Abstract
The Cdk8 kinase and associated proteins form a nonessential transcriptional repressor module of the Mediator in the budding yeast Saccharomyces cerevisiae. Genetic analyses of this module have demonstrated functions ranging from environmental responses in budding yeast to organogenesis and development in worms, flies, and zebrafish. Here we have investigated the function of mammalian Cdk8 using mice harboring a gene trap insertion at the Cdk8 locus inactivating this kinase. No phenotypes were noted in heterozygote Cdk8+/− mice, but intercrossing these did not produce homozygous Cdk8−/− offspring. Developmental analysis demonstrated a requirement for Cdk8 prior to implantation at embryonic days 2.5 to 3.0. Cdk8−/− preimplantation embryos had fragmented blastomeres and did not proceed to compaction. As Cdk8 deficiency in cultured metazoan cells did not affect cell viability, the results suggest that transcriptional repression of genes critical for early-cell-fate determination underlies the requirement of Cdk8 in embryogenesis.
Eukaryotic mRNA transcription depends on the interaction of a set of general transcription factors that regulate basal transcription from core promoters and a wide range of gene-specific transcription factors that bind to distal regulatory elements. A critical coactivator complex required for this interaction has been identified in both biochemical and genetic approaches and has been termed the Mediator (3, 23, 45). The Mediator is composed of more than 20 evolutionarily conserved proteins that have been divided into the core Mediator (with head, middle, and tail submodules) and a distinct, reversibly associating Cdk8 submodule (6).
Genetic analyses of core Mediator subunits in mammalian systems are consistent with their identified roles in yeast. Med21/Srb7 is one of 10 core Mediator subunits required for viability in the budding yeast Saccharomyces cerevisiae (15) and has also been proposed to be required for autonomous cell growth in mammals (48). Med1 is not required for viability in budding yeast (15), and Med1/TRAP220-deficient embryos survive until embryonic day 9.5 (E9.5), when they exhibit heart failure and neurological defects (20). Med24/TRAP100-deficient mice likewise do not develop beyond E10.0 and exhibit developmental defects in the cardiovascular system, central nervous system, and placenta (19).
In contrast to the core Mediator involved in transcriptional activation, the Cdk8 submodule is needed for transcriptional repression in vitro in both yeast and mammalian systems (33, 39, 41, 42). The Cdk8 submodule consists of Cdk8 and cyclin C (24, 26, 44), together with Med12/Srb8 and Med13/Srb9 (3, 4, 37), and the loss of any of these four subunits results in a similar upregulation of a subset of genes in budding yeast but has little effect on the viability of cells (17, 49). The molecular mechanism of transcriptional repression by Cdk8 has been well established in yeast, where a majority of the submodule-responsive genes are regulated by the transcription factors Ste12, Gcn4, and Msn2 and repressed through direct phosphorylation of these transcription factors by Cdk8, resulting in rapid transcription factor turnover or nuclear exclusion (7, 32).
The Cdk8 submodule is also important in development, as the submodule components Med12 and Med13 are required for the differentiation of several cell lineages and organ development in flies and worms (46, 54). In line with the role of the Cdk8 submodule in budding yeast, the function of Med12 and Med13 was also proposed to involve transcriptional regulation, in this case of the Wnt and Hedgehog pathways. As the functions of mammalian Cdk8 and other submodule components are uncharacterized in vivo, we investigated the function of mammalian Cdk8 by generating mice harboring a loss-of-function allele of Cdk8.
MATERIALS AND METHODS
Generation of Cdk8 mutant mice, husbandry, and embryo manipulation.
Embryonic stem (ES) cells (clone RRS314) containing a Cdk8 gene trap allele, designated according to JAX convention as Cdk8GT(pGT0Lxf)/Byg but here abbreviated to Cdk8−, were obtained from BayGenomics (40). Morula-ES cell aggregations were carried out by the Transgenic unit of the Meilahti Experimental Animal Center, University of Helsinki. Briefly, superovulated CD1 morulas were aggregated to ES cells containing the Cdk8 gene trap allele. Chimeric males were subsequently bred with CD1 females. Germ line transmission was indicated by coat color, and heterozygote mutant animals were identified by PCR genotyping. The staging of embryos was according to standard convention (E0.5 is noon on the day of the copulation plug). For the extraction of embryos, the uterine horns were removed postmortem. For midgestation expression analysis, embryos were fixed in paraformaldehyde and stained using standard LacZ-staining protocols (38). Preimplantation embryos were dissected from the oviducts at various stages as described previously (31). Mice were maintained on a mixed 129/Ola, CD1 background and in accordance with local regulations regarding the use of animals in research.
Genotyping.
In order to locate the exact integration of the gene trap insertion, overlapping PCR amplicons compatible with a gene trap-specific 5′ reverse primer were designed to cover intron 4 of Cdk8. One amplicon produced an RRS314 cell line-specific shorter product in addition to the expected wild-type product. The forward primer used to capture the mutant-specific amplicon was 5′-TGCAGTAACGAGAGGCAGTG-3′, the reverse primer of the gene trap was 5′-CACTCCAACCTCCGCAAACTC-3′, and the wild-type control amplicon reverse primer was 5′-GGACGTGCGTGATGTATCTG-3′. Purification and direct sequencing revealed the exact integration of the gene trap (not shown).
