Abstract
Enteric nervous system (ENS) development requires complex interactions between migrating neural crest-derived cells and the intestinal microenvironment. Although some molecules influencing ENS development are known, many aspects remain poorly understood. To identify additional molecules critical for ENS development, we used DNA microarray, quantitative real time PCR and in situ hybridization to compare gene expression in E14 and P0 aganglionic or wild type mouse intestine. 83 genes were identified with twofold higher expression in wild type than aganglionic bowel. ENS expression was verified for 39 of 42 selected genes by in situ hybridization. Additionally, nine identified genes had higher levels in aganglionic bowel than in WT animals suggesting that intestinal innervation may influence gene expression in adjacent cells. Strikingly, many synaptic function genes were expressed at E14, a time when the ENS is not needed for survival. To test for developmental roles for these genes, we used pharmacologic inhibitors of Snap25 or vesicle associated membrane protein (VAMP)/synaptobrevin and found reduced neural crest-derived cell migration and decreased neurite extension from ENS precursors. These results provide an extensive set of ENS biomarkers, demonstrate a role for SNARE proteins in ENS development and highlight additional candidate genes that could modify Hirschsprung’s disease penetrance.
Introduction
The enteric nervous system (ENS) is a complex network of neurons and glia within the bowel wall that is derived from multipotent neural crest cells (Gariepy, 2004; Gershon, 1997; Grundy and Schemann, 2005). As these cells migrate through the intestinal environment, they actively proliferate before differentiating into all of the different types of neurons and glia that populate the ENS. Once established, the ENS controls intestinal motility, regulates intestinal secretion, responds to sensory stimuli from the bowel wall, and controls intestinal blood flow.
A small number of genes are now known to influence specific aspects of ENS development (Gariepy, 2004; Gershon, 1997; Grundy and Schemann, 2005; Newgreen and Young, 2002a; Newgreen and Young, 2002b; Taraviras and Pachnis, 1999), but they are not sufficient to explain the complex developmental processes required to form the ENS. In particular, the molecular mechanisms that control ENS precursor migration and neurite extension remain poorly understood. One major barrier to progress in ENS biology is inadequate information about gene expression within the ENS and in the gut wall. Therefore in this report, we have used DNA microarray analysis and quantitative real-time polymerase chain reaction (qRT-PCR) to compare gene expression in normally innervated and aganglionic small bowel from E14 and newborn mice with Ret or Gfrα1 deficiency. These differential gene expression studies led to the identification of many genes expressed more strongly in the ENS than in surrounding cells, including a number of genes with a potential role in ENS precursor migration, neurite extension, cell adhesion, and transcription. Additional genes with intestinal epithelial expression were disregulated in the Ret−/− bowel.
From the identified genes, we were particularly interested in pursuing functional studies of molecules that might control cell migration or neurite extension. These studies are important since failure of ENS precursor migration causes distal intestinal aganglionosis (Hirschsprung’s disease) and extension of neurites from these ENS precursors is essential for forming an interconnected plexus of cells that controls intestinal function. Both of these processes also require complex changes in the cytoskeleton and the addition of membrane to the leading edge of the migrating cell or growing neurite (Park et al., 2002b; Pfenninger et al., 2003; Schmoranzer et al., 2003; Zakharenko and Popov, 1998). We were intrigued by the observation that every component of the synaptic machinery we investigated was present in the ENS at E14. While these proteins could help refine synaptic connections, we hypothesized instead that they might have a role in neurite extension or ENS precursor migration. This hypothesis was based on recent data suggesting that both SNARE (soluble N-ethylmaleimide–sensitive factor (NSF) attachment protein (SNAP) receptor) proteins and in some cases specific neurotransmitters may be important for cell migration or neurite growth (Pfenninger et al., 2003; Proux-Gillardeaux et al., 2005; Tayeb et al., 2005). SNAREs in particular form a large family of proteins that are essential for intracellular membrane trafficking events requiring membrane fusion (Ungar and Hughson, 2003). Membrane fusion in turn may be important at the leading edge of migrating cells or in the growth cone of neurites to add new membrane to specific regions of the cell. Both vesicle associated SNAREs (v-SNAREs) and their membrane target (t-SNAREs) were found in the developing ENS at E14.
To test the hypothesis that SNARE mediated vesicle fusion was essential for ENS precursor migration or neurite extension, we used the highly specific proteases Botulinum neurotoxin A (BoNT/A) (Blasi et al., 1993) to inhibit the t-SNARE Snap25 (synaptosomal-associated protein 25) and Tetanus neurotoxin (TeNT) (Schiavo et al., 1992) to inhibit the v-SNARE Vamp (vesicle associated membrane protein)/synaptobrevin. Both of these treatments delayed neural crest-derived cell migration into the distal bowel and reduced neurite growth in ENS precursors in vitro. In contrast, these toxins had no effect on cell survival or proliferation. Together, these observations provide an extensive set of genes that are prominently expressed in the developing ENS, highlight potential Hirschsprung’s disease modifier genes, and demonstrate novel roles for SNARE proteins in ENS development.
Materials and Methods
Microarray analysis
RNA was prepared using TRI reagent (Sigma, St Louis, MO) and purified by RNeasy mini kit (Qiagen, Hilden, Germany). Probes from three E14 WT and three Ret−/− littermate mouse bowel segments were hybridized to separate U74Av2, U74Bv2 and U74Cv2 arrays (2 genotypes × 3 mice/genotype × 3 different arrays/mouse = 18 arrays total, Affymetrix, Santa Clara, CA). These probes were prepared from whole mouse intestine including esophagus, stomach, small bowel and colon In addition, probes prepared from two WT and two mutant mouse small bowel segments (one Ret−/− and one Gfrα1−/−) at P0 were also hybridized to four separate U74Av2 arrays. All of the Ret−/− and Gfrα1−/− mice used for these studies had been bred into a C57BL/6 genetic background for at least 10 generations. Data were analyzed using Affymetrix MicroArray Suite 4.0 and GeneChip 3.1 Expression Analysis and Statistical Algorithms, dChip and Spotfire DecisionSite for functional genomics software. The complete methodology and full data sets are available at http://bioinformatics.wustl.edu and at http://www.ncbi.nlm.nih.gov/geo/.
qRT-PCR
Primers designed to generate short amplicons (50–100 bp, Tm about 60 °C) were synthesized by Integrated DNA Technologies Inc (IDT, Coralville, IA) and are listed in Supplemental Table 1. qRT-PCR was performed in triplicate for each cDNA with SYBR green PCR Master mix (Applied Biosystems, Foster City, CA) and the iCycler iQ (Bio-Rad, Hercules, CA). Control reactions were performed omitting reverse transcriptase from the cDNA synthesis. For each gene, qRT-PCR was performed with RNA from three individual WT and three individual aganglionic small bowel segments. The RNA content of samples was normalized based on GAPDH (glyceraldehyde-3-phosphate dehydrogenase) amplification. The threshold cycle (CT value) at which a significant increase in PCR product is first detected, was recorded. ΔCT = CT of gene of interest minus CT of GAPDH. For nine genes “fold changes” in RNA abundance between WT and aganglionic bowel were directly determined using standard dilution curves. For these genes, one cycle change in CT corresponded to a 2.1 +/− 0.2 (SEM) change in RNA dilution. To estimate the magnitude of the difference in expression for the other individual RNAs, the ΔΔCT (= ΔCT WT minus ΔCT for aganglionic bowel) was transformed to “fold change” = 2−ΔΔCT.
In situ hybridization
Wild type P0 C57BL/6 mice were perfused with cold 4 % paraformaldehyde (PFA), post fixed overnight at 4°C and then frozen in OCT before sectioning at 14 μm thickness. Slides were warmed to 25°C, baked 15 minutes at 50°C and then fixed again in 4% PFA for 20min at 25°C. After washing twice in diethylpyrocarbonate treated phosphate buffered saline (PBS-DEPC, 10mM) for 5 minutes, tissues were digested in Proteinase K (25 μg/mL) for 14–19 minutes in (50mM Tris pH 7.5, 5mM EDTA, DEPC treated water). Slides were then washed again in PBS-DEPC (2 × 5minutes), incubated in 4 % PFA for (15 minutes, 25°C), and rinsed in DEPC treated water. Tissues were then blocked with 0.2 % acetic anhydride/0.1 M triethanolamine (10 minutes, 25°C), washed in PBS-DEPC (5 minutes, 25°C), and pre-hybridized for 3hr at 65°C in pre-hybridization solution (50% formamide, 5x SSC, 1mg/ml Yeast tRNA, 100ug/ml Heparin, 1x Denhardt's Solution, 0.1% Tween 20 (Sigma P-1379), 0.1% CHAPS (Sigma C-3023), 5mM EDTA pH 8.0). Riboprobes (2 ng/mL final concentration) were then added to fresh pre-hybridization solution, slides were covered with coverslips and tissues were hybridized overnight at 65°C in humidified chamber. Following hybridization, tissues were washed in 1x SSC/50% Formamide at 65°C (3 × 30minutes), then twice in PBT (10mM PBS with 0.1% Triton X-100 and 2mg/mL BSA) for 20min at 25°C, and then blocked with PBT/20% NSS (normal sheep serum) for 1hr at 25°C. Hybridized probe was detected after incubation (overnight, 4°C) with an anti-digoxigenin antibody conjugated to alkaline phosphatase (Roche, 1:2000) in fresh blocking solution (PBT/20% NSS). Slides were then washed in PBT (3 × 30min, 25°C) followed by washing once in alkaline phosphatase (AP) buffer (100mM Tris pH 9.5, 50mM MgCl2, 100mM NaCl, 0.1% TWEEN 20) with levamisole (5mM, DakoCytomation) for 5min and once in AP buffer without levamisole. Finally slides were incubated in AP buffer with 3.5 μL/mL BCIP (0.35% final concentration) and 1.5 μL/mL NBT (0.15% final concentration) for 1–3 days in dark at 4°C, or until desired stain is attained. Plasmids used to generate most in situ probes are provided in Supplemental Table 2. For a few genes (Hes6, Hoxb5, Hoxa5, Pcgf1, Metrn, Tbx3), approximately 300 bp cDNA fragments were isolated by PCR and the cloned cDNA were used as riboprobe templates. Primers used to isolate these cDNA are listed at the end of Supplemental Table 1.
