Abstract
The molecular details linking integrin engagement to downstream cortactin (Ctn) tyrosine phosphorylation are largely unknown. In this report, we show for the first time that Fer and Ctn are potently tyrosine phosphorylated in response to hydrogen peroxide (H2O2) in a variety of cell types. Working with catalytically inactive fer and src/yes/fyn-deficient murine embryonic fibroblasts (ferDR/DR and syf MEF, respectively), we observed that H2O2-induced Ctn tyrosine phosphorylation is primarily dependent on Fer but not Src family kinase (SFK) activity. We also demonstrated for the first time that Fer is activated by fibronectin engagement and, in concert with SFKs, mediates Ctn tyrosine phosphorylation in integrin signaling pathways. Reactive oxygen species (ROS) scavengers or the NADPH oxidase inhibitor, diphenylene iodonium, attenuated integrin-induced Fer and Ctn tyrosine phosphorylation. Taken together, these findings provide novel genetic evidence that a ROS-Fer signaling arm contributes to SFK-mediated Ctn tyrosine phosphorylation in integrin signaling. Lastly, a migration defect in ferDR/DR MEF suggests that integrin signaling through the ROS-Fer-Ctn signaling arm may be linked to mechanisms governing cell motility. These data demonstrate for the first time an oxidative link between integrin adhesion and an actin-binding protein involved in actin polymerization.
Reactive oxygen species (ROS), such as superoxide anion radicals, hydrogen peroxide (H2O2), and the hydroxyl radical, are widely known as by-products of mitochondrial oxidative metabolism or as antimicrobial agents produced by immune cells during infection or inflammation (52). Excessive elevation of ROS as a result of abnormal mitochondrial oxidation or chronic inflammation has been implicated in the pathogenesis of a number of diseases such as atherosclerosis, pulmonary fibrosis, cancer, and various neurodegenerative disorders (52). Recent developments, however, suggest that ROS are not just by-products of oxygen metabolism but also act as second messengers in signal transduction (29). They have been shown to be produced by nonmitochondrial NADPH oxidases and/or by 5-lipooxygenase-dependent mechanisms in a variety of nonphagocytic cell types including endothelial cells and fibroblasts (6, 9, 41, 50).
Engagement of numerous receptor types including those of the growth factor (GF) and integrin receptor families leads to downstream ROS production (9, 17, 29). Importantly, signal propagation downstream of these receptors was shown to be dependent on ROS, as demonstrated by the signal-inhibitory effects of ROS scavengers, such as N-acetylcyteine (NAC), or NADPH oxidase inhibitors, such as diphenylene iodonium (DPI) (5, 9, 17, 49). This strongly suggested that ROS may be a key effector of GF- and integrin-induced morphological responses. In support of this, ROS have been implicated in regulating many cellular responses controlled by GF and integrin signaling cascades including adhesion, motility, actin and microtubule organization, proliferation, and invasion (3, 9, 22, 25, 28, 37, 39, 48). Thus, knowledge of how ROS regulate GF and integrin signaling mechanisms is necessary to understand how these pathways promote migration and invasion in cancer.
ROS have been shown to modulate protein function through oxidation of susceptible cysteine side chain moieties (46). A number of tyrosine kinases have been identified which contain cysteines susceptible to oxidation (18, 23). Perhaps more significant, however, is the identification of a susceptible cysteine thiolate anion present in the active-site signatures of protein tyrosine phosphatases (PTP) (46). Studies have shown that oxidative modification of this active-site cysteine is associated with reversible inactivation of a number of PTP involved in GF and integrin signaling including LMW-PTP, TC45, and PTP1B (9, 30, 35, 59). Hence, ROS-mediated inhibition of PTP may play a key role in enabling tyrosine kinase activity in GF and integrin signaling cascades.
GF receptor and integrin signaling have been shown to regulate actin polymerization through Arp2/3-mediated actin nucleation at the leading edges of motile cells. Arp2/3 is known to be stimulated by two principle nucleation promoting factors in eukaryotes (61). One of these nucleation promoting factors, Wiskott Aldrich syndrome protein (N-WASP), has a strong affinity for monomeric (G) actin and localizes to the leading edges of cells where it interacts with and activates Arp2/3 through its verprolin-cofilin-acidic homology domain (61). The other factor is the filamentous (F) actin-binding adaptor protein cortactin (Ctn). Ctn localizes to the edges of lamellipodia and filopodia of spreading or migrating cells where it directly interacts with and activates Arp2/3 through an N-terminal acidic domain (20, 61). The Ctn N-terminal acidic domain by itself is insufficient for either colocalization or activation of Arp2/3 but requires an adjacent F-actin-binding domain consisting of 6.5 tandemly repeated sequences (57, 61). In addition, Arp2/3 activation by Ctn is less potent than that of N-WASP; however, Ctn can synergize with N-WASP to enhance Arp2/3 activity (56). The exact relevance of Ctn-dependent Arp2/3 nucleation on F-actin networks at the leading edges of cells is unclear, but studies suggest that it is likely associated with the known requirement for Ctn in cell migration and invasion (21, 27, 32, 42).
Overexpression of Ctn promotes migration and invasion both in vitro and in vivo, although the mechanism by which Ctn does this remains unclear (21, 27, 32, 42). Several lines of evidence suggest that three tyrosine residues lying C terminal to the Ctn F-actin-binding domain play a key role in regulating Ctn-dependent migration (58). Epidermal growth factor (EGF) has been shown to be able to induce phosphorylation of two of these tyrosines—Y421 and Y466—in the lamellipodia and filopodia of spreading and migrating fibroblasts (20). Phosphorylation of these tyrosines was Rac dependent, required localization of Ctn to the cell cortex, and correlated with the activation of Src family kinase (SFK) (20). Ctn tyrosine phosphorylation was also reported in response to alphaVbeta1/3 integrin engagement on fibronectin (Fn)/vitronectin, although phosphorylation of Y421 and Y466 in this context was not specifically evaluated (1, 55). More importantly, however, studies have demonstrated that Ctn mutants containing phenylalanine mutations at Y421, Y466, and Y482 fail to potentiate cell motility and metastases (21, 27, 32, 42). This suggests that tyrosine phosphorylation at these sites may play a key role in regulating Ctn's ability to promote migration.
Fer and its homologue Fps/Fes comprise a unique two-member subfamily of cytoplasmic tyrosine kinases (19). Fer is structurally characterized by a central Src homology 2 domain and a C-terminal tyrosine kinase domain, and it is distinguished from members of the Src, Abl, Btk, Jak, Zap70, or Fak cytoplasmic tyrosine kinase subfamilies by N-terminal FCH (Fps/Fcs/Fer/Clp4 homology) and adjacent coiled-coil domains (4, 12). Although roles for Fer in oncogenesis and inflammation have been proposed, the molecular mechanisms by which Fer regulates these processes are unclear (2, 34a, 43). Biochemical studies suggest an important role for Fer in regulating the cytoskeleton through Ctn. GFs can induce Fer to interact with and tyrosine phosphorylate Ctn in an Src homology 2-dependent manner (24). Consistent with this, murine embryonic fibroblasts (MEF) derived from mice harboring catalytically inactive Fer (ferDR/DR) display reduced GF-induced Ctn tyrosine phosphorylation (13). In addition, the actin depolymerization agent latrunculin B was shown to induce Ctn phosphorylation at Y421 and Y466 (16). Interestingly, phosphorylation of these residues required the F-actin-binding region of Ctn and was dependent on an association between Fer and the Ctn N-terminal region harboring this actin-binding region. Using MEF derived from ferDR/DR and fyn-deficient mice, these studies concluded that Ctn tyrosine phosphorylation was mediated by actin depolymerization through a novel Fyn-Fer-dependent pathway.
In this study, we describe a novel adhesion pathway in which Fer transduces oxidative signals from integrin receptors to the actin cytoskeleton via tyrosine phosphorylation of Ctn. We provide novel genetic evidence that this pathway and a second pathway involving SFKs comprise two important routes leading to Ctn tyrosine phosphorylation downstream of integrins. Lastly, we report a migration defect in ferDR/DR MEF which may be linked to the requirement of ROS and Fer in integrin-mediated Ctn tyrosine phosphorylation.
MATERIALS AND METHODS
Cell culture.
Murine aortic endothelial cells (MAEC) and MEF were routinely cultured in Dulbecco's minimal essential medium (DMEM) (Invitrogen, Burlington, Ontario, Canada) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich). The normal mouse mammary epithelial cell line, HC-11, was cultured in RPMI supplemented with 10% FBS, 5 ng/ml EGF, and 10 μg/ml insulin (Sigma-Aldrich). The normal human mammary epithelial cell line, MCF-10A, was cultured in DMEM/F-12 (Invitrogen) supplemented with 5% donor horse serum, 20 ng/ml EGF, 10 μg/ml insulin, 0.5 μg/ml hydrocortisone, and 100 ng/ml cholera toxin (Sigma-Aldrich). Simian virus 40-large T-transformed, src/yes/fyn (syf) triple-deficient MEF and simian virus 40-large T-transformed wild-type (wt) MEF used as controls were cultured in DMEM supplemented with 10% FBS.
Isolation and immortalization of MEF.
Primary MEF were isolated as previously described from wt or fer-deficient (ferDR/DR) mice (13). These ferDR/DR mice were generated by targeting the fer locus with a kinase-inactivating mutation (D743R) in kinase subdomain IX. This mutation also destabilizes the FerDR protein (13). MEF were immortalized by infection with retrovirus encoding a short hairpin RNA against p53 (15). Heterogeneous populations of cells were then isolated by selection in puromycin, and these isolates were subsequently used in experiments between passages 12 and 35.
