Abstract
Background and purpose:
Clinical indications for erythropoietin (EPO) in the vascular system reach far beyond the treatment of anemia, but the development of EPO as a non-toxic agent rests heavily upon the cellular pathways controlled by EPO that require elucidation.
Experimental approach:
We modulated gene activity and examined cellular trafficking of critical pathways during oxidative stress that may work in concert with EPO to protect primary cerebral endothelial cells (ECs) during oxidative stress, namely protein kinase B (Akt1), 14-3-3 protein, the Forkhead transcription factor FOXO3a.
Key results:
Here, we show that preservation of ECs by EPO during oxygen-glucose deprivation (OGD) required the initial activation of the phosphatidylinositol 3-kinase (PI-3K) pathway through Akt1, since specific pharmacological blockade of Akt1 activity or gene silencing of Akt1 prevented EC protection by EPO. EPO subsequently involved a series of anti-apoptotic pathways to activate STAT3, STAT5, and ERK 1/2. Furthermore, EPO maintained the inhibitory phosphorylation and integrity of the ‘pro-apoptotic' transcription factor FOXO3a, promoted the binding of FOXO3a to 14-3-3 protein and regulated the intracellular trafficking of FOXO3a. Additionally, gene silencing of FOXO3a during OGD significantly increased EC survival, but did not synergistically improve cytoprotection by EPO, illustrating that EPO relied upon the blockade of the FOXO3a pathway.
Conclusions and implications:
Our work defines a novel cytoprotective pathway in ECs that involves PI-3 K, STAT3, STAT5, ERK 1/2, 14-3-3 protein and FOXO3a, which can be targeted for the development of EPO as a clinically effective and safe agent in the vascular system.
Keywords: apoptosis; endothelial cells; ERK; erythropoietin; Forkhead; FOXO3a; oxidative stress; phosphatidylinositol 3-kinase; 14-3-3 protein, STAT
Introduction
Erythropoietin (EPO), a 30.4-kDa glycoprotein, is currently approved for the treatment of anemia, but the potential roles for EPO have expanded far beyond the initial concept that EPO is required only for erythropoiesis. Early clinical studies in patients with anemia have shown that EPO administration can increase left ventricular ejection fraction and stroke volume (Goldberg et al., 1992). More recently, this work has been supported by randomized control studies with EPO administration in patients with congestive heart failure or diabetes combined with congestive heart failure that demonstrate improved cardiac output and a decrease in medical resource utilization (Silverberg et al., 2003).
At the cellular level, EPO plays a critical role in the vascular system through the maintenance of endothelial cell (EC) function (Chong et al., 2002, 2003a) and the induction of angiogenesis (Sakamaki, 2004; George et al., 2005; Maiese et al., 2005). Furthermore, by assuring EC integrity, EPO prevents ischemic cardiac death by reducing myocardial injury (Bullard et al., 2005), modulating cardiac remodeling (Miki et al., 2006) and reducing ventricular dysfunction (Parsa et al., 2003). Coupled to its ability to avert inflammation (Avasarala and Konduru 2005) and modulate phagocytic cell activity (Chong et al., 2003b, 2005a), EPO provides an effective cellular environment to foster EC protection.
Successful development of EPO as a safe and nontoxic (Hardee et al., 2006) therapeutic entity in the vascular system rests on the intricate pathways that drive cytoprotection by EPO. In particular, regulatory pathways that can become essential for cell survival, such as the phosphatidylinositol 3-kinase (PI 3-K) pathway through protein kinase B (Akt1) (Hardee et al., 2006), the signal transducer and activator of transcription (STAT) pathways involving STAT3 and STAT5 (Maiese et al., 2005) and the mitogen-activated protein kinases extracellular signal-related kinases 1/2 (ERK 1/2) may be critical for EPO cytoprotection. Additionally, the novel Akt1 substrate FOXO3a, a member of the mammalian Forkhead transcription factor family that regulates processes ranging from cell longevity to cell apoptosis (Chong et al., 2004b, 2005b; Wijchers et al., 2006), also plays a significant role in promoting EC survival during oxidative stress. We demonstrate here that vascular protection by EPO was limited to particular concentrations and to particular times – a ‘therapeutic window.' This protective effect was mediated by a novel sequential regulation of the PI 3-K pathway through Akt1, the activation of STAT3, STAT5, and ERK 1/2 and the subcellular trafficking of FOXO3a with its association with 14-3-3 protein, to block oxidative stress-induced apoptosis in ECs.
Methods
Cerebral microvascular EC cultures
Following our earlier protocols, vascular ECs were isolated from the cerebrum of adult male Sprague–Dawley rats by a modified collagenase/dispase-based digestion protocol (Lin and Maiese, 2001; Chong et al., 2002, 2003a, 2004a). The ECs were cultured in endothelial growth media consisting of M199E with 20% heat-inactivated fetal bovine serum, 2 mmol l−1 L-glutamine, 90 μg ml−1 heparin and 20 μg ml−1 EC growth supplement. Cells from the third passage were identified by positive direct immunocytochemistry for factor VIII-related antigen (Lin and Maiese 2001; Chong et al., 2002, 2003a, 2004a) and possessed characteristic spindle-shaped morphology with antigenic properties shown to resemble brain endothelium in vivo (Abbott et al., 1992).
Experimental treatments
Oxygen-glucose deprivation (OGD) in ECs was performed by replacing the media with glucose-free Hank's balanced salt solution containing 116 mmol l−1 NaCl, 5.4 mmol l−1. KCl, 0.8 mmol l−1 MgSO4, 1 mmol l−1 NaH2PO4, 0.9 mmol l−1 CaCl2, and 10 mg l−1 phenol red (pH 7.4) and cultures were maintained in an anoxic environment (95% N2 and 5% CO2) at 37°C. For treatments applied 1 h before OGD, application of EPO (R&D Systems, Minneapolis, MN, USA), D-3-deoxy-2-O-methyl-myo inositol 1-(R)-2-methoxy-3-(octadecyloxy) propyl hydrogen phosphate (SH-5), or D-2,3-dieoxy-myo inositol 1-(R)-2-methoxy-3-(octadecyloxy) propyl hydrogen phosphate (SH-6) (Alexis, San Diego, CA, USA) was continuous.
Cell survival and DNA fragmentation
EC injury was determined by bright-field microscopy using a 0.4% trypan blue dye exclusion method 24 h following OGD (Chong et al., 2004b). Genomic DNA fragmentation was determined by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay (Chong et al., 2002, 2003a, 2003b) with the 3′-hydroxy ends of cut DNA labeled with biotinylated 2′-deoxyuridine 5′-triphosphate (dUTP) using the enzyme terminal deoxytransferase (Promega, Madison, WI, USA) followed by streptavidin-peroxidase and visualized with 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA, USA).