Primary culture of immunoselected ENS precursors
Enteric neural crest were immunoselected from E12.5 embryonic CF1 mouse small bowel and colon using p75NTR antibody (Wu et al., 1999). Bowel was dissociated with collagenase (1mg/mL) and dispase (1mg/mL) to yield a single cell suspension. After p75NTR antibody (#9651, a generous gift of Dr. Moses Chao (Huber and Chao, 1995), 1:1000, 1 hour, 4°C) exposure in B27 (Invitrogen, NY, USA) supplemented Neurobasal medium, cells were incubated with goat anti-rabbit coupled paramagnetic beads (Miltenyi Biotec GmbH, 1:50, 1 hour, 4°C) before separating neural crest cells from unselected cells with a positive selection column (MACS Separation columns, Miltenyi Biotec GmbH). Immunoselected neural crest cells were plated at 700 cells/well in poly-D-lysine/laminin coated 8-well chamber slides (Biocoat, Fisher) and grown in Neurobasal medium supplemented with B27 and glial cell line-derived neurotrophic factor (GDNF, 50ng/mL). For some experiments, cultures of dissociated E12.5 small bowel cells were treated with 50 ng/ml TeNT (Munro et al., 2001) or 10−7 M BoNT/A (Gerona et al., 2000) (Sigma, St. Louis) six hours after plating. After an additional 48 hours in culture, cells were fixed for immunohistochemistry.
Organ culture analysis of neural crest migration
E11.5 CF1 mouse gut explants containing stomach, small bowel and colon were cultured in 500 μl (DMEM (Dulbecco’s Modified Eagle Medium), 10% fetal calf serum, penicillin/streptomycin) containing 10−7 M BoNT/A, 50ng/ml TeNT or vehicle (10 μL PBS) and were pinned to 2% agarose with 4-0 stainless steel filaments (Ethicon). This method, which is similar in principle to gut organ culture techniques previously described (Young et al., 1998), preserves the tubular gut while allowing organ growth and migration of ENS precursors within the gut wall. After 50 hours in a 37ºC, 5% CO2 incubator, tissues were fixed (4% paraformaldehyde, 30 minutes, 25°C) and then processed for whole-mount immunohistochemistry using TuJ1 (1:100) or Ret (1:100) antibodies (4°C overnight).
Immunohistochemistry
After fixation immunocytochemistry was performed with neuron specific beta III tubulin (TuJ1) antibody (Covance, CA, 1:1000, 4°C overnight), goat anti-Ret antibody (Neuromics Inc., MN, 1:200, at 4°C overnight), rabbit anti-phospho-Histone-3 (Upstate, 1:500, 4°C, overnight) or Sox2 antibody (Sigma, St. Louis, MO). Antibody binding was visualized with Alexa Fluor 350, 488, and 594 conjugated anti-goat and anti-rabbit secondary antibodies (Molecular Probes, 1:500, 25°C, 1 hour) and anti-rabbit FITC (fluorescein isothiocyanate) secondary antibody (Jackson ImmunoResearch, 1:500, 25°C, 2 hours). TUNEL (Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling) assays were performed as described (Srinivasan et al., 2005).
Whole mount acetylcholinesterase and NADPH (reduced nicotinamide adenine dinucleotide phosphate)-diaphorase staining
For α-N-catenin−/− and Protocadherin 15−/− mice, the gut including the esophagus, small bowel, and colon was opened along the mesenteric border, pinned flat onto Sylgard 184 (Dow Corning; Essex Brownell, Fort Wayne, IN) plates, fixed 30 minutes in 4 % paraformaldehyde, and then dissected to separate the muscular layer of the bowel (including the myenteric plexus) from the submucosa. Submucosal neurons in the small bowel and colon were visualized after acetylcholinesterase staining (Enomoto et al., 1998; Heuckeroth et al., 1999). Myenteric neurons and nerve fibers in the esophagus, small bowel and colon were also visualized using NADPH diaphorase staining (Aimi et al., 1993; Nemeth et al., 2001) by incubating the tissue in 1 mg/mL β-NADPH (Sigma, St. Louis, MO), 0.1 mg/mL nitroblue tetrazolium (Sigma), and 0.3 % Triton-X100 in 0.1 M sodium phosphate buffer (pH 7.4) at 37°C until ENS staining was seen (approximately 60 minutes). Specimens were then rinsed in PBS (3 × 10 minutes) and mounted on slides in 50% glycerol.
Results
Differential gene expression analysis in the WT and aganglionic bowel
The goal of this study was to identify additional genes that might critically regulate ENS morphogenesis. We initially used DNA microarray analysis to compare gene expression in WT and aganglionic (Ret−/−) mouse bowel at E14 using the full set of Affymetrix U74Av2, U74Bv2 and U74Cv2 arrays (36,698 probe sets, 18 arrays). Data was filtered to include genes “present” in WT, at least 1.2-fold more abundant in WT than aganglionic bowel and P values less than 0.05 (Supplemental Table 3). Four additional U74Av2 arrays were performed using P0 WT and aganglionic small bowel. To expand and validate these differential gene expression analyses, qRT-PCR was then performed to quantitatively measure P0 small bowel gene expression in three WT and three aganglionic Ret−/− newborn mice for 217 genes selected because of their roles in embryonic development and/or known functions in other parts of the nervous system. Some of the identified genes were present at below the detection level for the gene microarrays, but readily detected by qRT-PCR. Together these studies led to the identification of 71 genes expressed at two-fold or higher levels (P < 0.05) in P0 WT small bowel compared to aganglionic bowel (Table 1). An additional 12 genes were identified as 2-fold more abundant in WT than aganglionic bowel at E14 based solely on microarray data (Table 1). These genes include a variety of cell adhesion molecules (n = 11), protein kinases and phosphatases (n = 3), proteases and protease inhibitors (n = 4), receptors and ligands (n = 6), signal transduction proteins (n = 5), molecules involved in synaptic function (n = 7), structural proteins (n = 6), transcription factors (n = 22), and other miscellaneous genes (n = 19).
Table 1. Genes with higher abundance in innervated bowel than aganglionic bowel.
Each mRNA was more abundant in WT than in aganglionic bowel (P < 0.05). “Fold Δ” values were estimated by qRT-PCR as follows: Fold Δ = 2−ΔΔCT, where ΔΔCT is the difference in normalized crossing threshold for WT versus aganglionic bowel. “Fold Δ” values marked * were based on E14 gene microarray data. An “x” in columns labeled E14 or P0 indicates that expression was confirmed by in situ hybridization. “o” indicates signal was not detected in the ENS region. A more extensive searchable database of ENS gene expression is available at our website http://ensmutants.wustl.edu. This Microsoft Access gene expression database was created by combining the new information in this manuscript with data from previously published literature including 1249 articles containing the words “enteric neuron”. In addition, we examined in situ hybridization patterns in the www.genepaint.org database for 1275 genes expressed at E14.5. Of these in situ hybridization patterns reviewed, 66 genes (5%) had prominent expression within the region of the ENS similar to that seen in Figure 1. Thus, our gene enrichment strategy strongly selected for genes with high expression levels in the region of the ENS. 16/66 of the genes identified in the www.genepaint.org database had already been described in the ENS either in our studies for this manuscript or in the literature reviewed. The new database, which is the largest single public database of annotated ENS gene expression, currently contains 319 entries, is annotated by gene function and chromosomal localization, but does not attempt to extensively reference each of the 319 genes. This database will be maintained and updated on our website.
**Gene symbol is used for genes with long names. Full names are: Proprotein convertase subtilisin/kexin type 1 inhibitor (Pcsk1n); Serine (or cysteine) proteinase inhibitor, clade E, member 2 (Serpine 2); and Transmembrane protein with EGF-like and two follistatin-like domains 2 (Tmeff2); Nuclear receptor subfamily 0, group B, member 1 (Nr0b1); Ubiquitin carboxy-terminal hydrolase L1 (PGP 9.5, Uchl1); Sialyltransferase 8(alpha-2,8-sialyltransferase)C (Siat8c).
