Abstract
Two types of xylanases (1,4-beta-D-xylan xylanohydrolase, EC 3.2.1.8) were isolated from the culture filtrate of a thermophilic actinomycete, Streptomyces thermoviolaceus OPC-520. The enzymes (STX-I and STX-II) were purified by chromatography with DEAE-Toyopearl 650 M, CM-Toyopearl 650 M, Sephadex G-75, Phenyl-Toyopearl 650 M, and Mono Q HR. The purified enzymes showed single bands on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The molecular weights of STX-I and STX-II were 54,000 and 33,000, respectively. The pIs were 4.2 (STX-I) and 8.0 (STX-II). The optimum pH levels for the activity of STX-I and STX-II were pH 7.0. The optimum temperature for the activity of STX-I was 70 degrees C, and that for the activity of STX-II was 60 degrees C. The enzymes were completely inhibited by N-bromosuccinimide. The enzymes degraded xylan, producing xylose and xylobiose as the predominant products, indicating that they were endoxylanases. STX-I showed high sequence homology with the exoglucanase from Cellulomonas fimi (47% homology), and STX-II showed high sequence homology with the xylanase from Bacillus pumilus (46% homology).
Full text
PDF
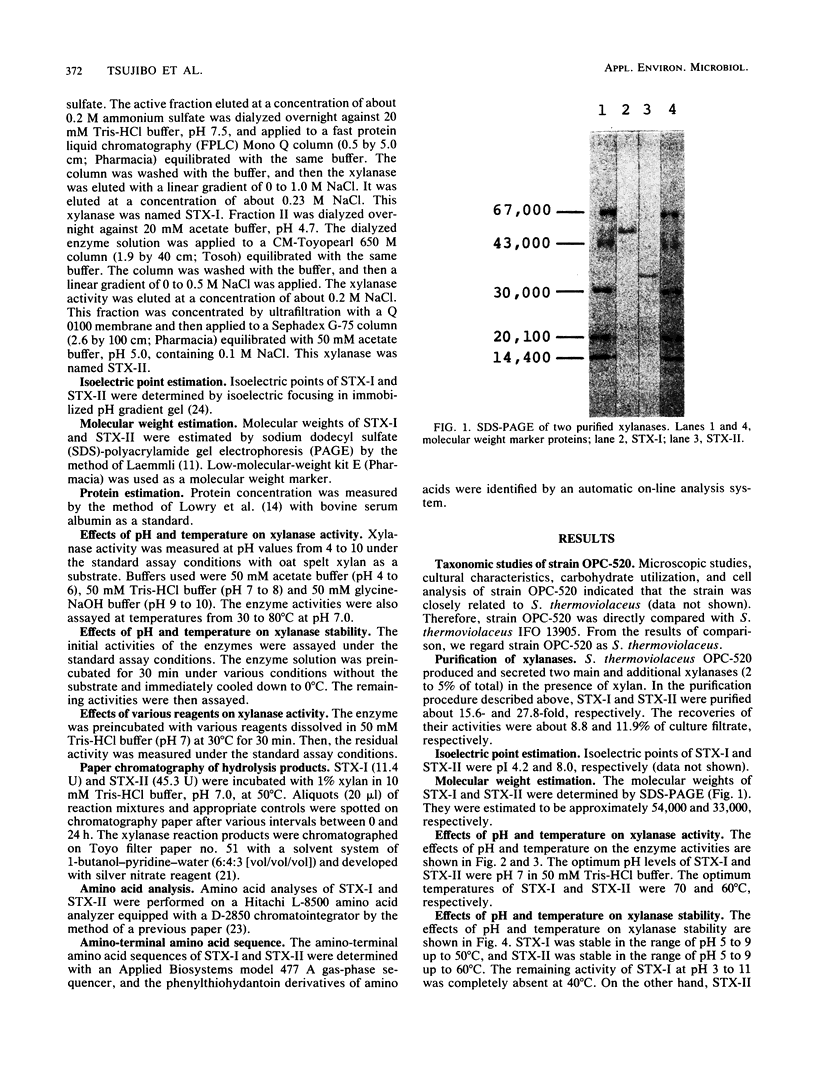
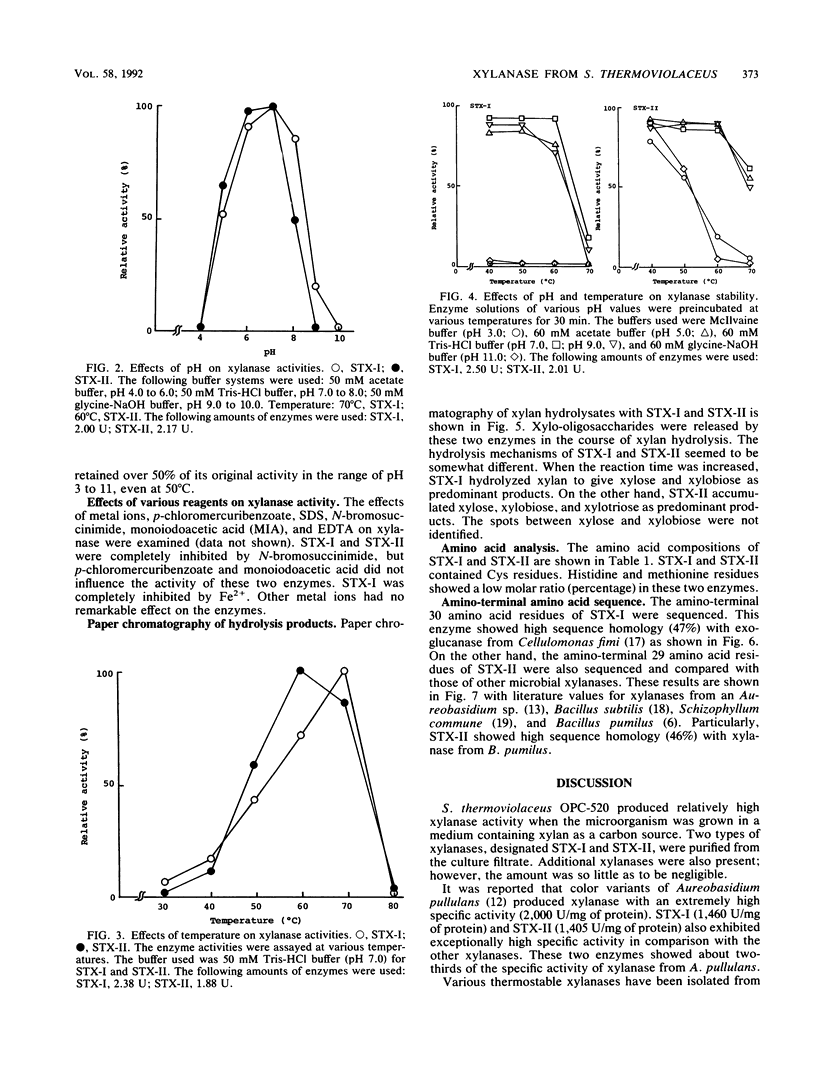
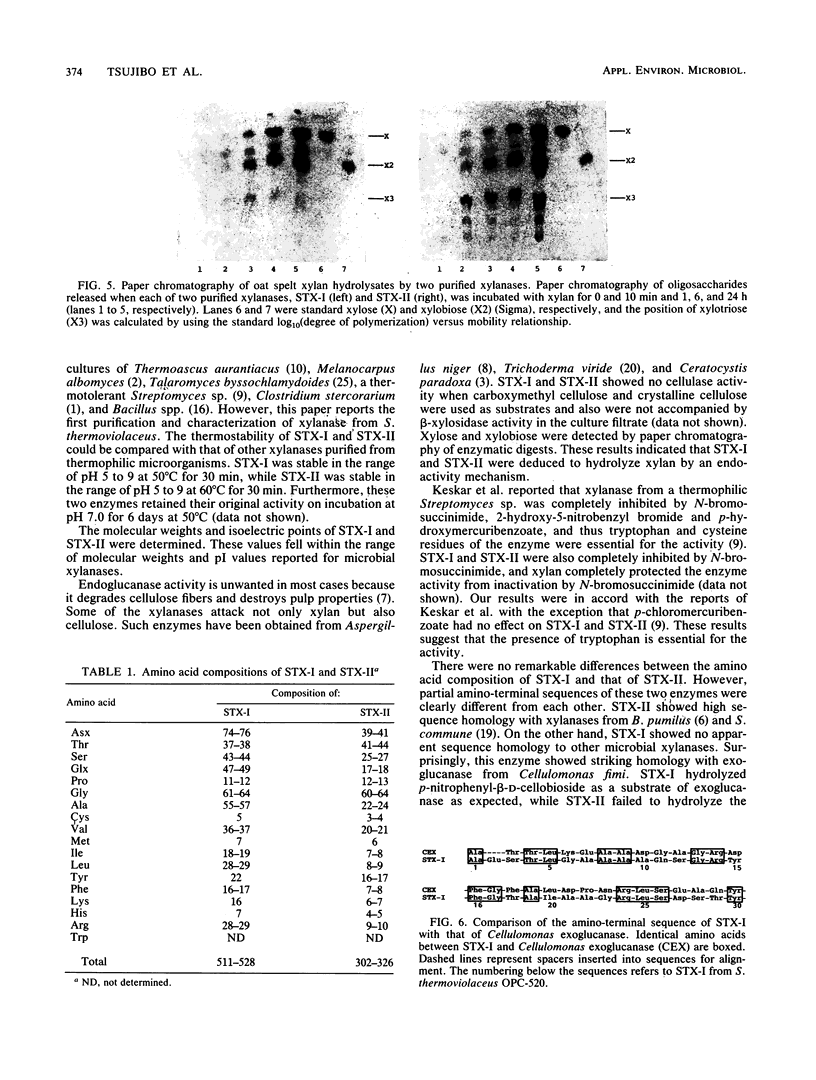

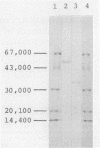
