Abstract
Calpains are ubiquitous calcium-regulated cysteine proteases that have been implicated in cytoskeletal organization, cell proliferation, apoptosis, cell motility, and hemostasis. Gene targeting was used to evaluate the physiological function of mouse calpain-1 and establish that its inactivation results in reduced platelet aggregation and clot retraction potentially by causing dephosphorylation of platelet proteins. Here, we report that calpain-1 null (Capn1−/−) platelets accumulate protein tyrosine phosphatase 1B (PTP1B), which correlates with enhanced tyrosine phosphatase activity and dephosphorylation of multiple substrates. Treatment of Capn1−/− platelets with bis(N,N-dimethylhydroxamido)hydroxooxovanadate, an inhibitor of tyrosine phosphatases, corrected the aggregation defect and recovered impaired clot retraction. More importantly, platelet aggregation, clot retraction, and tyrosine dephosphorylation defects were rescued in the double knockout mice lacking both calpain-1 and PTP1B. Further evaluation of mutant mice by the ferric chloride-induced arterial injury model suggests that the Capn1−/− mice are relatively resistant to thrombosis in vivo. Together, our results demonstrate that PTP1B is a physiological target of calpain-1 and suggest that a similar mechanism may regulate calpain-1-mediated tyrosine dephosphorylation in other cells.
Since the original identification of calpain activity, over three decades ago, there has been considerable interest in elucidating the physiological function of this protease system. Studies suggest a role for calpains in a wide variety of cellular processes, including cell motility, cell proliferation, apoptosis, cytoskeleton rearrangement, and hemostasis (21, 36). The term calpain activity generally refers to the combined effects of two cysteine proteases, which are calcium-dependent intracellular enzymes that are active at neutral pH. The current nomenclature designates these two enzymes as calpain-1 (μ-calpain or calpain I) and calpain-2 (m-calpain or calpain II). The calpains are ∼80-kDa polypeptides sharing ∼61% amino acid identity and are encoded by Capn1 and Capn2 genes (37). The two calpains each form a heterodimer with a 30-kDa regulatory subunit, encoded by the Capn4 gene (29), which has no catalytic activity. The enzyme activity of calpain-1 and calpain-2 is repressed by calpastatin, a specific endogenous protein inhibitor, present in large molar excess in various tissues (21). Calpain-1 is believed to be activated at micromolar calcium concentrations, whereas calpain-2 is activated at millimolar calcium concentrations in vitro (8, 21, 24). In the presence of calcium, limited autolysis of both calpains occurs, thus reducing their activation requirement for calcium (21). The precise mechanism of calpain activation by calcium remains poorly understood mainly because of uncertain local fluctuations in the calcium gradient from agonist-induced entry of extracellular calcium as well as calcium release from intracellular stores. Using an activity-based assay, previous studies have shown that both enzymes exist in most tissues with calpain-1 expression generally predominant in the hematopoietic compartment (25). On the basis of casein zymography measurements, we have demonstrated that calpain-1 is the sole active cysteine protease in mouse erythrocytes (3). In contrast, ∼80% of calpain activity in mouse platelets is contributed by calpain-1 with the remaining 20% derived from calpain-2 (3).
Functional studies of the calpain system have been facilitated by the use of synthetic inhibitors. However, as these synthetic inhibitors inhibit both calpains, they cannot identify the individual roles of calpain-1 and calpain-2. Both calpains degrade essentially the same set of substrates in vitro, and antibodies directed against calpain-1 cross-react with calpain-2 and vice versa. The endogenous inhibitor calpastatin, which inhibits both calpains, is not suitable for cell-based studies, as it does not cross the plasma membrane. Subsequent fusion of the minimum inhibitory sequence of calpastatin with a cell-permeable sequence led to the development of a calpastat reagent that functions as a cell-permeable inhibitor of both calpains (10). The use of calpeptin, a peptidyl aldehyde-based cell-permeable inhibitor of calpains, was seriously challenged with the demonstration that this inhibitor also inhibits several protein tyrosine phosphatases (PTPs) (33). Based on our knowledge thus far, there is no known synthetic inhibitor that can selectively inhibit either of the two calpains, suggesting that the development of experimental approaches that selectively ablate enzyme activity of individual calpains either systemically or in a tissue-specific manner is needed to explore their function in vivo.
Selective ablation of calpains in mice has been used to investigate the individual functions of calpains in vivo. Genetic disruption of the mouse Capn4 gene encoding the 30-kDa regulatory subunit resulted in early embryonic lethality, suggesting an essential requirement for both enzymes during mouse development (2, 39). We developed a calpain-1 null mouse model, which provided the first direct evidence for a functional role of calpain-1 in platelet aggregation and clot retraction pathways (3). The levels of calpain-2 and the 30-kDa regulatory subunit remained essentially unchanged in calpain-1 null mice, indicating that calpain-1 disruption does not alter their expression in vivo. Since calpain-1 disruption does not result in embryonic lethality, calpain-2 is likely to be the essential cysteine protease, or it compensates for calpain-1 function during embryonic development. Importantly, proteolysis of known calpain substrates, such as filamin (ABP 280), talin, and the β3 subunit of αIIbβ3 integrin, remained unaffected in calpain-1 null mouse platelets when examined at a high agonist concentration in the presence of calcium (3). These observations suggest that either these proteins are not physiological targets of platelet calpain-1 or that the presence of ∼20% calpain-2 is sufficient to compensate for the loss of calpain-1 activity. Interestingly, several proteins in calpain-1 null mouse platelets exhibited reduced tyrosine phosphorylation (3). Here, we present evidence that non-receptor protein tyrosine phosphatase 1B (PTP1B, also known as PTPN1) is a physiological target of calpain-1 in mouse platelets. The amount and activity of PTP1B are significantly increased in calpain-1 null mouse platelets and correlate with reduced tyrosine phosphorylation of platelet proteins, platelet aggregation, and clot retraction defects. More importantly, the platelet defects were rescued in double knockout mice lacking both calpain-1 and PTP1B. These studies suggest that calpain-1-dependent signaling pathways may serve as putative targets of therapeutic intervention in order to prevent pathogenic thrombosis in vivo.
MATERIALS AND METHODS
Antibodies and reagents.
Antiphosphotyrosine monoclonal antibody 4G10 was purchased from Upstate Biotechnology. Antibodies against PTP1B were obtained from Upstate Biotechnology and Santa Cruz Biotechnology (sc-1718 and sc-1719), respectively. The rabbit polyclonal antibody against PTP1B was kindly provided by Benjamin G. Neel. Antibodies against SH-PTP1 (sc-7289), SH-PTP2 (sc-7384), Fyn (sc-434AC), c-Yes (sc-14), and Syk (sc-573AC) were obtained from Santa Cruz Biotechnology. Src antibodies included an anti-v-Src monoclonal (OP07; Calbiochem), a phospho-specific anti-Src p418 (44-660G; Biosource), and a nonphospho-Src Tyr527 (2107; Cell Signaling). Focal adhesion kinase (FAK) antibodies included FAK (sc-557; Santa Cruz) and anti-FAK phospho-specific Tyr577 (ST1011; Oncogene Research Products). Antibodies specific for β3 integrin, phosphotyrosine-747, and tyrosine-759 were kindly provided by David Phillips. An antibody against calpain-1 was obtained from Chemicon International (MAB3104). An anti-β-actin antibody was purchased from Sigma (A-5441). The horseradish peroxidase-conjugated secondary antibodies were obtained from Upstate Biotechnology and Santa Cruz Biotechnology. Calcineurin activity was measured using a commercial kit (Calbiochem). Inhibitors MDL 28170 (a calpain inhibitor) (Aventis Pharmaceuticals), bis(N,N-dimethylhydroxamido)hydroxooxovanadate (DMHV) (Calbiochem), bovine thrombin (T-4648; Sigma), apyrase, prostaglandin E1 (PGE1), leupeptin, and aprotinin were purchased from Sigma. Raytide (PK04-22UG) was purchased from Oncogene Research Products. A PTP assay kit was obtained from Upstate Biotechnology. [α-32P]dCTP and [γ-32P]ATP were obtained from GE Healthcare. Thromboxane A2 agonist U46619 was purchased from Cayman Chemical, Ann Arbor, MI.
Generation of double knockout mice lacking calpain-1 and PTP1B.
Calpain-1 knockout (3) and PTP1B knockout (12) mice were mated to generate F1 heterozygotes. Two separate PCR-based strategies were developed to genotype PTP1B and calpain-1 mutant alleles. For calpain-1 knockout and wild-type (WT) alleles, CAL1F (5′-TGCACTCTAGTTCTGAGGCT-3′), CAL65 (5′-AGAGTGCACGAACACCAGCTT-3′), and Neo-RO-calpain (5′-TTAAGGGCCAGCTCATTCCT-3′) primers were used. CAL1F and CAL65 primers are targeted to an upstream intron and exon 4, respectively, whereas the Neo-RO-calpain primer is targeted to the neomycin cassette that was used to disrupt the WT allele in exon 4 within the calpain-1 genomic locus on chromosome 19. The WT allele will produce a 615-bp PCR product with CAL1F and CAL65 primers, whereas the calpain-1 mutant allele will generate a 415-bp product with CALF and Neo-RO-calpain primers. To distinguish PTP1B mutant and WT alleles, the PTP1B-F (5′-CGTTGACCTCTTTGCTTGGT-3′), PTP1B-R (5′-TTCTTCTTGCTGTGGCCAAT-3′), and Neo-R (5′-GCCAGAGGCCACTTGTGTAG-3′) primers were designed. The PTP1B-F and PTP1B-R primers are targeted to introns upstream of exon 4 and exon 5, respectively, whereas the Neo-R primer is targeted to the neomycin cassette used to disrupt exon 4 in the PTP1B genomic locus on chromosome 2 (12). The PTP1B-F and PTP1B-R primers are expected to generate a 900-bp product for the WT allele, and PTP1B-F and Neo-R primers will generate a 300-bp product for the disrupted allele. The PTP1B null mice are of pure C57B/6J genetic background, whereas the calpain-1 null mice are of mixed genetic background (∼85% C57B/6J and ∼15% SvJ129). Therefore, we predict that the double knockouts will be of a genetic background that is greater than 90% C57B/6J. The precise genetic composition of the double knockout mice was not established by the PCR analysis of linked loci. Nonetheless, we used WT mice of mixed genetic background, as well as a pure C57B/6J background, but did not find any differences in their platelet function under our experimental conditions.
