to endogenous mouse α (mα)-globin genes was separately analyzed by semiquantitative RT-PCR. The signals for hɛ-globin at 18 cycles and hγ/mα-globin at 12 cycles were quantified by PhosphorImager, and the ratios of hɛ/mα (top) and hγ/mα (bottom) were calculated (the mα signal at 12 cycles was set at 100%, and the values are normalized by transgene copy numbers). (B) Total RNA was prepared from the spleens of 1-month-old anemic mice. Samples were collected from two individuals from each line of TgM. Expression of human β (hβ)-globin compared to endogenous mα-globin genes was analyzed by semiquantitative RT-PCR. The signal for hβ/mα-globin at 12 cycles was quantified, and the ratio of hβ/mα was calculated (the mα signal at 12 cycles was set at 100%). (C) Total RNA was prepared from the fetal livers (14.5 dpc) derived from the intercross of male transgenic and female WT animals. Samples were collected from two independent litters of each mutant line. Expression of hγ- and hβ-globin compared to endogenous mα-globin genes was analyzed separately by semiquantitative RT-PCR. The signals for hγ-globin at 18 cycles and hβ/mα-globin at 12 cycles were quantified, and the ratios of hγ/mα (top) and hβ/mα (bottom) were calculated (the mα signal at 12 cycles was set at 100%). The averages ± SD from at least three independent experiments was calculated and are graphically depicted. Representative results are shown below each panel.
We then analyzed expression of the human β- and γ-globin genes in definitive erythroid cells. After 5 days of phenylhydrazine treatment, anemic spleens were collected from two animals (1 month old) from each line for RNA extraction and RT-PCR analysis (Fig. 3B). No significant difference was observed in β-globin gene expression between the HS1/λ and control TgM. Since expression levels of the γ-globin genes in the yolk sac and the β-globin gene in the adult spleen are much higher than that of the ɛ-globin gene in the yolk sac, we considered the possibility that only the gene with the lowest transcriptional activity would be affected by the mutation. We therefore collected fetal livers (14.5 dpc) from at least two litters of each line and analyzed β- and γ-globin gene expression (Fig. 3C). At this stage of development, the γ-globin genes are expressed at low levels in comparison to expression in the yolk sac and also in comparison to expression of the β-globin gene in the fetal liver. RT-PCR analysis showed that neither γ- nor β-globin gene expression was affected in the fetal livers of the HS1/λ mutant TgM. These results suggest that extending the distance, rather than a silencing function that can be associated with HS5, was responsible for attenuated ɛ-globin gene transcription.
Expression of the human β-like globin genes in the ɛ/λ TgM.
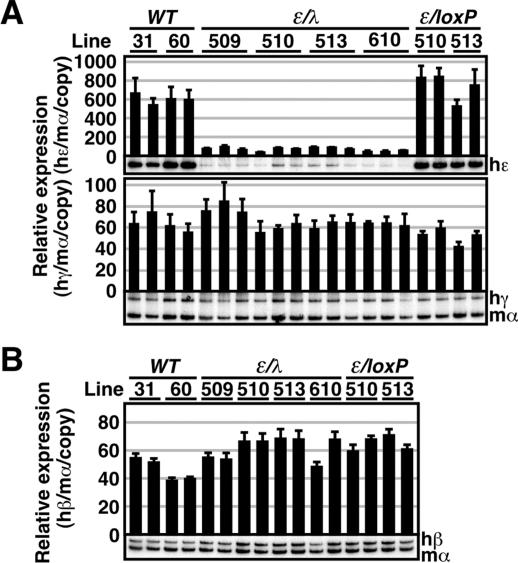
Although the cell transfection results demonstrated that inserting a ∼2-kbp DNA fragment 3′ to HS1 did not severely affect the activity of an uncharacterized nearby enhancer (Fig. 1C), this inhibitory effect might be revealed in an in vivo environment. We therefore generated a second construct, ɛ/λ, in which we inserted the floxed λ DNA at a different position 3′ to HS1 (Fig. 2B). RNA analysis of the ɛ/λ TgM revealed that expression of the human ɛ-globin gene in the yolk sac was severely attenuated by insertion of the 2.3-kbp λ DNA fragment (Fig. 4A, top). In contrast, γ-globin gene expression in the yolk sac, as well as β-globin gene expression in the adult spleen was not affected (Fig. 4A, bottom, and B). These results, considered together with the results from the HS1/λ TgM, clearly demonstrate that simply by extending the distance from the gene to the LCR, ɛ-globin gene transcription is severely and specifically attenuated in primitive erythroid cells.