Akt1, STAT3, STAT5, ERK 1/2 and FOXO3a Phosphorylation
Cells were homogenized and each sample (50 μg lane−1) was subjected to 7.5% (Akt1, STAT3, STAT5, FOXO3a) or 12.5% (ERK 1/2) sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis. The membranes were incubated with a mouse monoclonal antibody against phospho-Akt1 (Ser473, 1:1000, Active Motif, Carlsbad, CA, USA), a mouse monoclonal antibody against total Akt1 (1: 1000), a rabbit polyclonal antibody against phospho-STAT3 (Tyr705, 1:1000), a rabbit polyclonal antibody against total STAT3 (1:1000), a rabbit polyclonal antibody against phospho-STAT5 (Tyr694), a rabbit polyclonal antibody against total STAT5 (1:1000), a rabbit polyclonal antibody against phospho-ERK 1/2 (Thr202/Tyr204, 1: 1000), a rabbit polyclonal antibody against total ERK 1/2 (1:1000; all these antibodies were from Cell Signaling, Beverly, MA, USA), a goat polyclonal antibody against total FOXO3a (1:100), or a goat polyclonal antibody against phosphorylated FOXO3a (p-FOXO3a, Ser253, 1:100; both from Santa Cruz Biotechnologies, Santa Cruz, CA, USA). After washing, the membranes were incubated with a horseradish peroxidase (HRP) conjugated secondary antibody (goat anti-mouse immunoglobulin G (IgG), 1:2000 or rabbit anti-goat IgG, 1:2000). The antibody-reactive bands were revealed by chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and band density was performed using the NIH Image program (developed at the US National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
Akt Kinase activity
As described by Chong et al. (2004a), Akt1 activity was determined by using a commercially available nonradioactive Akt1 kinase assay kit with glycogen synthase kinase-3β (GSK-3β) fusion protein (Cell Signaling Technology, Beverly, MA, USA).
Gene silencing of Akt1 and FOXO3a with small interfering RNA
ECs were plated into 35 mm dishes or 24-well plates. To silence Akt1 gene expression, commercial reagents using the SMARTpool Akt1 small interfering RNA (siRNA) kit (Upstate, Lake Placid, NY, USA) was used. To silence FOXO3a gene expression, the following sequences were synthesized (Ambion, Austin, TX, USA): the FOXO3a target sequence 5′-AAATCTAACTCATCTGCAAGT-3′, the siRNA sense strand 5′-AUCUAACUCAUCUGCAAGUUU-3′ and the antisense strand 5′-ACUUGCAGAUG AGUUAGAUUU-3′. Transfection of siRNA duplexes were performed with oligofectamine reagent according to manufacturer guidelines (Invitrogen, Carlsbad, CA, USA). Experimental assays were performed 72 h post-transfection. For each siRNA assay, negative controls contain multiple siRNAs including the target siRNA and positive controls lack the target siRNA.
FOXO3a binding to protein 14-4-3
Cell lysates were precleared and total protein (200 μg) incubated with 2 μl of antibody against protein 14-3-3 (Chemicon, Temecula, CA, USA) overnight at 4°C. The complexes were collected with protein A/G-agarose beads, centrifuged and then prepared for FOXO3a Western analysis.
Immunocytochemistry for FOXO3a
Cells were fixed for either single or double staining with 50% methanol, 50% acetone, blocked with 1.5% normal horse serum and labeled with antibody (Vector laboratories, Burlingame, CA, USA). For specific double staining of FOXO3a and TUNEL, the 3′-hydroxy ends of cut DNA were labeled with biotinylated dUTP using an enzyme terminal deoxytransferase followed by fluorescence avidin (1:50). Cells were then incubated with rabbit anti-FOXO3a (1:100, Upstate, Lake Placid, NY, USA), then with biotinylated anti-rabbit IgG (1:50) followed by Texas Red streptavidin (1:50). Cells were washed in PBS, and then stained with 4,6-diamidino-2-phenylindole (DAPI) (Sigma, St Louis, MO, USA) for nuclear identification. Fluorescence imaging used the wavelengths of 565 nm (red) and 400 nm (DAPI).
Statistical analysis
For each experiment, the mean and standard error were determined. Statistical differences between groups were assessed by means of analysis of variance (ANOVA) from six replicate experiments with the post hoc Student's t-test.
Results
OGD leads to progressive EC injury
No significant toxicity over a 24 h period was present in cultures exposed to EPO alone (0.01–1000 ng ml−1) compared to EC survival in untreated control cultures (98±4%) (data not shown). As shown in Figure 1a, EC survival at 24 h was progressively reduced following OGD application for 6, 8 or 12 h. As an OGD exposure period of 8 h resulted in survival rate of approximately 35% (65% EC loss), this duration of exposure to OGD was used for the remainder of the experiments.
Figure 1.
EPO has a window of concentration and temporal administration for therapeutic effects during OGD. (a) OGD leads to progressive EC injury with increased exposure time (*P< 0.01 vs untreated control). (b) Representative images of ECs pretreated with EPO (10 ng ml−1). (c) Quantitation of EC survival with EPO (0.01–1000 ng ml−1) 1 h before exposure to OGD. Cell survival was assessed 24 h later (*P< 0.01 vs OGD). (d) ECs were treated with EPO (10 ng ml−1) at 2, 4, 6 and 12 h post-OGD exposure and cell survival was assessed 24 h later (*P< 0.01 vs OGD). (e and f) ECs were pretreated with EPO (10 ng ml−1) 1 h before exposure to OGD and DNA fragmentation was determined 24 h later using the TUNEL assay; representative images in (e) and quantitation of results in (f) (*P< 0.01 vs OGD). In all cases, control = untreated ECs.
EPO pre- and posttreatment prevents EC injury during OGD
Application of EPO (10 ng ml−1) 1 h before OGD, significantly reduced trypan blue uptake in ECs, illustrating preservation of intact membrane function and increased survival by EPO during OGD (Figure 1b). This concentration of EPO (10 ng ml−1) provided the maximal EC survival. Concentrations lower than 0.1 ng ml−1 or higher than 50 ng ml−1 did not improve EC survival during OGD (Figure 1c).
In posttreatment experiments (Figure 1d), ECs were treated with EPO (10 ng ml−1) at 2, 4, 6 and 12 h following OGD with cell survival determined at 24 h after OGD. EPO applied at 2 and 4 h following OGD exposure significantly increased EC survival but posttreatment with EPO at 6 and 12 h following OGD did not increase EC survival.
EPO blocks DNA fragmentation in ECs in the presence of OGD
Twenty-four hours following OGD exposure, apoptosis with chromatin condensation and nuclear fragmentation in ECs was present (Figure 1e). In contrast, ECs pretreated with EPO (10 ng ml−1) 1 h before OGD were without nuclear fragmentation. Quantitiative assays showed that OGD lead to a significant increase in DNA fragmentation, compared with untreated control cultures, but that EPO pretreatment significantly reduced this increased DNA fragmentation (Figure 1f).