| Gene name | Accession # | Fold Δ | E14 | P0 | Known | Comments (effect of mutation or function) |
|---|---|---|---|---|---|---|
| Cell Adhesion | ||||||
| Protocadherin 15 (Pcdh15) | AK034124 | 11.3 | x | KO: Deafness and vestibular dysfunction (Ames waltzer) | ||
| Protocadherin alpha 4 (Pcdha4) | D86916 | 7.5 | Thought to be involved in specificity of synaptic connections | |||
| Neural cell adhesion molecule 2 (Ncam2) | AF001287 | 4.4 | o | x | Homophilic adhesion molecule, expressed in olfactory system | |
| Integrin beta 2-like (Itgb2l) | AF051367 | 3.7 | Expressed primarily on neutrophils | |||
| Protocadherin alpha 10 (Pcdha10) | AB008183 | 3.6 | Thought to be involved in specificity of synaptic connections | |||
| Cadherin 6 (Cdh6) | D82029 | 2.8 | Expressed in restricted regions of CNS; KO abnormal kidneys | |||
| Cadherin 9 (Cdh9) | U69136 | 2.8 | ||||
| Junction cell adhesion molecule 3 (Jam3) | AI850297 | 2.7 | o | o | ||
| Cadherin 2 (Cdh2) | M31131 | 2.3 | o | x | KO: Abnormal heart, pancreas and CNS morphogenesis | |
| Semaphorin 3e (Sema3e) | Z80941 | 2.3 | Role in axon guidance | |||
| Immunoglobulin superfamily, member 4A (Igsf4a) | AF061260 | 2.2* | Homophilic cell adhesion molecule, regulates synapse formation | |||
| Kinases and phosphatases | ||||||
| Ret proto-oncogene (Ret) | X67812 | 6654.0 | x | KO: Hirschsprung | ||
| Doublecortin (Dcx) | AB011678 | 14.9 | x | x | KO: lissencephaly | |
| Protein tyrosine phosphatase, receptor type O (Ptpro) | U37465 | 3.0 | ||||
| Proteases and Inhibitors | ||||||
| Neuroserpin (Serpini1) | AJ001700 | 17.1 | x | x | KO: Familial encephalopathy | |
| (Pcsk1n)** | AI841733 | 4.0 | o | x | Inhibits prohormone convertase 1 | |
| (Serpine 2)** | X70296 | 2.5 | Influences neurite outgrowth, NMDA receptor function | |||
| A disintegrin and metalloprotease domain 23 (Adam23) | AB009673 | 2.1 | ||||
| Receptors Ligands | ||||||
| Fibroblast growth factor 13 (Fgf13) | AF020737 | 21.1 | o | x | Reduces infarct volume in cerebral ischemia model | |
| (Tmeff2)** | AB017270 | 7.8 | x | x | Hippocampal and mesencephalic neuron survival factor | |
| Fibroblast growth factor 6 (Fgf6) | M92416 | 4.0 | ||||
| Hepatocyte growth factor (Hgf) | X72307 | 2.3 | KO: Death in utero, multiple roles | |||
| Chemokine (C-C motif) receptor 9 (Ccr9) | AJ132336 | 2.2 | Primarily known to be expressed on T-cells | |||
| Fibroblast growth factor 5 (Fgf5) | M37823 | 2.0 | KO: Long hair growth (Angora mutation) | |||
| Signal Transduction | ||||||
| Collapsin response mediator protein 1 (Crmp1) | AB006714 | 24.3 | o | x | Role in axon growth and guidance | |
| Dihydropyrimidinase-like 2 (CRMP2) (Dpysl2) | X87817 | 6.0 | x | x | Role in axon growth and guidance | |
| N-myc downstream regulated gene 4 (Ndrg4) | AW121600 | 5.7 | o | x | ||
| Catenin alpha 2 (Catna2) | D25282 | 4.0 | x | x | KO: Cerebellar hypoplasia, abnormal hippocampal lamination | |
| RAS dexamethasone-induced 1 (Rasd1) | AF009246 | 2.0 | Nitric oxide effector protein | |||
| Synaptic Function | ||||||
| Synaptotagmin 1 (Syt1) | D37792 | 168.9 | x | x | Synaptic vesicle calcium sensor protein | |
| Reticulon 1 (Rtn1) | AW123115 | 128.0 | x | x | Involved in vesicle trafficking | |
| Synaptosomal-associated protein 25 (Snap25) | M22012 | 22.6 | x | x | Synaptic vesicle fusion protein on plasma membrane | |
| Ca2+dependent activator protein for secretion (Cadps) | D86214 | 13.9 | x | x | Probable role in calcium activated secretion | |
| Synaptosomal-associated protein 91 (Snap91) | M83985 | 9.0 | x | x | Synapse associated protein | |
| Secretogranin III (Scg3) | AV328553 | 3.9 | x | x | Secretory granule component | |
| Syntaxin binding protein 1 (Stxbp1) | D45903 | 2.5 | x | KO: Paralysis, essential for neurotransmitter secretion | ||
| Structural Proteins | ||||||
| Stathmin-like 3 (Stmn3) | AF069708 | 21.1 | x | x | Regulates microtubule dynamics | |
| Microtubule-associated protein tau (Mapt) | M18775 | 38.1 | Regulates microtubule dynamics | |||
| Internexin neuronal intermediate filament protein, alpha (Ina) | L27220 | 2.7* | Intermediate filament involved in neurite growth | |||
| Neurofilament 3, medium (Nef3) | AI849905 | 2.5* | ||||
| Stathmin-like 2 (Stmn2) | AI839868 | 2.4* | Regulates microtubule dynamics | |||
| Neurofilament, light polypeptide (Nefl) | M55424 | 2.3* | ||||
| Transcription Factors | ||||||
| Paired-like homeobox 2b (Phox2b) | Y14493 | 288.0 | x | KO: Hirschsprung, congental central hypoventillation | ||
| Achaete-scute complex homolog-like (Ascl1) | M95603 | 108.4 | x | Esophageal aganglionosis and other ENS anomalies | ||
| Distal-less homeobox 2 (Dlx2) | M80540 | 24.8 | o | x | Probably abnormal ENS, regulates neuronal differentiation | |
| SRY-box containing gene 2 (Sox2) | X94127 | 17.1 | o | x | KO: Anophthalmia, regulates neuronal differentiation | |
| POU domain, class 3, transcription factor 2 (Pou3f2) | NM_008899 | 13.0 | x | Regulates Schwann cell development | ||
| Forkhead box D3 (Foxd3) | AF067421 | 7.0 | KO: Embryonic lethality at E6.5 in mouse | |||
| Myelin transcription factor 1 (Myt1) | AF004294 | 6.5 | x | x | Regulates oligodendrocyte development | |
| Early growth response 1 (Egr1) | AV369921 | 5.8 | o | x | KO: Defect in late hippocampal LTP and synaptic plasticity | |
| Distal-less homeobox 1 (Dlx1) | U51000 | 5.8* | Regulates neuronal differentiation in cortex and hippocampus | |||
| Cyclin D binding myb-like transcription factor 1 (Dmtf1) | U70017 | 3.6 | o | o | Tumor suppressor, regulates cell cycle | |
| Neurogenic differentiation 1 (Neurod1) | U28068 | 3.5 | o | x | KO: Absent dentate gyrus granule cells, seizure, diabetes | |
| Activating transcription factor 3 (Atf3) | U19118 | 3.5 | Induced in response to injury | |||
| Paired box gene 4 (Pax4) | AB010557 | 3.4 | KO: Absent pancreatic beta and delta cells | |||
| Homeo box B5 (Hoxb5) | M26283 | 3.2 | x | x | ||
| Zinc Finger protein 146 (Zfp146) | AJ224763 | 3.1 | ||||
| Growth factor independent 1B (Gfi1b) | AF017275 | 3.0 | Primarily expressed in bone marrow and thymus | |||
| Homeo box A3 (Hoxa3) | Y11717 | 2.2 | Absent thymus, carotid body, abnormal cranial nerve axons | |||
| Neurogenin 2 (Neurog2) | Y07621 | 2.1 | KO: Missing subsets of sensory neurons | |||
| Homeo box A5 (Hoxa5) | Y00208 | 2.1 | KO: Lung, stomach and vertebral defects | |||
| Nuclear receptor subfamily 0, group B, member 1 (Nr0b1) | U41568 | 2.0 | KO: Adrenal hypoplasia, hypogonadism | |||
| Mesenchyme homeobox 2 (Meox2) | Z16406 | 2.0 | Regulates muscle development, expressed in smooth muscle | |||
| SRY-box containing gene 8 (Sox8) | AW214326 | 2.0* | Synergistic role with Sox10 in the ENS | |||
| Miscellaneous | ||||||
| Erythroid differentiation regulator 1 (Erdr1) | AI854606 | 128.0 | x | Induces hemoglobin synthesis | ||
| ELAV-like 4 (Hu antigen D) (Elavl4) | D31953 | 59.7 | x | x | Neuron specific RNA binding protein | |
| Neuron specific gene family member 2 (Nsg2) | U17259 | 42.2 | o | x | Present in Golgi apparatus of neurons | |
| Ganglioside-induced differentiation assoc prot (Gdap1) | Y17850 | 22.6 | Mutated in Charcot-Marie-Tooth | |||
| Synuclein, gamma (Sncg) | AF017255 | 18.4 | x | x | ||
| Growth associated protein 43 (Gap43) | AI841303 | 13.9 | x | x | Involved in neuron pathfinding | |
| Sulfotransferase family 4A, member 1 (Sult4a1) | AF059257 | 12.1 | x | x | Sulfotransferase | |
| Ca2+ channel, voltage-dep., gamma subunit 2 (Cacng2) | AF077739 | 10.3 | o | x | KO: Stargazer mouse, absence seizures, ataxia | |
| Ubiquitin carboxy-terminal hydrolase L1(PGP 9.5) (Uchl1) | AB025313 | 8.2 | x | KO: Gracile axonal dystrophy mouse, alters Parkinson's risk | ||
| Fatty acid binding protein 7, brain (Fabp7) | NM_021272 | 5.9 | x | x | Expressed in enteric glia | |
| Mab-21-like 1 (C. elegans) (Mab21l1) | AW047968 | 5.1* | Essential for eye development | |||
| Pancreatic lipase related protein 2 (Pnliprp2) | M30687 | 4.3 | x | x | Lipase expressed in pancreas, enterocytes and paneth cells | |
| Profilin 2 (Pfn2) | AW122536 | 3.0 | Regulates actin polymerization | |||
| Sialyltransferase 8(alpha-2,8-sialyltransferase)C (Siat8c) | X80502 | 3.0 | Ganglioside synthetic enzyme | |||
| Follistatin-like 5 (Fstl5) | AI850841 | 2.5* | ||||
| Nel-like 2 homolog (chicken) (Nell2) | AI838010 | 2.3* | Important in hippocampus dependent spatial learning | |||
| Vesicular membrane protein p24 (Vmp) | D83206 | 2.4 | ||||
| ELAV-like 2 (Hu antigen B) (Elavl2) | AW124188 | 2.4* | RNA binding protein | |||
| Kinesin family member 5C (Kif5c) | AI842555 | 2.1* | Microtubule motor, role in motor neuron maintenance |
To determine whether the qRT-PCR and gene microarray expression levels predict preferential gene expression in the ENS, 35 genes were targeted for in situ hybridization studies using P0 intestine: each had 2-fold or greater expression in WT than in mutant small bowel. For the majority of the genes analyzed (26/35), expression in the bowel wall was restricted to the region of the ENS (Figure 1 A and B, Table I). An additional 7/35 genes had expression in both the region of the ENS and in other cells of the gut wall. For one of these genes, Sox2, we directly confirmed ENS expression at P0 using double label immunohistochemistry (Figure 2). For 20 of these genes we also confirmed expression in the region of the ENS at E14 (Figure 1). For several additional genes (Pcsk1n, Fgf13, Ncam2, Cdh2, Dlx2, Nsg2, Dcx, Crmp1), expression is reported in the ENS at E14.5 at www.genepaint.org or in published literature (Inagaki et al., 2000). Finally, to determine whether small “fold change” values were likely to represent ENS expression, we also performed in situ hybridization on 7 genes with 1.2 to 1.6 fold higher levels in WT than in Ret−/− E14 bowel based on microarray analysis. For six of these seven genes, prominent ENS expression was seen, but expression was also detected in other cells of the bowel wall (Figure 1C). Together these data provide an extensive set of ENS biomarkers whose function merit additional evaluation. We have initiated functional studies for a few of the identified genes.
