Abstract
K63 polyubiquitin chains spatially and temporally link innate immune signaling effectors such that cytokine release can be coordinated. Crohn's disease is a prototypical inflammatory disorder in which this process may be faulty as the major Crohn's disease-associated protein, NOD2 (nucleotide oligomerization domain 2), regulates the formation of K63-linked polyubiquitin chains on the I kappa kinase (IKK) scaffolding protein, NEMO (NF-κB essential modifier). In this work, we study these K63-linked ubiquitin networks to begin to understand the biochemical basis for the signaling cross talk between extracellular pathogen Toll-like receptors (TLRs) and intracellular pathogen NOD receptors. This work shows that TLR signaling requires the same ubiquitination event on NEMO to properly signal through NF-κB. This ubiquitination is partially accomplished through the E3 ubiquitin ligase TRAF6. TRAF6 is activated by NOD2, and this activation is lost with a major Crohn's disease-associated NOD2 allele, L1007insC. We further show that TRAF6 and NOD2/RIP2 share the same biochemical machinery (transforming growth factor β-activated kinase 1 [TAK1]/TAB/Ubc13) to activate NF-κB, allowing TLR signaling and NOD2 signaling to synergistically augment cytokine release. These findings suggest a biochemical mechanism for the faulty cytokine balance seen in Crohn's disease.
Upon pathogen exposure, the innate immune system tailors the initial cytokine response such that the pathogen can be eradicated. While innate immune signaling pathways are essential for an effective immune response, dysregulation of these pathways can lead to immunologic and inflammatory diseases (23, 24, 29). As such, it is important to understand the biochemical mechanisms that regulate the strength and duration of the innate immune response.
Innate immune signaling initiates both extracellularly and intracellularly. Toll-like receptors (TLRs) are major extracellular pathogen receptors. Each TLR recognizes a component of a pathogen such as lipopolysaccharide (LPS) from gram-negative bacteria or lipoteichoic acid from gram-positive bacteria (23, 29). As a general mechanism, upon exposure to a pathogen, TLRs activate the IRAK kinases to activate the E3 ubiquitin ligase TRAF6. TRAF6 nucleates the transforming growth factor β-activated kinase 1 (TAK1) kinase complex such that it can phosphorylate and activate the I kappa kinase (IKK) signalosome (IKKα, IKKβ, and NF-κB essential modifier [NEMO]). This active IKK signalosome then induces NF-κB activation such that NF-κB-regulated cytokines can be transcriptionally regulated (12, 29).
One of the better-studied intracellular innate immune signaling pathways is the nuclear oligomerization domain 2 (NOD2) signaling system. Polymorphisms in NOD2 are responsible for approximately 15 to 30% of genetic Crohn's disease (7, 14, 27), an inflammatory disorder of the gastrointestinal (GI) tract that is characterized by a dysfunctional immune response to normal GI bacterial flora (24). NOD2 is activated by cytosolic exposure to a breakdown product of peptidoglycan (muramyl dipeptide [MDP]) (10, 13, 19). Upon activation, NOD2 binds to the scaffolding kinase, receptor interacting kinase 2 (RIP2 [RICK, CARDIAK]) to affect NF-κB signaling (27).
Both the TLR signaling pathway and the NOD signaling pathway are dependent on ubiquitination to affect NF-κB. TLR signaling utilizes TRAF6, a K63-specific E3 ubiquitin ligase, to activate NF-κB (17, 23, 29). Both the ubiquitin ligase activity of TRAF6 and its E2 ubiquitin ligase partners (Uev1a and Ubc13) are essential for TAK1 to phosphorylate and activate IKKβ (15, 28, 33). The NOD2 signaling pathway also requires ubiquitination. NOD2 activation stimulates the K63-linked ubiquitination of NEMO, and this ubiquitination event is required for optimal NF-κB signaling downstream of NOD2 activation. NOD2's induction of NEMO ubiquitination is dependent on the scaffolding kinase RIP2. Crohn's disease-associated polymorphisms of NOD2 both fail to induce ubiquitination of NEMO and fail to effectively bind to RIP2. RIP2 itself strongly induces the K63-linked ubiquitination of NEMO, and RIP2 expression is necessary for NOD2 to induce NEMO ubiquitination (1).
K63-linked ubiquitination is increasingly important in signal transduction pathways. Ubiquitin forms a C-terminal peptide linkage with a lysine of the target protein. Ubiquitin contains seven lysines, which can be targets of further ubiquitination to form a polyubiquitin chain. The ubiquitinated lysine forming the linkage for this chain helps to determine its function. Linkages on lysine 48 of ubiquitin target a protein for proteosomal degradation, while linkages on lysine 63 of ubiquitin are essential for NF-kB signaling (3, 9). Thus, both the ubiquitination of a target protein and the lysine specificity of the ubiquitin linkage help to determine the function of this posttranslational modification.
Synergy between TLR agonists and NOD2 agonists in both inflammatory and anti-inflammatory cytokine release has been demonstrated (20, 21, 31, 32, 34, 36, 37, 40); however, the biochemical basis for this synergy is not known. Given that K63-linked ubiquitination is required for effective TLR and NOD signaling, it is possible that ubiquitination of NEMO mediates cross talk between these signaling pathways. In this work, we investigate this possibility. We show that activation of the TLR4 pathway induces ubiquitination of lysine 285 (K285) on the IKK scaffolding protein, NEMO, and we show that this ubiquitination event as well as a second, inflammatory ubiquitination event on lysine 399 (K399) is required for optimal TLR-initiated NF-κB signaling. We show that a TLR-induced E3 ubiquitin ligase, TRAF6, causes ubiquitination of both K285 and K399 on NEMO and that NOD2 activation can cause activation of TRAF6. While NOD2 can activate TRAF6, small interfering RNA (siRNA) experiments indicate that there are additional E3 ligases that can substitute for TRAF6 in NOD2/RIP2-induced NEMO ubiquitination. This implies that joint activation of the TLR pathway and the NOD2/RIP2 pathway, a scenario that may occur during a cytosolically invasive bacterial infection, requires the utilization of common ubiquitin-dependent signaling machinery downstream of TRAF6 to synergistically enhance cytokine release. We present evidence for this hypothesis as purification of RIP2-induced NEMO complexes and TRAF6-induced NEMO complexes both contain the ubiquitin-activated TAK1/TAB kinase complex. TAK1 kinase activity is required for optimal RIP2-induced IKK activation, and the K63-specific E2s, Ubc13 and Uev1a, are required both for NEMO ubiquitination and for MDP-induced NF-κB activity. Finally, we show that TLR and NOD2 signaling is synergistic to affect cytokine release. Consistent with this finding, joint activation of the TLR and NOD2 pathways causes increased NF-κB activity and prolonged NEMO ubiquitination. Collectively, these findings suggest a biochemical mechanism to explain the synergy between the TLR and NOD signaling pathways.
MATERIALS AND METHODS
Cell culture, transfection, IPs, and Western blotting.
HEK-293 cells, NEMO-null mouse embryonic fibroblasts (MEFs), and THP-1 cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS) (Invitrogen) with antibiotic/antimycotic solution or in RPMI containing 10% FBS. RAW 264.7 cells were grown in Dulbecco's modified Eagle's medium containing 10% heat-inactivated FBS (Invitrogen). Calcium phosphate precipitation was utilized for transfection as described previously (1). For immunoprecipitation (IP)-ubiquitination assays, cell lysates were prepared using high-stringency radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 7.5], 150 mM to 1 M NaCl, 0.25% deoxycholate, 1% NP-40, 0.1% sodium dodecyl sulfate [SDS], 1 mM β-glycerophosphate, 2.5 mM sodium pyrophosphate, 1 mM EDTA, 1 mM EGTA, 1 mM sodium fluoride, 1 mM iodoacetimide, 5 mM N-ethylmaleimide, 0.5 μM calyculin, and protease inhibitor cocktail (Sigma) with fresh 1 mM phenylmethlysulfonyl fluoride. IPs were performed as described previously (1). For polyubiquitin pulldown assays, where indicated, THP-1 cells were treated with MDP (Invivogen) or with Pam3Cys4 (PC) (Invivogen) as indicated in the figure legends. Highly purified LPS from Escherichia coli (Invivogen) was used to stimulate TLR4 in MEFs or RAW macrophages (as indicated in the text). Polyubiquitin binding columns were obtained from Pierce Biotechnology, and assays were performed according to the manufacturer's specifications. Western blotting and SDS-polyacrylamide gel electrophoresis (SDS-PAGE) were performed as described previously (1).
Antibodies, plasmids, and reagents.