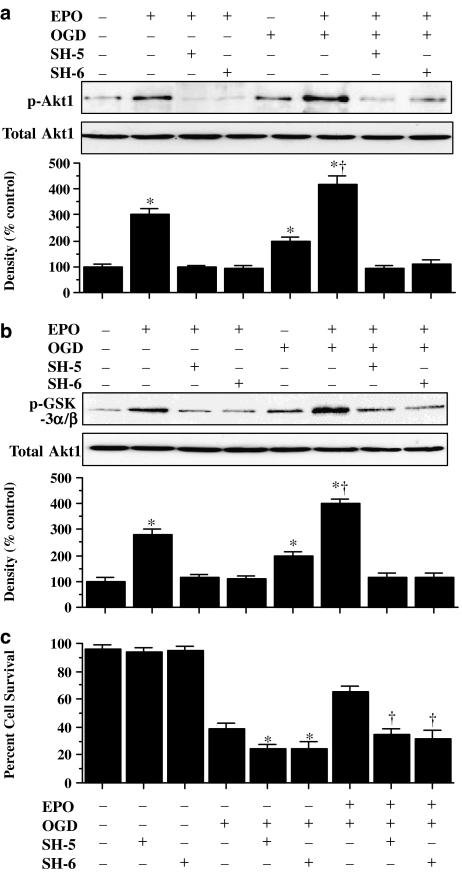
Protection of ECs by EPO during OGD requires Akt1 activity
Western blot assay was performed for phosphorylated Akt1 (p-Akt1) (activated form of Akt1) (Figure 2a) and direct assessment of Akt1 activity was performed through its specific GSK-3α/β substrate measured through the expression of phosphorylated (p)-GSK-3α/β (Figure 2b) 6 h following OGD. In Figure 2a and b, both OGD and EPO (10 ng ml−1) independently increased the expression of p-Akt1 or the activity of the p-GSK-3α/β substrate, but EPO, either alone or in the presence of OGD, elevated p-Akt1 expression and p-GSK-3α/β to a greater degree than application of OGD alone. This increased expression of p-Akt1 or p-GSK-3α/β activity was blocked by the specific Akt1 inhibitors SH-5 (20 μM) or SH-6 (20 μM) (Kozikowski et al., 2003).
Figure 2.
Cytoprotection by EPO requires the phosphorylation and activation of Akt1. (a and b) EC protein extracts (50 μg lane−1) were immunoblotted with antiphosphorylated-Akt1 (p-Akt1) (a) or with antiphosphorylated-GSK-3α/β (p-GSK-3α/β) (b) to assess Akt1 activity. Exposure to EPO (10 ng ml−1) or OGD significantly increased p-Akt1 and p-GSK-3α/β expression. Application of the specific Akt1 inhibitors SH-5 (20 μmol l−1) or SH-6 (20 μmol l−1) were sufficient to block the expression of p-Akt1 and p-GSK-3α/β in the presence of EPO during OGD (*P<0.01 vs control; †P<0.01 vs OGD). (c) At concentrations that block phosphorylation and activation of Akt1 during OGD, SH-5 (20 μM) or SH-6 (20 μM) applied 1 h before OGD significantly reduced the cytoprotective capacity of EPO (10 ng ml−1) (*P<0.01 vs OGD alone; †P<0.01 vs EPO with OGD). In all cases, control = untreated ECs.
In Figure 2c, application of EPO (10 ng ml−1) 1 h before OGD exposure significantly increased EC survival. Coapplication of SH-5 (20 μM) or SH-6 (20 μM), concentrations that were shown to block activation of Akt1 through phosphorylation (Figure 2a) or through p-GSK-3α/β substrate analysis (Figure 2b) significantly reduced the ability of EPO to protect ECs against OGD, suggesting that EPO required Akt1 activation to offer cytoprotection. When administered in the absence of OGD, SH-5 (20 μM) or SH-6 (20 μM) were not toxic to ECs, but did enhance injury during OGD, suggesting that endogenous Akt1 activation provides a small level of protection during toxic insults (Figure 2c).
Gene silencing of Akt1 eliminates the capacity of EPO to prevent apoptosis
Although pharmacological inhibitors can have their limitations in manipulating and assessing a protein's level of activity, we have used siRNA gene silencing of Akt1 to knockdown Akt1 activity specifically. ECs were transfected with Akt1 siRNA and the expression of total Akt1 and p-Akt1 was assessed through Western blot analysis (Figure 3a and b) as well as through p-GSK-3α/β substrate analysis as a measure of Akt1 activity (Figure 3c). Figure 3a illustrates that total Akt1 is expressed in untreated control ECs, but application of a negative control that contains multiple siRNAs including Akt1 or of the specific siRNA for Akt1 significantly reduced Akt1 expression. In addition, gene silencing of Akt1 during administration of EPO (10 ng ml−1) or during EPO (10 ng ml−1) with OGD exposure prevents phosphorylation of Akt1 (Figure 3b) and p-GSK-3α/β (Figure 3c). As shown in Figure 3d, OGD increased trypan blue staining and TUNEL labeling during OGD exposure. Transfection with siRNA for Akt1 was not toxic to ECs. As expected, EPO (10 ng ml−1) prevented cell injury assessed by trypan blue staining and cell apoptosis assessed by TUNEL labeling (Figure 3d), but this protection was lost with gene silencing of Akt1 (Figure 3d), illustrating that activation of Akt1 is essential for EPO to prevent EC injury and genomic DNA degradation.
Figure 3.
Gene silencing of Akt1 abolishes cytoprotection by EPO. (a–c) EC protein extracts (50 μg lane−1) were immunoblotted with antitotal Akt1 (a), antiphosphorylated-Akt1 (p-Akt1) (b) or antiphosphorylated-GSK-3α/β (p-GSK-3α/β) (c) to measure Akt1 activity. Exposure to EPO (10 ng ml−1) or OGD significantly increased p-Akt1 and p-GSK-3α/β expression. Transfection with Akt1 siRNA significantly reduced expression of total Akt1 (a), p-Akt1 during EPO (10 ng ml−1) (b) and p-GSK-3α/β during EPO (10 ng ml−1), OGD alone, or combined EPO (10 ng ml−1) with OGD (c) (*P<0.01 vs control untreated ECs; †P<0.01 vs corresponding band without siRNA). For (a), negative control with multiple siRNAs prevented total Akt1 expression, but a positive control lacking specific Akt1 siRNA did not alter total Akt1 expression. (d) Gene silencing with Akt1 siRNA significantly prevented EPO (10 ng ml−1) from blocking EC membrane injury assessed by trypan blue staining and genomic DNA degradation assessed by TUNEL (*P<0.01 vs OGD alone; †P<0.01 vs EPO/OGD without siRNA). Akt1 siRNA alone was not toxic.