Routine genotyping of mouse progeny from tail DNA samples was done by PCR using the following primers: for the Cdk8 wild-type allele, F1, 5′-GCAAAACAAGCAAGCAAACA-3′ and R1, 5′-CATGGCAGCATCTTCCAGTA-3′, and for the Cdk8 mutant allele, F2, 5′-CAGGCAGTTGTGCATTGTCA-3′, and R2, 5′-CGGAGCGGATCTCAAACTCT-3′.
The genotyping of preimplantation embryos was done using a multiplex nested PCR where the forward primer is shared and reverse primers are allele specific. The wild-type outer amplicon is 492 bp, and the mutant outer amplicon is 456 bp. The inner amplicons are 107 bp and 246 bp for the wild type and mutant, respectively. The common forward primers are 5′-TGCAGTAACGAGAGGCAGTG-3′ and 5′-CAGGCAGTTGTGCATTGTCA-3′ for the outer and inner amplicons, respectively. The reverse primers for the outer amplicons are 5′-AAGACCTCCCTCCAAGCAGT-3′ and 5′-TAGGACAAGAGGGCGAGACC-3′ for the wild-type and mutant alleles, respectively. The reverse primers for the inner amplicons are 5′-CATGGCAGCATCTTCCAGTA-3′ and 5′-CGGAGCGGATCTCAAACTCT-3′ for the wild-type and mutant alleles, respectively.
Southern blotting.
Genomic DNA was prepared from postmortem biopsy samples by standard protocols (36). Briefly, biopsy samples were incubated with a denaturing proteinase K lysis buffer at 56°C overnight. The aqueous phase was extracted two times using phenol extraction in combination with phase lock gel (Eppendorf). The extracted aqueous phase was then precipitated and resuspended in Tris-EDTA buffer. Ten micrograms of genomic DNA was digested overnight using a 5 U/μg restriction enzyme/DNA ratio. The digests were precipitated and separated on 0.8% agarose and transferred to Hybond N+ membranes by using capillary transfer. Hybridizations were carried out at 65°C in Rapid-hyb solution (Amersham Biosciences). The probe used was generated using a cloned intron fragment of the Cdk8 locus as template in a random-labeling reaction (Redi-Prime II; Amersham Biosciences). The cloning of the probe fragment was done by PCR cloning (Topo-TA kit; Invitrogen) of the fragment amplified by the following primers: 5′-TTTTAAGCAGCCATCCATCC-3′ and 5′-ACAAGCTCAGGCACCTTTGT-3′.
Plasmid constructs.
The Cdk8 and green fluorescent protein (GFP) templates used for double-stranded RNA (dsRNA) production were generated by PCR cloning (TOPO-TA kit; Invitrogen) by two-step amplification from Schneider S2 genomic DNA and pEGFP-N2 (Clontech) plasmid templates. The first-round PCR primers containing T7 primer-flanking sequences (bold) were F(Cdk8), CTATAGGGCGATGGCAAAGAATATGCC; R(Cdk8), CTATAGGGGTGGAGTATGTGGAGCGTT; F(GFP), CTATAGGGACGTAAACGGCCACAAGTTC; and R(GFP), CTATAGGGTGCTCAGGTAGTGGTTGTCG. First-round amplification was followed by a second amplification using T7 primers. The cloned inserts were verified by sequencing. These vectors were subsequently used as PCR templates for dsRNA production using a MegaScript T7 kit (Ambion) according to the manufacturer's instructions.
Plasmids encoding short hairpin RNAs (shRNAs) for Cdk8 and the control plasmid were obtained from the Biocentrum Helsinki BCH knockdown library, University of Helsinki (www.ltdk.helsinki.fi/sysbio). The control plasmid encodes an incomplete shRNA with a 4-bp deletion in the targeting sequence for Cdk8. The plasmid backbone, pENTR-H1-BgH, is a derivative of pENTR (Invitrogen) containing the H1 promoter and shRNA sequence cloned using BglII and HindIII sites. The mouse Cdk8 open reading frame was amplified by PCR from mouse embryo fibroblast cDNA template using primers CACC-ATG-GAC-TAT-GAC-TTT-AAA-GTG (translation start codon in bold) and TCA-GTA-CCG-ATG-TGT-CTG-ATG (translation stop codon in bold). The PCR product was cloned to pENTR/D-Topo (Invitrogen) according to the manufacturer's protocol and subsequently verified by sequencing. All clones of several independent reactions revealed a silent change (CCA→CCG) at codon 358 compared to the sequence NM 153599 (GenBank accession number). Subsequent transfer to pDEST47 vector (Invitrogen) was performed according to the manufacturer's protocol and verified by restriction digests.
Cell culture, transfections, and gene knockdown by RNA interference (RNAi) in S2 and 293FT cells.
ES cells were cultured according to standard protocols (31). Briefly, ES cells were maintained in KO Dulbecco's modified Eagle's medium (Invitrogen) on gelatinized plates in the presence of leukemia inhibitory factor (Chemicon) and passaged every 48 h.
293FT cells (Invitrogen) were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, l-glutamine, and penicillin-streptomycin. Transfections were performed using Lipofectamine plus (Invitrogen) according to the manufacturer's instructions. Cdk8 RNAi in mammalian cells was done by using transfection of plasmids encoding shRNA targeting Cdk8 or its control shRNA. In the stable transfection experiments, pCMV-β-gal (29) was cotransfected with a 1/20 (wt/wt) ratio to total DNA and used to normalize for transfection efficiency between samples (11). The selection of stably transfected clones was done using puromycin (1 μg/ml) for 12 days, using cotransfection of pRetroSuper (5) in a 1/10 (wt/wt) ratio of total DNA.