Anti-myc (9E10), antihemagglutinin (anti-HA) (rabbit), anti-TRAF2 (mouse), anti-TRAF6 (mouse), anti-RIP2 (rabbit), and anti-Omni (rabbit) antibodies were obtained from Santa Cruz Biotechnology. Anti-myc, anti-NEMO, anti-phospho-IKK, anti-IKK, anti-phospho-IκB, anti -IκB, anti-phospho-p38, and anti-total p38 were obtained from Cell Signaling Technology. To immunoprecipitate NEMO, monoclonal antibody clone C73-764 (BD Biosciences) was used. Omni-RIP2, HA-ubiquitin, myc-NEMO, myc-K399R NEMO, and myc-K285/399R were used as previously described. Omni-TRAF6 was generated from standard PCR cloning of IMAGE clone 5272008. The K285R NEMO site-directed mutant was generated by a modification of the QuikChange (Stratagene) protocol and was sequenced to verify identity. Wild-type (wt) TAK1 was obtained from the Harvard Institute of Proteomics and was subcloned into a FLAG-tagged expression vector (pcDNA3-FLAG). Site- directed mutagenesis was performed to generate the kinase-dead/dominant- negative TAK1. siRNA directed against TRAF6 and a control siRNA oligonucleotide were obtained from either Ambion or Dharmacon. siRNA directed against RIP2 was described previously (1).
Reporter assays.
Six-well dishes of NEMO-null MEFs (25, 26) or 293 cells were transfected using polyethyleneimine. All transfections contained the same amount of total DNA. Western blotting showed that all Omni-tagged constructs and NEMO constructs were expressed appropriately (data not shown). Transfection efficiency was standardized using the Renilla luciferase, and luciferase assays were performed according to the manufacturer's instructions (Promega).
Cytokine assays.
THP-1 cells were either left untreated or were treated with 10 μg/ml MDP, 1 μg/ml PC, or 10 μg/ml MDP plus 1 μg/ml PC for 16 h. A cytokine array (Ray Biotech, Inc.) was exposed to the conditioned medium, and the assay was then performed according to the manufacturer's instructions. For TaqMan quantitative reverse transcription-PCR (RT-PCR), total RNA was extracted from THP-1 cells 2 h after stimulation. Each PCR cycle was internally standardized to 18S RNA PCR.
RESULTS
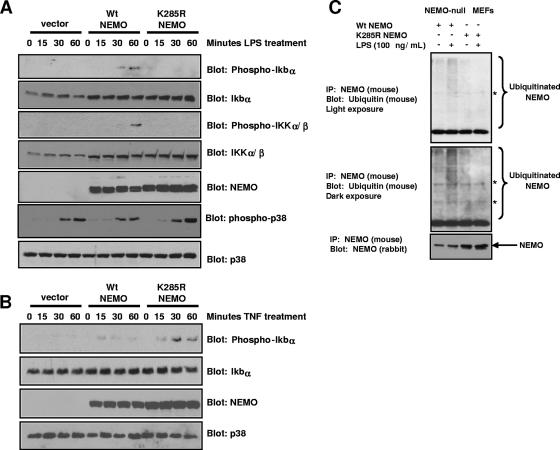
Intracellular and extracellular innate immune signaling systems must be cross-regulated to allow appropriate cytokine responses. We previously found that activation of the NOD2/RIP2 intracellular innate immune signaling pathway causes the K63-linked ubiquitination of a novel site (K285) on the IKK scaffolding protein, NEMO (1). Given this, we wanted to determine the effect of extracellular innate immune signaling pathways on K285 NEMO ubiquitination and the role of this ubiquitination event in mediating NF-κB signaling. To determine this ubiquitination site's effect on TLR signaling, NEMO-null MEFs (18, 25) were transduced with retrovirus expressing either empty vector, wt NEMO, or a form of NEMO that cannot be ubiquitinated on lysine 285 (K285R NEMO). Because MEFs have a well-described LPS/TLR4 response, selected pooled clones were exposed to 100 ng/ml of highly purified LPS (Invivogen) for 0, 15, 30, or 60 min. Western blotting showed that both K285R NEMO and wt NEMO were expressed in these stably transduced clones (Fig. 1A). Both active phospho-IKK and phospho-IκB were detected in wt NEMO-expressing MEFs weakly at 30 min and more strongly at 60 min. Neither the vector-only cell line nor the K285R NEMO cell line showed any activation of IKK or phosphorylation of IκB (Fig. 1A). As a control for LPS activity, phospho-p38 blotting showed similar activation between all three cell lines (Fig. 1A, bottom 2 panels). In contrast to these results, when the same cells were treated with TNF, both the wt NEMO-reconstituted cells and the K285R NEMO-reconstituted cells showed activation of IκB (Fig. 1B). Surprisingly, the TNF response was consistently stronger in the K285R NEMO-reconstituted cells. This finding suggests that NEMO ubiquitination may determine a level of specificity to NF-κB activation in response to inflammatory agonists. To correlate these findings with the ubiquitination of NEMO, the wt NEMO cell line and the K285R cell line were either left untreated or were treated with 100 ng/ml LPS for 45 min. NEMO was immunoprecipitated under stringent washing conditions (RIPA buffer with 0.1% SDS and 1 M NaCl) and Western blotting was performed. The wt NEMO cell line showed increased NEMO ubiquitination in the LPS-treated cells; however, the K285R NEMO cell line failed to show any NEMO ubiquitination despite consistently larger amounts of NEMO immunoprecipitated (Fig. 1C). While the LPS used in these experiments is commercially available as “highly purified” (Invivogen), we could not rule out contamination by the NOD2 agonist MDP. For this reason, we treated the same MEFs with MDP and did not see IKK activation or NEMO ubiquitination (data not shown). In addition, we transduced these MEFs with NOD2 retrovirus (cell line generation shown in Fig. S1 in the supplemental material) and saw only a very minimal response to MDP stimulation (data not shown). These findings suggest that in addition to limited (if any) NOD2 present in the MEFs, the MEFs used in this study lack the ability to transport MDP into the cytoplasm. Thus, our LPS response is unlikely to be due to contaminating MDP. Taken together, these findings suggest that the TLR4 agonist, LPS, requires ubiquitination of NEMO at lysine 285 to optimally activate NF-κB signaling.
FIG. 1.
NEMO ubiquitination at lysine 285 is required for optimal TLR4 signaling but not for optimal TNF signaling. (A) NEMO-null MEFs were infected with pBABE retrovirus expressing either vector alone, wt NEMO, or K285R NEMO. After 2 weeks of puromycin selection, clones (>1,000) were pooled and selected for an additional week. Each of these reconstituted cell lines was treated with 100 ng/ml of highly purified E. coli LPS (Invivogen) for the indicated time. Lysates were generated, and Western blots were performed using the indicated antibodies. As a control for LPS activity and as control for equivalent loading, phospho-p38 and total p38 Western blots were performed (bottom panels). (B) The vector-only NEMO-null MEFS, wt-NEMO reconstituted NEMO-null MEFs, and the K285R-reconstituted NEMO-null MEFs were treated with TNF for the indicated times. Lysates were generated, and Western blots were performed using the indicated antibodies. Again, as a loading control, a total p38 Western blot was performed (bottom panel). (C) LPS treatment causes K285 to be ubiquitinated. The wt and K285R-reconstituted NEMO-null MEF lines were either left untreated or were treated with LPS (100 ng/ml) for 45 min. NEMO IPs were performed under stringent conditions (RIPA buffer plus 0.1% SDS and 1 M NaCl). Western blots were then performed using either an antiubiquitin antibody (P4D1) or an anti-NEMO (rabbit) antibody. The asterisk indicates a cross-reactive band. No NEMO ubiquitination is present in the K285R-reconstituted cell line despite an approximate twofold increase in the amount of K285R NEMO immunoprecipitated (lower blot).