Figure 1. In situ hybridization.
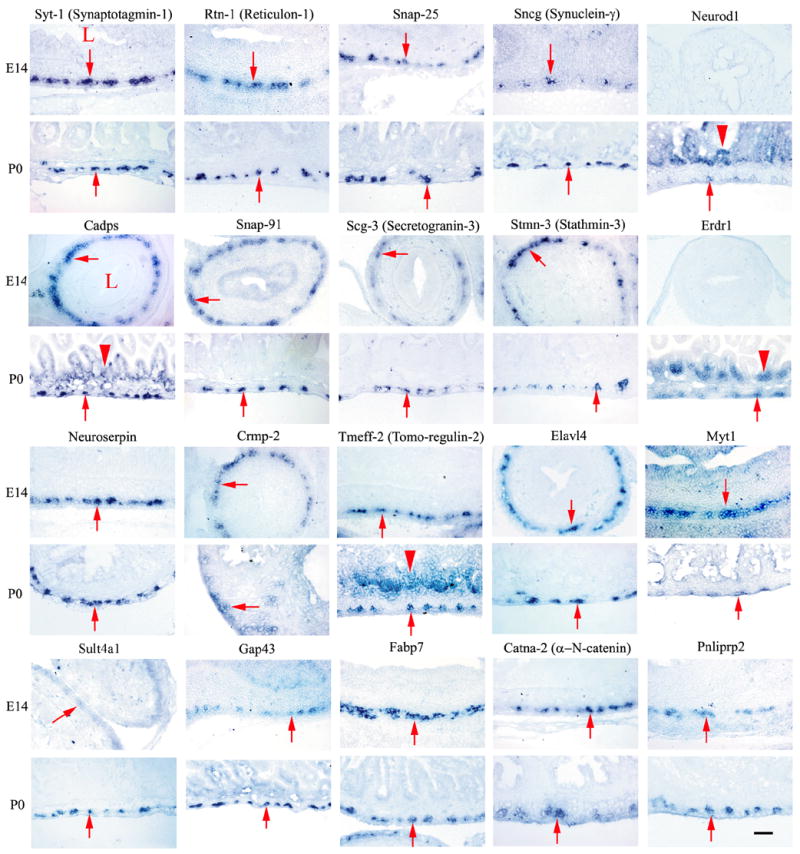
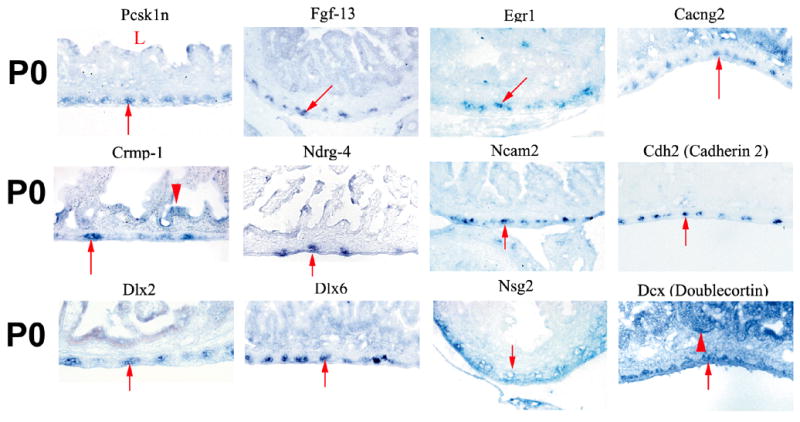
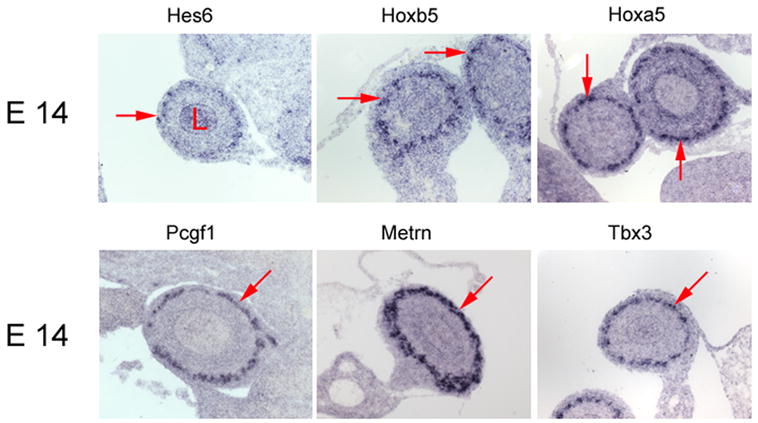
(A) Small bowel in situ hybridization at E14 and P0. Images were obtained from cross-sections of the bowel hybridized with probes for the genes indicated above each pair of images. Most of these genes are prominently expressed in the outer gut wall in the region of the developing enteric nervous system. A few genes (Neurod1, Tmeff2, Erdr1) are expressed more broadly in the gut wall including in the intestinal epithelium. (B) Small bowel in situ hybridization at P0. Images are shown for genes expressed at higher levels in WT than in aganglionic small bowel. (A, B) For these genes, “fold change” in expression between WT and aganglionic bowel as determined by qRT-PCR varies from 168.9-fold for Syt1 to 1.9-fold for Dlx6. (C) In situ hybridization at E14 for genes with 1.2 to 1.6 fold higher expression in WT than in Ret−/− bowel based on microarray analysis. (A–C) In each image, arrows point to the region of the ENS. “L” indicates the bowel lumen. Arrowheads indicate the intestinal epithelium for the few genes with prominent epithelial expression. Scale bar = 40 μm.
Figure 2. Sox2 is expressed in ENS precursors at P0.
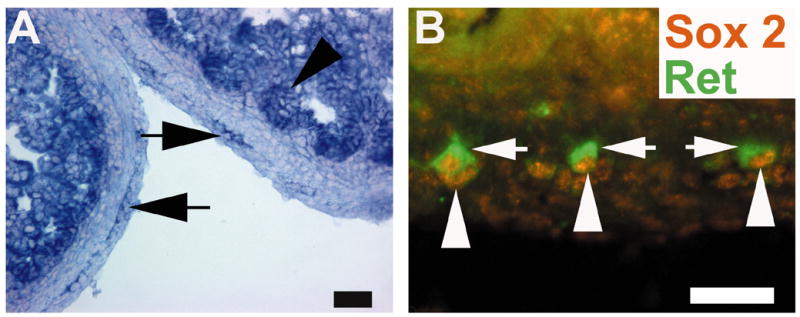
(A) Shows in situ hybridization for Sox2 at P0. There is prominent expression in the area of the ENS (arrows) and also in the intestinal epithelium (arrowhead). (B) Shows immunohistochemistry for Sox2 (orange) and Ret (green). This analysis confirms expression of Sox2 in the nucleus of Ret+ cells. Arrows point to Ret+ cytoplasmic staining in the ENS. Arrowheads point to Sox2+ nuclei in the same cells. Scale bar = 50 μm.
α-N-catenin and Protocadherin15 are not required for ENS development
Several genes essential for neuronal precursor migration within the central nervous system (CNS) were found in the developing ENS by in situ hybridization including Doublecortin (des Portes et al., 1998), Ncam1 (Rolf et al., 2002), N-Cadherin (Barami et al., 1994), α-N-Catenin (Park et al., 2002a), and Mapt (tau) (Takei et al., 2000). α-N-Catenin deficiency, for example, causes cerebellar and hippocampal neuronal migration defects (Park et al., 2002a), but function in the ENS was not known. To determine whether ENS precursor migration was abnormal in α-N-Catenin−/− mice, we examined the ENS by whole mount acetylcholinesterase and NADPH diaphorase staining. These methods allow improved visualization of the myenteric and submucosal plexus compared to sectioning and demonstrate clearly whether the distal bowel is aganglionic or hypoganglionic. These staining methods also demonstrate the organization of enteric ganglia and their fibers so that significant changes in ENS structure would be apparent. Although both acetylcholinesterase and NADPH diaphorase methods stain major populations of enteric neurons and provide a good screening approach to identify significant defects in ENS structure, subtle changes in ENS structure or function may be missed with these techniques. Nonetheless, no obvious intestinal aganglionosis or hypoganglionosis was found suggesting that ENS precursor migration does not depend on αN-Catenin−/−. (Supplemental Figure 1A, and at http://ensmutants.wustl.edu). ENS plexus organization also appeared normal in α-N-Catenin−/− mice.
Several other genes identified in the developing ENS have roles in CNS axon growth, axon pathfinding, and synaptic plasticity including protocadherins (Frank and Kemler, 2002), cadherins (Huntley, 2002), proteases (Parmar et al., 2002), Crmp2 (Inagaki et al., 2001) Gap43 (Donovan et al., 2002) and stathmin proteins (Liedtke et al., 2002). Their role in ENS development has not yet been explored. Analysis of ENS structure in protocadherin 15 deficient mice using the whole mount staining methods described above also failed to reveal an obvious defect in ENS structure (Supplemental Figure 1B, and at http://ensmutants.wustl.edu).