EPO activates STAT3, STAT5 and ERK 1/2 in ECs during OGD
In Figure 4a–c, Western blot assay was performed for phosphorylated STAT3 (p-STAT3), phosphorylated STAT5 (p-STAT5) and phosphorylated ERK 1/2 (activated forms of STAT3, STAT5 and ERK 1/2) 6 h following OGD. EPO (10 ng ml−1) given alone to ECs increased expression of p-STAT3 and p-STAT5 (Figure 4a and b). EPO (10 ng ml−1) in the presence of OGD elevated p-STAT3 and p-STAT5 expression, more than application of OGD alone (Figure 4a and b). In a similar manner, EPO (10 ng ml−1) in ECs alone or during OGD increased p-ERK 1/2 expression to a larger degree than OGD alone (Figure 4c).
Figure 4.
In the presence of OGD, EPO increases the activity and phosphorylation of STAT3, STAT5 and ERK 1/2 in ECs. (a–c) EC protein extracts (50 μg lane−1) were immunoblotted with antiphosphorylated-STAT3 (p-STAT3) (a), antiphosphorylated-STAT5 (p-STAT5) (b) or antiphosphorylated-ERK 1/2 (p-ERK 1/2) (c) to assess STAT3, STAT5 and ERK 1/2 activities 6 h following OGD. Exposure to EPO (10 ng ml−1) either alone or during OGD significantly increased p-STAT3, p-STAT5 and p-ERK 1/2 expression (*P<0.01 vs control). In all cases, control = untreated ECs.
EPO maintains the phosphorylation and integrity of p-FOXO3a during OGD through Akt1
Western blot assay was performed for phosphorylated FOXO3a at Ser253 (p-FOXO3a), the preferential phosphorylation site for Akt, as well as for the expression of total FOXO3a at 6 and 12 h following OGD (Figure 5a and b). OGD increased the expression of p-FOXO3a and total FOXO3a over a 6 h period when compared with control cultures (Figure 5a and b). At 12 h post-OGD exposure, expression of p-FOXO3a and total FOXO3a was much lower than it had been at 6 h and approached control levels (Figure 5a and b). In contrast, EPO (10 ng ml−1) alone or in combination with OGD exposure maintained the expression of p-FOXO3a and total FOXO3a over a 12 h course, suggesting that EPO can prevent the degradation of p-FOXO3a (Figure 5a and b). The capacity of EPO to phosphorylate FOXO3a and potentially maintain the integrity of FOXO3a is determined by Akt1, as application of the specific Akt1 inhibitors SH-5 (20 μM) or SH-6 (20 μM) blocked the phosphorylation of FOXO3a by EPO either when administered alone or in conjunction with OGD exposure (Figure 5c).
Figure 5.
EPO maintains the inhibitory phosphorylation of FOXO3a during OGD. (a and b) EC protein extracts (50 μg lane−1) were immunoblotted with antiphosphorylated-FOXO3a (p-FOXO3a, Ser253) (a) or antitotal FOXO3a (b) at 6 and 12 h following EPO (10 ng ml−1), OGD alone, or combined EPO (10 ng ml−1) with OGD. OGD led to the loss of p-FOXO3a (a) and the loss of total FOXO3a (b) at 12 h, but exposure to EPO (10 ng ml−1) maintained p-FOXO3a (a) and total FOXO3a (b) at 6 and 12 h following OGD (*P<0.01 vs OGD at 6 h). (c) At concentrations that block phosphorylation and activation of Akt1 during OGD, SH-5 (20 μM) or SH-6 (20 μM) applied 1 h before EPO (10 ng ml−1) or EPO (10 ng ml−1) combined with OGD significantly prevented the capacity of EPO to maintain the phosphorylation of p-FOXO3a at 6 h following OGD (*P<0.01 vs OGD alone; †P<0.01 vs EPO with OGD). In all cases, control = untreated ECs.
EPO relies upon Akt1 for the binding of FOXO3a to 14-3-3 protein in ECs during OGD
Akt 1 phosphorylation of FOXO3a leads to the association of FOXO3a with 14-3-3 protein and retention of FOXO3a in the cytoplasm (Chong et al., 2005b), rendering it ineffective to initiate apoptosis. We therefore assessed whether EPO altered the binding of FOXO3a to 14-3-3 protein during OGD by immunoprecipitation. EPO (10 ng ml−1) either alone or in conjunction with OGD exposure increased significantly the expression of FOXO3a in the lysate that was immunoprecipitated by anti-14-3-3 protein antibody (Figure 6a), suggesting that EPO increases the binding of FOXO3a to 14-3-3 protein. In contrast, binding of FOXO3a to 14-3-3 protein during EPO administration with or without OGD requires the presence of Akt1, as FOXO3a binding to 14-3-3 protein was abolished during gene silencing of Akt1 (Figure 6a).
Figure 6.
Through Akt1, EPO uses 14-3-3 protein to bind to FOXO3a and sequester FOXO3a in the cytoplasm during OGD. (a) EC protein extracts were immunoprecipitated 6 h after EPO or OGD exposure with anti-14-3-3 antibody and immunoblotted with FOXO3a. Lysates were from wild type and Akt1 siRNA transfected cells with EPO (10 ng ml−1) or combined EPO (10 ng ml−1) with OGD. Transfection with Akt1 siRNA significantly reduced expression of the FOXO3a/14-3-3 complex during EPO alone or during EPO combined with OGD. (b) EPO (10 ng ml−1) or combined EPO (10 ng ml−1) with OGD was followed at 6 h with immunofluorescent staining for FOXO3a (Texas-red). Nuclei of ECs were counterstained with DAPI. In merged images, cells with EPO alone or combined EPO and OGD with white arrows show EC nuclei with minimal FOXO3a staining (blue/white) and green arrows show EC cytoplasm with significant FOXO3a staining (red) in contrast to cells with OGD alone or Akt1 siRNA transfection with combined EPO and OGD with minimal FOXO3a staining (gray), demonstrating the inability of EPO to sequester FOXO3a in the cytoplasm during Akt1 gene silencing. (c) EPO prevents FOXO3a translocation to the nucleus during OGD, but this ability of EPO is lost during gene silencing of Akt1 (*P<0.01 vs OGD alone or Akt1 siRNA). Control = untreated ECs.
EPO requires Akt1 to sequester FOXO3a in the cytoplasm of ECs during OGD
Immunofluorescent staining for FOXO3a and DAPI nuclear staining were used to follow the subcellular translocation of FOXO3a in ECs during EPO and OGD exposure (Figures 6b and c). During OGD exposure alone, there was significant immunofluorescent staining for FOXO3a in the nucleus of ECs with minimal cytoplasmic staining. This was shown by the inability to detect significant DAPI nuclear staining in cells during merged OGD images since prominent FOXO3a staining was present in the nucleus (Figure 6b and c). In contrast, with administration of EPO (10 ng ml−1) with or without OGD exposure, FOXO3a was retained in the cytoplasm with minimal nuclear staining as shown with the clear presence of DAPI nuclear staining in merged images, illustrating that EPO retains FOXO3a in the cytoplasm at levels similar to or below control levels. However, the ability to sequester FOXO3a in the cytoplasm by EPO was lost on transfection with siRNA Akt1 (Figure 6b and c), further supporting the premise that EPO requires Akt1 to prevent FOXO3a translocation from the cytoplasm to the nucleus.