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dekker R. F., Richards G. N. Structures of the oligosaccharides from the enzymic hydrolysis of hemicellulose by a hemicellulase of Ceratocystis paradoxa. Carbohydr Res. 1975 Sep;43(2):335–344. doi: 10.1016/s0008-6215(00)83497-7. [DOI] [PubMed] [Google Scholar]
- Fall R., Phelps P., Spindler D. Bioconversion of xylan to triglycerides by oil-rich yeasts. Appl Environ Microbiol. 1984 May;47(5):1130–1134. doi: 10.1128/aem.47.5.1130-1134.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabski A. C., Jeffries T. W. Production, Purification, and Characterization of beta-(1-4)-Endoxylanase of Streptomyces roseiscleroticus. Appl Environ Microbiol. 1991 Apr;57(4):987–992. doi: 10.1128/aem.57.4.987-992.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John M., Schmidt B., Schmidt J. Purification and some properties of five endo-1,4-beta-D-xylanases and a beta-D-xylosidase produced by a strain of Aspergillus niger. Can J Biochem. 1979 Feb;57(2):125–134. doi: 10.1139/o79-016. [DOI] [PubMed] [Google Scholar]
- Keskar S. S., Srinivasan M. C., Deshpande V. V. Chemical modification of a xylanase from a thermotolerant Streptomyces. Evidence for essential tryptophan and cysteine residues at the active site. Biochem J. 1989 Jul 1;261(1):49–55. doi: 10.1042/bj2610049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandke K. M., Vithayathil P. J., Murthy S. K. Purification of xylanase, beta-glucosidase, endocellulase, and exocellulase from a thermophilic fungus, Thermoascus aurantiacus. Arch Biochem Biophys. 