Lentiviral and retroviral infection.
293T human embryonic kidney cells were cotransfected with the lentiviral packaging and envelope plasmids pCMVΔR8.91 and pMD.2G, respectively, and with the lentiviral transfer vector pWXLd, which encoded wt or kinase-inactivating fer sequences [fer(K592R)] fused to a DsRed fluorescent reporter (10). Viral supernatants were collected approximately 72 to 96 h posttransfection, filtered through 0.4-μm filters, and applied directly to syf triple-deficient or ferDR/DR MEF. Infection was allowed to proceed for 24 h, after which media was replaced with DMEM/10% FBS. Infected cell populations were used in experiments after 4 to 8 days of additional culture.
Retroviral infections.
293T human embryonic kidney cells were cotransfected with a pMSCVpac retroviral plasmid harboring a Fer-Myc-epitope fusion sequence and with the packaging plasmid, Φ-NX-ECOpac (11). Viral supernatants were collected, filtered, and applied to ferDR/DR MEF for 24 h in the presence of Polybrene (5 μg/ml). Heterogeneous populations of transduced cells expressing Fer-Myc were isolated by hygromycin selection for 2 weeks and frozen for subsequent use.
Adhesion experiments.
Adhesion experiments were performed as described previously (36). Briefly, subconfluent monolayers of wt or ferDR/DR MEF were serum starved for 12 to 16 h, detached by trypsin-treatment, and pooled in DMEM containing soya bean trypsin inhibitor (Sigma-Aldrich). Cells were then washed in DMEM and incubated in suspension for an additional 1 h at 37°C in bovine serum albumin-coated tubes. In some experiments, the H2O2 scavengers, NAC (25 mM) and 4,5-dihydroxy-1,3-benzenedisulfonic acid disodium salt monohydrate (Tiron; 20 mM), the NADPH oxidase inhibitor, DPI (25 μM), or the mitochondrial superoxide production inhibitor, rotenone (50 μM), were added during the incubation (5-7, 9, 45). MEF (wt or ferDR/DR) were then plated on tissue culture plates coated with Fn (10 μg/ml). Cells were allowed to adhere and were harvested postadhesion. Soluble cell lysates (SCL) were subsequently prepared by centrifugation at 18,000 × g, and the total protein concentration of each lysate was determined. SCL (100 to 200 μg) were routinely immunoprecipitated (IP) using rabbit antisera specific for Fer (13). In other experiments, SCL containing equivalent concentrations of total protein (5 to 20 μg) were immunoblotted (IB) with Fer antisera, with an anti-Ctn mouse monoclonal antibody (clone F411; Upstate Biotechnology, Charlottesville, VA), or with phosphospecific antibodies against pY421 or pY466 of Ctn (Chemicon International, Camarillo, CA). SCL were also probed with anti-pY397 Fak (BioSource, Camarillo, CA), anti-pY418Src (BioSource), anti-panSrc (Santa Cruz Biotechnologies Inc., Santa Cruz, CA), anti-pY118 paxillin (BioSource), antipaxillin (BD Biosciences, San Jose, CA), and anti-pY165 p130 Cas, anti-pY410 p130 Cas, anti-pY249 p130 Cas, and anti-p130 Cas (all from Cell Signaling, Danvers, MA).
ROS stimulations.
Subconfluent monolayers of wt or ferDR/DR MEF were serum starved for 12 to 16 h and stimulated with H2O2 (0 to 10 mM) for 0 to 60 min. In some experiments, cells were pretreated with the inhibitors described above for 1 h prior to stimulation with agonists. Cells were then harvested, total protein concentration was determined, and 100 to 200 μg or 5 to 20 μg of SCL were subjected to IP or IB, respectively.
Immunofluorescence.
MEF (wt and ferDR/DR) were plated on Fn-coated coverslips as described for the biochemical adhesion experiments. Thirty minutes postadhesion, cells were fixed with 2% formaldehyde, permeabilized with 0.5% Triton X-100, and subsequently stained with fluorescein isothiocyanate (FITC)-phalloidin and/or with the same Ctn, Fer, and Src antibodies utilized in the biochemical adhesion experiments. Cells were washed and mounted in Mowiol 4-88/glycerol solution (Calbiochem) before imaging by confocal microscopy.
Wound-healing assays.
Delta-T dishes (Bioptechs Inc., Butler, PA) were coated with a solution containing 5 μg/ml Fn in phosphate-buffered saline for 1 h at 37°C. Plates were rinsed with phosphate-buffered saline, and wt or ferDR/DR MEF were applied at a density of 1 × 106 cells per dish. MEF were allowed to adhere for 60 min at 37°C before scoring the confluent monolayer with a 200-μl plastic pipette tip. The dish was transferred to a heated stage (37°C; Bioptechs Inc.), and the denuded area was imaged at ×20 by differential interference contrast light microscopy every 4 min for 15 to 24 h. The rate of disappearance of the denuded area was measured in individual frames using Image Pro 5.1 and Image J 1.41 software.
Transwell migration assays.
Migration assays were performed using Fn (10 μg/ml)-coated, 8-μm-pore-size transwell inserts according to the manufacturer's instructions (BD Biosciences). Briefly, 5 × 104 cells were added to the top chambers of transwell inserts containing DMEM/10% FBS in the lower chamber. Cells were incubated at 37°C for 4 h, fixed with 3% (wt/vol) paraformaldehyde, and stained with 0.2% (wt/vol) crystal violet solution. The numbers of migrated cells present were counted and expressed as means ± standard deviations (SD).
Statistics.
Student's t tests and means were determined using Microsoft Excel (Microsoft, Mississisauga, Ontario, Canada) and two-way analysis of variance (ANOVA) analyses were determined using GraphPad Prism statistical analysis software (GraphPad Software Inc., San Diego, CA). t tests and ANOVA analyses of data sets with P values less than or equal to 0.05 were considered statistically significant. Where appropriate, data are expressed as means ± SD.
RESULTS
Fer is robustly phosphorylated in response to exogenous H2O2 in a variety of cell types.
Inducible Ctn tyrosine phosphorylation has been observed in endothelial cells treated with exogenous H2O2 (31). Since Ctn has been previously reported to be a substrate of Fer (13, 24), we postulated that Fer may mediate Ctn pY in the context of oxidative signaling. We tested this possibility by treating MAEC, breast epithelial cells (BEC), peritoneal macrophages (PM), and wt and ferDR/DR MEF with exogenous H2O2 and measured Fer phosphorylation by IP/IB analysis. The results showed that Fer was robustly activated after exogenous H2O2 treatment in MEF (Fig. 1A and B) and in MAEC, BEC, and PM (see Fig. S1A, B, and C in the supplemental material). However, Fer phosphorylation was entirely absent in MEF expressing kinase-inactive Fer, suggesting that H2O2-induced phosphorylation of wt Fer was dependent on its autophosphorylation activity (Fig. 1A and B).
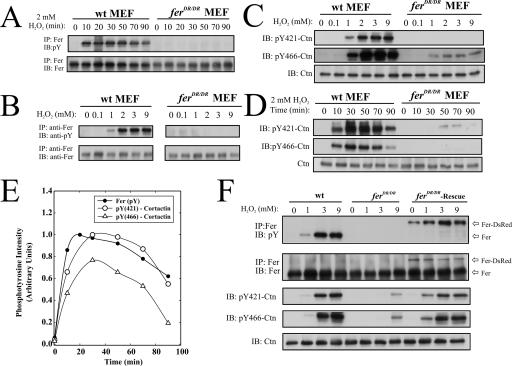
FIG. 1.
Fer and Ctn are robustly phosphorylated on tyrosine in response to H2O2 in wt but not ferDR/DR MEF. (A) Subconfluent monolayers of MEF were treated with 0 to 10 mM H2O2 for 0 to 90 min. Cells were then harvested and anti-Fer antibodies were used for IP analysis, followed by IB with anti-pY and anti-Fer antibodies, as indicated. (B) Fer phosphorylation was assessed as above in wt and ferDR/DR MEF after treatment with increasing concentrations of H2O2 for 10 min. (C and D) Total Ctn, pY421-Ctn, or pY466-Ctn were assessed by IB in lysates from subconfluent monolayers of wt and ferDR/DR MEF treated with 0 to 9 mM H2O2 for 10 min (C) or 2 mM H2O2 for 0 to 90 min (D). (E) Densitometric analyses of the phosphotyrosine profiles of Fer in panel A and Ctn at Y421 and Y466 in panel D. (F) Fer and Ctn phosphorylation were assessed by IP/IB or IB as described above after 10-min challenges with the indicated H2O2 concentrations in wt, ferDR/DR, or ferDR/DR MEF infected with lentivirus encoding a Fer-DsRed protein (ferDR/DR-Rescue).
We further explored the differences in kinetics and sensitivity of this H2O2 response between MEF and MAEC. Kinetic experiments revealed that Fer activity was sustained for 90 min in wt MEF (Fig. 1A) but was reversible in MAEC by about 5 min (see Fig. S1D in the supplemental material). Differences in the H2O2 sensitivity of Fer in these cell types were also apparent in dosing experiments. The sensitivity of Fer activation to H2O2 ranged from 0.5 to 2 mM in MEF and 0.1 to 10 mM in MAEC (see Fig. S1E and F in the supplemental material). These analyses suggest that Fer activity is strongly inducible by exogenous application of H2O2 and that the sensitivity and kinetics of this induction varies between fibroblasts and endothelial cells.