FIG. 4.
Expression of the human β-like globin genes in ɛ/λ TgM. (A) Total RNA was prepared from the yolk sacs of more than two embryos (9.5 dpc) from two independent litters of each mutant line. Expression of human ɛ (hɛ)- and human γ (hγ)-globin compared to endogenous mouse α (mα)-globin genes was separately analyzed by semiquantitative RT-PCR. (B) Total RNA was prepared from the spleens of 1-month-old anemic mice. Samples were collected from two individuals from each line of TgM. Expression of human β (hβ)-globin compared to endogenous mα-globin genes was analyzed by semiquantitative RT-PCR as described in the legend to Fig. 3.
Expression of the human β-like globin genes in the HS1/λ/Bepsi TgM.
The β-globin gene is highly active in definitive erythroid cells even though it is located 50 kbp away from, and is clearly dependent on, the LCR. Thus, it is possible that specific globin gene promoter sequences determine distance sensitivity or insensitivity with respect to the LCR. It has been reported that the LCR and the β-globin gene promoter region physically interact in definitive erythroid cells (3, 31). Furthermore, it was predicted that mouse and human β-globin gene loci form a higher-order chromatin architecture, dubbed an “active chromatin hub” (22, 23). The transcription factor EKLF, which is active both in primitive and definitive erythroid cells, interacts with LCR HSs as well as with the β-globin promoter and is required for active chromatin hub formation in the β-globin gene locus (8).
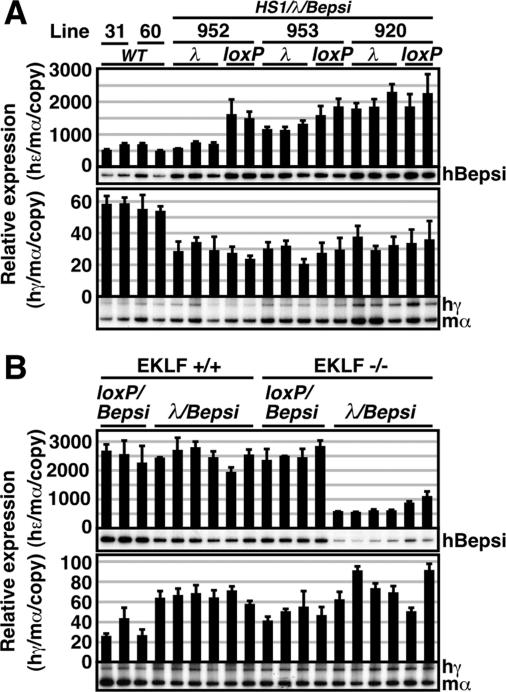
We have previously generated a mutant ɛ-globin promoter (Bepsi) by introducing a proximal CAC box, which binds to EKLF (and is also found in the β-globin promoter) and simultaneously disrupts two direct repeat elements, which silence ɛ-globin gene expression at the adult stage and are absent from the β promoter (Fig. 2C) (29). Thus, the ɛ-globin mutant Bepsi promoter sequences closely resemble those of the β-globin promoter. We have shown that the Bepsi promoter was aberrantly active in definitive erythroid cells and that this activity was EKLF dependent (29). To further analyze the mechanism that governs the observed LCR distance sensitivity of the ɛ-globin promoter, we asked if Bepsi mutant promoter activity is also sensitive to its distance from the LCR. In the absence of the λ DNA insertion 3′ to HS1, Bepsi promoter activity in HS1/loxP/Bepsi TgM was two- to threefold higher than that of the WT ɛ-globin promoter (in WT TgM) in primitive erythroid cells (Fig. 5A, top). Interestingly, introduction of the λ DNA 3′ to HS1 attenuated Bepsi promoter activity by 50%, 30%, and 0% in TgM lines 952, 953, and 920, respectively (Fig. 5A, top), in other words much less than the 90% attenuation observed in the HS1/λ TgM (Fig. 3A, top). Thus, Bepsi promoter activity was much less sensitive than the WT ɛ-globin gene promoter to its distance from the LCR.
FIG. 5.