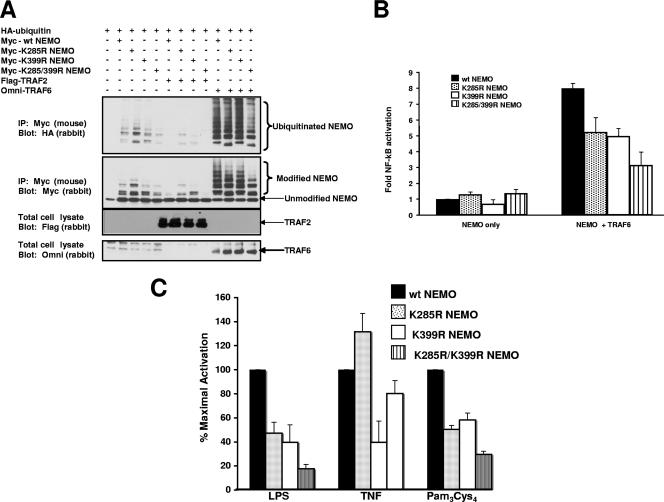
TLR signaling involves the activation of the K63 E3 ligase TRAF6 to ultimately help activate the NF-κB signaling pathway (3, 17). Given that TRAF6 is a K63-specific E3 ligase in the TLR4 system, we wanted to determine if TRAF6 induced the ubiquitination of K285 on NEMO. Because lysine 399 (K399) is also ubiquitinated on NEMO in response to inflammatory stimuli (30, 42), we generated NEMO mutants with the conservative (lysine to arginine) mutations at lysine 285 (K285R), lysine 399 (K399R), or both lysine 285 and lysine 399 (K285/399R). These mutations allow the conservation of charge but do not allow these sites to ubiquitinated. These forms of NEMO were transfected into 293 cells along with HA-tagged ubiquitin and either TRAF2 (as a negative control) or TRAF6. IPs were performed under stringent washing conditions, and Western blotting was performed. TRAF6 strongly induced the ubiquitination of NEMO, and the pattern of ubiquitination shifted substantially in every case in which lysine 285 was mutated to an arginine (Fig. 2A). Mutation of K399 alone had little effect on ubiquitination, but mutation of both sites resulted in both decreased intensity and a shift in the pattern of the ubiquitination species of NEMO. These results indicate that, when overexpressed, TRAF6 can enhance ubiquitination of NEMO at multiple sites, including K285 and K399.
FIG. 2.
TRAF6 ubiquitinates lysine 285 of NEMO, and this ubiquitination is required for optimal TRAF6-indued NF-κB activation. (A) myc-tagged wt NEMO or myc-tagged mutant NEMOs containing the conservative Lys-Arg mutations at two major NEMO ubiquitination sites (K285R, K399R, or K285/399R) were transfected into the cell with HA-tagged ubiquitin either alone or with TRAF2 or TRAF6. Transfected NEMO was immunoprecipitated and stringently washed. Western blotting was performed on the immunoprecipitate using either an anti-HA antibody (to detect ubiquitinated species) or an anti-NEMO antibody (upper two blots). To control for TRAF2 and TRAF6 expression, total cell lysates were Western blotted using antibodies directed against tagged TRAF2 (FLAG) or tagged TRAF6 (Omni) (lower two blots). (B) NEMO-null MEFs were transiently transfected with wt NEMO, K285R NEMO, K399R NEMO, or K285/399R NEMO as well as an NF-κB reporter construct, a cytomegalovirus (CMV)-driven Renilla luciferase construct (to control for transfection efficiency), and TRAF6 (as indicated). After 2 days, luciferase assays were performed. TRAF6 caused an approximate eightfold activation of the NF-κB reporter in the presence of wt NEMO, and this decreased approximately 40% in the presence of K285R NEMO. The combined K285R/K399R NEMO mutant caused TRAF6-induced NF-κB activity to decrease almost 70%. Each experiment was performed four times with similar results each time. Error bars are standard errors of the mean. (C) NEMO-null MEFs reconstituted with either wt NEMO, K285R NEMO, K399R NEMO, or K285/399R NEMO were transfected with and NF-κB reporter constructs and a CMV-driven Renilla luciferase contruct (to normalize transfection efficiency). Twenty-four hours after transfection, cells were treated with LPS (100 ng/ml), TNF (10 ng/ml), or PC (100 ng/ml) for 6 h. Luciferase assays were then performed. In both the LPS- and the PC-treated cells, diminished NF-κB activity was seen when cells were reconstituted with K285R or K399R NEMO with a greater effect with the double mutant. TNF-stimulated NF-κB activity was greatly diminished in the K399R NEMO-reconstituted cells but not in the K285R reconstituted cells. Error bars are SEM.
To show the functional significance of these ubiquitination events, we transiently transfected each of the forms of NEMO into NEMO-null MEFs and determined their effect on TRAF6-induced NF-κB activity. In the presence of wt NEMO, TRAF6 caused an approximately eightfold activation of NF-κB. Both K399R NEMO and K285R NEMO were impaired in their ability to mediate TRAF6-induced NF-κB activity. This K399R effect is in accordance with previously published results (42). The mutation of both K285R and K399R (K285 399R) substantially diminished TRAF6-induced NF-κB activity (Fig. 2B). The observation that mutation of K285 to arginine on NEMO completely blocks IκBα phosphorylation in response to LPS (Fig. 1A), but only partially blocks the NF-κB reporter response to TRAF6 overexpression (Fig. 2B), may be a consequence of nonphysiological ubiquitination events due to overexpression of TRAF6. Given this concern and to broaden the coverage of TLRs, we transfected the reconstituted NEMO cell lines with an NF-κB reporter construct and treated them with highly purified LPS, TNF, or the chemically synthesized TLR2 agonist PC. NF-κB activation was severely diminished under TLR4 or TLR2 stimulation with both K285R NEMO and K399R NEMO or the double mutant (Fig. 2C). In contrast, while TNF stimulation was reduced with K399R, it increased slightly in the presence of K285R and close-to-normal activity was seen in the presence of the K285R K399R double mutant (Fig. 2C). These findings closely mirror those seen in the signaling experiments of Fig. 1B, in which a higher TNF signal is seen in the K285R NEMO-reconstituted cells. In addition, because the recombinant (bacterially produced) TNF and the chemically synthesized PC show different results and because the PC and LPS agonists show similar NEMO ubiquitination dependence, it is unlikely that the effect that we see is due to a contaminant in the preparation of these agonists.
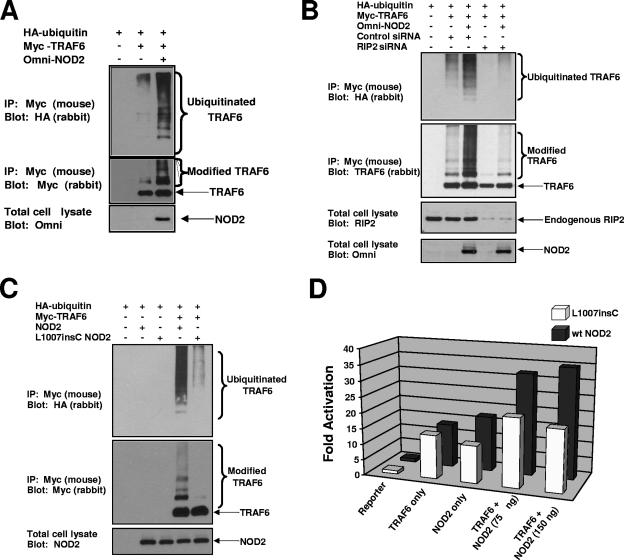
These experiments suggest that TRAF6 can cause NEMO ubiquitination on the same residue of NEMO (K285) as that induced by the NOD2/RIP2 complex. To determine whether activation of NOD2 could activate TRAF6, NOD2 was transfected into cells with myc-tagged TRAF6 and HA-tagged ubiquitin. TRAF6 was immunoprecipitated under stringent conditions, and Western blotting was performed. TRAF6 shows greatly enhanced ubiquitination in the presence of coexpressed NOD2 (Fig. 3A). To determine if this effect was due to NOD2's binding partner, RIP2, RIP2 expression was inhibited by siRNA and TRAF6 ubiquitination experiments were performed. Upon transfection of TRAF6 and NOD2 into control siRNA-transfected cells, NOD2 continued to cause activation of TRAF6, while in the RIP2 siRNA-transfected cells, activation was greatly diminished (Fig. 3B). These results suggest that NOD2 activation can induce TRAF6 activation and that this activation is dependent on RIP2.
FIG. 3.
