Abstract
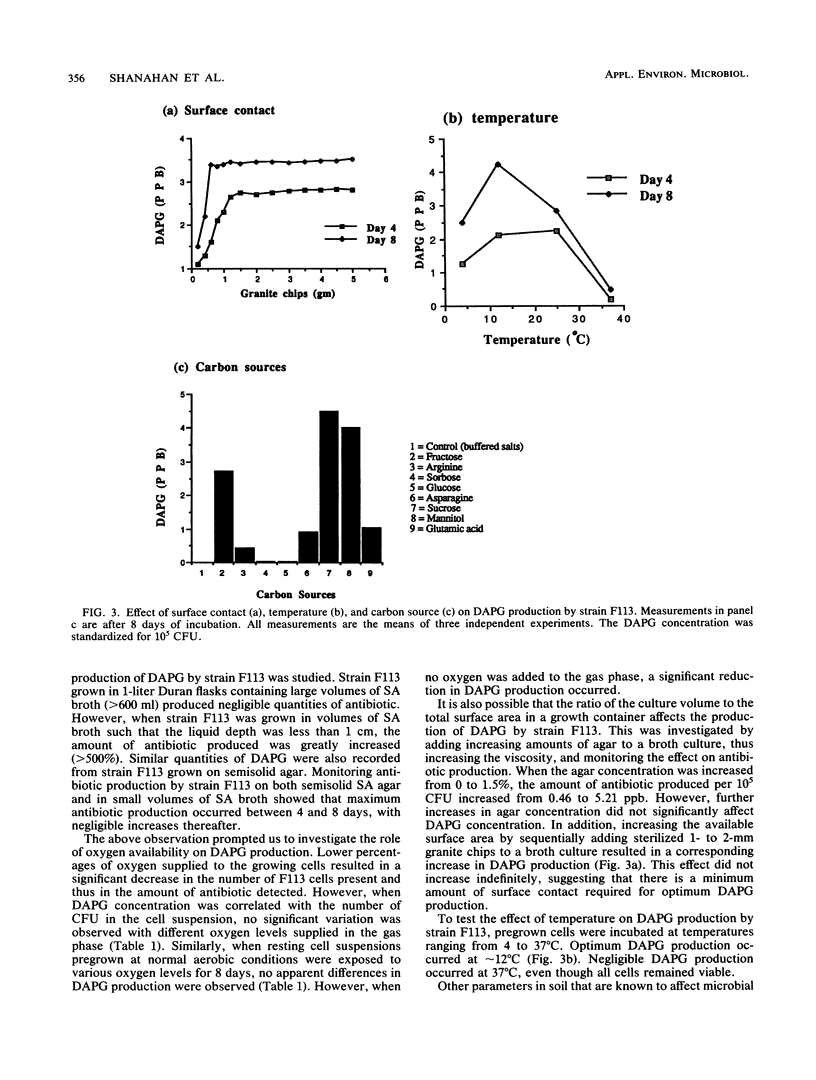
Pseudomonas sp. strain F113 was isolated from the rhizosphere of sugar beets and shown to inhibit a range of plant pathogenic fungi by producing an antibioticlike compound. An antibiotic-negative mutant strain, F113G22, was generated by transposon mutagenesis. This mutant has lost the ability to inhibit both bacterial and fungal microorganisms on high-iron medium. The antibioticlike compound was subsequently identified as 2,4-diacetylphloroglucinol (DAPG), and a high-pressure liquid chromatographic assay was developed for to detect it quantitatively in growth culture media and soil. The growth temperature had a direct bearing on DAPG production by strain F113, with maximum production at 12°C. The iron concentration, pH, and oxygen had no influence on DAPG production by strain F113 under the assay conditions used. However, a low ratio of culture volume to surface area available to the microbe in the growth container was critical for optimum DAPG production. Different types of carbon sources influenced DAPG production by strain F113 to various degrees. For example, sucrose, fructose, and mannitol promoted high yields of DAPG by strain F113, whereas glucose and sorbose resulted in very poor DAPG production.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brisbane P. G., Janik L. J., Tate M. E., Warren R. F. Revised structure for the phenazine antibiotic from Pseudomonas fluorescens 2-79 (NRRL B-15132). Antimicrob Agents Chemother. 1987 Dec;31(12):1967–1971. doi: 10.1128/aac.31.12.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibaldi J. A. Influence of temperature on the iron metabolism of a fluorescent pseudomonad. J Bacteriol. 1971 Mar;105(3):1036–1038. doi: 10.1128/jb.105.3.1036-1038.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb D. The production and role of antibiotics in soil. J Antibiot (Tokyo) 1976 Oct;29(10):987–1000. doi: 10.7164/antibiotics.29.987. [DOI] [PubMed] [Google Scholar]
- Hashimoto M., Hattori K. Isopyrrolnitrin: a metabolite from Pseudomonas. Bull Chem Soc Jpn. 1966 Feb;39(2):410–410. doi: 10.1246/bcsj.39.410. [DOI] [PubMed] [Google Scholar]
- Hashimoto M., Hattori K. Oxypyrrolnitrin: a metabolite of Pseudomonas. Chem Pharm Bull (Tokyo) 1966 Nov;14(11):1314–1316. doi: 10.1248/cpb.14.1314. [DOI] [PubMed] [Google Scholar]