Expression of the human β-like globin genes in the HS1/λ/Bepsi TgM. (A) Total RNA was prepared from the yolk sacs of more than two embryos (9.5 dpc) from two independent litters of each mutant line. Expression of human Bepsi (hBepsi)- and human γ (hγ)-globin compared to endogenous mouse α (mα)-globin genes was separately analyzed by semiquantitative RT-PCR. λ, HS1/λ/Bepsi; loxP, HS1/loxP/Bepsi. (B) HS1/λ/Bepsi TgM (line 920) was bred with the EKLF-null mouse to analyze β-like globin gene expression in the presence (EKLF+/+) or absence (EKLF−/−) of endogenous EKLF activity. Total RNA was prepared from the yolk sacs of more than two embryos (9.5 dpc) from two independent litters of the mutant line. Expression of hBepsi- and hγ-globin compared to endogenous mα-globin genes was separately analyzed by semiquantitative RT-PCR. See the legend to Fig. 3 for details.
Since one of the differences between Bepsi and WT ɛ-globin promoters is the presence or absence of a proximal CAC element, the high-affinity binding site for EKLF, we investigated whether the increased resistance of the Bepsi promoter to the distance from the LCR was due to EKLF activity. We used TgM line 920 for this purpose, because Bepsi promoter activity did not differ whether λ DNA was inserted or not in this line. We mated β-globin YAC TgM with EKLF-null mice to analyze the effects on Bepsi promoter activity of inserting λ DNA, in the presence (+/+) or absence (−/−) of endogenous EKLF (Fig. 5B).
In primitive erythroid cells of the EKLF+/+ mice, insertion of the λ DNA did not affect Bepsi promoter activity, as shown before in Fig. 5A. In the presence or absence of the EKLF, Bepsi promoter activities (in the HS1/loxP/Bepsi) did not differ significantly, meaning that Bepsi was two- to threefold more active than the WT ɛ-globin promoter. This demonstrates that the increase in Bepsi promoter activity is not due to EKLF binding (Fig. 5B, top) (29). However, insertion of the λ DNA severely attenuated Bepsi promoter activity in the absence of EKLF (HS1/λ/Bepsi in the EKLF−/− background) (Fig. 5B, top). This result strongly implies that EKLF binding to the Bepsi promoter is an important determinant of the distance insensitivity of the mutant promoter with respect to the LCR enhancer.
Expression of the human β-like globin genes in Bepsi-Inverted TgM.
We have previously shown that the Bepsi promoter was active in definitive erythroid cells when located proximal to the LCR (Fig. 6A, Bepsi) (29). Since we previously inverted the β-globin genes with respect to the LCR by means of in vivo Cre-loxP recombination (28), we decided to invert the Bepsi locus using the same strategy and to then test Bepsi-directed ɛ-globin gene expression when it was located in an LCR-distal position (Fig. 6A, Bepsi-Inverted). Analysis of Bepsi promoter activity in primitive erythroid cells revealed that this mutant gene was, in fact, expressed at low but significant levels when in the LCR-distal position (Fig. 6B, Bepsi-Inverted). Although the level is low, probably because of the highly active γ- and β-globin genes at more proximal positions relative to the LCR, it must be stressed that WT ɛ-globin gene transcription was not detected at this position in primitive erythroid cells (28). It is thus possible that introduction of the proximal CAC element into the ɛ-globin gene promoter allowed long-range interactions between the LCR enhancer and the Bepsi promoter.
FIG. 6.