Cortactin phosphorylation is dramatically reduced in H2O2-treated ferDR/DR MEF.
Having established that Fer is inducibly activated by H2O2, we explored our initial postulate that Fer may regulate Ctn tyrosine phosphorylation in oxidative pathways. In agreement with a published report by Li et al. (31), Ctn tyrosine phosphorylation at Y421 and Y466 was robustly inducible by H2O2 in endothelial cells (see Fig. S2A in the supplemental material) as well as in BEC (see Fig. S2B in the supplemental material). We also observed potent phosphorylation at both Y421- and Y466-Ctn across a wide range of H2O2 concentrations in wt MEF (Fig. 1C). The time course of this Ctn phosphorylation response induced by 2 mM H2O2 spanned 90 min and closely paralleled the time course of Fer activation by H2O2 (Fig. 1D and E, respectively). However in ferDR/DR MEF, we observed a severe defect in Ctn phosphorylation under identical conditions of exogenous H2O2 stimulation (Fig. 1C and D). To further substantiate the apparent role of Fer in Ctn phosphorylation, we used lentivirus encoding a Fer-DsRed fusion protein to rescue active Fer expression in ferDR/DR MEF. As expected, Fer-DsRed—which migrates slower on sodium dodecyl sulfate-polyacrylamide gel electrophoresis relative to Fer—was inducibly phosphorylated in response to H2O2 in ferDR/DR MEF (Fig. 1F), and Ctn tyrosine phosphorylation was almost completely rescued in ferDR/DR MEF expressing Fer-DsRed (Fig. 1F). These results strongly support the conclusion that Fer kinase activity is required for H2O2-induced Ctn tyrosine phosphorylation at residues Y421 and Y466.
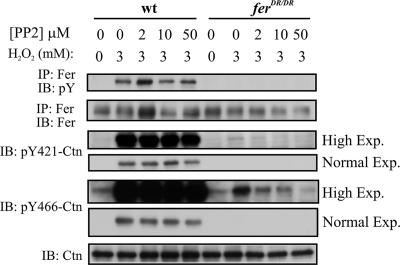
H2O2-induced cortactin tyrosine phosphorylation by Fer is largely independent of SFKs.
Fyn has been reported to act upstream of Fer in mediating actin depolymerization-induced Ctn tyrosine phosphorylation (16). To test whether Fyn or other Src family members function upstream of Fer in H2O2-induced Ctn tyrosine phosphorylation, we employed the SFK inhibitor PP2. Stimulation of MEF, with 3 mM H2O2 in the presence of PP2 concentrations as high as 50 μM, did not affect the level of Fer activation (Fig. 2, upper panel). This suggests that SFKs do not lie upstream of Fer in H2O2-induced signaling pathways. Consistent with this, PP2 did not appear to inhibit Y421-Ctn and Y466-Ctn phosphorylation in H2O2-stimulated wt MEF (Fig. 2, normal exposures). However, examination of highly exposed film revealed a faint Y421-Ctn- and Y466-Ctn-specific phosphosignal in ferDR/DR MEF which was dose-dependently abrogated by PP2 (Fig. 2, high exposures). As estimated by densitometry, the intensity of this residual signal was at least 2 orders of magnitude below that observed in wt MEF (data not shown). Taken together, these data suggest that SFKs do not play a prominent role upstream of Fer in H2O2-induced Ctn phosphorylation at residues Y421 and Y466.
FIG. 2.
SFKs do not play a major role in cortactin tyrosine phosphorylation downstream of H2O2. The effect of PP2 on H2O2-stimulated Fer and Ctn phosphorylation in wt or ferDR/DR MEF was tested by IP/IB or IB analyses. Both normal and high exposures of pY421-Ctn and pY466-Ctn are shown in order to accentuate the relative differences in H2O2-stimulated Ctn phosphorylation in wt and ferDR/DR MEF, as well as to demonstrate that low levels of H2O2-stimulated Ctn phosphorylation in ferDR/DR MEF are dose-dependently inhibited by PP2.
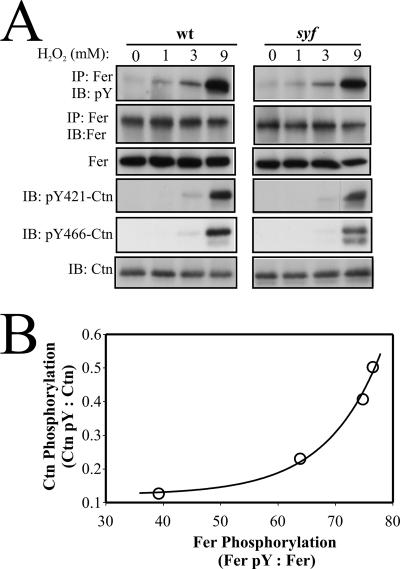
We further assessed the relative roles of Fer and SFKs in H2O2-induced Ctn tyrosine phosphorylation by comparing wt and src/yes/fyn (syf) triple-deficient MEF. In each cell type, the H2O2-induced profile of Fer activation closely paralleled that of Ctn tyrosine phosphorylation, with peak responses for both signaling proteins occurring at 9 mM H2O2 (Fig. 3A). More significantly, the relative levels of Fer and Ctn tyrosine phosphorylation in syf MEF were similar to those in wt MEF. This observation suggests that SFKs are not necessary for H2O2-induced Ctn tyrosine phosphorylation and that Fer might be directly responsible. This is consistent with the observation of an almost undetectable PP2-sensitive Ctn phosphotyrosine signal in H2O2-treated ferDR/DR MEF (Fig. 2).
FIG. 3.
H2O2-induced cortactin tyrosine phosphorylation in syf triple-deficient MEF correlates with Fer activation. (A) Tyrosine phosphorylation of Fer and Ctn was assessed by IP/IB (Fer) or IB (Ctn) in H2O2-treated wt and syf MEF. (B) A plot of Fer pY to Fer ratios versus Ctn pY to Ctn ratios from four independent experiments reveals a strong biphasic correlation in the level of activation of these proteins in response to H2O2.
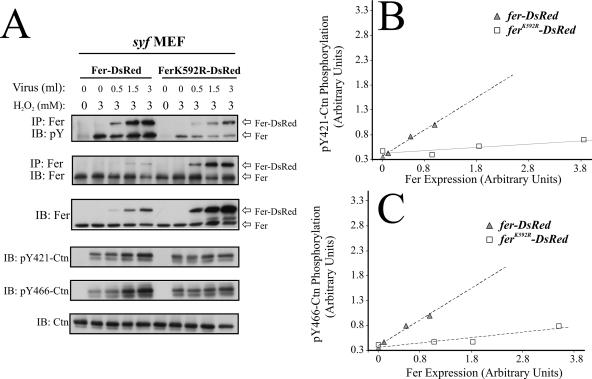
In subsequent experiments (data not shown), we observed progressive reductions in Fer expression levels in syf MEF which appeared to be associated with increased passaging. Interestingly, these reductions in Fer expression in syf MEF appeared to correlate with further reductions in H2O2-inducible Ctn tyrosine phosphorylation, suggesting a direct relationship between Fer activity and Ctn tyrosine phosphorylation under these conditions. This suspicion was partly affirmed by observation of a biphasic correlation between Ctn pY to Ctn and Fer pY to Fer ratios calculated from four independent experiments (Fig. 3B). To confirm this correlation, we expressed DsRed fusions of wt Fer and a kinase-inactive variant of Fer (FerK592R) in syf MEF using lentiviral-mediated transfer. Infection with increasing volumes of viral supernatant produced dose-dependent increases in Fer-DsRed and FerK592R-DsRed expression, although the latter was approximately 10-fold overexpressed compared to the former (Fig. 4A, panels 2 and 3). As expected, H2O2 induced Fer-DsRed-associated phosphotyrosine levels in syf MEF (Fig. 4A, panel 1). A minor increase in phosphotyrosine signal for FerK592R-DsRed was also observed in FerK592R-DsRed-expressing syf MEF which might be attributable to transphosphorylation reactions within Fer oligomeric complexes consisting of endogenous Fer and FerK592R-DsRed (12). Further support for heterogeneous Fer oligomer formation was indicated by an apparent inhibitory effect of increasing levels of FerK592R-DsRed on endogenous Fer activation in these cells (Fig. 4A, panel 1). More importantly, increasing Fer-DsRed expression in syf MEF correlated with increased Ctn tyrosine phosphorylation, while 10-fold greater levels of FerK592R-DsRed produced a comparably minor increase in Ctn tyrosine phosphorylation (Fig. 4A, panels 4 and 5). Quantification by densitometry confirmed a strong linear correlation (R2 > 0.95) between Fer-DsRed expression and increases in Ctn tyrosine phosphorylation at Y421 (Fig. 4B) and Y466 (Fig. 4C). Quantification also showed that the average increase in phosphorylation at these residues was approximately 8.2-fold greater than the increase of Ctn tyrosine phosphorylation in syf MEF expressing 10-fold more FerK592R-DsRed. The slight increase in Ctn tyrosine phosphorylation in syf MEF expressing FerK592R-DsRed (Fig. 4B and C) might be due to a small increase in Fer oligomeric populations containing at least some catalytically active endogenous Fer. Taken together, these data strongly implicate Fer as an essential mediator of Ctn phosphorylation in oxidative signaling.
FIG. 4.