Additional genes identified in the developing ENS have roles in CNS axon growth, axon pathfinding, and synaptic plasticity including protocadherins (Frank and Kemler, 2002), cadherins (Huntley, 2002), proteases (Parmar et al., 2002), Crmp2 (Inagaki et al., 2001) Gap43 (Donovan et al., 2002) and stathmin proteins (Liedtke et al., 2002). Their role in ENS development has not yet been explored.
Abnormal epithelial gene expression in Ret−/− mice
While pursuing the studies above we noticed some genes (Doublecortin, Erdr-1, Cadps, Tmeff2, and NeuroD1) are expressed in both the ENS and other cells within the bowel. Since several of these genes were identified based on markedly increased expression in WT compared to aganglionic bowel, we hypothesized that gene expression in the intestinal epithelium might be also be abnormal in Ret mutant mice despite the absence of detectable Ret expression within the intestinal epithelium. We therefore used in situ hybridization to examine the expression of one of these genes (Erythroid differentiation regulator 1, Erdr1) in WT and aganglionic newborn bowel. Although in situ hybridization is not as reliable as qRT-PCR for quantitative analysis of gene expression, these studies clearly demonstrated that the prominent Erdr1 expression in the intestinal epithelium and other cells of the bowel wall was reduced in Ret−/− mice compared to WT animals (Figure 3A and A).
Figure 3. Intestinal epithelial gene expression is abnormal in Ret−/− mice.
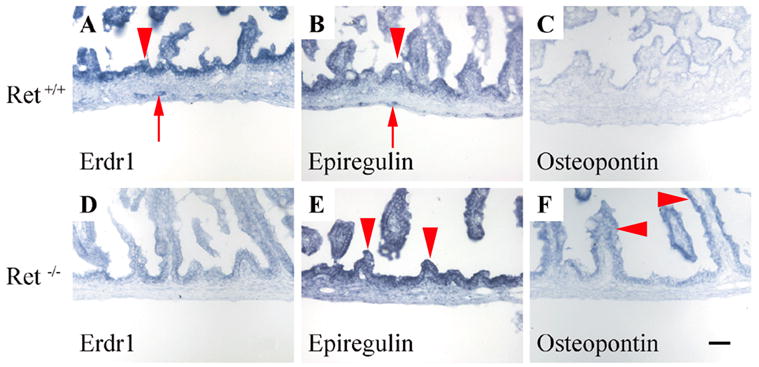
(A, D) In situ hybridization in P0 mouse small bowel demonstrates intense Erdr1 expression in WT mucosa (arrowhead) and detectable expression in the region of the ENS (arrow). In Ret−/− mice there is no expression of Erdr1 in the ENS region and significantly reduced expression in the mucosa and gut wall. (B, C, E, F) In contrast to Erdr1, expression of osteopontin and epiregulin were higher in Ret−/− mice than in WT littermates. Although epiregulin was detected in the ENS region (arrow) and at low levels in the epithelium (arrowhead) of P0 WT mice (B), increased epiregulin expression (arrowheads) occurs in the epithelium of Ret−/− (E) compared to WT mice. (C, F) Osteopontin epithelial expression (arrowheads) is also higher in Ret−/− than in WT intestinal epithelium. These findings were confirmed in three independent experiments. Scale bar = 40 μm.
To find additional evidence of abnormal intestinal epithelial gene expression in Ret−/− mice, we reviewed our GeneChip datasets to identify genes expressed at higher levels in the aganglionic bowel than in the WT bowel. Follow up qRT-PCR studies confirmed that mRNA levels for 9 genes (Table 2) were ≥ 2-fold higher in P0 Ret−/− compared to WT intestine. These genes include the cystic fibrosis transmembrane regulator (an intestinal epithelial chloride channel implicated in control of intestinal epithelial permeability and electrolyte transport) (McCole and Barrett, 2003; Musch et al., 2002), epiregulin (a protein that reduces intestinal susceptibility to colitis (Lee et al., 2004)) and osteopontin (a protein that contributes to and is a marker for colon cancer tumor progression) (Agrawal et al., 2003; Irby et al., 2004)). In situ hybridization studies of epiregulin and osteopontin mRNAs confirmed prominent expression outside of the ENS, and increased epithelial expression in Ret−/− mice compared to WT littermates (Figure 3 B, C, E, F). These results provide strong evidence that intestinal epithelial gene expression is abnormal in Ret−/− mice.
Table 2. Genes with higher abundance in aganglionic bowel than innervated bowel.
Fold change values were determined by qRT-PCR and based on standard dilution curves. P < 0.05 for each gene.
| Gene name | Accession # | Fold Δ |
|---|---|---|
| Secreted phosphoprotein 1 (Minopontin,osteopontin) | X13986 | 6.6 |
| Kininogen | NM_023125 | 4.2 |
| Serum albumin with line-1 repeat | X13060 | 4.0 |
| Perinatal skeletal muscle myosin heavy chain | M12289 | 3.5 |
| Epiregulin | AV231492 | 3.3 |
| Cystic fibrosis transmembrane conductance regulator | X72694 | 3.2 |
| Alpha 2-Heremans-Schmid-glycoprotein | AF025821 | 2.6 |
| Islet amyloid polypeptide | M25389 | 2.4 |
| Anterior restricted homeobox protein (Rpx) | U40720 | 2.3 |
Synaptic function and ENS development
Many of the identified genes are important for synaptic function including the SNARE complex proteins Snap25, Cadps and Snap91. All of these genes are expressed in the ENS at both P0 and E14. Although expression at P0 was expected to allow the ENS to function, expression at E14 suggested the possibility that they might play essential roles in ENS development. To test the hypothesis that SNARE-dependent vesicle fusion supports normal ENS development, E11.5 gut explants were maintained in culture for 50 hours with or without Botulinum toxin A (BoNT/A) to cleave the t-SNARE Snap25 (Blasi et al., 1993; Breidenbach and Brunger, 2004; Chen and Barbieri, 2006; Gerona et al., 2000) or Tetanus toxin (TeNT) to cleave the v-SNARE Vamp/synaptobrevin (Rossetto et al., 2001; Sakaba et al., 2005; Schiavo et al., 1992). These Clostridial toxins are highly specific proteases that cleave and inactivate their targets to block their function and inhibit synaptic activity. At the start of these culture experiments, enteric neural crest-derived cells, which migrate in a rostrocaudal direction through the gut wall, had just reached the ileocecal junction. During the time in culture, ENS precursors colonize the majority of the colon under control conditions (90 +/− 3 %, n = 7 explants). In contrast, both BoNT/A A or TeNT treatments slowed neural crest migration through the colon (Figure 4 AC, G), resulting in only 72 +/− 1 or 74 +/− 3 % colon innervation respectively (n = 4 BoNT/A and n = 9 TeNT treated explants; P < 0.006 versus control for each experimental group). When both toxins were used together there was no statistically significant additive effect compared to either toxin alone (66 +/− 3% colon innervation, n = 5; P > 0.05 compared to either single toxin) suggesting that the individual toxins fairly completely blocked SNARE mediated vesicle fusion. These experiments demonstrate novel and previously unsuspected roles for Snap25 and Vamp in ENS precursor migration.
Figure 4. Botulinum toxin A or Tetanus toxin treatment slows neural crest precursor migration into the colon and reduces neurite length.
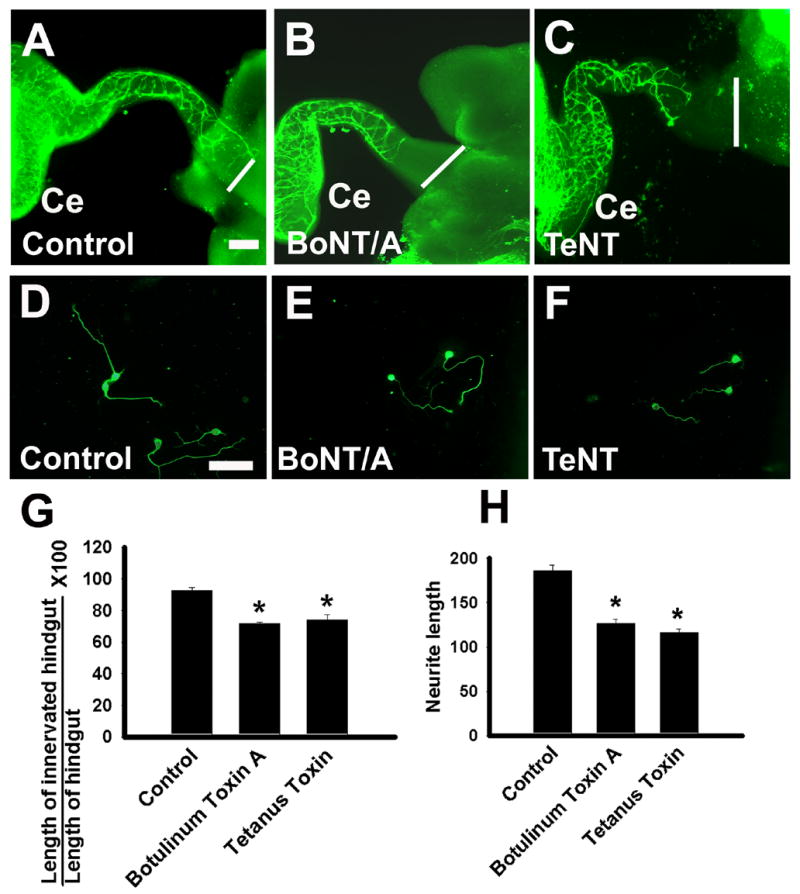
E11.5 mouse gut was incubated in organ culture for 50 hours with Botulinum toxin A (BoNT/A) (B), Tetanus toxin (TeNT) (C) or no added factor (A) before immunohistochemical staining for TuJ1 or Ret. Images show TuJ1 stained cells in the colon, but the extent of neural crest precursor migration was similar with either Ret or TuJ1 immunohistochemistry. The white line at the right side of the image corresponds to the end of the colon. (D–F) Neurite length was measured in developing enteric neurons grown at low density under control conditions (D) or with BoNT/A (E) or TeNT (F) for 48 hours. Cell bodies and neurites were visualized with TuJ1 immunohistochemistry. (G) Quantitative analysis of the percentage of the colon innervated demonstrates that both BoNT/A and TeNT treatment slow neural crest migration into the distal bowel (*P < 0.001). (E) Quantitative analysis of mean neurite length for the longest TuJ1+ neurite after culturing cells for 48 hours under control conditions, with BoNT/A, or with TeNT or demonstrates that BoNT/A and TeNT reduce neurite length (*P < 0.001). Scale Bar = 50 μm. Ce = cecum.