Drosophila Schneider S2 cell (Invitrogen) culture and RNAi knockdown protocols were performed as described previously (8) with minor modifications. Briefly, S2 cells were cultured at 22.5°C in Drosophila SFM (Gibco, United Kingdom) supplemented with 10% fetal bovine serum, l-glutamine, and penicillin-streptomycin. Cdk8 knockdown was done by adding 15 μg dsRNAs for Cdk8 (22) or GFP as control to 106 cells in 1 ml serum-free medium and incubating for 1 h at room temperature before adding medium to a total of 3 ml including supplements. To maintain efficient silencing of Cdk8, repeated RNAi treatments were performed as described above, coinciding with the counting of cells and dilution of cultures.
Western blotting.
Soluble protein extracts of ES cells from feeder-independent cultures or subconfluent 293FT cells were prepared. Cells were collected in lysis buffer (ELB plus inhibitors; 150 mM NaCl, 50 mM HEPES, pH 7.4, 5 mM EDTA, 0.1% CA-630 with 5 mM dithiothreitol, 12.5 mg/ml aprotinin, 0.5 mM phenylmethylsulfonyl fluoride-50 mM β-glycerophosphate, 5 μg/ml leupeptin) 48 h after transfection (293FT cells) or seeding (ES cells). Total protein extracts of Drosophila S2 cells were prepared by collecting cells in boiling Laemmli buffer (50 mM Tris, pH 6.8, 10% glycerol, 2% sodium dodecyl sulfate, 1 mM dithiothreitol). Forty micrograms of total protein in Laemmli buffer was separated by 7.5 to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subsequently transferred to a nitrocellulose membrane. Incubation with mouse monoclonal anti-β-galactosidase antibody (Promega) and subsequent enhanced chemiluminescence detection (Supersignal; Pierce) were performed according to the manufacturers' instructions. Cdk8 was detected by using anti-Cdk8 (human) antibodies (24), a kind gift from Erich Nigg (Max Planck Institute, Martinsried).
Image postprocessing.
For purposes of clarity in the presentation of the data, the Western blot images have been processed using the Level function of the Creative Suite software (Adobe Systems Inc.); the level changes were made to the entire image before cropping. Similarly, lanes have been reorganized as is indicated by the black bars seen in Fig. 3.
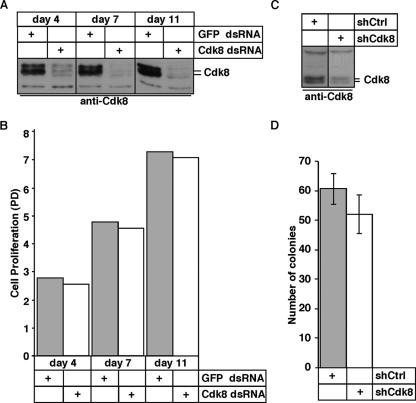
FIG. 3.
Proliferation of cultured metazoan cells is unaffected by Cdk8 depletion. (A) Efficiency of the Cdk8 knockdown (Cdk8 dsRNA) compared to that of the control (GFP dsRNA) in Schneider S2 cells was assessed by anti-Cdk8 Western blot analysis. (B) The proliferation rate of parallel S2 cultures was determined and is presented as population doublings (PD) over 11 days. (C) Anti-Cdk8 Western blot analysis of shRNA-mediated knockdown in transiently transfected 293FT cells. (D) The effect on proliferation of expressing shRNA-targeting Cdk8 in 293FT cells is determined as the number of drug-resistant colonies at 13 days posttransfection. The values are the average number ± SD of colonies/10 microscopic fields (10× objective, approximately 3.1 cm2) of three independent transfections normalized to pCMV-β-gal activity.
RESULTS AND DISCUSSION
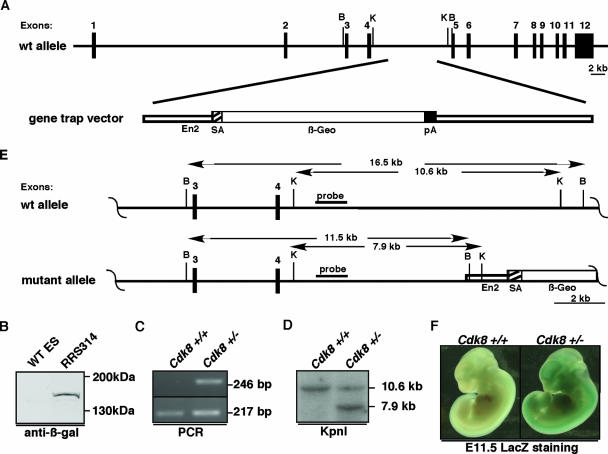
In order to study the in vivo functions of mammalian Cdk8, we used a murine ES cell line (RRS314) with a gene trap insertion in intron 4 of Cdk8 based on the sequencing of a fusion cDNA (Fig. 1A) (40). Consistent with this, we noted that the insertion of the gene trap leads to a fusion protein migrating at the predicted size of 163 kDa with 152 N-terminal amino acids of Cdk8 fused to 1,323 amino acids of the β-galactosidase-neomycin (β-Geo) selection marker (Fig. 1B) and lacking almost the entire kinase domain of Cdk8. Thus, this allele represents a loss-of-function allele of Cdk8 and, based on our knowledge of the interactions of Cdk8, probably represents a null allele. Supporting this, the Cdk8-β-Geo fusion protein has no kinase activity towards the recombinant glutathione S-transferase-carboxy-terminal domain when immunoprecipitated using an antibody against β-galactosidase (see Fig. S1 in the supplemental material). In order to characterize the genomic structure of this allele, we determined the precise integration site at the genomic level through a PCR mapping strategy and direct sequencing of the amplified genomic DNA (see Materials and Methods; data not shown). The information regarding the precise insertion site was then used to design allele-specific PCR genotyping reactions that distinguish wild-type and mutant alleles, as seen by the amplification of the 246-bp mutant product in the Cdk8+/− ES cells (Fig. 1C). Furthermore, we could confirm the sequence-based integration site by KpnI, BglI, and BamHI Southern blotting analysis (Fig. 1D and data not shown) using an external probe as outlined in Fig. 1E.