1989 Nov 1;274(2):491–500. doi: 10.1016/0003-9861(89)90462-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leathers T. D. Color Variants of Aureobasidium pullulans Overproduce Xylanase with Extremely High Specific Activity. Appl Environ Microbiol. 1986 Nov;52(5):1026–1030. doi: 10.1128/aem.52.5.1026-1030.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill G., Goh S. H., Warren R. A., Kilburn D. G., Miller R. C., Jr Structure of the gene encoding the exoglucanase of Cellulomonas fimi. Gene. 1986;44(2-3):325–330. doi: 10.1016/0378-1119(86)90197-6. [DOI] [PubMed] [Google Scholar]
- Paice M. G., Jurasek L., Carpenter M. R., Smillie L. B. Production, characterization, and partial amino acid sequence of xylanase A from Schizophyllum commune. Appl Environ Microbiol. 1978 Dec;36(6):802–808. doi: 10.1128/aem.36.6.802-808.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tsujibo H., Miyamoto K., Hasegawa T., Inamori Y. Amino acid compositions and partial sequences of two types of alkaline serine proteases from Nocardiopsis dassonvillei subsp. prasina OPC-210. Agric Biol Chem. 1990 Aug;54(8):2177–2179. [PubMed] [Google Scholar]
- Tsujibo H., Miyamoto K., Hasegawa T., Inamori Y. Purification and characterization of two types of alkaline serine proteases produced by an alkalophilic actinomycete. J Appl Bacteriol. 1990 Oct;69(4):520–529. doi: 10.1111/j.1365-2672.1990.tb01544.x. [DOI] [PubMed] [Google Scholar]