Platelet isolation.
Briefly, blood samples were collected from the inferior vena cava of WT, calpain-1 null (calpn1−/−), PTP1B null (PTP1B−/−), and double knockout (calpn1−/− PTP1B−/−) mice using an anticoagulant cocktail (acid citrate dextrose, apyrase [0.5 U/ml], and PGE1 [50 nM]). For each experiment, platelet-rich plasma was isolated from the pooled blood samples by centrifugation at 200 × g at room temperature for 10 min from five or six mice of each genotype. Platelets were isolated by gel filtration using a Sepharose 2B column, eluted with modified Tyrode's buffer (10.0 mM HEPES, 12 mM NaHCO3, pH 7.5, 137 mM NaCl, 2.5 mM KCl, 5 mM glucose, and 0.1% bovine serum albumin). The platelet count was adjusted to 2 × 108 to 5 × 108/ml, and the platelets were then allowed to rest for 30 min. Prior to experimental measurements, the platelet suspension was supplemented with 1.0 mM CaCl2 and 1.0 mM MgCl2.
Platelet aggregation.
The aggregation of gel-filtered mouse platelets (3 × 108 cells/ml) was measured by the turbidometric method (5) using a CHRONOG-LOG Whole Blood Aggregometer with stirring at 1,000 rpm. Platelet aggregation in each mouse strain was induced by stable thromboxane A2 mimetic, U46619, thrombin, and collagen.
Immunoblotting.
Western blotting of whole-platelet lysate and the cytoskeleton fraction was carried out using various antibodies. Gel-filtered platelets (3 × 108 cells/ml) were used in the resting and activated states in the presence of thrombin. The tyrosine phosphorylation status of platelet proteins was investigated using the 4G10 monoclonal antibody. The PTP1B protein was detected with antibodies targeted to its N and C termini. The time courses of SH-PTP1, SH-PTP2, c-Src, Syk, Fyn, Lyn, c-Yes, and FAK/Pyk2 were studied using the respective antibodies according to the manufacturer's directions. Phosphorylation of Src and FAK was examined in resting and thrombin (0.15 U/ml)-activated platelets under aggregating conditions.
Isolation of platelet cytoskeleton.
Platelets (5 × 108 cells/ml) were stimulated with thrombin for the indicated time intervals at 37°C with stirring. Platelets were lysed with a 0.5 volume of 3× lysis buffer (3% Triton X-100, 60 mM Tris-HCl, pH 7.4, 3 mM EGTA, 3 mM EDTA, and protease inhibitors) and gently agitated for 30 min at 4°C. Platelet lysate was centrifuged at 1,500 × g for 10 min to remove debris, and the cytoskeleton fraction was further sedimented by centrifugation at 15,000 × g for 5.0 min. The pellet was washed three times in the detergent-free lysis buffer, solubilized in sample buffer, and boiled for 5 min for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
Phosphatase assay.
Gel-filtered platelets (2 × 108 cells/ml) were activated with thrombin with stirring (1,000 rpm). Resting and activated platelets were lysed in a 0.5 volume of 3× concentrated lysis buffer (3% NP-40, 150 mM Tris-HCl, pH 7.4, 450 mM NaCl, 3.0 mM EGTA, 3.0 mM phenylmethylsulfonyl fluoride [PMSF], 15 μg/ml leupeptin, 15 μg/ml aprotinin) for 30 min at 4°C. For immunodepletion experiments, 6 μg of the rabbit polyclonal antibody was used to immunoprecipitate PTP1B from calpain-1 null platelet lysates followed by an incubation with protein G-conjugated agarose beads. Platelet lysates (20 μg) were incubated in a reaction mixture volume of 200 μl with the phosphatase assay buffer (25 mM HEPES, pH 7.2, 50 mM NaCl, 2.5 mM EDTA, 5.0 mM dithiothreitol). The reaction was started by the addition of specific substrates. Phosphatase activity was measured using p-nitrophenyl phosphate (pNPP) as a substrate following the company's protocol for the microplate format (Upstate Biotechnology). For experiments with sodium vanadate, phosphatase activity was measured in the presence of 1.0 mM sodium vanadate. Alternatively, free phosphate released by the phosphatase from a specific peptide substrate (TSTEPQpYQPGENL [where pY is phosphorylated tyrosine]) was quantified using the malachite green system (Upstate Biotechnology). Serine-threonine phosphatase activity was measured using a commercially available kit from Calbiochem.
PTP1B immune complex phosphatase assay.
Gel-filtered platelets (2 × 108 cells/ml) in Tyrode's buffer were stimulated with thrombin for 3.0 min at 37°C with stirring (1,000 rpm) and lysed in the ice-cold radioimmunoprecipitation assay lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 2.0 mM NaF, 4.0 mM EDTA, 4.0 mM benzamidine, 2.0 mM sodium pyrophosphate, 2.0 mM PMSF, 20 μg/ml leupeptin, 20 μg/ml aprotinin) for 30 min on ice. After centrifugation at 14,000 × g for 15 min at 4°C, lysates were precleared with protein G-agarose by rotation at 4°C for 1.0 h, and PTP1B was immunoprecipitated with 5.0 μg of goat anti-PTP1B (sc-1718; Santa Cruz Biotechnology) overnight at 4°C. Immune complexes were isolated by the addition of protein G-agarose (for 2.0 h at 4°C), washed three times in the phosphatase assay buffer, and resuspended in the same buffer. Samples were analyzed for the phosphatase activity using PTP assay kits (Upstate Biotechnology) per the manufacturer's directions.
Tyrosine kinase activity.
Platelet lysates were prepared in 3× NP-40 lysis buffer (3% NP-40, 150 mM Tris-HCl, pH 7.4, 450 mM NaCl, 3.0 mM EGTA, 3.0 mM PMSF, 15 μg/ml leupeptin, 15 μg/ml aprotinin, and 3.0 mM Na3VO4) and resuspended in 10 μl of the buffer solution containing 50 mM HEPES, pH 7.5, 0.1 mM EDTA, and 0.015% Brij 35. Protein kinase reactions were initiated by the addition of 10 μl of the ATP mixture containing 0.15 mM ATP, 30 mM MgCl2, and 10 μCi [γ-32P]ATP in the presence of 10 μl of Raytide (1.0 mg/ml in the kinase assay buffer). After incubation at 30°C for 30 min, peptide phosphorylation was stopped by the addition of 120 μl of 10% phosphoric acid, followed by the application of reaction mixture onto P-81 phosphocellulose filter paper. Papers were washed four times with 0.5% phosphoric acid and once with acetone, dried, and counted in a scintillation counter.
Bacterial expression of PTP1B.
Recombinant proteins of full-length PTP1B (435 amino acids) and C-terminally truncated PTP1B (t-PTP1B) (365 amino acids) fused to maltose binding protein (MBP) at their N termini were generated by PCR using human PTP1B cDNA as the template (kindly provided by Nick Tonks). Primers used for the subcloning of fragments into EcoRI and XbaI sites of pMal-c2X expression system (NEB) are designated as follows: PTP1B-F, 5′-CGGAATTCATGGAGATGGAAAAGGAG-3′; PTP1B-435-R, 5′-CCGTCTAGACTATGTGTTGCTGTTGAACAG-3′; and PTP1B-365-R, 5′-CCGTCTAGACTAACTCATGCTTTCGATGCC-3′. The expression of recombinant proteins was optimized in Escherichia coli TOP10 DH5α bacteria. The expected molecular mass of recombinant fusion protein for full-length PTP1B is ∼93.0 kDa (42 kDa contributed by MBP), whereas the truncated t-PTP1B migrates as a ∼86.0-kDa protein. Both PTP1B and t-PTP1B were expressed as soluble proteins in bacteria (see Fig. 7). Recombinant proteins were purified by affinity and ion-exchange chromatography. SDS-PAGE of full-length PTP1B and t-PTP1B showed breakdown products of smaller polypeptides. Further purification of intact polypeptides by ion-exchange chromatography on a Mono Q column/fast-performance liquid chromatography was performed using a linear salt gradient (0 to 500 mM NaCl). Although significant purification was achieved, the SDS-PAGE still showed some smaller polypeptides particularly in the full-length preparation of PTP1B. These lower-molecular-weight polypeptides reacted with an anti-MBP polyclonal antibody, indicating that the degradation occurred at the C termini of PTP1B polypeptides. A prominent truncated polypeptide present in the preparation of full-length PTP1B contains an intact tyrosine phosphatase domain as assessed by Western blotting using an anti-MBP antibody.
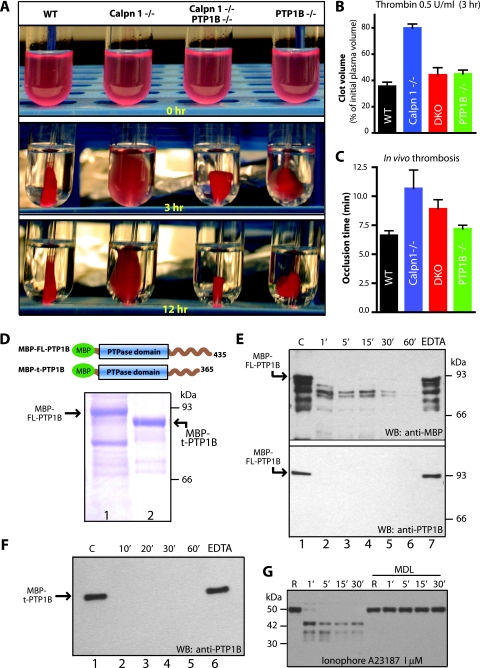
FIG. 7.