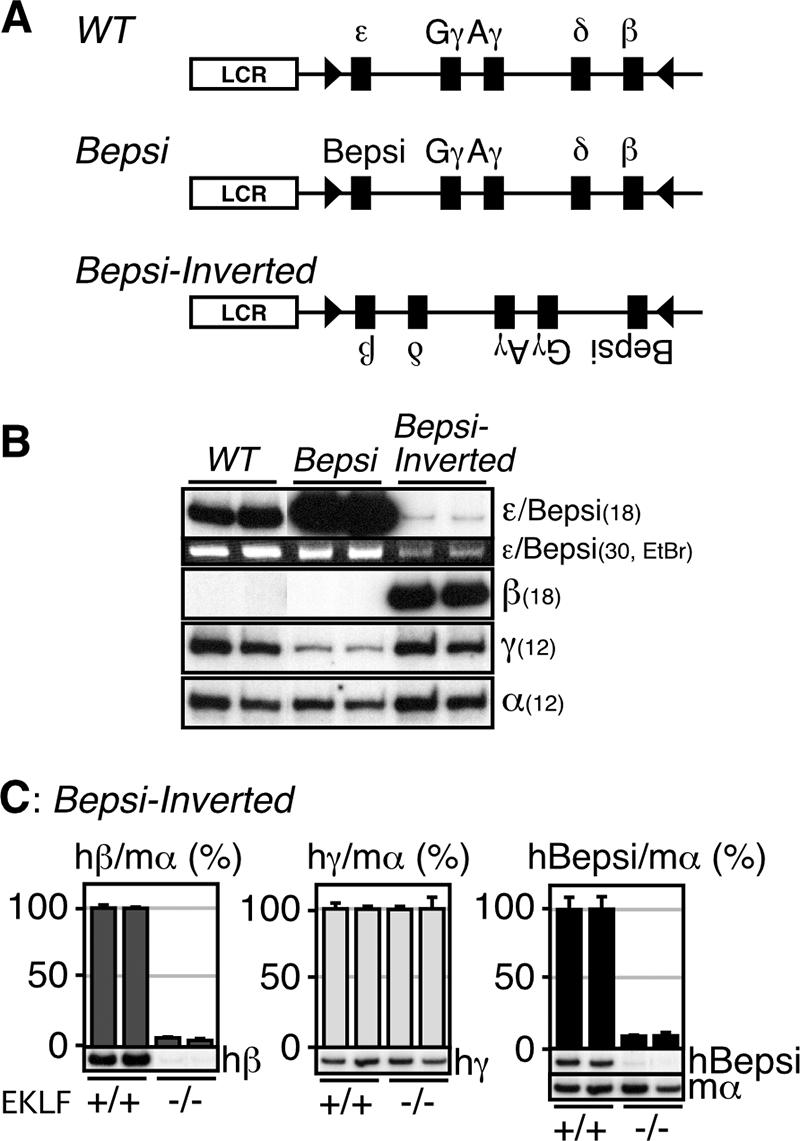
Expression of the human β-like globin genes in Bepsi-Inverted TgM. (A) Schematic representation of the Bepsi-Inverted locus. A pair of the loxP sites (arrowheads) was introduced into the human β-globin YAC in inverted orientation to generate a pseudo-WT locus (28). Then, the ɛ-globin promoter in the WT locus was mutated to generate the Bepsi locus. Following the establishment of TgM lines with the Bepsi YAC, the genes were inverted by mating with Cre-expressing TgM (Bepsi-Inverted). (B) Semiquantitative RT-PCR analysis of β-like globin genes expression in the yolk sacs of the mutant TgM. Amplified Bepsi transcript was also stained with the ethidium bromide (EtBr). PCR cycle numbers used are shown in parentheses. (C) Semiquantitative RT-PCR analysis of the β-like globin genes expression in the yolk sacs of the EKLF+/+ and EKLF−/− mice. The expression levels of the β-like globin genes in the EKLF+/+ background was set at 100. The average and SD and shown graphically for each animal. Representative RT-PCR results for human β (hβ)-, γ (hγ)-, Bepsi (hBepsi)-, and mouse α (mα)-globin in different EKLF mutant backgrounds (+/+ and −/−) are shown below each panel. See the legend to Fig. 3 for details.
To test whether there exists any requirement for EKLF activity in this long-range enhancer-promoter communication, we analyzed Bepsi promoter activity in the EKLF-null genetic background. As shown in our previous work (29), WT ɛ-globin gene transcription at the proximal position was partially dependent on EKLF activity in primitive erythroid cells. On the other hand, the EKLF dependency of the promoter activity was less obvious in the LCR-proximal Bepsi TgM (Fig. 5B) (29). Surprisingly, however, Bepsi promoter activity in primitive erythroid cells was completely dependent on the EKLF when it was located at the LCR-distal position in Bepsi-Inverted TgM (Fig. 6C, hBepsi). Thus, EKLF is absolutely required for Bepsi promoter activity at the LCR-distal position, while it is dispensable for the activity of the same promoter when the gene is in an LCR-proximal position. The complete loss of β-globin gene expression in the EKLF-null genetic background, even when it is in an LCR-proximal position, suggests that the β-globin promoter per se requires EKLF activity independent of the EKLF function that promotes LCR promoter communication from a distance (Fig. 6C). These results underscore the observation that EKLF is important for long-range gene activation in primitive erythroid cells.