FIG. 1.
Characterization of the Cdk8 gene trap allele. (A) Schematic representation of the gene trap insertion in the Cdk8 locus. En2, SA, and pA indicate the engrailed 2 sequence and its splice acceptor and the polyadenylation site of the gene trap vector. (B) Anti-β-galactosidase Western blot analysis showing the expression of the Cdk8-β-Geo mutant protein in the targeted (RRS314) ES cells. (C) PCR genotyping of Cdk8+/+ and Cdk8+/− ES cells. (D) Southern blotting using a 5′ flanking probe of KpnI-digested DNA samples of Cdk8+/− and Cdk8+/+ mice. (E) Map of the determined genomic structure of the Cdk8 gene trap allele. B (BglI) and K (KpnI) designate restriction sites used for Southern blotting analysis. The locations of the probes used in the Southern blots are indicated by the black bars. (F) Cdk8 expression is indicated by LacZ staining of paraformaldehyde-fixed embryos extracted at E11.5. The indicated genotypes were obtained by PCR genotyping of the yolk sac. wt, wild type.
Cdk8+/− mice, generated by morula aggregation using the described Cdk8+/− ES cell line (Materials and Methods), were normal, based on appearance and breeding capacity indistinguishable from those of wild-type littermates (data not shown). In order to analyze homozygous Cdk8−/− mice, the offspring of Cdk8+/− intercrosses were genotyped at weaning. Cdk8−/− animals were not observed, and the resulting 1:2 ratio of Cdk8+/+ and Cdk8+/− genotypes (Table 1) indicates that Cdk8 is required for normal mammalian development. In addition, the Mendelian ratio of wild-type and heterozygote mice strongly argues that the lethality in homozygous mice is not due to a dominant gain of function of the Cdk8 allele.
TABLE 1.
Genotyping of progeny from Cdk8 heterozygote intercrosses
| Developmental stage | No. with Cdk8 genotype
|
||
|---|---|---|---|
| +/+ | +/− | −/− | |
| Postnatal | 49 | 96 | 0 |
| E12.5 | 14 | 50 | 0 |
| E3.5 | 10 | 7 | 0 |
| E3.0 | 11 | 11 | 1 |
| E2.5 | 15 | 14 | 4 |
In order to analyze the developmental process where Cdk8 exerts the essential function, we turned to the midgestation period of mouse development, as the Cdk8 submodule has been shown to be involved in the organogenesis of worms, flies, and zebrafish (18, 35, 46, 54). First, we analyzed the expression of Cdk8 at midgestation, taking advantage of the fusion mRNA encoding β-galactosidase and controlled by the murine Cdk8 promoter. Widespread LacZ staining in E11.5 Cdk8+/− embryos (Fig. 1F) indicated ubiquitous expression of Cdk8 during mouse midgestation. In order to analyze the possible role of Cdk8 during midgestation, we examined embryos at E12.5 from Cdk8+/− intercrosses. The absence of Cdk8−/− animals among the embryos examined (Table 1) indicated a requirement of Cdk8 prior to midgestation. Importantly, the absence of empty deciduas or other signs of resorption indicated a critical function prior to implantation.
In order to analyze the apparent preimplantation requirement for Cdk8, we isolated embryos at E2.5, E3.0, and E3.5. The extracted embryos were photographed by using phase-contrast microscopy and subsequently genotyped by PCR. Cdk8−/− embryos were identified at E2.5 (Fig. 2A and Table 1), whereas we could find only one Cdk8−/− embryo at E3.0 (Table 1) and never past this stage (E3.5, Table 1). The apparent overrepresentation of wild-type embryos at E2.5 to E3.5 is not likely to be significant as it was not noted at later stages (Table 1). Inspection of the morphology of embryos at E2.5 revealed fragmented blastomeres in the Cdk8−/− mutants (Fig. 2B); notably, we could not find compacted Cdk8−/− embryos, indicating a requirement of Cdk8 prior to or during the 8-cell stage before compaction.
FIG. 2.