Correction of clot retraction defect in the double knockout mice. Platelet-rich plasma samples were pooled, and platelet counts were normalized. (A) Representative photographs of clot retraction induced by thrombin. (B) Quantification of fibrin clot retraction 3.0 h after thrombin and CaCl2 treatment. Clot volumes are expressed as a percentage of initial platelet-rich plasma volume. (C) Measurement of in vivo thrombosis by ferric chloride injury assay. Calpain-1 null mice are less susceptible to thrombus development. The mean plus SEM (error bar) for each group are shown. Data were analyzed using one-way analysis of variance by GraphPad prism software (P = 0.01). (D) Coomassie blue staining of recombinant PTP1B. Bacterially expressed full-length PTP1B (FL-PTP1B) (lane 1) and truncated PTP1B (t-PTP1B) (lane 2) were purified by affinity chromatography. The boundaries of each fusion protein with MBP at the N terminus are shown in the schematic diagram. (E) In vitro proteolysis of full-length recombinant PTP1B (FL-PTP1B) by commercial calpain-1 for indicated time intervals (from 1 min [1′] to 60 min [60′]) (see Materials and Methods for details). Lane 1 contains a negative control where calpain-1 was not added. Lanes 2 to 6 show various incubation times of PTP1B with calpain-1. Lane 7 shows incubation of PTP1B with calpain-1 in the presence of 2.0 mM EDTA for 60 min to inhibit calpain-1. The top blot was probed with a rabbit polyclonal antibody raised against MBP. The bottom blot was probed with a commercially available polyclonal antibody against PTP1B. WB, Western blotting. (F) In vitro proteolysis of truncated PTP1B by calpain-1. Lane 1 contains a negative control with no calpain-1, and lanes 2 to 5 show incubation times (from 10 min [10′] to 60 min [60′]) with calpain-1. Lane 6 shows incubation with calpain-1 in the presence of 2.0 mM EDTA for 60 min. Western blotting (WB) was carried out using an anti-PTP1B antibody. (G) Gel-filtered platelets (3 × 108/ml) from WT mice were incubated with either DMSO or calpain inhibitor MDL 28170 (350 μM) in DMSO for 30 min at room temperature. PTP1B proteolysis was initiated by incubation of platelets with calcium ionophore A23187, and PTP1B degradation was monitored by Western blotting using a polyclonal antibody directed against mouse PTP1B. This antibody, kindly provided by B. Neel, recognizes intact and cleaved forms of PTP1B under these conditions R, resting.
Proteolysis of PTP1B by calpain-1.
To test whether calpain-1 can degrade PTP1B completely, we performed in vitro proteolysis of full-length PTP1B (435 amino acids) and t-PTP1B (365 amino acids) fused to MBP. Proteins were incubated with purified calpain-1 (Sigma) in the protease buffer (50 mM HEPES, pH 7.4, 30 mM NaCl, 1.0 mM dithiothreitol, 100 μg/ml bovine serum albumin) in a reaction volume of 25 μl. Calpain-1 was activated by 10.0 mM CaCl2 or inhibited with 2.0 mM EDTA. Proteolysis was performed at 30°C and terminated at different time points, and Western blotting was performed using anti-MBP and anti-PTP1B antibodies. Platelets were incubated with MDL (350 μM) or dimethyl sulfoxide (DMSO) (negative control) for 30 min at room temperature. Calcium ionophore (1.0 μM) was added to activate platelets for 30 min under aggregating conditions. Platelet lysates were subjected to SDS-PAGE and Western blotting and probed with an anti-PTP1B antibody to examine the proteolysis of PTP1B.
Clot retraction.
Briefly, blood samples were collected from the inferior vena cava of WT, calpain-1 null (Calpn1−/−), PTP1B null (PTP1B−/−), double knockout (Calpn1−/− PTP1B−/−), and PTP1B null and calpain-1 heterozygotes (PTP1B−/− Calpn1+/−) in the anticoagulant cocktail (acid citrate dextrose, apyrase [0.5 U/ml], and PGE1 [50 nM]). Platelet-rich plasma was isolated from pooled blood samples by centrifugation at 200 × g at room temperature for 10 min. Platelet counts were normalized to ∼2.0 × 108/ml with platelet-poor plasma in each sample. In the borosilicate glass tubes, 200 μl of platelet-rich plasma from each sample was mixed with 750 μl of buffer (53 μM Na2HPO4, 12 μM KH2PO4, 1.0 mM CaCl2, pH 7.4). In each test tube, 1.0 μl of packed erythrocytes was added to generate color contrast for visualization of the clot. The addition of erythrocytes did not affect the extent of clot retraction as determined by parallel experiments without the addition of erythrocytes. Clot retraction was initiated and studied at two concentrations of thrombin, 1.0 U/ml and 0.5 U/ml. In experiments with DMHV, platelets were incubated with 50 μM DMHV at room temperature for 30 min before the initiation of clot retraction. Retracted clot volume was calculated by subtracting the volume of plasma left after the removal of the clot from the initial volume of plasma at each time point and expressed as a percentage of initial volume.
Thrombosis assay.
A previously described carotid artery thrombosis protocol was used with modification (15). Adult WT, calpain-1 null, double knockout, and PTP1B null mice (9 or 10 mice that were 10 to 12 weeks old and weighed ∼28.6 g) were anesthetized by inhaling a mixture of room air and isoflurane (4% during induction of anesthesia; 2 to 2.5% during maintenance of anesthesia), which was administered via a close-fitting nasal cone. The left common carotid artery was surgically exposed, and a miniature flow probe (model 0.5VB; Transonic Systems, Ithaca, NY) was placed on the surface of the artery. Sodium chloride solution (0.9%) was placed in the surgical wound to allow blood flow measurement, and baseline flow was recorded with a Transonic model T106 flow meter that interfaced with Windaq computer software (Dataq Instruments). Subsequently, the sodium chloride solution was removed from the wound and a piece of Whatman 1 M filter paper (2 by 1 mm) saturated with 10% FeCl3 (Fisher Scientific) was applied to the adventitial surface of the carotid artery, immediately proximal to the flow probe for 3.0 min. The filter paper was removed, and saline solution was placed in the wound to allow blood flow monitoring. The time to stable thrombotic occlusion (defined as lack of detectable blood flow for ≥1 min) after initiation of arterial injury with FeCl3 was recorded. The operator was blinded to mouse genotype while performing all experiments. Data are represented as mean occlusion time for each group ± standard error of the mean (SEM).
RESULTS
Reduced platelet aggregation and tyrosine phosphorylation in calpain-1 null mice.
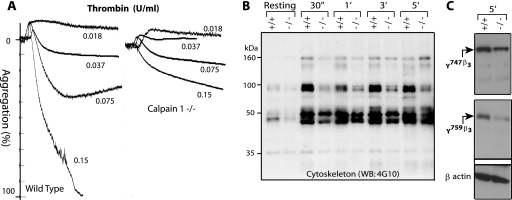
Previously, we have shown that the calpain-1 null mutation in mice leads to defective platelet aggregation in response to several agonists (3). We determined the dose dependence of thrombin activation and established that the thrombin-mediated platelet aggregation is defective in washed and stirred calpain-1 null platelets (Fig. 1A). A similar dose-dependent defect was observed in calpain-1 null mouse platelets when collagen was used as an agonist (data not shown). Our studies have shown reduced tyrosine phosphorylation of several platelet proteins, including the β3 subunit of αIIbβ3 integrin in the calpain-1 null mouse platelets (3). This conclusion was made when the whole-platelet protein lysate was tested using an antiphosphotyrosine antibody 4G10 (3). To investigate the status of tyrosine-phosphorylated proteins in the platelet cytoskeleton, we examined the phosphorylation of platelet cytoskeleton-associated proteins using the 4G10 antibody. In response to thrombin stimulation with stirring, tyrosine phosphorylation of several cytoskeleton-associated proteins was reduced in the calpain-1 null platelets (Fig. 1B). Phosphorylation of proteins migrating with molecular masses of ∼160, 97, and 45 kDa appears to be most affected in the calpain-1 null mouse platelet cytoskeleton (Fig. 1B). Although the identity of platelet proteins showing reduced tyrosine phosphorylation is currently unknown, we investigated the phosphorylation status of the β3 subunit of αIIbβ3 integrin that migrates at ∼100 kDa on SDS-polyacrylamide gels. Integrin αIIbβ3 is a major adhesion receptor for platelet outside-in signaling pathway, and the primary structure of the cytoplasmic domain of β3 subunit is identical in human and murine platelets. The β3 cytoplasmic domain contains two tyrosine residues at positions 747 and 759, and in vivo mutagenesis studies have shown that their phosphorylation is required for mouse platelet aggregation and clot retraction pathways (27). Using polyclonal antibodies specific for phosphotyrosines 747 and 759 of the β3 subunit, we evaluated its tyrosine phosphorylation in total platelet lysates from WT and calpain-1 knockout mice (Fig. 1C). Our results indicate that the tyrosine phosphorylation of both tyrosine residues in the β3 subunit at positions 747 and 759 is reduced ∼60 to 70% in calpain-1 null mouse platelets. These findings are consistent with our previous observations showing reduced tyrosine phosphorylation of the β3 subunit in calpain-1 null platelets under conditions where the β3 subunit was first immunoprecipitated and then examined with the 4G10 antibody (3). Together, these results indicate that the calpain-1 null mutation in mice leads to reduced tyrosine phosphorylation of several platelet cytoskeleton-associated proteins, including the β3 cytoplasmic domain of αIIbβ3 integrin.
