Abstract
The assembly of functional neural circuits in the developing brain requires neurons to extend axons to the correct targets. This in turn requires the navigating tips of axons to respond appropriately to guidance cues present along the axonal pathway, despite being cellular ‘outposts' far from the soma. Work over the past few years has demonstrated a critical role for local translation within the axon in this process in vitro, making axon guidance another process that requires spatially localized translation, among others such as synaptic plasticity, cell migration, and cell polarity. This article reviews recent findings in local axonal translation and discusses how new protein synthesis may function in growth cone guidance, with a comparative view toward models of local translation in other systems.
Keywords: axon guidance, growth cone, RNA-binding protein, translation
Introduction
In the developing nervous system, growing axons are guided to their correct targets by a motile structure at their tips called the growth cone, which turns in response to attractive or repulsive cues in the extracellular environment. Much progress has been made on identifying guidance cues and their receptors, including the four ‘classical' families, the netrins, ephrins, slits, and semaphorins, as well as many others. However, relatively little is known about the intracellular signaling mechanisms by which growth cones convert these signals into directional decisions. Recent studies have revealed that local axonal translation is involved in this process, helping to overturn the prevailing dogma that axons are incapable of protein synthesis (Koenig and Giuditta, 1999; Giuditta et al, 2002; Piper and Holt, 2004; Twiss and van Minnen, 2006).
Guidance cues induce chemotropic responses in growth cones in vitro; for example, bath application of repellents causes growth cones to withdraw filopodia and ‘collapse', while gradients of guidance cues can cause either attractive or repulsive turning. Initial experiments revealed that axons isolated from their cell body still exhibit appropriate chemotropic responses in vitro to netrin-1 and Semaphorin3A, but these responses are blocked by protein synthesis inhibitors, indicating that local translation within the axon is required (Campbell and Holt, 2001). Later studies identified other guidance cues that also require local axonal translation to induce chemotropic responses: Slit2 (Piper et al, 2006), pituitary adenylate cyclase-activating peptide (PACAP) (Guirland et al, 2003), brain-derived neurotrophic factor (BDNF) (Yao et al, 2006), and Engrailed-2 (Brunet et al, 2005). The requirement for local translation is found in both Xenopus and mammals (Campbell and Holt, 2001; Wu et al, 2005), though not for all guidance cues (see below). Notably, guidance cues induce growth cone responses very quickly in vitro—collapse within 10 min, and early signs of axon turning within 15–20 min. Correspondingly, guidance cues induce translation quickly as well, with translation initiation activated within 5 min and significant radiolabeled amino acid incorporation within 10 min (Campbell and Holt, 2001). Moreover, local translation is not required for axon extension (Eng et al, 1999; Campbell and Holt, 2001), even over 48 h (Blackmore and Letourneau, 2007), suggesting a specific role in responding to guidance cues.
Local translation is also involved in growth cone adaptation during chemotaxis. Growth cones undergo cycles of desensitization and resensitization that serve to continuously reset their sensitivity as they ‘climb up' a gradient, and the resensitization step (but not desensitization) requires protein synthesis (Ming et al, 2002; Piper et al, 2005). In addition, axons are often directed to intermediate targets or ‘guideposts' (e.g., the midline) en route to their final destination, and in order to get there but not stay there, they need to switch from being attracted to the intermediate target to being repelled. Local synthesis of new receptors may be a mechanism to rapidly change their responsiveness to guidance cues for the next leg of their journey (Brittis et al, 2002). Finally, once growth cones arrive at the target, they must respond to synaptogenic signals from the target cell, such as BDNF. Here, too, BDNF-induced potentiation of presynaptic neurotransmitter release requires local axonal translation (Zhang and Poo, 2002). Beyond development, local axonal translation has a role in axonal regeneration (Zheng et al, 2001; Verma et al, 2005; Twiss and van Minnen, 2006; Willis and Twiss, 2006). These initial studies raised several questions: what proteins are synthesized in axons, how is their synthesis regulated, and why is their de novo synthesis important for growth cone chemotropic responses?
Cue-induced axonal translation
An emerging theme is that guidance cues induce rapid translation of cytoskeletal proteins or regulators based on whether they are attractive or repulsive: proteins induced by attractive cues build up the cytoskeleton, whereas proteins induced by repulsive cues break it down. For example, an attractive gradient of netrin-1 or BDNF induces asymmetrical translation of β-actin in axonal growth cones within 5 min (Leung et al, 2006; Yao et al, 2006). Attractive turning toward netrin-1 or BDNF is prevented by morpholino-based blockade of β-actin translation or de-regulation of β-actin translation by antisense binding to the β-actin 3′ untranslated region (UTR). In contrast, the repellent Slit2 induces a protein synthesis-dependent increase in growth cone cofilin-1, an actin depolymerizing factor, within 5 min (Piper et al, 2006). Another repellent, Semaphorin3A (Sema3A), induces axonal synthesis of the small GTPase RhoA, which is required for Sema3A-induced growth cone collapse (Wu et al, 2005). RhoA mediates neurite retraction through regulation of the actin cytoskeleton (Luo, 2000). Finally, local translation of β-thymosin, an actin monomer sequestering protein, reduces neurite length in Lymnaea neurons (van Kesteren et al, 2006).
Since both attractive and repulsive chemotropic responses can require protein synthesis (Campbell and Holt, 2001), the identity of proteins whose synthesis is activated may help determine the polarity of the response. By this model, attractive and repulsive turning are not ‘mirror-symmetrical' phenomena where attractants promote extension on the ‘near' side while repellents promote extension on the ‘far' side, rather, attractants and repellents both act on the near side, but have opposite effects (Figure 1). In a similar example of attractive and repulsive turning involving distinct mechanisms, recent findings indicate that attractive, but not repulsive, turning requires asymmetrical exocytosis (Tojima et al, 2007). However, it remains to be seen whether repulsive gradients induce asymmetrical synthesis of proteins like RhoA, cofilin-1, or β-thymosin.