NOD2 overexpression activates TRAF6 in a manner dependent on RIP2, and this activity is lost with Crohn's disease-associated alleles of NOD2. (A) HEK 293 cells were transfected with HA-tagged ubiquitin, myc-tagged TRAF6 (1 μg) or wt NOD2 (3 μg). TRAF6 was immunopurified under stringent washing conditions (RIPA buffer plus 1 M NaCl). The immunoprecipitate was Western blotted using an HA polyclonal antibody (upper blot) to detect ubiquitin-conjugated species or a myc polyclonal antibody to ensure equivalent IP of transfected TRAF6 (middle blot). To control for NOD2 transfection, total cell lysates were subjected to Western blotting using an Omni antibody (lower blot). (B) 293 cells were transfected with an siRNA targeting RIP2 or a control siRNA 2 days before transfection with HA-ubiquitin, limiting amounts of NOD2 (0.5 μg) and/or myc-TRAF6 (1 μg). TRAF6 was immunopurified under stringent conditions, and Western blotting was performed using an HA-polyclonal antibody (upper blot) or a TRAF6 polyclonal antibody (2nd blot from the top). To control for NOD2 expression and endogenous RIP2 knockdown, total cell lysates were subjected to Western blotting using an Omni or a RIP2 antibody (bottom two blots). (C) myc-TRAF6 (1 μg) was transfected into cells with either Omni-tagged wt NOD2 (3 μg) or Omni-tagged L1007insC NOD2 (3 μg) in the presence of HA-ubiquitin. TRAF6 was immunoprecipitated under stringent washing conditions and was subjected to Western blotting with either an HA polyclonal antibody to detect ubiquitinated species (upper blot) or with a myc polyclonal antibody to ensure equivalent TRAF6 IP (middle blot). To control for equivalent NOD2 and L1007insC expression, total cell lysates were Western blotted using an antibody against the Omni tag. (D) An NF-κB reporter was transfected into 293 cells with a Renilla luciferase reporter to control for transfection efficiency. Cells were transfected with either 75 ng TRAF6, 75 or 150 ng wt NOD2, and/or 75 or 150 ng L1007insC NOD2. Twenty-four hours after transfection, luciferase assays were performed. The experiment was performed four times with similar results each time. Activation (n-fold) of the NF-κB reporter (with standard errors of the mean in parentheses) are as follows: TRAF6 (150 ng), 13.83 (1.24); NOD2 (150 ng), 17.48 (3.39); L1007insC (150 ng), 11.83 (2.08); TRAF6 (150 ng) plus NOD2 (75 ng), 32.3 (6.72); TRAF6 (150 ng) plus NOD2 (150 ng), 35.1 (8.48); TRAF6 (150 ng) plus L1007insC (75 ng), 21.76 (3.23); and TRAF6 (150 ng) plus L1007insC, 19.65 (5.51).
Since we have previously shown that NOD2-induced NEMO ubiquitination is dependent on RIP2 and that this is lost with the Crohn's disease-associated polymorphisms of NOD2 (1), we sought to determine whether the major Crohn's disease-associated polymorphism of NOD2 (L1007insC) lost the ability to activate TRAF6. Cells were transfected with myc-TRAF6, HA-ubiquitin, and either wt NOD2 or L1007insC NOD2. TRAF6 was immunoprecipitated, and Western blotting was performed using the indicated antibodies. Again, NOD2 strongly induced the ubiquitination of TRAF6, while this activity was greatly diminished in the L1007insC NOD2-transfected cells (Fig. 4A). In addition, when TRAF6 was cotransfected into cells with either NOD2 or L1007insC and NF-κB promoter activity was assayed, the additive effect on NF-κB activation was greatly diminished in the presence of L1007insC NOD2 (Fig. 4B). To determine the effect of an additional Crohn's disease-associated allele, D291N, on TRAF6's activation and as a control for a more general effect on the TRAF family of proteins, ubiquitination assays were performed using TRAF6 in the presence of wt NOD2, L1007insC NOD2, or D291N NOD2 or using TRAF2 in the presence of the same NOD2 variants. Again, NOD2 caused the strong ubiquitination of TRAF6, and this was lost when either the L1007insC allele or the D291N allele was used. NOD2 did cause minor ubiquitination of TRAF2; however, this was unaffected by the Crohn's disease-associated alleles (see Fig. S2 in the supplemental material). These findings suggest that NOD2 can activate TRAF6 in a disease-allele specific manner and suggest that the protein kinase RIP2 is required for this activity.
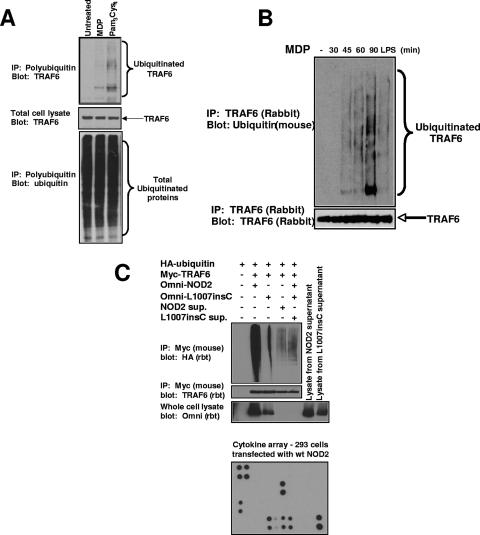
FIG. 4.
MDP causes the activation of endogenous TRAF6. (A) THP-1 cells were stimulated with either MDP (10 μg/ml) or PC (1 μg/ml) or were left untreated. Cells were lysed in RIPA buffer and were immunopurified using antiubiquitin-Sepharose beads (Pierce Biotech.). These antiubiquitin beads bind to polyubiquitin chains containing more than four ubiquitin molecules. The immunoprecipitated polyubiquitin proteins were subjected to SDS-PAGE, and Western blotting was performed using an anti-TRAF6 antibody (upper blot). The total cell lysates was also probed for TRAF6 expression as a control for equivalent starting amounts of TRAF6 (middle blot) and for equivalent amounts of precipitated ubiquitinated proteins (lower blot). (B) MDP stimulates the autoubiquitination of endogenous TRAF6. The mouse macrophage cell line RAW264.7 was left untreated or stimulated with MDP (10 μg/ml) or purified LPS (10 ng/ml) for the time periods indicated. Cells were lysed in RIPA buffer and were immunoprecipitated using anti-TRAF6 antibody (Santa Cruz). The immunoprecipitated polyubiquitinated proteins were subjected to SDS-PAGE, and Western blotting was performed using an antiubiquitin antibody (clone P4D1). The immunoprecipitates were also probed with an anti-TRAF6 antibody to ensure that equivalent amounts of TRAF6 were immunoprecipitated. (C) To determine if NOD2 caused TRAF6 activation indirectly through a paracrine or autocrine loop, NOD2 and the Crohn's disease-associated allele, L1007insC, were transfected into HEK293 cells. One millilter of the medium from these transfectants was subjected to cytokine array analysis (Ray Biotech; bottom panel) showing high levels of IL-8, MCP-1, angiopoietin, and VEGF. The relative positions of the cytokines are shown in Fig. S3 in the supplemental material. Three milliliters of this medium was then added to cells transfected with mouse myc-tagged TRAF6 and rabbit (rbt) HA-tagged ubiquitin. After 30 min, the cells were lysed and TRAF6 was immunoprecipitated. As a positive and negative control, TRAF6 was also cotransfected with wt NOD2 and L1007insC NOD2. Western blotting again showed a strong TRAF6 ubiquitination when wt NOD2 was cotransfected; however, a much weaker TRAF6 ubiquitination was seen when the cells were exposed to medium from cells transfected with wt NOD2. The overexposed blot is shown to highlight the difference. In addition, no change in TRAF6 ubiquitination was identified between cells exposed to media from cells transfected with wt NOD2 or L1007insC NOD2. sup., supernatant.
To test these findings in an endogenous manner, both THP-1 cells and RAW 264.7 macrophages were utilized. THP-1 cells were treated with the NOD2 agonist MDP. Lysates were generated and polyubiquitinated proteins were isolated using antiubiquitin columns (Pierce Biotechnology). Western blotting showed that both MDP and the TLR2 agonist PC could cause ubiquitination of TRAF6 (Fig. 4A, upper blot). The reverse experiment could also be performed in a separate cell line and in a time-dependent manner. RAW 264.7 cells were treated with MDP for the indicated times. Lysates were generated, and TRAF6 IPs were performed. Western blotting was performed using either an anti-TRAF6 or an antiubiquitin antibody. MDP induced TRAF6 ubiquitination in a time-dependent manner (Fig. 4B). These results could be due to an autocrine/paracrine effect whereby NOD2 activation (either by transfection in 293 cells or by MDP stimulation in monocytes/macrophages) induces the release of cytokine, which then causes activation of TRAF6. Because the time course of TRAF6 activation is relatively acute in the monocytes/macrophages (see Fig. 4B and Fig. 7E and F), this scenario is more likely in the transfected 293 cells. To test this possibility, 293 cells were transfected with NOD2 or with L1007insC. The next day, the medium was harvested from these cells and 1 ml of the medium was exposed to a cytokine array designed to test the cytokines present in the medium. In the NOD2-transfected cells, interleukin-8 (IL-8), macrophage chemoattractant protein 1 (MCP-1), angiopoietin, and vascular endothelial growth factor (VEGF) were at high levels (Fig. 4C, bottom blot; for the relative position of each cytokine see Fig. S3 in the supplemental material). None of these cytokines has been shown to signal through TRAF6. Because we could not rule out levels of IL-1 below our detection limits, we applied the remaining conditioned medium from either NOD2- or L1007insC-transfected cells to 293 cells which had been previously transfected with myc-tagged TRAF6 and HA-ubiquitin for 30 min. Weak, but detectable TRAF6 ubiquitination was present; however, the levels of this ubiquitination were similar between cells treated with medium from NOD2-transfected cells and cells treated with medium from L1007insC-transfected cells (Fig. 4C). In addition, to compare the levels of TRAF6 ubiquitination in cells exposed to NOD2-transfected medium to TRAF6 ubiquitination in cells expressing both TRAF6 and NOD2, cells were also cotransfected with myc-TRAF6, wt NOD2 and HA-ubiquitin, or myc-TRAF6, L1007insC NOD2, and HA-ubiquitin and the levels of these transfectants of TRAF6 ubiquitination were compared to those of the medium-treated cells. In this overexposed blot (Fig. 4C), cells in which TRAF6 and NOD2 were cotransfected showed much higher levels of TRAF6 ubiquitination than cells exposed to conditioned medium from NOD2-transfected cells (Fig. 4C). While we cannot conclusively rule out an autocrine/paracrine effect, our results suggest that NOD2's effect on TRAF6 is more likely to be direct.