FIG. 1.
Platelet aggregation and tyrosine phosphorylation of cytoskeletal proteins in calpain-1 null platelets. (A) Gel-filtered (3 × 108/ml) platelets were analyzed for platelet aggregation assay in response to different doses of thrombin. (B) Gel-filtered platelets (5 × 108/ml) from WT (+/+) and calpain-1 null (−/−) mice were activated by 0.15 U/ml of thrombin, and tyrosine phosphorylation of the cytoskeletal proteins was analyzed by Western blotting using the 4G10 antibody (WB: 4G10). The cytoskeleton extract was normalized after activation with thrombin for up to 5.0 min (5′) using β-actin as a control antibody. 30″, 30 s. (C) Tyrosine phosphorylation of the β3 subunit of αIIbβ3 integrin was evaluated after 5 min of thrombin activation (0.15 U/ml) of cells from WT (+/+) and calpain-1 null (−/−) mice using antiphosphotyrosine specific antibodies as described in the text. The antibody against β-actin served as a control.
Origin of reduced tyrosine phosphorylation in calpain-1 null platelets.
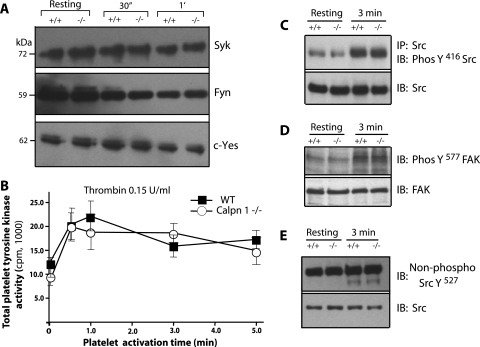
Calpains have been implicated in the regulation of several protein tyrosine kinases and phosphatases (7, 9, 14, 20, 22, 28, 32). To elucidate the basis of reduced tyrosine phosphorylation in calpain-1 null platelets, we first examined the status of tyrosine kinases that are known to serve as calpain substrates. In thrombin-stimulated mouse platelet lysates, immunoblotting of major platelet protein tyrosine kinases, such as pp125FAK, pp60src, pp72syk, Fyn, Lyn, and c-Yes revealed no discernible differences in the amounts of these proteins in WT and calpain-1 null platelets (Fig. 2). In addition, we measured total tyrosine kinase activity in thrombin-stimulated platelet lysates by monitoring the [γ-32P] phosphorylation of Raytide using an in vitro kinase assay. The time course of total tyrosine kinase activity was again indistinguishable between the WT and calpain-1 null mouse platelet lysates (Fig. 2B). Further immunoprecipitation and immunoblotting analysis indicated no difference in the phosphorylation of Src tyrosine-416 in the WT and calpain-1 null platelets (Fig. 2C). We also examined the phosphorylation status of FAK, a known Src substrate, at tyrosine-577. The FAK activation was comparable in the WT and calpain-1 null platelets (Fig. 2D). We also tested the dephosphorylation of tyrosine-527 in Src using an antibody specific for the dephosphorylated tyrosine-527 and found no difference in the activation of Src in the WT and calpain-1 null platelets (Fig. 2E). Together, these results suggest that Src activation is not altered in the calpain-1 null platelets under these experimental conditions. We did not examine the activation status of other individual tyrosine kinases; therefore, it remains possible that the activity of one or more of these and other tyrosine kinases may be altered by the calpain-1 null mutation. However, since the total tyrosine kinase activity in calpain-1 null platelet lysates is not measurably altered, we speculate that the individual activation of a tyrosine kinase, if any, must be subtle and transient under these conditions and is likely to make relatively little contribution to the total tyrosine kinase activity in the calpain-1 null mouse platelets.
FIG. 2.
Evaluation of protein tyrosine kinase activity in calpain-1 null platelets. (A) Gel-filtered (3 × 108/ml) platelets from WT (+/+) and calpain-1 null (−/−) mice were analyzed for the presence of different tyrosine kinases (Syk, Fyn, and c-Yes) by Western blotting with specific antibodies in the resting platelets and platelets activated by thrombin (0.15 U/ml) for defined time periods (30 s [30″ and 1 min [1′]). The Fyn antibody epitope is conserved in the Lyn tyrosine kinase and therefore can detect both tyrosine kinases. (B) Total protein tyrosine kinase activity was measured in the resting and thrombin-activated platelets. (C) Src tyrosine kinase was immunoprecipitated (IP) using a mouse monoclonal antibody, and the immunoprecipitated Src was probed with an antibody specific for phosphotyrosine-416 antibody (PhosY416 Src). The bottom gel shows the total amount of Src in the platelet lysate used to immunoprecipitate Src. IB, immunoblotting. (D) In the resting and thrombin-activated platelet lysates, FAK activation was analyzed using an antibody against phosphotyrosine 577 in FAK. The bottom gel shows the total amount of FAK in the platelet lysate. (E) Western blot analysis of Src in the resting and thrombin-activated platelet lysates. An antibody specific for the dephosphorylation site in Src at position 527 was used. The bottom gel shows the total amount of Src in platelet lysate.
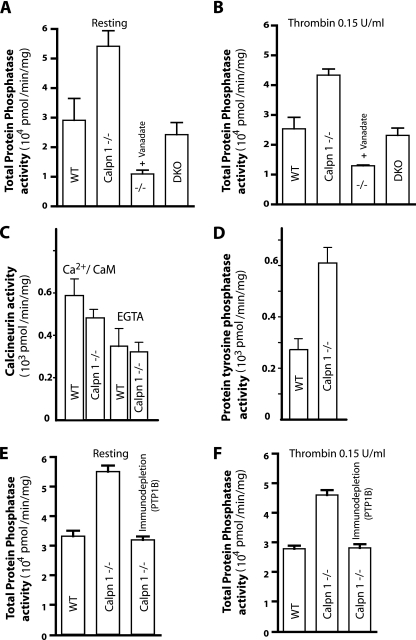
Next, we measured the total phosphatase activity in calpain-1 null mouse platelets. The calpain-1 null platelet lysate showed a significant increase in the total phosphatase activity compared to WT platelets in both resting and thrombin-activated conditions (Fig. 3A and B). This increase in total phosphatase activity did not originate from calcineurin, a major serine-threonine phosphatase that is known to serve as a substrate of calpains (38), since its activity was in fact slightly reduced in the calpain-1 null mouse platelets (Fig. 3C). Similarly, the calcium-calmodulin-independent serine-threonine phosphatase activity was not altered, suggesting that these phosphatases do not contribute to the total increase of phosphatase activity observed in the calpain-1 null mouse platelets (Fig. 3C). Further analysis suggested that the increased total phosphatase activity in calpain-1 null platelets is a consequence of the approximately twofold increase in total tyrosine phosphatase activity in the calpain-1 null platelets (Fig. 3D). Thus, the reduced tyrosine phosphorylation of platelet proteins in the calpain-1 null platelets correlates with an approximately twofold increase in the total tyrosine phosphatase activity as measured by the dephosphorylation of the synthetic phosphopeptide substrate (Fig. 3D).
FIG. 3.
Comparison of phosphatase activity in the resting and thrombin-activated platelets. (A and B) Platelet lysates from gel-filtered platelets were tested for total phosphatase activity. Inhibition of tyrosine phosphatases in calpain-1 null lysates was performed by incubation with 1.0 mM sodium vanadate in the phosphatase assay buffer. Total phosphatase activity in the double knockout (DKO) platelet lysate was included to demonstrate the rescue of enhanced phosphatase activity in calpain-1 null platelets. (C) The serine-threonine phosphatase activity of calcineurin was measured calorimetrically as described in the text. (D) Total protein tyrosine phosphatase (PTP) activity was measured in thrombin-activated platelets. (E and F) Immunodepletion of PTP1B was carried out with polyclonal antibodies against PTP1B using protein G-conjugated agarose beads. Resting and thrombin-activated platelet lysates were analyzed for total phosphatase by the pNPP hydrolysis assay in the WT, calpain-1 null, and calpain-1 null lysate that was immunodepleted as previously described.
PTP1B activity is elevated in calpain-1 null platelets.
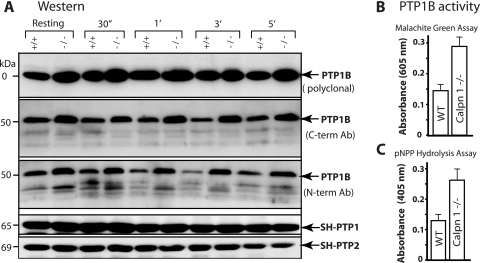
Since the calpain-1 null mouse platelets exhibit an approximately twofold increase in tyrosine phosphatase activity, an expectation was that the calpain-1 mutation might have affected a key tyrosine phosphatase. PTP1B, SH-PTP1, and SH-PTP2 are the major platelet tyrosine phosphatases that are known to serve as substrates for calpains and are believed to modulate tyrosine dephosphorylation of platelet proteins (13, 14, 19). The protein content of SH-PTP1 and SH-PTP2 was unaltered in the calpain-1 null mouse platelets as assessed by Western blotting using commercially available antibodies (Fig. 4A). In contrast, an approximately twofold increase in the amount of PTP1B protein was observed in calpain-1 null platelets in resting and thrombin-activated platelets (Fig. 4A). We performed immunoblotting using three different antibodies directed against the N and C termini of PTP1B. Multiple truncated forms of PTP1B, as detected by antibodies directed against the N terminus of PTP1B, were observed in both WT and calpain-1 null platelets. Both the full-length PTP1B (∼50 kDa) and its truncated forms were increased in the calpain-1 null platelets compared to WT platelets. The presence of truncated PTP1B forms suggests that either calpain-2 or another protease can cleave PTP1B in the absence of calpain-1. We measured the levels of PTP1B transcripts in nonplatelet cells by Northern blotting but did not find any significant differences (data not shown). However, at this stage, we cannot rule out the possibility that the calpain-1 mutation exerts differential effects on PTP1B gene transcription in megakaryocytes or other cells, thus contributing to the increased level of PTP1B protein in vivo.