Since both cell migration and neurite extension require the addition of membrane to the leading edge of the cell or neurite, we next investigated whether BoNT/A or TeNT treatment influenced neurite growth in cultured ENS precursors. Cultures of dissociated E12.5 small intestine including ENS precursors were grown in GDNF containing media in the presence or absence of BoNT/A or TeNT for 48 hours. The length of the longest neurite was measured (Figure 4 D–F, H). These studies demonstrated a significant reduction in neurite length after both BoNT/A and TeNT treatment (Figure 4 H, control = 186 +/− 7 μm, BoNT/A = 127 +/− 4 μm, TeNT = 116 +/− 4 μm, P < 0.001 versus control for each experimental group; n > 155 neurites measured in each group). Thus SNARE function appears to be important for both neurite growth and migration of ENS precursors.
To be certain that these results did not occur because of changes in cell viability or proliferation under the culture conditions, cell death of immunoselected ENS precursors was measured by TUNEL histochemistry (n = 3 separate experiments) and cell proliferation was measured by H3P/Ret double label immunohistochemistry (n = 3 separate experiments). In addition, for cells grown in isolated culture, all of the Ret expressing cells in the well were counted. These studies confirmed that under the experimental conditions BoNT/A and TeNT did not alter cell death (%TUNEL positive/Ret+ cells: control = 1.3 +/− 0.5%, BoNT/A = 1.3 +/− 0.7%, TeNT = 1.3 +/− 0.4%, n > 6400 cells in each group), cell proliferation (H3PRet/Ret expressing cells × 100: control = 20 +/− 2%, BoNT/A = 20 +/− 2%, TeNT = 21 +/− 2%, n > 1500 cells in each group) or the number of Ret expressing cells/well (control = 2216 +/− 90, BoNT/A = 2159 +/− 80, TeNT = 2141 +/− 34, P > 0.4 versus control). Thus, inhibiting SNARE function by cleaving either of two different proteins (Snap25 or Vamp/synaptobrevin) alters ENS precursor migration and neurite extension without affecting cell survival or proliferation.
Discussion
Although remarkable progress has been made defining the molecular mechanisms of ENS development, the known genes cannot adequately explain many aspects of ENS morphogenesis. In particular, mechanisms governing neurite extension, axon pathfinding, neuronal precursor migration, and neuronal subtype identity remain poorly understood. Furthermore, information about gene expression within the ENS is difficult to find in the medical literature. To begin to address these problems we initiated differential gene expression and in situ hybridization analyses to identify additional genes that might regulate ENS morphogenesis. Our new data provide an extensively annotated database of genes expressed in the developing ENS. In addition, we created a more comprehensive searchable electronic database by combining our new data with other available information. This growing database (available at http://ensmutants.wustl.edu/) contains 319 entries and represents the single most detailed source of information about gene expression within the ENS currently available. By organizing and annotating this information, we expect to spur novel avenues of investigation and facilitate identification of genes mutated in patients with Hirschsprung’s disease or intestinal pseudo-obstruction syndromes. Indeed, these studies led to our own investigations into a-N-catenin, protocadherin 15, Snap25, and Vamp/synaptobrevin in the developing ENS. Many other genes expressed in the ENS also deserve additional investigation. For example, Tmeff1 (www.genepaint.org), Edg2 (Segura et al., 2004), and Ccr9 were identified by our studies and extensive literature review as genes expressed in the ENS that map to previously described Hirschsprung’s disease susceptibility loci at 9q31 (Bolk et al., 2000) and 3p21 (Gabriel et al., 2002). The gene doublecortin, may also deserve additional evaluation since as an X-linked gene required for cortical neuron precursor migration, doublecortin could modify Hirschsprung’s disease penetrance and contribute to the 4:1 male/female ratio in Hirschsprung’s disease.
Transcription factors and ENS development
A small number of transcription factors are currently known to influence ENS development (Phox2b, Mash1, Sox10, Pax3, Tlx2, and Sox8) (Blaugrund et al., 1996; Lang et al., 2000; Maka et al., 2005; Pattyn et al., 1999; Shirasawa et al., 1997; Southard-Smith et al., 1999). Our studies identified seven additional transcription factors expressed within the region of the bowel containing the ENS (Dlx1, Dlx2, Dlx6, Myt1, Egr1, Neurod1 and Sox2). Dlx2 had previously been demonstrated in E12.5 mouse ENS and Dlx2 mutant mice have reduced intestinal peristalsis (Qiu et al., 1995). In contrast, Sox2 is important for maintaining CNS neural stem cells (Graham et al., 2003), but is turned off when neural crest are specified and had not been previously described in the ENS. Because transcription factor expression critically determines neuronal subtype identity in other regions of the nervous system, we anticipate that many other transcriptional regulators will be required to establish all of the distinct neuronal subtypes essential for normal ENS function. Nonetheless, these new data more than double the number of transcription factors previously known to be expressed within the ENS and suggest new directions for investigation into ENS morphogenesis.
Ret signaling and epithelial gene expression
Since Ret is not expressed in intestinal epithelium, we were surprised to find altered intestinal epithelial gene expression in Ret−/− mice. Indeed, some genes with abnormal epithelial expression critically influence intestinal epithelial function. This could in part explain the development of intestinal inflammation when the ENS is damaged (Bush et al., 1998). While these data suggest the possibility that normal intestinal epithelial development requires the ENS, additional studies will be needed to exclude other possibilities.
Differential gene expression studies led to the identification of novel roles for SNARE proteins in ENS development
In our analysis of 217 genes by qRT-PCR, only a few mRNAs were at least 100-fold more abundant in WT than aganglionic bowel (Ret, Phox2b, and Mash1, Synaptotagmin 1, Reticulon 1 and Erythroid differentiation regulator (Erdr1)). Ret, Phox2b, and Mash1 are already known to play critical roles in ENS development suggesting that the other highly differentially expressed genes might also be essential for ENS development or function.
Synaptotagmin 1 is a calcium sensor important for fast neurotransmitter release at synapses by accelerating SNARE dependent membrane fusion (Yoshihara and Montana, 2004). Reticulon 1 also binds to SNARE proteins and is implicated in vesicle trafficking including regulated exocytosis (Steiner et al., 2004). Remarkably, many other genes important for synaptic function were also expressed in the ENS at both P0 and E14. For example, Snap25 is essential for evoked synaptic transmission, but not for CNS neuronal process growth in vivo (Molnar et al., 2002; Washbourne et al., 2002). Cadps is also essential for calcium stimulated exocytosis (Grishanin et al., 2004) while Snap91 is found in the presynaptic terminals and promotes endocytosis (Morgan et al., 1999). These observations suggested that regulated transmitter release or SNARE mediated vesicle fusion is important for ENS development long before enteric neurons are required for intestinal motility. Our new data demonstrating dramatic effects on ENS precursor migration and neurite extension by inhibiting Snap25 or Vamp support this hypothesis.
These data add to a recent, but growing literature implicating both vesicle fusion and synaptic function in neuronal precursor migration, differentiation, and the establishment of an interconnected network of neurons. 5-HT2B receptor activation, for example, promotes differentiation of fetal enteric neurons in vitro (Fiorica-Howells et al., 2000) and synaptic activity influences synapse development (Murphy, 2003) and dendritic morphology (Wong and Ghosh, 2002) in other regions of the nervous system. These results are also consistent with the recent observation that SNARE function is required for the migration of CHO-K1 and MDCK cells (Proux-Gillardeaux et al., 2005; Tayeb et al., 2005) and that nitric oxide and cyclic nucleotides regulate ENS precursor migration in insect embryos (Haase and Bicker, 2003). Furthermore, neurotransmitters have been implicated in neuronal precursor migration in other regions of the embryo including effects of PACAP (pituitary adenylate cyclase-activating peptide) (Falluel-Morel et al., 2005), glutamate (Kim et al., 2005) and acetylcholine (Fucile et al., 2004) on cerebellar granule cells, GABA (gamma-aminobutyric acid) in the cerebral cortex (Bolteus and Bordey, 2004; Lopez-Bendito et al., 2003), and serotonin in GnRH (gonadotropin releasing hormone) producing neurons (Giacobini et al., 2004; Pronina et al., 2003). Together these data suggest that SNARE dependent exocytosis is important for ENS precursor migration and neurite extension. Determining whether these SNARE proteins are needed for release of specific neurotransmitters that secondarily affect ENS precursor migration and neurite extension will require additional investigation.
Overall these findings provide many new avenues for investigating ENS development by identifying a number of growth factors, kinases, cell adhesion molecules and transcription factors not previously known to be expressed within the ENS. Furthermore, we have demonstrated that blocking Snap25 or Vamp reduces neural crest precursor migration into the distal bowel and decreases neurite extension in cultured ENS precursors. The function of many other genes newly identified as expressed in the ENS can be examined further in vivo using mutant mice and efficiently pursued ex vivo using ENS precursors in primary culture. By more broadly investigating genes that are important for ENS structure and function, we may identify additional Hirschsprung’s disease modifiers or genes that result in human dysmotility syndromes.
Supplementary Material
Supplemental Table 1: Primers used for qRT-PCR and primers used to clone cDNA fragments for Hes6, Hoxb5, Hoxa5, Pcgf1, Metrn, Tbx3 in situ hybridization probe preparation.
Supplemental Table 2: Plasmids used for preparation of the in situ probes.
Supplemental Table 3: Genes identified by microarray analysis of E14 small bowel as 1.2-fold or more abundant in WT than in aganglionic bowel.