DISCUSSION
Enhancer-promoter communication is important for developmentally regulated gene expression, especially within clustered gene loci. Structural organization of the β-globin locus apparently plays a role in determining gene activity in primitive erythroid cells, since the ɛ-globin gene was silenced while the β-globin gene was activated when we inverted the gene order relative to the LCR (28). We interpreted this result to mean that proximally located β- and γ-globin genes more favorably interact with the LCR in the inverted locus, excluding the ɛ-globin gene from competition. Considering the current results, however, the cause of ɛ-globin gene silencing in the inverted locus appears more likely to be due to its increased distance from the LCR and the absence of high-affinity EKLF binding sites in its promoter. An extension of this interpretation would be that β-globin gene silencing in primitive erythroid cells might also be due to its distance from the LCR in the native locus. However, the fact that the proximal CAC site, which made the ɛ-globin promoter insensitive to distance from the LCR, already exists in the β-globin promoter and that murine adult βmaj and βmin genes were activated by two- to threefold in primitive erythroid cells after the elimination of the more LCR-proximal ɛy and βh1 genes (14) is inconsistent with this notion. At this point, whether the distance-sensitive feature of the promoters is gene or developmental stage specific is obscure. Analysis of β-globin gene expression in primitive erythroid cells of TgM carrying disrupted ɛ- and γ-globin genes in the human locus would clarify this issue.
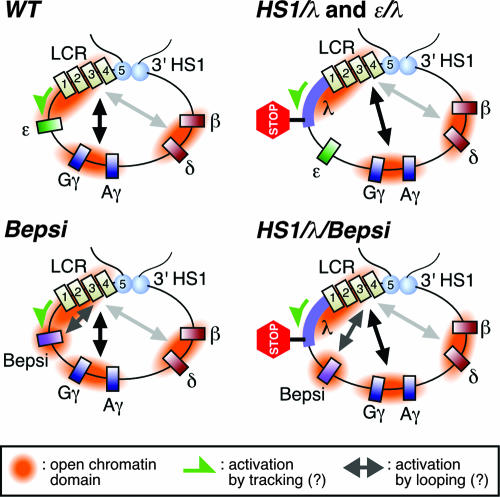
According to the looping model, gene activity is in part determined by looping efficiency, which depends on the flexibility of chromatin fiber, distance between enhancer and promoter, and the affinities of interactions between promoter-bound and enhancer-bound protein complexes (12, 18). Although smaller DNA loops are energetically favored, a moderate increase in the distance of interacting regions would not make a dramatic difference in looping efficiency if the distances were relatively large (several kilobase pairs) (6, 26). Whereas interactions between the β-globin gene and the LCR are well established (23), it is currently unknown whether the ɛ-globin gene interacts with the LCR in primitive erythroid cells. Because the distance between the 3′ end of HS1 and the ɛ-globin gene is 5.7 kbp, it is difficult to imagine that increasing this distance to 8.0 (5.7 + 2.3) kbp would diminish looping efficiency by more than 10-fold. Therefore, we infer that the ɛ-globin gene may be activated by a distance-sensitive, nonlooping mechanism in primitive erythroid cells.
Chromatin immunoprecipitation data from definitive erythroid cells suggest that erythroid-specific transcription factor NF-E2 and RNA Pol II are initially attached to the LCR and may then be delivered to the adult β-globin promoter region (2). Because of a lack of detectable Pol II enrichment within the intervening embryonic ɛ- and γ-globin gene region, which is marked by hypoacetylated histones, it has been postulated that Pol II is transferred via a looping mechanism to the adult β-globin gene in definitive erythroid cells. In primitive erythroid cells, however, such a delivery may be accomplished by a tracking (or facilitated tracking) mechanism, and the intergenic transcription observed across the β-globin locus may be a by-product of this process (25). Ling and colleagues recently demonstrated that transcripts initiating within the LCR are relatively small (20). Thus, extending the distance between the LCR and the ɛ-globin gene may lead to premature dissociation of Pol II before it reaches the ɛ-globin gene.