Figure 1.
Hypothesis for cue-induced asymmetrical synthesis of cytoskeletal proteins. A guidance cue gradient causes an asymmetrical activation of translation initiation, ‘opening the gates' to translation asymmetrically. mRNAs are selected for translation according to whether the guidance cue is attractive or repulsive, which may also depend on the internal state of the growth cone. For an attractive guidance cue, proteins that promote actin assembly are asymmetrically synthesized (green dots), whereas for a repulsive guidance cue, proteins that promote actin disassembly (red dots) are asymmetrically synthesized.
In addition to rapid synthesis of cytoskeletal proteins for immediate chemotropic responses, axons also locally translate transmembrane and secreted proteins to modulate future responsiveness, most likely over a longer timescale. The EphA2 receptor is upregulated in the post-midline segment of chick commissural axons, as is a translational reporter controlled by the 3′UTR of EphA2, suggesting that signals at the midline induce axons to locally translate EphA2 to change responsiveness to guidance cues after crossing (Brittis et al, 2002). Recent studies show that dorsal root ganglion (DRG) neurons translate κ-opioid receptor locally in axons, in response to KCl depolarization (Bi et al, 2006), and at least in the cell body in response to netrin-1 (Tsai et al, 2006), although it remains unclear what function local modulation of opioid responsivity serves. Finally, after the axon arrives at the target, synapse formation between Aplysia sensory and motor neurons requires local translation in the presynaptic terminal of sensorin (Lyles et al, 2006), a secreted neuropeptide that regulates presynaptic growth and synapse stabilization in an autocrine response (Hu et al, 2004).
These findings present a general cell biological puzzle with regard to how new proteins are processed. As the growth cone travels to its target, it increases the distance between itself and the cell body and becomes a cytoplasmic ‘outpost' remote from the Golgi apparatus. Ultrastructural studies have not reported the classical signs of protein processing machinery in axons, such as membrane-bound ribosomes or Golgi stacks, although they have identified ribosomes and smooth endoplasmic reticulum (ER) (Tennyson, 1970; Yamada et al, 1971; Bunge, 1973; Zheng et al, 2001). However, ribosomes might be bound to membranes rarely, if axons only synthesize membrane proteins occasionally, and axonal protein export machinery may exhibit non-classical morphology, as is seen in the primitive eukaryote Giardia lamblia (Lujan et al, 1995). ER markers have been detected immunocytochemically in DRG axons (Willis et al, 2005) and both ER and Golgi markers are seen in Aplysia neurites (Lyles et al, 2006), but it is not yet clear whether these markers are localized to intracellular membranes. Moreover, vertebrates show sharper distinctions between axons and dendrites than invertebrates, and there are as yet no published accounts of axonal Golgi complex in vertebrates.
Regulation of translation in the growth cone
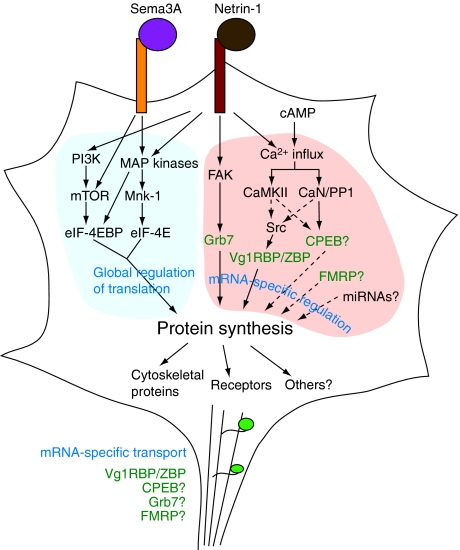
Protein synthesis can be regulated at a global level by translation initiation, and at an mRNA-specific level by transport, repression, and activation by RNA-binding proteins and microRNAs. We suggest that guidance cues activate translation initiation to ‘open the gates' to translation, and use mRNA-specific regulation by RNA-binding proteins and microRNAs to select the appropriate proteins to synthesize for a given guidance cue response (Figures 1 and 2).
Figure 2.
A model for regulation of translation in axonal growth cones. Guidance cues induce global activation of cap-dependent translation by activating translation initiation factors (left, ‘global activation of translation'). This activation is largely ‘permissive,' as translation of most mRNAs is also controlled by RNA-binding proteins and possibly microRNAs. By regulating these factors, different guidance cues—modulated by the internal state of the growth cone (e.g., cAMP levels)—can cause different effects by activating the translation of different mRNAs (right, ‘mRNA-specific regulation'). Translation is also regulated by the differential transport of specific mRNAs to the growth cone, which is also controlled by RNA-binding proteins (bottom, ‘mRNA-specific transport'). Dotted lines indicate hypothetical connections.
Global regulation of translation is achieved by translation initiation factors. Eukaryotic initiation factor 4E (eIF-4E) binds the 5′ cap of mRNAs and is the rate-limiting factor for cap-dependent translation. Hypophosphorylated eIF-4E-binding protein (eIF-4EBP) sequesters eIF-4E, preventing the recruitment of the rest of the translation initiation complex, while phosphorylation of eIF-4EBP releases eIF-4E, thus activating translation (Gebauer and Hentze, 2004). Netrin-1 and Sema3A induce phosphorylation of eIF-4EBP via MAP kinases and mammalian target of rapamycin (mTOR) (Campbell and Holt, 2001, 2003), and a netrin-1 gradient induces asymmetrical eIF-4EBP phosphorylation (Leung et al, 2006). Guidance cues also activate eIF-4E by phosphorylation, via MAP kinases and Mnk-1 (Campbell and Holt, 2003; Piper et al, 2006). Similar mechanisms have been described in local dendritic translation (Klann and Dever, 2004). Once translation is activated, how does the growth cone select which mRNAs to translate?