FIG. 4.
Characterization of PTP1B in mouse platelets. (A) Gel-filtered platelets from WT (+/+) and calpain-1 null (−/−) mice were activated with 0.15 U/ml of thrombin, and the amount of PTP1B was examined using three different antibodies (Ab) targeted to defined epitopes at the C and N termini (C-term and N-term) of PTP1B. Western blot analysis of SH-PTP1 and SH-PTP2 indicated no changes in their protein level and served as an internal control. 30″, 30 s; 1′, 1 min. (B and C) Gel-filtered platelets were activated with 0.15 U/ml of thrombin, and PTP1B was immunoprecipitated using a polyclonal antibody directed against the N terminus of PTP1B. The data show PTP1B enzyme activity in the mouse platelets activated for 3.0 min by thrombin.
To determine whether the observed approximately twofold increase in PTP1B protein correlates with increased tyrosine phosphatase activity in the calpain-1 null platelet lysates, we performed an immunocomplex phosphatase assay. The PTP1B protein was immunoprecipitated from thrombin-stimulated platelet lysates, and its tyrosine phosphatase activity was measured by two different methods. The free phosphate released from the TSTEOQpYQPGENL substrate was quantified using the malachite green assay (Fig. 4B). Alternatively, the hydrolysis of pNPP was measured spectroscopically (Fig. 4C). In both assays, the calpain-1 null platelets showed an approximately twofold increase in the PTP1B enzyme activity that correlated with the approximately twofold increase in the amount of PTP1B protein. To determine whether the increase in the total phosphatase activity was attributed solely to PTP1B, we immunodepleted PTP1B from the calpain-1 null platelet lysates, which resulted in the quantitative removal of the tyrosine phosphatase activity under resting and thrombin-activated conditions (Fig. 3E and F). Similarly, the calpain-1 null platelets were treated with sodium vanadate, a generic inhibitor of tyrosine phosphatases, which, as expected, reduced the total tyrosine phosphatase activity (Fig. 3A and B). Together, these results suggest that the increased tyrosine phosphatase activity in the calpain-1 null platelets is due to the increased amount of PTP1B protein.
Pharmacological inhibitors modulate platelet signaling in calpain-1 null platelets.
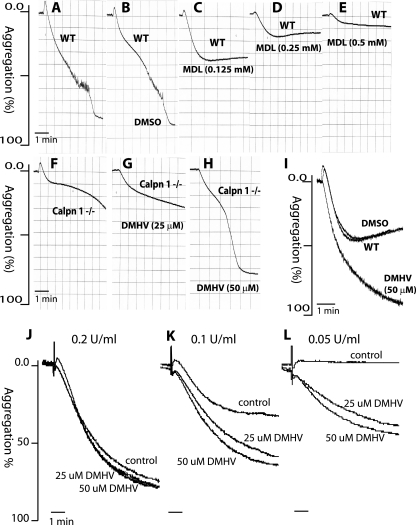
We examined the effects of MDL 28170, a calpain inhibitor, on platelet aggregation from WT mice. Gel-filtered platelets were incubated with MDL 28170 for 30 min at room temperature, followed by the initiation of aggregation by thrombin at 0.15 U/ml with stirring (Fig. 5A to E). MDL 28170 inhibited WT mouse platelet aggregation in a dose-dependent manner (Fig. 5C to E), which is consistent with published studies (6, 10). Since reduced platelet aggregation in calpain-1 null platelets correlates with increased PTP1B activity, we examined the effect of DMHV, an inhibitor of tyrosine phosphatases, including PTP1B, on calpain-1 null platelet aggregation. We first titrated DMHV concentration using thrombin-activated calpain-1 null platelets (Fig. 5F to H). The DMHV inhibitor at 50 μM completely corrected the aggregation defect in calpain-1 null platelets at 0.15 U/ml of thrombin activation (Fig. 5H). Next, we tested the effect of 50 μM DMHV on WT mouse platelet aggregation but did not observe any effect at 0.15 U/ml of thrombin activation (data not shown). Interestingly, the treatment with 50 μM DMHV enhanced the extent of WT mouse platelet aggregation at a lower concentration (0.075 U/ml) of thrombin (Fig. 5I). Similar results were obtained when human platelets treated with DMHV were tested for platelet aggregation in response to different doses of thrombin (Fig. 5J to L). The effect of DMHV on clot retraction in the calpain-1 null mice was also examined. Treatment of 50 μM DMHV with the calpain-1 null platelet-rich plasma at 0.5 U/ml of thrombin corrected the clot retraction defect to a level similar to that of WT mice (data not shown). Together, our results indicate that DMHV, a tyrosine phosphatase inhibitor, can correct platelet aggregation and clot retraction defects in the calpain-1 null mice, thus suggesting that PTP1B may be a key mediator of calpain-1 signaling in mouse platelets. Nonetheless, the possibility that the DMHV exerted its effects by inhibiting some unknown tyrosine phosphatases in mouse platelets remained. This critical issue was addressed using a genetic approach as outlined below.
FIG. 5.
Effects of pharmacological inhibitors of calpain-1 and PTP1B on platelet aggregation. Gel-filtered platelets were analyzed for platelet aggregation in response to synthetic inhibitors of calpains and PTP1B. The platelet aggregation was induced by 0.15 U/ml of thrombin. (A to E) WT platelets (A), WT platelets incubated with vehicle DMSO (B), and WT platelets incubated with the indicated concentrations of MDL (C to E). (F) Platelet aggregation response of calpain-1 null platelets. It is noteworthy that at 25 μM DMHV, although the final extent of platelet aggregation was not altered in calpain-1 null mice (G), the overall shape of the aggregation tracing without DMHV appears to be distinct (F). (H) The reduced platelet aggregation in calpain-1 null mice was rescued by incubation with 50 μM DMHV, a pharmacological inhibitor of tyrosine phosphatases, including PTP1B. (I) In the WT mouse platelets, 50 μM DMHV enhanced platelet aggregation at 0.075 U/ml thrombin activation. (J to L) Washed human platelets (2 × 108/ml) were incubated with 25 μM and 50 μM DMHV for 30 min at room temperature for aggregation response to thrombin activation.
Generation of double knockout mice for calpain-1 and PTP1B.
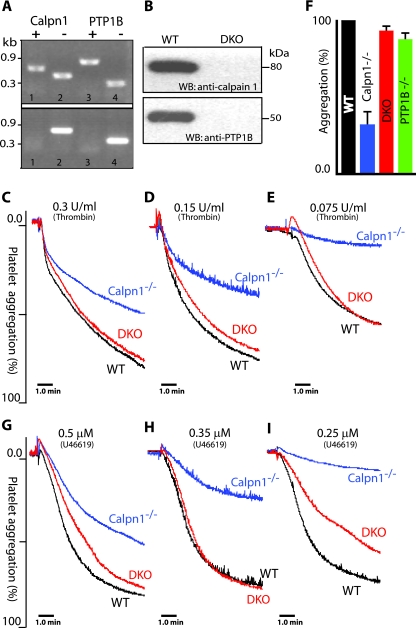
If the elevated level of PTP1B were responsible for the observed defects of platelet aggregation and clot retraction pathways in calpain-1 null mice, one would anticipate a correction of these defects in mice where the genes of both calpain-1 and PTP1B have been inactivated. To accomplish this goal, we first developed a new PCR-based assay to screen the genomic DNA of progeny produced from the mating of calpain-1 and PTP1B null mice (Fig. 6A). The characterization of PTP1B null mice used in our study has been previously described (12). Another mouse model of PTP1B deficiency has also been described (26). The successful generation of calpain-1 and PTP1B double knockout mice was confirmed by the PCR assay (Fig. 6A). Individual inactivation of calpain-1 and PTP1B genes does not exhibit any deleterious effects on mouse embryonic development (3, 12). The double knockout mice for calpain-1 and PTP1B null alleles were born according to a normal Mendelian ratio and are fertile with no obvious developmental defects. Western blotting was used to confirm that the double knockout mice were null for both calpain-1 and PTP1B proteins. Previously, we have shown that mature erythrocytes are the only cells that exclusively contain calpain-1 (3). All commercially available antibodies against calpain-1 cross-react with calpain-2 under our conditions; therefore, Western blotting of platelets and other tissues could not be used to establish the absence of calpain-1 in the double knockout mice. Since mouse erythrocytes contain only calpain-1, we performed Western blotting on the red cell membranes prepared from the double knockout erythrocytes. Both calpain-1 and PTP1B proteins were detected in the mature erythrocytes of WT mice and were completely eliminated from the double knockout mice (Fig. 6B). Together, these results confirm the development of double knockout mice lacking both calpain-1 and PTP1B.
FIG. 6.
Generation of double knockout mice lacking calpain-1 and PTP1B and correction of the platelet aggregation defect. (A) Genomic DNA was analyzed for the corresponding genotype by PCR. Mice heterozygous (top gel) for both calpain-1 (lanes 1 and 2) and PTP1B (lanes 3 and 4), and double knockout mice (bottom gel) for calpain-1 (lane 2) and PTP1B (lane 4) are shown. (B) Western blot (WB) analysis of erythrocyte membrane lysates with anti-calpain-1 and anti-PTP1B antibodies. The erythrocytes were from WT and double knockout (DKO) mice. (C to I) Correction of platelet aggregation defect in the double knockout (DKO) mice. Gel-filtered platelets were analyzed for platelet aggregation response with thrombin (C to E) and with U46619, a stable thromboxane A2 analogue (G to I). (F) Quantification of platelet aggregation at various concentrations of thrombin in the WT, calpain-1 null (Calpn1−/−), double knockout (DKO), and PTP1B null (PTP1B−/−) mice (P < 0.05). For statistical analysis, the extent of platelet aggregation in the WT mouse platelets at each dose was taken as 100%. The platelet aggregation response at comparable doses in the mutant platelets was normalized to their respective dose in the WT platelets. Each bar graph shows the mean platelet aggregation plus SEM for a mouse genotype. Data were analyzed using one-way analysis of variance by GraphPad Prism software.