FIG. 7.
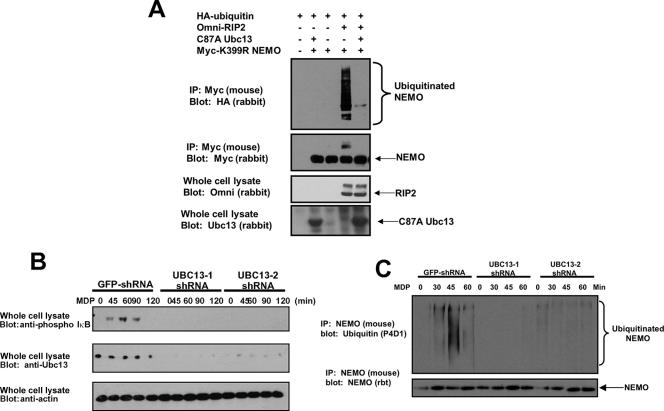
RIP2 utilizes the same E2 as TRAF6 to cause NEMO ubiquitination. (A) RIP2 was transfected into 293 cells with the dominant-negative E2 ligase Ubc13 with myc-tagged K399R NEMO and HA-tagged ubiquitin. NEMO was immunoprecipitated, and Western blotting was performed using the indicated antibodies. (B) MDP-induced NF-κB activation is dependent on the expression of the E2-conjugating enzyme Ubc13. The mouse macrophage cell line RAW 264.7 was infected with lentiviruses containing two separate shRNA sequences specific for Ubc13 (Open Biosystems) or green fluorescent protein (GFP) as a control, and stable lines were selected. These lines were left untreated or stimulated with MDP (10 μg/ml) for the time periods indicated, and NF-κB activation was measured by probing with a phospho-IκBα antibody (Cell Signaling). Ubc13 and β-actin levels were determined by immunoblotting with anti-Ubc13 or anti-β-actin (Sigma) antibodies. (C) To determine whether NEMO ubiquitination was also decreased in these Ubc13-knockdown cell lines, either the control shRNA line or the two Ubc13 cell lines were treated with MDP for 0, 30, 45, or 60 min. NEMO was immunoprecipitated under stringent washing conditions, and Western blotting was performed using either an anti-NEMO antibody or an antiubiquitin antibody. NEMO ubiquitination was significantly decreased in the Ubc13-knockdown cell lines. rbt, rabbit.
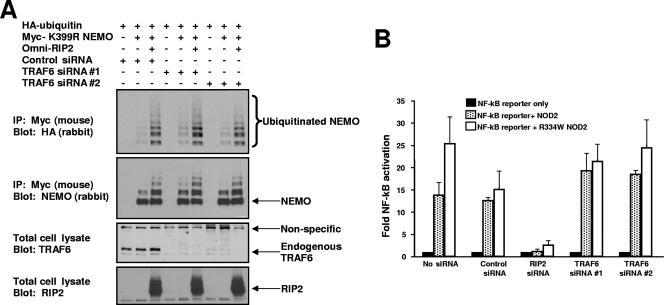
The above data suggest that NOD2 can activate TRAF6 to affect NEMO ubiquitination. TRAF6 is a member of a family (currently containing seven members) of K63-specific E3 ubiquitin ligases that help coordinate inflammatory signaling, not only via TLRs, but also via TNF, CD40L, TRAIL, and other agonists (4). Because of this complexity, we sought to determine whether TRAF6 was the only E3 ubiquitin ligase responsible for NOD2/RIP2-induced NEMO ubiquitination. To this end, we inhibited the expression of TRAF6 using two separate siRNAs. RIP2, K399R NEMO, and HA-tagged ubiquitin were transfected into cells previously transfected with either control siRNA or with TRAF6 siRNA. In these sets of experiments, K399R NEMO was utilized due to greatly decreased basal ubiquitination of NEMO (shown previously in reference 1). TRAF6 expression was greatly inhibited (Fig. 5A), but RIP2-induced NEMO ubiquitination continued to be strong. To determine whether TRAF6 was required for NOD2-indued NF-κB activation, TRAF6 was again inhibited by siRNA. Either NOD2 or the active Blau syndrome form of NOD2 (R334W) was transfected into cells, and NF-κB activation was assayed. Inhibition of TRAF6 expression did not significantly affect NF-κB activation, while inhibition of RIP2 expression greatly decreased NOD2-induced NF-κB activation (Fig. 5B). Given that there are now seven published TRAF proteins and given that other E3 ligases have been reported to induce ubiquitination of NEMO (25, 29), it is not unexpected that while NOD2 can activate TRAF6, TRAF6 loss can be compensated for by other E3 ligases.
FIG. 5.
TRAF6 is not the only E3 ubiquitin ligase responsible for NOD2/RIP2-induced NEMO ubiquitination. (A) TRAF6 expression was inhibited by siRNA (second blot from bottom) using two siRNAs designed to be unique to TRAF6, and RIP2-induced NEMO ubiquitination assays were performed as described previously (1). (B) TRAF6 or RIP2 expression was inhibited by siRNA, and NOD2-induced NF-κB activation was assayed (300 ng NOD2, 200 ng NF-κB reporter, 200 ng Renilla luciferase).
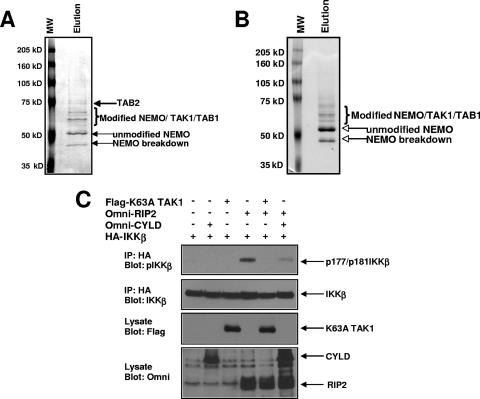
Because TRAF6 was not absolutely required for NOD2/RIP2-dependent NEMO ubiquitination, we considered a model in which the NOD2/RIP2 complex and TRAF6 utilize the same ubiquitin-dependent signaling components to activate IKK. To determine this, we took a biochemical approach. NEMO was immunopurified from cells transfected with either TRAF6 or RIP2. NEMO was eluted from the anti-myc- coupled protein G beads, and the eluate was subjected to SDS-PAGE followed by Coomassie staining. Stained bands were excised and subjected to mass spectrometry analysis. In both the TRAF6-transfected cells (Fig. 6A) and the RIP2-transfected cells (Fig. 6B), the TAK1/TAB kinase complex was present in the NEMO purification. This complex is activated by K63-linked ubiquitin chains (33), and the binding to ubiquitinated NEMO would represent a mechanism by which TAK1 would have proximity to IKK such that it could phosphorylate IKK's activation loop. To test RIP2's dependence on TAK1 to activate IKK, HA-tagged IKK was transfected into cells with RIP2 and/or kinase-dead TAK1 (K63A). As a control for ubiquitin dependence, the transfection was also performed with the K63 deubiquitinase, CYLD, an inhibitor of RIP2-induced IKK activation (1). IKK was immunoprecipitated, and Western blotting was performed utilizing an antibody that recognizes the phosphorylated activation loop of IKK. Figure 6C shows that RIP2 activates IKK and that this activation is strongly inhibited by coexpression of kinase-dead TAK1. This set of experiments suggests that while TRAF6 is not the only E3 ligase downstream of the NOD2/RIP2 complex, TRAF6 and NOD2/RIP2 share a common ubiquitin-dependent signaling complex (TAK1/TAB) to activate IKK.
FIG. 6.