Increasing Fer expression in syf MEF directly increased the levels of H2O2-induced Ctn tyrosine phosphorylation. (A) syf MEF were infected with increasing amount of lentiviruses encoding Fer-DsRed or kinase-dead FerK592R-DsRed. The cells were then challenged with H2O2, and assessed by IP/IB or IB for Fer and Ctn expression and tyrosine phosphorylation. (B and C) The blots in panel A were analyzed by densitometry, and pY421-Ctn to Ctn and pY466-Ctn to Ctn ratios were expressed as functions of Fer-DsRed and FerK592R-DsRed expression levels. The data show a linear correlation between pY421-Ctn phosphorylation and both Fer-DsRed and FerK592R-DsRed expression (B; R2 = 0.9786 and 0.8565, respectively). A linear correlation between pY466-Ctn phosphorylation and Fer-DsRed and FerK592R-DsRed expression was also observed (C; R2 = 0.9913 and 0.7935, respectively). The responses of pY421-Ctn and pY466-Ctn tyrosine phosphorylation to expression of Fer-DsRed in syf MEF were calculated to be 10.5- and 5.8-fold greater, respectively, than the responses of phosphorylation at these residues to expression of FerK592R-DsRed.
Fer is phosphorylated downstream of cell substratum-initiated signaling.
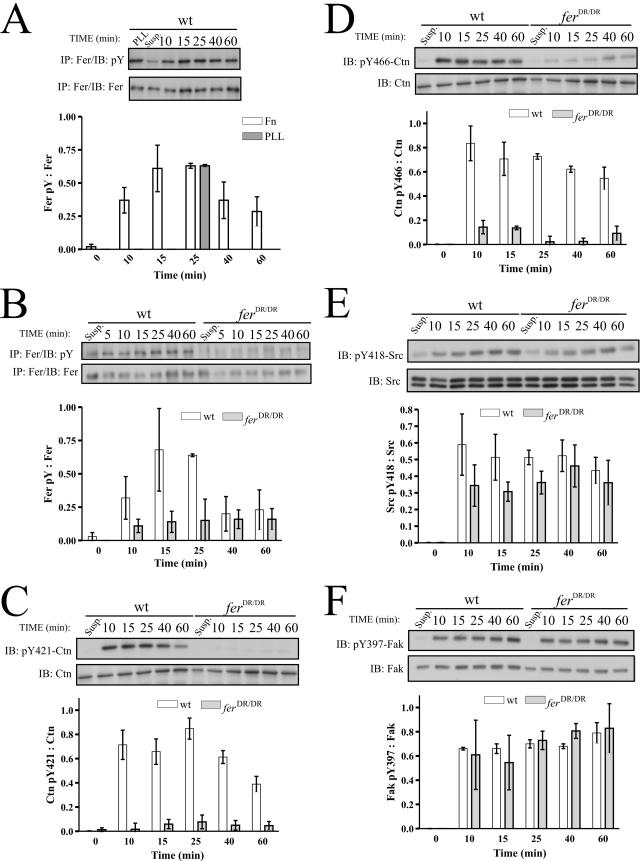
It was recently reported that Fak and Src activation were dependent on NADPH oxidase-mediated generation of ROS downstream of integrin engagement on Fn (9). We therefore hypothesized that Fer may be activated in integrin-mediated Fak signaling by a mechanism involving upstream generation of ROS. To test this hypothesis we examined whether Fer is tyrosine phosphorylated in response to adhesion on Fn on both tissue culture-treated and bacterial plates. Serum-starved MEF were plated on Fn, and newly adhered cells were harvested at time points between 0 and 60 min and subjected to IP/IB analysis. These experiments revealed that cell adhesion can induce tyrosine phosphorylation of Fer both on Fn-coated tissue culture plates (Fig. 5A) and on Fn-coated bacterial plates (data not shown). On average, Fn-induced Fer phosphorylation peaked around 15 to 25 min and declined slightly thereafter (Fig. 5A). As expected, Fn-induced Fer phosphorylation was dramatically compromised in ferDR/DR MEF, although a faint phosphotyrosine signal was still visible (Fig. 5B). These data demonstrate for the first time that Fer is directly activated in integrin signaling pathways. Interestingly, Fer activation was also observed when cells were plated on poly-l-lysine (PLL) (Fig. 5A), suggesting a role for other adhesion receptor systems in substratum-induced Fer activation.
FIG. 5.
Cortactin tyrosine phosphorylation is dependent on cell substratum-induced Fer tyrosine kinase activity. (A) wt MEF were plated on Fn or PLL for the indicated times and harvested. IP/IB analyses showed that Fer is tyrosine phosphorylated in response to Fn and PLL plating. Statistical analyses of each time point showed that Fn-induced Fer tyrosine phosphorylation was significantly different from the level of Fer phosphorylation in suspended cells (P = 9.3 × 10−9 − 0.046). (B) wt and ferDR/DR MEF were plated on Fn for 0 to 60 min. IP/IB analyses showed that Fer was inducibly phosphorylated in response to cell adhesion in wt but not fer-deficient MEF. Two-way ANOVA analyses showed a statistically significant difference in Fer activation in wt and ferDR/DR MEF (P = 0.0058). (C to F) Soluble cell lysates were prepared from cells harvested after adhesion to Fn for the indicated times. IB analysis using phosphospecific antibodies against pY421-Ctn (C) and pY466-Ctn (D) showed that both sites were strongly phosphorylated in wt but not ferDR/DR MEF in response to substratum adhesion (two-way ANOVA; P = 10−4 and 10−5, respectively). IB analyses using phosphospecific antibodies against pY418 of Src (E) and pY397 of Fak (F) showed that the kinetic phosphorylation profiles of these key adhesion proteins were normal in adhering ferDR/DR MEF (two-way ANOVA; P = 0.08 and 0.96, respectively).
Fer phosphorylates cortactin downstream of integrin signaling.
Earlier reports have shown that integrin engagement on Fn induces Ctn tyrosine phosphorylation (1, 55). It therefore followed that Fer might be an upstream regulator of Ctn in integrin-mediated signaling pathways. wt and ferDR/DR MEF were plated on Fn, and Ctn tyrosine phosphorylation was tested in soluble lysate preparations using phosphospecific antibodies against pY421- and pY466-Ctn. Consistent with earlier studies, Ctn phosphorylation was indeed observed in wt cells in response to Fn adhesion (1, 55). Strikingly however, Ctn phosphorylation at Y421 and Y466 was significantly compromised in ferDR/DR MEF, suggesting that Fer is an upstream regulator of Ctn tyrosine phosphorylation at residues Y421 and Y466 during integrin-mediated adhesion (Fig. 5C and D).
Activation of the adhesion signaling proteins Fak, Src, paxillin, and p130Cas is not dependent on Fer kinase activity.
The role of Fak, Src, paxillin, and p130Cas in Fn-induced integrin signaling has been well established (26). We tested whether Fer activation in adhering cells correlated with the activation profiles of these signaling proteins by comparing their kinetics of phosphorylation in wt and ferDR/DR MEF. Using phosphospecific antibodies, we observed that the kinetics of phosphorylation of the major Fak autophosphorylation site, Y397, were similar in wt and ferDR/DR MEF (Fig. 5F). Activation of Src, as assessed by its autophosphorylation at Y418, exhibited a delayed activation trend in ferDR/DR MEF, but the curve was not significantly different from that of the wt (P = 0.08) (Fig. 5E). In agreement with normal Src activation, previously identified downstream targets of Src such as Y118 of paxillin and Y165/Y410/Y249 of p130Cas were normal in ferDR/DR MEF (see Fig. S3A and B in the supplemental material, respectively). Thus, phosphorylation of Fak, Src, p130Cas, and paxillin during integrin-mediated signaling is independent of Fer activity.
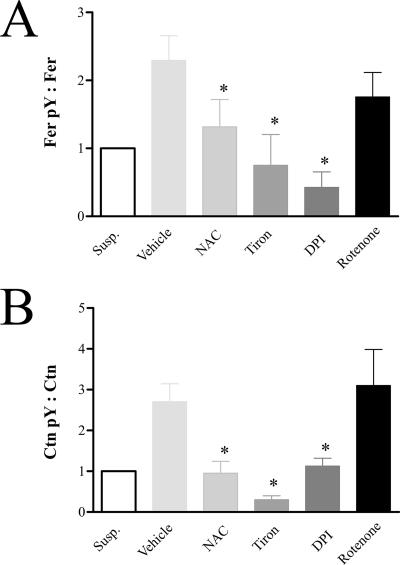
Fer-regulated cortactin phosphorylation is linked to upstream redox signals generated during integrin-mediated adhesion.
A recent report showed that ROS are essential in integrin signaling mediated by Fak and Src (9). To test whether Fer-regulated Ctn phosphorylation is also dependent on oxidative signals generated by integrins, wt MEF were pretreated with the ROS scavengers, NAC and Tiron, or the NADPH oxidase inhibitor, DPI, and subsequently plated on Fn. Adhered MEF were then harvested and examined for levels of Fer and Ctn tyrosine phosphorylation. IP/IB analysis showed that NAC, Tiron, and DPI inhibited both Fer and Ctn tyrosine phosphorylation in response to Fn adhesion (Fig. 6; also see Fig. S6 in the supplemental material). In contrast, the mitochondrial superoxide production inhibitor, rotenone, had only modest effects on Fer and Ctn tyrosine phosphorylation. These results suggest that Fer and Ctn tyrosine phosphorylation (like that of Fak and Src) are dependent on upstream ROS production by membrane NADPH oxidases during integrin-mediated signaling.
FIG. 6.