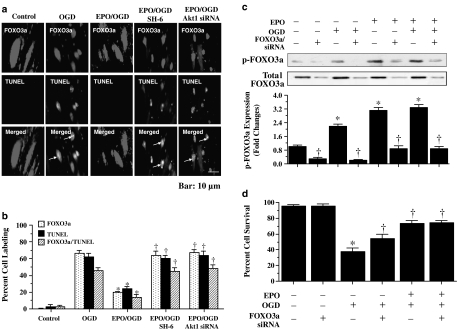
FOXO3a nuclear translocation correlates directly with nuclear DNA degradation in ECs during OGD
To examine the relationship between FOXO3a nuclear translocation and the induction of apoptosis, we assessed colocalization of FOXO3a and TUNEL with immunofluorescent double staining. Following OGD exposure, merged images confirmed nuclear staining for both FOXO3a and TUNEL, indicative of active proapoptotic transcriptional activity for FOXO3a leading to nuclear DNA degradation (Figure 7a and b). In contrast, EPO (10 ng ml−1) administered 1 h before OGD significantly reduced the number of ECs that stained positive for FOXO3a and TUNEL in the same cells (Figure 7a and b). The colocalization of FOXO3a with TUNEL in the same ECs dramatically increased, comparable to OGD exposure alone, during application of the Akt1 inhibitor SH-6 (20 μM) or during gene silencing of Akt1 (Figure 7a and b), illustrating the essential role of Akt1 in blocking the proapoptotic properties of FOXO3a.
Figure 7.
EPO modulates intracellular trafficking of FOXO3a to block nuclear DNA degradation and EPO cytoprotection parallels EC survival levels during FOXO3a gene silencing. (a) Immunofluorescent double staining for FOXO3a and TUNEL was performed at 6 h after OGD. EPO (10 ng ml−1) during OGD prevents nuclear DNA degradation and FOXO3a nuclear translocation in the same ECs with no overlap of staining in merged images. In contrast, white arrows in merged images show both nuclear FOXO3a and TUNEL staining (yellow) in ECs with OGD alone, with combined EPO/OGD and inhibition of Akt1 activity (SH-6, 20 μM), or with combined EPO/OGD and Akt1 siRNA gene silencing, illustrating that EPO requires Akt1 to prevent FOXO3a nuclear translocation that leads to apoptotic DNA degradation. (b) EPO (10 ng ml−1) during OGD prevented FOXO3a and TUNEL nuclear staining in the same ECs, but this ability of EPO is lost during combined EPO/OGD with inhibition of Akt1 activity (SH-6, 20 μM) or with combined EPO/OGD and Akt1 siRNA (*P<0.01 vs OGD; †P<0.01 vs EPO/OGD). (c) EC protein extracts (50 μg lane−1) were immunoblotted with antiphosphorylated-FOXO3a (p-FOXO3a, Ser253) at 6 h following with EPO (10 ng ml−1), OGD alone or combined EPO (10 ng ml−1) with OGD in lysates from wild type and FOXO3a siRNA transfected cells. Transfection with FOXO3a siRNA significantly reduced expression of p-FOXO3a and total FOXO3a during EPO alone, OGD alone and combined EPO with OGD (*P<0.01 vs untreated control; †P<0.01 vs corresponding band without siRNA). (d) Gene silencing with FOXO3a siRNA significantly increased survival during OGD, but lead to similar survival level during combined EPO with OGD without a synergistic increase, suggesting that EPO required the prevention of FOXO3a activity for cytoprotection (*P<0.01 vs untreated control; †P<0.01 vs OGD alone).
Gene silencing of FOXO3a improves survival during OGD and parallels protection with EPO administration
ECs were transfected with FOXO3a siRNA and the expression of p-FOXO3a was observed through Western blot analysis (Figure 7c). Gene silencing of FOXO3a during OGD exposure alone or during administration of EPO (10 ng ml−1) with or without OGD significantly reduced or eliminated the expression of p-FOXO3a and total FOXO3a (Figure 7c). Following transfection of ECs with FOXO3a siRNA and exposure to OGD, EC survival was increased relative to that during OGD alone (Figure 7d), demonstrating that elimination of FOXO3a can enhance EC survival during OGD exposure. Interestingly, EPO (10 ng ml−1) treatment with OGD in ECs transfected with FOXO3a siRNA yielded a similar survival to EPO (10 ng ml−1) with OGD in wild-type cells, without a synergistic increase, suggesting that EPO relied upon the prevention of FOXO3a activity to exert cytoprotection (Figure 7d).
Discussion and conclusions
EPO has been shown to modulate several vital functions in the vascular system that include progenitor stem cell maturation, angiogenesis and cellular protection, but it is the elucidation of the cytoprotective pathways that govern the properties of EPO, which will be critical for the development of effective and safe strategies for a number of disease entities (Sakamaki, 2004; Maiese et al., 2005). We have shown that administration of EPO in a concentration range from 1 to 50 ng ml−1 with either 1 h pretreatment or within 4 h posttreatment protected significantly ECs against cellular membrane injury and apoptotic genomic DNA degradation during OGD. Although experimental injury models are limited in their ability to replicate clinical pathology and the injury model of OGD is no exception to this rule, cytoprotection by EPO is a robust finding and has been already demonstrated in a number of injury paradigms that involve hypoxic ischemia (Chong et al., 2002, 2003b; Yu et al., 2005; Liu et al., 2006; Meloni et al., 2006; Wei et al., 2006), excitotoxicity (Yamasaki et al., 2005), infection (Kaiser et al., 2006), free radical exposure (Chong et al., 2003a, 2003c; Yamasaki et al., 2005), neurodegeneration (McLeod et al., 2006; Pregi et al., 2006) and amyloid toxicity (Chong et al., 2005a). Cytoprotection by EPO also extends to neuronal cells to afford protection of the neurovascular unit during both experimental and clinical cerebral vascular disease (Dzietko et al., 2004; Maiese et al., 2004; Demers et al., 2005; Wei et al., 2006).
EPO is unusual in its ability to block both early and late phases of apoptotic cell injury especially in the vascular system that requires modulation of subsequent inflammatory and thrombotic injury (Parsa et al., 2003; Chong et al., 2003b, 2005a). The treatment parameters that we observed for EPO were similar to those for other in vitro cell culture models (Chong et al., 2002; van der Meer et al., 2004; Wright et al., 2004; Bullard et al., 2005) and in vivo animal or clinical studies (Bullard et al., 2005; George et al., 2005; Namiuchi et al., 2005; Spandou et al., 2006). Our cytoprotective concentration range for EPO beginning with 1 ng ml−1 parallels endogenous EPO serum levels in patients with cardiac or renal disease that have been associated with potential EPO cellular protection (Mason-Garcia et al., 1990; Namiuchi et al., 2005). It is also of note that serum EPO levels in healthy individuals are approximately 0.1 ng ml−1, but treatment with EPO can significantly elevate plasma EPO levels well above the 1 ng ml−1 range, similar to experimental in vitro work, and confer beneficial results (Sohmiya et al., 1998; Bierer et al., 2006).