RIP2 and TRAF6 utilize the TAK1/TAB complex of proteins to induce NEMO ubiquitination and IKK activation. (A) myc-tagged wt NEMO and Omni-tagged TRAF6 were transfected into cells. wt NEMO was immunopurified via four washes with modified RIPA buffer, four washes with modified RIPA buffer containing 1 M NaCl, four washes with phosphate-buffered saline, and four washes with modified RIPA buffer. The immunoprecipitate was eluted from the beads using 1% SDS at 60°C. SDS-PAGE was performed followed by Coomassie staining (left panel). Stained bands were excised and subjected to mass spectrometry analysis. In addition to modified forms of NEMO, the TAK1/TAB complex was present in the NEMO binding complexes. MW, molecular mass markers. (B) myc-tagged wt NEMO and Omni-tagged RIP2 were transfected into cells. The experiment was performed as described for panel A. Again, in addition to modified forms of NEMO, the TAK1/TAB complex was present in the NEMO binding complex. (C) To determine TAK1's role in RIP2-induced IKK activation, HA-tagged IKKβ was transfected into 293 cells with Omni-tagged RIP2, FLAG-tagged kinase-dead TAK1 (K63A), or, as a control, Omni-tagged CYLD. IKK was immunoprecipitated, and Western blotting was performed using the indicated antibodies. Kinase-dead TAK1 reduced RIP2-induced IKK activation to essentially undetectable levels.
Given NOD2/RIP2's dependence on the TAK1/TAB complex and given that TRAF6 and TAK1 also require the K63-specific E2s Ubc13 and Uev1a to activate NF-κB (5, 8, 38, 39), we sought to determine whether the NOD2/RIP2 complex also required Ubc13 to signal to NF-κB. To this end, we transfected K399R NEMO (again to minimize background NEMO ubiquitination) with RIP2, HA-tagged ubiquitin, and/or dominant-negative Ubc13 (C87A). K399R NEMO was immunoprecipitated, and Western blotting was performed. RIP2 induced the ubiquitination of NEMO, and expression of dominant-negative Ubc13 strongly inhibited this ubiquitination (Fig. 7A). To determine the in vivo effect of loss of Ubc13 on NOD2/RIP2-induced NF-κB activation, two cell lines (RAW 264.7) stably expressing separate short hairpin RNAs (shRNAs) designed to inhibit the expression of Ubc13 were generated. These cells were treated with the NOD2 agonist MDP for the indicated times, and lysates were generated. Western blotting was performed with the indicated antibodies. Figure 7B shows that both of these cell lines have limited expression of endogenous Ubc13. In both of these cell lines, MDP-induced NF-κB activity was substantially lower as judged by phospho-IκB in the Ubc13-knockdown cells (Fig. 7B). The same cell lines were then treated with MDP for 0, 30, 45, and 60 min. NEMO was immunoprecipitated under stringent washing conditions, and Western blotting was performed to determine whether Ubc13 loss correlated with lack of NEMO ubiquitination. In both of the Ubc13-knockdown cell lines, NEMO ubiquitination was substantially decreased, while NEMO ubiquitination was intact in the control cell line (Fig. 7C). Collectively, these results suggest that TRAF6 and NOD2/RIP2 share a common E2 complex to ubiquitinate NEMO and activate NF-κB.
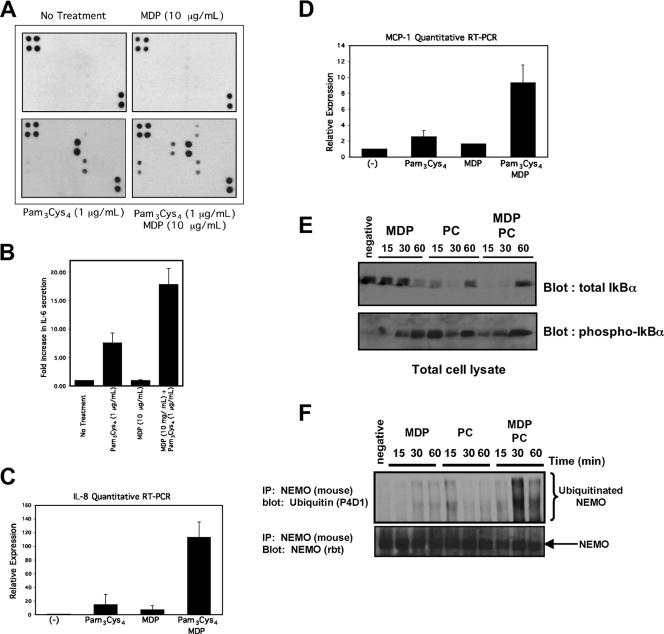
If extracellular innate immune signaling (via TRAF6) and intracellular innate immune signaling (via NOD2/RIP2) utilize common ubiquitin-dependent molecular scaffolds to regulate cytokine release, then these two innate immune pathways could synergize to coordinate cytokine release. To test this, we utilized the chemically synthesized TLR2 agonist PC for experiments in THP-1 monocytes (a human cell line with high expression of NOD2) (11). PC has the advantage of being chemically synthesized such that there is no contamination with MDP. We stimulated THP-1 cells with PC and/or the NOD2 agonist MDP. After exposure to these agonists, medium was collected and subjected to cytokine array analysis. Each of the 40 cytokines and their relative positions on the array are shown in Fig. S3 in the supplemental material. With no treatment, there was little cytokine release from the THP-1 cells. MDP caused only minor IL-8 release (Fig. 8A, right top panel). This result is consistent with previous studies that have shown that MDP only causes slight cytokine release (19, 30, 36, 39). PC caused IL-8 and RANTES to be strongly upregulated, while treatment with both PC and MDP caused release of GRO, IL-6, MCP-1, and, to a lesser extent, TNF (Fig. 8A, lower panels). Because we were concerned about the linearity of cytokine array analysis, these findings were further quantified by IL-6 enzyme-linked immunosorbent assays (ELISAs), which also showed an additive effect of PC and MDP (Fig. 8B), and by TaqMan real-time RT-PCR, which showed additive and synergistic increases of MCP-1 and IL-8 mRNA (Fig. 8C and D). To correlate these findings with NF-κB activation, macrophages were treated with 500 ng/ml PC, 10 μg/ml MDP, or both PC and MDP for 15, 30, or 60 min. NF-κB activation was monitored by IκB degradation and subsequent transcriptional activation. By itself, MDP caused a small amount of IκB degradation that peaked at 60 min. PC caused IκB degradation at 15 min, with undetectable IκB at 30 min. When both MDP and PC were added, there was no detectable IκB 15 min after treatment and there was increased IκB at 60 min (Fig. 8E). The phospho-IκB blot was consistent with these results. MDP alone caused phosphorylation of IκB at 30 and 60 min, the time when total IκB begins to decrease. PC caused earlier phosphorylation of IκB. When MDP and PC were added at the same time, there was no detectable phospho-IκB at 15 min due to the enhanced IκB degradation. In addition, the amount of IκB transcriptionally upregulated by 60 min was higher, as was the residual IKK activity (reflected by phospho-IκB) (Fig. 8E). To correlate these findings with NEMO ubiquitination, cells were treated with MDP, PC, or both MDP and PC for 0, 15, 30, and 60 min. Lysates were generated, and NEMO was immunoprecipitated. MDP alone caused NEMO ubiquitination at 30 and 60 min, while PC treatment caused a large degree of NEMO ubiquitination at 15 min but more limited NEMO ubiquitination at later time points (Fig. 8F). When MDP and PC were added together, NEMO ubiquitination occurred earlier (relative to MDP treatment alone) and was significantly prolonged (Fig. 8F). The results of these signaling experiments (Fig. 8E and F) correlate very well with the cytokine expression results of Fig. 8A to D and suggest that rather than serving solely as a driver of an inflammatory response, MDP may be serving to modulate TLR signaling. These findings also provide further evidence for cross talk between extracellular and intracellular bacterial sensing systems.
FIG. 8.