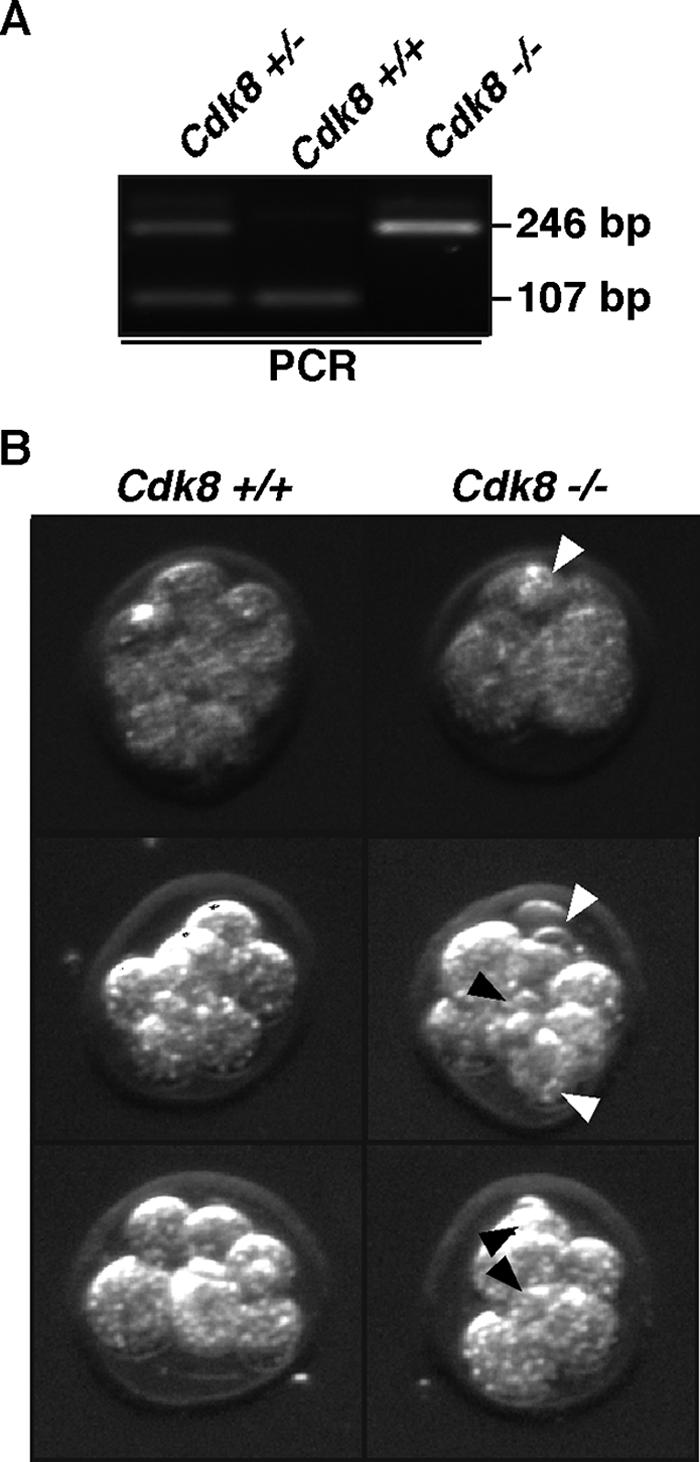
Cdk8 is required at E2.5. (A) Embryos isolated at E2.5 of Cdk8+/− intercrosses were genotyped using nested PCR. E2.5 is the last stage of embryogenesis where Cdk8−/− embryos can readily be isolated. Molecular size markers are indicated on the right. (B) Phase-contrast photomicrographs of Cdk8+/+ and Cdk8−/− embryos at E2.5. Black and white arrowheads indicate fragmented blastomeres.
In an effort to rescue the loss of Cdk8 in preimplantation embryos, we microinjected synthetic, capped mRNA encoding Cdk8 and enhanced GFP flanked by the untranslated regions of the Xenopus globin gene (25) into the cytoplasm of zygotes isolated from timed Cdk8+/− intercrosses using superovulated females (see materials and methods in the supplemental material). As indicated by the robust expression of enhanced GFP in the injected embryos (see Fig. S2A and B in the supplemental material), this method allows the expression of injected synthetic mRNAs in all cells of the embryo throughout preimplantation development. Surprisingly, although we observed progression to morula and blastocyst stages in both noninjected controls and mRNA-injected embryos (see Fig. S2A and B in the supplemental material), we could not find Cdk8−/− embryos after 4 days of culture (see Fig. S2C and D in the supplemental material). The ratio of Cdk8+/+ and Cdk8+/− embryos (n = 37 and n = 62, respectively; see Fig. S2D in the supplemental material) suggests that Cdk8−/− embryos were lost during the embryo extraction and injection procedure. In an attempt to verify this hypothesis, we managed to obtain the genotypes of a limited number of the runted noninjected embryos, revealing one of Cdk8−/− genotype (see Fig. S2C, embryo 12, in the supplemental material). Thus, while Cdk8−/− embryos can develop in utero to a stage prior to compaction, Cdk8−/− embryos extracted from superovulated females have a runted appearance and reduced viability already at E0.5 when cultured in vitro.
The lethality of Cdk8−/− embryos prior to compaction was unexpected, as this early lethality is rare and typically associated with genes involved in functions required for cell viability, such as Xpd (10), Rad51 (47), Sfrs3 (21), and Incenp (9). Cdk8, on the other hand, has been demonstrated to be dispensable for the viability of cells in budding yeast (17, 49) and the fission yeast Schizosaccharomyces pombe (52). In order to examine the requirement of Cdk8 for cell autonomous growth in metazoan cells, we performed knockdown of Cdk8 in Drosophila S2 cells using long dsRNAs. A single dose of dsRNA against Cdk8 efficiently downregulated Cdk8 at the protein level compared to control (GFP)-treated samples (Fig. 3A, day 4). Sustained knockdown of Cdk8, ensured by repeated Cdk8 and GFP dsRNA treatments on days 4 and 7 (Fig. 3A, days 7 and 11), had no effect on the proliferation of Drosophila S2 cells (Fig. 3B). In addition, shRNA-mediated knockdown of Cdk8, as seen in transient transfections of cultured mammalian 293FT cells analyzed by Western blotting (Fig. 3C), does not decrease the ability to form drug-resistant colonies in stable transfections (Fig. 3D). Taken together, these data provide evidence for Cdk8 not being required for autonomous cell growth or survival. This conclusion is consistent with the results of large knockdown screens of cultured Drosophila cells (1, 2) and human cells (30); also, slime mold (27, 43) and wall cress (51) Cdk8 are not essential for cell viability. Interestingly, a recent report discussing null alleles of Drosophila Cdk8 and Cyclin C also found that neither is required for the viability of cells, while they were essential for organismal development, similar to our results (28).