H2O2 scavengers and the NADPH oxidase inhibitor, DPI, inhibit Fer and cortactin phosphorylation induced by cell substratum engagement. (A) Fn-induced tyrosine phosphorylation of Fer at 25 min (expressed as a ratio of Fer pY to Fer in IP/IB analysis) was attenuated by the H2O2 scavengers NAC and Tiron and the NADPH oxidase inhibitor, DPI (*, P = 0.04, 0.01, and 0.002, respectively). The mitochondrial superoxide production inhibitor rotenone caused a partial inhibitory effect on Fer pY, although this change was not statistically significant. (B) Fn-induced phosphorylation of Ctn at 25 min (expressed as a ratio of pY421-Ctn to total Ctn) was also attenuated by NAC, Tiron, and DPI (*, P = 0.0044, 0.0007, and 0.0046, respectively). Rotenone had no effect on adhesion-induced Ctn phosphorylation.
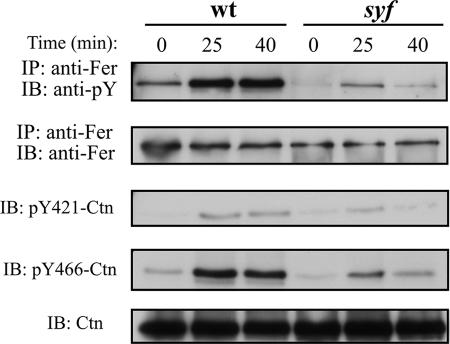
SFKs promote integrin-induced Fer and Ctn tyrosine phosphorylation.
The SFK inhibitor, PP2, has previously been shown to inhibit integrin-induced Ctn tyrosine phosphorylation (1). This suggests that in addition to Fer, SFKs may also be required for Ctn tyrosine phosphorylation downstream of integrins. To test this, wt and syf MEF were plated on Fn, and Fer activation and Ctn tyrosine phosphorylation were assessed as before. Our results showed that Fer and Ctn tyrosine phosphorylation are reduced in syf MEF relative to that of wt MEF (Fig. 7). The kinetics of activation of these proteins in syf MEF were very similar. This is consistent with the data in Fig. 5, which show a strong dependence of Ctn phosphorylation on Fer kinase activity in integrin signaling. Importantly, the observed reduction in Ctn tyrosine phosphorylation in syf MEF provides the first genetic confirmation of SFK involvement in Ctn tyrosine phosphorylation downstream of integrins. Moreover, reduced Fer phosphorylation in this genetic background suggests that SFK can act upstream of Fer in integrin signaling pathways leading to Ctn tyrosine phosphorylation.
FIG. 7.
SFKs promote Fer and Ctn tyrosine phosphorylation downstream of integrins. wt and syf MEF were plated on Fn and subsequently analyzed by IP/IB. Reduced Fer activation and Ctn phosphorylation at residues Y421 and Y466 are observed in syf MEF in response to adhesion on Fn at 25 min. This reduction is most pronounced at 40 min.
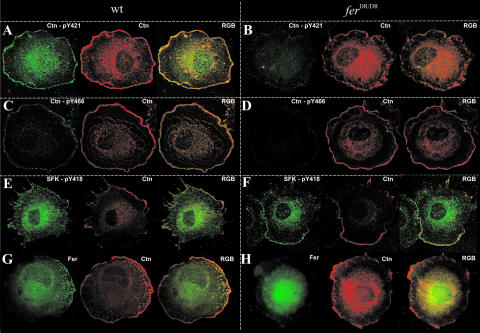
Deficient Ctn tyrosine phosphorylation in the periphery of adhering ferDR/DR MEF.
We further evaluated Ctn tyrosine phosphorylation in spreading wt and ferDR/DR MEF using confocal immunofluorescence microscopy. Tyrosine phosphorylation of Ctn at Y421 and at Y466 was detected in most wt cells and was largely restricted to the periphery, though some signal was also evident in the perinuclear/cytoplasmic region. On the other hand, Ctn-pY421 and Ctn-pY466 staining was either weakly detected or completely undetectable in ferDR/DR MEF (Fig. 8, compare panels A and C with panels B and D). Fer staining in wt MEF was predominantly perinuclear/cytoplasmic, although localized punctate patterns directly at and/or proximal to the plasma membrane were observed in some but not all cells. In contrast, Fer staining was markedly diffuse in ferDR/DR MEF and overall staining intensity was reduced in peripheral regions (Fig. 8G and H). In line with this, Fer-Ctn colocalization was most readily observed in wt MEF in regions at or adjacent to the membrane (Fig. 8G). Not unexpectedly, SFK-pY418 staining colocalized with Ctn at the plasma membrane in both wt and ferDR/DR MEF (Fig. 8E and F). These results correlated well with biochemical results showing aberrant Ctn phosphorylation and normal Src activation in adhering ferDR/DR cells (Fig. 5).
FIG. 8.
Ctn tyrosine phosphorylation is compromised in ferDR/DR fibroblasts. MEF were allowed to adhere to Fn-coated surfaces and fixed after 30 min. Cells were stained with antibodies against total Ctn (red) and Fer (green) and with phosphospecific antibodies against pY421 or pY466 of Ctn (green) or against pY418 of Src (green), as indicated. (A to D) Ctn staining was observed at the cell periphery and in the perinuclear region in both wt (A and C) and ferDR/DR (B and D) MEF. Consistent with the biochemical adhesion data in Fig. 5, phosphorylation at residues Y421 and Y466 of Ctn was detected in wt fibroblasts (A and C) but was undetectable or only faintly visible in ferDR/DR fibroblasts (B and D). (E and F) Phosphorylation of residue Y418 of Src, Yes, and Fyn was observed in peripheral regions of both wt and ferDR/DR MEF. (G and H) Cytoplasmic staining of Fer was observed in both wt and ferDR/DR MEF. Localization of Fer in the periphery and its colocalization with Ctn in these regions were more predominant in wt MEF.
Fer kinase activity is required for migration in vitro.
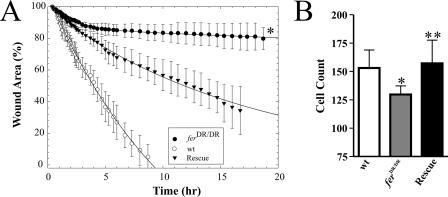
Ctn tyrosine phosphorylation is required for cell migration and invasion (21, 32, 33). We therefore reasoned that impaired integrin-mediated Ctn phosphorylation in ferDR/DR MEF may impair the ability of these cells to migrate. This led us to compare the migrations of wt and ferDR/DR MEF into denuded wound areas using time-lapse videomicroscopy. Open wound areas were calculated at regular time intervals during wound closure and plotted as a function of time (Fig. 9A). wt MEF wound-closure profiles indicated a rapid and consistent progress of cells into the wound, with complete closure occurring within 9 to 10 h (Fig. 9A; also see movie S1 in the supplemental material). In contrast, ferDR/DR MEF progressed only minimally past the initial wound interface at this time, with only about 20% wound closure after 18 h (Fig. 9A; also see movie S2 in the supplemental material). Introduction of Fer-DsRed into ferDR/DR MEF by lentivirus transduction partially rescued the impaired rate of wound closure seen in ferDR/DR MEF, as evidenced by 60% wound closure within 18 h (Fig. 9A; see movie S3 in the supplemental material). These data strongly suggest a requirement for Fer kinase activity in MEF migration. Reduced migration of ferDR/DR MEF was also observed in transwell migration assays. Retroviral-mediated expression of a Fer-Myc fusion protein in ferDR/DR MEF fully rescued the transwell migration defect, further supporting a requirement for Fer kinase activity in migration (Fig. 9B). The differential degrees of rescue of migration defects in ferDR/DR MEF observed between the wound-healing and transwell assays might reflect different degrees of rescue achieved by the lentiviral and retroviral systems (see Fig. S4 in the supplemental material, compare panels A and B with C).
FIG. 9.
ferDR/DR MEF have defects in migration in vitro. (A and B) Migration assays of wt, ferDR/DR, and ferDR/DR MEF rescued by lentiviral-mediated expression of Fer-DsRed (A; Rescue) or retroviral-mediated expression of Fer-Myc (B; Rescue). (A) Detailed kinetic profiles of scrape-wound areas (expressed as percentages of the initial wound area ± SD) for wt, ferDR/DR, and fer-Rescue MEF over 18 h. Initial wound areas, expressed as means ± SD, were 161,447 ± 20,659 μm2 for wt MEF, 176,044 ± 16,763 μm2 for ferDR/DR MEF, and 154,958 ± 9,317 μm2 for fer-Rescue MEF. Kinetic migration profiles of ferDR/DR MEF show a severe delay in wound closure which is partially rescued by expression of Fer-DsRed (*, P = 0.0001). (B) Relative migration in transwell assays of wt MEF, ferDR/DR MEF, and ferDR/DR MEF rescued by retroviral-mediated expression of epitope-tagged Myc-Fer. ferDR/DR MEF exhibited impaired migration in transwell assays which was rescued by retroviral transduction of a Fer-Myc fusion protein (*, P = 0.0019; **, P = 0.0036). The y axis represents the average number of migrated cells per insert acquired from four ×20 fields of view. Three transwell inserts were used per cell type, per experiment.