MDP, a NOD2 agonist, and PC, a TLR2 agonist, synergize to increase cytokine release, and this correlates with a prolonged NF-κB activation and with prolonged NEMO ubiquitination. (A) MDP and PC synergize to increase specific cytokine expression. THP-1 cells were either left untreated or were treated with 10 μg/ml MDP, 1 μg/ml PC, or 10 μg/ml MDP plus 1 μg/ml PC overnight. The medium was collected and subjected to cytokine array analysis (Ray Biotech, Inc.). The position of each of the 40 cytokines on the array is indicated in Fig. S3 in the supplemental material. In this analysis, there was limited cytokine release in either the untreated or the MDP-treated THP-1 cells (upper two panels). PC caused strong upregulation of IL-8 and RANTES and weaker upregulation of GRO (macrophage inhibitory protein β/γ [MIPβ/γ]) (lower left panel). Cells treated with MDP plus PC showed strong upregulation of IL-8, RANTES, MCP-1, IL-6, and GRO (MIPβ/γ) and weaker upregulation of TNF-α (lower right panel). (B) IL-6 ELISA performed under identical conditions shows an additive effect of PC and MDP. (C and D) Quantitative TaqMan RT-PCR was performed on THP-1 cells 2 h after stimulation with 500 ng/ml PC, 1 μg/ml MDP, or 500 ng/ml PC plus 1 μg/ml MDP. Total RNA from each sample was extracted and equalized. Within the RT-PCR cycle, each sample was then internally standardized to the 18S RNA present in that sample. Relative expression levels (with standard errors of the mean) are presented. (E) Macrophages were treated with PC (500 ng/ml), 10 μg/ml MDP, or both PC and MDP for 15, 30, or 60 min. Lysates were generated, and Western blots were performed. Phospho-IκB is shown in the lower blot. This blot was stripped and reprobed for total IκB (upper blot). (F) To correlate the synergy seen at the cytokine level with NEMO ubiquitination, macrophages were treated with 10 μg/ml MDP, 200 ng/ml PC, or both for 15, 30, or 60 min. In addition, one plate of cells was left untreated. At the indicated time, NEMO was immunopurified under stringent washing conditions and Western blotting was performed. MDP caused a slower and more prolonged NEMO ubiquitination, while PC caused a stronger, more acute NEMO ubiquitination (upper blot). When both MDP and PC were added, the NEMO ubiquitination was both more pronounced and more prolonged.
DISCUSSION
In this work, we have found that the TLRs and NOD2 utilize a common ubiquitin-dependent signaling complex (TAK1/TAB/ubc13) that centers on ubiquitination of both lysine 285 (K285) and lysine 399 (K399) on NEMO. We showed that TLR2 and TLR4 require ubiquitination of these lysines on NEMO to optimally signal (Fig. 1 and 2C), and we showed that TRAF6 could cause ubiquitination of this site (Fig. 2). NOD2 activation led to TRAF6 ubiquitination both in vivo and in vitro (Fig. 3 and 4), and this activity was lost in the presence of the most common Crohn's disease-associated NOD2 allele, L1007insC (Fig. 4), as well as in an additional Crohn's disease-associated allele, D291N (see Fig. S2 in the supplemental material). While NOD2 activated TRAF6, TRAF6 was not absolutely required for NOD2/RIP2-induced NEMO ubiquitination (Fig. 5), suggesting a redundancy among E3 ligases targeting NEMO. Despite this, biochemical purification showed that both TRAF6 and NOD2/RIP2 utilize the TAK1/TAB complex to cause IKK activation (Fig. 6) and that NOD2/RIP2 utilizes the same E2 ligase (Ubc13) to cause NEMO ubiquitination and NF-κB activation (Fig. 7). The net result is a synergy between TLR and NOD2 signaling at the level of NEMO ubiquitination to influence cellular cytokine release (Fig. 7).
Our findings suggest that while NOD2 activation may play a role as a driver of the innate immune response, under certain circumstances NOD2 also helps to coordinate innate immune signaling from the TLRs (27, 35). This model is attractive, as a cell would first need to identify and respond to a pathogen in the extracellular environment via the TLRs. This would allow the cell to initiate an innate immune response against the pathogen before intracellular infection. Two scenarios could then develop. First, the initial TLR innate immune response may be inadequate, and in the case of an invasive bacterium, the bacterium could then invade the cell. Once in the cytosol, it would be detected by the NOD2/RIP2 complex, which would then augment the TLR response by causing either quantitatively more K285 and K399 NEMO ubiquitination or a longer duration of NEMO ubiquitination. In an alternative model, the initial TLR response might be adequate and the bacterium may be phagocytosed. In this scenario, the NOD2/RIP2 complex might react to a dead bacterium to also induce anti-inflammatory cytokines such as IL-12 (34, 36) to decrease the innate immune response. In fact, both of these models are supported by the literature (20, 21, 31, 32, 34, 36, 37, 40), suggesting that NOD2 modulates the innate immune response to both increase acute inflammatory cytokines and to also repress further cytokine release through the release of IL-12 in an autocrine/paracrine loop (34, 36). This model would suggest that Crohn's disease results from a dysregulated coupling of cytokine release in response to innate immune stimulation.
NOD2 activation and TLR activation have previously been shown to be nonredundant in response to the intracellular pathogen Mycobacterium tuberculosis (6), and RIP2 has been shown to be nonessential for TLR signaling (22). While the NOD and TLR signaling systems are separable (6, 22), both signaling systems respond to bacteria, and as such, their signaling pathways must contain cross talk mechanisms such that an appropriate cytokine balance is released from an infected cell. K63-linked polyubiquitination is well situated to couple these innate immune responses. It is a rapidly occurring posttranslational modification that can serve to initiate binding between signaling proteins (3, 9). Ubiquitination of NEMO occurs at low stoichiometry, and as such, a small increase in NEMO ubiquitination can amplify the downstream NF-κB signal. In addition, K63-linked ubiquitination is rapidly reversible, a trait that allows the innate immune response to be down-regulated once a cytokine response is no longer required. This feature is highlighted by the fact that the two known K63 deubiquitinases, A20 and CYLD, both deubiquitinate NEMO and, when knocked out in vivo, lead to inflammatory bowel disease (2, 16, 41). These findings, coupled with our own in vitro work, suggest that K63-linked ubiquitination is a key feature regulating the strength and duration of the innate immune response in the GI tract.
Our best signaling model for the integration between the TLRs and NOD2 is presented in Fig. 9. Extracellular exposure to a pathogen activates TRAF6, which then ubiquitinates K285 and K399 on NEMO. This ubiquitinated NEMO then is able to nucleate the TAK1/TAB complex, as this complex has been shown both to bind directly to polyubiquitin chains and to require ubiquitin to function as an active kinase (5, 28, 33). TAK1 then phosphorylates the activation loop of the IKKs to induce IκB degradation and NF-κB transcriptional activity. Should the pathogen become intracellular, NOD2 would then be activated, increasing TRAF6's quantitative activity or its duration of activity, in addition to activating additional E3 ligases to allow greater/longer IKK activation via TAK1 nucleation. Despite our findings, many unknowns remain in this model. What are the additional NOD2-dependent E3 ligases? How do A20 and CYLD contribute to the temporal regulation of NEMO ubiquitination and IKK activation, and are their activities altered in Crohn's disease? Does the NOD2/RIP2 complex cause additional posttranslational modifications (phosphorylation, sumoylation, etc.) on NEMO, and what role do these play in NF-κB signaling? Finally, our work suggests that K285 ubiquitination antagonizes TNF-induced NF-κB activation. This finding implies that NEMO ubiquitination may coordinate inflammatory signaling. All of these questions will be important to answer in the future to help understand the pathophysiology of Crohn's disease.
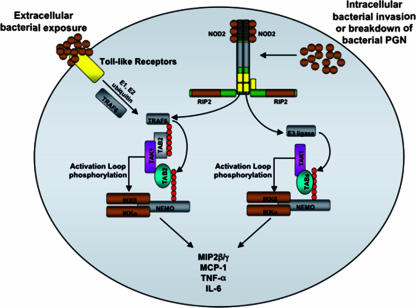
FIG. 9.
Model of the molecular synergy of TLR and NOD2 signaling converging on NEMO ubiquitination. In this model, TLRs are activated by extracellular bacteria to activate TRAF6. TRAF6 then causes ubiquitination of NEMO, which then allows nucleation of the TAB/TAK1 complex to ubiquitinated NEMO. This induced proximity of TAK1 to the IKKs allows TAK1 to phosphorylate the activation loop of the IKKs. Separately, the poorly controlled infection moves intracellularly to activate the NOD2/RIP2 complex to further activate TRAF6 as well as other, redundant E3 ligases, which activate to enhance NEMO ubiquitination and increase both the amount and duration of TAK1/TAB loading on NEMO. PGN, peptidoglycan.
Supplementary Material
Acknowledgments
We gratefully acknowledge Michael Karin (UCSD) and Tak Mak (Toronto) for providing NEMO-null cell lines, Gabriel Nunez (University of Michigan) for wt NOD2 and R334Q NOD2 constructs, and Zhijian Chen (UT Southwestern) for the C87A Ubc13 construct. We thank members of the Cantley lab for critical comments regarding these studies. We thank Reginald Gray (CWRU) for technical help and Clifford Harding (CWRU) for helpful discussions.
This work was supported by the Burroughs Wellcome Career Award for Biomedical Scientists (D.W.A.) and NIH grants 1K08 AI53819-01A1 (D.W.A.) and GM56203 (L.C.C.).