mRNA-specific regulation is achieved in part by which mRNAs are present in the growth cone. Neurotrophins induce specific transport of β-actin, peripherin, and vimentin mRNAs into axonal growth cones (Zhang et al, 1999, 2001; Willis et al, 2005). mRNA localization is even regulated within the growth cone, a structure only 5–10 μm across: a gradient of BDNF induces asymmetrical localization of β-actin mRNA to the side experiencing more BDNF (Yao et al, 2006). mRNAs are typically localized by cis-acting sequences at their 5′ or 3′UTRs. β-actin mRNA localization is controlled by a so-called ‘zipcode' in the 3′UTR (Kislauskis et al, 1994), and the RhoA 3′UTR drives axonal localization of a reporter mRNA (Wu et al, 2005).
mRNA transport and translation are coupled and regulated by RNA-binding proteins, which transport mRNAs in ‘granules', large ribonucleoprotein (RNP) complexes that hold mRNAs repressed at the initiation or elongation stage (Sossin and DesGroseillers, 2006). To activate translation of the transported mRNA, for example, in response to guidance cues, RNA-binding proteins typically release the RNA cargo to polysomes (Krichevsky and Kosik, 2001). Currently, the best studied axonal RNA-binding protein is zipcode-binding protein (ZBP), which binds to β-actin mRNA through the 3′UTR zipcode (Ross et al, 1997). The Xenopus homolog of ZBP, Vg1RBP, colocalizes with β-actin mRNA in axons, and moves asymmetrically within growth cones upon exposure to a netrin-1 or BDNF gradient (Leung et al, 2006; Yao et al, 2006). Biochemically, ZBP has been shown to repress β-actin translation until Src phosphorylates it, making it release β-actin mRNA, and thus activating β-actin translation (Huttelmaier et al, 2005). However, in vivo, BDNF actually increases ZBP colocalization with β-actin mRNA in growth cones, concomitant with an increase in β-actin translation (Yao et al, 2006); it may be that ZBP remains associated with translating polysomes after releasing its repressive grip on β-actin mRNA.
Several other RNA-binding proteins have also been proposed to regulate axonal translation. Grb7 represses κ-opioid receptor translation by binding to the 5′UTR of kor mRNA; netrin-1 stimulation causes focal adhesion kinase to phosphorylate Grb7, which then releases kor mRNA, allowing κ-opioid receptor translation (Tsai et al, 2007). In addition, axonal translation of EphA2 receptor requires a cytoplasmic polyadenylation element (CPE) sequence (UUUUUAU) in the 3′UTR (Brittis et al, 2002), suggesting a role for CPE-binding protein (CPEB), which has been shown to regulate dendritic translation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Richter, 2007). Finally, the RNA-binding protein Fragile X Mental Retardation Protein (FMRP) has recently been found in axonal growth cones, where it is required for growth cone motility (Antar et al, 2006). FMRP regulates local translation in dendrites (Zalfa et al, 2006) and the 3′UTR of RhoA mRNA contains a possible binding site for FMRP (Wu et al, 2005).
RNA interference (RNAi) and microRNAs may also play a role in mRNA-specific regulation of axonal translation. Functional RNAi machinery is present in axons (Hengst et al, 2006) and the RhoA 3′UTR contains miRNA-binding sequences (Wu et al, 2005). In addition, miRNAs have been implicated in regulating local translation in dendrites, a system conceptually similar to axonal growth cones (see below). The microRNA miR-134 regulates dendritic spine morphology by inhibiting translation of Lim kinase 1, a protein that regulates actin dynamics by inhibiting cofilin (Schratt et al, 2006). In Drosophila, CaMKII translation in olfactory projection neuron dendrites is suppressed by miRNAs, until neuronal activity stimulates proteasomal degradation of RNA-induced silencing complex (RISC) proteins, relieving repression of CaMKII translation (Ashraf et al, 2006). RNAi is partly carried out in distinct RNA granules called processing bodies, or P bodies, which degrade mRNAs and store repressed mRNAs (Anderson and Kedersha, 2006); it is not yet clear whether axonal growth cones contain P bodies.
How might attractive versus repulsive guidance cues induce translation of different proteins? One hint comes from attraction and repulsion induced by Ca2+ release, a candidate integrator of growth cone signals. Artificial asymmetrical elevation of Ca2+ levels causes growth cone attraction or repulsion if the Ca2+ release is moderate or small, respectively (Zheng, 2000; Henley and Poo, 2004; Gomez and Zheng, 2006). Likewise, Ca2+-dependent guidance cues like netrin-1, BDNF, and myelin-associated glycoprotein (MAG) induce Ca2+ release in the growth cone, and the polarity of the growth cone's response can be reversed by modulating the size of this Ca2+ release (Hong et al, 2000; Henley et al, 2004). Indeed, polarity reversals induced by substrate molecules (Hopker et al, 1999) and cyclic nucleotides (Song et al, 1998) appear to act through modulation of Ca2+-induced Ca2+ release from internal stores (Ooashi et al, 2005). Attraction induced by moderate Ca2+ release is mediated by CaMKII, while repulsion induced by small Ca2+ release is mediated by calcineurin (CaN)-phosphatase-1 (PP1) (Wen et al, 2004), suggesting that the balance of activity between CaMKII and CaN/PP1 may act as a ‘switch' between attraction and repulsion. Interestingly, in hippocampal neurons, CaMKII phosphorylates CPEB and thereby activates CPE-dependent translation (Atkins et al, 2004), and during cell cycle progression, the CPEB-binding translational inhibitor maskin is dephosphorylated by CaN, inducing translational repression (Cao et al, 2006), raising the intriguing possibility that selective translation of CPE-containing mRNAs may mediate the Ca2+ ‘switch'. Also, Src can be asymmetrically activated or inhibited by attractive or repulsive Ca2+-dependent cues, respectively (Yao et al, 2006), suggesting that Vg1RBP/ZBP and its target mRNAs, like β-actin, could help mediate the Ca2+ ‘switch'. These conjectures are supported by findings that protein synthesis is required downstream of Ca2+ influx for both attractive and repulsive Ca2+-induced turning (Yao et al, 2006). However, it should be noted that some guidance cues that act through local translation, such as Sema3A and PACAP, do not rely on Ca2+ (Song et al, 1998; Guirland et al, 2003; Wen et al, 2004).