DISCUSSION
We report here for the first time that exogenous H2O2 can induce potent activation of Fer in a number of different cell lines, including MAEC, murine HC-11 BEC, human MCF-10A BEC, and peritoneal macrophages (data not shown). The potency of this activation varied from cell type to cell type, with endothelial cells exhibiting the most sensitivity. In the latter, Fer activation was detected with H2O2 concentrations ranging from 100 μM to 10 mM, while in fibroblasts, Fer activation was detected at concentrations ranging from 500 μM to 9 mM. Final cellular H2O2 concentrations are about one-tenth of the exogenously applied concentration (47). According to this estimation, Fer is activated at intracellular H2O2 levels ranging from 0.01 to 1 mM, which falls within the 0.1 to 1.0 mM range estimated to be generated by GF receptors and well within the range generated by integrins, the latter being up to fivefold higher (9, 49). Thus, Fer may be a mediator of oxidative signaling arising from GF and integrin receptors in a variety of cell types.
In agreement with an earlier report (31), we confirm that H2O2 can induce Ctn tyrosine phosphorylation in endothelial cells; and further, we report that this response also occurs in MEF and BEC lines. Temporal patterns of H2O2-induced Ctn phosphorylation paralleled those of Fer activation, suggesting that a distinct redox-responsive Fer-Ctn signaling axis regulates actin dynamics in a variety of cell types. The existence of such a pathway was strongly supported by data showing barely detectable, residual H2O2-induced Ctn phosphotyrosine signal in ferDR/DR MEF (Fig. 1). This residual Ctn phosphotyrosine signal was inhibited by PP2, suggesting that it was mediated by SFK (Fig. 2). This relatively small contribution by SFK was unexpected, given the prominent involvement of Src and its family member Fyn in Ctn tyrosine phosphorylation (34). Examination of H2O2-induced Ctn tyrosine phosphorylation in syf-deficient MEF showed comparable levels of Ctn tyrosine phosphorylation in wt and syf MEF when Fer expression levels were equivalent in these genotypes (Fig. 3). However, in experiments where Fer expression levels in syf MEF was reduced, relative to levels in wt cells, a corresponding drop in H2O2-induced Ctn tyrosine phosphorylation was also observed. This reduction in Fer expression in syf MEF was found to be associated with prolonged passaging. However, when Fer expression levels in these extensively passaged syf MEF were restored using lentiviral transduction, we observed a dose-dependent increase in H2O2-induced Ctn tyrosine phosphorylation (Fig. 4). This titratable effect demonstrated that a direct correlation exists between H2O2-induced Fer activation and Ctn tyrosine phosphorylation in the absence of SFKs. These results concurred with observations of severely diminished H2O2-induced Ctn tyrosine phosphorylation in ferDR/DR MEF (Fig. 1), and together these results support a unique, SFK-independent role for Fer in mediating Ctn tyrosine phosphorylation in response to ROS.
We also report here that Fer tyrosine phosphorylation is induced by Fn and PLL adhesion, suggesting that Fer is activated by integrin-dependent and -independent cell adhesion mechanisms. Several considerations support an integrin-mediated component of Fer activation: (i) Fer activation was observed in response to adhesion on Fn-coated bacterial culture plates (data not shown); (ii) activation of fundamental components of integrin-mediated signaling, such as Src, Fak, paxillin, and p130Cas, were observed in the same experiments; (iii) the observation of Ctn tyrosine phosphorylation in response to Fn plating is consistent with previous reports of integrin-induced Ctn tyrosine phosphorylation observed after plating on Fn- or alpha5beta1/3-integrin-coated plates (55); and lastly (iv), we observed Ctn tyrosine phosphorylation in response to plating on PLL (data not shown), which is in agreement with a previous study (1). Therefore, these data provide the first evidence of Fer activation in integrin signaling pathways.
Interestingly, knockdown of a Caenorhabditis elegans Fer-related kinase-1 (Frk-1) resulted in an adhesion-related defect in epithelial migration and enclosure of the embryo. This defect was rescued by mammalian FerT (a testis-specific isoform of Fer) or by a kinase-dead Frk-1 mutant (44). This suggested that Fer family members may also have kinase-independent adhesive functions which might be conserved in mammals. The Drosophila Fer homolog was also shown to be important for dorsal closure (40); and interestingly, this defect was accentuated when both DFer and the Drosophila Src42A gene were mutated, suggesting that in the fly, Fer and SFKs cooperate in regulating epithelial cell migration and morphological interactions.
Our results suggest that Fer activation downstream of integrins requires ROS since oxidant scavengers or inhibition of NADPH oxidase with DPI compromised Fer and Ctn Y421/Y466 phosphorylation. This activation was specific to NADPH oxidases since the mitochondrial oxidase inhibitor rotenone failed to inhibit integrin-induced Fer and Ctn phosphorylation. Inhibition of Fer and Ctn tyrosine phosphorylation by DPI, however, was only partial, indicating that a 5-lipooxgenase-dependent ROS generation mechanism previously shown to signal downstream of integrins may also be involved (9). At present, it is unclear how upstream H2O2 activates Fer. Preliminary data suggest that activation of Fer by H2O2 is not direct, because we were unable to detect inducible Fer autophosphorylation or glutathione S-transferase-Ctn tyrosine phosphorylation by treating Fer-immune complexes with H2O2 (see Fig. S5 in the supplemental material). This suggests that Fer may be activated as a result of ROS-mediated inhibition of upstream PTP. Taken together, these data suggest a novel oxidant pathway which links upstream ROS production by integrins to the actin cytoskeleton via Fer and Ctn. To our knowledge, this is the first report to demonstrate that oxidants can link upstream integrin signaling to an actin-binding protein involved in the regulation of actin polymerization and migration (38). These data strongly substantiate a growing body of evidence supporting a central role for ROS in regulating cell motility (38).
Earlier studies have shown that integrin-induced Ctn tyrosine phosphorylation is PP2 sensitive and is compromised in Fak−/− MEF (1), suggesting that Fak and Src are key players in integrin pathways leading to Ctn phosphorylation. Our observation of compromised integrin-induced Ctn tyrosine phosphorylation in ferDR/DR MEF (Fig. 5C and D) further suggests that Fer is an additional player in this process. Fak and SFK autophosphorylation were normal in adhering ferDR/DR MEF cells, implying that Fer lies downstream of Fak and SFKs in pathways leading to Ctn tyrosine phosphorylation (Fig. 10). This was confirmed in adhesion experiments with syf MEF which showed a reduction of both Fer and Ctn tyrosine phosphorylation (Fig. 7). Hence, Src and Fak lie upstream of Fer in integrin signaling, an ordering which is consistent with a requirement for Fyn upstream of Fer in Ctn tyrosine phosphorylation induced by actin depolymerization (16). Taken together, these results provide the first genetic evidence for a requirement of ROS, SFK, and Fer in integrin-induced Ctn phosphorylation and directly implicate residues Y421 and Y466 of Ctn as key downstream targets of Fer in integrin signaling (Fig. 10).
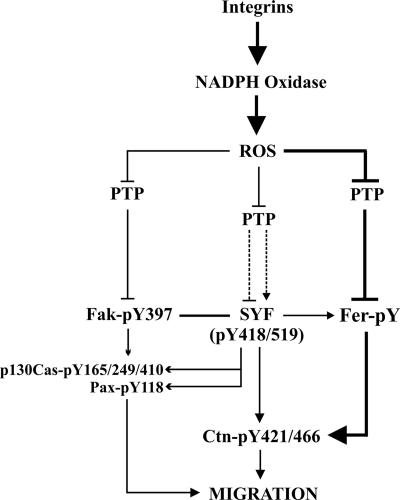
FIG. 10.
Model of Fer involvement in integrin-induced Ctn phosphorylation. Ligand engagement of integrins leads to activation of NADPH oxidase and production of ROS, which in turn leads to inhibition of PTP. PTP inhibition is involved in the inactivation of SFKs at the plasma membrane and plays a complex role in SYF regulation, including both stimulatory and inhibitory dephosphorylation at Y519 and Y418, respectively (51). LMW-PTP inhibits Fak (9), and we propose that PTP may also be involved in the inhibition of Fer. Collectively, integrin-induced ROS production correlates with Fer, Fak, and SYF activation, and subsequent phosphorylation of Ctn, which in turn contributes to the regulation of cell migration. This model predicts that Fer can act both independently of SFK and Fak and downstream from them during oxidative signaling from integrins to Ctn. It emphasizes a novel ROS-Fer-Ctn signaling arm involved in the regulation of cell migration downstream of integrin signaling pathways.
SFKs have been shown to be inactivated early in response to exogenous H2O2, specifically at focal adhesions and the plasma membrane (51). Their activity eventually recovers within 40 to 60 min and coincides with recovery of PTP activity from early oxidative inhibition. This suggests that SFK may not play a prominent role downstream of oxidants at early time points. On the other hand, Fer, which demonstrated strong H2O2 responsiveness as early as 10 min, independently of SFK (Fig. 2 and 3), showed peak activities 15 to 25 min post-Fn adhesion (Fig. 5A). We speculate therefore that Fer may phosphorylate Ctn in an oxidant-dependent manner at early stages of integrin signaling, while SFK may be oxidatively inhibited during this period. This is consistent with a stronger signal for Fer and Ctn tyrosine phosphorylation at earlier (25 min) rather than later (40 min) times in adhering syf MEF (Fig. 7). Taken together, these data emphasize the importance of an upstream ROS-dependent Fer-Ctn pathway early during integrin signaling.