- Imanaka H., Kousaka M., Tamura G., Arima K. Studies on pyrrolnitrin, a new antibiotic. 3. Structure of pyrrolnitrin. J Antibiot (Tokyo) 1965 Sep;18(5):207–210. [PubMed] [Google Scholar]
- James D. W., Jr, Gutterson N. I. Multiple antibiotics produced by Pseudomonas fluorescens HV37a and their differential regulation by glucose. Appl Environ Microbiol. 1986 Nov;52(5):1183–1189. doi: 10.1128/aem.52.5.1183-1189.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda N., Ishizaki N., Inoue N., Oshima M., Handa A. DB-2073, a new alkylresorcinol antibiotic. I. Taxonomy, isolation and characterization. J Antibiot (Tokyo) 1975 Dec;28(12):935–942. doi: 10.7164/antibiotics.28.935. [DOI] [PubMed] [Google Scholar]
- O'Sullivan D. J., O'Gara F. Delivery system for creation of one-step in vivo lac gene fusions in Pseudomonas spp. involved in biological control. Appl Environ Microbiol. 1988 Nov;54(11):2877–2880. doi: 10.1128/aem.54.11.2877-2880.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi T. K., Borokov A. V. Antibioticheskie svoistva 2,4-diatsetilflorogliutsina, produtsiruemogo Pseudomonas fluorescens shtamm 26-o. Antibiotiki. 1970 Jan;15(1):19–21. [PubMed] [Google Scholar]
- Reddy T. K., Khudiakov Ia P., Borovkov A. V. Pseudomonas fluorescens shtamm 26-o--produtsent fitotoksicheskikh veshchestv. Mikrobiologiia. 1969 Sep-Oct;38(5):909–913. [PubMed] [Google Scholar]
- Schroth M. N., Hancock J. G. Disease-suppressive soil and root-colonizing bacteria. Science. 1982 Jun 25;216(4553):1376–1381. doi: 10.1126/science.216.4553.1376. [DOI] [PubMed] [Google Scholar]
- Strunz G. M., Wall R. E., Kelly D. J. Phloroglucinol derivatives from Aeromonas hydrophila. J Antibiot (Tokyo) 1978 Nov;31(11):1201–1202. doi: 10.7164/antibiotics.31.1201. [DOI] [PubMed] [Google Scholar]
- Thomashow L. S., Weller D. M., Bonsall R. F., Pierson L. S. Production of the antibiotic phenazine-1-carboxylic Acid by fluorescent pseudomonas species in the rhizosphere of wheat. Appl Environ Microbiol. 1990 Apr;56(4):908–912. doi: 10.1128/aem.56.4.908-912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wratten S. J., Wolfe M. S., Andersen R. J., Faulkner D. J. Antibiotic metabolites from a marine pseudomonad. Antimicrob Agents Chemother. 1977 Mar;11(3):411–414. doi: 10.1128/aac.11.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]