Why is local translation used for growth cone turning?
Local axonal translation as a mechanism for growth cone guidance may be puzzling at first glance. To cite one example, β-actin translation seems unlikely to have a substantial impact on actin polymerization, given that in migrating fibroblasts the rate of β-actin translation is only 7% or less of the rate of consumption of actin monomers by actin polymerization (Condeelis and Singer, 2005), and given the large supply of pre-existing actin monomers and the varied array of actin-binding proteins that regulate actin polymerization (dos Remedios et al, 2003). In this section, we propose possible rationales for local axonal translation.
Macromolecular crowding and protein turnover
Why regulate protein activity by translation rather than post-translational modulations like phosphorylation? From a strictly theoretical standpoint, cells have limited volume, and it has been estimated that 20–30% of that volume is occupied by macromolecules (Ellis, 2001); further crowding might slow diffusion or alter reaction rates unacceptably. Since an mRNA can be a template for theoretically unlimited translation, it may be more efficient in the face of this biophysical limit to store mRNA rather than inactive proteins. Indeed, netrin-1-induced turning requires both translation and proteasomal protein degradation (Campbell and Holt, 2001), suggesting a constant turnover of proteins that tightly regulates the levels of specific proteins. A similar recycling of proteins may occur in synaptic plasticity: translation inhibitors and proteasomal inhibitors each block long-term potentiation (LTP), while both applied together do not (Fonseca et al, 2006).
RNA flexibility
In addition, regulation of proteins by mRNA translation rather than protein modification provides more flexibility, because the activity of a protein can be regulated by arbitrary mRNA sequences rather than constituent domains of the protein. Indeed, proteins do not always contain the information necessary for their localization (see discussion of tau, below). Moreover, alternative splicing can create mRNAs with different regulatory sequences. Cytoplasmic mRNA splicing has been demonstrated in anucleate platelets (Denis et al, 2005) and isolated dendrites (Glanzer et al, 2005). One can speculate that axonal mRNA splicing might provide an additional layer of regulation for axonally translated proteins.
Decentralization
A corollary of the idea that proteins sometimes need to be regulated at the mRNA translation level is that proteins should be formed locally. Axonal growth cones are often far from the cell body, and it would be temporally and energetically inefficient to wait for protein delivery from the soma, not to mention that in very long axons, the protein might not even survive the journey (Alvarez et al, 2000). Indeed, growth cones can navigate correctly even when the soma has been removed, both in vivo and in vitro (Harris et al, 1987; Campbell and Holt, 2001), suggesting that the ‘devolution' of decision making from the soma to the growth cone is a likely function for local axonal translation.
Axonal fate
Local translation has long been known to play a role in cell polarity, for example in anterior–posterior axis determination in Drosophila oocytes (Johnstone and Lasko, 2001). In this case, local translation is important for localizing transcription factors and hence for fate determination in daughter cells. However, one may also consider polarity in differentiated cells as ‘fate determination' of cellular compartments, for example in specifying neurites as axons or dendrites. Axonal targeting of tau mRNA by its 3′UTR is required for axonal targeting of tau protein (Aronov et al, 2001). Tau binds to microtubules and promotes microtubule assembly (Johnson and Stoothoff, 2004), and plays a role in forming and maintaining an axonal phenotype (Caceres and Kosik, 1990), perhaps by inducing specifically axonal microtubule organization. As tau associates with all microtubules, axonal translation of tau mRNA may be required to prevent mislocalization of nascent tau protein and hence disruption of neuronal polarity (Aronov et al, 2001). This suggests that other axonally translated proteins may also be required for the expression or maintenance of axonal (rather than dendritic) fate.
‘Microdomains' and asymmetry
In the case of β-actin or other cytoskeletal proteins, the large amount of pre-existing protein suggests that local translation of cytoskeletal proteins regulates not the presence or absence of protein, but site of translation. This is supported by findings that guidance cue gradients induce asymmetrical translation of β-actin (Leung et al, 2006; Yao et al, 2006), and that local translation is required for directional turning, not elongation (Campbell and Holt, 2001). The rate-limiting step in actin polymerization is nucleation, and the concentrated local synthesis of β-actin in a confined cellular compartment could contribute to actin nucleation (see also next paragraph). Asymmetrical actin nucleation would lead to asymmetrical filopodial and lamellopodial protrusion and eventually turning. A similar mechanism has been proposed for β-actin translation at the leading edge of motile cells (Shestakova et al, 2001; Condeelis and Singer, 2005), a system intuitively akin to motile growth cones (Figure 3). Interestingly, it has been suggested that the source of Ca2+ influx—through the plasma membrane or from internal stores—controls the polarity of the growth cone response (Ooashi et al, 2005), and Gomez and Zheng (2006) have highlighted the potential importance of Ca2+ ‘microdomains,' local Ca2+ signals generated by a cluster of Ca2+ channels, where the Ca2+ sensor is less than 1 μm from the Ca2+ channels. It can be envisaged that Ca2+ microdomains regulate similar microdomains of protein synthesis.
Figure 3.
Comparison of models of stimulus-induced local translation in axon guidance, cell migration, and synaptic plasticity. mRNAs are transported to and within the growth cone (A), to the leading edge of migrating cells (B), and into dendrites and dendritic spines (C). Impinging signals stimulate translation of specific mRNAs, resulting in the formation of new proteins (green dots) in the appropriate location, thus changing the morphology or function of a localized subcellular compartment. Note that local translation occurs on a similar spatial scale across these systems, in subcellular compartments of the order of microns.