An alternative role for Cdk8 that would be consistent with the observed early embryonic lethality of the Cdk8−/− embryos is provided by the analysis of Cdk8 submodule mutant flies and worms, where Cdk8 submodule members appear to regulate specific developmental pathways, such as the Wnt and Hedgehog pathways, involved in differentiation and cell fate determination (28, 46, 54). In this regard, it is interesting to note that the blastomeres at the 2-cell stage of the developing mouse embryo already have biased cell fates (13, 14, 34, 53).
Active transcriptional regulation has been suggested to be an important molecular mechanism for differentiation events before the compacted 8-cell stage, based on transcription profiling (16, 50), where bursts of transcription are noted between the 2-cell and 8-cell stages. Cdk8 can function as a transcriptional repressor in cultured mammalian cells (12), and the repression of transcription observed between the 2-cell and 8-cell stages is correlated with an induction of Cdk8 transcription just prior to compaction (50), raising the interesting notion that Cdk8 is important for transcriptional repression during preimplantation development.
To summarize, these observations suggest that the developmental arrest of Cdk8-deficient embryos is due to transcriptional deregulation of developmentally critical genes and that this underlies the unexpected very early requirement of Cdk8 in murine development prior to compaction observed in this study.
Supplementary Material
Acknowledgments
This study was supported by Biocentrum Helsinki, the Finnish Cancer Organization, Sigrid Juselius Foundation, and the Academy of Finland.
We thank Eero Lehtonen, Kirsi Sainio, Maria-Elena Torres Padilla, and the members of the Mäkelä lab, especially Tea Vallenius, for fruitful discussions, Anou Londesborough for instructions in embryo manipulation, and Kirsi Salonen for transgenic work.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
Published ahead of print on 9 July 2007.
REFERENCES
- 1.Bettencourt-Dias, M., R. Giet, R. Sinka, A. Mazumdar, W. G. Lock, F. Balloux, P. J. Zafiropoulos, S. Yamaguchi, S. Winter, R. W. Carthew, M. Cooper, D. Jones, L. Frenz, and D. M. Glover. 2004. Genome-wide survey of protein kinases required for cell cycle progression. Nature 432:980-987. [DOI] [PubMed] [Google Scholar]
- 2.Bjorklund, M., M. Taipale, M. Varjosalo, J. Saharinen, J. Lahdenpera, and J. Taipale. 2006. Identification of pathways regulating cell size and cell-cycle progression by RNAi. Nature 439:1009-1013. [DOI] [PubMed] [Google Scholar]
- 3.Bjorklund, S., and C. M. Gustafsson. 2005. The yeast Mediator complex and its regulation. Trends Biochem. Sci. 30:240-244. [DOI] [PubMed] [Google Scholar]
- 4.Borggrefe, T., R. Davis, H. Erdjument-Bromage, P. Tempst, and R. D. Kornberg. 2002. A complex of the Srb8, -9, -10, and -11 transcriptional regulatory proteins from yeast. J. Biol. Chem. 277:44202-44207. [DOI] [PubMed] [Google Scholar]
- 5.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2:243-247. [DOI] [PubMed] [Google Scholar]
- 6.Chadick, J. Z., and F. J. Asturias. 2005. Structure of eukaryotic Mediator complexes. Trends Biochem. Sci. 30:264-271. [DOI] [PubMed] [Google Scholar]
- 7.Chi, Y., M. J. Huddleston, X. Zhang, R. A. Young, R. S. Annan, S. A. Carr, and R. J. Deshaies. 2001. Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev. 15:1078-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemens, J. C., C. A. Worby, N. Simonson-Leff, M. Muda, T. Maehama, B. A. Hemmings, and J. E. Dixon. 2000. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA 97:6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutts, S. M., K. J. Fowler, B. T. Kile, L. L. Hii, R. A. O'Dowd, D. F. Hudson, R. Saffery, P. Kalitsis, E. Earle, and K. H. Choo. 1999. Defective chromosome segregation, microtubule bundling and nuclear bridging in inner centromere protein gene (Incenp)-disrupted mice. Hum. Mol. Genet. 8:1145-1155. [DOI] [PubMed] [Google Scholar]
- 10.de Boer, J., I. Donker, J. de Wit, J. H. Hoeijmakers, and G. Weeda. 1998. Disruption of the mouse xeroderma pigmentosum group D DNA repair/basal transcription gene results in preimplantation lethality. Cancer Res. 58:89-94. [PubMed] [Google Scholar]
- 11.Eustice, D. C., P. A. Feldman, A. M. Colberg-Poley, R. M. Buckery, and R. H. Neubauer. 1991. A sensitive method for the detection of beta-galactosidase in transfected mammalian cells. BioTechniques 11:739-743. [PubMed] [Google Scholar]
- 12.Fryer, C. J., J. B. White, and K. A. Jones. 2004. Mastermind recruits CycC:CDK8 to phosphorylate the Notch ICD and coordinate activation with turnover. Mol. Cell 16:509-520. [DOI] [PubMed] [Google Scholar]