Correction of platelet aggregation defect in the double knockout mice.
Previously, we have shown that the mouse calpain-1 null mutation leads to reduced platelet aggregation in response to several agonists, such as thrombin, ADP, collagen, and calcium ionophore (3). To test our hypothesis that defective platelet aggregation might be regulated by the elevated level of PTP1B in calpain-1 null mice, platelet aggregation was measured in the double knockout mice. We compared the platelet aggregation defect of calpain-1 null mice with the WT, double knockout, and PTP1B null mice. The thrombin-mediated platelet aggregation defect of calpain-1 null mice was rescued in the double knockout mice lacking both calpain-1 and PTP1B in a dose-dependent manner with stirring (Fig. 6C to E). Under similar experimental conditions, the thrombin-induced platelet aggregation response of PTP1B null mice was comparable to that of WT and double knockout mice (Fig. 6F). These results are consistent with a recently published study where no defect in platelet aggregation was observed in the PTP1B null platelets upon activation by ADP and PAR4 receptor-activating peptide (1). For logistical reasons of maintaining mice of four different genotypes and to keep our focus on the characterization of calpain-1 mutation, we did not evaluate platelet aggregation phenotype of PTP1B null mice with other agonists.
Next, we investigated whether the thromboxane A2 receptor-mediated platelet aggregation is defective in the calpain-1 null mice. U46619, a stable thromboxane analogue, was used to induce platelet aggregation under conditions similar to those used with thrombin. The U46619-induced platelet aggregation was also blocked in the calpain-1 null mouse platelets in a dose-dependent manner, and this defect was rescued in the double knockout mice lacking both calpain-1 and PTP1B (Fig. 6G to I). The thrombin-induced platelet aggregation in the double knockout mice was rescued even at low doses of thrombin, whereas the U46619-mediated rescue appears to be incomplete at lower U46619 concentrations, particularly in the secondary part of the platelet aggregation curve.
Correction of clot retraction defect in the double knockout mice.
The reversal of clot retraction defect in the calpain-1 null platelet-rich plasma by DMHV suggested that PTP1B and/or a related phosphatase is the key regulator of calpain-1 function during outside-in signaling in mouse platelets. To validate these findings in vivo, we tested the double knockout mice lacking both calpain-1 and PTP1B for clot retraction phenotype and compared this phenotype with those of single mutants. The clot retraction defect in the calpain-1 null mice was rescued in the double knockout mice lacking both calpain-1 and PTP1B (Fig. 7A and B). In addition, we compared the double knockout mice with PTP1B null mice that were heterozygous for the calpain-1 mutation (PTP1B−/− Calpn1+/−). Again, the results were consistent with the PTP1B null mice (data not shown). In agreement with a recently published study (1), we also observed a mild clot retraction defect in the PTP1B null mice (Fig. 7A). Together, these results indicate that calpain-1 regulates clot retraction phenotype in the mouse platelet-rich plasma, primarily through the activity of PTP1B.
Correction of tyrosine phosphorylation defect in the double knockout mice.
We have previously shown that calpain-1 null platelets exhibit reduced tyrosine phosphorylation of several platelet proteins, including β3 integrin, in response to various agonists (3). The reduced tyrosine phosphorylation of platelet proteins correlates with increased PTP1B in the calpain-1 null platelets (Fig. 4). To investigate this observation further, we examined the tyrosine phosphorylation status of thrombin-activated gel-filtered platelets from double knockout mice under resting as well as aggregated conditions. A comparison of the time course of tyrosine phosphorylation between WT, calpain-1 null, and double knockout platelets indicates that the tyrosine phosphorylation defect in calpain-1 null platelets was reversed in the double knockout platelets (data not shown). The reduced tyrosine phosphorylation of platelet protein p100 is relatively more prominent at the 0.5-min time point and is rescued in the double knockout mice (data not shown). Similarly, the reduced tyrosine phosphorylation of p74, p125, and p160 polypeptides was also rescued in the double knockout mouse platelets. We also investigated the tyrosine phosphorylation status of β3 integrin in the double knockout platelets using antibodies directed against tyrosine-747 and tyrosine-759 (data not shown). Again, the reduced tyrosine phosphorylation of β3 integrin in the calpain-1 null mice was rescued in the double knockout platelets. This result suggests that phosphorylated β3 integrin is a substrate of PTP1B in mouse platelets but does not resolve the issue of whether this effect is direct or indirect. Together, our results indicate that the tyrosine phosphorylation defect in the calpain-1 null mouse platelets is corrected in the double knockout mice lacking both calpain-1 and PTP1B proteins.
Calpain-1 null mice are less susceptible to thrombosis in vivo.
We evaluated age- and gender-matched WT, calpain-1 null, double knockouts lacking both calpain-1 and PTP1B, and PTP1B null mice in a carotid artery injury model that has been previously demonstrated to induce platelet-rich thrombi (15). The mean time required to form a completely occlusive thrombus in calpain-1 null mice after initiation of arterial injury (10.7 ± 1.6 min; n = 9) was significantly greater than that of WT mice (6.6 ± 0.4 min; n = 9; P = 0.02), suggesting that complete deficiency of calpain-1 suppresses thrombus development in vivo (Fig. 7C). The mean occlusion time in the PTP1B null mice (7.1 ± 0.3 min; n = 10; P = 0.3) is similar to that of the WT mice as assessed by the same carotid artery thrombosis assay (Fig. 7C). Interestingly, the mean occlusion time in double knockout mice lacking both calpain-1 and PTP1B (8.9 ± 0.8 min; n = 9) was lower than in calpain-1 null mice; however, this value was statistically different from the corresponding value of WT mice, suggesting that the reduced propensity to thrombosis in calpain-1 null mice is mediated at least in part by PTP1B.
Proteolysis of PTP1B by calpain-1.
In order to examine the proteolysis of PTP1B by calpain-1, we expressed recombinant forms of full-length and truncated PTP1B (Fig. 7D). Both forms of PTP1B were degraded by purified calpain-1 in vitro (Fig. 7E and F). The full-length PTP1B was degraded completely upon 60 min of incubation with calpain-1 (Fig. 7E, lane 6). Full-length PTP1B (93 kDa) degraded rapidly at an early time point (Fig. 7E, lane 2), thus releasing several truncated polypeptides. These lower-molecular-weight truncated polypeptides, ranging from 78 to 80 kDa, were initially resistant to proteolysis but were ultimately degraded completely by calpain-1 (Fig. 7E, lane 6). Since these truncated polypeptides reacted with an anti-MBP antibody, it is likely that these polypeptides originated from the truncation of the C terminus of full-length PTP1B. This prediction was further confirmed by Western blotting using a polyclonal antibody specific for the C terminus of PTP1B. This antibody detected a single 93-kDa band of PTP1B and did not recognize any truncated polypeptides (Fig. 7E, bottom gel). Consistent with these observations, our results confirm that PTP1B is cleaved into the 42-kDa form and multiple lower-molecular-weight fragments upon treatment of WT mouse platelets with 1.0 μM calcium ionophore A23187 (Fig. 7G). Pretreatment of mouse platelets with MDL, a peptidyl inhibitor of both calpains, completely abolished PTP1B proteolysis under these conditions (Fig. 7G). Together, these results support the hypothesis that calpain-1 modulates PTP1B function by sequential degradation of the full-length polypeptide to differentially regulate tyrosine phosphatase activity in a time- and site-dependent manner.
DISCUSSION
This report presents evidence that demonstrates that the reduced tyrosine phosphorylation in calpain-1 null platelets is due to the increased amount of PTP1B, a major tyrosine phosphatase. The generation of double knockout mice null for calpain-1 and PTP1B suggests that calpain-1 regulates mouse platelet signaling by modulating tyrosine phosphorylation of key platelet proteins. The rescue of the platelet aggregation, clot retraction, and tyrosine phosphorylation defects in the double knockout mice further supports the notion that PTP1B is the major physiological target of calpain-1 in mouse platelets. Calpain activity has been implicated in a number of platelet functions, including cytoskeletal reorganization, shape change regulation, granule/vesicle release, and clot retraction pathways (3, 6, 10, 11, 13, 16, 17, 34, 35). Our previous findings which show that the major calpain substrates are still cleaved in calpain-1 null mouse platelets raise the possibility that either calpain-2 or an unknown protease is the likely source of substrate degradation in calpain-1 null platelets (3). The calpain inhibitors used in nearly all published studies on platelet calpains inhibit both calpains, as well as other enzymes including cathepsins and phosphatases (10, 33). We believe that our in vivo mouse models begin to offer, for the first time, a definitive clue to the physiological function(s) of calpain-1 in mouse platelets.
The aggregation defect in calpain-1 null platelets upon activation with thrombin and other agonists suggests that calpain-1 may integrate the cross talk between signaling pathways mediated by various agonists under conditions of stirring. The reduced tyrosine phosphorylation and clot retraction phenotype in the calpain-1 null mice is consistent with a similar phenotype observed in the diYF mice where both tyrosines of β3 integrin were replaced by phenylalanines (27). It is noteworthy that unlike diYF and β3 integrin-deficient mice (23), the calpain-1 null mice do not show any significant bleeding phenotype (3), thus implying that a phosphorylation-independent pathway also contributes to the signaling function of calpain-1 in mouse platelets. Alternatively, partial tyrosine phosphorylation of the β3 subunit of αIIbβ3 integrin in calpain-1 null platelets might be sufficient to overcome the bleeding phenotype as observed in the diYF and β3 integrin-deficient mice. The reduced tyrosine phosphorylation of β3 integrin in the calpain-1 null mice was rescued in the double knockout platelets, further supporting the notion that phosphorylated β3 integrin is a physiological substrate of PTP1B in mouse platelets.
