Abstract
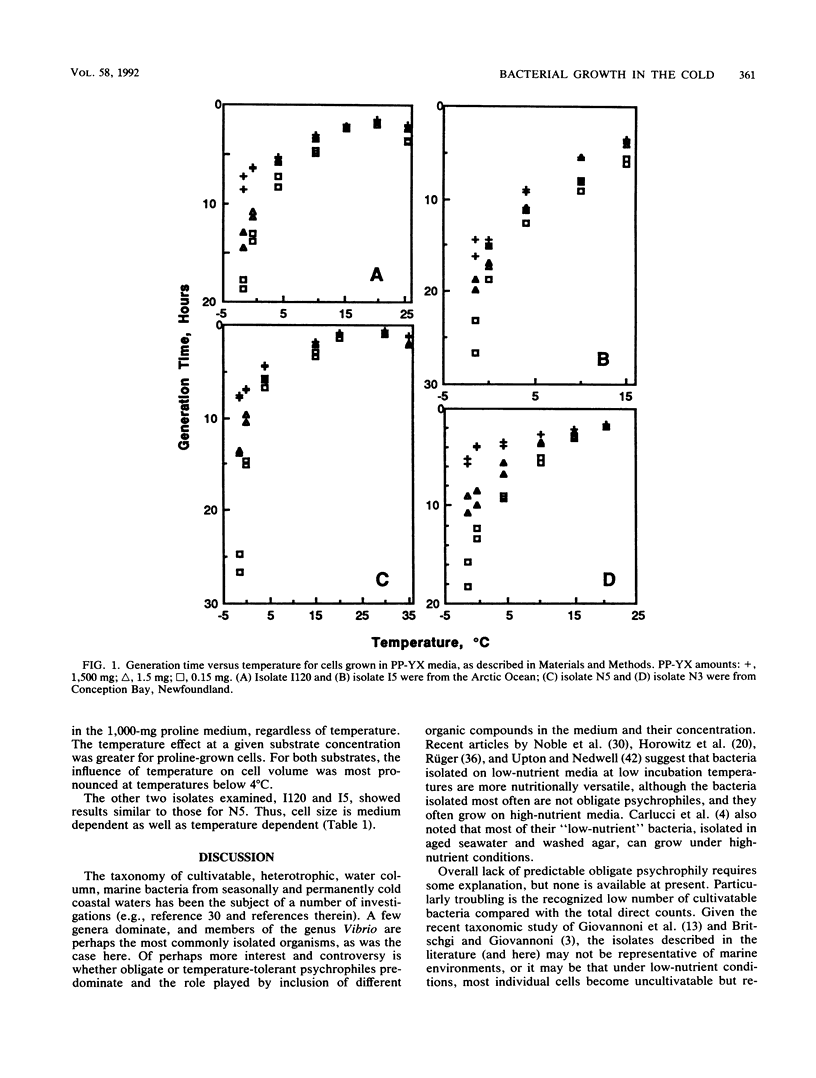
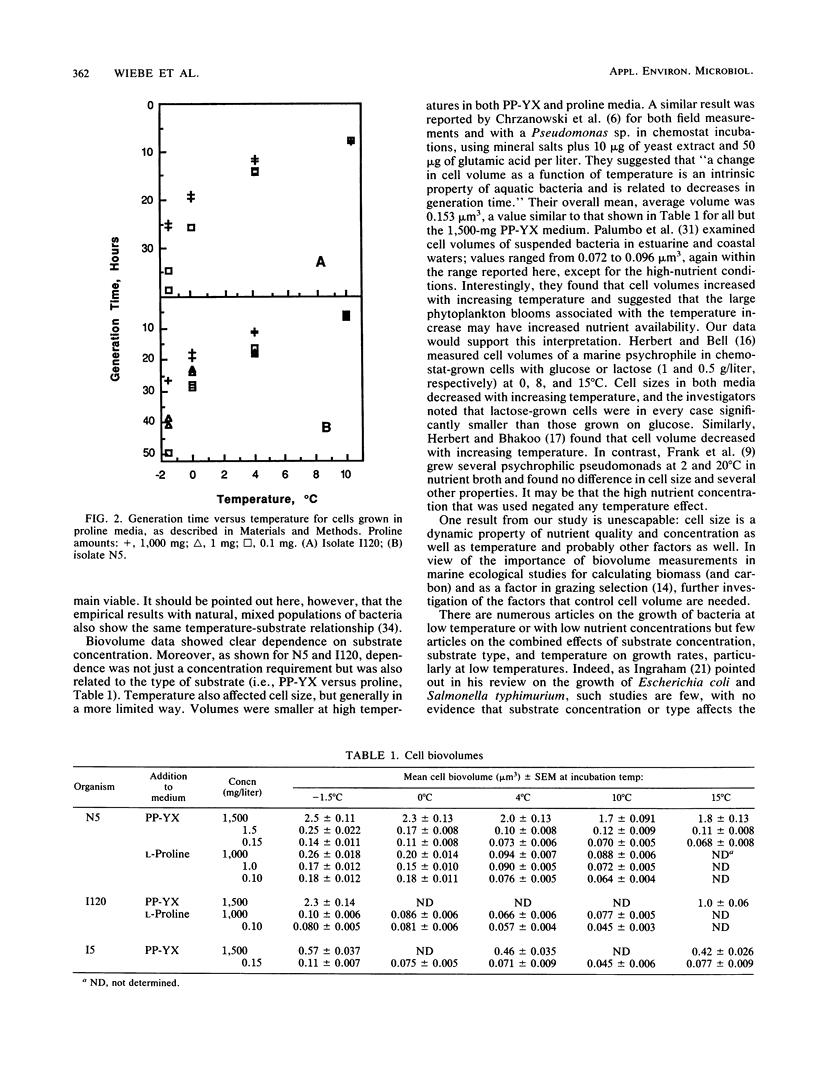
Growth responses and biovolume changes for four facultatively psychrophilic bacterial isolates from Conception Bay, Newfoundland, and the Arctic Ocean were examined at temperatures from - 1.5 to 35°C, with substrate concentrations of 0.15, 1.5, and 1,500 mg of proteose peptone-yeast extract per liter. For two cultures, growth in 0.1, 1.0, and 1,000 mg of proline per liter was also examined. At 10 to 15°C and above, growth rates showed no marked effect of substrate concentration, while at - 1.5 and 0°C, there was an increasing requirement for organic nutrients, with generation times in low-nutrient media that were two to three times longer than in high-nutrient media. Biovolume showed a clear dependence on substrate concentration and quality; the largest cells were in the highest-nutrient media. Biovolume was also affected by temperature; the largest cells were found at the lowest temperatures. These data have implications for both food web structure and carbon flow in cold waters and for the effects of global climate change, since the change in growth rate is most dramatic at the lowest temperatures.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki T. The effect of temperature shifts on protein synthesis by the psychrophilic bacterium Vibrio sp. strain ANT-300. J Gen Microbiol. 1991 Apr;137(4):817–826. doi: 10.1099/00221287-137-4-817. [DOI] [PubMed] [Google Scholar]
- Britschgi T. B., Giovannoni S. J. Phylogenetic analysis of a natural marine bacterioplankton population by rRNA gene cloning and sequencing. Appl Environ Microbiol. 1991 Jun;57(6):1707–1713. doi: 10.1128/aem.57.6.1707-1713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank H. A., Reid A., Santo L. M., Lum N. A., Sandler S. T. Similarity in several properties of psychorophilic bacteria grown at low and moderate temperatures. Appl Microbiol. 1972 Oct;24(4):571–574. doi: 10.1128/am.24.4.571-574.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni S. J., Britschgi T. B., Moyer C. L., Field K. G. Genetic diversity in Sargasso Sea bacterioplankton. Nature. 