- 13.Fujimori, T., Y. Kurotaki, J. Miyazaki, and Y. Nabeshima. 2003. Analysis of cell lineage in two- and four-cell mouse embryos. Development 130:5113-5122. [DOI] [PubMed] [Google Scholar]
- 14.Gardner, R. L. 2001. Specification of embryonic axes begins before cleavage in normal mouse development. Development 128:839-847. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson, C. M., and T. Samuelsson. 2001. Mediator—a universal complex in transcriptional regulation. Mol. Microbiol. 41:1-8. [DOI] [PubMed] [Google Scholar]
- 16.Hamatani, T., M. G. Carter, A. A. Sharov, and M. S. Ko. 2004. Dynamics of global gene expression changes during mouse preimplantation development. Dev. Cell 6:117-131. [DOI] [PubMed] [Google Scholar]
- 17.Holstege, F. C., E. G. Jennings, J. J. Wyrick, T. I. Lee, C. J. Hengartner, M. R. Green, T. R. Golub, E. S. Lander, and R. A. Young. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95:717-728. [DOI] [PubMed] [Google Scholar]
- 18.Hong, S. K., C. E. Haldin, N. D. Lawson, B. M. Weinstein, I. B. Dawid, and N. A. Hukriede. 2005. The zebrafish kohtalo/trap230 gene is required for the development of the brain, neural crest, and pronephric kidney. Proc. Natl. Acad. Sci. USA 102:18473-18478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito, M., H. J. Okano, R. B. Darnell, and R. G. Roeder. 2002. The TRAP100 component of the TRAP/Mediator complex is essential in broad transcriptional events and development. EMBO J. 21:3464-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito, M., C. X. Yuan, H. J. Okano, R. B. Darnell, and R. G. Roeder. 2000. Involvement of the TRAP220 component of the TRAP/SMCC coactivator complex in embryonic development and thyroid hormone action. Mol. Cell 5:683-693. [DOI] [PubMed] [Google Scholar]
- 21.Jumaa, H., G. Wei, and P. J. Nielsen. 1999. Blastocyst formation is blocked in mouse embryos lacking the splicing factor SRp20. Curr. Biol. 9:899-902. [DOI] [PubMed] [Google Scholar]
- 22.Kiger, A. A., B. Baum, S. Jones, M. R. Jones, A. Coulson, C. Echeverri, and N. Perrimon. 2003. A functional genomic analysis of cell morphology using RNA interference. J. Biol. 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kornberg, R. D. 2005. Mediator and the mechanism of transcriptional activation. Trends Biochem. Sci. 30:235-239. [DOI] [PubMed] [Google Scholar]
- 24.Leclerc, V., J. P. Tassan, P. H. O'Farrell, E. A. Nigg, and P. Leopold. 1996. Drosophila Cdk8, a kinase partner of cyclin C that interacts with the large subunit of RNA polymerase II. Mol. Biol. Cell 7:505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemaire, P., N. Garrett, and J. B. Gurdon. 1995. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell 81:85-94. [DOI] [PubMed] [Google Scholar]
- 26.Liao, S. M., J. Zhang, D. A. Jeffery, A. J. Koleske, C. M. Thompson, D. M. Chao, M. Viljoen, H. J. van Vuuren, and R. A. Young. 1995. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature 374:193-196. [DOI] [PubMed] [Google Scholar]
- 27.Lin, H. H., M. Khosla, H. J. Huang, D. W. Hsu, C. Michaelis, G. Weeks, and C. Pears. 2004. A homologue of Cdk8 is required for spore cell differentiation in Dictyostelium. Dev. Biol. 271:49-58. [DOI] [PubMed] [Google Scholar]
- 28.Loncle, N., M. Boube, L. Joulia, C. Boschiero, M. Werner, D. L. Cribbs, and H. M. Bourbon. 2007. Distinct roles for Mediator Cdk8 module subunits in Drosophila development. EMBO J. 26:1045-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacGregor, G. R., and C. T. Caskey. 1989. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 17:2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacKeigan, J. P., L. O. Murphy, and J. Blenis. 2005. Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nat. Cell Biol. 7:591-600. [DOI] [PubMed] [Google Scholar]
- 31.Nagy, A., M. Gertsenstein, K. Vintersten, and R. Behringer. 2003. Manipulating the mouse embryo, a laboratory manual, 3rd ed., vol. 1. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 32.Nelson, C., S. Goto, K. Lund, W. Hung, and I. Sadowski. 2003. Srb10/Cdk8 regulates yeast filamentous growth by phosphorylating the transcription factor Ste12. Nature 421:187-190. [DOI] [PubMed] [Google Scholar]
- 33.Pavri, R., B. Lewis, T. K. Kim, F. J. Dilworth, H. Erdjument-Bromage, P. Tempst, G. de Murcia, R. Evans, P. Chambon, and D. Reinberg. 2005. PARP-1 determines specificity in a retinoid signaling pathway via direct modulation of mediator. Mol. Cell 18:83-96. [DOI] [PubMed] [Google Scholar]
- 34.Piotrowska, K., F. Wianny, R. A. Pedersen, and M. Zernicka-Goetz. 2001. Blastomeres arising from the first cleavage division have distinguishable fates in normal mouse development. Development 128:3739-3748. [DOI] [PubMed] [Google Scholar]