Distinct properties of nascent proteins
Nascent proteins are presumably free of post-translational modifications that may mark ‘older' proteins. For example, β-actin can be arginylated, which prevents actin filament clustering (Karakozova et al, 2006), or glutathionylated, which restricts actin polymerization (Wang et al, 2001). However, both arginylation and glutathionylation are thought to be reversible, like most post-translational modifications, and it is unclear why cells should make new proteins rather than simply removing post-translational modifications, as with dephosphorylation. New proteins should also lack the random oxidative damage that presumably accumulates on older proteins; however, the fact that axon outgrowth does not require local axonal translation suggests that proteins transported from the cell body are ‘fresh' enough to function properly.
A more conceptually appealing possibility lies in chaperones, which associate with nascent proteins to assist proper folding (Hartl and Hayer-Hartl, 2002). The chaperone prefoldin aids β-actin folding by stabilizing intermediate folding states and transfers nascent β-actin to cytosolic chaperonin (CCT) (Hansen et al, 1999), which also catalyzes β-actin folding (Pappenberger et al, 2006) and associates with F-actin (Grantham et al, 2002). It has been suggested that CCT binding is required to aid actin polymerization by stabilizing vulnerable intermediates between the monomeric and polymerized states and protecting them from inappropriate aggregation in the crowded in vivo environment (Grantham et al, 2002). This model could explain why new translation of β-actin is required in cell motility and growth cone turning. Intriguingly, several chaperones are locally translated in injury-conditioned DRG axons (Willis et al, 2005). Perhaps analogous to local dendritic synthesis of translation factors to increase dendritic translation capacity (Tsokas et al, 2005), local synthesis of chaperones may support the continued function of newly translated proteins.
Comparison with local dendritic translation and synaptic plasticity
At the other end of the neuron in the post-synaptic compartment, dendritic protein synthesis and its role in synaptic plasticity has been the focus of intense study over the last 20 years (Sutton and Schuman, 2005). Many informative parallels can be drawn with axonal protein synthesis, as both axonal growth cones and dendritic spines are cellular compartments distant from the soma that need to respond quickly and autonomously to impinging signals. Just as axonal growth cones change shape in response to guidance cues to turn and guide the axon, dendritic spines change shape in response to stimulation to modulate synaptic efficacy (Figure 3), a process that involves local translation of proteins such as FMRP, Lim kinase, CaMKII, β-actin, and postsynaptic density-95 (Grossman et al, 2006; Schratt et al, 2006). Long-term potentiation and depression (LTP and LTD) might be considered analogous to attractive and repulsive turning, and a Ca2+-dependent kinase/phosphatase balance similar to that suggested above in growth cones has been proposed to underlie the switch between LTP and LTD (Lisman and Zhabotinsky, 2001). In addition, translation-dependent re-sensitization of axonal growth cones (Ming et al, 2002; Piper et al, 2005) resembles translation-dependent homeostatic regulation of synaptic strength by mini-EPSP frequency (Sutton et al, 2006), suggesting that local translation is a general mechanism for maintaining a dynamic range of responsivity. Beyond potential parallels in axonal and dendritic translation already described, other regulatory mechanisms described in dendrites will likely prove important in axons as well, such as regulation of internal ribosomal entry (Pinkstaff et al, 2001) and mRNA stability (Perrone-Bizzozero and Bolognani, 2002).
Axonal translation may provide insights for synaptic plasticity as well. Studies on local translation in synaptic plasticity in vertebrates have hitherto focused on the post-synaptic side. However, activity-dependent refinement of synapses requires presynaptic responses to retrograde signals like BDNF (Schmidt, 2004). In vitro synapse formation in Aplysia (Lyles et al, 2006) and Xenopus (Zhang and Poo, 2002) requires translation in the presynaptic compartment. Moreover, recent evidence suggests that presynaptic translation may be required for LTD at the corticostriatal synapse in brain slices cut to exclude pre-synaptic cell bodies, although glial protein synthesis has not been excluded (Yin et al, 2006). Although the focus on axonal translation thus far has been on developing axons, adult mammalian axons are also capable of protein synthesis (Piper and Holt, 2004). In light of these initial studies, it may be time to consider a role in synaptic plasticity for local translation in mature vertebrate presynaptic terminals.
Future directions and questions
Future work on axonal translation will be aided by new tools to block translation of specific genes of interest in axons only, such as microfluidic compartmentalized cultures (Taylor et al, 2005) and axonal application of siRNA (Hengst et al, 2006). Axonal translation can be visualized using photoconvertible fluorescent reporters such as Kaede (Leung et al, 2006; Raab-Graham et al, 2006), or tetracysteine tags and the biarsenial dyes FlAsH and ReAsH (Rodriguez et al, 2006). It will be important, although technically challenging due to the small amounts of material obtainable from axons, to develop screens to uncover whole populations of proteins translated in response to specific guidance cues, which may form ‘functionally coherent' groups (Ule and Darnell, 2006), such as ‘attractive' or ‘repulsive' proteins. If such functionally coherent groups exist, it will be interesting to see whether each group is associated with a specific RNA-binding protein. It will also be interesting to investigate whether axons contain distinct types of RNA granules such as stress granules and P bodies (Kiebler and Bassell, 2006), and what roles these diverse granules may play in axon guidance.