1990 May 3;345(6270):60–63. doi: 10.1038/345060a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez J. M., Sherr E. B., Sherr B. F. Size-selective grazing on bacteria by natural assemblages of estuarine flagellates and ciliates. Appl Environ Microbiol. 1990 Mar;56(3):583–589. doi: 10.1128/aem.56.3.583-589.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. B., Shafer D., Ryan T., Pope D. H., Lowery H. K. Bacterioplankton in antarctic ocean waters during late austral winter: abundance, frequency of dividing cells, and estimates of production. Appl Environ Microbiol. 1983 May;45(5):1622–1632. doi: 10.1128/aem.45.5.1622-1632.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert R. A., Bell C. R. Growth characteristics of an obligately psychrophilic Vibrio sp. Arch Microbiol. 1977 Jun 20;113(3):215–220. doi: 10.1007/BF00492028. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krambeck C., Krambeck H. J., Overbeck J. Microcomputer-assisted biomass determination of plankton bacteria on scanning electron micrographs. Appl Environ Microbiol. 1981 Jul;42(1):142–149. doi: 10.1128/aem.42.1.142-149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. K., Dickie P. M. Temperature characteristics of photosynthetic and heterotrophic activities: seasonal variations in temperate microbial plankton. Appl Environ Microbiol. 1987 Oct;53(10):2282–2295. doi: 10.1128/aem.53.10.2282-2295.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S. F. Direct acridine orange counting of bacteria preserved with acidified lugol iodine. Appl Environ Microbiol. 1986 Sep;52(3):602–604. doi: 10.1128/aem.52.3.602-604.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A. V., Ferguson R. L., Rublee P. A. Size of suspended bacterial cells and association of heterotrophic activity with size fractions of particles in estuarine and coastal waters. Appl Environ Microbiol. 1984 Jul;48(1):157–164. doi: 10.1128/aem.48.1.157-164.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy L. R., Deibel D. Temperature regulation of bacterial activity during the spring bloom in newfoundland coastal waters. Science. 1986 Jul 18;233(4761):359–361. doi: 10.1126/science.233.4761.359. [DOI] [PubMed] [Google Scholar]
- Privalov P. L. Cold denaturation of proteins. Crit Rev Biochem Mol Biol. 1990;25(4):281–305. doi: 10.3109/10409239009090612. [DOI] [PubMed] [Google Scholar]