To determine the basis of reduced tyrosine phosphorylation of platelet proteins in calpain-1 null mice, we first evaluated the status of tyrosine kinases in mutant platelets, since several tyrosine kinases serve as calpain substrates in human platelets (7, 9, 28, 32). In principle, one would expect that the inactivation of calpain-1 gene is unlikely to diminish the activity of tyrosine kinases; however, the possibility that the limited cleavage of tyrosine kinases or an adaptor protein triggers an activation cascade that is silenced in the calpain-1 null mice remained. Our results indicate that the total tyrosine kinase activity remains unaffected by the inactivation of the calpain-1 gene in mouse platelets (Fig. 2). Consistent with this observation, the protein amounts of major tyrosine kinases were not altered in both resting and activated calpain-1 null platelets. The activation of Src and FAK was also comparable in WT and calpain-1 null platelets. However, the possibility that the enzyme activity of some other tyrosine kinases could be modulated by calpain-1 in a way that was not detectable under our experimental settings remains.
Our finding indicating PTP1B as the major physiological target of calpain-1 in mouse platelets is consistent with several previous reports that PTP1B is cleaved by calpains in platelets and other cells (19, 22). According to one model of PTP1B regulation, limited cleavage of the carboxyl terminus of PTP1B by calpains releases the enzyme from its docking site at the endoplasmic reticulum (18). The truncated soluble PTP1B exhibits twofold-enhanced enzyme activity and translocates to the plasma membrane to initiate multiple dephosphorylation reactions (19). Other published studies indicate that calpains may also regulate protein dephosphorylation by alternate mechanisms. For example, Falet et al. have shown that calpain activation, and hence the formation of shorter forms of platelet PTP1B, may not be sufficient to account for the significant increase in tyrosine phosphatase activity observed in platelets treated with calcium ionophore A23187 (14). Another study by Pasquet et al. demonstrated complete proteolysis of PTP1B in A23187-treated platelets, and this cleavage of PTP1B correlated with the activation of calpains (30). Recently, it has been shown that full-length PTP1B is a fully active enzyme under conditions where calpains are not activated, suggesting that PTP1B exhibits enzyme activity without the limited C-terminal cleavage by calpains (1). Indeed, a very recent study compared the enzyme activity of full-length and C-terminally truncated PTP1B by in vitro assays and demonstrated that the two forms of PTP1B exhibit identical enzyme activity against pNPP, a synthetic soluble substrate (31). Together, these studies indicate that both full-length and truncated forms of PTP1B are active enzymes and can contribute to the dephosphorylation of platelet proteins under specified experimental conditions.
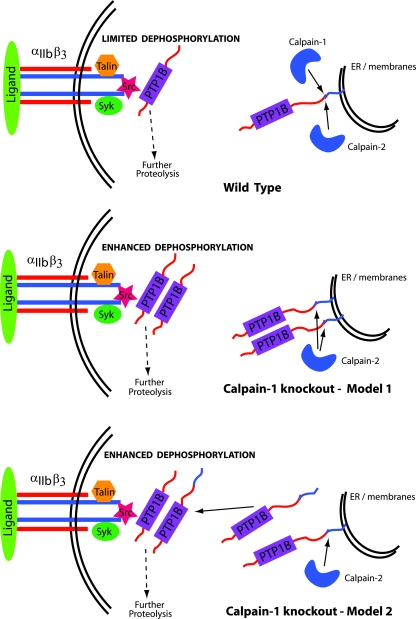
Our results indicate that the combination of increased amounts of full-length PTP1B and the truncated 42-kDa polypeptide of PTP1B contributes to the enhanced tyrosine phosphatase activity in calpain-1 null platelets. Since the resting calpain-1 null platelets contain an elevated level of PTP1B, it is likely that the mutant platelets inherited the elevated level of PTP1B from megakaryocytes. Although the precise mechanism of how calpain-1 regulates PTP1B protein synthesis remains unknown at this stage, one may speculate that the lack of calpain-1 increases PTP1B by affecting either transcription or translation or by some as yet unknown mechanism not directly related to platelet activation. Increased basal PTP1B in calpain-1 null platelets might cause some of it to avoid binding to the endoplasmic reticulum membrane and translocate to the plasma membrane, thereby affecting integrin signaling by dephosphorylating multiple substrates (Fig. 8). According to this model, calpains regulate PTP1B activity by first cleaving its C terminus, followed by the complete degradation of the truncated PTP1B under certain conditions. Upon activation, both calpain-1 and calpain-2 cleave PTP1B, thus releasing it from the pool of intracellular membranes. Once released, the truncated PTP1B translocates to the plasma membrane to dephosphorylate multiple substrates, including β3 integrin and other cytoskeletal proteins. As the outside-in signaling sets in, further proteolysis of truncated PTP1B occurs by calpains, thus limiting the excessive dephosphorylation of substrates to maintain a homeostatic balance between the kinases and phosphatases. The mechanism of how the ratio of two PTP1B forms is maintained, the differential effects of calpain-1 mutation on the kinetics of PTP1B translocation, substrate specificity, and the route of their complete degradation are issues that would now be experimentally testable with the further characterization of calpain-1 null and double knockout mice as reported here.
FIG. 8.
Proposed model for the regulation of PTP1B by calpains. See text for details. ER, endoplasmic reticulum.
The rescue of the platelet aggregation, clot retraction, and tyrosine phosphorylation defects in the double knockout mice strongly suggests a critical role for PTP1B in the regulation of calpain-1-mediated signaling in mouse platelets. It is noteworthy that the clot retraction phenotype in PTP1B null mice and double knockout mice is not identical (Fig. 7), indicating the contribution of additional regulatory mechanisms in the absence of calpain-1. Similarly, the PTP1B null mice do not show any measurable effect on platelet aggregation (1), yet we observed an enhancing effect of DMHV, a general inhibitor of tyrosine phosphatases, on the aggregation of WT mouse platelets as well as human platelets at low doses of thrombin activation. This result suggests that DMHV presumably inhibits PTP1B, as well as other tyrosine phosphatases in mouse platelets under these conditions. Interestingly, physiological evaluation of double knockout mice by the ferric chloride-induced arterial injury in vivo thrombosis assay revealed a partial rescue of the thrombosis defect in calpain-1 null mice, again implying that factors other than PTP1B are likely to contribute to the development of thrombosis in vivo. Recently, Arias-Salgado et al. (1) investigated platelet integrin signaling in PTP1B knockout mice under nonaggregating conditions and reported that PTP1B functions as a positive regulator of outside-in signaling by dephosphorylating c-Src tyrosine 529 and c-Src activation. They also reported decreased platelet spreading and clot retraction but no effect on platelet aggregation in PTP1B null mice. Consistent with these observations, our findings also did not show any measurable difference in the platelet aggregation of PTP1B null mice, and the clot retraction defect we observed in the PTP1B null mice was more pronounced at low agonist concentrations. In contrast to the reduced tyrosine phosphorylation of platelet proteins reported in PTP1B null platelets (1), our data show reduced tyrosine phosphorylation of multiple proteins in calpain-1 null mouse platelets, which contain an elevated level of PTP1B. Although the precise reason behind these opposing findings is not yet clear, the presence of excess PTP1B in calpain-1 null platelets may dephosphorylate multiple tyrosine residues in c-Src and/or other tyrosine kinases, thus suppressing their tyrosine kinase activity in vivo. It is also important to emphasize here that our experiments were performed with mouse platelets under aggregating conditions where both inside-out and outside-in signaling components are operational. Future studies will determine whether the effects of increased PTP1B activity in calpain-1 null platelets are mediated through the activation of c-Src and Csk dissociation from the integrin complex under conditions that promote selective induction of outside-in signaling without a contribution from the inside-out signaling component.
The PTP1B null mice are known to display increased insulin sensitivity and obesity resistance (12, 26), thus making the pharmacological inhibition of PTP1B a major therapeutic goal of many ongoing efforts in both academia and pharmaceutical companies. The two independent mouse models of PTP1B deficiency that currently exist (12, 26), as well as the development of tissue-specific PTP1B knockouts (4), may not be suitable for testing the efficacy of novel synthetic inhibitors of PTP1B. Our calpain-1 null mice offer the first model system where the elevated PTP1B level in circulating platelets could provide a unique experimental tool to test the selectivity of PTP1B inhibitors in vivo.
Acknowledgments
We thank M. Azam for help with some Western blotting experiments, David Phillips and Jeanne Manganello for their generous gift of phosphotyrosine-specific antibodies against β3 integrin, Ben Neel for PTP1B antibody, and Brian P. Kennedy of Merck Frosst Canada for kindly sharing the PTP1B null mice. We are particularly grateful to Guy LeBreton for his continued guidance and advice during the course of these studies and Xiaoping Du and Stephen Lam for providing technical advice and sharing resources for the platelet experiments. Finally, we deeply appreciate helpful suggestions from Ronald Dubreuil, Sean Quigley, Toshihiko Hanada, Xuerong Li, Anwar Khan, Dena Inempolidis, and members of our laboratory for improving the presentation of data in the manuscript.
This work was supported by HL 51445 grant from the National Institutes of Health.
Footnotes
Published ahead of print on 18 June 2007.