It is unclear why guidance cues that appear to have the same effect have different requirements for protein synthesis. For example, Sema3A, lysophosphatidic acid, and EphB2 all cause retinal growth cone collapse, but only Sema3A requires local translation (Campbell and Holt, 2001; Mann et al, 2003). Moreover, RhoA activity is also required for translation-independent LPA-induced neurite retraction (Yuan et al, 2003), suggesting that RhoA does not necessarily have to be translated to induce collapse. One possible rationale is that translation-requiring guidance cues might take higher (or lower) priority than non-translation-requiring guidance cues, when growth cones encounter multiple guidance cues at once. Another explanation is that growth cones in vitro have a limited repertoire of ‘behaviors'—turning, outgrowth, collapse, branching—making in vitro assays too crude to distinguish specific in vivo behaviors that differentially require protein synthesis. Local translation may be required for behaviors such as topographic mapping and target selection, which do not have obvious in vitro analogies. Although recent studies have identified roles for translation and translational regulators in axon guidance and arborization in vivo in Drosophila (Chihara et al, 2007; Lee et al, 2007), the role of local axonal translation in axon guidance in vivo remains unknown. Answering this important question will require new tools such as translational inhibitors that act only in axons. A final area of interest is to understand what special, functionally important properties new, axonally synthesized proteins have, a question for which this review has provided only speculative answers.
References
- Alvarez J, Giuditta A, Koenig E (2000) Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype, with a critique of slow transport theory. Prog Neurobiol 62: 1–62 [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N (2006) RNA granules. J Cell Biol 172: 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ (2006) Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci 32: 37–48 [DOI] [PubMed] [Google Scholar]
- Aronov S, Aranda G, Behar L, Ginzburg I (2001) Axonal tau mRNA localization coincides with tau protein in living neuronal cells and depends on axonal targeting signal. J Neurosci 21: 6577–6587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf SI, McLoon AL, Sclarsic SM, Kunes S (2006) Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell 124: 191–205 [DOI] [PubMed] [Google Scholar]
- Atkins CM, Nozaki N, Shigeri Y, Soderling TR (2004) Cytoplasmic polyadenylation element binding protein-dependent protein synthesis is regulated by calcium/calmodulin-dependent protein kinase II. J Neurosci 24: 5193–5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J, Tsai NP, Lin YP, Loh HH, Wei LN (2006) Axonal mRNA transport and localized translational regulation of {kappa}-opioid receptor in primary neurons of dorsal root ganglia. Proc Natl Acad Sci USA 26: 9743–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore M, Letourneau PC (2007) Protein synthesis in distal axons is not required for axon growth in the embryonic spinal cord. Dev Neurobiol 67: 976–986 [DOI] [PubMed] [Google Scholar]
- Brittis PA, Lu Q, Flanagan JG (2002) Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell 110: 223–235 [DOI] [PubMed] [Google Scholar]
- Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris W, Prochiantz A, Holt C (2005) The transcription factor Engrailed-2 guides retinal axons. Nature 438: 94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge MB (1973) Fine structure of nerve fibers and growth cones of isolated sympathetic neurons in culture. J Cell Biol 56: 713–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A, Kosik KS (1990) Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature 343: 461–463 [DOI] [PubMed] [Google Scholar]
- Campbell DS, Holt CE (2001) Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 32: 1013–1026 [DOI] [PubMed] [Google Scholar]
- Campbell DS, Holt CE (2003) Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron 37: 939–952 [DOI] [PubMed] [Google Scholar]
- Cao Q, Kim JH, Richter JD (2006) CDK1 and calcineurin regulate Maskin association with eIF4E and translational control of cell cycle progression. Nat Struct Mol Biol 13: 1128–1134 [DOI] [PubMed] [Google Scholar]
- Chihara T, Luginbuhl D, Luo L (2007) Cytoplasmic and mitochondrial protein translation in axonal and dendritic terminal arborization. Nat Neurosci 10: 828–837 [DOI] [PubMed] [Google Scholar]
- Condeelis J, Singer RH (2005) How and why does beta-actin mRNA target? Biol Cell 97: 97–110 [DOI] [PubMed] [Google Scholar]
- Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, Tolley E, Kraiss LW, McIntyre TM, Zimmerman GA, Weyrich AS (2005) Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell 122: 379–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ (2003) Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev 83: 433–473 [DOI] [PubMed] [Google Scholar]
- Ellis RJ (2001) Macromolecular crowding: obvious but underappreciated. Trends Biochem Sci 26: 597–604 [DOI] [PubMed] [Google Scholar]
- Eng H, Lund K, Campenot RB (1999) Synthesis of beta-tubulin, actin, and other proteins in axons of sympathetic neurons in compartmented cultures. J Neurosci 19: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca R, Nagerl UV, Bonhoeffer T (2006) Neuronal activity determines the protein synthesis dependence of long-term potentiation. Nat Neurosci 9: 478–480 [DOI] [PubMed] [Google Scholar]
- Gebauer F, Hentze MW (2004) Molecular mechanisms of translational control. Nat Rev Mol Cell Biol 5: 827–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuditta A, Kaplan BB, van Minnen J, Alvarez J, Koenig E (2002) Axonal and presynaptic protein synthesis: new insights into the biology of the neuron. Trends Neurosci 25: 400–404 [DOI] [PubMed] [Google Scholar]
- Glanzer J, Miyashiro KY, Sul JY, Barrett L, Belt B, Haydon P, Eberwine J (2005) RNA splicing capability of live neuronal dendrites. Proc Natl Acad Sci USA 102: 16859–16864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TM, Zheng JQ (2006) The molecular basis for calcium-dependent axon pathfinding. Nat Rev Neurosci 7: 115–125 [DOI] [PubMed] [Google Scholar]