- 35.Rau, M. J., S. Fischer, and C. J. Neumann. 2006. Zebrafish Trap230/Med12 is required as a coactivator for Sox9-dependent neural crest, cartilage and ear development. Dev. Biol. 296:83-93. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook, J., and D. W. Russell. 2001. Molecular cloning, a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 37.Samuelsen, C. O., V. Baraznenok, O. Khorosjutina, H. Spahr, T. Kieselbach, S. Holmberg, and C. M. Gustafsson. 2003. TRAP230/ARC240 and TRAP240/ARC250 Mediator subunits are functionally conserved through evolution. Proc. Natl. Acad. Sci. USA 100:6422-6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlaeger, T. M., Y. Qin, Y. Fujiwara, J. Magram, and T. N. Sato. 1995. Vascular endothelial cell lineage-specific promoter in transgenic mice. Development 121:1089-1098. [DOI] [PubMed] [Google Scholar]
- 39.Spahr, H., O. Khorosjutina, V. Baraznenok, T. Linder, C. O. Samuelsen, D. Hermand, T. P. Makela, S. Holmberg, and C. M. Gustafsson. 2003. Mediator influences Schizosaccharomyces pombe RNA polymerase II-dependent transcription in vitro. J. Biol. Chem. 278:51301-51306. [DOI] [PubMed] [Google Scholar]
- 40.Stryke, D., M. Kawamoto, C. C. Huang, S. J. Johns, L. A. King, C. A. Harper, E. C. Meng, R. E. Lee, A. Yee, L. L'Italien, P. T. Chuang, S. G. Young, W. C. Skarnes, P. C. Babbitt, and T. E. Ferrin. 2003. BayGenomics: a resource of insertional mutations in mouse embryonic stem cells. Nucleic Acids Res. 31:278-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun, X., Y. Zhang, H. Cho, P. Rickert, E. Lees, W. Lane, and D. Reinberg. 1998. NAT, a human complex containing Srb polypeptides that functions as a negative regulator of activated transcription. Mol. Cell 2:213-222. [DOI] [PubMed] [Google Scholar]
- 42.Taatjes, D. J., A. M. Naar, F. Andel III, E. Nogales, and R. Tjian. 2002. Structure, function, and activator-induced conformations of the CRSP coactivator. Science 295:1058-1062. [DOI] [PubMed] [Google Scholar]
- 43.Takeda, K., T. Saito, and H. Ochiai. 2002. A novel Dictyostelium Cdk8 is required for aggregation, but is dispensable for growth. Dev. Growth Differ. 44:213-223. [DOI] [PubMed] [Google Scholar]
- 44.Tassan, J. P., M. Jaquenoud, P. Leopold, S. J. Schultz, and E. A. Nigg. 1995. Identification of human cyclin-dependent kinase 8, a putative protein kinase partner for cyclin C. Proc. Natl. Acad. Sci. USA 92:8871-8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas, M. C., and C. Chiang. 2006. The general transcription machinery and general cofactors. Crit. Rev. Biochem. Mol. Biol. 41:105-178. [DOI] [PubMed] [Google Scholar]
- 46.Treisman, J. 2001. Drosophila homologues of the transcriptional coactivation complex subunits TRAP240 and TRAP230 are required for identical processes in eye-antennal disc development. Development 128:603-615. [DOI] [PubMed] [Google Scholar]
- 47.Tsuzuki, T., Y. Fujii, K. Sakumi, Y. Tominaga, K. Nakao, M. Sekiguchi, A. Matsushiro, Y. Yoshimura, and T. Morita. 1996. Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc. Natl. Acad. Sci. USA 93:6236-6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tudor, M., P. J. Murray, C. Onufryk, R. Jaenisch, and R. A. Young. 1999. Ubiquitous expression and embryonic requirement for RNA polymerase II coactivator subunit Srb7 in mice. Genes Dev. 13:2365-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van de Peppel, J., N. Kettelarij, H. van Bakel, T. T. Kockelkorn, D. van Leenen, and F. C. Holstege. 2005. Mediator expression profiling epistasis reveals a signal transduction pathway with antagonistic submodules and highly specific downstream targets. Mol. Cell 19:511-522. [DOI] [PubMed] [Google Scholar]
- 50.Wang, Q. T., K. Piotrowska, M. A. Ciemerych, L. Milenkovic, M. P. Scott, R. W. Davis, and M. Zernicka-Goetz. 2004. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev. Cell 6:133-144. [DOI] [PubMed] [Google Scholar]
- 51.Wang, W., and X. Chen. 2004. HUA ENHANCER3 reveals a role for a cyclin-dependent protein kinase in the specification of floral organ identity in Arabidopsis. Development 131:3147-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watson, P., and J. Davey. 1998. Characterization of the Prk1 protein kinase from Schizosaccharomyces pombe. Yeast 14:485-492. [DOI] [PubMed] [Google Scholar]
- 53.Weber, R. J., R. A. Pedersen, F. Wianny, M. J. Evans, and M. Zernicka-Goetz. 1999. Polarity of the mouse embryo is anticipated before implantation. Development 126:5591-5598. [DOI] [PubMed] [Google Scholar]
- 54.Yoda, A., H. Kouike, H. Okano, and H. Sawa. 2005. Components of the transcriptional Mediator complex are required for asymmetric cell division in C. elegans. Development 132:1885-1893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.