It has been proposed that active genes are organized into “open” chromatin domains, which are characterized by hyperacetylated histones (2, 5, 9). One function associated with enhancers may be the establishment of open chromatin, which is a prerequisite for subsequent gene activation (17). Chromatin immunoprecipitation analysis of a model construct, in which the LCR-HS2 enhancer was linked to an ɛ-globin gene, in transfected human erythroid K562 cells revealed that the LCR enhancer caused widespread histone hyperacetylation, including intervening, as well as gene coding, sequences (16). It is likely that histone hyperacetylation initiated at HS2 spreads from the LCR to the gene (15). The spreading mechanism leading to the formation of a hyperacetylated chromatin domain may be impaired in TgM containing insertions between the LCR and the ɛ-globin gene (Fig. 7). As a consequence, the chromatin domain encompassing the ɛ-globin gene and its promoter would remain hypoacetylated. If this is the case, the ɛ-globin gene does not participate in competing with the LCR's “transcription enhancing” activity (10). Since EKLF interacts with histone acetyltransferase-containing proteins, such as p300, CREB-binding protein (CBP), and p300/CBP-associated factor (35), local histone modification could take place at the Bepsi promoter region in Bepsi TgM. This regional formation of an open chromatin domain would allow Bepsi promoter activation by the LCR enhancer, even when λ DNA is inserted between the LCR and the ɛ-globin gene in HS1/λ/Bepsi TgM. However, this hypothesis is inconsistent with data showing that the LCR is dispensable for the establishment of an open chromatin structure across the entire murine β-globin gene locus (10).
FIG. 7.
Two modes of LCR-promoter communication (tracking and looping) may control developmental stage- and/or gene-specific activation within the human β-globin locus. The 5′ and 3′ HSs (light blue ovals and numbered rectangles) may be involved in forming higher order chromatin architecture within the β-globin locus, one part of which is a powerful enhancer (the LCR). In primitive erythroid cells, the ɛ-globin gene may be activated by the LCR via tracking mechanism (WT, green arrow) and therefore displays sensitivity to the linear distance from the LCR (HS1/λ and ɛ/λ). Introduction of the CAC (presumptive EKLF binding) site into and/or disruption of the direct repeat sites from the ɛ-globin promoter (Bepsi) may allow it to be activated by means of a looping mechanism (arrow). Thus, the Bepsi promoter is highly active even after the λ DNA is inserted (HS1/λ/Bepsi). Predicted open chromatin domains are indicated.
Taking the above-mentioned issues into consideration, we propose the following model (Fig. 7). According to our current results, ɛ-globin gene activation in the WT locus involves a distance-sensitive mechanism(s), possibly including Pol II delivery via tracking and/or progressive chromatin opening initiated by the LCR. Introduction of the CAC site into the ɛ-globin promoter creates an alternative distance-insensitive mechanism (looping) for activating the Bepsi promoter (Bepsi versus HS1/λ/Bepsi). This new path apparently requires EKLF, since the Bepsi promoter becomes distance sensitive in the absence of EKLF. One mechanism may be that EKLF binds to the Bepsi promoter and recruits a “facilitator” protein, which then mediates loop formation. While metazoan enhancers can influence gene expression from remote positions, yeast enhancers (upstream activating sequences) are less flexible in terms of distance to the target promoters. Although human ɛ- and β-globin genes descended from a common ancestral gene, their developmental specificity in transcription is significantly different (27). In order to allow promoter-enhancer communication over long distances, some metazoan promoters, such as the promoter of the β-globin gene, might have developed the ability to recruit proteins that facilitate long-range chromosomal interactions.
One remaining issue to be resolved is why the γ-globin genes are distance insensitive with respect to the LCR (Fig. 3A and 4A). Similar to the ɛ-globin promoter, γ-globin genes do not carry high-affinity EKLF binding sites in their promoters (7). However, other transcription factors, such as GATA-1 (33), could be involved in locally opening chromatin structure and/or facilitating loop formation during γ-globin gene activation.
Based on the data presented here, we propose that mechanisms of enhancer-mediated transcriptional activation of proximal and distal genes might be fundamentally different. In this respect, proper spacing between cis-linked enhancers and promoters could also participate in establishing the specificity of enhancer-promoter communications.
Acknowledgments
We thank Jörg Bungert (University of Florida) for critical reading of the manuscript.
This work was supported by research grants from the NIH (HL24415 to J.D.E. and O.T.); the 21st Century COE Program (to A.F.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT); and a grant-in-aid for Scientific Research in Priority Areas (MEXT; to K.T. and A.F.) and Young Scientists (category A, MEXT; to K.T.).