Integrin-induced Fer activation and Ctn phosphorylation are on the whole compromised in syf MEF relative to wt MEF, suggesting a general contribution of SFK in integrin-induced Fer and Ctn tyrosine phosphorylation. This contribution to Ctn tyrosine phosphorylation could be mediated directly (58) or indirectly, through Fer (16). Our observations of reduced integrin-induced Fer and Ctn phosphorylation in syf MEF supports the possibility that SFK signaling through Fer constitutes a second significant route to Ctn (Fig. 7). Alternatively, SFK contributions may involve a feedback loop from Fer through which ROS-induced Fer activation could promote SFK-mediated Ctn tyrosine phosphorylation. Such a feedback effect, however, is likely to be minor in light of observations of normal Fn-induced SFK activation in ferDR/DR MEF (Fig. 5E). It is unclear to what extent SFK-mediated contributions to Ctn phosphorylation are oxidant dependent. Such a dependency is likely to be complex, as H2O2-induced inhibition of upstream PTP could result in both positive and negative regulation of SFK activity. As discussed above, studies suggest that at least initially, H2O2 negatively regulates SFK activation (51). Oxidants, however, may play a greater role in SFK activation at later stages; this is supported by a slight dependence of Src phosphorylation on integrin-mediated ROS generation 45 min after Fn adhesion (9). Importantly, this dependence is partial, suggesting that oxidant-independent mechanisms also play a role in SFK activation during integrin signaling. Further studies will be needed to clarify the interplay between Src and Fer and the role of upstream oxidants in integrin-induced Ctn tyrosine phosphorylation.
Several lines of evidence exist which link the migration defect in ferDR/DR MEF to reduced integrin-induced Ctn tyrosine phosphorylation in these cells. Aside from the obvious association between integrin signaling pathways and cell motility, the most important line of evidence linking this pathway to the motility defect is the known requirement for Ctn in migration and invasion. Studies have shown that RNA interference-mediated Ctn knockdown impairs migration and invasion in vitro (27). Further, overexpression of wt, but not Y421F/Y66F/Y486F Ctn mutants, strongly potentiated migration and invasion both in vitro and in vivo (21, 27, 32, 42). The ferDR/DR MEF migration defect can also be linked to a large body of evidence which supports a role for ROS as a central regulator of cell motility and invasion (38). Studies have shown that DPI treatment of cells significantly reduces the rate of incorporation of actin monomers at fast-growing barbed ends (38). Similarly, derivatives of the NADPH oxidase inhibitor apocynin can attenuate the migration and invasion of BEC (25). Perhaps most significantly, ROS are strongly generated by cells at wound margins and are localized within lamellipodia and membrane ruffles (38). Hence, regulation of Fer and Ctn tyrosine phosphorylation by ROS may underlie the migration defect in ferDR/DR MEF.
EGF has been shown to induce Ctn phosphorylation at Y421 and Y466 at the cell periphery of spreading and migrating cells (20). Our results provide new evidence that integrin engagement also induces Ctn phosphorylation at Y421 and Y466 near the plasma membrane; and moreover, we show that Fer colocalizes with peripheral Ctn under these conditions (Fig. 8). But how does integrin-induced Ctn tyrosine phosphorylation at the plasma membrane impact migration? Focal complexes at the plasma membrane contain Fak, beta3-integrin, paxillin, and vinculin (60). Fn adhesion can induce Rac-dependent Arp2/3 recruitment to vinculin which has been implicated in linking actin polymerization machinery to integrins (14). As Ctn can bind to and colocalize with F-actin and Arp2/3 at the plasma membrane (8, 53), we speculate that Ctn may be recruited to vinculin-containing focal complexes where it may promote new adhesion assembly as previously described (8). The observed colocalization of Fer and active SFK with Ctn at the periphery (Fig. 8) suggests that tyrosine phosphorylation of Ctn may regulate new focal complex assembly at the periphery. Moreover, NADPH oxidase components have recently been identified within focal complexes, suggesting that locally generated ROS may regulate Fer and Ctn phosphorylation within new adhesions at the protruding edge (54). Studies are under way to address whether ROS, Fer, and Ctn regulate focal complex dynamics at the leading edges of migrating cells.
Supplementary Material
Acknowledgments
We thank Brad Webb and Alan Mak for generously providing purified glutathione S-transferase-Ctn.
This study was funded by a grant from the Canadian Institutes of Health Research. This work was supported by an operating grant from the Canadian Institute of Health Research.
Footnotes
Published ahead of print on 2 July 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Agerer, F., S. Lux, A. Michel, M. Rohde, K. Ohlsen, and C. R. Hauck. 2005. Cellular invasion by Staphylococcus aureus reveals a functional link between focal adhesion kinase and cortactin in integrin-mediated internalisation. J. Cell Sci. 118:2189-2200. [DOI] [PubMed] [Google Scholar]
- 2.Allard, P., A. Zoubeidi, L. T. Nguyen, S. Tessier, S. Tanguay, M. Chevrette, A. Aprikian, and S. Chevalier. 2000. Links between Fer tyrosine kinase expression levels and prostate cell proliferation. Mol. Cell. Endocrinol. 159:63-77. [DOI] [PubMed] [Google Scholar]
- 3.Arnold, R. S., J. Shi, E. Murad, A. M. Whalen, C. Q. Sun, R. Polavarapu, S. Parthasarathy, J. A. Petros, and J. D. Lambeth. 2001. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc. Natl. Acad. Sci. USA 98:5550-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aspenstrom, P. 1997. A Cdc42 target protein with homology to the non-kinase domain of FER has a potential role in regulating the actin cytoskeleton. Curr. Biol. 7:479-487. [DOI] [PubMed] [Google Scholar]
- 5.Bae, Y. S., S. W. Kang, M. S. Seo, I. C. Baines, E. Tekle, P. B. Chock, and S. G. Rhee. 1997. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272:217-221. [PubMed] [Google Scholar]
- 6.Bae, Y. S., J. Y. Sung, O. S. Kim, Y. J. Kim, K. C. Hur, A. Kazlauskas, and S. G. Rhee. 2000. Platelet-derived growth factor-induced H(2)O(2) production requires the activation of phosphatidylinositol 3-kinase. J. Biol. Chem. 275:10527-10531. [DOI] [PubMed] [Google Scholar]
- 7.Blouin, E., L. Halbwachs-Mecarelli, and P. Rieu. 1999. Redox regulation of beta2-integrin CD11b/CD18 activation. Eur. J. Immunol. 29:3419-3431. [DOI] [PubMed] [Google Scholar]
- 8.Bryce, N. S., E. S. Clark, J. L. Leysath, J. D. Currie, D. J. Webb, and A. M. Weaver. 2005. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr. Biol. 15:1276-1285. [DOI] [PubMed] [Google Scholar]
- 9.Chiarugi, P., G. Pani, E. Giannoni, L. Taddei, R. Colavitti, G. Raugei, M. Symons, S. Borrello, T. Galeotti, and G. Ramponi. 2003. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J. Cell Biol. 161:933-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole, L. A., R. Zirngibl, A. W. Craig, Z. Jia, and P. Greer. 1999. Mutation of a highly conserved aspartate residue in subdomain IX abolishes Fer protein-tyrosine kinase activity. Protein Eng. 12:155-162. [DOI] [PubMed] [Google Scholar]
- 11.Craig, A. W., and P. A. Greer. 2002. Fer kinase is required for sustained p38 kinase activation and maximal chemotaxis of activated mast cells. Mol. Cell. Biol. 22:6363-6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig, A. W., R. Zirngibl, and P. Greer. 1999. Disruption of coiled-coil domains in Fer protein-tyrosine kinase abolishes trimerization but not kinase activation. J. Biol. Chem. 274:19934-19942. [DOI] [PubMed] [Google Scholar]
- 13.Craig, A. W., R. Zirngibl, K. Williams, L. A. Cole, and P. A. Greer. 2001. Mice devoid of Fer protein-tyrosine kinase activity are viable and fertile but display reduced cortactin phosphorylation. Mol. Cell. Biol. 21:603-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeMali, K. A., and K. Burridge. 2003. Coupling membrane protrusion and cell adhesion. J. Cell Sci. 116:2389-2397. [DOI] [PubMed] [Google Scholar]
- 15.Dirac, A. M., and R. Bernards. 2003. Reversal of senescence in mouse fibroblasts through lentiviral suppression of p53. J. Biol. Chem. 278:11731-11734. [DOI] [PubMed] [Google Scholar]
- 16.Fan, L., C. Di Ciano-Oliveira, S. A. Weed, A. W. Craig, P. A. Greer, O. D. Rotstein, and A. Kapus. 2004. Actin depolymerization-induced tyrosine phosphorylation of cortactin: the role of Fer kinase. Biochem. J. 380:581-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finkel, T. 1999. Signal transduction by reactive oxygen species in non-phagocytic cells. J. Leukoc. Biol. 65:337-340. [DOI] [PubMed] [Google Scholar]