- Grantham J, Ruddock LW, Roobol A, Carden MJ (2002) Eukaryotic chaperonin containing T-complex polypeptide 1 interacts with filamentous actin and reduces the initial rate of actin polymerization in vitro. Cell Stress Chaperones 7: 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AW, Aldridge GM, Weiler IJ, Greenough WT (2006) Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci 26: 7151–7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirland C, Buck KB, Gibney JA, DiCicco-Bloom E, Zheng JQ (2003) Direct cAMP signaling through G-protein-coupled receptors mediates growth cone attraction induced by pituitary adenylate cyclase-activating polypeptide. J Neurosci 23: 2274–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen WJ, Cowan NJ, Welch WJ (1999) Prefoldin-nascent chain complexes in the folding of cytoskeletal proteins. J Cell Biol 145: 265–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WA, Holt CE, Bonhoeffer F (1987) Retinal axons with and without their somata, growing to and arborizing in the tectum of Xenopus embryos: a time-lapse video study of single fibres in vivo. Development 101: 123–133 [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295: 1852–1858 [DOI] [PubMed] [Google Scholar]
- Hengst U, Cox LJ, Macosko EZ, Jaffrey SR (2006) Functional and selective RNA interference in developing axons and growth cones. J Neurosci 26: 5727–5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley J, Poo MM (2004) Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol 14: 320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JR, Huang KH, Wang D, Poo MM (2004) Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron 44: 909–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M (2000) Calcium signalling in the guidance of nerve growth by netrin-1. Nature 403: 93–98 [DOI] [PubMed] [Google Scholar]
- Hopker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C (1999) Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature 401: 69–73 [DOI] [PubMed] [Google Scholar]
- Hu JY, Goldman J, Wu F, Schacher S (2004) Target-dependent release of a presynaptic neuropeptide regulates the formation and maturation of specific synapses in Aplysia. J Neurosci 24: 9933–9943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttelmaier S, Zenklusen D, Lederer M, Dictenberg J, Lorenz M, Meng X, Bassell GJ, Condeelis J, Singer RH (2005) Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature 438: 512–515 [DOI] [PubMed] [Google Scholar]
- Johnson GV, Stoothoff WH (2004) Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci 117: 5721–5729 [DOI] [PubMed] [Google Scholar]
- Johnstone O, Lasko P (2001) Translational regulation and RNA localization in Drosophila oocytes and embryos. Annu Rev Genet 35: 365–406 [DOI] [PubMed] [Google Scholar]
- Karakozova M, Kozak M, Wong CC, Bailey AO, Yates JR III, Mogilner A, Zebroski H, Kashina A (2006) Arginylation of beta-actin regulates actin cytoskeleton and cell motility. Science 313: 192–196 [DOI] [PubMed] [Google Scholar]
- Kiebler MA, Bassell GJ (2006) Neuronal RNA granules: movers and makers. Neuron 51: 685–690 [DOI] [PubMed] [Google Scholar]
- Kislauskis EH, Zhu X, Singer RH (1994) Sequences responsible for intracellular localization of beta-actin messenger RNA also affect cell phenotype. J Cell Biol 127: 441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann E, Dever TE (2004) Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci 5: 931–942 [DOI] [PubMed] [Google Scholar]
- Koenig E, Giuditta A (1999) Protein-synthesizing machinery in the axon compartment. Neuroscience 89: 5–15 [DOI] [PubMed] [Google Scholar]
- Krichevsky AM, Kosik KS (2001) Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron 32: 683–696 [DOI] [PubMed] [Google Scholar]
- Lee S, Nahm M, Lee M, Kwon M, Kim E, Zadeh AD, Cao H, Kim HJ, Lee ZH, Oh SB, Yim J, Kolodziej PA, Lee S (2007) The F-actin-microtubule crosslinker Shot is a platform for Krasavietz-mediated translational regulation of midline axon repulsion. Development 134: 1767–1777 [DOI] [PubMed] [Google Scholar]
- Leung KM, van Horck FP, Lin AC, Allison R, Standart N, Holt CE (2006) Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat Neurosci 9: 1247–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Zhabotinsky AM (2001) A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron 31: 191–201 [DOI] [PubMed] [Google Scholar]
- Lujan HD, Marotta A, Mowatt MR, Sciaky N, Lippincott-Schwartz J, Nash TE (1995) Developmental induction of Golgi structure and function in the primitive eukaryote Giardia lamblia. J Biol Chem 270: 4612–4618 [DOI] [PubMed] [Google Scholar]
- Luo L (2000) Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci 1: 173–180 [DOI] [PubMed] [Google Scholar]
- Lyles V, Zhao Y, Martin KC (2006) Synapse formation and mRNA localization in cultured Aplysia neurons. Neuron 49: 349–356 [DOI] [PubMed] [Google Scholar]
- Mann F, Miranda E, Weinl C, Harmer E, Holt CE (2003) B-type Eph receptors and ephrins induce growth cone collapse through distinct intracellular pathways. J Neurobiol 57: 323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC, Poo MM (2002) Adaptation in the chemotactic guidance of nerve growth cones. Nature 417: 411–418 [DOI] [PubMed] [Google Scholar]
- Ooashi N, Futatsugi A, Yoshihara F, Mikoshiba K, Kamiguchi H (2005) Cell adhesion molecules regulate Ca2+-mediated steering of growth cones via cyclic AMP and ryanodine receptor type 3. J Cell Biol 170: 1159–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenberger G, McCormack EA, Willison KR (2006) Quantitative actin folding reactions using yeast CCT purified via an internal tag in the CCT3/gamma subunit. J Mol Biol 360: 484–496 [DOI] [PubMed] [Google Scholar]
- Perrone-Bizzozero N, Bolognani F (2002) Role of HuD and other RNA-binding proteins in neural development and plasticity. J Neurosci Res 68: 121–126 [DOI] [PubMed] [Google Scholar]
- Pinkstaff JK, Chappell SA, Mauro VP, Edelman GM, Krushel LA (2001) Internal initiation of translation of five dendritically localized neuronal mRNAs. Proc Natl Acad Sci USA 98: 2770–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Anderson R, Dwivedy A, Weinl C, van Horck F, Leung KM, Cogill E, Holt C (2006) Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron 49: 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Holt C (2004) RNA translation in axons. Annu Rev Cell Dev Biol 20: 505–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Salih S, Weinl C, Holt CE, Harris WA (2005) Endocytosis-dependent desensitization and protein synthesis-dependent resensitization in retinal growth cone adaptation. Nat Neurosci 8: 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Graham KF, Haddick PC, Jan YN, Jan LY (2006) Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science 314: 144–148 [DOI] [PubMed] [Google Scholar]