Footnotes
Published ahead of print on 4 June 2007.
REFERENCES
- 1.Bell, A. C., A. G. West, and G. Felsenfeld. 1999. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98:387-396. [DOI] [PubMed] [Google Scholar]
- 2.Bulger, M., T. Sawado, D. Schubeler, and M. Groudine. 2002. ChIPs of the beta-globin locus: unraveling gene regulation within an active domain. Curr. Opin. Genet. Dev. 12:170-177. [DOI] [PubMed] [Google Scholar]
- 3.Carter, D., L. Chakalova, C. S. Osborne, Y. F. Dai, and P. Fraser. 2002. Long-range chromatin regulatory interactions in vivo. Nat. Genet. 32:623-626. [DOI] [PubMed] [Google Scholar]
- 4.Choi, O. R., and J. D. Engel. 1988. Developmental regulation of beta-globin gene switching. Cell 55:17-26. [DOI] [PubMed] [Google Scholar]
- 5.Dean, A. 2006. On a chromosome far, far away: LCRs and gene expression. Trends Genet. 22:38-45. [DOI] [PubMed] [Google Scholar]
- 6.Dillon, N., T. Trimborn, J. Strouboulis, P. Fraser, and F. Grosveld. 1997. The effect of distance on long-range chromatin interactions. Mol. Cell 1:131-139. [DOI] [PubMed] [Google Scholar]
- 7.Donze, D., T. M. Townes, and J. J. Bieker. 1995. Role of erythroid Kruppel-like factor in human gamma- to beta-globin gene switching. J. Biol. Chem. 270:1955-1959. [DOI] [PubMed] [Google Scholar]
- 8.Drissen, R., R. J. Palstra, N. Gillemans, E. Splinter, F. Grosveld, S. Philipsen, and W. de Laat. 2004. The active spatial organization of the beta-globin locus requires the transcription factor EKLF. Genes Dev. 18:2485-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engel, J. D., and K. Tanimoto. 2000. Looping, linking, and chromatin activity: new insights into beta-globin locus regulation. Cell 100:499-502. [DOI] [PubMed] [Google Scholar]
- 10.Epner, E., A. Reik, D. Cimbora, A. Telling, M. A. Bender, S. Fiering, T. Enver, D. I. Martin, M. Kennedy, G. Keller, and M. Groudine. 1998. The beta-globin LCR is not necessary for an open chromatin structure or developmentally regulated transcription of the native mouse beta-globin locus. Mol. Cell 2:447-455. [DOI] [PubMed] [Google Scholar]
- 11.Farrell, C. M., A. G. West, and G. Felsenfeld. 2002. Conserved CTCF insulator elements flank the mouse and human beta-globin loci. Mol. Cell. Biol. 22:3820-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley, K. P., and J. D. Engel. 1992. Individual stage selector element mutations lead to reciprocal changes in beta- vs. epsilon-globin gene transcription: genetic confirmation of promoter competition during globin gene switching. Genes Dev. 6:730-744. [DOI] [PubMed] [Google Scholar]
- 13.Fraser, P. 2006. Transcriptional control thrown for a loop. Curr. Opin. Genet. Dev. 16:490-495. [DOI] [PubMed] [Google Scholar]
- 14.Hu, X., S. Eszterhas, N. Pallazzi, E. E. Bouhassira, J. Fields, O. Tanabe, S. A. Gerber, M. Bulger, J. D. Engel, M. Groudine, and S. Fiering. 2006. Transcriptional interference among the murine β-like globin genes. Blood 109:2210-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, A., and A. Dean. 2004. Developmental stage differences in chromatin subdomains of the beta-globin locus. Proc. Natl. Acad. Sci. USA 101:7028-7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, A., and A. Dean. 2003. A human globin enhancer causes both discrete and widespread alterations in chromatin structure. Mol. Cell. Biol. 23:8099-8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krumm, A., L. Madisen, X. J. Yang, R. Goodman, Y. Nakatani, and M. Groudine. 1998. Long-distance transcriptional enhancement by the histone acetyltransferase PCAF. Proc. Natl. Acad. Sci. USA 95:13501-13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, Q., G. Barkess, and H. Qian. 2006. Chromatin looping and the probability of transcription. Trends Genet. 22:197-202. [DOI] [PubMed] [Google Scholar]