As in other studies that have assessed EPO cytoprotection (Chong et al., 2003a; Nishihara et al., 2006; Spandou et al., 2006; Li et al., 2006c), we show that EPO exhibits a narrow window of concentration and time for application to achieve therapeutic effects . This ‘therapeutic window' for EPO may be determined by several mechanisms. For example, EPO administration can induce formation of anti-EPO antibodies that block further biological function of EPO at any concentration level (Casadevall et al., 2002). In addition, activation of the EPO receptor by EPO can affect proteasomal and lysosomal activity that degrades and internalizes the EPO receptor, thus downregulating EPO signal transduction pathways (Walrafen et al., 2005).
The PI 3-K pathway through Akt1 pathway plays a prominent role for cellular protection in a number of disorders that can involve ischemia, hypoxia and free radical-induced oxidative stress (Chong et al., 2002, 2003b; Bahlmann et al., 2004; Miki et al., 2006; Li et al., 2006b) and without exception, EPO cytoprotective capacity appears to rely directly upon Akt1. Exogenous EPO administration can enhance Akt activation in endothelial progenitor cells (Urao et al., 2006) as well as in differentiated ECs (Bahlmann et al., 2004). In ECs, modulation of Akt1 is critical not only for increased cell survival by EPO, but also for EPO to modulate downstream substrates of Akt1. We show that endogenous activation of Akt1 provides a minimum level of protection during OGD, as application of the specific Akt1 inhibitors SH-5 or SH-6 increased EC injury. However, EPO by itself, increased the phosphorylation and the activation of Akt1 to a significantly greater degree than OGD alone, suggesting that EPO employs Akt1 activation to protect cells against oxidative stress. Loss of EC protection with EPO during the application of specific Akt1 inhibitors or the gene silencing of Akt1 further confirmed the premise that Akt1 was essential for EPO to protect against apoptotic injury in ECs.
Interestingly, EPO can require Akt1 activation, but also work in concert with other substrates of the Akt1 pathway, such as nuclear factor-κB (Xu et al., 2005; Chong et al., 2005a; Spandou et al., 2006) and caspase 9 (Chong et al., 2003a). This knowledge led us to examine whether other cellular pathways linked to EPO were activated in ECs during OGD. In particular, EPO has been shown in other cell systems to lead not only the activation of STAT 3 (Parsa et al., 2003), STAT 5 (Um and Lodish 2006; Menon et al., 2006b) and ERK 1/2 (Bullard et al., 2005; Menon et al., 2006a), but also to rely possibly on these pathways for cell development and cell protection. We demonstrated that during OGD, EPO activated STAT3, STAT5 and ERK 1/2 as shown by the increased phosphorylation of these proteins, suggesting that EPO may require these cellular pathways to confer EC cytoprotection during oxidative stress.
We subsequently investigated another major Akt1 substrate, namely the ‘proapoptotic' transcription factor FOXO3a that also may modulate cell survival, erythroid maturation and EPO signaling (Kashii et al., 2000; Chong et al., 2005b, 2006). FOXO3a is a member of the mammalian Forkhead family that function as transcription factors by preferentially binding to the core consensus DNA sequence 5′-TTGTTTAG-3′, the Forkhead response element. In the absence of inhibitory Akt1 phosphorylation, Forkhead transcription factors are activated, translocate to the nucleus, and can control a variety of functions that involve cell-cycle progression, cell longevity and apoptosis (Lehtinen et al., 2006; Li et al., 2006a). Phosphorylation of FOXO3a by Akt1 leads to the association of FOXO3a with 14-3-3 protein. As a result of this association with 14-3-3 protein, FOXO3a is retained in the cell cytoplasm and rendered ineffective for transcriptional activity that would otherwise promote apoptotic activity (Chong et al., 2005b).
We identified four vital functions of EPO that employ Akt1 and can prevent FOXO3a from initiating apoptosis in ECs. First, we demonstrated that OGD initially increased the inhibitory phosphorylation of FOXO3a within 6 h following OGD, but over the course of 12 h, expression of phosphorylated FOXO3a was lost. In contrast, EPO, in conjunction with Akt1, maintained inhibitory phosphorylation of FOXO3a and the integrity of both phosphorylated FOXO3a and total FOXO3a over this time. Given that proteolysis of FOXO3a has been shown to occur during cellular stress, yielding amino-terminal (Nt) fragments that may further precipitate cellular injury (Charvet et al., 2003), EPO may derive its cytoprotective capacity through the posttranslational, inhibitory phosphorylation of FOXO3a, as well as through the prevention of total and phosphorylated FOXO3a degradation. Second, we showed that EPO promoted the binding of FOXO3a to 14-3-3 protein, which serves to retain FOXO3a in the cytoplasm of cells and prevent the induction of apoptosis (Chong et al., 2005b). This modulation by 14-3-3 protein also was dependent on Akt1, since FOXO3a binding to 14-3-3 protein is lost during gene silencing of Akt1. Third, EPO had the capacity to sequester FOXO3a in the cytoplasm through an Akt1-dependent pathway. This modulation of FOXO3a intracellular trafficking, retaining FOXO3a in the cytoplasm, blocked apoptotic death in ECs, as nuclear translocation of FOXO3a during OGD exposure directly correlates with apoptotic nuclear DNA degradation. Fourth, the presence of FOXO3a during OGD leads to apoptotic EC injury, but gene silencing of FOXO3a as well as the application of EPO with FOXO3a siRNA leads to similar survival levels without a synergistic increase, further illustrating that EPO relied significantly on the prevention of FOXO3a activity to promote protection in ECs during OGD.
Apoptotic injury in vascular ECs influences the course of many disorders that can involve the hematopoietic, cardiac and nervous systems of the body. New therapeutic strategies that can identify cellular pathways leading to EC apoptosis and target these mechanisms for protection of ECs are critical. Our work is the first to elucidate a unique series of functionally integrated pathways for vascular protection by EPO that employs the PI 3-K pathway of Akt1, STAT3, STAT5, ERK 1/2, 14-3-3 protein and FOXO3a. Employing agents such as EPO offers a unique investigative advantage to identify the earliest stages of EC injury during oxidative stress that can lay the foundation for the fruitful development of clinically efficacious and safe therapies for vascular disorders.
Acknowledgments
This research was supported by the following grants (KM): American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS (P30 ES06639) and NIH NINDS/NIA (NS053946).