Footnotes
Published ahead of print on 11 June 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abbott, D. W., A. Wilkins, J. M. Asara, and L. C. Cantley. 2004. The Crohn's disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr. Biol. 14:2217-2227. [DOI] [PubMed] [Google Scholar]
- 2.Boone, D. L., E. E. Turer, E. G. Lee, R. C. Ahmad, M. T Wheeler, et al. 2004. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5:1052-1060. [DOI] [PubMed] [Google Scholar]
- 3.Chen, Z. J. 2005. Ubiquitin signaling in the NF-kB pathway. Nat. Cell Biol. 7:758-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung, J. Y., Y. C. Park, H. Ye, and H. Wu. 2002. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J. Cell Sci. 115:679-688. [DOI] [PubMed] [Google Scholar]
- 5.Deng, L., C. Wang, E. Spencer, L. Yang, A. Braun, et al. 2000. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103:351-361. [DOI] [PubMed] [Google Scholar]
- 6.Ferwerda, G., S. E. Girardin, B. J. Kullberg, L. Le Bourhis, D. J. de Jong, et al. 2005. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 1:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fritz, J. H., R. L. Ferrero, D. J. Philpott, and S. E. Girardin. 2006. Nod-like proteins in immunity, inflammation and disease. Nat. Immunol. 7:1250-1257. [DOI] [PubMed] [Google Scholar]
- 8.Fukushima, T., S. Matsuzawa, C. L. Kress, J. M. Bruey, M. Krajewska, S. Lefebvre, J. M. Zapata, Z. Ronai, and J. C. Reed. 2007. Ubiquitin-conjugating enzyme Ubc13 is a critical component of TNF receptor-associated factor (TRAF)-mediated inflammatory responses. Proc. Natl. Acad. Sci. USA 104:6371-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao, M., and M. Karin. 2005. Regulating the regulators: control of protein ubiquitination and ubiquitin-like modifications by extracellular stimuli. Mol. Cell 19:581-593. [DOI] [PubMed] [Google Scholar]
- 10.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869-8872. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez, O., C. Pipaon, N. Inohara, A. Fontalba, Y. Ogura, F. Prosper, G. Nunez, and J. L. Fernandez-Luna. 2002. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J. Biol. Chem. 277:41701-41705. [DOI] [PubMed] [Google Scholar]
- 12.Hayden, M. S., and S. Ghosh. 2004. Signaling to NF-kB. Genes Dev. 18:2195-2224. [DOI] [PubMed] [Google Scholar]
- 13.Inohara, N., Y. Ogura, A. Fontalba, O. Gutierrez, F. Pons, et al. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 278:5509-5512. [DOI] [PubMed] [Google Scholar]
- 14.Inohara, N., M. Chamaillard, C. McDonald, and G. Nunez. 2005. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 74:355-383. [DOI] [PubMed] [Google Scholar]
- 15.Kanayama, A., R. B. Seth, L. Sun, C. K. Ea, et al. 2004. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol. Cell 15:535-548. [DOI] [PubMed] [Google Scholar]
- 16.Lee, E. G., D. L. Boone, S. Chai, S. L. Libby, M. Chien, J. P. Lodolce, and A. Ma. 2000. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 289:2350-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lomaga, M. A., W. C. Yeh, I. Sarosi, G. S. Duncan, C. Furlonger, et al. 1999. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13:1015-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makris, C., V. L. Godfrey, G. Krahn-Senftleben, T. Takahashi, et al. 2000. Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol. Cell 5:969-979. [DOI] [PubMed] [Google Scholar]
- 19.McDonald, C., N. Inohara, and G. Nunez. 2005. Peptidoglycan signaling in innate immunity and inflammatory disease. J. Biol. Chem. 280:20177-20180. [DOI] [PubMed] [Google Scholar]
- 20.Netea, M. G., B. J. Kullberg, D. J. de Jong, B. Franke, T. Sprong, T. H. Naber, J. P. Drenth, and J. W. Van der Meer. 2004. NOD2 mediates anti-inflammatory signals induced by TLR2 ligands: implications for Crohn's disease. Eur. J. Immunol. 34:2052-2059. [DOI] [PubMed] [Google Scholar]
- 21.Netea, M. G., G. Ferwerda, D. J. de Jong, T. Jansen, et al. 2005. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J. Immunol. 174:6518-6523. [DOI] [PubMed] [Google Scholar]
- 22.Park, J. H., Y. G. Kim, C. McDonald, T. D. Kanneganti, M. Hasegawa, M. Body-Malapel, N. Inohara, and G. Nunez. 2007. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J. Immunol. 178:2380-2386. [DOI] [PubMed] [Google Scholar]
- 23.Pasare, C., and R. Medzhitov. 2004. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 6:1382-1387. [DOI] [PubMed] [Google Scholar]
- 24.Podolsky, D. K. 2002. Inflammatory bowel disease. N. Engl. J. Med. 8:417-429. [DOI] [PubMed] [Google Scholar]
- 25.Rudolph, D., W. C. Yeh, A. Wakeham, B. Rudolph, D. Nallainathan, J. Potter, A. J. Elia, and T. W. Mak. 2000. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 14:854-862. [PMC free article] [PubMed] [Google Scholar]
- 26.Ruefli-Brasse, A. A., D. M. French, and V. M. Dixit. 2003. Regulation of NF-kappaB-dependent lymphocyte activation and development by paracaspase. Science 302:1581-1584. [DOI] [PubMed] [Google Scholar]
- 27.Strober, W., P. J. Murray, A. Kitani, and T. Watanabe. 2006. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 6:9-21. [DOI] [PubMed] [Google Scholar]
- 28.Sun, L., L. Deng, C. K. Ea, Z. P. Xia, and Z. J. Chen. 2004. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell 7:289-301. [DOI] [PubMed] [Google Scholar]
- 29.Takeda, K., and S. Akira. 2004. TLR signaling pathways. Semin. Immunol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 30.Tang, E. D., C. Y. Wang, Y. Xiong, and K. L. Guan. 2003. A role for NF-kappaB essential modifier/IkappaB kinase-gamma (NEMO/IKKgamma) ubiquitination in the activation of the IkappaB kinase complex by tumor necrosis factor-alpha. J. Biol. Chem. 278:37297-37305. [DOI] [PubMed] [Google Scholar]
- 31.Uehara, A., S. Yang, Y. Fujimoto, K. Fukase, et al. 2005. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell. Microbiol. 7:53-61. [DOI] [PubMed] [Google Scholar]
- 32.van Heel, D. A., S. Ghosh, K. A. Hunt, C. G. Mathew, A. Forbes, D. P. Jewell, and R. J. Playford. 2005. Synergy between TLR9 and NOD2 innate immune responses is lost in genetic Crohn's disease. Gut 54:1553-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, C., L. Deng, M. Hong, G. R. Akkaraju, J. Inoue, and Z. J. Chen. 2001. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412:346-351. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe, T., A. Kitani, P. J. Murray, and W. Strober. 2004. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat. Immunol. 5:800-808. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe, T., A. Kitani, and W. Strober. 2005. NOD2 regulation of Toll-like receptor responses and the pathogenesis of Crohn's disease. Gut 54:1515-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe, T., A. Kitani, P. J. Murray, Y. Wakatsuki, I. J. Fuss, and W. Strober. 2006. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity 25:473-485. [DOI] [PubMed] [Google Scholar]
- 37.Wolfert, M. A., T. F. Murray, G. J. Boons, and J. N. Moore. 2002. The origin of the synergistic effect of muramyl dipeptide with endotoxin and peptidoglycan. J. Biol. Chem. 277:39179-39186. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto, M., S. Sato, T. Saitoh, H. Sakurai, S. Uematsu, et al. 2006. Pivotal function of Ubc13 in thymocyte TCR signaling. J. Immunol. 177:7520-7524. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto, M., T. Okamoto, K. Takeda, S. Sato, H. Sanjo, et al. 2006. Key function for the Ubc13 E2 ubiquitin-conjugating enzyme in immune receptor signaling. Nat. Immunol. 7:962-970. [DOI] [PubMed] [Google Scholar]
- 40.Yang, S., R. Tamai, S. Akashi, O. Takeuchi, S. Akira, S. Sugawara, and H. Takada. 2001. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect. Immun. 69:2045-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, J., B. Stirling, S. T. Temmerman, C. A. Ma, I. J. Fuss, J. M. Derry, and A. Jain. 2006. Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J. Clin. Investig. 116:3042-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou, H., I. Wertz, K. O'Rourke, M. Ultsch, S. Seshagiri, M. Eby, W. Xiao, and V. M. Dixit. 2004. Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature 424:167-171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.