Supplemental Figure 1: Whole mount acetylcholinesterase and NADPH diaphorase staining of the ENS in adult α-N-catenin−/− and Protocadherin 15−/− mice. (A, B) Small bowel, esophagus and colon segments from mice deficient in α-N-catenin (A) or Protocadherin 15 (B) were stained using acetylcholinesterase or NADPH diaphorase methods. These representative images showing enteric ganglion cells and their fibers are similar to the appearance of the ENS in WT mice. Scale bar = 50 μm.
Acknowledgments
We thank Dr. Jeffrey Milbrandt, Dr. Louis Muglia, and Dr. Jonathan Gitlin for helpful comments on the manuscript. We especially appreciate the assistance of Dr. Jeffrey Gordon for additional guidance on the manuscript. We also thank Moses Chao for sharing valuable reagents. In addition, we thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri, for the use of the Multiplexed Gene Analysis Core for performing the microarray hybridization experiments. The Siteman Cancer Center is supported in part by an NCI Cancer Center Support Grant (P30 CA91842). ROH was supported by grants from the NIH (DK64592, DK057038, and DK068371). HE was supported by RIKEN and MEXT "The Project for Realization of Regenerative Medicine".
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal D, Chen T, Irby R, Quackenbush J, Chambers AF, Szabo M, Cantor A, Coppola D, Yeatman TJ. Osteopontin identified as colon cancer tumor progression marker. C R Biol. 2003;326:1041–3. doi: 10.1016/j.crvi.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Aimi Y, Kimura H, Kinoshita T, Minami Y, Fujimura M, Vincent SR. Histochemical localization of nitric oxide synthase in rat enteric nervous system. Neuroscience. 1993;53:553–60. doi: 10.1016/0306-4522(93)90220-a. [DOI] [PubMed] [Google Scholar]
- Barami K, Kirschenbaum B, Lemmon V, Goldman SA. N-cadherin and Ng-CAM/8D9 are involved serially in the migration of newly generated neurons into the adult songbird brain. Neuron. 1994;13:567–82. doi: 10.1016/0896-6273(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Sudhof TC, Niemann H, Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365:160–3. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Blaugrund E, Pham TD, Tennyson VM, Lo L, Sommer L, Anderson DJ, Gershon MD. Distinct subpopulations of enteric neuronal progenitors defined by time of development, sympathoadrenal lineage markers, and Mash-1-dependence. Development. 1996;122:309–320. doi: 10.1242/dev.122.1.309. [DOI] [PubMed] [Google Scholar]
- Bolk S, Pelet A, Hofstra RMW, Angrist M, Salomon R, Croaker D, Buys CHCM, Lyonnet S, Chakravarti A. A human model for multigenic inheritance: phenotypic expression in Hirschsprung disease requires both the RET gene and a new 9q31 locus. Proc Natl Acad Sci. 2000;97:268–273. doi: 10.1073/pnas.97.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–31. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach MA, Brunger AT. Substrate recognition strategy for botulinum neurotoxin serotype A. Nature. 2004;432:925–9. doi: 10.1038/nature03123. [DOI] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- Chen S, Barbieri JT. Unique substrate recognition by botulinum neurotoxins serotypes A and E. J Biol Chem. 2006;281:10906–11. doi: 10.1074/jbc.M513032200. [DOI] [PubMed] [Google Scholar]
- des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrie A, Gelot A, Dupuis E, Motte J, Berwald-Netter Y, Catala M, Kahn A, Beldjord C, Chelly J. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Donovan SL, Mamounas LA, Andrews AM, Blue ME, McCasland JS. GAP-43 is critical for normal development of the serotonergic innervation in forebrain. J Neurosci. 2002;22:3543–52. doi: 10.1523/JNEUROSCI.22-09-03543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EMJ, Milbrandt J. GFRα1 deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- Falluel-Morel A, Vaudry D, Aubert N, Galas L, Benard M, Basille M, Fontaine M, Fournier A, Vaudry H, Gonzalez BJ. Pituitary adenylate cyclase-activating polypeptide prevents the effects of ceramides on migration, neurite outgrowth, and cytoskeleton remodeling. Proc Natl Acad Sci U S A. 2005;102:2637–42. doi: 10.1073/pnas.0409681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorica-Howells E, Maroteaux L, Gershon MD. Serotonin and 5-HT2b receptor in the development of enteric neurons. J Neuroscience. 2000;20:294–305. doi: 10.1523/JNEUROSCI.20-01-00294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Kemler R. Protocadherins. Curr Opin Cell Biol. 2002;14:557–62. doi: 10.1016/s0955-0674(02)00365-4. [DOI] [PubMed] [Google Scholar]
- Fucile S, Renzi M, Lauro C, Limatola C, Ciotti T, Eusebi F. Nicotinic cholinergic stimulation promotes survival and reduces motility of cultured rat cerebellar granule cells. Neuroscience. 2004;127:53–61. doi: 10.1016/j.neuroscience.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Salomon R, Pelet A, Angrist M, Amiel J, Fornage M, Attie-Bitach T, Olson JM, Hofstra R, Buys C, Steffann J, Munnich A, Lyonnet S, Chakravarti A. Segregation at three loci explains familial and population risk in Hirschsprung disease. Nat Genet. 2002;31:89–93. doi: 10.1038/ng868. [DOI] [PubMed] [Google Scholar]
- Gariepy CE. Developmental disorders of the enteric nervous system: genetic and molecular bases. J Pediatr Gastroenterol Nutr. 2004;39:5–11. doi: 10.1097/00005176-200407000-00003. [DOI] [PubMed] [Google Scholar]
- Gerona RR, Larsen EC, Kowalchyk JA, Martin TF. The C terminus of SNAP25 is essential for Ca(2+)-dependent binding of synaptotagmin to SNARE complexes. J Biol Chem. 2000;275:6328–36. doi: 10.1074/jbc.275.9.6328. [DOI] [PubMed] [Google Scholar]
- Gershon M. Genes and lineages in the formation of the enteric nervous system. Current Opinion in Neurobiology. 1997;7:101–109. doi: 10.1016/s0959-4388(97)80127-4. [DOI] [PubMed] [Google Scholar]
- Giacobini P, Kopin AS, Beart PM, Mercer LD, Fasolo A, Wray S. Cholecystokinin modulates migration of gonadotropin-releasing hormone-1 neurons. J Neurosci. 2004;24:4737–48. doi: 10.1523/JNEUROSCI.0649-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–65. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Grishanin RN, Kowalchyk JA, Klenchin VA, Ann K, Earles CA, Chapman ER, Gerona RR, Martin TF. CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron. 2004;43:551–62. doi: 10.1016/j.neuron.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Grundy D, Schemann M. Enteric nervous system. Curr Opin Gastroenterol. 2005;21:176–82. doi: 10.1097/01.mog.0000153315.28327.6e. [DOI] [PubMed] [Google Scholar]
- Haase A, Bicker G. Nitric oxide and cyclic nucleotides are regulators of neuronal migration in an insect embryo. Development. 2003;130:3977–87. doi: 10.1242/dev.00612. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EM, Jr, Milbrandt J. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. 1999;22:253–263. doi: 10.1016/s0896-6273(00)81087-9. [DOI] [PubMed] [Google Scholar]
- Huber LJ, Chao MV. Mesenchymal and neuronal cell expression of the p75 neurotrophin receptor gene occur by different mechanisms. Dev Biol. 1995;167:227–38. doi: 10.1006/dbio.1995.1019. [DOI] [PubMed] [Google Scholar]
- Huntley GW. Dynamic aspects of cadherin-mediated adhesion in synapse development and plasticity. Biol Cell. 2002;94:335–44. doi: 10.1016/s0248-4900(02)00003-5. [DOI] [PubMed] [Google Scholar]
- Inagaki H, Kato Y, Hamajima N, Nonaka M, Sasaki M, Eimoto T. Differential expression of dihydropyrimidinase-related protein genes in developing and adult enteric nervous system. Histochem Cell Biol. 2000;113:37–41. doi: 10.1007/s004180050005. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N, Nishimura T, Amano M, Kaibuchi K. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4:781–2. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- Irby RB, McCarthy SM, Yeatman TJ. Osteopontin regulates multiple functions contributing to human colon cancer development and progression. Clin Exp Metastasis. 2004;21:515–23. doi: 10.1007/s10585-004-2873-4. [DOI] [PubMed] [Google Scholar]
- Kim PM, Aizawa H, Kim PS, Huang AS, Wickramasinghe SR, Kashani AH, Barrow RK, Huganir RL, Ghosh A, Snyder SH. Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci U S A. 2005;102:2105–10. doi: 10.1073/pnas.0409723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D, Chen F, Milewski R, Li J, Lu MM, Epstein JA. Pax3 is required for enteric ganglia formation and functions with Sox10 to modulate expression of c-Ret. J Clinical Investigation. 2000;106:963–971. doi: 10.1172/JCI10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Pearsall RS, Das S, Dey SK, Godfrey VL, Threadgill DW. Epiregulin is not essential for development of intestinal tumors but is required for protection from intestinal damage. Mol Cell Biol. 2004;24:8907–16. doi: 10.1128/MCB.24.20.8907-8916.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Leman EE, Fyffe RE, Raine CS, Schubart UK. Stathmin-deficient mice develop an age-dependent axonopathy of the central and peripheral nervous systems. Am J Pathol. 2002;160:469–80. doi: 10.1016/S0002-9440(10)64866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Lujan R, Shigemoto R, Ganter P, Paulsen O, Molnar Z. Blockade of GABA(B) receptors alters the tangential migration of cortical neurons. Cereb Cortex. 2003;13:932–42. doi: 10.1093/cercor/13.9.932. [DOI] [PubMed] [Google Scholar]