REFERENCES
- 1.Arias-Salgado, E. G., F. Haj, C. Dubois, B. Moran, A. Kasirer-Friede, B. C. Furie, B. Furie, B. G. Neel, and S. J. Shattil. 2005. PTP-1B is an essential positive regulator of platelet integrin signaling. J. Cell Biol. 170:837-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur, J. S. C., J. S. Elce, C. Hegadorn, K. Williams, and P. A. Greer. 2000. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol. Cell. Biol. 20:4474-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azam, M., S. S. Andrabi, K. E. Sahr, L. Kamath, A. Kuliopulos, and A. H. Chishti. 2001. Disruption of the mouse μ-calpain gene reveals an essential role in platelet function. Mol. Cell. Biol. 21:2213-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bence, K. K., M. Delibegovic, B. Xue, C. Z. Gorgun, G. S. Hotamisligil, B. G. Neel, and B. B. Kahn. 2006. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat. Med. 12:917-924. [DOI] [PubMed] [Google Scholar]
- 5.Born, G. V. 2005. Light on platelets. J. Physiol. 568:713-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brass, L. F., and S. J. Shattil. 1988. Inhibition of thrombin-induced platelet activation by leupeptin. Implications for the participation of calpain in the initiation of platelet activation. J. Biol. Chem. 263:5210-5216. [PubMed] [Google Scholar]
- 7.Carragher, N. O., B. Levkau, R. Ross, and E. W. Raines. 1999. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J. Cell Biol. 147:619-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cong, J., D. E. Goll, A. M. Peterson, and H. P. Kapprell. 1989. The role of autolysis in activity of the Ca2+-dependent proteinases (mu-calpain and m-calpain). J. Biol. Chem. 264:10096-10103. [PubMed] [Google Scholar]
- 9.Cooray, P., Y. Yuan, S. M. Schoenwaelder, C. A. Mitchell, H. H. Salem, and S. P. Jackson. 1996. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem. J. 318:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croce, K., R. Flaumenhaft, M. Rivers, B. Furie, B. C. Furie, I. M. Herman, and D. A. Potter. 1999. Inhibition of calpain blocks platelet secretion, aggregation, and spreading. J. Biol. Chem. 274:36321-36327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du, X., T. C. Saido, S. Tsubuki, F. E. Indig, M. J. Williams, and M. H. Ginsberg. 1995. Calpain cleavage of the cytoplasmic domain of the integrin beta 3 subunit. J. Biol. Chem. 270:26146-26151. [DOI] [PubMed] [Google Scholar]
- 12.Elchebly, M., P. Payette, E. Michaliszyn, W. Cromlish, S. Collins, A. L. Loy, D. Normandin, A. Cheng, J. Himms-Hagen, C. C. Chan, C. Ramachandran, M. J. Gresser, M. L. Tremblay, and B. P. Kennedy. 1999. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283:1544-1548. [DOI] [PubMed] [Google Scholar]
- 13.Ezumi, Y., H. Takayama, and M. Okuma. 1995. Differential regulation of protein-tyrosine phosphatases by integrin alpha IIb beta 3 through cytoskeletal reorganization and tyrosine phosphorylation in human platelets. J. Biol. Chem. 270:11927-11934. [DOI] [PubMed] [Google Scholar]
- 14.Falet, H., S. Pain, and F. Rendu. 1998. Tyrosine unphosphorylated platelet SHP-1 is a substrate for calpain. Biochem. Biophys. Res. Commun. 252:51-55. [DOI] [PubMed] [Google Scholar]
- 15.Farrehi, P. M., C. K. Ozaki, P. Carmeliet, and W. P. Fay. 1998. Regulation of arterial thrombolysis by plasminogen activator inhibitor-1 in mice. Circulation 97:1002-1008. [DOI] [PubMed] [Google Scholar]
- 16.Fox, J. E. 2001. Cytoskeletal proteins and platelet signaling. Thromb. Haemostasis 86:198-213. [PubMed] [Google Scholar]
- 17.Fox, J. E., C. D. Austin, C. C. Reynolds, and P. K. Steffen. 1991. Evidence that agonist-induced activation of calpain causes the shedding of procoagulant-containing microvesicles from the membrane of aggregating platelets. J. Biol. Chem. 266:13289-13295. [PubMed] [Google Scholar]
- 18.Frangioni, J. V., P. H. Beahm, V. Shifrin, C. A. Jost, and B. G. Neel. 1992. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell 68:545-560. [DOI] [PubMed] [Google Scholar]
- 19.Frangioni, J. V., A. Oda, M. Smith, E. W. Salzman, and B. G. Neel. 1993. Calpain-catalyzed cleavage and subcellular relocation of protein phosphotyrosine phosphatase 1B (PTP-1B) in human platelets. EMBO J. 12:4843-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gil-Henn, H., G. Volohonsky, and A. Elson. 2001. Regulation of protein-tyrosine phosphatases alpha and epsilon by calpain-mediated proteolytic cleavage. J. Biol. Chem. 276:31772-31779. [DOI] [PubMed] [Google Scholar]
- 21.Goll, D. E., V. F. Thompson, H. Li, W. Wei, and J. Cong. 2003. The calpain system. Physiol. Rev. 83:731-801. [DOI] [PubMed] [Google Scholar]
- 22.Gulati, P., B. Markova, M. Gottlicher, F. D. Bohmer, and P. A. Herrlich. 2004. UVA inactivates protein tyrosine phosphatases by calpain-mediated degradation. EMBO Rep. 5:812-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodivala-Dilke, K. M., K. P. McHugh, D. A. Tsakiris, H. Rayburn, D. Crowley, M. Ullman-Cullere, F. P. Ross, B. S. Coller, S. Teitelbaum, and R. O. Hynes. 1999. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Investig. 103:229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapprell, H. P., and D. E. Goll. 1989. Effect of Ca2+ on binding of the calpains to calpastatin. J. Biol. Chem. 264:17888-17896. [PubMed] [Google Scholar]
- 25.Kawasaki, H., and S. Kawashima. 1996. Regulation of the calpain-calpastatin system by membranes. Mol. Membr. Biol. 13:217-224. [DOI] [PubMed] [Google Scholar]
- 26.Klaman, L. D., O. Boss, O. D. Peroni, J. K. Kim, J. L. Martino, J. M. Zabolotny, N. Moghal, M. Lubkin, Y. B. Kim, A. H. Sharpe, A. Stricker-Krongrad, G. I. Shulman, B. G. Neel, and B. B. Kahn. 2000. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 20:5479-5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Law, D. A., F. R. DeGuzman, P. Heiser, K. Ministri-Madrid, N. Killeen, and D. R. Phillips. 1999. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature 401:808-811. [DOI] [PubMed] [Google Scholar]
- 28.Mukhopadhyay, S., A. S. Ramars, H. D. Ochs, and D. Dash. 2001. Bruton's tyrosine kinase is a substrate of calpain in human platelets. FEBS Lett. 505:37-41. [DOI] [PubMed] [Google Scholar]
- 29.Ohno, S., S. Minoshima, J. Kudoh, R. Fukuyama, Y. Shimizu, S. Ohmi-Imajoh, N. Shimizu, and K. Suzuki. 1990. Four genes for the calpain family locate on four distinct human chromosomes. Cytogenet. Cell Genet. 53:225-229. [DOI] [PubMed] [Google Scholar]
- 30.Pasquet, J. M., J. Dachary-Prigent, and A. T. Nurden. 1998. Microvesicle release is associated with extensive protein tyrosine dephosphorylation in platelets stimulated by A23187 or a mixture of thrombin and collagen. Biochem. J. 333:591-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picha, K. M., S. S. Patel, S. Mandiyan, J. Koehn, and L. P. Wennogle. 2007. The role of the C-terminal domain of protein tyrosine phosphatase-1B in phosphatase activity and substrate binding. J. Biol. Chem. 282:2911-2917. [DOI] [PubMed] [Google Scholar]
- 32.Satoh, K., Y. Ozaki, N. Asazuma, Y. Yatomi, Q. Ruomei, K. Kuroda, L. Yang, and S. Kume. 1996. Differential mobilization of tyrosine kinases in human platelets stimulated with thrombin or thrombin receptor agonist peptide. Biochem. Biophys. Res. Commun. 225:1084-1089. [DOI] [PubMed] [Google Scholar]
- 33.Schoenwaelder, S. M., and K. Burridge. 1999. Evidence for a calpeptin-sensitive protein-tyrosine phosphatase upstream of the small GTPase Rho. A novel role for the calpain inhibitor calpeptin in the inhibition of protein-tyrosine phosphatases. J. Biol. Chem. 274:14359-14367. [DOI] [PubMed] [Google Scholar]
- 34.Schoenwaelder, S. M., Y. Yuan, P. Cooray, H. H. Salem, and S. P. Jackson.1997. Calpain cleavage of focal adhesion proteins regulates the cytoskeletal attachment of integrin alphaIIbbeta3 (platelet glycoprotein IIb/IIIa) and the cellular retraction of fibrin clots. J. Biol. Chem. 272:1694-1702. [DOI] [PubMed] [Google Scholar]
- 35.Schoenwaelder, S. M., Y. Yuan, and S. P. Jackson. 2000. Calpain regulation of integrin alpha IIb beta 3 signaling in human platelets. Platelets 11:189-198. [DOI] [PubMed] [Google Scholar]
- 36.Sorimachi, H., S. Ishiura, and K. Suzuki. 1997. Structure and physiological function of calpains. Biochem. J. 328:721-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki, K., H. Sorimachi, T. Yoshizawa, K. Kinbara, and S. Ishiura. 1995. Calpain: novel family members, activation, and physiologic function. Biol. Chem. Hoppe-Seyler 376:523-529. [DOI] [PubMed] [Google Scholar]
- 38.Tallant, E. A., L. M. Brumley, and R. W. Wallace. 1988. Activation of a calmodulin-dependent phosphatase by a Ca2+-dependent protease. Biochemistry 27:2205-2211. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerman, U. J., L. Boring, J. H. Pak, N. Mukerjee, and K. K. Wang. 2000. The calpain small subunit gene is essential: its inactivation results in embryonic lethality. IUBMB Life 50:63-68. [DOI] [PubMed] [Google Scholar]