- Richter JD (2007) CPEB: a life in translation. Trends Biochem Sci 32: 279–285 [DOI] [PubMed] [Google Scholar]
- Rodriguez AJ, Shenoy SM, Singer RH, Condeelis J (2006) Visualization of mRNA translation in living cells. J Cell Biol 175: 67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AF, Oleynikov Y, Kislauskis EH, Taneja KL, Singer RH (1997) Characterization of a beta-actin mRNA zipcode-binding protein. Mol Cell Biol 17: 2158–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JT (2004) Activity-driven sharpening of the retinotectal projection: the search for retrograde synaptic signaling pathways. J Neurobiol 59: 114–133 [DOI] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME (2006) A brain-specific microRNA regulates dendritic spine development. Nature 439: 283–289 [DOI] [PubMed] [Google Scholar]
- Shestakova EA, Singer RH, Condeelis J (2001) The physiological significance of beta -actin mRNA localization in determining cell polarity and directional motility. Proc Natl Acad Sci USA 98: 7045–7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M (1998) Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 281: 1515–1518 [DOI] [PubMed] [Google Scholar]
- Sossin WS, DesGroseillers L (2006) Intracellular trafficking of RNA in neurons. Traffic 7: 1581–1589 [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM (2006) Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125: 785–799 [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM (2005) Local translational control in dendrites and its role in long-term synaptic plasticity. J Neurobiol 64: 116–131 [DOI] [PubMed] [Google Scholar]
- Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL (2005) A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Methods 2: 599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennyson VM (1970) The fine structure of the axon and growth cone of the dorsal root neuroblast of the rabbit embryo. J Cell Biol 44: 62–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojima T, Akiyama H, Itofusa R, Li Y, Katayama H, Miyawaki A, Kamiguchi H (2007) Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat Neurosci 10: 58–66 [DOI] [PubMed] [Google Scholar]
- Tsai NP, Bi J, Loh HH, Wei LN (2006) Netrin-1 signaling regulates de novo protein synthesis of kappa opioid receptor by facilitating polysomal partition of its mRNA. J Neurosci 26: 9743–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai NP, Bi J, Wei LN (2007) The adaptor Grb7 links netrin-1 signaling to regulation of mRNA translation. EMBO J 26: 1522–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P, Grace EA, Chan P, Ma T, Sealfon SC, Iyengar R, Landau EM, Blitzer RD (2005) Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci 25: 5833–5843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twiss JL, van Minnen J (2006) New insights into neuronal regeneration: the role of axonal protein synthesis in pathfinding and axonal extension. J Neurotrauma 23: 295–308 [DOI] [PubMed] [Google Scholar]
- Ule J, Darnell RB (2006) RNA binding proteins and the regulation of neuronal synaptic plasticity. Curr Opin Neurobiol 16: 102–110 [DOI] [PubMed] [Google Scholar]
- van Kesteren RE, Carter C, Dissel HM, van Minnen J, Gouwenberg Y, Syed NI, Spencer GE, Smit AB (2006) Local synthesis of actin-binding protein beta-thymosin regulates neurite outgrowth. J Neurosci 26: 152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P, Chierzi S, Codd AM, Campbell DS, Meyer RL, Holt CE, Fawcett JW (2005) Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci 25: 331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, Mieyal JJ, Chock PB (2001) Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem 276: 47763–47766 [DOI] [PubMed] [Google Scholar]
- Wen Z, Guirland C, Ming GL, Zheng JQ (2004) A CaMKII/calcineurin switch controls the direction of Ca(2+)-dependent growth cone guidance. Neuron 43: 835–846 [DOI] [PubMed] [Google Scholar]
- Willis D, Li KW, Zheng JQ, Chang JH, Smit A, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL (2005) Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci 25: 778–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, Twiss JL (2006) The evolving roles of axonally synthesized proteins in regeneration. Curr Opin Neurobiol 16: 111–118 [DOI] [PubMed] [Google Scholar]
- Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, Jaffrey SR (2005) Local translation of RhoA regulates growth cone collapse. Nature 436: 1020–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Spooner BS, Wessells NK (1971) Ultrastructure and function of growth cones and axons of cultured nerve cells. J Cell Biol 49: 614–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Sasaki Y, Wen Z, Bassell GJ, Zheng JQ (2006) An essential role for beta-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nat Neurosci 9: 1265–1273 [DOI] [PubMed] [Google Scholar]
- Yin HH, Davis MI, Ronesi JA, Lovinger DM (2006) The role of protein synthesis in striatal long-term depression. J Neurosci 26: 11811–11820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan XB, Jin M, Xu X, Song YQ, Wu CP, Poo MM, Duan S (2003) Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat Cell Biol 5: 38–45 [DOI] [PubMed] [Google Scholar]
- Zalfa F, Achsel T, Bagni C (2006) mRNPs, polysomes or granules: FMRP in neuronal protein synthesis. Curr Opin Neurobiol 16: 265–269 [DOI] [PubMed] [Google Scholar]
- Zhang HL, Eom T, Oleynikov Y, Shenoy SM, Liebelt DA, Dictenberg JB, Singer RH, Bassell GJ (2001) Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron 31: 261–275 [DOI] [PubMed] [Google Scholar]
- Zhang HL, Singer RH, Bassell GJ (1999) Neurotrophin regulation of beta-actin mRNA and protein localization within growth cones. J Cell Biol 147: 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Poo MM (2002) Localized synaptic potentiation by BDNF requires local protein synthesis in the developing axon. Neuron 36: 675–688 [DOI] [PubMed] [Google Scholar]
- Zheng JQ (2000) Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature 403: 89–93 [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Kelly TK, Chang B, Ryazantsev S, Rajasekaran AK, Martin KC, Twiss JL (2001) A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci 21: 9291–9303 [DOI] [PMC free article] [PubMed] [Google Scholar]