- 19.Li, Q., K. R. Peterson, X. Fang, and G. Stamatoyannopoulos. 2002. Locus control regions. Blood 100:3077-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling, J., B. Baibakov, W. Pi, B. M. Emerson, and D. Tuan. 2005. The HS2 enhancer of the beta-globin locus control region initiates synthesis of non-coding, polyadenylated RNAs independent of a cis-linked globin promoter. J. Mol. Biol. 350:883-896. [DOI] [PubMed] [Google Scholar]
- 21.Nuez, B., D. Michalovich, A. Bygrave, R. Ploemacher, and F. Grosveld. 1995. Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature 375:316-318. [DOI] [PubMed] [Google Scholar]
- 22.Palstra, R. J., B. Tolhuis, E. Splinter, R. Nijmeijer, F. Grosveld, and W. de Laat. 2003. The beta-globin nuclear compartment in development and erythroid differentiation. Nat. Genet. 35:190-194. [DOI] [PubMed] [Google Scholar]
- 23.Patrinos, G. P., M. de Krom, E. de Boer, A. Langeveld, A. M. Imam, J. Strouboulis, W. de Laat, and F. G. Grosveld. 2004. Multiple interactions between regulatory regions are required to stabilize an active chromatin hub. Genes Dev. 18:1495-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perkins, A. C., K. M. Gaensler, and S. H. Orkin. 1996. Silencing of human fetal globin expression is impaired in the absence of the adult beta-globin gene activator protein EKLF. Proc. Natl. Acad. Sci. USA 93:12267-12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plant, K. E., S. J. Routledge, and N. J. Proudfoot. 2001. Intergenic transcription in the human beta-globin gene cluster. Mol. Cell. Biol. 21:6507-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ringrose, L., S. Chabanis, P. O. Angrand, C. Woodroofe, and A. F. Stewart. 1999. Quantitative comparison of DNA looping in vitro and in vivo: chromatin increases effective DNA flexibility at short distances. EMBO J. 18:6630-6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamatoyannopoulos, G., and A. W. Neinhuis. 1994. Hemoglobin switching, p. 107-155. In G. Stamatoyannopoulos, A. W. Neinhuis, P. Majerus, and H. Varmus (ed.), The molecular basis of blood diseases, 2nd ed. W. B. Saunders Co., New York, NY.
- 28.Tanimoto, K., Q. Liu, J. Bungert, and J. D. Engel. 1999. Effects of altered gene order or orientation of the locus control region on human beta-globin gene expression in mice. Nature 398:344-348. [DOI] [PubMed] [Google Scholar]
- 29.Tanimoto, K., Q. Liu, F. Grosveld, J. Bungert, and J. D. Engel. 2000. Context-dependent EKLF responsiveness defines the developmental specificity of the human epsilon-globin gene in erythroid cells of YAC transgenic mice. Genes Dev. 14:2778-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanimoto, K., A. Sugiura, A. Omori, G. Felsenfeld, J. D. Engel, and A. Fukamizu. 2003. Human beta-globin locus control region HS5 contains CTCF- and developmental stage-dependent enhancer-blocking activity in erythroid cells. Mol. Cell. Biol. 23:8946-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tolhuis, B., R. J. Palstra, E. Splinter, F. Grosveld, and W. de Laat. 2002. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol. Cell 10:1453-1465. [DOI] [PubMed] [Google Scholar]
- 32.Tuan, D., S. Kong, and K. Hu. 1992. Transcription of the hypersensitive site HS2 enhancer in erythroid cells. Proc. Natl. Acad. Sci. USA 89:11219-11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vakoc, C. R., D. L. Letting, N. Gheldof, T. Sawado, M. A. Bender, M. Groudine, M. J. Weiss, J. Dekker, and G. A. Blobel. 2005. Proximity among distant regulatory elements at the beta-globin locus requires GATA-1 and FOG-1. Mol. Cell 17:453-462. [DOI] [PubMed] [Google Scholar]
- 34.Wijgerde, M., J. Gribnau, T. Trimborn, B. Nuez, S. Philipsen, F. Grosveld, and P. Fraser. 1996. The role of EKLF in human beta-globin gene competition. Genes Dev. 10:2894-2902. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, W., and J. J. Bieker. 1998. Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc. Natl. Acad. Sci. USA 95:9855-9860. [DOI] [PMC free article] [PubMed] [Google Scholar]