- 18.Giannoni, E., F. Buricchi, G. Raugei, G. Ramponi, and P. Chiarugi. 2005. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol. Cell. Biol. 25:6391-6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greer, P. A. 2002. Closing in on the biological functions of Fps/Fes and Fer. Nat. Rev. Mol. Cell Biol. 3:278-289. [DOI] [PubMed] [Google Scholar]
- 20.Head, J. A., D. Jiang, M. Li, L. J. Zorn, E. M. Schaefer, J. T. Parsons, and S. A. Weed. 2003. Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol. Biol. Cell 14:3216-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, C., J. Liu, C. C. Haudenschild, and X. Zhan. 1998. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J. Biol. Chem. 273:25770-25776. [DOI] [PubMed] [Google Scholar]
- 22.Irani, K., Y. Xia, J. L. Zweier, S. J. Sollott, C. J. Der, E. R. Fearon, M. Sundaresan, T. Finkel, and P. J. Goldschmidt-Clermont. 1997. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 275:1649-1652. [DOI] [PubMed] [Google Scholar]
- 23.Kato, M., T. Iwashita, K. Takeda, A. A. Akhand, W. Liu, M. Yoshihara, N. Asai, H. Suzuki, M. Takahashi, and I. Nakashima. 2000. Ultraviolet light induces redox reaction-mediated dimerization and superactivation of oncogenic Ret tyrosine kinases. Mol. Biol. Cell 11:93-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, L., and T. W. Wong. 1998. Growth factor-dependent phosphorylation of the actin-binding protein cortactin is mediated by the cytoplasmic tyrosine kinase FER. J. Biol. Chem. 273:23542-23548. [DOI] [PubMed] [Google Scholar]
- 25.Klees, R. F., P. C. De Marco, R. M. Salasznyk, D. Ahuja, M. Hogg, S. Antoniotti, L. Kamath, J. S. Dordick, and G. E. Plopper. 2006. Apocynin derivatives interrupt intracellular signaling resulting in decreased migration in breast cancer cells. J. Biomed. Biotechnol. 2006:87246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klinghoffer, R. A., C. Sachsenmaier, J. A. Cooper, and P. Soriano. 1999. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18:2459-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowalski, J. R., C. Egile, S. Gil, S. B. Snapper, R. Li, and S. M. Thomas. 2005. Cortactin regulates cell migration through activation of N-WASP. J. Cell Sci. 118:79-87. [DOI] [PubMed] [Google Scholar]
- 28.Kundu, N., S. Zhang, and A. M. Fulton. 1995. Sublethal oxidative stress inhibits tumor cell adhesion and enhances experimental metastasis of murine mammary carcinoma. Clin. Exp. Metastasis 13:16-22. [DOI] [PubMed] [Google Scholar]
- 29.Lander, H. M. 1997. An essential role for free radicals and derived species in signal transduction. FASEB J. 11:118-124. [PubMed] [Google Scholar]
- 30.Lee, S. R., K. S. Kwon, S. R. Kim, and S. G. Rhee. 1998. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J. Biol. Chem. 273:15366-15372. [DOI] [PubMed] [Google Scholar]
- 31.Li, Y., J. Liu, and X. Zhan. 2000. Tyrosine phosphorylation of cortactin is required for H2O2-mediated injury of human endothelial cells. J. Biol. Chem. 275:37187-37193. [DOI] [PubMed] [Google Scholar]
- 32.Li, Y., M. Tondravi, J. Liu, E. Smith, C. C. Haudenschild, M. Kaczmarek, and X. Zhan. 2001. Cortactin potentiates bone metastasis of breast cancer cells. Cancer Res. 61:6906-6911. [PubMed] [Google Scholar]
- 33.Liu, J., C. Huang, and X. Zhan. 1999. Src is required for cell migration and shape changes induced by fibroblast growth factor 1. Oncogene 18:6700-6706. [DOI] [PubMed] [Google Scholar]
- 34.Lua, B. L., and B. C. Low. 2005. Cortactin phosphorylation as a switch for actin cytoskeletal network and cell dynamics control. FEBS Lett. 579:577-585. [DOI] [PubMed] [Google Scholar]
- 34a.McCafferty, D. M., A. W. Craig, Y. A. Senis, and P. A. Greer. 2002. Absence of Fer protein-tyrosine kinase exacerbates leukocyte recruitment in response to endotoxin. J. Immunol. 168:4930-4935. [DOI] [PubMed] [Google Scholar]
- 35.Meng, T. C., D. A. Buckley, S. Galic, T. Tiganis, and N. K. Tonks. 2004. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J. Biol. Chem. 279:37716-37725. [DOI] [PubMed] [Google Scholar]
- 36.Miranti, C. K. 2002. Application of cell adhesion to study signaling networks. Methods Cell Biol. 69:359-383. [DOI] [PubMed] [Google Scholar]
- 37.Moldovan, L., N. I. Moldovan, R. H. Sohn, S. A. Parikh, and P. J. Goldschmidt-Clermont. 2000. Redox changes of cultured endothelial cells and actin dynamics. Circ. Res. 86:549-557. [DOI] [PubMed] [Google Scholar]
- 38.Moldovan, L., K. Mythreye, P. J. Goldschmidt-Clermont, and L. L. Satterwhite. 2006. Reactive oxygen species in vascular endothelial cell motility. Roles of NAD(P)H oxidase and Rac1. Cardiovasc. Res. 71:236-246. [DOI] [PubMed] [Google Scholar]
- 39.Mori, K., M. Shibanuma, and K. Nose. 2004. Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res 64:7464-7472. [DOI] [PubMed] [Google Scholar]
- 40.Murray, M. J., C. M. Davidson, N. M. Hayward, and A. H. Brand. 2006. The Fes/Fer non-receptor tyrosine kinase cooperates with Src42A to regulate dorsal closure in Drosophila. Development 133:3063-3073. [DOI] [PubMed] [Google Scholar]
- 41.Park, H. S., S. H. Lee, D. Park, J. S. Lee, S. H. Ryu, W. J. Lee, S. G. Rhee, and Y. S. Bae. 2004. Sequential activation of phosphatidylinositol 3-kinase, βPix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol. Cell. Biol. 24:4384-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel, A. S., G. L. Schechter, W. J. Wasilenko, and K. D. Somers. 1998. Overexpression of EMS1/cortactin in NIH3T3 fibroblasts causes increased cell motility and invasion in vitro. Oncogene 16:3227-3232. [DOI] [PubMed] [Google Scholar]
- 43.Paulson, R., J. Jackson, K. Immergluck, and J. M. Bishop. 1997. The DFer gene of Drosophila melanogaster encodes two membrane-associated proteins that can both transform vertebrate cells. Oncogene 14:641-652. [DOI] [PubMed] [Google Scholar]
- 44.Putzke, A. P., S. T. Hikita, D. O. Clegg, and J. H. Rothman. 2005. Essential kinase-independent role of a Fer-like non-receptor tyrosine kinase in Caenorhabditis elegans morphogenesis. Development 132:3185-3195. [DOI] [PubMed] [Google Scholar]
- 45.Radisky, D. C., D. D. Levy, L. E. Littlepage, H. Liu, C. M. Nelson, J. E. Fata, D. Leake, E. L. Godden, D. G. Albertson, M. A. Nieto, Z. Werb, and M. J. Bissell. 2005. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436:123-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhee, S. G., Y. S. Bae, S. R. Lee, and J. Kwon. 2000. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. STKE 2000:PE1. [DOI] [PubMed]
- 47.Stone, J. R., and S. Yang. 2006. Hydrogen peroxide: a signaling messenger. Antioxid. Redox Signal. 8:243-270. [DOI] [PubMed] [Google Scholar]
- 48.Storz, P. 2005. Reactive oxygen species in tumor progression. Front. Biosci. 10:1881-1896. [DOI] [PubMed] [Google Scholar]
- 49.Sundaresan, M., Z. X. Yu, V. J. Ferrans, K. Irani, and T. Finkel. 1995. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270:296-299. [DOI] [PubMed] [Google Scholar]
- 50.Sundaresan, M., Z. X. Yu, V. J. Ferrans, D. J. Sulciner, J. S. Gutkind, K. Irani, P. J. Goldschmidt-Clermont, and T. Finkel. 1996. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem. J. 318(Pt. 2):379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang, H., Q. Hao, S. A. Rutherford, B. Low, and Z. J. Zhao. 2005. Inactivation of SRC family tyrosine kinases by reactive oxygen species in vivo. J. Biol. Chem. 280:23918-23925. [DOI] [PubMed] [Google Scholar]
- 52.Thannickal, V. J., and B. L. Fanburg. 2000. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L1005-L1028. [DOI] [PubMed] [Google Scholar]
- 53.Uruno, T., J. Liu, P. Zhang, Y. Fan, C. Egile, R. Li, S. C. Mueller, and X. Zhan. 2001. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 3:259-266. [DOI] [PubMed] [Google Scholar]
- 54.Ushio-Fukai, M. 2006. Localizing NADPH oxidase-derived ROS. Sci. STKE 2006:re8. [DOI] [PubMed]
- 55.Vuori, K., and E. Ruoslahti. 1995. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J. Biol. Chem. 270:22259-22262. [DOI] [PubMed] [Google Scholar]
- 56.Weaver, A. M., A. V. Karginov, A. W. Kinley, S. A. Weed, Y. Li, J. T. Parsons, and J. A. Cooper. 2001. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 11:370-374. [DOI] [PubMed] [Google Scholar]
- 57.Weed, S. A., A. V. Karginov, D. A. Schafer, A. M. Weaver, A. W. Kinley, J. A. Cooper, and J. T. Parsons. 2000. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J. Cell Biol. 151:29-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weed, S. A., and J. T. Parsons. 2001. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene 20:6418-6434. [DOI] [PubMed] [Google Scholar]
- 59.Xu, D., I. I. Rovira, and T. Finkel. 2002. Oxidants painting the cysteine chapel: redox regulation of PTPs. Dev. Cell. 2:251-252. [DOI] [PubMed] [Google Scholar]
- 60.Zaidel-Bar, R., C. Ballestrem, Z. Kam, and B. Geiger. 2003. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 116:4605-4613. [DOI] [PubMed] [Google Scholar]
- 61.Zettl, M., and M. Way. 2001. New tricks for an old dog? Nat. Cell Biol. 3:E74-E75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.