- Maka M, Stolt CC, Wegner M. Identification of Sox8 as a modifier gene in a mouse model of Hirschsprung disease reveals underlying molecular defect. Dev Biol. 2005;277:155–69. doi: 10.1016/j.ydbio.2004.09.014. [DOI] [PubMed] [Google Scholar]
- McCole DF, Barrett KE. Epithelial transport and gut barrier function in colitis. Curr Opin Gastroenterol. 2003;19:578–82. doi: 10.1097/00001574-200311000-00011. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Lopez-Bendito G, Small J, Partridge LD, Blakemore C, Wilson MC. Normal development of embryonic thalamocortical connectivity in the absence of evoked synaptic activity. J Neurosci. 2002;22:10313–23. doi: 10.1523/JNEUROSCI.22-23-10313.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Zhao X, Womack M, Prasad K, Augustine GJ, Lafer EM. A role for the clathrin assembly domain of AP180 in synaptic vesicle endocytosis. J Neurosci. 1999;19:10201–12. doi: 10.1523/JNEUROSCI.19-23-10201.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro P, Kojima H, Dupont JL, Bossu JL, Poulain B, Boquet P. High sensitivity of mouse neuronal cells to tetanus toxin requires a GPI-anchored protein. Biochem Biophys Res Commun. 2001;289:623–9. doi: 10.1006/bbrc.2001.6031. [DOI] [PubMed] [Google Scholar]
- Murphy TH. Activity-dependent synapse development: changing the rules. Nat Neurosci. 2003;6:9–11. doi: 10.1038/nn0103-9. [DOI] [PubMed] [Google Scholar]
- Musch MW, Clarke LL, Mamah D, Gawenis LR, Zhang Z, Ellsworth W, Shalowitz D, Mittal N, Efthimiou P, Alnadjim Z, Hurst SD, Chang EB, Barrett TA. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase. J Clin Invest. 2002;110:1739–47. doi: 10.1172/JCI15695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth L, Yoneda A, Kader M, Devaney D, Puri P. Three-dimensional morphology of gut innervation in total intestinal aganglionosis using whole-mount preparation. J Pediatr Surg. 2001;36:291–5. doi: 10.1053/jpsu.2001.20693. [DOI] [PubMed] [Google Scholar]
- Newgreen D, Young HM. Enteric nervous system: development and developmental disturbances--part 1. Pediatr Dev Pathol. 2002a;5:224–47. doi: 10.1007/s10024-001-0142-y. [DOI] [PubMed] [Google Scholar]
- Newgreen D, Young HM. Enteric nervous system: development and developmental disturbances--part 2. Pediatr Dev Pathol. 2002b;5:329–49. doi: 10.1007/s10024-001-0142-y. [DOI] [PubMed] [Google Scholar]
- Park C, Falls W, Finger JH, Longo-Guess CM, Ackerman SL. Deletion in Catna2, encoding alpha N-catenin, causes cerebellar and hippocampal lamination defects and impaired startle modulation. Nat Genet. 2002a;31:279–84. doi: 10.1038/ng908. [DOI] [PubMed] [Google Scholar]
- Park HT, Wu J, Rao Y. Molecular control of neuronal migration. Bioessays. 2002b;24:821–7. doi: 10.1002/bies.10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar PK, Coates LC, Pearson JF, Hill RM, Birch NP. Neuroserpin regulates neurite outgrowth in nerve growth factor-treated PC12 cells. J Neurochem. 2002;82:1406–15. doi: 10.1046/j.1471-4159.2002.01100.x. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–70. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- Pfenninger KH, Laurino L, Peretti D, Wang X, Rosso S, Morfini G, Caceres A, Quiroga S. Regulation of membrane expansion at the nerve growth cone. J Cell Sci. 2003;116:1209–17. doi: 10.1242/jcs.00285. [DOI] [PubMed] [Google Scholar]
- Pronina T, Ugrumov M, Adamskaya E, Kuznetsova T, Shishkina I, Babichev V, Calas A, Tramu G, Mailly P, Makarenko I. Influence of serotonin on the development and migration of gonadotropin-releasing hormone neurones in rat foetuses. J Neuroendocrinol. 2003;15:549–58. doi: 10.1046/j.1365-2826.2003.01029.x. [DOI] [PubMed] [Google Scholar]
- Proux-Gillardeaux V, Gavard J, Irinopoulou T, Mege RM, Galli T. Tetanus neurotoxin-mediated cleavage of cellubrevin impairs epithelial cell migration and integrin-dependent cell adhesion. Proc Natl Acad Sci U S A. 2005;102:6362–7. doi: 10.1073/pnas.0409613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M, Bulfone A, Martinez S, Meneses JJ, Shimamura K, Pedersen RA, Rubenstein JL. Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes Dev. 1995;9:2523–38. doi: 10.1101/gad.9.20.2523. [DOI] [PubMed] [Google Scholar]
- Rolf B, Bastmeyer M, Schachner M, Bartsch U. Pathfinding errors of corticospinal axons in neural cell adhesion molecule-deficient mice. J Neurosci. 2002;22:8357–62. doi: 10.1523/JNEUROSCI.22-19-08357.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetto O, Seveso M, Caccin P, Schiavo G, Montecucco C. Tetanus and botulinum neurotoxins: turning bad guys into good by research. Toxicon. 2001;39:27–41. doi: 10.1016/s0041-0101(00)00163-x. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Stein A, Jahn R, Neher E. Distinct kinetic changes in neurotransmitter release after SNARE protein cleavage. Science. 2005;309:491–4. doi: 10.1126/science.1112645. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–5. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schmoranzer J, Kreitzer G, Simon SM. Migrating fibroblasts perform polarized, microtubule-dependent exocytosis towards the leading edge. J Cell Sci. 2003;116:4513–9. doi: 10.1242/jcs.00748. [DOI] [PubMed] [Google Scholar]
- Segura BJ, Zhang W, Cowles RA, Xiao L, Lin TR, Logsdon C, Mulholland MW. Lysophosphatidic acid stimulates calcium transients in enteric glia. Neuroscience. 2004;123:687–93. doi: 10.1016/j.neuroscience.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Shirasawa S, Yunker AM, Roth KA, Brown GA, Horning S, Korsmeyer SJ. Enx (Hox11L1)-deficient mice develop myenteric neuronal hyperplasia and megacolon. Nature Medicine. 1997;3:646–650. doi: 10.1038/nm0697-646. [DOI] [PubMed] [Google Scholar]
- Southard-Smith EM, Angrist M, Ellison JS, Agarwala R, Baxevanis AD, Chakravarti A, Pavan WJ. The Sox10(Dom) mouse: modeling the genetic variation of Waardenburg-Shah (WS4) syndrome. Genome Res. 1999;9:215–25. [PubMed] [Google Scholar]
- Srinivasan S, Anitha M, Mwangi S, Heuckeroth RO. Enteric neuroblasts require the phosphatidylinositol 3-kinase/Akt/Forkhead pathway for GDNF-stimulated survival. Mol Cell Neurosci. 2005;29:107–19. doi: 10.1016/j.mcn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Steiner P, Kulangara K, Sarria JC, Glauser L, Regazzi R, Hirling H. Reticulon 1-C/neuroendocrine-specific protein-C interacts with SNARE proteins. J Neurochem. 2004;89:569–80. doi: 10.1111/j.1471-4159.2004.02345.x. [DOI] [PubMed] [Google Scholar]
- Takei Y, Teng J, Harada A, Hirokawa N. Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J Cell Biol. 2000;150:989–1000. doi: 10.1083/jcb.150.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraviras S, Pachnis V. Development of the mammalian enteric nervous system. Current Opinion in Genetics and Development. 1999;9:321–327. doi: 10.1016/s0959-437x(99)80048-3. [DOI] [PubMed] [Google Scholar]
- Tayeb MA, Skalski M, Cha MC, Kean MJ, Scaife M, Coppolino MG. Inhibition of SNARE-mediated membrane traffic impairs cell migration. Exp Cell Res. 2005;305:63–73. doi: 10.1016/j.yexcr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Ungar D, Hughson FM. SNARE protein structure and function. Annu Rev Cell Dev Biol. 2003;19:493–517. doi: 10.1146/annurev.cellbio.19.110701.155609. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Bendito G, Molnar Z, Becher MW, Valenzuela CF, Partridge LD, Wilson MC. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002;5:19–26. [Google Scholar]
- Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3:803–12. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Chen JX, Rothman TP, Gershon MD. Inhibition of in vitro enteric neuronal development by endothelin-3: mediation by endothelin B receptors. Development. 1999;126:1161–1173. doi: 10.1242/dev.126.6.1161. [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Montana ES. The synaptotagmins: calcium sensors for vesicular trafficking. Neuroscientist. 2004;10:566–74. doi: 10.1177/1073858404268770. [DOI] [PubMed] [Google Scholar]
- Young HM, Hearn CJ, Ciampoli D, Southwell BR, Brunet JF, Newgreen DF. A single rostrocaudal colonization of the rodent intestine by enteric neuron precursors is revealed by the expression of Pho2b, Ret, and p75 and by explants grown under the kidney capsule or in organ tissue. Developmental Biology. 1998;202:67–84. doi: 10.1006/dbio.1998.8987. [DOI] [PubMed] [Google Scholar]
- Zakharenko S, Popov S. Dynamics of axonal microtubules regulate the topology of new membrane insertion into the growing neurites. J Cell Biol. 1998;143:1077–86. doi: 10.1083/jcb.143.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Primers used for qRT-PCR and primers used to clone cDNA fragments for Hes6, Hoxb5, Hoxa5, Pcgf1, Metrn, Tbx3 in situ hybridization probe preparation.
Supplemental Table 2: Plasmids used for preparation of the in situ probes.
Supplemental Table 3: Genes identified by microarray analysis of E14 small bowel as 1.2-fold or more abundant in WT than in aganglionic bowel.
Supplemental Figure 1: Whole mount acetylcholinesterase and NADPH diaphorase staining of the ENS in adult α-N-catenin−/− and Protocadherin 15−/− mice. (A, B) Small bowel, esophagus and colon segments from mice deficient in α-N-catenin (A) or Protocadherin 15 (B) were stained using acetylcholinesterase or NADPH diaphorase methods. These representative images showing enteric ganglion cells and their fibers are similar to the appearance of the ENS in WT mice. Scale bar = 50 μm.