Abbreviations
- Akt1
protein kinase B
- EC
endothelial cell
- EPO
erythropoietin
- ERK 1/2
mitogen-activated protein kinases extracellular signal-related kinases 1/2
- GSK-3β
glycogen synthase kinase-3β
- OGD
oxygen-glucose deprivation
- PI 3-K
phosphatidylinositol 3-kinase
- SH-5
D-3-deoxy-2-O-methyl-myo inositol 1-(R)-2-methoxy-3-(octadecyloxy) propyl hydrogen phosphate
- SH-6
D-2,3-dideoxy-myo inositol 1-(R)-2-methoxy-3-(octadecyloxy) propyl hydrogen phosphate
- STAT3
signal transducer and activator of transcription 3
- STAT5
signal transducer and activator of transcription 5
- TUNEL
terminal deoxynucleotidyl transferase nick end labeling
Conflict of interest
The authors state no conflict of interest.
References
- Abbott NJ, Hughes CC, Revest PA, Greenwood J. Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood–brain barrier. J Cell Sci. 1992;103:23–37. doi: 10.1242/jcs.103.1.23. [DOI] [PubMed] [Google Scholar]
- Avasarala JR, Konduru SS. Recombinant erythropoietin down-regulates IL-6 and CXCR4 genes in TNF-alpha-treated primary cultures of human microvascular endothelial cells: implications for multiple sclerosis. J Mol Neurosci. 2005;25:183–189. doi: 10.1385/JMN:25:2:183. [DOI] [PubMed] [Google Scholar]
- Bahlmann FH, Song R, Boehm SM, Mengel M, von Wasielewski R, Lindschau C, et al. Low-dose therapy with the long-acting erythropoietin analogue darbepoetin alpha persistently activates endothelial Akt and attenuates progressive organ failure. Circulation. 2004;110:1006–1012. doi: 10.1161/01.CIR.0000139335.04152.F3. [DOI] [PubMed] [Google Scholar]
- Bierer R, Peceny MC, Hartenberger CH, Ohls RK. Erythropoietin concentrations and naurodevelopmental outcome in preterm infants. Pediatrics. 2006;118:e635–e640. doi: 10.1542/peds.2005-3186. [DOI] [PubMed] [Google Scholar]
- Bullard AJ, Govewalla P, Yellon DM. Erythropoietin protects the myocardium against reperfusion injury in vitro and in vivo. Basic Res Cardiol. 2005;100:397–403. doi: 10.1007/s00395-005-0537-4. [DOI] [PubMed] [Google Scholar]
- Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian J-J, Martin-Dupont P, et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med. 2002;346:469–475. doi: 10.1056/NEJMoa011931. [DOI] [PubMed] [Google Scholar]
- Charvet C, Alberti I, Luciano F, Jacquel A, Bernard A, Auberger P, et al. Proteolytic regulation of Forkhead transcription factor FOXO3a by caspase-3-like proteases. Oncogene. 2003;22:4557–4568. doi: 10.1038/sj.onc.1206778. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, cytochrome c, and caspase-9 form the critical elements for cerebral vascular protection by erythropoietin. J Cereb Blood Flow Metab. 2003a;23:320–330. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003b;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Akt1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-x(L) and caspase 1, 3, and 9. Exp Cell Res. 2004a;296:196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005a;2:387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005b;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Group I metabotropic receptor neuroprotection requires Akt and its substrates that govern FOXO3a, Bim, and beta-catenin during oxidative stress. Curr Neurovasc Res. 2006;3:107–117. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003c;71:659–669. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004b;24:728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- Demers EJ, McPherson RJ, Juul SE. Erythropoietin protects dopaminergic neurons and improves neurobehavioral outcomes in juvenile rats after neonatal hypoxia-ischemia. Pediatr Res. 2005;58:297–301. doi: 10.1203/01.PDR.0000169971.64558.5A. [DOI] [PubMed] [Google Scholar]
- Dzietko M, Felderhoff-Mueser U, Sifringer M, Krutz B, Bittigau P, Thor F, et al. Erythropoietin protects the developing brain against N-methyl-D-aspartate receptor antagonist neurotoxicity. Neurobiol Dis. 2004;15:177–187. doi: 10.1016/j.nbd.2003.10.006. [DOI] [PubMed] [Google Scholar]
- George J, Goldstein E, Abashidze A, Wexler D, Hamed S, Shmilovich H, et al. Erythropoietin promotes endothelial progenitor cell proliferative and adhesive properties in a PI 3-kinase-dependent manner. Cardiovasc Res. 2005;68:299–306. doi: 10.1016/j.cardiores.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Goldberg N, Lundin AP, Delano B, Friedman EA, Stein RA. Changes in left ventricular size, wall thickness, and function in anemic patients treated with recombinant human erythropoietin. Am Heart J. 1992;124:424–427. doi: 10.1016/0002-8703(92)90608-x. [DOI] [PubMed] [Google Scholar]
- Hardee ME, Arcasoy MO, Blackwell KL, Kirkpatrick JP, Dewhirst MW. Erythropoietin biology in cancer. Clin Cancer Res. 2006;12:332–339. doi: 10.1158/1078-0432.CCR-05-1771. [DOI] [PubMed] [Google Scholar]
- Kaiser K, Texier A, Ferrandiz J, Buguet A, Meiller A, Latour C, et al. Recombinant human erythropoietin prevents the death of mice during cerebral malaria. J Infect Dis. 2006;193:987–995. doi: 10.1086/500844. [DOI] [PubMed] [Google Scholar]
- Kashii Y, Uchida M, Kirito K, Tanaka M, Nishijima K, Toshima M, et al. A member of Forkhead family transcription factor, FKHRL1, is one of the downstream molecules of phosphatidylinositol 3-kinase-Akt activation pathway in erythropoietin signal transduction. Blood. 2000;96:941–949. [PubMed] [Google Scholar]
- Kozikowski AP, Sun H, Brognard J, Dennis PA. Novel PI analogues selectively block activation of the pro-survival serine/threonine kinase Akt. J Am Chem Soc. 2003;125:1144–1145. doi: 10.1021/ja0285159. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Cell life versus cell longevity: the mysteries surrounding the NAD(+) precursor nicotinamide. Curr Med Chem. 2006a;13:883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res. 2006b;3:187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Takemura G, Okada H, Miyata S, Maruyama R, Li L, et al. Reduction of inflammatory cytokine expression and oxidative damage by erythropoietin in chronic heart failure. Cardiovasc Res. 2006c;71:684–694. doi: 10.1016/j.cardiores.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J Cereb Blood Flow Metab. 2001;21:262–275. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Liu R, Suzuki A, Guo Z, Mizuno Y, Urabe T. Intrinsic and extrinsic erythropoietin enhances neuroprotection against ischemia and reperfusion injury in vitro. J Neurochem. 2006;96:1101–1110. doi: 10.1111/j.1471-4159.2005.03597.x. [DOI] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled. Trends Pharmacol Sci. 2004;25:577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason-Garcia M, Beckman BS, Brookins JW, Powell JS, Lanham W, Blaisdell S, et al. Development of a new radioimmunoassay for erythropoietin using recombinant erythropoietin. Kidney Int. 1990;38:969–975. doi: 10.1038/ki.1990.299. [DOI] [PubMed] [Google Scholar]
- McLeod M, Hong M, Mukhida K, Sadi D, Ulalia R, Mendez I. Erythropoietin and GDNF enhance ventral mesencephalic fiber outgrowth and capillary proliferation following neural transplantation in a rodent model of Parkinson's disease. Eur J Neurosci. 2006;24:361–370. doi: 10.1111/j.1460-9568.2006.04919.x. [DOI] [PubMed] [Google Scholar]
- Meloni BP, Tilbrook PA, Boulos S, Arthur PG, Knuckey NW. Erythropoietin preconditioning in neuronal cultures: signaling, protection from in vitro ischemia, and proteomic analysis. J Neurosci Res. 2006;83:584–593. doi: 10.1002/jnr.20755. [DOI] [PubMed] [Google Scholar]
- Menon MP, Fang J, Wojchowski DM. Core erythropoietin receptor signals for late erythroblast development. Blood. 2006a;107:2662–2672. doi: 10.1182/blood-2005-02-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon MP, Karur V, Bogacheva O, Bogachev O, Cuetara B, Wojchowski DM. Signals for stress erythropoiesis are integrated via an erythropoietin receptor-phosphotyrosine-343-Stat5 axis. J Clin Invest. 2006b;116:683–694. doi: 10.1172/JCI25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Miura T, Yano T, Takahashi A, Sakamoto J, Tanno M, et al. Alteration in erythropoietin-induced cardioprotective signaling by postinfarct ventricular remodeling. J Pharmacol Exp Ther. 2006;317:68–75. doi: 10.1124/jpet.105.095745. [DOI] [PubMed] [Google Scholar]
- Namiuchi S, Kagaya Y, Ohta J, Shiba N, Sugi M, Oikawa M, et al. High serum erythropoietin level is associated with smaller infarct size in patients with acute myocardial infarction who undergo successful primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;45:1406–1412. doi: 10.1016/j.jacc.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Nishihara M, Miura T, Miki T, Sakamoto J, Tanno M, Kobayashi H, et al. Erythropoietin affords additional cardioprotection to preconditioned hearts by enhanced phosphorylation of glycogen synthase kinase-3 beta. Am J Physiol Heart Circ Physiol. 2006;291:H748–H755. doi: 10.1152/ajpheart.00837.2005. [DOI] [PubMed] [Google Scholar]
- Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, et al. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pregi N, Vittori D, Perez G, Leiros CP, Nesse A. Effect of erythropoietin on staurosporine-induced apoptosis and differentiation of SH-SY5Y neuroblastoma cells. Biochim Biophys Acta. 2006;1763:238–246. doi: 10.1016/j.bbamcr.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Sakamaki K. Regulation of endothelial cell death and its role in angiogenesis and vascular regression. Curr Neurovasc Res. 2004;1:305–315. doi: 10.2174/1567202043362072. [DOI] [PubMed] [Google Scholar]
- Silverberg DS, Wexler D, Blum M, Tchebiner JZ, Sheps D, Keren G, et al. The effect of correction of anaemia in diabetics and non-diabetics with severe resistant congestive heart failure and chronic renal failure by subcutaneous erythropoietin and intravenous iron. Nephrol Dial Transplant. 2003;18:141–146. doi: 10.1093/ndt/18.1.141. [DOI] [PubMed] [Google Scholar]
- Sohmiya M, Kakiba T, Kato Y. Therapeutic use of continuous subcutaneous infusion of recombinant human erythropoietin in malnourished predialysis anemic patients with diabetic nephropathy. Eur J Endocrinol. 1998;139:367–370. doi: 10.1530/eje.0.1390367. [DOI] [PubMed] [Google Scholar]
- Spandou E, Tsouchnikas I, Karkavelas G, Dounousi E, Simeonidou C, Guiba-Tziampiri O, et al. Erythropoietin attenuates renal injury in experimental acute renal failure ischaemic/reperfusion model. Nephrol Dial Transplant. 2006;21:330–336. doi: 10.1093/ndt/gfi177. [DOI] [PubMed] [Google Scholar]
- Um M, Lodish HF. Antiapoptotic effects of erythropoietin in differentiated neuroblastoma SH-SY5Y cells require activation of both the STAT5 and AKT signaling pathways. J Biol Chem. 2006;281:5648–5656. doi: 10.1074/jbc.M510943200. [DOI] [PubMed] [Google Scholar]
- Urao N, Okigaki M, Yamada H, Aadachi Y, Matsuno K, Matsui A, et al. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98:1405–1413. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- van der Meer P, Lipsic E, Henning RH, de Boer RA, Suurmeijer AJ, van Veldhuisen DJ, et al. Erythropoietin improves left ventricular function and coronary flow in an experimental model of ischemia–reperfusion injury. Eur J Heart Fail. 2004;6:853–859. doi: 10.1016/j.ejheart.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Walrafen P, Verdier F, Kadri Z, Chretien S, Lacombe C, Mayeux P. Both proteasomes and lysosomes degrade the activated erythropoietin receptor. Blood. 2005;105:600–608. doi: 10.1182/blood-2004-03-1216. [DOI] [PubMed] [Google Scholar]
- Wei L, Han BH, Li Y, Keogh CL, Holtzman DM, Yu SP. Cell death mechanism and protective effect of erythropoietin after focal ischemia in the whisker-barrel cortex of neonatal rats. J Pharmacol Exp Ther. 2006;317:109–116. doi: 10.1124/jpet.105.094391. [DOI] [PubMed] [Google Scholar]
- Wijchers PJ, Burbach JP, Smidt MP. In control of biology: of mice, men and Foxes. Biochem J. 2006;397:233–246. doi: 10.1042/BJ20060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GL, Hanlon P, Amin K, Steenbergen C, Murphy E, Arcasoy MO. Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. FASEB J. 2004;18:1031–1033. doi: 10.1096/fj.03-1289fje. [DOI] [PubMed] [Google Scholar]
- Xu B, Dong GH, Liu H, Wang YQ, Wu HW, Jing H. Recombinant human erythropoietin pretreatment attenuates myocardial infarct size: a possible mechanism involves heat shock protein 70 and attenuation of nuclear factor-kappaB. Ann Clin Lab Sci. 2005;35:161–168. [PubMed] [Google Scholar]
- Yamasaki M, Mishima HK, Yamashita H, Kashiwagi K, Murata K, Minamoto A, et al. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Res. 2005;1050:15–26. doi: 10.1016/j.brainres.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Yu YP, Xu QQ, Zhang Q, Zhang WP, Zhang LH, Wei EQ. Intranasal recombinant human erythropoietin protects rats against focal cerebral ischemia. Neurosci Lett. 2005;387:5–10. doi: 10.1016/j.neulet.2005.07.008. [DOI] [PubMed] [Google Scholar]